Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a progressive pulmonary vascular disease characterized by pulmonary artery stenosis or obstructions resulting from insufficient thrombus resolution. Chemokine (C‐X‐C motif) ligand 10 (CXCL10) is a chemokine that contributes to the pathogenesis of many autoimmune diseases and cancers. The present study aims to investigate the levels of CXCL10 in patients with CTEPH throughout balloon pulmonary angioplasty (BPA) and its correlation with the improvement of pulmonary hemodynamics. Plasma CXCL10 levels were measured in 38 CTEPH patients with 100 BPA sessions and in 28 healthy controls. Correlations between CXCL10 and pulmonary hemodynamics were investigated. Receiver operating characteristic (ROC) curves were plotted to display the diagnostic value and the predictive ability for perioperative complications of CXCL10 and CXCL10‐related models. Nomograms were plotted to visualize the diagnostic value and the predictive ability for perioperative complications of CXCL10 and CXCL10‐related models. CXCL10 levels are higher in CTEPH patients compared with healthy controls (36.5 [95% confidence interval {CI}: 25.0–51.1] vs. 14.8 [95% CI: 11.1–30.9], p < 0.0001) and decreased significantly after BPA treatment (36.5 [95% CI: 25.0–51.1] vs. 24.7 [95% CI: 17.2–36.6], p < 0.0005). Preoperative CXCL10 levels positively correlated with mean right atrial pressure (r = 0.25), systolic pulmonary artery pressure (PAP; r = 0.28), diastolic PAP (r = 0.33), mean PAP (r = 0.36), pulmonary vascular resistance (r = 0.31), and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP; r = 0.46). Furthermore, plasma CXCL10 levels adjusting for age and sex displayed a sensitivity of 86.0% and a specificity of 67.9% for discriminating CTEPH patients from healthy controls. Preoperative CXCL10 levels, in combination with NT‐proBNP, predicted perioperative complications with a sensitivity of 100.0% and a specificity of 46.9% as displayed in ROC analysis. In conclusion, circulating CXCL10 might contribute to the evaluation of disease severity in CTEPH patients and be useful to evaluate the treatment effect of BPA. Future studies are warranted to further study the relationship between pulmonary hemodynamics and circulating CXCL10.
Keywords: balloon pulmonary angioplasty, chronic thromboembolic pulmonary hypertension, complications, CXCL10, diagnosis
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is a progressive pulmonary vascular disease characterized by pulmonary artery stenosis or obstructions resulting from insufficient thrombus resolution and vascular remodeling. 1 Elevated pulmonary vascular resistance (PVR), along with severe pulmonary hypertension, might eventually lead to right heart failure and death if left untreated. 2 Due to the nonspecific symptoms throughout the course of the disease and the infrequent use of lung ventilation/perfusion scintigraphy, CTEPH is likely underdiagnosed and diagnosis is delayed; 1 thus, the search for biomarkers facilitating diagnosis and predictors of outcome has never come to an end.
The mechanism of pulmonary hypertension in CTEPH is multifactorial and recent insights have revealed an important role of inflammation in the development and progression of the disease. 3 As compared with healthy controls, a variety of abnormally elevated autoimmune and inflammatory markers have been found in patients with CTEPH, and some of them, such as C‐reactive protein, reduced after pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA). 3 , 4 , 5 , 6 Furthermore, surgical tissues harvested from patients who underwent PEA showed a significant upregulation of inflammatory markers, such as interleukin‐6, monocyte chemoattractant protein‐1, and chemokine (C‐X‐C motif) ligand 10 (CXCL10), as compared with lung tissue from healthy controls. 7 Interestingly, recent results showed that circulating CXCL10, also known as interferon‐γ‐induced protein‐10, a chemokine that appears to contribute to the pathogenesis of many autoimmune diseases and cancers, 8 was negatively correlated with baseline exercise capacity and cardiac output (CO) in patients with CTEPH. 7 CXCL10 is a ligand of CXCR3 secreted by several cell types, including T lymphocytes, neutrophils, monocytes, endothelial cells, and fibroblasts. 8 Previous studies have shown that CXCL10 causes endothelial dysfunction in human pulmonary artery endothelial cells while facilitating fibroblasts migration, 7 , 9 which might contribute to the formation of unresolved thromboembolic material and the progression of vascular remodeling.
Although PEA remains the treatment of choice for patients with CTEPH, 10 BPA has emerged as a novel endovascular treatment for patients ineligible for PEA due to distal disease or the presence of comorbidities. 11 Performed in staged procedures, BPA dilates narrowed or obstructed segmental and subsegmental pulmonary arteries, significantly improving patients' pulmonary hemodynamics, exercise capacity, and right heart function, with a relatively low rate of complications. 12 , 13 Potential changes of CXCL10 levels in patients with CTEPH throughout BPA treatment and its relation to the improvement of pulmonary hemodynamics and exercise capacity have never been investigated. Our hypothesis is that abnormally upregulated circulating CXCL10 levels in CTEPH patients might decrease after BPA treatment, which might be correlated with patients' improving pulmonary hemodynamics.
The present study aims to evaluate the diagnostic value of CXCL10 in patients with CTEPH; to investigate the levels of CXCL10 throughout BPA‐staged procedures and its correlation with pulmonary hemodynamics and exercise capacity in patients with CTEPH; and to investigate the possibility of pre‐BPA CXCL10 levels, in combination with N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and pulmonary hemodynamics parameters, as the predictors of BPA perioperative complications.
METHODS
The present study was approved by the institutional review board of the First Affiliated Hospital of our Medical University (No. 202172). Written informed consent was obtained from all participants in this study.
Study population
Thirty‐eight consecutive patients with CTEPH, who underwent at least two BPA sessions from January 2019 to August 2021 in the First Affiliated Hospital of Guangzhou Medical University, were included. A total of 100 BPA sessions were evaluated. Patients with inoperable CTEPH were diagnosed by a multidisciplinary CTEPH team based on the current guideline. 10 In addition, 28 sex‐ and age‐matched healthy controls who had normal echocardiographic and electrocardiography findings, and attended their routine clinical examination, were included.
Patient evaluation and right heart catheterization (RHC)
Upon admission, World Health Organization (WHO) functional class, 6 min walk distance (6MWD), and serum level of NT‐proBNP were evaluated in all CTEPH patients. RHC was performed using a Swan–Ganz catheter before and immediately after each BPA procedure. Hemodynamic parameters, including pulmonary artery pressure (PAP), pulmonary arterial wedge pressure, mean right atrial pressure (RAP), pulmonary arterial oxygen saturation, and mixed venous oxygen saturation were measured. CO was assessed by the thermodilution method and cardiac index (CI) and PVR were calculated based on previous measurements.
BPA
We performed BPA as a staged procedure in a standard manner as described previously. 14 Briefly, BPA was performed via right femoral venous access. A 70 cm 7‐French vascular sheath was inserted into the vein, through which a 6‐French guiding catheter was advanced into the target pulmonary artery. We selected target vessels appropriate for angioplasty and target lesions with webs, filling defects, or complete occlusion, based on selective pulmonary angiography and optical coherence tomography. A 0.014 in guidewire was passed through the target lesion and the target lesion was dilated to an appropriate size by multiple balloon inflations manually using semicompliant balloons (diameter range 2.0–6.0 mm) depending on vessel diameter. Generally, 2–14 segmental or subsegmental arteries were treated in each session according to patient condition and the amount of contrast media given (<300 ml). Two BPA sessions were performed at 1–2 months intervals until mean PAP below 30 mmHg was achieved and/or when all accessible lesions have been treated.
BPA‐related complications
In this study, perioperative complications related to BPA were defined as follows: (1) hemoptysis; (2) severe cough with an increase in heart rate and/or a decrease of oxygen saturation, requiring supplemental oxygenation; (3) reperfusion pulmonary edema (RPO), defined as chest X‐ray opacities in the segment treated with BPA; (4) the use of mechanical ventilation or extracorporeal membrane oxygenation; and (5) renal dysfunction.
Blood samples
Blood samples of all BPA sessions were prospectively collected from all CTEPH patients when undergoing pre‐BPA RHC. Venous blood samples of healthy controls were prospectively collected when undergoing routine clinical examination. All blood samples were collected using 10 ml EDTA BD Vacutainer tubes and underwent the same procedure. Blood samples were left for 30 min at room temperature and then centrifuged at 1000g for 10 min. Plasma was collected, aliquoted, and stored at −80°C until used.
A total of 100 blood samples from 38 CTEPH patients were collected before each BPA session and a total of 28 blood samples from 28 healthy controls were included for analysis. Specifically, as all blood samples of CTEPH patients were collected before each BPA procedure, the blood sample of the first session in each patient represents his/her baseline condition, whereas blood samples from the second session represent the condition after the first sessions and so on.
Enzyme‐linked immunosorbent assay (ELISA)
Plasma CXCL10 levels were quantified in the ELISA method using a Human IP‐10 ELISA Kit (LAIZEE BIOTECH CO, China). For CXCL10 quantification by ELISA, 100 µl of plasma sample was used. All ELISA procedures were performed according to the standard protocol provided by the manufacturer.
Statistical analysis
Continuous variables are expressed as mean ± SD or median and interquartile range according to variable distribution. Categorical variables, such as gender, WHO functional class, and incidence of complications, were expressed as numbers and percentages, and were compared using the χ 2 test for independence or Fisher's exact test. Differences in continuous variables, such as CXCL10 levels and hemodynamic parameters, were compared using the independent Student's t test for normally distributed variables and the Mann–Whitney U test for non‐normally distributed variables, and the Wilcoxon matched‐pairs signed‐rank test as appropriate. When comparing multiple groups, the Kruskal–Wallis tests were performed followed by multiple comparisons. The Spearman test was performed to assess the associations between CXCL10 and pulmonary hemodynamics. Receiver operating characteristic (ROC) curves were plotted to display the diagnostic value, as well as the predictive ability for perioperative complications of CXCL10 and CXCL10‐related models. Optimal thresholds and their corresponding sensitivity and specificity were based on Youden's index. Delong's test was used to compare the area under the ROC curves (AUCs) of two ROC curves. Multivariable logistic regression analysis with the “Enter” method was performed to derive predicted probabilities from different CXCL10‐related models for subsequent ROC analysis. Nomograms were built based on the results of multivariate analysis and through the rms package in R version 4.1.2 (http://www.r-project.org/). The maximum score of each variable was set as 100. The performance of the nomogram was measured based on the Harrel concordance index (C‐index). Bootstraps of 1000 resamples were set and calibration curves were calculated by the regression analysis.
All statistical analyses were performed using R (Version 4.1.2), IBM SPSS Statistics (Version 25.0), and MedCalc (Version 20.011). A p < 0.05 was considered statistically significant.
RESULTS
Population characteristics
The clinical features of the patients with CTEPH and healthy controls are reported in Table 1. In 38 CTEPH patients, the mean age was 63 ± 11 years old and 29 of them (76%) were female. A total of 100 BPA sessions were performed (2 [2,3] sessions/patient) and the interval between each BPA session was 70 (34,117) days. Half of the patients had a WHO functional class of Ⅲ or Ⅳ (19, 50%) at the time of the first BPA session. Pulmonary arterial hypertension (PAH) targeted therapy was administrated to 23 (60.5%) patients and all patients received anticoagulant therapy with warfarin or new oral anticoagulants, including Rivaroxaban and Dabigatran. In 28 healthy controls, the mean age was 59 ± 6 years old and 18 of them (64%) were female.
Table 1.
Baseline characteristics of CTEPH patients and healthy controls
| CTEPH patients | Healthy controls | p | |
|---|---|---|---|
| Subjects (n) | 38 | 28 | |
| Age (years) | 63 ± 11 | 59 ± 6 | 0.13 |
| Female (n, %) | 29 (76%) | 18 (64%) | 0.286 |
| BPA sessions/patient | 2 (2–3) | ||
| Total BPA sessions | 100 | ||
| Interval between BPA (days) | 70 (34–117) | ||
| Exercise capacity | |||
| WHO functional class (Ⅰ–Ⅱ/Ⅲ–Ⅳ, %) | 50%/50% | ||
| 6MWD (m) | 369 ± 81 | ||
| Pulmonary hemodynamics | |||
| Mean RAP (mmHg) | 6 (4–10) | ||
| Systolic PAP (mmHg) | 80 ± 27 | ||
| Diastolic PAP (mmHg) | 25 ± 10 | ||
| Mean PAP (mmHg) | 44 ± 14 | ||
| PVR (Wood Unit) | 9.8 (7.2–17.1) | ||
| Cardiac output (L/min) | 3.3 (2.5–4.5) | ||
| Cardiac index (L/min*m2) | 2.0 (1.6–3.0) | ||
| PASO2 (%) | 61 ± 9 | ||
| MVSO2 (%) | 94 (91–97) | ||
| NT‐proBNP (pg/ml) | 838 (217–2114) | ||
| PAH‐targeted therapy (n, %) | 23 (60.5%) | ||
| Riociguat | 13 (34.2) | ||
| Macitentan | 2 (5.3) | ||
| Treprostinil | 7 (18.4) | ||
| Tadalafil | 3 (7.9) | ||
| Sildenafil | 3 (7.9) | ||
| Ambrisentan | 2 (5.3) | ||
| Anticoagulation (n, %) | 38 (100%) | ||
| Warfarin | 8 (21.1) | ||
| Rivaroxaban | 28 (73.7) | ||
| Dabigatran | 2 (5.3) |
Note: Data are presented as median and interquartile range or n (%).
Abbreviations: 6MWD, 6 min walk distance; CTEPH, chronic thromboembolic pulmonary hypertension; MVSO2, mixed venous oxygen saturation; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAH, pulmonary arterial hypertension; PAP, pulmonary artery pressure; PASO2, pulmonary arterial oxygen saturation; PVR, pulmonary vascular resistance; RAP, right atrium pressure; WHO, World Health Organization.
Plasma CXCL10 levels are higher in CTEPH patients and decreased after BPA
Improvements in pulmonary hemodynamics and exercise capacity in patients with CTEPH undergoing BPA are shown in Table 2. Plasma CXCL10 levels were evaluated in patients with CTEPH undergoing BPA and healthy controls. As compared with healthy controls, patients with CTEPH showed a higher level of CXCL10 at baseline (Figure 1a). Furthermore, CXCL10 levels decreased gradually as BPA treatment proceeded(Figure 1c). As compared with the baseline, CXCL10 levels decreased significantly after BPA treatment (Figure 1a). A significant decrease was also found when comparing patients' CXCL10 levels at baseline and at their last BPA sessions in matched pairs (Figure 1b). In a subgroup analysis, no significant difference in baseline CXCL10 levels was observed between patients who received PAH‐targeted therapy and those who did not (Figure 2a). Similarly, CXCL10 levels decreased significantly after BPA treatment in both groups.
Table 2.
Comparison of CXCL10, NT‐proBNP, pulmonary hemodynamics, and exercise capacity between baseline and last BPA in CTEPH patients
| Baseline | Last BPA | p | |
|---|---|---|---|
| CXCL10 (pg/ml) | 36.5 (25.0–51.1) | 24.7 (17.2–36.7) | 0.005 |
| 0.0004a | |||
| NT‐proBNP (pg/ml) | 838 (217–2114) | 267 (49–1471) | 0.005 |
| Pulmonary hemodynamics | |||
| Mean RAP (mmHg) | 6 (4–10) | 5 (3–9) | 0.401 |
| Systolic PAP (mmHg) | 80 ± 27 | 68 ± 24 | 0.051 |
| Diastolic PAP (mmHg) | 25 ± 10 | 21 ± 8 | 0.051 |
| Mean PAP (mmHg) | 44 ± 14 | 38 ± 12 | 0.028 |
| PVR (Wood Unit) | 9.8 (7.2–17.1) | 6.7 (4.8–9.9) | 0.005 |
| Cardiac output (L/min) | 3.3 (2.5–4.5) | 4.1 (3.5–5.1) | 0.026 |
| Cardiac index (L/min*m2) | 2.0 (1.6–3.0) | 0.027 | |
| Exercise capacity | |||
| WHO functional class (Ⅰ–Ⅱ/Ⅲ–Ⅳ, %) | 50%/50% | 16%/84% | 0.002 |
| 6MWD (m) | 369 ± 80 | 376 ± 101 | 0.786 |
Note: Data are presented as median and interquartile range or n (%).
Abbreviations: 6MWD, 6 min walk distance; BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; CXCL10, chemokine (C‐X‐C motif) ligand 10; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; RAP, right atrium pressure; WHO, World health organization.
Wilcoxon matched‐pairs signed‐rank test.
Figure 1.
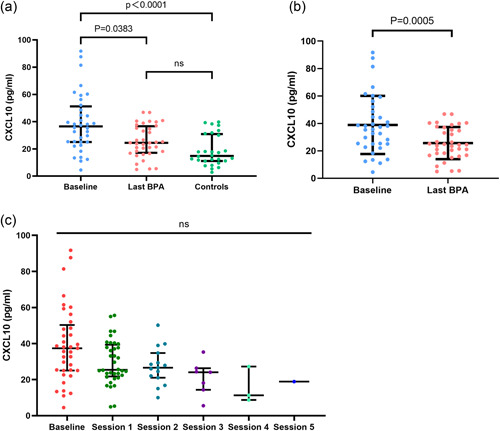
Plasma chemokine (C‐X‐C motif) ligand 10 (CXCL10) levels of healthy controls and chronic thromboembolic pulmonary hypertension (CTEPH) patients undergoing balloon pulmonary angioplasty (BPA). (a)Plasma CXCL10 levels of CTEPH patients at baseline, at the last BPA session, and of healthy controls. (2) CTEPH patients' plasma CXCL10 levels at baseline and at their last BPA sessions in matched pairs. (3) Changes of plasma CXCL10 levels during BPA treatment.
Figure 2.
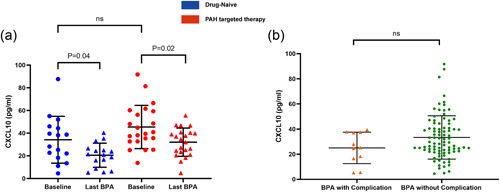
Subgroup analysis of chemokine (C‐X‐C motif) ligand 10 (CXCL10) levels in chronic thromboembolic pulmonary hypertension (CTEPH) patients and balloon pulmonary angioplasty (BPA) sessions. (a) Comparison of CXCL10 levels between patients who received pulmonary arterial hypertension (PAH)‐targeted therapy and those who did not, at baseline and at the last BPA session (23 patients with PAH‐targeted therapy vs. 15 patients without PAH‐targeted therapy). (b) Comparison of preoperative CXCL10 levels between BPA sessions with complications and those without complications (89 sessions without complications vs. 11 sessions with complications).
Preoperative CXCL10 levels are significantly correlated with pulmonary hemodynamics in CTEPH patients undergoing BPA
We performed a Spearman analysis between preoperative CXCL10 levels and pulmonary hemodynamics in patients with CTEPH undergoing BPA (Figure 3). There is a significant positive correlation between CXCL10 and mean RAP (r = 0.25), systolic PAP (r = 0.28), diastolic PAP (r = 0.33), mean PAP (r = 0.36), PVR (r = 0.31), and NT‐proBNP (r = 0.46). However, there is no correlation demonstrated between CXCL10 and CO, CI, or 6MWD. Moreover, no correlations were demonstrated between baseline CXCL10 and baseline pulmonary hemodynamics in patients with CTEPH (Figure S1).
Figure 3.
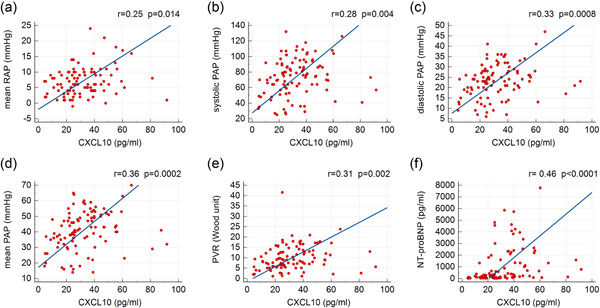
Correlations of preoperative plasma chemokine (C‐X‐C motif) ligand 10 (CXCL10) levels with (a) mean right atrial pressure (RAP), (b) systolic pulmonary artery pressure (PAP), (c) diastolic PAP, (d) mean PAP, (e) pulmonary vascular resistance (PVR), and (f) N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) undergoing balloon pulmonary angioplasty (n = 100 sessions).
ROC analysis of CXCL10 for diagnosis of CTEPH
Next, we performed an ROC analysis to assess the ability of CXCL10 for discriminating CTEPH patients from healthy controls (Table 3). CXCL10 alone displayed an AUC of 0.80 (95% confidence interval [CI] 0.69–0.89), with a sensitivity of 81.4% and a specificity of 71.4% under the optimal cutoff value of 18.6 pg/ml (Figure 4a). After adjusting for age and sex with a logistic regression model, subsequent ROC analysis showed an AUC of 0.83 (95% CI: 0.72–0.91) with a sensitivity of 86.0%, a specificity of 67.9%, and a negative predictive value of 76% under the optimal cutoff value of 0.452 (Figure 4a,b). A nomogram was built based on the results of univariate analysis. The probability of CTEPH was obtained based on the bottom point scale of the nomogram (Figure 4c). The calibration plots on bootstrap resampling validation are illustrated in Figure S2a. The C‐index for the prediction of CTEPH was 0.82.
Table 3.
ROC analyses of CXCL10 for diagnosis of CTEPH
| ROC analysis of CXCL10 for diagnosis of CTEPH | AUC (95% CI) | Cutoff value (pg/ml) | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|
| CXCL10 | 0.80 (0.69–0.89) | >18.6 | 81.4 | 71.4 | ||
| Multiple logistic regression model and ROC analysis | OR (95% CI) | p | AUC (95% CI) | Cutoff value (probability) | Sensitivity (%) | Specificity (%) |
| CXCL10 (pg/ml) | 1.092 | <0.0001 | 0.83 (0.72–0.91) | > 0.452 | 86.0 | 67.9 |
| (1.042–1.144) | ||||||
| Age (year) | 1.015 | 0.65 | ||||
| (0.952–1.083) | ||||||
| Sex (female) | 1.481 | 0.55 | ||||
| (0.413–5.313) | ||||||
Abbreviations: AUC, area under the curve; CI, confidence interval; CTEPH, chronic thromboembolic pulmonary hypertension; CXCL10, chemokine (C‐X‐C motif) ligand 10; OR, odds ratio; ROC, receiver operating characteristic curve.
Figure 4.
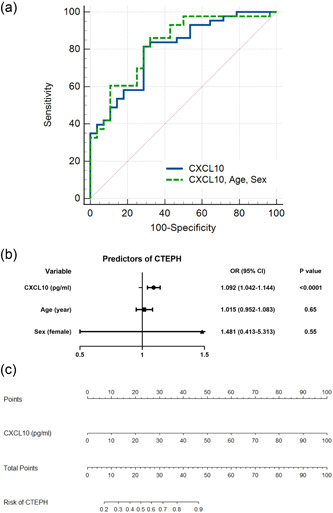
Diagnostic value of chemokine (C‐X‐C motif) ligand 10 (CXCL10) in patients with chronic thromboembolic pulmonary hypertension (CTEPH). (a) Receiver operating characteristic curve analysis of CXCL10 for diagnosis of CTEPH. (b) Forest plot of the multivariate logistic regression of CXCL10 adjusting for sex and age. (c) A nomogram for differentiating CTEPH patients from healthy controls.
ROC analyses of preoperative CXCL10 and related models for prediction of perioperative complications
In the present study, in 100 BPA sessions, perioperative complications were noted in 11 sessions. Hemoptysis, severe cough, and RPO were noted in 6 (6.0%), 1 (1.0%), and 4 (4.0%) BPA sessions, respectively (Table 4). No patients suffered renal dysfunction postprocedure, or required mechanical ventilation or extracorporeal membrane oxygenation. No significant difference in preoperative CXCL10 levels was observed between BPA sessions with complications and those without (Figure 2b). ROC analysis was performed to assess the ability of preoperative CXCL10 and related models for the prediction of perioperative complications in BPA sessions (Table 5). CXCL10 alone displayed an AUC of 0.62 (95% CI: 0.52–0.72), with a sensitivity of 100.0% and a specificity of 31.5% under the optimal cutoff value of 39.4 pg/ml. Next, six logistic regression models, derived from a series of combinations of CXCL10, NT‐proBNP, mPAP, and PVR, were fitted with subsequent ROC analysis (Table T1). Of them, two logistic regression models, with the most optimal AUC in the subsequent ROC analysis to assess the predictive value of CXCL10 and NT‐proBNP (Model 1), and CXCL10, NT‐proBNP, mean PAP, and PVR (Model 2) are displayed (Table 5 and Figure 5a,b). Two nomograms were built based on the results of multivariate analysis of Model 1 and Model 2 (Figure 5c,d). The probability of complications was obtained based on the bottom point scale of the nomogram. Model 1 displayed an AUC of 0.73 (95% CI: 0.63‐0.82), with a sensitivity of 100.0%, a specificity of 46.9%, and a negative predictive value of 100% under the optimal cutoff value (>0.08). Model 2 displayed an AUC of 0.75 (95% CI: 0.65–0.84), with a sensitivity of 100.0%, a specificity of 50.0%, and a negative predictive value of 100% under the optimal cutoff value (>0.084). However, Model 2 was not superior to Model 1 in terms of AUC (p = 0.17). The calibration plots on bootstrap resampling validation are illustrated in Figure S2b,c. The C‐index of Model 1 and Model 2 for prediction of complications were 0.73 and 0.75, respectively.
Table 4.
Complications of BPA
| Complications (%) | Number of sessions | Number of patients |
|---|---|---|
| Total | 11 (11.0) | 9 (23.7) |
| Hemoptysis | 6 (6.0) | 5 (13.2) |
| Severe cough | 1 (1.0) | 1 (2.6) |
| RPO | 4 (4.0) | 4 (10.5) |
| Use of mechanical ventilation | 0 | 0 |
| Use of ECMO | 0 | 0 |
| Renal dysfunction | 0 | 0 |
Abbreviations: BPA, balloon pulmonary angioplasty; ECMO, extracorporeal membrane oxygenation; RPO, reperfusion pulmonary edema.
Table 5.
ROC analyses of preoperative CXCL10 and related models for prediction of perioperative complications in BPA sessions
| ROC analysis of variables for prediction of complications | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|
| CXCL10 (pg/ml) | 0.62 (0.52–0.72) | ≤39.4 | 100.0 | 31.5 | ||
| NT‐proBNP (pg/ml) | 0.56 (0.45–0.67) | >1982 | 45.5 | 79.0 | ||
| Mean PAP (mmHg) | 0.54 (0.44–0.64) | >43 | 63.6 | 59.6 | ||
| PVR (Wood unit) | 0.64 (0.54–0.73) | >12.2 | 63.6 | 72.7 | ||
| Multiple logistic regression models and ROC analysis | OR (95% CI) | p | AUC (95% CI) | Cutoff value (probability) | Sensitivity (%) | Specificity (%) |
| Model 1 | ||||||
| CXCL10 (pg/ml) | 0.931 | 0.03 | 0.73 (0.63–0.82)a | >0.080 | 100.0 | 46.9 |
| (0.875–0.991) | ||||||
| NT‐proBNP (pg/ml) | 1.0004 | 0.02 | ||||
| (1.00006–1.0007) | ||||||
| Model 2 | ||||||
| CXCL10 (pg/ml) | 0.934 | 0.04 | 0.75 (0.65–0.84)b | >0.084 | 100.0 | 50.0 |
| (0.875–0.997) | ||||||
| NT‐proBNP (pg/ml) | 1.0004 | 0.06 | ||||
| (1.0000–1.0008) | ||||||
| Mean PAP (mmHg) | 0.993 | 0.88 | ||||
| (0.912–1.082) | ||||||
| PVR (wood unit) | 1.022 | 0.77 | ||||
| (0.880–1.187) | ||||||
Abbreviations: AUC, area under curve; BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; CXCL10, chemokine (C‐X‐C motif) ligand 10; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; ROC, receiver operating characteristic.
Statistical significance (p < 0.01).
Statistical significance (p < 0.001).
Figure 5.
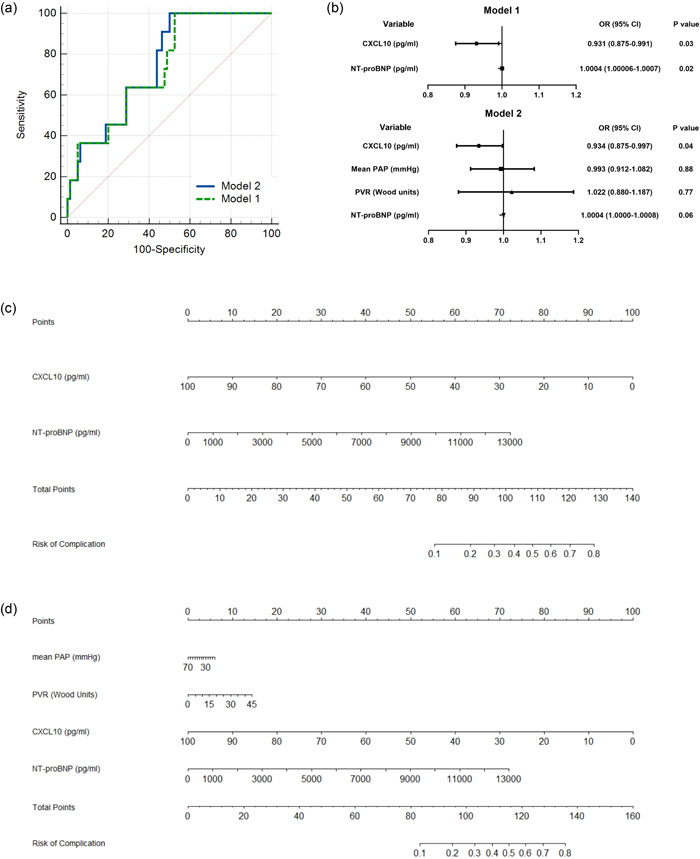
Preoperative chemokine (C‐X‐C motif) ligand 10 (CXCL10) and related models for prediction of balloon pulmonary angioplasty (BPA)‐related complications. (a) Receiver‐operating characteristic curve (ROC) analysis of Model 1 and Model 2 for prediction of complications in BPA. (b) Forest plot of the multivariate logistic regression of Model 1 and Model 2. Nomograms of (c) Model 1 and (d) Model 2 for predicting complications in BPA. NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance.
DISCUSSION
The results of this study show that abnormally upregulated circulating CXCL10 levels in CTEPH patients decreased significantly after BPA treatment. In addition, preoperative CXCL10 levels are significantly correlated with improving pulmonary hemodynamics in CTEPH patients who underwent BPA, indicating a gradual decrease of circulating CXCL10 as pulmonary hemodynamics improve. Furthermore, plasma CXCL10 levels, after adjusting for age and sex, displayed a sensitivity of 86.0% and a specificity of 67.9% under the optimal cutoff value for discriminating CTEPH patients from healthy controls. Preoperative CXCL10 levels, combined with NT‐proBNP, predicted perioperative complications in BPA sessions with a sensitivity of 100.0%, a specificity of 46.9%, and a negative predictive value of 100% under the optimal cutoff value as displayed in the ROC analysis. Further, a nomogram model was developed to predict perioperative complications in BPA sessions based on preoperative circulating CXCL10 and NT‐proBNP levels, revealing the ability of combined circulating biomarkers for the prediction of possible perioperative complications.
Inflammation has been recognized as an important component in the pathophysiology of CTEPH. 2 Recent research has revealed a significantly increased level of inflammatory mediators, including interleukin‐6 and CXCL10, both in PEA tissues and in serums from patients with CTEPH. 7 In accordance with previous research, we detected increased circulating CXCL10 levels in patients with CTEPH as compared with sex‐ and age‐matched healthy controls. Further ROC analysis showed that CXCL10 predicted CTEPH risk to some extent, revealing the potential role of CXCL10 as a diagnostic biomarker in CTEPH. Further, a simple diagnostic nomogram was developed based on circulating CXCL10 levels to help discriminate CTEPH patients from healthy controls. The efficacy of the nomogram was supported by the C‐index (0.82) and the calibration curve.
CXCL10 is reported to activate and recruit CXCR3+ immune cells and is strongly upregulated in many inflammatory diseases and cancers. 8 Interestingly, CXCL10, which is secreted by endothelial cells, potentiates the adhesion of T lymphocytes to the endothelium, 15 promotes the migration of CXCR3+ cells to the lung, 16 and has been shown to play an important role in the development of PAH and CTEPH. 7 , 16 , 17 CXCL10 disrupts calcium homeostasis in normal human pulmonary artery endothelial cells and causes endothelial dysfunction as observed by Zabini et al., 9 which might take part in the progression of vascular remodeling in CTEPH. More recent research by Zabini et al. 7 found a significant increase of human pulmonary arterial adventitial fibroblast migration as exposed to CXCL10, which could be diminished by a CXCR3‐blocking antibody, indicating the possible involvement of the CXCL10/CXCR3 axis in the formation of unresolved thromboembolic material.
We have, for the first time, observed a significant decrease in CXCL10 levels in patients with CTEPH, who underwent BPA, which was correlated with improved pulmonary hemodynamics and decreased NT‐proBNP. Our results revealed a potential improvement in inflammatory status in patients with CTEPH as pulmonary hemodynamics improved gradually by interventional treatment. The connection between pulmonary hemodynamics and inflammation has long been postulated. Classical inflammatory mediators, such as C‐reactive protein and tumor necrosis factor‐α, were found to be elevated in patients with CTEPH and decreased after PEA. 4 , 18 Interestingly, recent insights have found that cyclical hydrostatic pressure triggered robust upregulation of a variety of proinflammatory genes, including CXCL10, in mice macrophages through PIEZO1 signaling. 19 However, in the present study, unable to determine the origin of abnormally upregulated CXCL10, we can only speculate that elevated CXCL10 levels were related to dysregulated pulmonary hemodynamics in patients with CTEPH, and that BPA decrease circulating CXCL10 levels (along with improved inflammatory status) as a consequence of improving pulmonary hemodynamics.
NT‐proBNP is commonly used as a marker for decompensation of the right ventricle. 20 We also observed a significant positive correlation of circulating CXCL10 with NT‐proBNP (r = 0.46), but not with CO or CI, in patients who underwent BPA. Moreover, no correlation was found between 6MWD and CXCL10. We consider that pathophysiological changes precede functional and exercise improvement, as functional and exercise capacity takes time to improve after BPA. Taken together, we propose CXCL10 as a potential biomarker for the evaluation of disease severity in patients with CTEPH and a noninvasive predictor of improved pulmonary hemodynamics and right heart function in patients undergoing BPA.
During the past decade, the efficacy of BPA treatment on pulmonary hemodynamics and exercise capacity has been proved in a series of studies worldwide. 21 Consideration has been focusing on the refinement of BPA procedure and patients' evaluation in the aim of improving pulmonary hemodynamics as effectively as possible while avoiding perioperative complications. 22 Research by Inami et al. 23 has identified BNP, mean PAP, and PVR, as significantly related to the occurrence of RPO. Complications tend to occur in BPA sessions performed in patients with worse baseline hemodynamics and higher BNP. 23 In the present study, we also investigate the ability of preoperative CXCL10 and related models for the prediction of perioperative complications. We found that preoperative CXCL10, combined with NT‐proBNP, predicted perioperative complications in BPA sessions with high sensitivity (100.0%) but low specificity (46.9%) under the optimal cutoff value as displayed in ROC analysis. In the nomogram model based on the results of multivariate analysis, both CXCL10 and NT‐proBNP were important predictors of perioperative complications. However, both Model 1 and Model 2 displayed high sensitivity but relatively low specificity, which were not sufficiently high to accurately predict perioperative complications. Besides, the success of a BPA session, performed effectively without complication, is mainly dependent on the operators' experience, lesions characteristics, and patients' baseline hemodynamics. 11 , 23 Thus findings from noninvasive biomarkers should be compared parallelly with hemodynamics examination and radiographic imaging findings of pre‐BPA.
The present study was performed in one single center with a relatively small number of patients and BPA sessions. Therefore, the findings in this study needed to be externally validated in future multicenter studies with a larger cohort. Besides, 60% of patients were in PAH‐targeted therapy before the first BPA session, so we could not preclude the influence of PAH‐targeted therapy on CXCL10 levels during BPA treatment. However, all these patients included in the present study have been in stable PAH‐targeted therapy for over 3 months, and our subgroup analysis showed no significant difference in baseline CXCL10 levels between patients who received PAH‐targeted drugs and those who did not, indicating the comparability of the two groups. In addition, the hemodynamics effect of BPA treatment in the present study was not so apparent as the results achieved in the European centers and Japanese centers, 12 , 13 because we compared the patients' baseline hemodynamics with that achieved in the patients' last BPA session, but not at follow‐up. It is well known that hemodynamics effect and functional improvement take time to develop after BPA. Besides, as most patients in the present study have not finished their BPA treatment, we are unable to collect pulmonary hemodynamics data and circulating CXCL10 levels at follow‐up. Lastly, we have not studied the diagnostic value of CXCL10 for differentiating patients with CTEPH from patients with other types of pulmonary hypertension, or patients with inflammatory diseases, as previous studies have found that CXCL10 levels are also elevated in patients with PAH and connective tissue diseases. 7 , 18 , 24 However, for patients with confirmed CTEPH, circulating CXCL10 might contribute to the evaluation of disease severity and be useful to evaluate the treatment effect of BPA.
CONCLUSION
Collectedly, our study shows that abnormally upregulated circulating CXCL10 levels in CTEPH patients decreased significantly after BPA treatment. In addition, preoperative CXCL10 levels significantly correlated with improving pulmonary hemodynamics and NT‐proBNP, as CTEPH patients underwent BPA. Circulating CXCL10 might contribute to the evaluation of disease severity in patients with CTEPH and be useful to evaluate the treatment effect of BPA. A comprehensive evaluation of circulating noninvasive biomarkers, including CXCL10 and NT‐proBNP, might facilitate the successful operation of BPA. External validation is warranted to further study the relationship between pulmonary hemodynamics and circulating CXCL10, and to investigate the origin of abnormal upregulation of CXCL10 in CTEPH patients.
AUTHOR CONTRIBUTIONS
Cheng Hong and Jianmin Lu are the guarantors of the manuscript and take responsibility for the content of this manuscript. Cheng Hong and Jianmin Lu contributed to the design of the study. Riken Chen, Haimin Liu, and Haiming Chen were involved in the data analysis. Xiaofeng Wu, Wenliang Guo, Zijie Huang, and Huizhao Liao contributed to the acquisition of primary data. Cheng Hong and Jianmin Lu wrote the initial draft of the manuscript. Riken Chen and Haiming Chen contributed significantly to the revision of the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
The present study was approved by the institutional review board of the First Affiliated Hospital of Guangzhou Medical University (number 202172). Written informed consent was obtained from all participants in this study.
Supporting information
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENT
Non declared. This study was funded by the Zhongnanshan Medical Foundation of Guangdong Province, China (Grant number ZNSA‐2020013), the State Key Laboratory of Respiratory Diseases, China (Grant number SKLRD‐OP‐202107), and the Natural Science Foundation of Guangdong Province, China (Grant number 2021A1515011373).
Hong C, Lu J, Chen R, Liu H, Chen H, Wu X, et al. CXCL10 levels in diagnosis and improved hemodynamics in patients with chronic thromboembolic pulmonary hypertension undergoing balloon pulmonary angioplasty. Pulmonary Circulation. 2022;12:e12091. 10.1002/pul2.12091
Cheng Hong, Jianmin Lu, and Riken Chen contributed equally to this work.
REFERENCES
- 1. Kim NH, Delcroix M, Jais X, Madani MM, Matsubara H, Mayer E, Ogo T, Tapson VF, Ghofrani HA, Jenkins DP. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;Jan 24 53(1):1801915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simonneau G, Torbicki A, Dorfmüller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;Mar 29 26(143):160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quarck R, Wynants M, Verbeken E, Meyns B, Delcroix M. Contribution of inflammation and impaired angiogenesis to the pathobiology of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2015;Aug 46(2):431–43. [DOI] [PubMed] [Google Scholar]
- 4. Quarck R, Nawrot T, Meyns B, Delcroix M. C‐reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;Apr 7 53(14):1211–8. [DOI] [PubMed] [Google Scholar]
- 5. Kriechbaum SD, Rudolph F, Wiedenroth CB, Mielzarek L, Haas M, Guth S, Hamm CW, Mayer E, Liebetrau C, Keller T. Pregnancy‐associated plasma protein A–a new indicator of pulmonary vascular remodeling in chronic thromboembolic pulmonary hypertension? Respir Res. 2020;Aug 3 21(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cabbar AT, Değertekin MM, Şimşek MA, et al. Evaluation of asymmetric dimethylarginine levels in patients with chronic thromboembolic pulmonary hypertension undergoing pulmonary endarterectomy. Heart Lung Circ. 2021;Jun 12 S1443‐9506(21):00552–7. [DOI] [PubMed] [Google Scholar]
- 7. Zabini D, Heinemann A, Foris V, Nagaraj C, Nierlich P, Bálint Z, Kwapiszewska G, Lang IM, Klepetko W, Olschewski H, Olschewski A. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur Respir J. 2014;Oct 44(4):951–62. [DOI] [PubMed] [Google Scholar]
- 8. Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H, Lenz HJ. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation ‐ A target for novel cancer therapy. Cancer Treat Rev. 2018;Feb 63:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zabini D, Nagaraj C, Stacher E, Lang IM, Nierlich P, Klepetko W, Heinemann A, Olschewski H, Bálint Z, Olschewski A. Angiostatic factors in the pulmonary endarterectomy material from chronic thromboembolic pulmonary hypertension patients cause endothelial dysfunction. PLoS One. 2012;7(8):e43793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;Jan 1 37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 11. Lang I, Meyer BC, Ogo T, Matsubara H, Kurzyna M, Ghofrani HA, Mayer E, Brenot P. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;Mar 29 26(143):160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aoki T, Sugimura K, Tatebe S, Miura M, Yamamoto S, Yaoita N, Suzuki H, Sato H, Kozu K, Konno R, Miyata S, Nochioka K, Satoh K, Shimokawa H. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo‐embolic pulmonary hypertension: long‐term effects and procedure‐related complications. Eur Heart J. 2017;Nov 7 38(42):3152–59. [DOI] [PubMed] [Google Scholar]
- 13. Brenot P, Jaïs X, Taniguchi Y, Garcia Alonso C, Gerardin B, Mussot S, Mercier O, Fabre D, Parent F, Jevnikar M, Montani D, Savale L, Sitbon O, Fadel E, Humbert M, Simonneau G. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur Respir J. 2019;May 18 53(5):1802095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin JL, Chen HM, Lin FC, Li JY, Xie CX, Guo WL, Huang XF, Hong C. Application of DynaCT angiographic reconstruction in balloon pulmonary angioplasty. Eur Radiol. 2020;Dec 30(12):6950–57. [DOI] [PubMed] [Google Scholar]
- 15. Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon‐inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;Jun 1 177(6):1809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heresi GA, Aytekin M, Newman J, Dweik RA. CXC‐chemokine ligand 10 in idiopathic pulmonary arterial hypertension: marker of improved survival. Lung. 2010;Jun 188(3):191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George PM, Oliver E, Dorfmuller P, Dubois OD, Reed DM, Kirkby NS, Mohamed NA, Perros F, Antigny F, Fadel E, Schreiber BE, Holmes AM, Southwood M, Hagan G, Wort SJ, Bartlett N, Morrell NW, Coghlan JG, Humbert M, Zhao L, Mitchell JA. Evidence for the involvement of type I interferon in pulmonary arterial hypertension. Circ Res. 2014;Feb 14 114(4):677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langer F, Schramm R, Bauer M, Tscholl D, Kunihara T, Schäfers HJ. Cytokine response to pulmonary thromboendarterectomy. Chest. 2004;Jul 126(1):135–41. [DOI] [PubMed] [Google Scholar]
- 19. Solis AG, Bielecki P, Steach HR, Sharma L, Harman C, Yun S, de Zoete MR, Warnock JN, To S, York AG, Mack M, Schwartz MA, Dela Cruz CS, Palm NW, Jackson R, Flavell RA. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;Sep 573(7772):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weber T, Auer J, Eber B. The diagnostic and prognostic value of brain natriuretic peptide and aminoterminal (nt)‐pro brain natriuretic peptide. Curr Pharm Des. 2005;11(4):511–25. [DOI] [PubMed] [Google Scholar]
- 21. Zhang L, Bai Y, Yan P, He T, Liu B, Wu S, Qian Z, Li C, Cao Y, Zhang M. Balloon pulmonary angioplasty vs. pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: a systematic review and meta‐analysis. Heart Fail Rev. 2021;Jul 26(4):897–917. [DOI] [PubMed] [Google Scholar]
- 22. Kataoka M, Inami T, Kawakami T, Fukuda K, Satoh T. Balloon pulmonary angioplasty (percutaneous transluminal pulmonary angioplasty) for chronic thromboembolic pulmonary hypertension: a Japanese perspective. JACC Cardiovasc Interv. 2019;Jul 22 12(14):1382–88. [DOI] [PubMed] [Google Scholar]
- 23. Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Taguchi H, Fukuda K, Yoshino H, Satoh T. Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv. 2013;Jul 6(7):725–36. [DOI] [PubMed] [Google Scholar]
- 24. Rabquer BJ, Tsou PS, Hou Y, Thirunavukkarasu E, Haines GK, Impens AJ, Phillips K, Kahaleh B, Seibold JR, Koch AE. Dysregulated expression of MIG/CXCL9, IP‐10/CXCL10 and CXCL16 and their receptors in systemic sclerosis. Arthritis Res Ther. 2011;Feb 8 13(1):R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.


