Abstract
Objective
The relationship between Toll like receptor 4(TLR4) gene Asp299Gly polymorphism and diabetic microvascular complications (DMI) is unclear. Therefore, the aim of this meta analysis was to explore the relationship between TLR4 Asp299Gly polymorphism and DMI.
Methods
System search PubMed, Web of science, Springer, Cochrane library, ELSEVIER, Wanfang database, VIP, CNKI, a case–control study of the correlation between TLR4 gene Asp299Gly polymorphism and DMI published before June 2020 was collected.
Results
We included 6 articles, a total of 11 studies involving patients with type 2 diabetes mellitus (T2DM) complicated by microvascular complications 1834 cases, without corresponding microvascular complications 4069 cases. TLR4 gene Asp299Gly polymorphism increased the risk of microvascular complications in T2DM (dominant model OR = 1.52, 95% CI 1.10–2.09, p = 0.01; allelic model OR = 1.42, 95% CI 1.02–1.96, p = 0.04). Subgroup analysis by race and different type of microvascular complications, we found that TLR4 gene Asp299Gly polymorphism was associated with increased risk of microvascular complications in the Caucasian population (dominant model OR = 1.69, 95% CI 1.22–2.35, P = 0.002; allelic model OR = 1.56, 95% CI 1.10–2.21, P = 0.01) and increased the risk of retinopathy in patients with T2DM(dominant model OR = 1.81, 95% CI 1.04–3.14, P = 0.03; allelic model OR = 1.77, 95% CI 1.05–2.98, P = 0.03).
Conclusion
TLR4 gene Asp299Gly polymorphism was associated with increased risk of microvascular complications in patients with T2DM, especially diabetic retinopathy (DR).
Keywords: Toll like receptor 4 (TLR4); Asp299Gly(rs4986790, 896A > G); Gene polymorphism; Diabetic microvascular complications (DMI); Meta analysis
Introduction
Diabetic microvascular complications (DMI) are the most common chronic complications of diabetes, which are the main causes of disability and death in diabetic patients [1], including diabetic retinopathy, diabetic nephropathy and diabetic neuropathy [2]. Many risk factors play an important role in the onset of DMI, such as course of diabetes, blood glucose control level, BMI and age [3, 4]. However, DMI are a complex metabolic disease composed of multiple factors including genetic and environmental factors, its specific pathogenesis is still unclear, causing difficult in diagnosis and treatment. Due to the high morbidity and mortality of DMI, early diagnosis and treatment are particularly important. Recent studies have shown that some macromolecules such as homocysteine (HCY), troponin T (TnT) and asymmetric dimethylarginine (ADMA) play an important part in the pathogenesis of diabetic vascular diseases, which have good clinical diagnostic value and can be considered as diagnostic markers for diabetic vascular complications [5–7]. These macromolecules are more widely used in diagnosis of diabetic macrovascular complications, however, are less in DMI. Therefore, the discovery of biomarkers for the diagnosis of DMI requires further investigation. A cross-sectional retrospective study involving 90 patients showed that the expression of TLR4 in Diabetic polyneuropathy (DPN) patients was significantly higher than that in T2DM patients and healthy controls, and the up-regulation of TLR4 level would significantly increase the risk of DPN, which suggest that TLR4 may be a potential and sensitive diagnostic biomarker for diabetic neuropathy [8].
TLR4 gene, encodes TLR4 protein, located on chromosome 9Q32-33 and its variations can alter TLR4's recognition and interaction functions [9]. TLR4 is considered a key member of the TLRs families, as a highly conserved pattern recognition receptor, which can bind to specific ligands that induces activation of nuclear factor-κB, leading to an increase in downstream proinflammatory cytokines and adhesion molecules, involved in the occurrence of inflammatory responses by MyD88 dependent or non-dependent signaling pathways [10, 11]. The polymorphism of TLR4Asp299Gly gene may affect ligand binding, folding efficiency, cell surface expression and protein stability, while the polymorphism of Thr399Ile gene has little effect [12].
Recently, studies on the relationship between TLR4 gene polymorphism and type 2 diabetes mellitus (T2DM) and its complications have attracted extensive attention. Yin et al. indicated that TLR4 gene Asp299Gly and Thr399Ile polymorphisms are not associated with increased T2DM risk through a meta-analysis [13], whereas Chang et al. pointed out some problems that still exist or need to be improved in the meta-analysis of Yin et al. [14]. Several studies have shown that TLR4 gene polymorphism is associated with the occurrence of DMI, among which the research on TLR4Asp299Gly is the hotspot [15–24]. Khaghanzadeh et al. [15] showed that c.1196C > T and 896A > G variants might increase the risk of DR by involving in the dysregulation of serum lipid levels and hyperglycemia. Buraczynska et al. [16] concluded that the G(rs4986790) polymorphism of TLR4Asp299Gly allele increased the risk of DR. That is to say, a significant increase of G allele frequency was observed in DR compared to those without it in T2DM (OR = 2.12, 95% CI (1.43–3.12), p = 0.0002). Yet Balistreri et al. [17] revealed that there was no correlation between rs4986790 gene polymorphism and the occurrence of DR and diabetic neuropathy. In view of the conclusions on the correlation between p.Asp299Gly and DMI are not consistent, so we performed this meta analysis to further study the relationship between them, to determine whether TLR4 gene polymorphism can be considered as a risk factor or diagnostic marker for DMI.
Materials and methods
Literature retrieval methods
The electronic databases PubMed, Web of science, Springer link, Cochrane library, ELSEVIER, Wanfang database, VIP, CNKI were searched using the following terms: “(TLR4 or toll-like receptor-4) and (gene or polymorphism or allele or genotype or variant or variation or mutation) and (diabetic or diabetes) and (nephropathy or retinopathy or neuropathy)”. All literature is limited to Chinese or English published before June 20 2020 ranging from 2000 to 2020 (last research was updated on May 2020). At the same time, the literature was traced, and the literature related to this study was searched according to the abstract and the full text was obtained.
Inclusion and exclusion criteria
Inclusion criteria: (1) study of the relationship between TLR4Asp299Gly gene polymorphism and diabetic microvascular complications(including diabetic retinopathy, diabetic nephropathy and diabetic neuropathy); (2) case–control study; (3) population studies; (4) the original text provides the frequency of the genotype or allele or can be extrapolated; (5) the control group was type 2 diabetes patients without corresponding microvascular complications; (6) sufficient data are available to estimate OR values and 95 percent confidence intervals; (7) the control group met the law of genetic balance. Exclusion criteria: (1) critical literature, case reports, conference abstracts, letters etc.; (2) non-case–control studies; (3) the original text does not provide raw data or data is insufficient.
Data extraction
All the data are extracted independently by the two authors according to the set retrieval strategy and the inclusion criteria, the dissenting literature was judged by the third author and finally reached an agreement. The data extracted in the literature included the first author, year of publication, country, race, type of diabetic microvascular complications, sample size of case group and control group, distribution of genotypes and alleles. In addition, whether the control group included in the study complied with Hardy–Weinberg balance (HWE) was also mentioned in this paper.
Quality evaluation
The literature quality assessment scale of case–control studies, namely Newcastle-Ottawascale (NOS), was used for evaluation, including the selection of study subjects, comparability between groups and measurement of exposure factors. The scores ranged from 0 to 9, and the higher the score, the better the quality of the literature.
Statistical analysis
Genetic balance test was carried out for each genotype distribution in the control group. The HWE P < 0.05 was considered significant. Review Manager 5.3 statistical software was used to analyze different models of TLR4Asp299Gly loci(dominant model, allelic model, recessive model and additive model) in case group and control group. That is, the relationship between TLR4Asp299Gly gene polymorphism and diabetic microvascular complications was estimated by ORs and its 95% confidence interval under different genetic models: dominant model(G/G + A/G vs A/A), allelic model(G vs A), recessive model(G/G vs A/G + A/A), additive model(G/G vs A/A). Judging the heterogeneity by I2 and P, if the heterogeneity is slight (P ≥ 0.1, I2 < 50), the fixed effect model was used. When there is obvious heterogeneity (P < 0.1, I2 > 50%), the possible source of heterogeneity is subgroup analysis or sensitivity analysis, and heterogeneity is eliminated as far as possible. If the reason of heterogeneity is not found, random effect model is used for combined statistical analysis. The final result was statistically significant with P < 0.05.
Results
Study characteristics
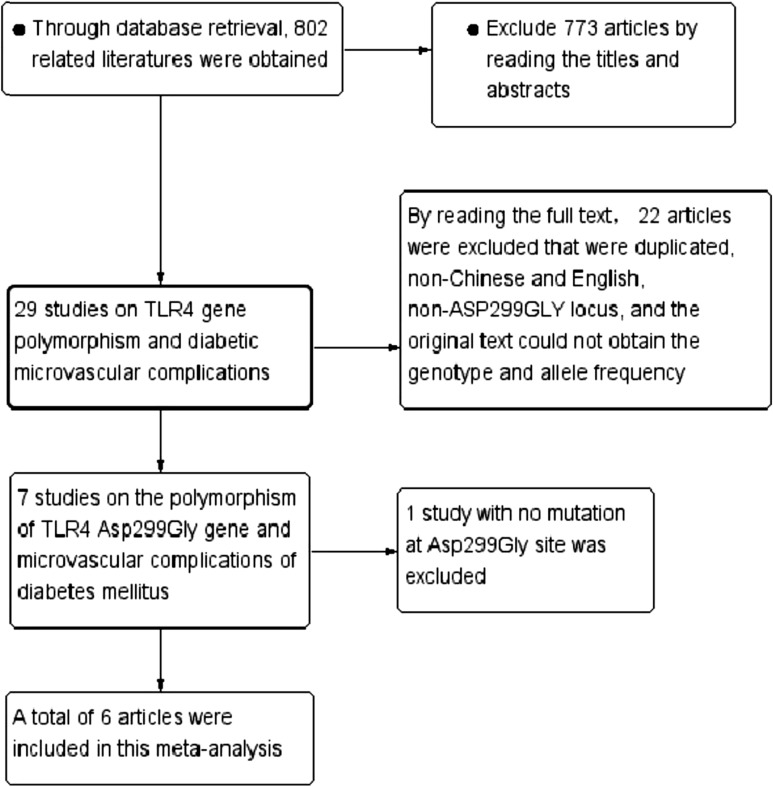
The study selection process is detailed in Fig. 1. According to the searching strategy, 802 potentially eligible articles were identified in our initial search. We reviewed the titles and abstracts, 773 articles were excluded. By reading the full text to exclude duplicate, non-English, non-Asp299Gly locus, original text cannot obtain genotype and allele frequency excluded 22, 7 articles were initially included [15–20, 24]. Through data collation, 1 literature with no mutation in Asp299Gly site was not included in this meta analysis [24]. Finally, 6 articles that met the inclusion criteria were included in this study [15–20], including 11 case–control studies. The main characteristics of the selected studies are listed in Table 1, with a total of 11 studies of 1834 cases and 4069 controls for investigating the association between DMI and TLR4Asp299Gly polymorphism. Ethnic groups among these studies were as follows: 2 were Asians involving 478 population and 4 were Caucasians with 5425 population.
Fig. 1.
The flow chart of literature search and study selection
Table 1.
Characteristics of studies included in this meta-analysis
| Author | Year | Country | Ethnicity | Language | Case | Control | Research method | Score |
|---|---|---|---|---|---|---|---|---|
| Buraczynska [16] | 2016 | Poland | Caucasian | English | 368 | 722 | RFLP-PCR | 9 |
| Balistreri [17] | 2014 | Italy | Caucasian | English | 48 | 319 | RFLP-PCR | 9 |
| Khaghanzadeh [15] | 2020 | Iran | Asian | English | 11 | 89 | SSP-PCR | 8 |
| Balistreri* [17] | 2014 | Italy | Caucasian | English | 112 | 255 | RFLP-PCR | 9 |
| Buraczynska* [16] | 2016 | Poland | Caucasian | English | 342 | 748 | RFLP-PCR | 9 |
| Buraczynska* [18] | 2009 | Poland | Caucasian | English | 352 | 512 | RFLP-PCR | 9 |
| Singh* [19] | 2014 | India | Asian | English | 128 | 250 | RFLP-PCR | 9 |
| Zaharieva * [20] | 2017 | Bulgaria | Caucasian | English | 10 | 75 | PCR | 8 |
| Balistreri** [17] | 2014 | Italy | Caucasian | English | 74 | 293 | RFLP-PCR | 9 |
| Buraczynska**[16] | 2016 | Poland | Caucasian | English | 302 | 788 | RFLP-PCR | 9 |
| Zaharieva** [20] | 2017 | Bulgaria | Caucasian | English | 87 | 18 | PCR | 8 |
*Diabetic retinopathy; **diabetic neuropathy; without*diabetic nephropathy. PCR: Polymerase chain reaction; SSP-PCR: Polymerase chain reaction sequence specific primers; PCR–RFLP: PCR-restriction fragment length polymorphism
NOS score results showed that the overall quality of the literature included in the study was high. The distribution of genotypes and alleles in the case group and the control group and the results of genetic balance were shown in Table 2, indicating that the studies included are in accordance with the law of genetic balance.
Table 2.
TLR4Asp299Gly distribution of genotypes and alleles
| Author | Year | Genotype (case/control) | Allele (case/control) | HWE | |||
|---|---|---|---|---|---|---|---|
| AA | AG | GG | A | G | (Y/N) | ||
| Balistreri [17] | 2014 | 43/293 | 5/26 | 0/0 | 91/612 | 5/26 | Y |
| Buraczynska [16] | 2016 | 329/658 | 37/61 | 2/3 | 695/1377 | 41/67 | Y |
| Khaghanzadeh [15] | 2020 | 10/71 | 1/18 | 0/0 | 21/160 | 1/18 | Y |
| Balistreri* [17] | 2014 | 102/234 | 10/21 | 0/0 | 214/489 | 10/21 | Y |
| Buraczynska* [16] | 2016 | 292/695 | 48/50 | 2/3 | 632/1440 | 52/56 | Y |
| Buraczynska* [18] | 2009 | 309/487 | 40/24 | 3/1 | 658/998 | 6/26 | Y |
| Singh* [19] | 2014 | 99/188 | 28/61 | 1/1 | 226/437 | 30/63 | Y |
| Zaharieva* [20] | 2017 | 7/7 | 3/4 | 0/0 | 17/146 | 3/4 | Y |
| Balistreri** [17] | 2014 | 63/273 | 11/20 | 0/0 | 137/566 | 11/20 | Y |
| Buraczynska** [16] | 2016 | 273/714 | 27/71 | 2/3 | 573/1499 | 31/77 | Y |
| Zaharieva** [20] | 2017 | 80/17 | 7/1 | 0/0 | 167/35 | 7/1 | Y |
*Diabetic retinopathy; **diabetic neuropathy; without*diabetic nephropathy; AA: AA genotype; AG: AG genotype; GG: GG genotype; A: A allele; G: G allele; HWE: Hardy–Weinberg equilibrium; Y: Yes; N: No
Meta-analysis results
The relationship between TLR4Asp299Gly gene polymorphism and DMI
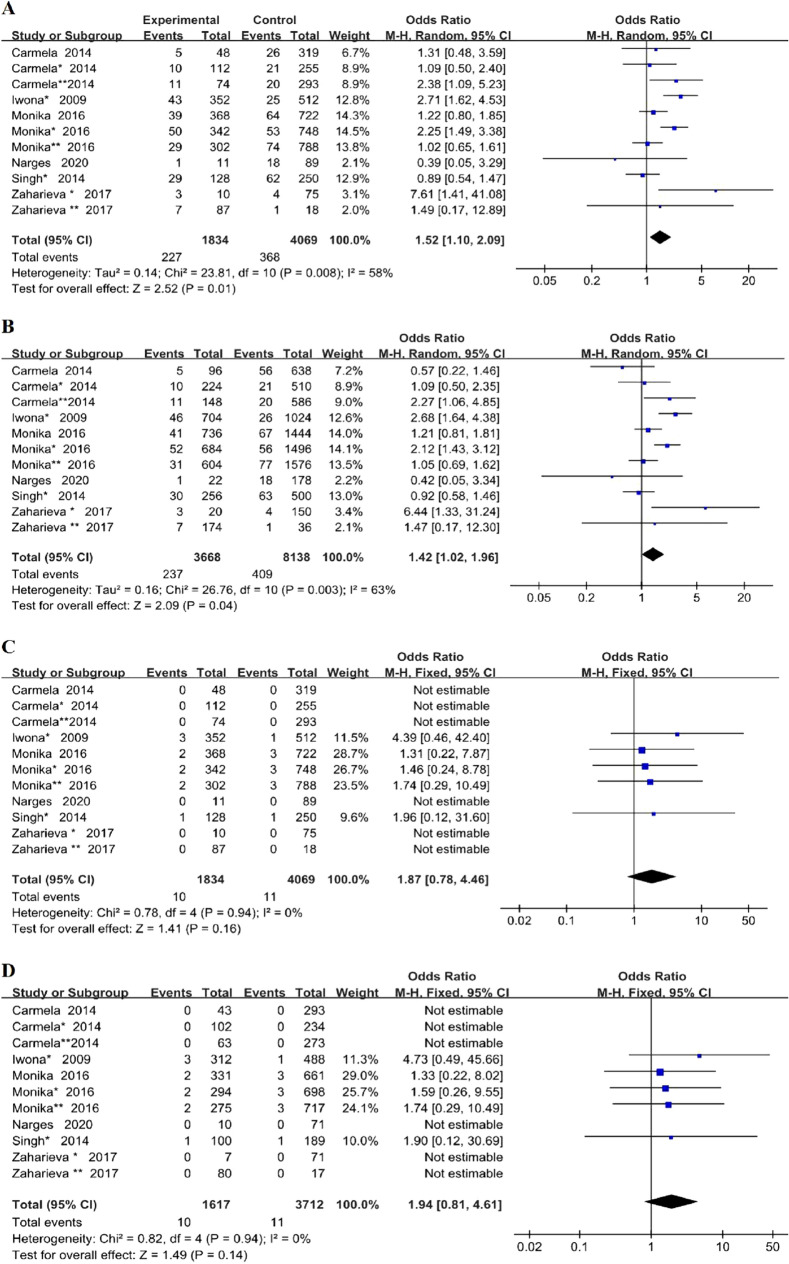
Heterogeneity is significant among included studies in the dominant model and allelic model (P = 0.008, I2 = 58%; P = 0.003, I2 = 63% respectively), but not in the recessive model and additive model (P = 0.94, I2 = 0%; P = 0.94, I2 = 0% respectively). So we chose the random effects model while we analyze the dominant model and the allele model and used fixed-effects model to analyze the data of recessive model and additive model, as shown in Fig. 2 (A: dominant model, B: allele model, C: recessive model, D: additive model).
Fig. 2.
Forest plots of the meta-analysis for TLR4Asp299Gly gene polymorphism associated with DMI in different genetic model. A Dominant model: G/G + A/G vs A/A; B allelic model: G allele vs A allele; C recessive model: G/G vs A/G + A/A; D additive model: G/G vs A/A
As shown in Fig. 2, our meta-analysis showed that TLR4Asp299Gly gene polymorphism was associated with increased risk of DMI under dominant model (OR = 1.52, 95% CI (1.10–2.09), p = 0.01) and allelic model (OR = 1.42, 95% CI (1.02–1.96), p = 0.04). However, there was no significant correlation between this gene polymorphism and DMI either using a recessive model (OR = 1.87, 95% CI (0.78–4.46), p = 0.16) or additive model (OR = 1.94, 95% CI (0.81–4.61), p = 0.14).
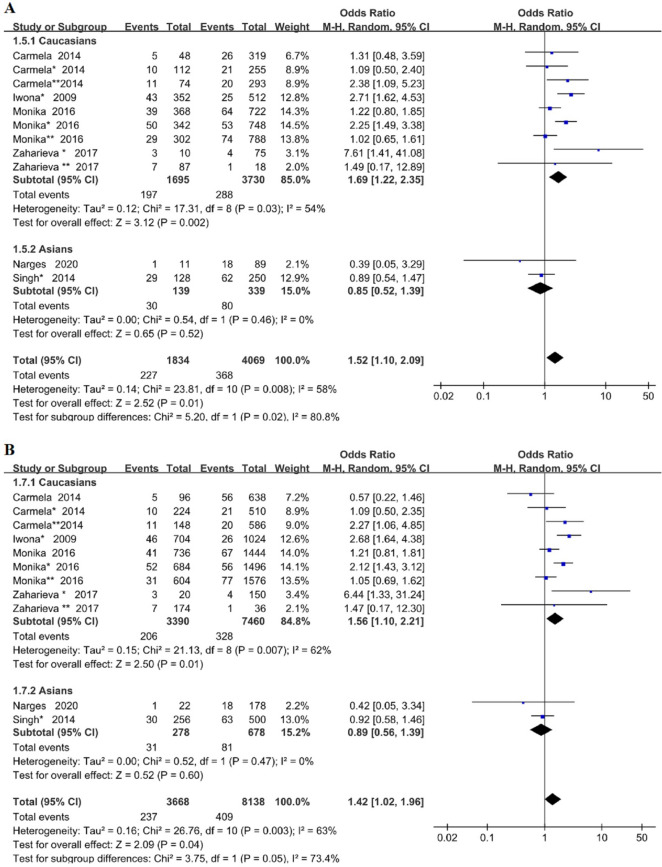
Subgroup analysis
Subgroup analysis was performed by different ethnic groups, we can draw the conclusion that TLR4Asp299Gly gene polymorphism increased the risk of microvascular complications in Caucasian patients with T2DM (dominant model OR = 1.69, 95% CI (1.22–2.35), P = 0.002; allelic model OR = 1.56, 95% CI (1.10–2.21), P = 0.01). However, we found no correlation in the Asian population (dominant model OR = 0.85, 95% CI (0.52–1.39), P = 0.52; allelic model OR = 0.89, 95% CI (0.56–1.39), P = 0.60), as shown in Fig. 3 (A: dominant model; B: allele model). This study showed that there were racial differences in TLR4Asp299Gly gene polymorphism.
Fig. 3.
Forest plots of the meta-analysis for TLR4Asp299Gly gene polymorphism associated with DMI in different genetic model after stratification analysis by ethnicity. A Dominant model: G/G + A/G vs A/A; B allelic model: G allele vs A allele
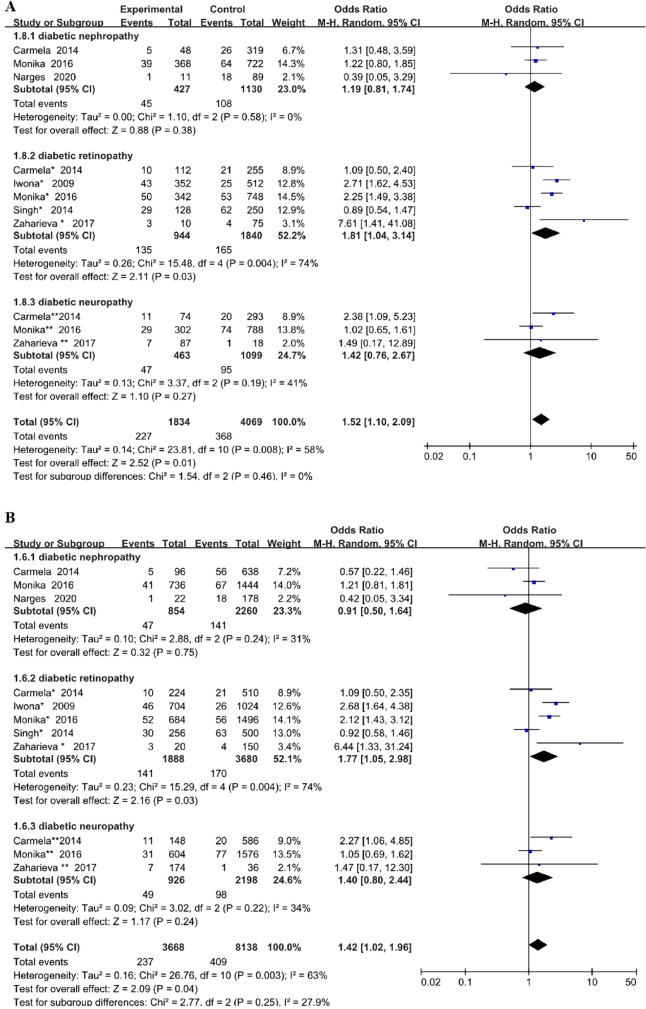
With regard to the specific type of DMI, we also conducted the subgroup analysis stratified by DR or DN or diabetic neuropathy. The results showed that TLR4Asp299Gly genetic polymorphism increased the risk of retinopathy in T2DM under dominant model and allelic model (OR = 1.81, 95% CI (1.04–3.14), P = 0.03; OR = 1.77, 95% CI (1.05–2.98), P = 0.03 respectively). But no significant association was found between DN or diabetic neuropathy and this gene polymorphism (DN: dominant model OR = 1.19, 95% CI (0.81–1.74), P = 0.38; allelic model OR = 0.91, 95% CI (0.50–1.64), P = 0.75; diabetic neuropathy: dominant model OR = 1.42, 95% CI (0.76–2.67), P = 0.27; allelic model OR = 1.40, 95% CI (0.80–2.44), P = 0.24), as shown in Fig. 4 (A: dominant model, B: allele model). Meta-analysis of the associations of TLR4Asp299Gly polymorphism with DMI risk was shown in Table 3, in which we include subgroups, test of association, test of heterogeinity.
Fig. 4.
Forest plots of the meta-analysis for TLR4Asp299Gly gene polymorphism associated with DMI after stratification analysis by different types of microvascular complications in different genetic model. A Dominant model: G/G + A/G vs A/A; B allelic model: G allele vs A allele
Table 3.
Meta-analysis of the associations of TLR4Asp299Gly polymorphism with DMI risk
| Genetic model | Comparison | Test of association | Test of heterogeneity | ||
|---|---|---|---|---|---|
| OR (95% CI) | P-value | P | I2 (%) | ||
| Dominant model | G/G + A/G vs A/A | 1.52 (1.10–2.09) | 0.01 | 0.008 | 58.0 |
| Allelic model | G vs A | 1.42 (1.02–1.96) | 0.04 | 0.003 | 63.0 |
| Recessive model | G/G vs A/G + A/A | 1.87 (0.78–4.46) | 0.16 | 0.94 | 0.0 |
| Additive model | G/G vs A/A | 1.94 (0.81–4.61) | 0.14 | 0.94 | 0.0 |
| Subgroups | |||||
| Ethnicity | |||||
| Caucasians | G/G + A/G vs A/A | 1.69 (1.22–2.35) | 0.002 | 0.03 | 54.0 |
| G vs A | 1.58 (1.10–2.21) | 0.01 | 0.007 | 62.0 | |
| Asians | G/G + A/G vs A/A | 0.85 (0.52–1.39) | 0.52 | 0.46 | 0.0 |
| G vs A | 0.89 (0.56–1.39) | 0.60 | 0.47 | 0.0 | |
| Difference of DMI types | |||||
| Diabetic retinopathy | G/G + A/G vs A/A | 1.81 (1.04–3.14) | 0.03 | 0.004 | 74.0 |
| G vs A | 1.77 (1.05–2.98) | 0.03 | 0.004 | 74.0 | |
| Diabetic nephropathy | G/G + A/G vs A/A | 1.19 (0.81–1.74) | 0.38 | 0.58 | 0.0 |
| G vs A | 0.91 (0.50–1.64) | 0.75 | 0.24 | 31.0 | |
| Diabetic neuropathy | G/G + A/G vs A/A | 1.42 (0.76–2.67) | 0.27 | 0.19 | 41.0 |
| G vs A | 1.40 (0.80–2.44) | 0.24 | 0.22 | 34.0 | |
Bold values indicate P-value < 0.05, that is, the combined effect size is statistically significant
Sensitivity analysis
The method of one-by-one exclusion was used to conduct sensitivity analysis, and the results showed that the combined OR value and 95% CI were not significantly affected in the whole population under the dominant model, indicating that the meta results were stable and reliable under the analysis of this model. And under the allelic model analysis one by one to eliminate are included in each of the study results show that OR value and 95% CI had essential changes after the merger, so we have to cut out the significant change in merger results in 5 cases of study (Carmela * * 2014, Iwona *2009, Monika2016, Monika * 2016, Zaharieva * 2017), the merge OR = 0.95, 95% CI (0.72, 1.25), P = 0.70, heterogeneity, P = 0.81, I2 = 0. We found significant changes in OR value and 95%CI, and the heterogeneity disappeared, suggesting that the heterogeneity may be caused by the excluded studies.
Publication bias
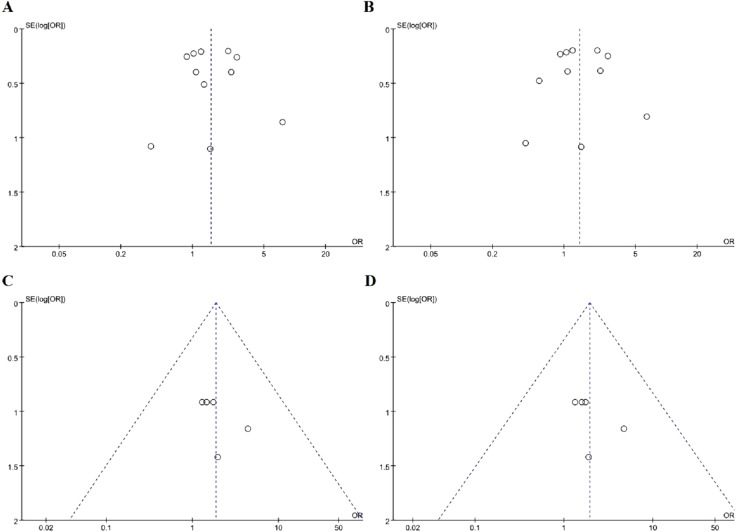
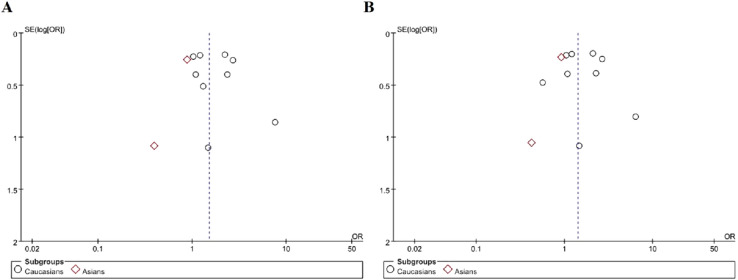
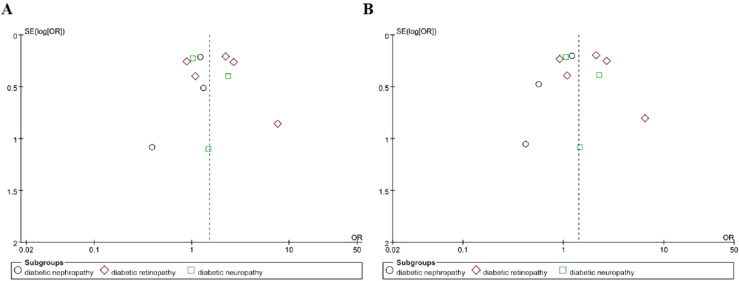
Funnel plots was used to evaluate publication bias among included studies. The included studies in the funnel plots are basically symmetrical around the center line, suggesting that there was no significant publication bias among included studies, as shown in Figs. 5, 6 and 7.
Fig. 5.
Funnel plots for TLR4Asp299Gly gene polymorphism and DMI risk in different genetic model. A Dominant model: G/G + A/G vs A/A); B allelic model: G allele vs A allele; C recessive model: G/G vs A/G + A/A; D additive model: G/G vs A/A
Fig. 6.
Funnel plots for TLR4Asp299Gly gene polymorphism and DMI risk in different genetic model after stratification analysis by ethnicity. A Dominant model: G/G + A/G vs A/A; B allelic model: G allele vs A allele
Fig. 7.
Funnel plots for TLR4Asp299Gly gene polymorphism and DMI risk in different genetic model after stratification analysis by specific type of DMI. A Dominant model: G/G + A/G vs A/A; B allelic model: G allele vs A allele
Discussion
TLR4, as one of the key members of the earliest and most clearly studied Toll-like Receptors (TLRs) family, consists of extracellular domain, transmembrane domain and intracellular domain. The extracellular domain of 22 leucine-rich repeats mediates Lipopolysaccharide (LPS) recognition and receptor dimerization, while the intracellular domain is structurally similar to interleukin 1(TIR1) and plays an important role in downstream signal transduction [25]. TLR4 is mainly distributed on the surface of B lymphocytes, monocytes/macrophages, smooth muscle cells, dendritic cells and other cells [26]. As a highly conserved pattern recognition receptor, it can interact with endogenous ligands such as Heat shock protein(HSP), fibrinogen, High Mobility Group Box 1 protein(HMGB1), Oxidized low density lipoprotein(OX-LDL) and exogenous ligands such as LPS released by Gram-negative bacteria [27–29]. The activation of Nuclear factor-kappa B(NF-κB) was induced by regulating the dependent or independent signaling pathways of Myeloid differentiation factor 88(MyD88) [30]. TLR4/MyD88/NF-κB signaling pathway leads to the increase of downstream pro-inflammatory cytokines and adhesion molecules, participate in the occurrence of inflammatory reactions [31], and cause the pathological changes such as vascular extravasation or increased vascular permeability, vascular obstruction, and degeneration and pathological changes of neovascularization, thus leading to the occurrence and development of DMI [32, 33]. Activation of the TLR4 signaling pathway mediates the inflammatory response, which is associated with TLR4 gene polymorphism [34]. Genetic variation of TLR4 can alter TLR4’s recognition and interaction functions, and then, change the immune response [9]. It has been reported that the replacement of the conserved Asp with Gly at position 299 theoretically cause disruption of the alpha helix protein structure, leading to extended beta strain, which is less functional [35]. As one of the most common variants of TLR4, Asp299Gly gene polymorphism is known to alter the extracellular domain of the receptor, resulting in hyporesponsiveness to LPS, attenuating the TLR4 signaling pathway and reducing the inflammatory response to Gram-negative pathogens [36, 37]. In addition, Zaharieva et al. also noted that it is mainly Asp299Gly rather than Thr399Ile polymorphism that leads to peptide structural modifications, changes in ligand-receptor interactions, post-receptor signal transduction, and further cytokine production [18].
Several studies have shown that TLR4 gene polymorphism is closely related to the occurrence and development of DMI [15–24]. Multiple loci of the TLR4 gene polymorphisms such as rs4986791, rs1927911, rs1927914, rs10759931, are associated with DMI. What’s more, the relationship between TLR4Asp299Gly gene polymorphism and DMI is a hot topic [15–21, 23]. The mutant of TLR4Asp299Gly/Thr399Ile will change the structure of TLR4 itself, leading to problems in ligand binding. This structural/functional irregularity seems ultimately to lead to a blunt immune response, such as the reduction of IgA production against microbial targets and the decrease of functional TLR4 levels [38]. The results of our meta analysis covering a total of 1834 cases and 4069 controls showed that TLR4Asp299Gly gene polymorphism increased the risk of DMI under dominant model and allele model (OR = 1.52, 95% CI (1.10–2.09), p = 0.01; OR = 1.42, 95% CI (1.02–1.96), p = 0.04 respectively). However, we found that there was no correlation between them under the recessive model and additive model (OR = 1.87, 95% CI (0.78–4.46), p = 0.16; OR = 1.94, 95% CI (0.81–4.61), p = 0.14 respectively). The conclusion of Carmela, CS Aioanei and Gottfried were contradictory to our results, which may be attributed to the ethnic difference of the included population and the difference of DMI types [17, 21, 23].
So it is necessary to perform subgroup analysis, we found that the significant association was observed in DR subgroup under dominant model and allele model (OR = 1.81, 95% CI (1.04–3.14), P = 0.03; OR = 1.77, 95% CI (1.05–2.98), P = 0.03 respectively) from subgroup analysis of the difference of DMI types. Nevertheless, no significant association was observed between TLR4Asp299Gly gene polymorphism and DN and diabetic neuropathy subgroup (p > 0.05). The studies of Buraczynska et al. [16], Buraczynska et al. [18], and Zaharieva [20] were consistent with our results, which further confirmed that TLR4Asp299Gly gene polymorphism was significantly associated with increased risk of DR. Therefore, we can infer that although DR, DN and diabetic neuropathy were included in microvascular complications, there still exists some differences in the pathogenesis.
To our knowledge, this is the first meta-analysis to investigate the relationship between TLR4Asp299Gly gene polymorphism and DMI. The results showed that TLR4Asp299Gly gene polymorphism increased the risk of retinopathy in patients with T2DM(dominant model OR = 1.81, 95% CI (1.04–3.14), P = 0.03; allelic model OR = 1.77, 95% CI (1.05–2.98), P = 0.03), but there was no correlation between this gene polymorphism and DN or diabetic neuropathy(DN: dominant model OR = 1.19, 95% CI (0.81–1.74), P = 0.38; allelic model OR = 0.91, 95% CI (0.50–1.64), P = 0.75; diabetic neuropathy: dominant model OR = 1.42, 95% CI (0.76–2.67), P = 0.27; allelic model OR = 1.40, 95% CI (0.80–2.44), P = 0.24). Hence, we speculated that the expression of TLR4 or its ligands in retinal vessels, glomerular microvessels and neural tissues may exist tissue specificity, and TLR4/MyD88/NF-κB inflammatory response regulated by signal channels may have the greatest effect on retinal vessels. From this we can put forward a hypothesis: TLR4Asp299Gly gene polymorphism may be a susceptibility indicator of DR and the detection of this susceptibility gene can be used as a clinical diagnostic index, which may be a new target for drug therapy of DR in the future.
A number of studies have found that distribution of TLR4Asp299Gly site is population specificity. African populations have the highest Asp299Gly mutation rates, and then Caucasians, and the probability of mutation in Asian population, especially in Chinese Han population, is very low [39–41]. The study in Zhao et al. [42] found no genetic variation in Asp299Gly and Thr399Ile in Chinese Han populations, which is consistent with Huayin Cai’s findings [11]. Because TLR4Asp299Gly gene polymorphism varies widely among races, we performed a subgroup analysis of different ethnic groups in terms of Caucasian and Asian populations. The results showed that TLR4Asp299Gly gene polymorphism was associated with increased risk of DMI in Caucasian patients with T2DM under dominant model and allele model (dominant model OR = 1.69, 95% CI (1.22–2.35), P = 0.002; allelic model OR = 1.56, 95% CI (1.10–2.21), P = 0.01). This gene polymorphism may be a risk factor for DMI in the Caucasian population. Therefore, it may be used as a target for early prevention and treatment. But in the Asian population we found no correlation (dominant model OR = 0.85, 95% CI (0.52–1.39), P = 0.52; allelic model OR = 0.89, 95% CI (0.56–1.39), P = 0.60). This further confirmed that there are racial differences in TLR4Asp299Gly gene polymorphism. It's worth noting that the absence of GG genotype of TLR4Asp299Gly in some studies [15, 17, 20], So not all studies were included in recessive model and additive model analysis, which may have a certain impact on our final results.
Furthermore, we performed sensitivity analysis. The results showed that the corresponding OR value did not change significantly after the combination under the dominant model, which suggested that the overall results were statistically robust under this genetic model. However, the merge OR value and 95% CI (OR = 0.95, 95% CI (0.72–1.25), P = 0.70; heterogeneity: P = 0.81, I2 = 0) were significantly altered under the allelic model and the heterogeneity disappeared, suggesting that the heterogeneity among the meta-included studies may be attributed to these studies which are excluded by us. At the same time, we can conclude that there was no correlation between TLR4Asp299Gly gene polymorphism and DMI in the allelic model after sensitivity analysis.
In order to explain the results obtained better, some limitations of this meta-analysis should not be ignored. Firstly, DMI are a complex metabolic disease with multiple genetic and environmental factors. This meta analysis does not take into account the effects of other loci of TLR4 gene or other susceptible genes polymorphisms and environmental factors. Secondly, some studies have shown that glycated albumin(GA) can be used as a predictor of diabetes, its microvascular complications and cardiovascular prognosis compared with fasting glucose(FPG) and glycated hemoglobin(HbA1c), and it would be more convincing if GA was detected in our meta-included studies[43, 44]. Thirdly, there was heterogeneity among the studies included in the dominant and allelic model analysis. The heterogeneity was not completely eliminated by subgroup analysis with different race and difference of DMI types and sensitivity analysis.
Conclusions
In conclusion, this meta analysis showed that TLR4Asp299Gly gene polymorphism was associated with increased risk of DMI under dominant model, while no significant correlation was observed under allelic model, recessive model and additive model. TLR4Asp299Gly gene polymorphism may be considered as one of the risk factors for DMI. More importantly, our study have shown that this gene polymorphism increased the risk of DR in patients with T2DM, but not DN or diabetic neuropathy. Therefore, the detection of TLR4Asp299Gly can be used as a clinical diagnostic index and may be a new target for drug therapy of DR in the future.
Acknowledgements
Thanks to Mr. Matthew from Lanzhou University for polishing our English manuscript.
Abbreviations
- TLR4
Toll like receptor 4
- MyD88
Myeloid differentiation factor 88
- NF-KB
Nuclear factor-kappa B
- PCR
Polymerase chain reaction
- SSP-PCR
Polymerase chain reaction sequence specific primers
- PCR–RFLP
PCR-restriction fragment length polymorphism
- HWE
Hardy–Weinberg equilibrium
- DMI
Diabetic microvascular complications
- DR
Diabetic retinopathy
- DN
Diabetic nephropathy
- DPN
Diabetic polyneuropathy
- HCY
Homocysteinemia
- TnT
Troponin T
- ADMA
Asymmetric dimethylarginine
- GA
Glycated albumin
- BMI
Body mass index
- FBG
Fasting blood glucose
- HbA1c
Hemoglobin A1c
- TIR1
Interleukin 1
- LPS
Lipopolysaccharide
- HSP
Heat shock protein
- HMGB1
High Mobility Group Box 1 protein
- OX-LDL
Oxidized low density lipoprotein
Author contributions
YZ conceived the present study, participated in its design and was a major contributor in writing the manuscript. HLi, CW analyzed and interpreted the data regarding the relationship between TLR4Asp299Gly gene polymorphism and diabetic microvascular complications. HLv and SF jointly conceived the present study, participated in the coordination, helped to draft the manuscript and contributed to the interpretation of the results. We are grateful for the support from The First Hospital of Lanzhou University and The First Clinical Medical College of Lanzhou University. All authors read and approved the final manuscript.
Funding
This work was supported by Clinical Medical Research Center of Endocrine Diseases of Gansu Province (20JR10FA667), Science and Technology Planning project of Lanzhou Chengguan District (2021-2-7) and Special Funds of Science and Technology Development of the Chinese Central Government to guide Local in 2020.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential competing interests with respect to the authorship and/or publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuqi Zhang, Email: 1149826934@qq.com.
Huanhuan Li, Email: 1076251202@qq.com.
Chenyi Wang, Email: 18165104105@163.com.
Haihong Lv, Email: haihonglv@126.com.
Songbo Fu, Email: 1041685970@qq.com.
References
- 1.Girach A, Manner D, Porta M. Diabetic microvascular complications: can patients at risk be identified? A review. Int J Clin Pract. 2006;60(11):1471–1483. doi: 10.1111/j.1742-1241.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raman R, Gupta A, Krishna S, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic microvascular complications in newly diagnosed type II diabetes mellitus Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study (SN-DREAMS, report 27) J Diabetes Complicat. 2012;26(2):123–128. doi: 10.1016/j.jdiacomp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Bellia C, Bivona G, Scazzone C, Ciaccio M. Association between homocysteinemia and metabolic syndrome in patients with cardiovascular disease. Ther Clin Risk Manag. 2007;3(6):999–1001. [PMC free article] [PubMed] [Google Scholar]
- 6.Resl M, Vila G, Heinzl M, et al. Changes in the prognostic values of modern cardiovascular biomarkers in relation to duration of diabetes mellitus. J Diabetes Complicat. 2021;35(9):107990. doi: 10.1016/j.jdiacomp.2021.107990. [DOI] [PubMed] [Google Scholar]
- 7.Zinellu A, Sotgia S, Porcu P, et al. Carotid restenosis is associated with plasma ADMA concentrations in carotid endarterectomy patients. Clin Chem Lab Med. 2011;49(5):897–901. doi: 10.1515/CCLM.2011.121. [DOI] [PubMed] [Google Scholar]
- 8.Zhu T, Meng Q, Ji J, et al. Toll-like receptor 4 and tumor necrosis factor-alpha as diagnostic biomarkers for diabetic peripheral neuropathy. Neurosci Lett. 2015;585:28–32. doi: 10.1016/j.neulet.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Liang R. Quantitative assessment of TLR4 gene polymorphisms and T2DM risk: a meta-analysis. Mol Genet Genomic Med. 2020;8(10):e1466. doi: 10.1002/mgg3.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobias P, Curtiss LK. Thematic review series: the immune system and atherogenesis. Paying the price for pathogen protection: toll receptors in atherogenesis. J Lipid Res. 2005;46(3):404–411. doi: 10.1194/jlr.R400015-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Cai H, Cai J, Tao G. Association of toll-like receptor 4 polymorphisms with type 2 diabetes mellitus. APMIS. 2013;121(7):605–611. doi: 10.1111/apm.12027. [DOI] [PubMed] [Google Scholar]
- 12.Ohto U, Yamakawa N, Akashi-Takamura S, et al. Structural analyses of human Toll-like receptor 4 polymorphisms D299G and T399I. J Biol Chem. 2012;287(48):40611–40617. doi: 10.1074/jbc.M112.404608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin YW, Wang Q, Sun QQ, Hu AM, Liu HL. Toll-like receptor 4 gene Asp299Gly and Thr399Ile polymorphisms in type 2 diabetes mellitus: a meta-analysis of 15,059 subjects. Diabetes Res Clin Pract. 2015;107(3):338–347. doi: 10.1016/j.diabres.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Chang WW, Zhang L, Jin YL, Yao YS. Toll-like receptor 4 gene Asp299Gly and Thr399Ile polymorphisms in type 2 diabetes mellitus: a meta-analysis of 15,059 subjects: need for clarification of data in a recent meta-analysis. Diabetes Res Clin Pract. 2015;110(3):e31–32. doi: 10.1016/j.diabres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Khaghanzadeh N, Naderi N, Pournasrollah N, Farahbakhsh E, Kheirandish M, Samiei A. TLR4 polymorphisms (896A>G and 1196C>T) affect the predisposition to diabetic nephropathy in type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2020;13:1015–1021. doi: 10.2147/DMSO.S238942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buraczynska M, Zukowski P, Ksiazek K, Wacinski P, Dragan M. The effect of Toll-like receptor 4 gene polymorphism on vascular complications in type 2 diabetes patients. Diabetes Res Clin Pract. 2016;116:7–13. doi: 10.1016/j.diabres.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Balistreri CR, Bonfigli AR, Boemi M, et al. Evidences of+896 A/G TLR4 Polymorphism as an indicative of prevalence of complications in T2DM patients. Mediat Inflamm. 2014;2014. [DOI] [PMC free article] [PubMed]
- 18.Buraczynska M, Baranowicz-Gaszczyk I, Tarach J, Ksiazek A. Toll-like receptor 4 gene polymorphism and early onset of diabetic retinopathy in patients with type 2 diabetes. Hum Immunol. 2009;70(2):121–124. doi: 10.1016/j.humimm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Singh K, Kant S, Singh VK, Agrawal NK, Gupta SK, Singh K. Toll-like receptor 4 polymorphisms and their haplotypes modulate the risk of developing diabetic retinopathy in type 2 diabetes patients. Mol Vis. 2014;20:704–713. [PMC free article] [PubMed] [Google Scholar]
- 20.Zaharieva ET, Kamenov ZA, Savov AS. TLR4 polymorphisms seem not to be associated with prediabetes and type 2 diabetes but predispose to diabetic retinopathy; TLR4 polymorphisms in glucose continuum. Endocr Regul. 2017;51(3):137–144. doi: 10.1515/enr-2017-0014. [DOI] [PubMed] [Google Scholar]
- 21.Aioanei CS, Ilies RF, Bala C, et al. The role of adiponectin and toll-like receptor 4 gene polymorphisms on non-proliferative retinopathy in type 2 diabetes mellitus patients. A case-control study in Romanian Caucasians patients. Acta Endocrinol. 2019;5(1):32–38. doi: 10.4183/aeb.2019.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng D, Wang J, Pan J, et al. Lack of association between TLR4 genetic polymorphisms and diabetic nephropathy in a Chinese population. Biomed Res Int. 2014;2014:704167. doi: 10.1155/2014/704167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudofsky G, Jr, Reismann P, Witte S, et al. Asp299Gly and Thr399Ile genotypes of the TLR4 gene are associated with a reduced prevalence of diabetic neuropathy in patients with type 2 diabetes. Diabetes Care. 2004;27(1):179–183. doi: 10.2337/diacare.27.1.179. [DOI] [PubMed] [Google Scholar]
- 24.ZHANG HM. Study on the correlation between TLR4, RAGE gene polymorphism and diabetes mellitus in Wuhan Han population and the correlation between TLR4 expression and obesity in human white adipose tissue [D]. Huazhong University of Science and Technology, 2008.
- 25.Gay NJ, Gangloff M. Structure and function of Toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Zhu Y, Huang X, et al. Association between TLR2 and TLR4 Gene polymorphisms and the susceptibility to inflammatory bowel disease: a meta-analysis. PLoS ONE. 2015;10(5):e0126803. doi: 10.1371/journal.pone.0126803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87(6):989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 29.Rallabhandi P, Bell J, Boukhvalova MS, et al. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177(1):322–323. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 30.Tian J, Zhao Y, Wang L, et al. Role of TLR4/MyD88/NF-κB signaling in heart and liver-related complications in a rat model of type 2 diabetes mellitus. J Int Med Res. 2021;49(3):300060521997590. doi: 10.1177/0300060521997590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yehualashet AS. Toll-like receptors as a potential drug target for diabetes mellitus and diabetes-associated complications. Diabetes Metab Syndr Obes. 2020;13:4763. doi: 10.2147/DMSO.S274844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2(2):96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79(8 Suppl):1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- 34.Shukur W, Alyaqubi K, Dosh R, et al. Association of Toll-like receptors 4 (TLR4) gene expression and polymorphisms in patients with severe asthma. J Med Life. 2021;14(4):544–548. doi: 10.25122/jml-2021-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibrat JF, Garnier J, Robson B. Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs. J Mol Biol. 1987;198(3):425–443. doi: 10.1016/0022-2836(87)90292-0. [DOI] [PubMed] [Google Scholar]
- 36.Konda N, Kaur I, Garg P, Chakrabarti S, Willcox MDP. Toll-like receptor gene polymorphisms in patients with keratitis. Cont Lens Anterior Eye. 2021;44(3):101352. doi: 10.1016/j.clae.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Balistreri CR, Grimaldi MP, Chiappelli M, et al. Association between the polymorphisms of TLR4 and CD14 genes and Alzheimer's disease. Curr Pharm Des. 2008;14(26):2672–2677. doi: 10.2174/138161208786264089. [DOI] [PubMed] [Google Scholar]
- 38.Manolakis AC, Kapsoritakis AN, Tiaka EK, et al. TLR4 gene polymorphisms: evidence for protection against type 2 diabetes but not for diabetes-associated ischaemic heart disease. Eur J Endocrinol. 2011;165(2):261–267. doi: 10.1530/EJE-11-0280. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Jiang Z, Huang J, Meng Q, Coh P, Tao L. The association between toll-like receptor 4 polymorphisms and diabetic retinopathy in Chinese patients with type 2 diabetes. Br J Ophthalmol. 2015;99(9):1301–1305. doi: 10.1136/bjophthalmol-2015-306677. [DOI] [PubMed] [Google Scholar]
- 40.Yuan M, Xia J, Ma L, Xiao B, Yang Q. Lack of the toll-like receptor 4 gene polymorphisms Asp299Gly and Thr399ile in a Chinese population. Int J Neurosci. 2010;120(6):415–420. doi: 10.3109/00207451003778736. [DOI] [PubMed] [Google Scholar]
- 41.Mockenhaupt FP, Cramer JP, Hamann L, et al. Toll-like receptor (TLR) polymorphisms in African children: common TLR-4 variants predispose to severe malaria. Proc Natl Acad Sci USA. 2006;103(1):177–182. doi: 10.1073/pnas.0506803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng PL, Eng HL, Chou MH, You HL, Lin TM. Genetic polymorphisms of viral infection-associated Toll-like receptors in Chinese population. Transl Res. 2007;150(5):311–318. doi: 10.1016/j.trsl.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Bellia C, Zaninotto M, Cosma C, et al. Clinical usefulness of glycated albumin in the diagnosis of diabetes: results from an Italian study. Clin Biochem. 2018;54:68–72. doi: 10.1016/j.clinbiochem.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Bellia C, Cosma C, Lo Sasso B, et al. Glycated albumin as a glycaemic marker in patients with advanced chronic kidney disease and anaemia: a preliminary report. Scand J Clin Lab Invest. 2019;79(5):293–297. doi: 10.1080/00365513.2019.1613673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.