Abstract
The worldwide impact of the ongoing COVID-19 pandemic on public health has made imperative the discovery and development of direct-acting antivirals aimed at targeting viral and/or host targets. SARS-CoV-2 3C-like protease (3CLpro) has emerged as a validated target for the discovery of SARS-CoV-2 therapeutics because of the pivotal role it plays in viral replication. We describe herein the structure-guided design of highly potent inhibitors of SARS-CoV-2 3CLpro that incorporate in their structure novel spirocyclic design elements aimed at optimizing potency by accessing new chemical space. Inhibitors of both SARS-CoV-2 3CLpro and MERS-CoV 3CLpro that exhibit nM potency and high Safety Indices have been identified. The mechanism of action of the inhibitors and the structural determinants associated with binding were established using high-resolution cocrystal structures.
Graphical Abstract

INTRODUCTION
Severe Acute Respiratory Syndrome coronavirus-2 (SARS-CoV-2), the causative agent of coronavirus disease (COVID-19), is an enveloped, single stranded, positive-sense RNA β-coronavirus in the family Coronaviridae.1–3 SARS-CoV-2 infections are continuing to have a major impact on public health worldwide despite the availability of vaccines,4–5 and this is further exacerbated by the limited armamentarium of effective countermeasures that can be deployed to combat the virus, including emerging and re-emerging strains, underscoring the urgent need for the development of small-molecule therapeutics and prophylactics.6–9
The SARS-CoV-2 genome (~30 kb) encodes multiple structural (spike (S), envelope (E), membrane (M), and nucleocapsid (N)) and nonstructural proteins.1,10 The homotrimeric spike protein plays a critical role in viral attachment, fusion and entry by binding to the receptor binding domain of the host receptor (ACE2), followed by the furin-, transmembrane serine protease 2-, and cathepsin L-mediated fusion of viral and endosomal membranes, and the release of viral RNA into the cytosol.11–13 The replicase is expressed by two open reading frames that encode two large polyproteins (pp1a and pp1ab) which are processed by the 3C-like protease (3CLpro) and papain-like protease (PLpro), to generate mature structural and nonstructural proteins. The 3CLpro, also called main protease (Mpro), is an induced-fit enzyme with an extended binding cleft, a Cys-His catalytic dyad, and a primary substrate specificity for a P1 Gln residue and a preference for a P2 Leu.14–15 The enzyme is essential for viral replication; consequently, it is an attractive validated target for the development of direct-acting antivirals.16–22 SARS-CoV-2 3CLpro has been under intense investigation for the development of SARS-CoV-2 therapeutics by us23–29 and others.18,30–40 The rationale underlying the targeting of SARS-CoV-2 3CLpro is further buttressed by the first time demonstration of clinical efficacy by a feline coronavirus 3CLpro inhibitor.27–28 We report herein the results of preliminary studies related to the structure-guided design of potent inhibitors of SARS-CoV-2 3CLpro (Figure 1/general structure I) that incorporate in their structure a spirocyclic component as a design element to optimally exploit new chemical space in the active site of the protease.
Figure 1.

General structures of spirocyclic (I) and azetidine (II) inhibitors
RESULTS AND DISCUSSION
Inhibitor design rationale.
There is an array of advantages accrued through the judicious use of spirocycles in drug design, including improved physicochemical and PK characteristics, structural novelty, reduced conformational flexibility, and the capture of favorable binding interactions by probing and exploiting poorly-explored regions of chemical space.41–43 Importantly, the structural motifs embodied in spirocycles make possible the rigorous control of the spatial disposition of exit vectors; consequently, it was envisaged that the attachment of a suitably-decorated spirocycle capable of engaging in favorable binding interactions with the S4 subsite of SARS-CoV-2 3CLpro to a recognition element that is congruent with the known substrate specificity of the enzyme (a Leu-Gln surrogate fragment), can be leveraged to yield a molecule (Figure 1/general structure I) with high inhibitory prowess. The validity of the approach and the design of the inhibitors was further facilitated by the availability and use of high resolution cocrystal structures.16–18, 24–26 Finally, for comparative purposes, a series of azetidine-derived inhibitors (Figure 1/general structure II) were also synthesized and evaluated in biochemical and cell-based assays.
Chemistry
The inhibitors were readily synthesized by attaching a spirocyclic alcohol to a Leu-Gln surrogate fragment incorporating an aldehyde warhead or latent aldehyde bisulfite adduct. The spirocyclic and azetidine-based precursor alcohols were either commercially available or readily synthesized using commercially available ketone or carboxylic acid precursors. The appropriate spirocyclic and azetidine alcohol inputs (Figure 2) were treated with N, N’-disuccinimidyl carbonate (DSC)44, followed by coupling of the resulting mixed carbonate to amino alcohol A. Dess-Martin periodinane oxidation of dipeptidyl alcohol a generated the desired aldehydes b which were subsequently transformed into the corresponding aldehyde bisulfite adducts c (Scheme 1).45
Figure 2.

Alcohol precursors to 2-azaspiro [3.3]-, 6-azaspiro [3.5]-, 6-azaspiro [3.4]-, 2-azaspiro [3.4]- and azetidine-derived inhibitors
Scheme 1. General Synthesis of Inhibitors 1–18b/c.

a DSC/TEA/ACN/RT/4h,b A/TEA/DCM/RT/3h, c DMP/DCM/150C/3h, d NaHSO3/EtOAc/EtOH/500C/3h.
Biochemical Studies
The inhibitory activity of compounds 1–18b/c toward SARS-CoV-2 3CLpro in biochemical assays,23–25,28 as well as the cytotoxicity of the compounds, were determined and the results are listed in Tables 1 and 2. For comparative purposes, the interaction of a select number of compounds with MERS-CoV 3CLpro was also investigated.23 The IC50 and CC50 values of GC376 are included in Table 1. Selected compounds were tested in a cell-based assay against SARS-CoV-2 as described in the experimental section. The IC50 values, EC50 values for a select number of inhibitors, and the CC50 values in CRFK cells26 are summarized in Tables 1 and 2 and they are the average of at least three replicates.
Table 1.
IC50 values of spirocyclic inhibitors 1–11b/c against SARS-CoV-2 3CLpro and MERS-CoV 3CLpro, and CC50 values.

| |||||
|---|---|---|---|---|---|
| Compound Code | R | Z | IC50(μM)* | CC50 (μM) | |
| SARS-CoV-2 3CLpro | MERS-CoV 3CLpro | ||||
| 1b |

|
-CHO | 3.30±0.28 | 1.50±0.42 | >100 |
| 1c | -CH(OH)SO3Na | 0.85±0.07 | 0.28±0.11 | >100 | |
| 2b |

|
-CHO | 1.15±0.49 | 0.35±0.07 | >100 |
| 2c | -CH(OH)SO3Na | 0.65±0.07 | 0.55±0.07 | >100 | |
| 3b |

|
-CHO | 0.26±0.04 | 0.21±0.04 | >100 |
| 3c | -CH(OH)SO3Na | 0.26±0.05 | 0.13±0.03 | >100 | |
| 4b |

|
-CHO | 0.68±0.04 | 0.22±0.03 | >100 |
| 4c | -CH(OH)SO3Na | 0.76±0.08 | 0.16±0.01 | >100 | |
| 5b |

|
-CHO | 0.45±0.05 | 0.70±0.14 | >100 |
| 5c | -CH(OH)SO3Na | 0.75±0.07 | 0.75±0.07 | >100 | |
| 6b |

|
-CHO | 1.25±0.07 | 1.01±0.27 | >100 |
| 6c | -CH(OH)SO3Na | 1.20±0.14 | 1.30±0.14 | >100 | |
| 7b # |

|
-CHO | 0.36±0.06 | 1.20±0.14 | >100 |
| 7c # | -CH(OH)SO3Na | 0.29±0.02 | 0.95±0.21 | >100 | |
| 8b |

|
-CHO | 0.38±0.04 | 0.65±0.21 | >100 |
| 8c | -CH(OH)SO3Na | 0.41±0.01 | 0.52±0.12 | >100 | |
| 9b |

|
-CHO | 0.35±0.07 | 0.70±0.14 | >100 |
| 9c | -CH(OH)SO3Na | 0.29±0.06 | 0.61±0.13 | >100 | |
| 10b |

|
-CHO | 0.24±0.01 | 0.37±0.05 | >100 |
| 10c | -CH(OH)SO3Na | 0.24±0.03 | 0.33±0.04 | >100 | |
| 11b |

|
-CHO | 0.32±0.05 | 0.56±0.06 | >100 |
| 11c | -CH(OH)SO3Na | 0.39±0.03 | 0.63±0.18 | >100 | |
| GC376 |

|
-CH(OH)SO3Na | 0.41±0.07 | 0.25±0.10 | > 100 |
Mean ± SD of at least 3 replicates.
The EC50 values of the aldehyde and bisulfite salt adduct were determined to be 0.09±0.01 μM* and 0.08±0.02 μM*, respectively.
Table 2.
IC50 values of azetidine inhibitors 12–18b/c against SARS-CoV-2 3CLpro and MERS-CoV 3CLpro, and CC50 values.
| Compound Code | R | Z | IC50 (μM)* | CC50 (μM) | |
|---|---|---|---|---|---|
| SARS-CoV-2 3CLpro | MERS-CoV 3CLpro | ||||
| 12b |

|
-CHO | 2.50±0.28 | 1.65±0.64 | >100 |
| 12c | -CH(OH)SO3Na | 3.05±0.35 | 2.55±0.92 | >100 | |
| 13b |
|
-CHO | 3.65±0.64 | 3.45±1.20 | >100 |
| 13c | -CH(OH)SO3Na | 2.50±0.57 | 4.30±0.28 | >100 | |
| 14b# |

|
-CHO | 0.41±0.04 | 0.49±0.04 | >100 |
| 14c # | -CH(OH)SO3Na | 0.50±0.14 | 0.44±0.06 | >100 | |
| 15b |

|
-CHO | 0.83±0.04 | 0.28±0.11 | >100 |
| 15c | -CH(OH)SO3Na | 0.76±0.08 | 0.18±0.01 | >100 | |
| 16b |

|
-CHO | 0.52±0.14 | 0.19±0.04 | >100 |
| 16c | -CH(OH)SO3Na | 0.49±0.02 | 0.17±0.03 | >100 | |
| 17b |

|
-CHO | 4.95±0.49 | 1.40±0.14 | >100 |
| 17c | -CH(OH)SO3Na | 4.05±0.78 | 1.35±0.21 | >100 | |
| 18b |

|
-CHO | 0.33±0.04 | 0.35±0.01 | > 100 |
| 18c | -CH(OH)SO3Na | 0.34±0.01 | 0.37±0.06 | > 100 | |
Mean ± SD of at least 3 replicates.
The EC50 values of the aldehyde and bisulfite salt adduct were determined to be 0.38±0.07 (μM)* and 0.43±0.16 (μM)*, respectively.
We have previously reported EC50 values determined by the natural infection of SARS-CoV-2 in Vero E6 cells26 as well as a cell-based assay with two plasmids expressing SARS-CoV-2 3CLpro and luciferase fused with the 3CLpro cleavage site (VRLQS) in cells 25. While the latter system is a safe and fast BSL-2 based assay, EC50 values were relatively higher than those by natural infection of SARS-CoV-2 in Vero E6 cells. In this study, we used another BSL2 cell-based replicon assay in 293T cells, mimicking the natural cycle of SARS-CoV-2 replication.46 As a control, we used GC376 and the EC50 was calculated at 0.027±0.01 μM in the assay, which is comparable to the value (0.02 μM in 293T cells) previously reported with the same system.46 Four compounds were selected for the determination of EC50 values, and inhibition curves by each compound were consistent with a dose-dependent mode and R2 > 0.9 (Figure 3). The selected compounds were potent SARS-CoV-2 inhibitors with EC50 values ranging from 0.08 to 0.43 μM (Tables 1 and 2). These were correlated well with IC50 values.
Figure 3.

Inhibition curves of selected compounds, 7b, 7c, 14b and 14c in the cell-based SARS-CoV-2 replicon assay.
X-Ray Crystallographic Studies
In order to gain insight and understanding into the binding of the spirocyclic inhibitors to the active site of the protease, as well as identify the structural determinants associated with binding, high-resolution cocrystal structures of SARS-CoV-2 3CLpro and MERS-CoV 3CLpro were obtained in complex with spirocyclic and azetidine-derived inhibitors. For all structures described below, the electron density was consistent with both the R and S enantiomers at the stereocenter formed by covalent attachment of the Sγ atom of Cys 145 or Cys 148 in SARS-CoV-2 3CLpro and MERS-CoV 3CLpro, respectively. Therefore, the alternate conformations were modeled as each enantiomer with 0.5 occupancy.
Azetidine-derived inhibitor bound structures.
In the case of the azetidine inhibitor 14c, the active site contained prominent difference electron density consistent with the inhibitor covalently bound to Cys 148 and Cys 145 in each subunit (Figures 4A and 4B). Inhibitor 14c forms the typical hydrogen bonds to MERS-CoV 3CLpro and SARS-CoV-2 3CLpro (Figures 4C and 4D) along with an additional contact to the backbone nitrogen atom of Ala 191 in the case of SARS-CoV-2 3CLpro. This places the inhibitor deep within the S4 subsites as shown in Figures S1A and S1B. Superposition of the two structures revealed similar binding modes although the azetidine rings are rotated in the S4 subsite approximately 90° relative to one another (Figure S1C).
Figure 4.

Binding mode of the azetidine-derived inhibitor 14c to MERS-CoV 3CLpro (A and C) and SARS-CoV-2 3CLpro (B and D). Fo-Fc omit map (green mesh) contoured at 3σ (A and B). Hydrogen bond interactions (dashed lined) (C and D). PDB IDs: 14c with MERS-CoV 3CLpro (7T41), 14c with SARS-CoV-2 3CLpro (7T4B).
2-Azaspiro [3.3]-derived inhibitor bound structures.
Similar to the azetidine inhibitors above, difference electron density consistent with inhibitors 2c, 3c and 4c bound in the SARS-CoV-2 3CLpro active site covalently to Cys 145 (Figure 5 A–C). For 2c, the spirocyclic portion of the inhibitor that binds in the S4 subsite appears to adopt two conformations based on the electron density (Figure 5A). However, the isopropyl groups were disordered in both conformations. Inhibitor 3c, also adopted two conformations (Figure 5B) but the benzyl ring at the terminal end was disordered and could not be modeled. Interestingly, 4c appeared to adopt one conformation in the spirocyclic region of the inhibitor (Figure 5C) although electron density for the methyl sulfonyl group was not present, which indicated a certain degree of disorder in this region. It may be that the larger isopropyl and benzyl groups in 2c and 3c respectively, interact transiently with different regions in the S4 subsite and result in the observed dual conformations in the spirocycle relative to 4c. The inhibitors form the typical hydrogen bonds to the protein (Figure 5D–F) with an additional polar contact observed between the carbonyls of 2c and Leu 167 (Figure 5D). The diverse conformational differences in these inhibitors allows the spirocyclic portion of the compounds to cover a wide region of space within the S4 subsite as shown in Figure S2. Overall, superposition of these structures revealed a high degree of similarity in the ligand conformations. However, as evident in Figure 6, a large degree of motion is present in the spirocyclic region of the compounds with the largest span covering 8.5 Å in the case of inhibitor 2c.
Figure 5.

Binding modes of 2-azaspiro [3.3] inhibitors 2c (A and D), 3c (B and E) and 4c (C and F) with SARS-CoV-2 3CLpro. Fo-Fc omit map (green mesh) contoured at 3σ (A-C). Hydrogen bond interactions (dashed lined) (D-F). PDB IDs: 2c (7T42), 3c (7T43), 4c (7T44).
Figure 6.

Superposition of 2c (blue), 3c (gold) and 4c (green) inhibitors bound to SARS-CoV-2 3CLpro highlighting the broad conformations in the spirocyclic regions. PDB IDs: 2c (7T42), 3c (7T43), 4c (7T44).
6-Azaspiro [3.5]-derived inhibitor bound structures.
Interestingly, the spirocyclic inhibitors that contained the larger 6-membered nitrogen heterocycle did not display the same degree of disorder observed for 2c, 3c and 4c, which contain the 4-membered rings. This was revealed by the structure determination of 7c, 8c, 9c, 10c and 11c in complex with SARS-CoV-2 3CLpro in which the electron density was well-defined for the majority of these inhibitors (Figure 7A–C and Figure S3 A–B). These inhibitors form similar hydrogen bond interactions with the protein that are typically observed which include His 41, His 163, His 164, Glu 166, Gln 189 and bifurcated H-bonds between Glu 166 and Phe 140 and the NH of the δ-lactam ring (Figure 7 D–F and Figure S3 C–D). However, the structure with 9c adopts an additional polar contact (2.81 Å) between the carbonyl and the backbone carbonyl of Pro 168 (Figure 7E).
Figure 7.

Binding modes of 6-azaspiro [3.5] inhibitors 8c (A and D), 9c (B and E) and 10c (C and F) with SARS-CoV-2 3CLpro. Fo-Fc omit map (green mesh) contoured at 3σ (A-C). Hydrogen bond interactions (dashed lined) (D-F). PDB IDs: 8c (7T46), 9c (7T48), 10c (7T49).
Notably, the methyl sulfonyl group of 10c is in proximity to Pro 168 but too far to form an interaction (3.4 Å). The interaction between Pro 168 and 9c results in the movement (~2.6 Å) of a nearby loop that includes Leu 167, Pro 168 and Thr 169 relative to the other structures, such as 10c (Figure 8A). Overall, the structures with 7c, 8c and 11c adopt very similar binding modes (Figure 8B) in which the terminal ends of the inhibitors are positioned between a cleft formed by Glu 166 and Pro 168 (Figure S4 A–C). Inhibitor 10c is in an intermediate position as it is closer to Pro 168 within the hydrophobic ridge of the S4 subsite and 9c is the extreme case in which the benzyl ring is located on top of this ridge (Figure S4 D–E). As a whole, these inhibitors occupy a wide range of space within the S4 subsite spanning approximately 9.5 Å (Figure 8B). Notably, the extended length of the azaspiro[3.5] inhibitors relative to the azaspiro[3.3] compounds permits further engagement with the hydrophobic cleft of the S4 subsite. Presumably, this “locks” the azaspiro[3.5] inhibitors in a stable conformation and precludes the compounds from adopting multiple conformations (see Figures S2 and S4).
Figure 8.

Comparison of 6-azaspiro [3.5] inhibitors complexed with SARS-CoV-2 3CLpro. Superposition of 9c (coral) and 10c (gray) in complex with SARS-CoV-2 3CLpro. The protein residues are colored gold and magenta for 9c and 10c respectively (A). Superposition of 7c (green), 8c (cyan), 9c (coral), 10c (gray) and 11c (pink) (B). PDB IDs: 7c (7T45), 8c (7T46), 9c (7T48), 10c (7T49), 11c (7T4A).
Similarly, the structures of MERS-CoV 3CLpro with 8c, 9c and 10c yielded well-defined electron density overall (Figure 9 A–C) although the benzyl ring was disordered in 9c. The inhibitors form the typical array of hydrogen bond interactions with the protein, including Glu 169, His 41, His 166 and bifurcated H-bonds between Glu 169 and Phe 143 and the NH of the δ-lactam ring of the inhibitor (Figure 9 D–F). For the structure with 9c, an additional polar contact with the backbone carbonyl of Ala 171 (3.07 Å) positions the molecule in the S4 subsite in a similar pose as observed for 8c (Figure S5 A–B). Although the carbonyl in the structure of 8c is in a similar orientation as 9c, the distance to the backbone carbonyl of Ala 171 is much larger (4.07 Å). The binding mode of 10c differs from 8c and 9c in that the methyl sulfonyl group is positioned deeper within the S4 subsite (Figure S5 C) and is positioned 3.4 Å from His 194 potentially forming a salt bridge like interaction. The superimposed structures of MERS-CoV 3CLpro in complex with 8c, 9c and 10c are shown in Figure S6 show that these inhibitors span a space within the S4 subsite of approximately 8.0 Å. Collectively, the structural studies suggest that the use of spirocycles with different exit vectors are well-suited to exploiting new chemical space in and around the S4 subsite.
Figure 9.

Binding modes of 6-azaspiro [3.5] inhibitors 8c (A and D), 9c (B and E) and 10c (C and F) with MERS-CoV 3CLpro. Fo-Fc omit map (green mesh) contoured at 3σ (A-C). Hydrogen bond interactions (dashed lined) (D-F). PDB IDs: 8c (7T3Y), 9c (7T3Z), 10c (7T40).
Structure-Activity Relationships
A representative series of spirocyclic inhibitors derived from 2-azaspiro[3.3]-, 2-azaspiro[3.4]-, 6-azaspiro[3.4]-, and 6-azaspiro[3.5]-spirocycles displaying different exit vectors were synthesized and evaluated in biochemical and cell-based assays. It is evident from the results shown in Table 1 that the synthesized compounds generally display high inhibitory activity toward SARS-CoV-2 3CLpro and MERS-CoV 3CLpro, with the IC50 values of most of the inhibitors in the submicromolar range. Furthermore, the compounds are devoid of cytotoxic effects. The IC50 values of spirocycles 7b and 3b were found to be >9-fold and nearly 13-fold lower than that of compound 1b, respectively, suggesting that directional and recognition effects associated with the nature of the spirocycle and R group, respectively, are important in enhancing potency. The importance of exit vectors is also evident in comparing the relative potency of aldehyde inhibitors 1b, 5b and 6b which are derived from different spirocycles. The potency of compounds 8b, 9b, 10b and 11b was high and remained invariant to the nature of the R group. Several of the inhibitors were found to be broadly active against both SARS-CoV-2 3CLpro and MERS-CoV 3CLpro, suggesting a high likelihood for identifying a broad-spectrum preclinical candidate. The EC50 values of the aldehyde and corresponding bisulfite adduct pairs tested, were comparable, and one pair was in the nM range (Table 1, compounds 7b/7c). The Safety Index (SI), defined as CC50/EC50, for the compounds was very high (~1250). The results shown in Table 1 are congruent with the crystallographic studies (vide supra) and validate the use of spirocyclic inhibitors in exploring and exploiting new chemical space in the S4 region of SARS-CoV-2 3CLpro.
In the azetidine series, biochemical evaluation of the synthesized azetidine inhibitors revealed that the compounds were fairly potent against both SARS-CoV 3CLpro and MERS-CoV 3CLpro (Table 2). The IC50 values of compounds 14b/14c having an extra methylene group were >6-fold better than those of the 12b/12c pair. Furthermore, in the series of compounds 14b, 15b, 16b and 17b, potency was found to be sensitive to the nature of the group attached to the azetidine nitrogen, with compound 14b being 12-fold more potent than 17b and with an EC50 value of 0.38 μM. We previously harnessed the benefits accrued through deuteration by demonstrating that deuterated variants of GC-376 have enhanced antiviral activity and display efficacy in a fatal mouse model (K18-hACE2 mice) of SARS-CoV-2 infection.26 Thus, the effect of deuteration on pharmacological activity was investigated by determining the IC50 values of a representative deuterated aldehyde and bisulfite adduct pair 18b/18c. These were found to be comparable to those of the corresponding non-deuterated compounds 14b/14c. Although not established in the present studies, it is anticipated that deuterated variants of inhibitors reported herein will likely display improved PK characteristics.47 These dipeptidyl compounds, including GC376, have inhibitory activity against Cathepin L,62 and thus they could act as entry inhibitors against SARS-CoV-2. When we examined if 7b/7c and 14b/14c could inhibit the entry of SARS-CoV-2 using a pseudotyped lentivirus with S,63 the inhibition was moderate with EC50 values in the 2–10 μM range. Of note, because the EC50s listed in Tables 1–2 were determined with the SARS-CoV-2 replicon system46 which bypasses entry events, the inhibitory action was likely due to blocking 3CLpro.
CONCLUSIONS
There is currently a need for the development of direct-acting antivirals to complement the use of vaccines and biologics for the treatment of COVID-19. In this study we have sought to exploit the directional and stereochemical control afforded by spirocycles to optimize potency. The results indicate that the incorporation of spirocyclic elements embellished with appropriate recognition moieties, combined with structural information gained from cocrystal structures, into the design of process, has resulted in the identification of highly effective broad-spectrum inhibitors of SARS-CoV-2 3CLpro and MERS-CoV 3CLpro, with EC50 values and Safety Indices in the 0.08–0.43 μM and 1250–233 range, respectively. The structural determinants associated with binding and the mechanism of action involving participation of the catalytic dyad Cys145 and His41 and the formation of a tetrahedral adduct, were elucidated using X-ray crystallography. These studies provide a solid foundation for conducting further preclinical studies.
EXPERIMENTAL SECTION
General
Reagents and dry solvents were purchased from various chemical suppliers (Advanced ChemBlocks, Sigma-Aldrich, Acros Organics, Chem-Impex, TCI America, Oakwood chemical, APExBIO, SynQuest, Fisher and Bachem) and were used as obtained. The synthesized compounds were purified using flash chromatography and silica gel (230–450 mesh) (Sorbent Technologies, Atlanta, GA). Normal phase chromatography was performed on a Teledyne ISCO CombiFlash system using RediSep normal phase silica cartridges (35–70 μm particle size range). Thin layer chromatography was performed using Analtech silica gel plates. Visualization was accomplished using UV light and/or iodine. 1H NMR spectra were recorded in CDCl3 or DMSO-d6 using a Varian XL-400 spectrometer. Chemical shifts and coupling constants are reported in parts per million and hertz, respectively. The following abbreviations are used to describe splitting patterns: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad.
The purity of the inhibitors was determined by absolute qNMR analysis using a Bruker AV III 500 NMR spectrometer equipped with a CPDUL CRYOprobe and CASE autosampler (the University of Kansas Nuclear Magnetic Resonance Laboratory). Dimethyl sulfone TraceCERT® was used as the internal calibrant. High resolution mass spectrometry (HRMS) was performed at the Wichita State University Mass Spectrometry lab using an Orbitrap Velos Pro mass spectrometer (ThermoFisher, Waltham, MA) equipped with an electrospray ion source. The purity of the compounds in the b-series (aldehydes) was found to be ≥ 90% and that of the c-series (bisulfite adducts) was found to be ≥ 95%. Note: the generated aldehydes are prone to facile racemization involving the α-carbon of the aldehyde group. The protocol used to minimize racemization included fast and rigorous work up (<1 h) and rapid flash chromatography (silica gel/ethyl acetate/hexane gradient; <1 h). This protocol invariably yields aldehydes with racemization in the 0–5% range. With certain aldehydes, attainment of low racemization resulted in lower than 95% purity due to incomplete removal of Dess-Periodinane byproducts.
Synthesis of compounds
Preparation of compounds 1–18a. General procedure.
To a solution of alcohol (1 eq) (Table 1) in anhydrous acetonitrile (10 mL/g alcohol) was added N,N’-disuccinimidyl carbonate (1.2 eq) and TEA (3.0 eq) and the reaction mixture was stirred for 4h at room temperature. The solvent was removed in vacuo and the residue was dissolved in ethyl acetate (40 mL/g alcohol). The organic phase was washed with saturated aqueous NaHCO3 (2 × 20 mL/g alcohol), followed by brine (20 mL/g alcohol). The organic layers were combined and dried over anhydrous Na2SO4, filtered and concentrated in vacuo to yield the mixed carbonate which was used in the next step without further purification. To a solution of Leu-Gln surrogate amino alcohol A (1.0 eq) in dry methylene chloride (10 mL/g of amino alcohol) was added TEA (1.5 eq) and the reaction mixture was stirred for 20 min at room temperature (solution 1). In a separate flask, the mixed carbonate was dissolved in dry methylene chloride (10 mL/g of carbonate) (solution 2). Solution 1 was added to solution 2 and the reaction mixture was stirred 3h at room temperature. Methylene chloride was added to the organic phase (40 mL/g of carbonate) and then washed with saturated aqueous NaHCO3 (2 × 20 mL/g alcohol), followed by brine (20 mL/g alcohol). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The resultant crude product was purified by flash chromatography (hexane/ethyl acetate) to yield dipeptidyl alcohol a as a white solid.
Preparation of compounds 1–18b. General procedure.
To a solution of dipeptidyl alcohol a (1 eq) in anhydrous dichloromethane (100 mL/g dipeptidyl alcohol) kept at 0–5 °C under a N2 atmosphere was added Dess-Martin periodinane reagent (3.0 eq) and the reaction mixture was stirred for 3 h at 15–20 °C. The organic phase was washed with 10% aq Na2S2O3 (2 × 100 mL/g dipeptidyl alcohol), followed by saturated aqueous NaHCO3 (2 × 100 mL/g dipeptidyl alcohol), distilled water (2 × 100 mL/g dipeptidyl alcohol), and brine (100 mL/g dipeptidyl alcohol). The organic phase was dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The resulting crude product was purified by flash chromatography (hexane/ethyl acetate) to yield aldehyde b as a white solid.
tert-Butyl 6-((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)-2-azaspiro[3.3]heptane-2-carboxylate (1b).
1H NMR (500 MHz, DMSO-d6) δ 9.38 (d, J = 6.9 Hz, 1H), 8.44 (d, J = 7.6 Hz, 1H), 7.53 (s, 1H), 7.33 (d, J = 8.1 Hz, 1H), 4.74 – 4.60 (m, 1H), 4.08 – 3.89 (m, 2H), 3.81 (d, J = 26.2 Hz, 4H), 3.19 – 3.04 (m, 2H), 2.30 – 2.02 (m, 7H), 1.98 – 1.74 (m, 2H), 1.71 – 1.38 (m, 3H), 1.36 (s, 9H), 0.86 (ddd, J = 14.0, 10.5, 6.4 Hz, 6H). Yield (74%). HRMS m/z: [M+Na]+ Calculated for C25H40N4NaO7 531.2795; Found 531.2776.
2-Isobutyryl-2-azaspiro[3.3]heptan-6-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (2b).
Yield (24%). 1H NMR (400 MHz, cdcl3) δ 9.58 (s, 1H), 6.69 (s, 1H), 5.88 (s, 1H), 5.68 (s, 1H), 5.23 – 4.79 (m, 2H), 4.38 – 4.09 (m, 2H), 4.02 – 3.89 (m, 2H), 3.78 – 3.66 (m, 2H), 3.63 – 3.54 (m, 2H), 3.51 – 3.24 (m, 4H), 2.69 – 2.19 (m, 2H), 2.19 – 1.98 (m, 1H), 1.98 – 1.37 (m, 5H), 1.19 – 1.10 (m, 6H), 1.03 – 0.79 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C24H38N4NaO6 501.2689; Found 501.2672.
2-(2-Phenylacetyl)-2-azaspiro[3.3]heptan-6-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (3b).
Yield (69%). 1H NMR (400 MHz, cdcl3) δ 9.46 (s, 1H), 8.95 (d, J = 5.1 Hz, 1H), 7.40 – 7.22 (m, 5H), 6.61 (s, 1H), 5.87 (s, 1H), 5.17 (d, J = 8.5 Hz, 1H), 4.94 – 4.86 (m, 1H), 4.35 – 4.25 (m, 1H), 4.25 – 4.17 (m, 1H), 3.63 – 3.53 (m, 2H), 3.53 – 3.39 (m, 4H), 3.37 – 3.29 (m, 2H), 2.51 – 2.35 (m, 2H), 2.33 – 2.10 (m, 2H), 2.09 – 1.96 (m, 1H), 1.94 – 1.62 (m, 5H), 1.57 – 1.44 (m, 1H), 1.01 – 0.89 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C28H38N4NaO6 549.2689; Found 549.2675.
2-(Methylsulfonyl)-2-azaspiro[3.3]heptan-6-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (4b).
Yield (16%). 1H NMR (400 MHz, cdcl3) δ 9.45 (s, 1H), 8.12 (s, 1H), 6.67 (s, 1H), 6.24 (s, 1H), 5.03 – 4.79 (m, 1H), 4.23 (t, J = 11.4 Hz, 1H), 4.00 – 3.87 (m, 1H), 3.71 – 3.54 (m, 4H), 3.44 – 3.16 (m, 6H), 2.99 (s, 3H), 2.52 – 2.28 (m, 2H), 2.26 – 1.71 (m, 3H), 1.68 – 1.45 (m, 3H), 1.05 – 0.78 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C21H34N4NaO7S 509.2046; Found 509.1988.
tert-Butyl 2-((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)-6-azaspiro[3.4]octane-6-carboxylate (5b).
Yield (88%). 1H NMR (400 MHz, cdcl3) δ 9.49 (s, 1H), 8.34 (s, 1H), 6.06 (s, 1H), 5.28 – 5.17 (m, 1H), 5.02 – 4.89 (m, 1H), 4.38 – 4.12 (m, 2H), 3.47 – 3.19 (m, 6H), 2.55 – 2.29 (m, 4H), 2.19 – 1.80 (m, 7H), 1.80 – 1.61 (m, 2H), 1.61 – 1.49 (m, 1H), 1.45 (s, 9H), 1.01 – 0.89 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H42N4NaO7 545.2951; Found 545.2931.
tert-Butyl 6-((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)-2-azaspiro[3.4]octane-2-carboxylate (6b).
Yield (67%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (d, J = 2.0 Hz, 1H), 7.71 – 7.44 (m, 2H), 7.16 (dt, J = 51.0, 7.3 Hz, 1H), 5.01 – 4.83 (m, 1H), 4.65 (t, J = 5.6 Hz, 1H), 4.19 (td, J = 7.7, 4.0 Hz, 1H), 4.07 – 3.84 (m, 1H), 3.87 – 3.49 (m, 5H), 3.39 – 3.03 (m, 3H), 2.75 – 2.70 (m, 1H), 2.32 – 1.94 (m, 3H), 1.94 – 1.69 (m, 4H), 1.69 – 1.57 (m, 3H), 1.37 (s, 9H), 0.95 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H42N4NaO7 545.2951; Found 545.2928.
tert-Butyl 2-((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)-7-azaspiro[3.5]nonane-7-carboxylate (7b).
Yield (67%). 1H NMR (500 MHz, DMSO-d6) δ 7.63 (s, 1H), 7.51 (d, J = 6.9 Hz, 1H), 7.22 – 7.16 (m, 1H), 4.86 – 4.78 (m, 1H), 4.07 – 3.91 (m, 2H), 3.28 – 3.03 (m, 6H), 2.30 – 2.02 (m, 4H), 1.89 – 1.77 (m, 2H), 1.75 – 1.50 (m, 4H), 1.49 – 1.40 (m, 7H), 1.40 (s, 9H), 0.93 – 0.80 (m, 6H). HRMS m/z: [M+H]+ Calculated for C27H45N4O7 537.3288; Found 537.3257.
7-Isobutyryl-7-azaspiro[3.5]nonan-2-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (8b).
Yield (55%). 1H NMR (400 MHz, cdcl3) δ 9.49 (s, 1H), 8.33 (s, 1H), 6.21 – 6.13 (m, 1H), 5.29 – 5.22 (m, 1H), 5.01 – 4.93 (m, 1H), 4.32 (s, 2H), 3.61 – 3.45 (m, 2H), 3.44 – 3.28 (m, 4H), 2.82 – 2.69 (m, 1H), 2.54 – 2.27 (m, 5H), 2.12 – 1.93 (m, 2H), 1.92 – 1.81 (m, 3H), 1.79 – 1.63 (m, 1H), 1.56 (s, 5H), 1.10 (d, J = 6.7 Hz, 6H), 0.97 (d, J = 6.2 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C26H42N4NaO6 529.3002; Found 529.2985.
7-(2-Phenylacetyl)-7-azaspiro[3.5]nonan-2-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (9b).
Yield (87%). 1H NMR (400 MHz, cdcl3) δ 9.48 (s, 1H), 8.32 (s, 1H), 7.35 – 7.15 (m, 5H), 6.19 (d, J = 13.8 Hz, 1H), 5.28 – 5.21 (m, 1H), 5.00 – 4.86 (m, 1H), 4.37 – 4.10 (m, 2H), 3.72 (s, 2H), 3.59 – 3.44 (m, 2H), 3.42 – 3.23 (m, 4H), 2.52 – 2.34 (m, 2H), 2.34 – 2.18 (m, 2H), 2.12 – 1.90 (m, 1H), 1.90 – 1.76 (m, 3H), 1.74 – 1.60 (m, 1H), 1.58 – 1.43 (m, 4H), 1.41 – 1.32 (m, 3H), 0.96 (d, J = 6.1 Hz, 6H). HRMS m/z: [M+H]+ Calculated for C30H43N4O6 555.3182; Found 555.3156.
7-(Methylsulfonyl)-7-azaspiro[3.5]nonan-2-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (10b).
Yield (62%). 1H NMR (400 MHz, cdcl3) δ 9.49 (s, 1H), 8.33 (d, J = 5.8 Hz, 1H), 6.10 (s, 1H), 5.25 (d, J = 8.6 Hz, 1H), 5.03 – 4.89 (m, 1H), 4.31 (s, 2H), 3.44 – 3.29 (m, 2H), 3.21 – 3.00 (m, 4H), 2.76 (s, 3H), 2.55 – 2.20 (m, 4H), 2.09 – 1.80 (m, 4H), 1.69 (td, J = 12.3, 7.5 Hz, 7H), 1.54 (t, J = 8.8 Hz, 1H), 0.97 (d, J = 6.2 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C23H38N4NaO7S 537.2359; Found 537.2341.
7-Cyano-7-azaspiro[3.5]nonan-2-yl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (11b).
Yield (53%). 1H NMR (400 MHz, cdcl3) δ 9.49 (s, 1H), 8.36 (d, J = 5.7 Hz, 1H), 5.95 (s, 1H), 5.21 (d, J = 8.3 Hz, 1H), 5.04 – 4.89 (m, 1H), 4.38 – 4.25 (m, 2H), 3.45 – 3.30 (m, 2H), 3.19 – 3.08 (m, 4H), 2.56 – 2.22 (m, 4H), 2.01 – 1.81 (m, 4H), 1.77 – 1.62 (m, 7H), 1.61 – 1.48 (m, 1H), 0.97 (d, J = 5.8 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C23H35N5NaO5 484.2536; Found 484.2522.
tert-Butyl 3-((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)azetidine-1-carboxylate (12b).
Yield (74%). 1H NMR (500 MHz, DMSO-d6) δ 7.78 (s, 1H), 7.68 – 7.61 (m, 1H), 7.54 – 7.47 (m, 1H), 5.01 – 4.90 (m, 1H), 4.19 – 4.05 (m, 2H), 4.05 – 3.61 (m, 4H), 3.26 – 3.04 (m, 2H), 2.27 – 2.02 (m, 3H), 1.86 – 1.71 (m, 2H), 1.70 – 1.39 (m, 4H), 1.38 – 1.34 (m, 9H), 0.92 – 0.79 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C22H36N4NaO7 491.2482; Found 491.2461.
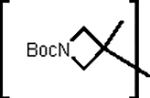
tert-Butyl 3-methyl-3-((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)azetidine-1-carboxylate (13b).
Yield (76%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (d, J = 4.9 Hz, 1H), 8.45 (d, J = 7.8 Hz, 1H), 7.63 (s, 1H), 7.50 (d, J = 7.7 Hz, 1H), 4.22 (ddd, J = 11.6, 7.7, 3.9 Hz, 1H), 4.08 – 3.93 (m, 1H), 3.88 (d, J = 9.3 Hz, 2H), 3.78 (d, J = 9.4 Hz, 2H), 3.23 – 3.02 (m, 2H), 2.34 – 2.07 (m, 2H), 1.96 – 1.84 (m, 1H), 1.63 (ddt, J = 16.1, 11.8, 6.3 Hz, 3H), 1.55 (s, 3H), 1.46 (qd, J = 8.4, 3.9 Hz, 2H), 1.37 (s, 9H), 0.93 – 0.82 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H38N4NaO7 505.2638; Found 505.2621.
tert-Butyl 3-(((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)methyl)azetidine-1-carboxylate (14b).
Yield (90%). 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 8.45 (d, J = 7.5 Hz, 1H), 7.63 (s, 1H), 7.40 (d, J = 7.9 Hz, 1H), 4.21 – 3.99 (m, 3H), 3.92 – 3.82 (m, 2H), 3.62 – 3.52 (m, 2H), 3.21 – 3.05 (m, 2H), 2.83 – 2.72 (m, 2H), 2.34 – 2.06 (m, 2H), 1.95 – 1.83 (m, 2H), 1.70 – 1.56 (m, 3H), 1.52 – 1.42 (m, 1H), 1.37 (s, 9H), 0.92 – 0.83 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H38N4NaO7 505.2638; Found 505.2609.
(1-(2-Phenylacetyl)azetidin-3-yl)methyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (15b).
Yield (63%). 1H NMR (400 MHz, cdcl3) δ 9.46 (s, 1H), 8.73 – 8.66 (m, 1H), 7.40 – 7.16 (m, 5H), 6.38 (d, J = 32.7 Hz, 1H), 6.14 (d, J = 22.3 Hz, 1H), 5.32 (d, J = 16.0 Hz, 1H), 4.35 – 3.89 (m, 4H), 3.81 – 3.65 (m, 2H), 3.60 – 3.43 (m, 2H), 3.40 – 3.13 (m, 4H), 2.57 – 2.19 (m, 2H), 2.06 (s, 1H), 1.98 – 1.77 (m, 2H), 1.75 – 1.61 (m, 2H), 1.57 – 1.45 (m, 1H), 1.03 – 0.82 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H36N4NaO6 523.2533; Found 523.2518.
(1-(Bicyclo[2.2.1]heptane-2-carbonyl)azetidin-3-yl)methyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (16b).
Yield (75%). 1H NMR (400 MHz, cdcl3) δ 9.47 (s, 1H), 8.85 (s, 1H), 6.28 (d, J = 42.6 Hz, 1H), 6.10 (d, J = 32.7 Hz, 1H), 5.32 – 5.28 (m, 1H), 4.45 – 3.98 (m, 5H), 3.94 – 3.70 (m, 1H), 3.69 – 3.52 (m, 2H), 3.51 – 3.16 (m, 3H), 2.69 – 2.56 (m, 1H), 2.56 – 2.21 (m, 5H), 1.96 – 1.67 (m, 6H), 1.66 – 1.46 (m, 2H), 1.43 – 1.23 (m, 3H), 1.18 (q, J = 8.3 Hz, 1H), 0.97 (d, J = 5.6 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C26H40N4NaO6 527.2846; Found 527.2837.
(1-(Methylsulfonyl)azetidin-3-yl)methyl ((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamate (17b).
Yield (14%). 1H NMR (400 MHz, cdcl3) δ 9.48 (s, 1H), 8.28 (d, J = 7.5 Hz, 1H), 6.56 (s, 1H), 5.54 (s, 1H), 4.46 – 3.91 (m, 2H), 3.90 – 3.73 (m, 2H), 3.70 – 3.10 (m, 4H), 3.02 – 2.71 (m, 2H), 2.57 – 2.16 (m, 3H), 2.16 – 1.78 (m, 1H), 1.75 – 1.48 (m, 3H), 1.46 – 1.36 (m, 2H), 1.26 (s, 3H), 1.08 – 0.78 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C19H32N4NaO7S 483.1890; Found 483.1832.
tert-Butyl 3-(((((S)-4-methyl-1-oxo-1-(((S)-1-oxo-3-((R)-2-oxopyrrolidin-3-yl)propan-2-yl)amino)pentan-2-yl)carbamoyl)oxy)methyl-d2)azetidine-1-carboxylate (18b).
Yield (80%). 1H NMR (400 MHz, dmso) δ 9.40 (s, 1H), 8.45 (d, J = 7.6 Hz, 1H), 7.64 (s, 1H), 7.40 (d, J = 7.9 Hz, 1H), 4.23 – 4.13 (m, 1H), 4.11 – 3.98 (m, 1H), 3.94 – 3.79 (m, 2H), 3.63 – 3.52 (m, 2H), 3.21 – 3.05 (m, 2H), 2.77 (d, J = 5.6 Hz, 1H), 2.38 – 2.07 (m, 2H), 1.95 – 1.83 (m, 1H), 1.72 – 1.58 (m, 3H), 1.52 – 1.39 (m, 2H), 1.36 (s, 9H), 0.87 (dd, J = 10.2, 6.6 Hz, 6H). HRMS m/z: [M+Na]+ Calculated for C23H36D2N4NaO7 507.2764; Found 507.2768.
Preparation of compounds 1–18c. General procedure.
To a solution of dipeptidyl aldehyde b (1 eq) in ethyl acetate (10 mL/g of dipeptidyl aldehyde) was added absolute ethanol (5 mL/g of dipeptidyl aldehyde) with stirring, followed by a solution of sodium bisulfite (1 eq) in water (1 mL/g of dipeptidyl aldehyde). The reaction mixture was stirred for 3 h at 50 °C. The reaction mixture was allowed to cool to room temperature and then vacuum filtered. The solid was thoroughly washed with absolute ethanol and the filtrate was dried over anhydrous sodium sulfate, filtered, and concentrated to yield a white solid. The white solid was stirred with dry ethyl ether (3 × 10 mL/g of dipeptidyl aldehyde), followed by careful removal of the solvent using a pipette and dried using a vacuum pump for 2 h to yield dipeptidyl bisulfite adduct c as a white solid.
Sodium (2S)-2-((S)-2-((((2-(tert-butoxycarbonyl)-2-azaspiro[3.3]heptan-6-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (1c).
Yield (56%). 1H NMR (400 MHz, DMSO-d6) δ 7.52 (d, J = 9.3 Hz, 1H), 7.44 (s, 1H), 7.18 (d, J = 8.2 Hz, 1H), 5.71 (d, J = 5.9 Hz, 1H), 4.74 – 4.59 (m, 2H), 4.08 – 3.58 (m, 5H), 3.23 – 2.99 (m, 2H), 2.29 – 1.94 (m, 4H), 1.91 – 1.71 (m, 1H), 1.69 – 1.38 (m, 7H), 1.35 (s, 9H), 0.91 – 0.79 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C25H41N4Na2O10S 635.2339; Found 635.2379.
Sodium (2S)-1-hydroxy-2-((S)-2-((((2-isobutyryl-2-azaspiro[3.3]heptan-6-yl)oxy)carbonyl)amino)-4-methylpentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (2c).
Yield (69%). 1H NMR (400 MHz, DMSO-d6) δ 7.85 (s, 1H), 7.64 (s, 1H), 7.19 (s, 1H), 5.80 – 5.66 (m, 1H), 4.90 – 4.60 (m, 2H), 4.28 – 3.81 (m, 2H), 3.81 – 3.59 (m, 2H), 3.25 – 2.98 (m, 4H), 2.47 – 2.34 (m, 1H), 2.34 – 1.75 (m, 7H), 1.75 – 1.29 (m, 4H), 1.01 (d, J = 6.8 Hz, 6H), 0.95 – 0.77 (m, 6H). HRMS m/z: [M+H]+ Calculated for C24H40N4NaO9S 583.2413; Found 583.2675.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((2-(2-phenylacetyl)-2-azaspiro[3.3]heptan-6-yl)oxy)carbonyl)amino)pentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (3c).
Yield (97%). 1H NMR (400 MHz, DMSO-d6) δ 8.41 – 8.32 (m, 1H), 8.21 (dd, J = 13.7, 7.3 Hz, 1H), 7.47 (d, J = 3.9 Hz, 1H), 7.35 – 7.14 (m, 5H), 5.55 (dd, J = 188.2, 6.3 Hz, 1H), 4.86 – 4.70 (m, 1H), 4.07 – 3.87 (m, 2H), 3.86 – 3.54 (m, 2H), 3.49 – 3.42 (m, 2H), 3.42 – 3.31 (m, 4H), 3.29 – 2.99 (m, 2H), 2.32 – 1.85 (m, 6H), 1.70 – 1.49 (m, 2H), 1.49 – 1.39 (m, 2H), 0.93 – 0.79 (m, 6H). HRMS m/z: [M+H]+ Calculated for C28H40N4NaO9S 631.2853; Found 631.2413.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((2-(methylsulfonyl)-2-azaspiro[3.3]heptan-6-yl)oxy)carbonyl)amino)pentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (4c).
Yield (90%). 1H NMR (400 MHz, DMSO-d6) δ 7.63 (s, 1H), 7.37 (d, J = 8.0 Hz, 1H), 7.19 – 7.15 (m, 1H), 5.73 – 5.67 (m, 1H), 5.01 – 4.78 (m, 2H), 4.78 – 4.59 (m, 1H), 4.09 – 3.67 (m, 6H), 3.23 – 2.98 (m, 4H), 2.91 (s, 3H), 2.38 – 2.06 (m, 4H), 2.06 – 1.76 (m, 2H), 1.73 – 1.53 (m, 1H), 1.53 – 1.33 (m, 1H), 0.98 – 0.78 (m, 6H). HRMS m/z: [M+H]+ Calculated for C21H36N4NaO10S2 591.1770; Found 591.1647.
Sodium (2S)-2-((S)-2-((((6-(tert-butoxycarbonyl)-6-azaspiro[3.4]octan-2-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (5c).
Yield (22%). 1H NMR (400 MHz, DMSO-d6) δ 7.63 (s, 1H), 7.53 (s, 1H), 7.24 – 7.20 (m, 1H), 5.73 – 5.68 (m, 1H), 4.86 – 4.77 (m, 1H), 4.07 – 3.77 (m, 2H), 3.67 – 3.38 (m, 4H), 3.28 – 2.95 (m, 6H), 2.37 – 2.20 (m, 2H), 2.20 – 2.05 (m, 1H), 2.05 – 1.88 (m, 2H), 1.88 – 1.74 (m, 3H), 1.74 – 1.45 (m, 2H), 1.39 (s, 9H), 0.92 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H43N4Na2O10S 649.2496; Found 649.2458.
Sodium (2S)-2-((2S)-2-((((2-(tert-butoxycarbonyl)-2-azaspiro[3.4]octan-6-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (6c).
Yield (7%). 1H NMR (400 MHz, DMSO-d6) δ 7.63 – 7.38 (m, 2H), 7.30 – 7.03 (m, 1H), 5.30 (dt, J = 54.1, 5.9 Hz, 1H), 5.00 – 4.81 (m, 1H), 4.66 (t, J = 5.6 Hz, 1H), 4.02 – 3.86 (m, 2H), 3.83 – 3.48 (m, 4H), 3.37 – 2.97 (m, 3H), 2.29 – 1.96 (m, 3H), 1.96 – 1.67 (m, 5H), 1.67 – 1.48 (m, 4H), 1.37 (s, 9H), 0.94 – 0.77 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H43N4Na2O10S 649.2496; Found 649.2454.
Sodium (2S)-2-((S)-2-((((7-(tert-butoxycarbonyl)-7-azaspiro[3.5]nonan-2-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (7c).
Yield (87%). 1H NMR (400 MHz, DMSO-d6) δ 7.57 – 7.50 (m, 1H), 7.45 (s, 1H), 7.28 (dd, J = 35.4, 8.4 Hz, 1H), 5.33 (dd, J = 56.7, 6.1 Hz, 1H), 4.88 – 4.77 (m, 2H), 4.42 – 4.10 (m, 1H), 4.07 – 3.76 (m, 4H), 3.27 – 3.00 (m, 6H), 2.36 – 1.85 (m, 4H), 1.85 – 1.66 (m, 1H), 1.65 – 1.50 (m, 1H), 1.43 (d, J = 14.3 Hz, 6H), 1.38 (s, 9H), 0.89 – 0.79 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C27H45N4Na2O10S 663.2652; Found 663.2690.
Sodium (2S)-1-hydroxy-2-((S)-2-((((7-isobutyryl-7-azaspiro[3.5]nonan-2-yl)oxy)carbonyl)amino)-4-methylpentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (8c).
Yield (80%). 1H NMR (400 MHz, DMSO-d6) δ 7.63 (s, 1H), 7.46 (s, 1H), 7.36 – 7.28 (m, 1H), 5.42 (dd, J = 64.4, 6.1 Hz, 1H), 4.87 – 4.81 (m, 1H), 4.52 – 4.12 (m, 2H), 4.09 – 3.80 (m, 2H), 3.22 – 2.97 (m, 4H), 2.88 – 2.79 (m, 2H), 2.37 – 2.18 (m, 3H), 2.18 – 1.96 (m, 1H), 1.96 – 1.68 (m, 3H), 1.68 – 1.32 (m, 8H), 1.00 – 0.94 (m, 6H), 0.92 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C26H43N4Na2O9S 633.2546; Found 633.2526.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((7-(2-phenylacetyl)-7-azaspiro[3.5]nonan-2-yl)oxy)carbonyl)amino)pentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (9c).
Yield (68%). 1H NMR (400 MHz, DMSO-d6) δ 7.60 – 7.49 (m, 2H), 7.45 (s, 1H), 7.35 – 7.11 (m, 5H), 5.38 (dd, J = 60.0, 6.1 Hz, 1H), 4.86 – 4.73 (m, 2H), 4.44 – 4.12 (m, 1H), 4.06 – 3.77 (m, 4H), 3.71 – 3.61 (m, 4H), 3.22 – 2.99 (m, 2H), 2.35 – 2.03 (m, 4H), 2.03 – 1.79 (m, 1H), 1.78 – 1.65 (m, 1H), 1.63 – 1.49 (m, 1H), 1.48 – 1.27 (m, 7H), 0.91 – 0.79 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C30H43N4Na2O9S 681.2546; Found 681.2522.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((7-(methylsulfonyl)-7-azaspiro[3.5]nonan-2-yl)oxy)carbonyl)amino)pentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (10c).
Yield (71%). 1H NMR (400 MHz, DMSO-d6) δ 7.62 (d, J = 9.3 Hz, 1H), 7.45 (s, 1H), 7.38 – 7.31 (m, 1H), 5.41 (dd, J = 73.2, 6.1 Hz, 1H), 4.88 – 4.76 (m, 1H), 4.28 – 3.76 (m, 2H), 3.21 – 2.91 (m, 6H), 2.83 (s, 3H), 2.35 – 1.98 (m, 3H), 1.96 – 1.68 (m, 4H), 1.67 – 1.50 (m, 6H), 1.49 – 1.32 (m, 2H), 1.14 – 1.01 (m, 1H), 0.91 – 0.78 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H39N4Na2O10S2 641.1903; Found 641.1874.
Sodium (2S)-2-((S)-2-((((7-cyano-7-azaspiro[3.5]nonan-2-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (11c).
Yield (74%). 1H NMR (400 MHz, DMSO-d6) δ 8.43 (d, J = 7.6 Hz, 1H), 7.63 (s, 1H), 7.30 (d, J = 8.0 Hz, 1H), 4.86 – 4.76 (m, 1H), 4.26 – 4.08 (m, 1H), 4.06 – 3.80 (m, 1H), 3.40 – 3.24 (m, 2H), 3.22 – 3.00 (m, 4H), 2.36 – 2.02 (m, 4H), 1.95 – 1.63 (m, 2H), 1.62 – 1.49 (m, 7H), 1.49 – 1.30 (m, 2H), 1.15 – 1.02 (m, 2H), 0.96 – 0.76 (m, 6H). HRMS m/z: [M+H]+ Calculated for C23H37N5NaO8S 566.2260; Found 566.2238.
Sodium (2S)-2-((S)-2-((((1-(tert-butoxycarbonyl)azetidin-3-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (12c).
Yield (64%). 1H NMR (400 MHz, DMSO-d6) δ 7.66 (d, J = 11.1 Hz, 2H), 7.58 – 7.42 (m, 1H), 5.01 – 4.90 (m, 2H), 4.71 – 4.64 (m, 1H), 4.23 – 3.84 (m, 3H), 3.84 – 3.51 (m, 2H), 3.19 – 3.04 (m, 2H), 2.34 – 2.01 (m, 2H), 2.00 – 1.73 (m, 1H), 1.71 – 1.43 (m, 5H), 1.38 (s, 9H), 0.92 – 0.81 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C22H37N4Na2O10S 595.2026; Found 595.1995.
Sodium (2S)-2-((S)-2-((((1-(tert-butoxycarbonyl)-3-methylazetidin-3-yl)oxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (13c).
Yield (33%). 1H NMR (400 MHz, DMSO-d6) δ 7.64 (d, J = 10.0 Hz, 1H), 7.58 – 7.35 (m, 2H), 4.29 – 4.10 (m, 1H), 4.08 – 3.86 (m, 3H), 3.77 – 3.69 (m, 3H), 3.18 – 2.98 (m, 2H), 2.37 – 2.04 (m, 2H), 2.02 – 1.77 (m, 1H), 1.77 – 1.50 (m, 6H), 1.48 – 1.34 (m, 11H), 0.93 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H39N4Na2O10S 609.2183; Found 609.2160.
Sodium (2S)-2-((S)-2-((((1-(tert-butoxycarbonyl)azetidin-3-yl)methoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (14c).
Yield (57%). 1H NMR (400 MHz, DMSO-d6) δ 7.59 (dd, J = 9.2, 5.5 Hz, 1H), 7.43 (s, 1H), 7.36 – 7.23 (m, 1H), 5.34 (dd, J = 69.8, 6.1 Hz, 1H), 4.14 – 4.01 (m, 2H), 4.01 – 3.76 (m, 3H), 3.62 – 3.47 (m, 2H), 3.20 – 2.98 (m, 3H), 2.87 – 2.67 (m, 1H), 2.24 – 2.06 (m, 3H), 2.04 – 1.80 (m, 1H), 1.72 – 1.48 (m, 3H), 1.46 – 1.39 (m, 1H), 1.37 (s, 9H), 0.92 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H39N4Na2O10S 609.2183; Found 609.2205.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((1-(2-phenylacetyl)azetidin-3-yl)methoxy)carbonyl)amino)pentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (15c).
Yield (92%). 1H NMR (400 MHz, DMSO-d6) δ 8.16 (s, 1H), 7.64 (s, 1H), 7.52 – 7.44 (m, 1H), 7.34 – 7.14 (m, 5H), 4.22 (d, J = 6.5 Hz, 2H), 4.14 – 3.79 (m, 4H), 3.72 – 3.54 (m, 2H), 3.50 – 3.38 (m, 2H), 3.23 – 3.00 (m, 4H), 2.38 – 1.95 (m, 3H), 1.93 – 1.72 (m, 1H), 1.72 – 1.53 (m, 2H), 1.53 – 1.30 (m, 2H), 0.92 – 0.80 (m, 6H). HRMS m/z: [M+H]+ Calculated for C26H38N4NaO9S 605.2257; Found 605.2698.
Sodium (2S)-2-((2S)-2-((((1-(bicyclo[2.2.1]heptane-2-carbonyl)azetidin-3-yl)methoxy)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (16c).
Yield (71%). 1H NMR (400 MHz, DMSO-d6) δ 7.78 (s, 1H), 7.64 (s, 1H), 7.38 (s, 1H), 4.18 (s, 1H), 4.13 – 3.79 (m, 3H), 3.72 – 3.54 (m, 2H), 3.26 – 2.96 (m, 4H), 2.70 – 2.55 (m, 1H), 2.36 – 2.02 (m, 4H), 2.00 – 1.76 (m, 1H), 1.75 – 1.32 (m, 9H), 1.29 – 1.19 (m, 4H), 1.19 – 1.00 (m, 2H), 0.93 – 0.77 (m, 6H). HRMS m/z: [M+H]+ Calculated for C26H42N4NaO9S 609.2570; Found 609.3013.
Sodium (2S)-1-hydroxy-2-((S)-4-methyl-2-((((1-(methylsulfonyl)azetidin-3-yl)methoxy)carbonyl)amino)pentanamido)-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (17c).
Yield (88%). 1H NMR (400 MHz, DMSO-d6) δ 7.64 (s, 1H), 7.16 (s, 1H), 6.96 (s, 1H), 4.67 (s, 2H), 4.29 – 3.83 (m, 5H), 3.81 – 3.52 (m, 3H), 3.24 – 2.96 (m, 2H), 2.36 – 2.02 (m, 2H), 1.95 – 1.73 (m, 1H), 1.59 (s, 4H), 1.37 (s, 3H), 1.24 (s, 1H), 0.97 – 0.77 (m, 6H). HRMS m/z: [M+H]+ Calculated for C19H34N4NaO10S2 565.1614; Found 565.1878.
Sodium (2S)-2-((S)-2-((((1-(tert-butoxycarbonyl)azetidin-3-yl)methoxy-d2)carbonyl)amino)-4-methylpentanamido)-1-hydroxy-3-((R)-2-oxopyrrolidin-3-yl)propane-1-sulfonate (18c).
Yield (71%). 1H NMR (400 MHz, dmso) δ 7.68 – 7.56 (m, 1H), 7.50 – 7.42 (m, 1H), 7.39 – 7.23 (m, 1H), 5.42 (dd, J = 73.8, 6.2 Hz, 1H), 4.28 – 3.96 (m, 2H), 3.94 – 3.75 (m, 2H), 3.68 – 3.50 (m, 2H), 3.16 – 3.00 (m, 2H), 2.83 – 2.70 (m, 1H), 2.27 – 1.80 (m, 4H), 1.66 – 1.47 (m, 2H), 1.47 – 1.40 (m, 2H), 1.37 (s, 9H), 0.93 – 0.80 (m, 6H). HRMS m/z: [M+Na]+ Calculated for C23H37D2N4Na2O10S 611.2308; Found 611.2258.
Biochemical Studies
Enzyme assays and inhibition studies.
Cloning and expression of the 3CL protease of SARS-CoV-2 and FRET enzyme assays.
The codon-optimized cDNA of full length of 3CLpro of SARS-CoV-2 (GenBank number MN908947.3) fused with sequences encoding 6 histidine at the N-terminal was synthesized by Integrated DNA (Coralville, IA). The synthesized gene was subcloned into the pET-28a(+) vector. The expression and purification of SARS-CoV-2 3CLpro were conducted following a standard procedure described previously.23,28,29
Briefly, a stock solution of an inhibitor was prepared in DMSO and diluted in assay buffer comprised of 20 mM HEPES buffer, pH 8, containing NaCl (200 mM), EDTA (0.4 mM), glycerol (60%), and 6 mM dithiothreitol (DTT). The SARS-CoV-2 protease was mixed with serial dilutions of inhibitors 1–18b/c or with DMSO in 25 μL of assay buffer and incubated at 37°C for 1 h, followed by the addition of 25 μL of assay buffer containing substrate (FAM-SAVLQ/SG-QXL®520, AnaSpec, Fremont, CA). The substrate was derived from the cleavage sites on the viral polyproteins of SARS-CoV. Fluorescence readings were obtained using an excitation wavelength of 480 nm and an emission wavelength of 520 nm on a fluorescence microplate reader (FLx800; Biotec, Winoosk, VT) 1 h following the addition of substrate. Relative fluorescence units (RFU) were determined by subtracting background values (substrate-containing well without protease) from the raw fluorescence values, as described previously.29 The dose-dependent FRET inhibition curves were fitted with a variable slope by using GraphPad Prism software (GraphPad, La Jolla, CA) in order to determine the IC50 values of the compounds. To assess if the compounds have a broad-spectrum activity to other coronaviruses, they were also examined against MERS-CoV 3CLpro as described before.23
Antiviral Assays/Cell-based inhibition assays.
To assess antiviral effects of selected compounds (dissolved in DMSO) in cell culture, the SARS-CoV-2 replicon system with pSMART-T7-scv2-replicon (pSMART® BAC V2.0 Vector Containing the SARS-CoV-2, Wuhan-Hu-1 Non-Infectious Replicon) was used.46 The synthetic SARS-CoV-2 replicon RNA was prepared from the pSMART-T7-scv2-replicon as described,47 and the Neon Electroporation system (ThermoFisher, Chicago, IL) was used for the RNA electroporation to 293T cells. After the electroporation, cells were incubated with DMSO (0.1%) or each compound at 2, 0.5, 0.1 and 0.02 uM for 30 hr, and luciferase activities were measured for antiviral effects. The dose-dependent inhibition curve for each compound was prepared and the 50% effective concentration (EC50) values were determined by GraphPad Prism software using a variable slope (GraphPad, La Jolla, CA).
Nonspecific cytotoxic effects/Measurement of in vitro cytotoxicity.
Confluent cells grown in 96-well plates were incubated with various concentrations (1 to 100 μM) of each compound for 72 h. Cell cytotoxicity was measured by a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI), and the CC50 values were calculated using a variable slope by GraphPad Prism software. The in vitro Safety Index was calculated by dividing the CC50 by the EC50.
X-ray Crystallographic Studies
Crystallization and Data Collection.
Purified MERS-CoV 3CLpro and SARS-CoV-2 3CLpro in 100mM NaCl, 20mM Tris pH 8.0 were concentrated to 10 mg/mL (0.3 mM) for crystallization screening. Stock solutions of the inhibitors were prepared in DMSO at 100 mM and the complexes with the 3CL proteases were prepared by adding 2 mM of each compound and incubating the complexes on ice for 1 hour. All crystallization experiments were setup using an NT8 drop-setting robot (Formulatrix Inc.) and UVXPO MRC (Molecular Dimensions) sitting drop vapor diffusion plates at 18 °C. 100 nL of protein and 100 nL crystallization solution were dispensed and equilibrated against 50 uL of the latter. Crystals of the MERS-CoV 3CLpro complexes were obtained from the following conditions. Index HT screen (Hampton Research) 9c: condition E7 (30% (w/v) PEG 550 MME, 100 mM Hepes pH 7.5, 50 mM magnesium chloride), 8c: condition F7 (20% (w/v) PEG 3350, 100 mM Bis-Tris pH 6.5, 200 mM ammonium sulfate) and 10c: condition F5 (17% (w/v) PEG 10000, 100 mM Bis-Tris pH 5.5, 100 mM ammonium acetate). Proplex HT screen (Molecular Dimensions) 14c: condition E2 (25% (w/v) PEG 3350, 100 mM Hepes pH 7.5, 200 mM magnesium chloride). Crystals of the SARS-CoV-2 3CLpro complexes were obtained from the following conditions. PACT screen (Molecular Dimensions) 2c: condition C2 (25% (w/v) PEG 1500, 100 mM PCTP pH 5.0), 3c: condition C1 (25% (w/v) PEG 1500, 100 mM PCTP pH 4.0), 11c: condition E1 (20 % (w/v) PEG 3350, 20 mM sodium/postassium phosphate) and 10c: condition D4 (25 % (w/v) PEG 1500, 100 MMT pH 7.0), Index HT screen (Hampton Research) 4c: condition F5 (17% (w/v) PEG 10000, 100 mM Bis-Tris pH 5.5, 100 mM ammonium acetate), 8c: condition F10 (25 % (w/v) PEG 3350, 100 mM Bis-Tris pH 5.5, 200 mM NaCl), 14c: condition F11 (25% (w/v) PEG 3350, 100 mM Bis-Tris pH 6.5, 200 mM sodium chloride), 9c: condition G4 (20% (w/v) PEG 3350, 100 mM Hepes pH 7.5, 200 mM lithium sulfate) and Berkeley screen (Rigaku Reagents) 7c: condition B6 (20% (w/v) PEG 3350, 200 mM sodium fluoride). Cryoprotectants containing 80% crystallant and 20% (v/v) PEG 200 were layered onto the drop, samples were harvested and stored in liquid nitrogen. For MERS-CoV 3CLpro in complex with 9c, the crystallization solution served as the cryoprotectant. X-ray diffraction data were collected at the Advanced Photon Source beamline 17-ID (IMCA-CAT) and National Synchrotron Light Source-II, beamline 19-ID (NYX).
Structure Solution and Refinement.
Intensities were integrated using XDS48–49 via Autoproc50 and the Laue class analysis and data scaling were performed with Aimless51. Structure solution was conducted by molecular replacement with Phaser52 using a previously determined inhibitor bound structures of MERS-CoV (5WKK) and SARS-CoV-2 3CLpro (PDB 6XMK) as the search models. Structure refinement and manual model building were conducted with Phenix53 and Coot54, respectively. Disordered side chains were truncated to the point for which electron density could be observed. Structure validation was conducted with Molprobity55 and figures were prepared using the CCP4MG package.56 Crystallographic data are provided in Tables S1 and S2. 57–61
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported in part by grants from the National Institutes of Health (NIH) (grants R01 AI109039 and AI161085 to K.O.C). Use of the IMCA-CAT beamline 17-ID at the Advanced Photon Source was supported by the companies of the Industrial Macromolecular Crystallography Association through a contract with Hauptman-Woodward Medical Research Institute. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-06CH11357. This research used the Biological Microdiffraction Facility beamline 19-ID (NYX) at the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. Support for the NMR instrumentation was provided by NIH Shared Instrumentation Grant # S10RR024664 and NSF Major Research Instrumentation Award # 1625923. Use of the University of Kansas Protein Structure Laboratory was supported by a grant from the National Institute of General Medical Sciences (P30GM110761) of the NIH. The Center for BioMolecular Structure (CBMS) is primarily supported by the National Institutes of Health, National Institute of General Medical Sciences (NIGMS) through a Center Core P30 Grant (P30GM133893), and by the DOE Office of Biological and Environmental Research (KP1605010). The following reagent was obtained through BEI Resources, NIAID, NIH: pSMART® BAC V2.0 Vector Containing the SARS-Related Coronavirus 2, Wuhan-Hu-1 Non-Infectious Replicon, NR-54972.
ABBREVIATIONS USED
- CC50
50% cytotoxic concentration in cell-based assays
- CDI
carbonyl diimidazole
- CPE
cytopathic effects
- DMSO
dimethyl sulfoxide
- DMP
Dess-Martin periodinane
- DSC
N,N’-disuccinimidyl carbonate
- DTT
dithiothreitol
- EC50
the 50% effective concentration in cell culture
- GESAMT
general efficient structural alignment of macromolecular targets
- IC50
the 50% inhibitory concentration in the enzyme assay
- MME
monomethyl ether
- MNV
murine norovirus
- MOI
multiplicity of infection
- ORF
open reading frame
- PK
pharmacokinetics
- RMSD
root mean square deviation
- TCID50
the 50% tissue culture infectious dose
- TEA
Triethyl amine
- XDS
X-ray detector software
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Supplemental information - comparison of 14c bound to MERS-CoV 3CLpro and SARS-CoV-2 3CLpro, comparison of azaspiro [3.3] inhibitors 2c, 3c, 4c bound to SARS-CoV-2 3CLpro, binding modes of azaspiro [3.5] inhibitors 7c, 11c with SARS-CoV-2 3CLpro, comparison of azaspiro [3.5] inhibitors 7c, 8c, 11c, 10c, 9c bound to SARS-CoV-2 3CLpro, comparison of azaspiro [3.5] inhibitors 8c, 9c, 10c with MERS-CoV 3CLpro, crystallographic data for SARS-CoV-2 3CLpro inhibitor complexes, and absolute qNMR data. (.docx)
Molecular formula strings – SMILES codes (.csv)
PDB Validation reports for new X-ray crystal structures (.pdf)
Accession Codes
Coordinates and structure factors for complexes with the following with inhibitors were deposited to the Worldwide Protein Databank (wwPDB) with the accession codes: MERS-CoV 3CLpro complexes: 8c (7T3Y), 9c (7T3Z), 10c (7T40), 14c (7T41) and SARS-CoV-2 3CLpro complexes: 2c (7T42), 3c (7T43), 4c (7T44), 7c (7T45), 8c (7T46), 9c (7T48), 10c (7T49), 11c (7T4A), 14c (7T4B). Authors will release the atomic coordinates upon article publication.
REFERENCES
- 1.Perlman S, Masters PS; Coronaviridae: The Viruses and Their Replication, in Fields Virology: Emerging Viruses; Howley PM, Knipe DM, Whelan S, Eds.; Wolters Kluwer: Wichita, 2020; Vol. 1, pp 410–448. [Google Scholar]
- 2.Wu F; Zhao S; Yu B; Chen Y-M; Wang W; Song Z-G; Hu Y; Tao Z-W; Tian J-H; Pei Y-Y; Yuan M-L; Zhang Y-L; Dai F-H; Liu Y; Wang Q-M; Zheng J-J; Xu L; Holmes EC; Zhang Y-Z A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N; Zhang D; Wang W; Li X; Yang B; Song J; Zhao X; Huang B; Shi W; Lu R; Niu P; Zhan F; Ma X; Wang D; Xu W; Wu G; Gao GF; Tan W A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2020, 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu B; Guo H; Zhou P; Shi Z-L Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison AG; Lin T; Wang P Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020, 40, 1100–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh AK; Brindisi M; Shahabi D; Chapman ME; Mesecar AD Drug development and medicinal chemistry efforts toward SARS-Coronavirus and Covid-19 therapeutics. Chem. Med. Chem 2020, 15, 907–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil C; Ginex T; Maestro I; Nozal V; Barrado-Gil L; Cuesta-Geijo MA; Urquiza J; Ramírez D; Alonso C; Campillo NE, Martinez A, COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020, 63, 12359–12386. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A; Tiwari S; Deb MK; Marty JL, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): A global pandemic and treatments strategies. Intl J. Antimicrob. Agents 2020, 56, 106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannalire R; Cerchia C; Beccari AR; Di Leva FS; Summa V, Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: State of the art and future opportunities. J. Med. Chem. [online early access]. DOI: 10.1021/acs.jmedchem.0c01140. Published online: Nov 13, 2020. 10.1021/acs.jmedchem.0c01140 (accessed Nov 13, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimoto FK; The proteins of Severe Acute Respiratory Coronavirus-2 (SARS-CoV-2 or nCOVID-19), the cause of COVID-19. Protein J. 2020, 39, 198–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirano T; Murakami M; COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity 2020, 52, 731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tai W; He L; Zhang X; Pu J; Voronin D; Jiang S; Zhou Y; Du L Characterization of the receptor binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y-W; Chao T-L; Li C-L; Chiu M-F; Kao H-C; Wang S-H; Pang Y-H; Lin C-H; Tsai Y-M; Lee W-H; Tao M-H; Ho T-C; Wu P-Y; Jang L-T; Chen P-J; Chang S-Y; Yeh S-H Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell. Rep. 2020, 33, 108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The nomenclature used is that of Schechter, I. and Berger, A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Com 2012, 425, 497–502, where the residues on the N-terminus side of the peptide bond that is cleaved are designated as P1-Pn and those on the C-terminus side are designated P1-P1’. The corresponding active site subsites are designated S1-Sn and S1-Sn’. P1 is the primary substrate specificity residue and P1-P1’ is the scissile bond. [DOI] [PubMed] [Google Scholar]
- 15.Rut W; Groborz K; Zhang L; Sun X; Zmudzinski M; Pawlik B; Młynarski W; Hilgenfeld R; Drag M Substrate specificity profiling of SARS-CoV-2 main protease enables design of activity-based probes for patient-sampling imaging. bioRxiv, [online early access]. DOI: 10.1101/2020.03.07.981928 Published online: Mar 7, 2020. 10.1101/2020.03.07.981928. (accessed Mar 7, 2020). [DOI] [Google Scholar]
- 16.Zhang L; Lin D; Sun X; Curth U; Drosten C; Sauerhering L; Becker S; Rox K; Hilgenfeld R, Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Z; Du X; Xu Y; Deng Y; Liu M; Zhao Y; Zhang B; Li X; Zhang L; Peng C, Duan Y; Yu J; Wang L; Yang K; Liu F; Jiang R; Yang X; You T; Liu X; Yang X; Bai F; Liu H; Liu X; Guddat LW; Xu W; Xiao G; Qin C; Shi Z; Jiang H; Rao Z; Yang H, Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [DOI] [PubMed] [Google Scholar]
- 18.Dai W; Zhang B; Jiang X-M; Su H; Li J; Zhao Y; Xie X; Jin Z; Peng J; Liu F, Li C; Li Y; Bai F; Wang H; Cheng X; Cen X; Hu S; Yang X; Wang J,; Liu X; Xiao G; Jiang H; Rao Z; Zhang L-K; Xu Y; Yang H; Liu H Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullrich S; Nitsche C, The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020, 30, 127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J; Hu L; Huang X; Wang C; Zhang Z; Wang Y; Zhang D; Ye W, Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: Insights from structures of protease and inhibitors. Intl. J. Antimicrob. Agents 2020, 56, 106055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rut W; Lv Z; Zmudzinski M; Patchett S; Nayak D; Snipas SJ; El Oualid F; Huang TT; Bekes M; Drag M; Olsen SK Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti–COVID-19 drug design. Sci. Adv. 2020, 6, eabd4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerjee R; Perera L; Tillekeratne LMV Potential SARS-CoV-2 main protease inhibitors. Drug. Discov. Today 2021, 26, 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathnayake AD; Zheng J; Kim Y; Perera KD; Mackin S; Meyerholz DK; Kashipathy MM; Battaile KP; Lovell S; Perlman S, 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV–infected mice. Science Transl. Med. 2020, 12, eabc5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dampalla CS; Kim Y; Bickmeier N; Rathnayake AD; Nguyen HN; Zheng J; Kashipathy MM; Baird MA; Battaile KP; Lovell S; Perlman S; Chang KO; Groutas WC Structure-guided design of conformationally-constrained cyclohexane inhibitors of severe acute respiratory syndrome coronavirus-2 3CL protease. J. Med. Chem. 2021, 64, 10047–10058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dampalla CS; Rathnayake AD; Perera KD; Jesri A-RM; Nguyen HN; Miller MJ; Thurman HA; Zheng J; Kashipathy MM; Battaille KP; Lovell S; Perlman S; Kim Y; Groutas WC; Chang KO Structure-guided design of potent inhibitors of SARS-CoV-2 3CL protease: structural, biochemical, and cell-based studies. J. Med. Chem. 2021, 64, 17846–17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dampalla CS; Zheng J; Perera KD; Wong L-YR; Meyerholz DK; Nguyen HN; Kashipathy MM; Battaile KP; Lovell S; Kim Y; Perlman S; Groutas WC; Chang K-O . Post-infection treatment with a protease inhibitor increases survival of mice with a fatal SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA, 2021,118, e2101555118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen NC; Kim Y; Liu H; Galasiti Kankanamalage AC; Eckstrand C; Groutas WC; Bannasch M; Meadows JM; Chang K-O, Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg 2018, 20, 378–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y; Liu H; Galasiti Kankanamalage AC; Weerasekara S; Hua DH; Groutas WC; Chang K-O; Pedersen NC, Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathogens 2016, 12, e1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y; Lovell S; Tiew K-C; Mandadapu SR; Alliston KR; Battaile KP; Groutas WC; Chang K-O, Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 2012, 86, 11754–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao J; Li Y-S; Zeng R; Liu F-L; Luo R-H; Huang C; Wang Y-F; Zhang J; Quan B; Shen C; Mao X,; Liu X; Sun W; Yang W; Ni X; Wang K; Xu L; Duan Z-L; Zou Q-C; Zhang H-L; Qu W; Long Y-H-P; Li M-H; Yang R-C; Liu X; You J; Zhou Y; Yao R; Li W-P; Liu J-M; Chen P; Liu Y; Lin G-F; Yang X; Zou J; Li L; Hu Y; Lu G-W; Li W-M; Wei Y-Q; Zheng Y-T; Lei J; Yang S, SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 2021, 371, 1374–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai B; Belovodskiy A; Hena M; Kandadai AS; Joyce MA; Saffran HA; Shields JA; Khan MB; Arutyunova E; Lu J; Bajwa SK; Hockman D; Fischer C; Lamer T; Vuong W; van Belkum MJ; Gu Z; Lin F; Du Y; Xu J; Rahim M; Young HS; Vederas JC; Tyrrell DL; Lemieux MJ; Nieman JA Peptidomimetic α-acyloxymethylketone warheads with six-membered lactam P1 glutamine mimic: SARS-CoV-2 3CL protease inhibition, coronavirus antiviral activity, and in vitro biological stability. J. Med. Chem. [online early access]. DOI: 10.1021/acs.jmedchem.1c00616. Published online: Jul 9, 2021. 10.1021/acs.jmedchem.1c00616 (accessed Jul 9, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh AK; Raghavaiah J; Shahabi D; Yadav M; Anson BJ; Lendy EK; Hattori S; Higashi-Kuwata N; Mitsuya H; Mesecar AD Indole chloropyridinyl ester-derived SARS-CoV-2 3CLpro inhibitors: enzyme inhibition, antiviral efficacy, structure-activity relationship and X-ray structural studies. J. Med. Chem. 2021, 64, 14702–14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konno S; Kobayashi K; Senda M; Funai Y; Seki Y; Tamai I; Schakel L; Sakata K; Pillaiyar T; Taguchi A; Taniguchi A; Gutschow M; Muller CE; Takeuchi K; Hirohama M; Kawaguchi A; Kojima M; Senda T; Shirasaka Y; Kamitani W; Hayashi Y 3CL protease inhibitors with an electrophilic acylketone moiety as anti-SARS-CoV-2 agents. J. Med. Chem. [online early access]. DOI: 10.1021/acs.jmedchem.1c00665. Published online: Jul 27, 2021. 10.1021/acs.jmedchem.1c00665 (accessed Jul 27, 2021). [DOI] [PubMed] [Google Scholar]
- 34.Li L; Chenna BC; Yang KS; Cole TR; Goodall ZT; Giardini M; Moghadamchargari Z; Hernandez EA; Gomez J; Calvet CM; Bernatchez JA; Mellott DM; Zhu J; Rademacher A; Thomas D; Blankenship LR; Drelich A; Laganowsky A; Tseng C-TK; Liu WR; Wand AJ; Cruz-Reyes J; Siqueira-Neto J; Meek TD Self-masked aldehyde inhibitors: a novel strategy for inhibiting cysteine proteases. J. Med. Chem. 2021, 64, 11267–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SH; Goins CM; Arya T; Shin W-J; Maw J; Hooper A; Sonawane DP; Porter MR; Bannister BE; Crouch RD; Lindsey AA; Lakatos G; Martinez SR; Alvarado J; Akers WS; Wang NS; Jung JU; Macdonald JD; Stauffer SR Structure-based optimization of ML300-derived, noncovalent inhibitors targeting the Severe Acute Respiratory Syndrome Coronavirus 3CL protease (SARS-CoV-3CLpro). J. Med. Chem. [online early access]. DOI: 10.1021/acs.jmedchem.1c00598. Published online: Aug 4, 2021. 10.1021/acs.jmedchem.1c00598 (accessed Aug 4, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breidenbach J; Lemke C; Pillaiyar T; Schakel L; Al Hamwi G; Diett M; Gedschold R; Geiger N; Lopez V; Mirza S; Namasivayam V; Schiedel AC; Sylvester K; Thimm D; Vielmuth C; Vu LP; Zyulina M; Bodem J; Gutschow M; Muller CE Targeting the main protease of SARS-CoV-2: from the establishment of a high throughput screening to the design of tailored inhibitors. Angew. Chem. Int. Ed. Engl, 2021, 60, 10423–10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshmukh MG; Ippolito JA; Zhang C-H; Stone EA; Reilly RA; Miller SJ; Jorgensen WL; Anderson KS Structure-guided design of a perampanel-derived pharmacophore targeting the SARS-CoV-2 main protease. Structure. 2021, 29, 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen DR; Allerton CMN; Anderson AS; Aschenbrenner L; Avery M; Berritt S; Boras B; Cardin RD; Carlo A; Coffman KJ; Dantonio A; Di L; Eng H; Ferre R; Gajiwala KS; Gibson SA; Greasley SE; Hurst BL; Kadar EP; Kalgutkar AS; Lee JC; Lee J; Liu W; Mason SW; Noell S; Novak JJ; Obach RS; Ogilvie K; Patel NC; Pettersson M; Rai DK; Reese MR; Sammons MF; Sathish JG; Singh RSP; Steppan CM; Stewart AE; Tuttle JB; Updyke L; Verhoest PR; Wei L; Yang Q; Zhu Y An oral SARS-0CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021, 374, 1586–1593. [DOI] [PubMed] [Google Scholar]
- 39.Boras B; Jones RM; Anson BJ; Arenson D; Aschenbrenner L; Bakowski MA; Beutler N; Binder J; Chen E; Eng H; Hammond H; Hammond J; Haupt RE; Hoffman R; Kadar EP; Kania R; Kimoto E; Kirkpatrick MG; Lanyon L; Lendy EK; Lillis JR; Logue J; Luthra SA; Ma C; Mason SW; McGrath ME; Noell S; Obach RS; O’ Brien MN; O’Connor R; Ogilvie K; Owen D; Pettersson M; Reese MR; Rogers TF; Rosales R; Rossulek MI; Sathish JG; Shirai N; Steppan C; Ticehurst M; Updyke LW; Weston S; Zhu Y; White KM; García-Sastre A; Wang J; Chatterjee AK; Mesecar AD; Frieman MB; Anderson AS; Allerton C Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID 19. Nat. Commun. 2021, 12, 6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman RL; Kania RS; Brothers MA; Davies JF; Ferre RA; Gajiwala KS; He M; Hogan RJ; Kozminski K; LI LY; Lockner JW; Lou J; Marra MT; Mitchell LJ Jr; Murray BW; Nieman JA; Noell S; Planken SP; Rowe T; Ryan K; Smith III GJ; Solowiej JE; Steppan CM; Taggart B Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 2020, 63, 12725–12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carreira EM; Fessard TC Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Revs 2014, 114, 8257–8322. [DOI] [PubMed] [Google Scholar]
- 42.Talele TT Opportunities for tapping into three-dimensional chemical space through a quaternary carbon. J. Med. Chem. 2020, 63, 13291–13315. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y; Tice CM; Singh SB The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh AK; Duong TT; McKee SP; Thompson WJ, N, N’-Disuccinimidyl carbonate: A useful reagent for alkoxycarbonylation of amines. Tetrahedron Lett. 1992, 33, 2781–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kjell DP; Slattery BJ; Semo MJ, A novel, nonaqueous method for regeneration of aldehydes from bisulfite adducts. J. Org. Chem. 1999, 64, 5722–5724. [DOI] [PubMed] [Google Scholar]
- 46.He X; Quan S; Xu M; Rodriguez S; Goh SL; Wei J; Fridman A; Koeplinger KA; Carroll SS; Grobler JA; Espeseth AS; Olsen DB; Hazudas DJ; Wang D Generation of SARS-CoV-2 reporter replicon for high-throughput antiviral screening and testing. Proc. Natl. Acad. Sci. USA. 2021, 118, e2025866118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pirali T; Serafini M; Cargnin S; Genazzani AA, Applications of deuterium in medicinal chemistry. J. Med. Chem. 2019, 62, 5276–5297. [DOI] [PubMed] [Google Scholar]
- 48.Kabsch W, Automatic indexing of rotation diffraction patterns. J. Applied. Crystallogr. 1988, 21, 67–72. [Google Scholar]
- 49.Kabsch W, XDS. Acta. Cryst. 2010, D66, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vonrhein C; Flensburg C; Keller P; Sharff A; Smart O; Paciorek W; Womack T; Bricogne G, Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. Section D: Biolog. Crystallogr. 2011, 67, 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans PR, An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. Section D: Biolog. Crystallogr. 2011, 67, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCoy AJ; Grosse-Kunstleve RW; Adams PD; Winn MD; Storoni LC; Read RJ, Phaser crystallographic software. J. Applied Crystallogr. 2007, 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams PD; Afonine PV; Bunkóczi G; Chen VB; Davis IW; Echols N; Headd JJ; Hung L-W; Kapral GJ; Grosse-Kunstleve RW, PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Section D: Biolog. Crystallogr. 2010, 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emsley P; Lohkamp B; Scott WG; Cowtan K, Features and development of Coot. Acta Crystallogr. Section D: Biolog. Crystallogr. 2010, 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen VB; Arendall WB; Headd JJ; Keedy DA; Immormino RM; Kapral GJ; Murray LW; Richardson JS; Richardson DC, MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. Section D: Biolog. Crystallogr. 2010, 66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potterton L; McNicholas S; Krissinel E; Gruber J; Cowtan K; Emsley P; Murshudov GN; Cohen S; Perrakis A; Noble M, Developments in the CCP4 molecular-graphics project. Acta Crystallogr. Section D: Biolog. Crystallogr. 2004, 60, 2288–2294. [DOI] [PubMed] [Google Scholar]
- 57.Evans P Scaling and assessment of data quality, Acta Crystallogr. Sect D: Biol. Crystallogr. 2006, 62, 72–82. [DOI] [PubMed] [Google Scholar]
- 58.Diederichs K; Karplus PA Improved R-factors for diffraction data analysis in macromolecular crystallography, Nat Struct Biol. 1997, 4, 269–275. [DOI] [PubMed] [Google Scholar]
- 59.Weiss MS Global indicators of X-ray data quality, J. Appl. Crystallogr. 2001, 34 130–135. [Google Scholar]
- 60.Karplus PA; Diederichs K Linking crystallographic model and data quality, Science 2012, 336, 1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans P Biochemistry. Resolving some old problems in protein crystallography, Science 2012, 336, 986–987. [DOI] [PubMed] [Google Scholar]
- 62.Sacco MD; Ma C; Lagarias P; Gao A; Townsend JA; Meng X; Dube P; Zhang X; Hu Y; Kitamura N; Hurst B; Tarbet B; Marty MT; Kolocouris A; Xiang Y; Chen Y; Wang J Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M(pro) and cathepsin L. Sci Adv 2020, 6, eabe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y; Gaudreault N; Meekins DA; Perera K; Bold D; Trujillo JD; Morozov I; McDowell CD, Chang KO, Richt RA Effects of Spike Mutations in SARS-CoV-2 variants of concern on human or animal ACE2-mediated virus entry and neutralization, bioRxiv 2021, 457627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


