Abstract
Calcium imaging using GCaMP indicators and miniature microscopes has been used to image cellular populations during long timescales and in different task phases, as well as to determine neuronal circuit topology and organization. Because the hippocampus (HPC) is essential for tasks of memory, spatial navigation, and learning, calcium imaging of large populations of HPC neurons can provide new insight on cell changes over time during these tasks. All reported HPC in vivo calcium imaging experiments have been done in mouse. However, rats have many behavioral and physiological experimental advantages over mice. In this paper, we present the first (to our knowledge) in vivo calcium imaging from CA1 HPC in freely moving male rats. Using the UCLA Miniscope, we demonstrate that, in rat, hundreds of cells can be visualized and held across weeks. We show that calcium events in these cells are highly correlated with periods of movement, with few calcium events occurring during periods without movement. We additionally show that an extremely large percent of cells recorded during a navigational task are place cells (77.3 ± 5.0%, surpassing the percent seen during mouse calcium imaging), and that these cells enable accurate decoding of animal position and can be held over days with consistent place fields in a consistent spatial map. A detailed protocol is included, and implications of these advancements on in vivo imaging and place field literature are discussed.
SIGNIFICANCE STATEMENT In vivo calcium imaging in freely moving animals allows the visualization of cellular activity across days. In this paper, we present the first in vivo Ca2+ recording from CA1 hippocampus (HPC) in freely moving rats. We demonstrate that hundreds of cells can be visualized and held across weeks, and that calcium activity corresponds to periods of movement. We show that a high percentage (77.3 ± 5.0%) of imaged cells are place cells, and that these place cells enable accurate decoding and can be held stably over days with little change in field location. Because the HPC is essential for many tasks involving memory, navigation, and learning, imaging of large populations of HPC neurons can shed new insight on cellular activity changes and organization.
Keywords: Ca1, calcium imaging, hippocampus, miniscopes, place cells, rats
Introduction
The explosion in the use of in vivo calcium imaging has allowed the visualization of hundreds of identified neurons over long time periods (Grienberger and Konnerth, 2012; Rickgauer et al., 2014; Hamel et al., 2015; Resendez et al., 2016; Aharoni and Hoogland, 2019; Aharoni et al., 2019; Zhou et al., 2019). Calcium (Ca2+) imaging using GCaMP calcium indicators and miniature microscopes has been used to image cellular populations during long timescales (Dombeck et al., 2010; Ziv et al., 2013; Cai et al., 2016; Sheintuch et al., 2017, 2020; Hainmueller and Bartos, 2018; Kinsky et al., 2020) and in different task phases (Zhang and Li, 2018; Jimenez et al., 2020; Shuman et al., 2020), as well as to determine neuronal circuit topography and organization (Klaus et al., 2017; Wang et al., 2017; Guo et al., 2020; Shin et al., 2020; Wirtshafter and Disterhoft, 2022).
The hippocampus (HPC) has been studied with cellular resolution using in vivo electrophysiology, but this technique does not allow the same cells to be definitively followed over days, nor does it allow the visualization of cellular organization within the structure (O'Keefe, 1976; Wilson and McNaughton, 1993; McEchron and Disterhoft, 1999; Leutgeb et al., 2004; Schimanski et al., 2013). In vivo calcium imaging is particularly well suited for imaging the rodent HPC, as the orientation of the horizontal cell layer permits imaging of large numbers of neurons with insertion of a 1 mm diameter or smaller lens (Guo et al., 2020; Kinsky et al., 2020; Sheintuch et al., 2020; Stefanini et al., 2020). Because the HPC is essential for many tasks involving memory (Scoville and Milner, 1957; Squire, 1992; Eichenbaum et al., 1999; Hasselmo and McClelland, 1999; Ferbinteanu and Shapiro, 2003; Ferbinteanu et al., 2006; Smith and Mizumori, 2006; Vann, 2013; Cai et al., 2016; Josselyn and Tonegawa, 2020), spatial navigation (Foster et al., 2000; Rosenzweig et al., 2003; McNaughton et al., 2006; Foster and Knierim, 2012; Moser et al., 2017; Avigan et al., 2020), and learning (Moyer et al., 1990; Chan et al., 2001; Ito et al., 2005; Andrzejewski and Ryals, 2016), Ca2+ imaging of large populations of HPC neurons can shed new insight on cell changes and organization over time during these tasks.
To our knowledge, all reports of hippocampal in vivo calcium imaging have been done in mouse (although calcium imaging has been used to visualize other structures in rat; Scott et al., 2018; Anner et al., 2020; Hart et al., 2020; Pritchard et al., 2021). Using calcium imaging in rat HPC requires optimizing a number of methodological parameters, including aspiration and lens implantation techniques, to account for the increased thickness of the rat alveus compared with mouse which makes focusing on the CA1 cell layer difficult (Swanson, 2004; Routh et al., 2009; Franklin and Paxinos, 2013). However, rats have many basic experimental advantages over mice. First, rats are able to learn and perform more complex tasks than mice, and employ more advanced strategies, and are thus suited for a wider variety of behavioral experiments (Whishaw, 1995; Frick et al., 2000; Whishaw et al., 2001; Cressant et al., 2007; Rosenfeld and Ferguson, 2014; Hok et al., 2016). Second, compared with mouse physiology, rat physiology for a number of conditions and disorders (including addiction, aging, and schizophrenia, among others) more closely mirrors human physiology (Iannaccone and Jacob, 2009; Vengeliene et al., 2014; Ellenbroek and Youn, 2016; Carter et al., 2020). Third, rats are less stressed by handling than mice, thus introducing less variability in experimental procedures (Buerge and Weiss, 2004).
Rats are also an advantageous organism to use for calcium imaging. Because of their larger size, rats are able to support larger implants, including a wider diameter imaging lens, larger and heavier cameras that may be available in the future, and more combinations with electrophysiology implants. This advantage will thereby enable the recording of a greater number of cells (Voigts et al., 2013, 2020; Aharoni and Hoogland, 2019). Additionally, rat hippocampal neurons are less excitable than those in mice (Routh et al., 2009; Hok et al., 2016), and rats are less prone to postoperative seizures than mice (Hunt et al., 2009, 2010; Bolkvadze and Pitkänen, 2012). Rats may therefore, compared with mice, be at a decreased risk of the seizures (and potentially to cell death) seen in mice after prolonged GCaMP expression (Tian et al., 2009; Grienberger and Konnerth, 2012; Resendez et al., 2016; Steinmetz et al., 2017; Yang et al., 2018).
Although in vivo Ca2+ imaging would appear to hold substantial promise for the study of HPC in freely moving rats, there is currently no direct evidence that it will work in this system. To our knowledge, there are no published studies employing in vivo Ca2+ imaging in rat HPC, likely partially because of the number of methodological changes necessary to adapt the technique from mice to rats. In this paper, we present reports of in vivo Ca2+ recording from CA1 HPC in freely moving rats. We demonstrate that hundreds of cells are reliably visualized and held across weeks using this technique, and that calcium activity corresponds to periods of movement. We show that during a navigational task involving retrieving food reward on a linear track, a uniquely high percentage (77.3 ± 5.0%) of imaged cells are place cells (as compared with calcium imaging studies in mouse and electrophysiology studies in rat and mouse), and that these place cells enable accurate decoding and can be held stably over days with little change in field location. A detailed protocol for this technique, including notes on the numerous parameter changes needed to use Ca2+ in rats, is included in Materials and Methods, and implications of these advancements are discussed.
Materials and Methods
Subject details
All procedures were performed within Northwestern Institutional Animal Care and Use Committee and NIH guidelines. Five male Fischer 344 × Brown Norway rats (275–325 g) were sourced from National Institute on Aging colony at Charles River Laboratories, injected with AAV9-GCaMP7c, implanted with a 2-mm GRIN lens and run on a linear maze (Fig. 1). Animals were individually housed in an animal facility with a 12/12 h light/dark cycle.
Figure 1.
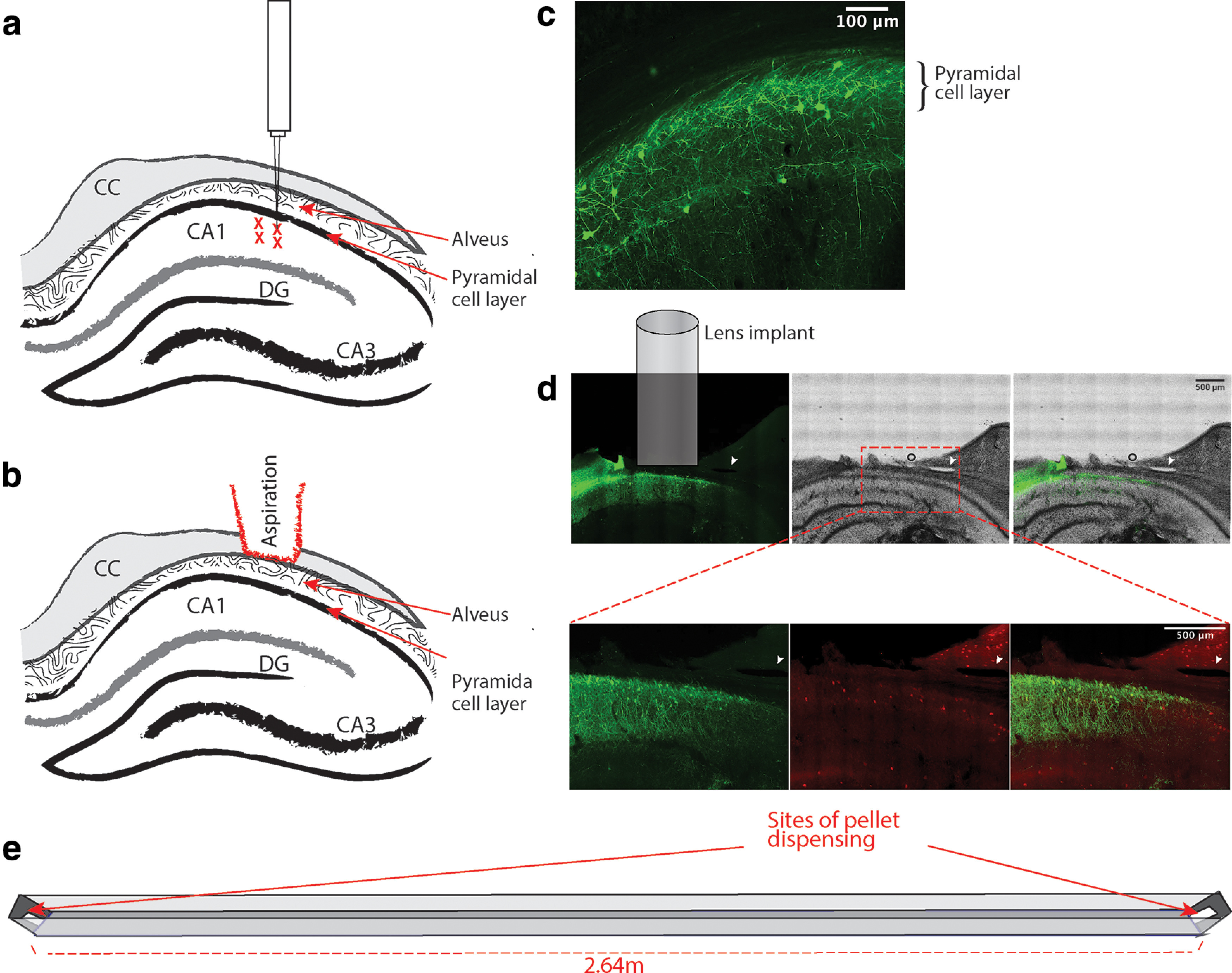
GCaMP is expressed in the CA1 cell layer of rat HPC. a, Diagram showing injection locations of AAV9-Syn-GCaMP7c, right below the CA1 cell layer. There are four total injection sites, with 0.6 μl injected at each site (CC = corpus callosum, DG = dentate gyrus). b, Diagram showing level of aspiration in brain tissue. The entire corpus callosum is aspirated until the vertical striations of the alveus are visible. c, GCaMP expression in the HPC, taken with a confocal microscope. The CA1 cell layer is labeled. d, Cellular GCaMP expression is primarily localized to principal cells in the pyramidal call layer. All images are from the same section; arrow indicates a space in the tissue that was used for alignment and that can be followed in all images. Top, A comparison of GCaMP labeling (left) with cresyl violet staining (middle) performed sequentially in the same tissue slice. An overlay of the two images (right) shows that cellular GCaMP expression is highest in the pyramidal cell layer with some labeled dendrites and cell bodies extending into the stratum radiatum and oriens. The outlined area in the middle image roughly corresponds to the area imaged in the bottom panel. Bottom, GCaMP labeling (left) and labeling of PV+ interneurons using immunohistochemistry (center). The overlay (right) indicates only a minority of GCaMP cells in the stratum pyramidale expressed PV. The majority of PV+ cells appear to be located slightly ventral to the cellular GCaMP labeling. e, Diagram of linear track. The animal would run back and forth between the two sides to receive a pellet reward.
Method details
GCaMP7c injection and lens implantation
Rats were anesthetized with isoflurane (induction 4%, maintenance 1–2%). Following anesthesia, ear bars, and skull leveling, the skull was cleaned and a craniotomy site was marked above CA1 (stereotaxic coordinates bregma AP −4.00 mm, ML 3.00 mm). The skull was scored around the craniotomy site using a razor blade to provide texture for future application of epoxy. A craniotomy was made with a 2-mm trephine around the marked site and dura was removed with a bent 30-gauge needle. A Hamilton syringe (Gastight 1700 Series Syringes, Hamilton, Model 1705 Small RN Syringe, lolume = 50 µl, point style = 3, gauge = 22 s, needle length = 50.8 mm) loaded with AAV9-GCaMP7c (obtained from Viogene Biosciences, packaged AAV9 of pGP-AAV-syn-jGCaMP7c-WPRE, titer 1.73 × 1013 GC/ml) was lowered straight down to ∼0.2 mm past the CA1 cell layer (approximate coordinates bregma AP −4.00 mm, ML 3 mm, DV 2.95 mm relative to skull). A total of 0.6 μl of virus were injected over 12 min using an automated stereotaxic Injector. The syringe was then raised 0.2 mm (bregma AP −4.00 mm, ML 3 mm, DV 2.75 mm) and another 0.6 μl of virus were injected over 12 min (Fig. 1a). The syringe was then left for 10 min before slowly raising to the skull.
We used a computerized stereotaxic injection system that allowed us to specify the coordinates of the injection. Because of the imprecision of craniotomies and imperfectly level skulls, often the initial injection at the specified sub-cortical coordinates did not fall in the center of the craniotomy. To increase and maximize the spread of viral injection, we completed a second injection. The process of lowering the syringe and doing two injections was repeated so that the two syringe “lowering points” were evenly spread throughout the craniotomy site. There was thus a total of four injections of 0.6 μl each (2.4 μl total): two injections per each brain entrance site, each at different depths (Fig. 1a).
Following injection, four skull screws were screwed into the skull: one rostral to bregma above the same hemisphere as the craniotomy, two between bregma and λ on the hemisphere opposite the craniotomy, and one behind λ on the hemisphere with the craniotomy. Under a dissecting microscope, tissue from the craniotomy was then aspirated using a vacuum pump and 25-gauge needle that had been previously blunted using a Dremel drill. Tissue was quickly aspirated until the start of horizontal striations could be seen in the craniotomy hole. Care was then taken to “level” the hole and make sure aspiration was at an even depth. The horizontal striations of the corpus callosum were then carefully and slowly aspirated until the vertical striations of the alveus were visible in the entirety of the craniotomy hole (Fig. 1b). If the aspiration hole was bleeding, a small twisted piece of gel foam was inserted into the hole for ∼5 min and then removed.
Notes differentiating this method from mouse Ca2+ imaging. We found the best AAV for expression in rats was AAV9. Because of rat's lower seizure threshold and, anecdotally noted, lower GCaMP expression than in mice, four injection sites appear both necessary and sufficient for ideal GCaMP expression. The injection volumes are much larger than those used in mice (Ziv et al., 2013; Cai et al., 2016), which is made possible because of the larger hippocampal area and the technique of injecting below the CA1 cell layer, instead of on it. Unlike in mice, where just the cortex or most dorsal part of the corpus callosum is aspirated (Cai et al., 2016; Schoenfeld et al., 2021), it is important that the entire corpus callosum be completely aspirated to get very close to the alveus.
A 2-mm GRIN lens (obtained from Go!Foton, CLH lens, 2.00-mm diameter, 0.448 pitch, working distance 0.30 mm, 550-nm wavelength) was then held on the end of a pipette using suction from the vacuum pump. The pipette was placed in the stereotaxic instrument which was zeroed with the bottom of the lens on bregma. The lens was then moved to the center of the craniotomy and lowered to a depth of ∼3 mm from skull surface. The lens was then fixed in place with UV curing epoxy and the vacuum pump was turned off and the pipette holding the lens removed. The epoxied lens was then secured to the skull screws using dental acrylic. Animals were then given buprenorphine (0.05 mg/kg) and 20-ml saline, taken off anesthesia, and allowed to recover in a clean cage placed on a heat pad.
Notes differentiating this method from mouse Ca2+ imaging. A much larger lens (2-mm diameter as opposed to 0.5- or 1.0-mm diameter) lens can be used in rats because of their larger size. Of high importance: to be as close to the CA1 layer as possible, the lens is inserted further down (about 0.5 mm) lower than the cell layer. This serves, presumably, to temporarily compress the alveus to make sure the alveus is pressed against the grin lens; this allows for in focus imaging of the CA1. We saw no permanent compression of the CA1 cell layer (Fig. 1c) or any behavioral deficits that are associated with HPC compression (Shim et al., 2003; Chen et al., 2017). We had zero visualization of cells unless the lens was deeply inserted. Additionally, unlike in mouse implantation surgeries, we did not have success using meloxicam after surgery as it appeared to cause excessive bleeding around the lens.
Four to six weeks after surgery, animals were again anesthetized with isoflurane and checked for cells expressing GCaMP. If expression was seen, the animal's lens was base-plated. The baseplate was attached using UV-curing epoxy and dental acrylic.
Notes differentiating this method from mouse Ca2+ imaging. During base plating, it gets progressively harder to see cells the longer the animal is under anesthesia. Additionally, while a few cells will appear to be bright circles in the captured image, cells do not appear to be flashing at all while the rat is under isoflurane, even after a tail pinch or loud noise (techniques often used to incite a calcium burst during mouse base plating). We recommend focusing on the bright, not flashing cells while base plating. The entire field of view should become progressively brighter as the animal wakes up. We recommend not imaging for experiments within 24 h of isoflurane exposure and base plating.
Behavioral training
Before training, animals were food deprived to 85% body weight and maintained at this weight throughout the experiment. Implanted animals were trained on a 2.64-m Med Associates linear track with pellet dispensers at either end. Animals learned to run back and forth between the sides of the track and received a grain pellet at alternate ends. Each pellet received was considered a trial. The fasted animals were able to achieve over 120 trials in 30 min. (Animals were fed after daily training to maintain weight.) Six sessions were selected for analysis for each animal, with first and last recorded sessions automatically included. When not being run, animals were housed in individual cages with a 12/12 h light/dark cycle.
Six sessions from each animal were selection for analysis. Although each animal was run for at least seven sessions, occasionally an animal would refuse to run or run slowly (often the day following a run with a lot of pellets received). For the animal run the least number of times, only six sessions exceeded 50 trials per run. For consistency across analysis, we therefore selected the six sessions with the most trials to analyze from each animal. On average, the four animals ran 100.3 ± 33.1 trials per session, with the individual animals averaging 94.7 ± 35.3, 85.5 ± 24.0, 114.5 ± 19.8, and 106.2 ± 41.2 trials per session.
Because the runs with the most trials were selected for analysis, trials were not always consecutive. If the first analyzed session is considered day 1, the analyzed sessions were as follows for each animal (as numbered by days on a calendar; animals were not necessarily run every day because of scheduling and equipment troubleshooting):
Animal 1: days 1, 2, 3, 7, 8, 9 (run across 9 d)
Animal 2: days 1, 2, 6, 7, 9, 13 (run across 13 d)
Animal 3: days 1, 2, 11, 14, 15, 16 (run across 16 d)
Animal 4: days 1, 2, 10, 14, 15, 16 (run across 16 d)
Calcium imaging
One photon calcium imaging was done using UCLA V4 Miniscopes (Silva, 2017; Aharoni and Hoogland, 2019), assembled with standard lens configurations (two 3-mm diameter, 6-mm FL achromat lens used in the objective module and one 4-mm diameter, 10-mm FL achromat lens used in the emission module). We tested Ca2+ recording when one of the objective module lenses was replaced by a 3-mm diameter, 9-mm FL achromat lens, but no improvement in cell visualization was noted. Gain was set at high and Miniscope LED intensity varied between 10 and 38.
Notes differentiating this method from mouse Ca2+ imaging. Base fluorescence appears lower in rats as compared with mice. While we have been advised that, using Miniscopes, gain can often be on “low” for mouse imaging, we found it necessary to have gain on “high” to visualize the highest number of cells.
GCaMP visualization in slice and processing of tissue sections
Following conclusion of the study, animals were anesthetized and perfused with PBS then 10% formalin. The brain was then sliced into 60-μm coronal sections. For visualization as seen in Figure 1c, sections were mounted, let dry, and coverslipped with Vectashield. Sections were then imaged with a confocal microscope.
For GCaMP and PV+ visualization in Figure 1d, all solutions were prepared in PBS containing 0.05% sodium azide and 2% normal donkey serum. Free-floating sections were rinsed in PBS three times for a total of at least 30 min between incubation steps. Tissue sections were sequentially placed in (1) 0.2% Triton X-100, 90 min; (2) mouse monoclonal anti-parvalbumin (PV) 2000× (Millipore), 48 h; (3) rabbit anti-GFP 2000× (AbCam), 24 h; (4) biotinylated goat anti-rabbit serum 200× (Jackson ImmunoResearch), 90 min; (5) a cocktail containing Cy3-conjugated donkey anti-mouse serum 200× (Jackson ImmunoResearch) and FITC-conjugated avidin 200× (Jackson ImmunoResearch), 90 min. Sections were coverslipped with a 9:1 mixture of glycerin and PBS containing 0.2% p-phenylenediamine and examined with a confocal microscope.
Cresyl violet visualization in Figure 1d was completed using the same sections as seen in GFP and PV+ imaging. Sections were aligned based on interior landmarks such as blood vessels and tissue spaces (some mild deformation of the neocortex occurred during processing of some sections). For staining, we used 0.1% cresyl violet solution containing 1% acetic acid. Sections were sequentially placed in (1) histoclear, 5 min; (2) 100% alcohol, 2 min; (3), 95% alcohol, 1 min; (4) 70% alcohol, 1 min; (5) distilled water, 2 min; (6) cresyl violet solution, 12 min; (7) distilled water, quick rinses; (8) acid alcohol; 1.5 min; (9) distilled water; 0.5 min; (10) 70% alcohol, 0.5 min; (11) 95% alcohol, 0.5 min; (12) 100% alcohol, 0.5 min; (13) histoclear, 2 min. Sections were then immediately coverslipped with Permount and examined with a confocal microscope.
Quantification and statistical analysis
Means are expressed as mean ± the SD. Overall averages are computed by finding average per animal then averaging those values. All analysis code is available at https://github.com/hsw28/ca_imaging.
Position and speed analysis
Position was sampled by an overhead camera at 30 Hz. Position tracking was done postrecording using Bonsai (Lopes et al., 2015). Speed was determined by taking the hypotenuse of the coordinates of the point one before and one after the time point of interest. Speed was then smoothed using a Gaussian kernel of 1 s and speed was converted from pixels/s to cm/s.
Video preprocessing
Videos were recorded with Miniscope software at 15 frames/s and 752 × 480 pixels. All video processing was done using the MATLAB-based CIATAH software (Ahanonu, 2018; Corder et al., 2019) and processing files are available at https://github.com/hsw28/ca_imaging/. Videos were down sampled in space (3× using bi-linear interpolation) and time (2×). The video was normalized by subtracting the mean value of each frame from the frame. Each frame was then spatially filtered (normalized) using a bandpass FFT filter (70–100 cycles/pixel). The video was then motion corrected to a chosen reference frame using TurboReg (Thévenaz et al., 1998). Videos were then converted to relative florescence (dF/F0), where F0 was the mean over the entire video.
Cell identification
Cells were first automatically identified using the MATLAB-based CIATAH software (Ahanonu, 2018; Corder et al., 2019), using the CNMF-E method (Zhou et al., 2018). Processing files are available at https://github.com/hsw28/ca_imaging/. Images were filtered with a Gaussian kernel of width three pixels. Neuron diameter was set at a pixel size of 13. The threshold for merging neurons was set at a calcium trace correlation of 0.65, and neurons were merged if their distances were smaller than two pixels and they had highly correlated spatial shapes (correlation >0.8) and small temporal correlations (correlation <0.4).
All cells identified using CNMF-E were then scored as neurons or not by a human scorer. Scoring was also done within the MATLAB-based CIATAH software (Ahanonu, 2018; Corder et al., 2019) in a MATLAB GUI. Human scoring was done while visualizing an activity trace, average Ca2+ waveform, a cropped movie montage of the candidate cell's Ca2+ events, and a maximum projection of all cells on which the candidate cell was highlighted. The relative fluorescence (ΔF/F0) local maxima of each identified cell were considered calcium event times.
Place cell identification and spiking characteristics
Place cells were identified using two criteria. First, cells must have a mean firing rate >0.01 Hz during periods of movement (>12 cm/s; Wirtshafter and Wilson, 2019, 2020). Second, the mutual information (MI) computed in either direction of travel must be >95% of MI scores computed 500 times from shuffled cells (Kinsky et al., 2018). To compute MI, the track was divided lengthwise into 4-cm bins, the firing rate of each cell and the occupancy of the animal were found for each bin. Rate and occupancy were smoothed with a 2-cm Gaussian kernel. MI was computed during periods of movement in each direction of travel as follows (Olypher et al., 2003; Kinsky et al., 2018; Wirtshafter and Wilson, 2020):
Place field location was determined by the location of maximum firing rate after binning position into 4-cm bins. Speed modulation for individual cells was determined by computing average event rate when the animal was moving ≤5 and ≥12 cm/s; a cell was considered speed modulated if the rate at ≥12 cm/s was larger. At the population level, speed modulation was determined by examining population event rate versus speed. Cross correlations between speed and event rate were computed by binning speed and rate into 100-ms bins.
Position decoding
Position was decoded using all recorded cells in a session and was decoded for times when the rat was moving (velocity >12 cm/s). The track was split in 10-cm segments and the time window of decoding was 0.5 s with nonoverlapping time bins. Decoding was done using Bayesian decoding (Zhang et al., 1998):
Accuracy decoding was determined by randomly shuffling firing per position bins and re-decoding. The difference between actual and decoded position was then determined per time bin and compared with decoded shuffled data.
Validation across days and within session
Validation was done using the MATLAB-based CIATAH software (Ahanonu, 2018; Corder et al., 2019). Videos underwent three rounds of registration comprised of Turboreg image rotation (Thévenaz et al., 1998). An image binarization threshold of 40% of the images' maximum value was used to remove background noise and axons and dendrites. A distance threshold of a maximum of five pixels was used to match cells across sessions, with a minimum 2-D correlation coefficient of 0.5. All sessions were aligned to the fourth recording session.
Results
To initiate Ca2+ imaging, we injected AAV9-Syn-GCaMP7c below the CA1 cell layer in five male Fischer 344 × Brown Norway rats (Fig. 1a; see Materials and Methods; Nathanson et al., 2009). After injection, in the same surgery, cortex and corpus collosum were aspirated through a craniotomy until the alveus was clearly visible (Fig. 1b; see Materials and Methods). A 2-mm diameter GRIN lens was inserted into the craniotomy and cemented in place. Three animals were checked for GCaMP expression four weeks after surgery, and all three animals showed expression and were subsequently affixed with a base plate to secure the position of the Miniscope. The other two animals were checked six weeks after surgery and showed expression and were base plated. After the completion of the experiment, animals were killed and expression of GCaMP in the CA1 pyramidal cell layer was verified (Figs. 1c,d). Based on a comparison with cresyl violet staining of the hippocampal region, cellular GCaMP expression appeared to be located primarily in the CA1 principal cell layer (Fig. 1d). Although contributions of interneurons to this study cannot be ruled out, antibody staining for PV+ interneurons revealed that only a minority of GCaMP cells in the stratum pyramidale expressed PV (Fig. 1d), consistent with observations that ∼3% of cells in the pyramidal layer are interneurons (Aika et al., 1994).
Animals were then food deprived to 85% body weight and trained on a 2.64-m linear track (Fig. 1e). Animals were rewarded with grain pellets on alternating ends of the linear track and quickly learned to run back and forth on the track. A visit to one reward well signified a trial. Animals were run for a minimum of 30 min or 50 trials, whichever came later, with total track time not to exceed 1 h per session. Cells were recorded for 7 d for one animal, 11 d for one animal, and 16 d for two animals (n = 4 rats, one of the five animals was eliminated when the implant was lost).
The six sessions with the most trials run were analyzed from each animal (average trials run = 100.3 ± 33.1; see Materials and Methods), with first and last recorded sessions automatically included; sessions were not always consecutive (see Materials and Methods). These sessions were analyzed for cellular activity using the CNMF-E method implemented by the CIATAH software package (Ahanonu, 2018; Zhou et al., 2018; Corder et al., 2019; see Materials and Methods). A total of 5761 neurons were recorded over the 24 sessions, with a maximum of 428 cells from a single session (mean of 239.6 ± 90.0 cells; Fig. 2a–d). The number of cells recorded from each animal across sessions was relatively constant (Fig. 2c).
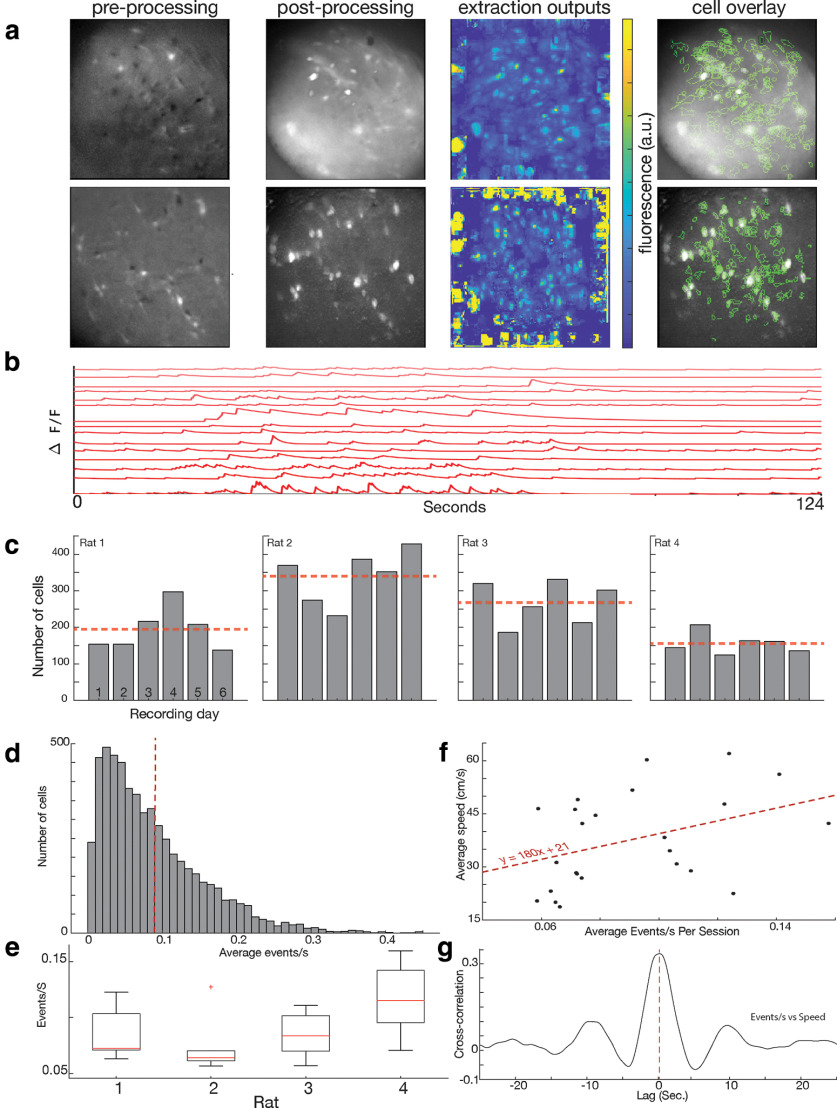
Figure 2.
Hundreds of cells can be captured in a single Ca2+ imaging session. a, Examples of data from two (top and bottom rows) recording sessions. First column, Example image from preprocessing. Second column, Image postprocessing (see Materials and Methods). Third column. All cell extractions, before manual cell sorting. Fourth column, Postprocessing image overlayed with cell outlines identified after manual sorting. b, Example traces from 15 cells taken during 124 s of calcium imaging. c, Number of cells imaged per session for each rat. Dotted red line indicates the average for each animal. d, Histogram showing average rate (events/s) for individual cells. Dotted red line indicates the average (0.090 ± 0.019 events/s). e, The average calcium event rate (events/s) for individual rats (averages: 0.084 ± 0.024, 0.074 ± 0.026, 0.085 ± 0.020, and 0.116 ± 0.032 events/s). f, Correlation of average calcium event rate with average animal running speed. Average events per second in a session is positively correlated with the animal's average speed in that session (p < 0.05). g, An example from one session for the cross-correlation at different lags between the number of calcium events that occur in a second and the animal's speed. In this example, the maximum cross-correlation value at 0 s was ∼0.33, and the maximum value occurred at approximately 0.1 s. On average, the cross-correlation at a lag of 0 s was 1.8 ± 0.08, although the average maximum cross-correlation was 0.2 ± 0.7 at an average lag of +0.47 ± 0.54 s, indicating that calcium events reliably followed changes in speed, as opposed to vice versa.
We then determined average calcium events/s across animals to be 0.090 ± 0.019 events/s, with individual animals averaging 0.084 ± 0.024, 0.074 ± 0.026, 0.085 ± 0.020, and 0.116 ± 0.032 events/s. (Fig. 2d,e). Some difference in average calcium event rate could be accounted for by differences in the base line speeds of the animals; there was a significant positive correlation between the animal's average speed and the average events/s for each session (F(22) = 4.2, p = 0.05; Fig. 2f). Because speed is highly variable on a moment-to-moment basis, we also completed a cross-correlation between event rate and animal speed. In every session, we saw a positive cross-correlation between the animal's speed and the number of calcium events across the population. The average cross-correlation at a lag of 0 s was 1.8 ± 0.08, although the average maximum cross-correlation was 0.2 ± 0.7 at an average lag of +0.47 ± 0.54 s, indicating that calcium events reliably followed changes in speed, as opposed to vice versa (Fig. 2g).
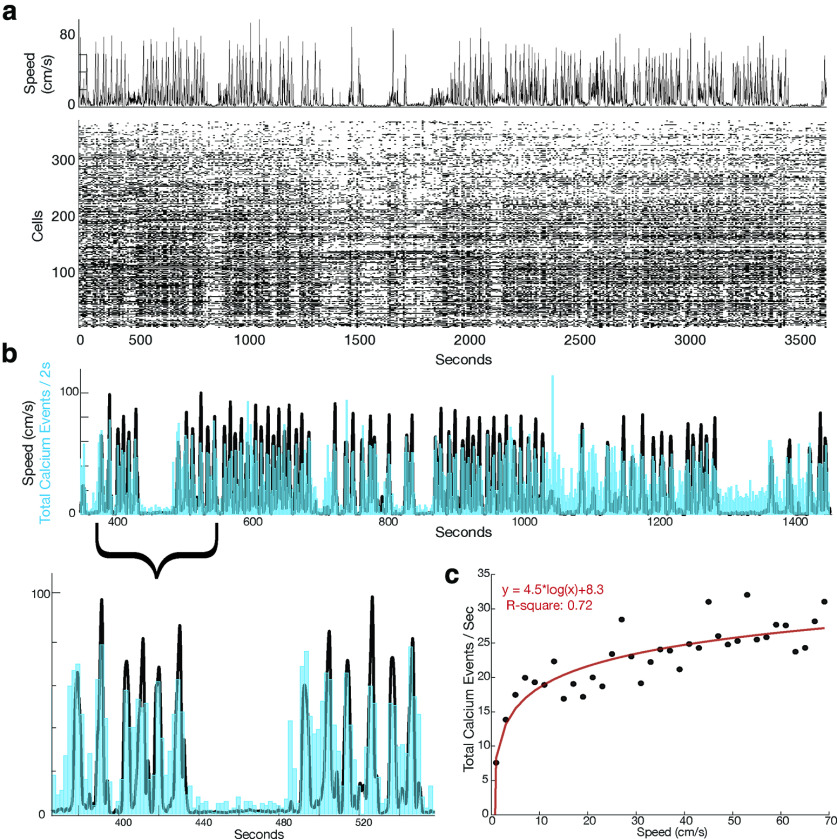
Significantly, calcium activity appeared to be heavily modulated by the animal's activity, with periods of higher activity when the animal was running rather than stationary on the track (Fig. 3). The biggest contributor to a change in event rate was transition from no movement to movement, with very few events occurring during periods of no movement (Fig. 3c).
Figure 3.
Calcium events are correlated with periods of movement. a, Raster plot of cell firing during linear track running. Top, The animal's speed. Bottom, Raster of peak calcium transients of 352 identified cells. b, Graph showing an example of speed (plotted in black) overlayed with event rate (in blue). Bottom panel is a zoom in on a smaller time period. c, An example logarithmic correlation of animal speed against calcium events/s.
We then determined whether there were place cells on any of the trials. A cell was considered to be a place cell if the MI computed in either direction of travel was >95% of MI scores computed 500 times after shuffling the times of the calcium events that occur when the animal is moving (Ziv et al., 2013; Kinsky et al., 2018). Based on this criterion all trials contained place cells (Fig. 3a). An average of 77.3 ± 5.0%, of all cells recorded on the track were place cells (Fig. 3), with individual rat averages at 71.6 ± 10.6%, 77.3 ± 1.2%, 76.4 ± 9.5%, and 83.7 ± 7.1%. We additionally determined the percentage of place cells if we only analyzed cells that had an average calcium events rate of >0.01 hz (Stefanini et al., 2020). This additional criterion yielded an even higher percentage of place cells, with 88.3 ± 4.4% of these cells being classified as place cells (Fig. 3a).
We extended these results using an additional criterion previously used to determine the percentage of place cells using calcium imaging (Stefanini et al., 2020). We determined the percentage of cells that would be place cells if the MI value exceeded the mean plus 3 SDs of a shuffled distribution. Using this criterion, we found that 64.3 ± 5.1% of all cells were place cells, with individual animals at 59.5 ± 12.0%, 65.5 ± 2.3%, 61.2 ± 14%, and 71.0 ± 12.1%. If we applied the 0.01-Hz threshold for cell inclusion to this group, 73.4 ± 4.3% of cells were place cells, with individual animals at 68.0 ± 11.8%, 77.6 ± 5.6%, 77.0 ± 14.6%, and 76.0 ± 13.0%.
We wished to further investigate the properties of these place cells and to confirm that cells were increasing their firing rate in place fields as is typical for pyramidal cells; as opposed to interneurons that mark spatial location by a substantial decrease in firing (Ego-Stengel and Wilson, 2007; Wilent and Nitz, 2007; Dombeck et al., 2010). We examined the place cells' maximum event rate during movement (presumably occurring in the place field) with their average firing rate during movement. The mean maximum rate was 0.15 events/s, and the mean average rate was 0.02 events/s. On average, cells increased firing 7.4× from their average firing rate during movement to their maximum firing rate (Fig. 4d). We next determined whether speed modulation occurred in the cells which display place fields. We compared whether speed-modulated neurons increased their in-field firing rate as much as nonspeed-modulated neurons. We found no significant difference in firing rate increases between speed-modulated and nonmodulated neurons (t test(4023) = 0.7102, p > 0.05), nor were the distributions of increases different (Kolmogorov–Smirnov test p > 0.05): both speed-modulated and nonmodulated cells increased their firing rates, on average, >7× from baseline at the center of their place fields.
Figure 4.
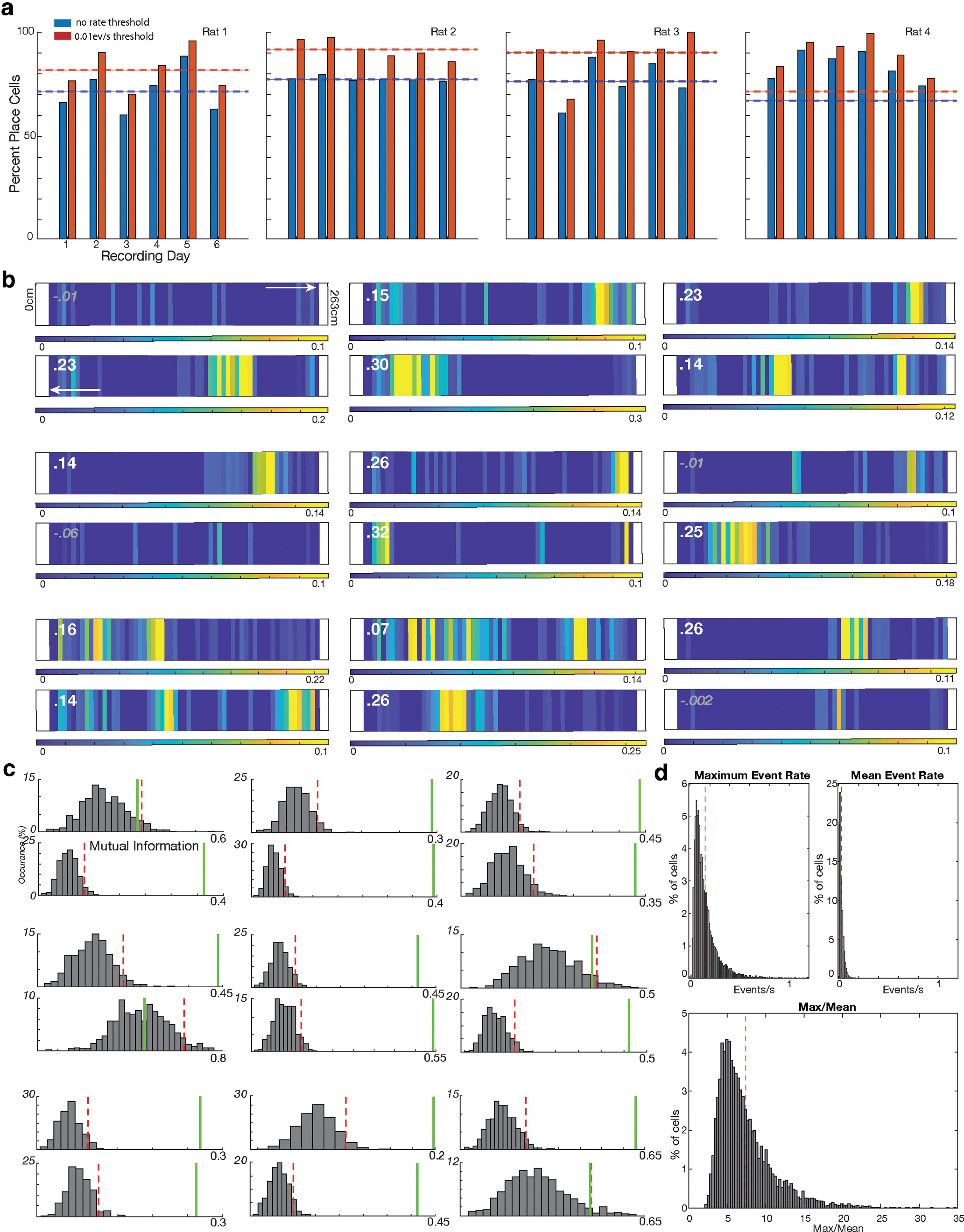
A high percentage of place cells are recorded on the linear track using Ca2+ imaging. a, Graph showing the percentage of place cells per session for each rat (blue). Analysis was repeated including only cells with a firing rate >0.01 Hz during movement (red). Dotted lines indicate the average for each animal. b, Nine example place cells. A firing rate map in both directions of travel is provided for each cell, with the top map corresponding with running to the right, and the bottom map corresponding with running to the left. Note the colorbar scales may be different for each direction of travel. The difference in MI scores between the cell and the top 95% of shuffled data (actual MI-shuffled MI) is indicated in the left corner. If the actual MI was >95% of shuffled data, the number is printed in bold white. c, Distribution of directional MI scores after shuffling cells seen in Figure 3b. Event data were shuffled 500 times and the top 95% of MI scores were determined from shuffled data (red dotted line). If the actual MI score (green line) was greater than the upper 95% MI score of shuffled data, the cell was considered to be a place cell. d, Top left, Place field maximum event rate during movement (dotted red line indicates average of 0.15 events/s). Top right, Average firing rate during movement (red line is average at 0.02 events/s). Bottom, Increase from average to maximum rate (max/mean). On average, cells increased firing 7.4× from their average firing rate during movement to their maximum firing rate (dotted red line indicates average).
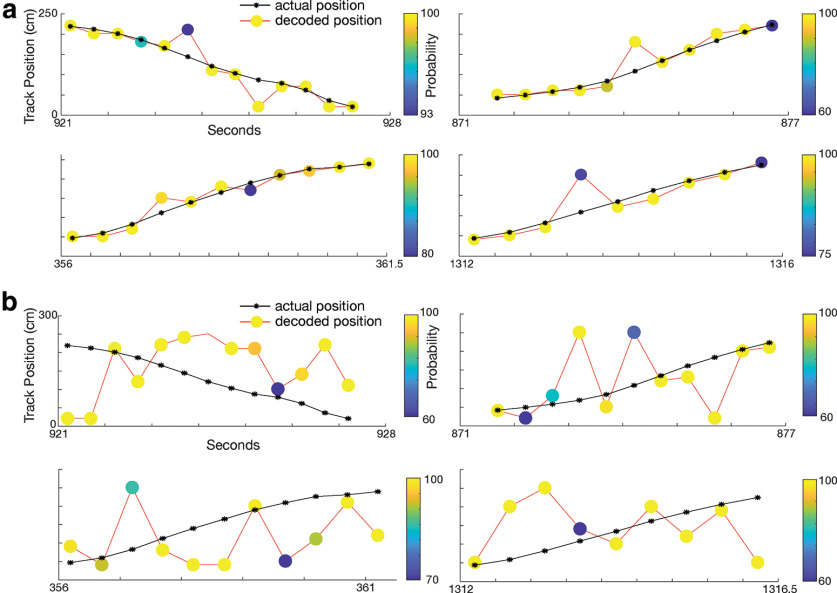
We then aimed to determine whether place cell firing was sufficient to decode the position of the animal. We split the track in 10-cm segments and used Bayesian decoding to determine the position of the animal in nonoverlapping 0.5-s increments (Zhang et al., 1998). We found that the best positional decoding was accurate to a median error of 4.3 cm (1.6% of the 2.64-m track). Across all animals, accuracy ranged from a median of 4.3–13.6 cm (decoding errors were below 10 cm in 23 sessions and above 10 cm in one session) with a median error of 5.9 cm (Fig. 5a). To confirm that decoding accuracy was better than chance we shuffled firing rate per position bins and, using the shuffled data, decoded position. Median error for shuffled data ranged from 59.1 to 77.6 cm, which was significantly worse than nonshuffled data (Kruskal–Wallis test, χ2(47) = 35.3, p = 2.9 × 10−9; Fig. 5b).
Figure 5.
Place cells are sufficient to decode the animal's position. a, Four decoding examples as the animal traverses the track. Black line indicates the animal's actual position, with the stars indicated sample point. The colored circles connected by the red line indicate decoded position. The color of the circles indicates decoding certainty. b, Same as a, but decoding is using shuffled units. Decoding is much less accurate using shuffled data.
We then aimed to determine whether the same cells could be held over the duration of our recordings (six recording sessions, spanning 7, 11, or 16 d). 40% of the cells imaged on day 1 of the 7-d session were also present on day 7. Of the rat run for 11 d, 31% of the cells present on day 1 were also present on day 11. For the two rats run 16 d, 28% and 50% of cells, respectively, that were present on day 1 were also present on day 16. Across all four animals, cells that were imaged on day 1 of recording had, on average, a 72 ± 6% chance of being imaged on at least one subsequent day of recording (Fig. 6a). [Of note, while the camera was placed in the same approximate position each day, thus allowing landmarks (such as blood vessels) to be followed throughout the study, we did not attempt to perfectly align landmarks or focal planes across days; instead, recordings each day were made to optimize total cell number in each day as proof of method.]
Figure 6.
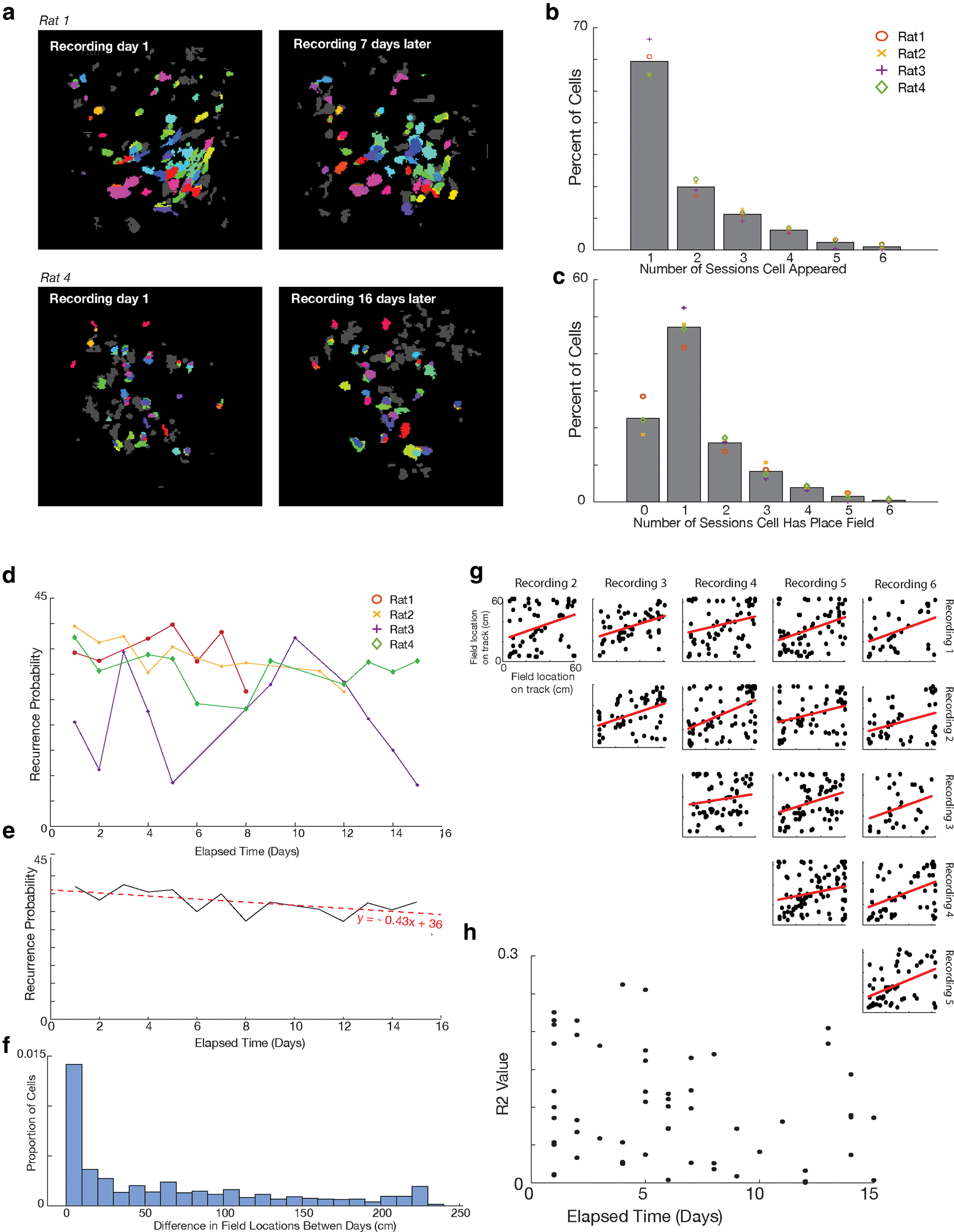
Individual cells and their place fields can be maintained across sessions. a, Cells can be maintained across sessions weeks apart. Examples from two animals of data taken on the first and final days of recording. Cells are color-matched from the first to last day. Cells in gray represent cells with no matches between the 2 d. Of note, while the camera was placed in the same approximate position each day, thus allowing landmarks (such as blood vessels) to be followed throughout the study, we did not attempt to perfectly align landmarks or focal planes across days; instead recordings each day were made to optimize total cell number in each day as proof of method. b, Percent of cells that appeared in different numbers of sessions. Symbols represent averages for individual animals. There was no significant difference in distribution of values between animals (nonparametric k-sample Anderson–Darling test, rank statistic: −1.48, p > 0.05). c, Percent of cells that had place fields in different numbers of sessions. Symbols represent averages for individual animals. There was no significant difference in distribution of values between animals (nonparametric k-sample Anderson–Darling test, rank statistic: −1.59, p > 0.05). d, The recurrence probability of a cell based on the number of days between recordings. Recordings from rat 3 were from found to be from two different focal planes. e, Average recurrence probability across days, excluding rat 3. For every day passing, a cell has a 0.43% less chance of reoccurring. f, Change in place field location between recording sessions. Included all sessions recorded for an animal; sessions did not need to be adjacent. g, A comparison of place field locations for one rat across all six recording sessions (15 comparisons). For all 15 comparisons using this animal, place field locations were highly correlated between the sessions. Each point indicates the place field of a single cell identified in the two sessions indicated. Red lines on the graphs indicate a p < 0.05 using an F test. Across all four animals, we found field locations to be consistently correlated, with 93% of session comparisons having a significantly positive correlation between place field locations (F test, p < 0.05). h, There was no correlation (p > 0.05) between r2 value of place field correlations and days elapsed between sessions.
We then looked to determine how often a cell appeared in each session. Across all cells, 59.4 ± 5.4% of cells were visible in only one session, 19.9 ± 2.4 were visible in two, 11.2 ± 1.5 in three, 6.3 ± 0.8 in four, 2.3 ± 1.3 in five, and 1.0 ± 0.9 were in all six sessions (Fig. 6b). We then looked at place cells that were repeated across sessions. As stated, 22.6 ± 4.3% of cells did not have place cells in any session. Of the 77.4% of cells that did have place fields, 61.0 ± 5.7% (47.2% of total cells) had fields in only one session, 20.6 ± 2.0 (15.9% of total) had fields in two sessions, 10.8 ± 2.4% (8.3% of total) had fields in three sessions, 5.1 ± 0.7% (4.9% of total) had fields in four sessions, 2.0 ± 1.4% (1.5% of total) had fields in five sessions, and 0.5 ± 0.5 (0.4%of total) had fields in all six sessions (Fig. 6c). Place cell recurrence was not statistically different from the recurrence of all cells (paired t test(5) = 2.04 × 10−15, p = 1).
We then determined the recurrence probability of a cell based on the number of days apart the recording took place (Fig. 6d). Three of our animals had relatively consistent recurrence probabilities, while one animal (animal 3) was highly variable. Going back to the recordings, we saw that recordings from this animal came from two different focal planes, causing the recording of two different cell populations. Excluding this animal, there was a modest trend across days for a decreasing recurrence probability, with the probability of a cell reoccurring decreasing by 0.43% per day separating the recording periods (Fig. 6e).
We then determined whether a cell's place field remained in the same location during days when the place cell reoccurred. We found that, across all days of recording, the mean SD of place field locations was ∼36 cm (∼13% of track length), with the individual animals at 39, 36, 32, and 37 cm. We compared these distances with distances if place field locations were shuffled, and we repeated shuffling 500 times. All four animals had a smaller deviation between fields than would be expected by chance (p < 0.05 for all animals). Between individual days (including nonadjacent days), the change in field distance was highly variable at 65 ± 71 cm, with a median of 40 cm and a large concentration of values around 0 cm (Fig. 6f).
Although fields across days were more consistent than would have been expected by random chance, we had not yet determined whether the animal had a consistent map across all recording days (Ziv et al., 2013), or whether it switched between multiple maps (Sheintuch et al., 2020). To determine this, we compared place field location for cells in a session against the place field locations of the same cells in another session. To do this, for each cell, we plotted the location of the place field for that session against the place field location of the same cell for another session. We did this comparison for every cell that had a place field in more than one session. If place field mapping was similar from session to session, we would expect the plotted field locations of one session to have a strong positive correlation with the locations from another session. Of the pairs of sessions that had >10 place cells shared between sessions (57 out of 60 total session pairs; 15 session pairs for rat 1, 15 for rat 2, 14 for rat 3, and 13 for rat 4), 53 of these pairs (93%) had a significant positive correlation (F test, p < 0.05) between the place field locations, with no evidence of switching between maps across days (Fig. 6g). We found that correlations between field locations did not decay as a function of the number of days between sessions (Fig. 6h).
Discussion
In this manuscript, we have demonstrated calcium imaging recordings can be performed in the CA1 region of the HPC in freely moving rats. These recordings can capture hundreds of cells in a single recording session, including a high number and percentage of place cells sufficient to decode the animal's position to within centimeters of accuracy, and that place cell field location is consistent across days. Calcium imaging in rat will allow for more behaviorally complex and translational experiments than have been done in mouse.
Measuring spatial content and categorizing place cells
We chose to use MI as the measure of spatial content for multiple reasons. First, it has precedence for use in both calcium imaging (Ziv et al., 2013; Meshulam et al., 2017; Kinsky et al., 2018, 2020; Stefanini et al., 2020) and electrophysiology studies (Jun et al., 2020; Table 1). Second, threshold measures commonly used in electrophysiology that depend on firing rate (such as “a cell is a place cell if firing rate, as measured by electrophysiology, exceeds 2 Hz for a 10 cm area”; Rich et al., 2014; Table 1) do not transfer well to calcium imaging experiments as, using currently available GCaMPs, calcium events are captured much less frequently than spikes in electrophysiology (typically, ∼0.02–0.06 events/s is the mean rate observed during 1p calcium imaging, vs >0.5–1.5 spikes/s in electrophysiology, a 25-fold difference, see Table 1). Third, other measures of information content, such as bits/spike or bits/s (Table 1), tend to be highly sensitive to changes in firing rate, including baseline firing rate (Souza et al., 2018), as compared with MI. Specifically, cells with lower baseline firing rates are favored to have higher baseline bits/spike or bits/s values (Souza et al., 2018; Wirtshafter and Wilson, 2020), which makes it not a useful metric with which to compare values from calcium imaging with data collected from electrophysiology. Additionally, MI is advantageous in that it is a better predictor of decoding accuracy than bits/spike and bits/s, and thus may better capture spatial informational content (Souza et al., 2018). The criteria we used to identify place cells were as or more stringent than previously used criteria in calcium imaging that identified a significantly lower percentage of place fields (Table 1).
Table 1.
Comparison of place cell findings in previous studies in mice and rats
| Study | Method | Species | Task | Mean spike rate or Ca event rate | % place cells | How place cells were determined |
|---|---|---|---|---|---|---|
| Guzowski et al. (1999) | IEG | Rat | Cylinder and platform exploration | NA | 38–39% | ARC detection |
| Witharana et al. (2016) | IEG | Rat | Environmental exposure and circular track | NA | 22–73%, mean 33 ± 18% | Homer1a and ARC detection |
| Talbot et al. (2018) | Ephys | Mouse | Open field | 1.14 ± 0.12 spikes/s for all pyramidal cells 1.49 ± 0.15 spikes/s for place cells 2.61 ± 0.2 spikes/s mean in-field rate |
47.8% | Overall discharge rate was 0.1–2 AP/s, the spatial coherence was >0.4, and the information content was >0.4 bits/spike |
| Jun et al. (2020) | Ephys | Mouse | Open field and linear track | ∼0.8 spikes/s mean rate ∼3.5 spikes/s maximum rate |
50% | MI >95% of shuffled data |
| Maurer et al. (2006) | Ephys | Rat | Circular track | Not noted | ∼43% on smaller track ∼68% on larger track |
Place field has a single cycle (360%) phase precession |
| Wirtshafter and Wilson (2020) | Ephys | Rat | Double sided T maze | ∼0–5 Hz in field | 75.2% exceeded 0.8 bits/spike 63% had at least one place field and exceeded bits/spike threshold |
(1) Bits/s >0.8 (2) Firing in a region must be greater than 2 SDs greater than the mean firing rate and place field area must be at least 15 cm long |
| Fenton et al. (2008) | Ephys | Rat | Forage for food in cylinder and chamber | 0.91–1.01 spikes/s depending on environment 2–2.5 spikes/s mean in-field rate |
64% in smaller cylinder 72% in 6× larger chamber |
Cell coherence was >0.25 and information content was >0.5 bits/AP, and if the cell had at least one place field |
| Markus et al. (1994) | Ephys | Rat | 8-arm radial maze forced choice | 0.56 spikes/s in young animals | 37% | Bits/spike cutoff of 2.29 |
| Rich et al. (2014) | Ephys | Rat | 48-m-long track | Up to ∼0.7 spikes/s during running, highly correlated with # of place fields | 65% at 48 m (range 46–77%) | A place field was defined as ≥15 contiguous cm of rate map in where firing rate >2 Hz |
| Meshulam et al. (2017) | 2p calcium imaging | Mouse | VR linear track | Not noted | ∼30%, range 28–41% | MI above 80% of shuffled |
| Ziv et al. (2013) | 1p calcium imaging | Mouse | Linear track | ∼0–0.5 events/s | 20% | MI above 95% of shuffled |
| Stefanini et al. (2020) | 1p calcium imaging | Mouse | Open field | 0.06 ± 0.04 ev/s | MI mean + 3σ: ∼4% for all cells ∼10% with 0.1 ev/s cutoff >95% shuffled MI ∼12% for all cells ∼22% with 0.1 ev/s cutoff |
MI > mean + 3σ of the shuffled distribution Also compared with MI above 95% of shuffled |
| Kinsky et al. (2018) | 1p calcium imaging | Mouse | Square and octagon tracks | Not noted | Unspecified | (1) Had ≥5 calcium transients during the session (2) MI above 95% of shuffled |
| Kinsky et al. (2020) | 1p calcium imaging | Mouse | Continuous alternation | 0.02–0.03 ev/s (mean ∼40–50 events in ∼30-m session) | Unspecified | (1) Had ≥5 calcium transients during the session (2) MI above 95% of shuffled |
| This work | 1p calcium imaging | Rat | Linear track | 0.02 events/s during movement only 0.15 events/s mean maximum in-field rate |
>95% shuffled MI 77.3% for all cells 88.3% with 0.01 ev/s cutoff MI mean + 3σ: 64.3% for all cells 73.4% with 0.01 ev/s cutoff |
MI above 95% of shuffled Also computed MI > mean + 3σ of the shuffled distribution |
Based on anatomic data (Fig. 1d), it is reasonable to conclude that the cells we recorded from were primarily pyramidal cells in the stratum pyramidale. GCaMP expression appears to be most densely expressed in pyramidal cells in the cell layer (Fig. 1d; although dendritic processes and occasional cells can be viewed extending into the radiatum and oriens; Fig. 1c,d). Further, many of the labeled cells displayed an obviously pyramidal morphology (Fig. 1c) and only a small proportion of total GCaMP-labeled cells expressed PV, a result consistent with reports that only ∼3% of cells in the stratum pyramidale are interneurons (Aika et al., 1994).) The majority of double labeled cells were in the stratum radiatum, or on the border between the stratum pyramidale and the stratum radiatum, and would likely be too deep and too obscured to be captured by the implanted lens and Miniscope. (Even including the radiatum, double labeled cells constitute a minority of GCaMP-labeled neurons.)
In addition to anatomic and morphologic data, the cells we imaged had calcium event patterns characteristic of pyramidal cells. It is well established that pyramidal place cells increase their firing rate in their place field, while interneurons that express place specificity do so by decreasing their firing rate. Both ways of specifying place presence would result in an elevated MI score. However, given that the vast majority of cells we observed displayed an increased rate of calcium events at specific locations (Fig. 4d), it is likely that these cells are pyramidal excitatory cells. The percentage of place cells recorded in the present study are comparable to those seen in electrophysiology (Table 1), where interneurons can be more easily separated from pyramidal cells based on spike morphology and frequency, suggesting that we may be imaging from a similar population of cells.
These considerations suggest that any contribution of interneurons to the observed effects would, at most, be very small.
Differences in place cell findings between electrophysiology, intermediate early gene (IEG) studies, and calcium imaging
The percentage of hippocampal CA1 cells found to be place cells using calcium recordings (77.3 ± 5.0%) was also very slightly above the upper range of findings from electrophysiological data in rats, which report that 35–75% of CA1 pyramidal cells recorded on a navigational task are place cells (the number varies widely depending on task parameters, with studies using a linear track task reporting the highest number of place cells; Table 1; Markus et al., 1994; Fenton et al., 2008; Wirtshafter and Wilson, 2020). There are multiple possible reasons related to recording technology as to why our calcium imaging experiments detected a higher percentage of place cells than traditional electrophysiology experiments: the difference likely resulted from electrophysiology capturing (as compared with calcium imaging) both a lower number of cells with active place fields detected (a smaller numerator) and a larger number of cells without active place fields detected (a larger denominator). First, in electrophysiology experiments with moveable electrodes (such as tetrodes), electrodes are typically positioned while rats are inside a “sleep box” or other nontraining apparatus until cells are detected. The animal is then placed on a track for experimentation. This practice optimizes cell detection for place cells that are active in the “sleep box” (both place cells with fields in the sleep box, and place cells that are reactivated during ripples during sleep), rather than cells that have place fields on the track. Adjusting and recording in a sleep box therefore increases the number of total cells recorded that do not have fields on the track and therefore reduces the place cell percentage. Because calcium imaging uses a fixed lens location and images a large number of cells at once, recording is not optimized for a particular environment. This may result in the recording of a higher percentage of active place cells on the recording apparatus. (Because of logistic constraints in this study, we did not complete any recording while the animal was not on the track.) Second, while an effort is made in electrophysiology to discard times on the track when the animal is not moving, and thus to remove corresponding spiking during ripples, this removal is never perfectly done. The recording of ripples during electrophysiology has two effects: the recording of cells that show replay on the track but do not have place fields on the track and, second, the recording of “extra-field” ripple spiking from cells with fields on the track. This “extra-field” spiking can cause place fields to seem less defined, and cause corresponding distortion of measures of spatial information, such as MI and bits/spike. This, in turn, may cause these cells not to be classified as place cells. Conversely, in calcium imaging, very few to no calcium events are picked up during periods of no movement (see later, Discussion; Fig. 3), so both of these issues (increasing the count of cells without fields on the track, and the distortion of spatial information for place cells) are avoided. Third, while current GCaMPs, such as the GCaMP7c used in this study, have improved single spike resolution compared with past GCaMPs, they still fall short of the single spike resolution of electrophysiology (Tian et al., 2009; Chen et al., 2013; Podor et al., 2015; Shemesh et al., 2020). This property of calcium indicators causes individual spikes, such as those that may occur on the track outside a cell's place field, to be missed, therefore biasing calcium imaging toward fast cell activity that only occurs in the confines of a place field. Calcium imaging therefore, again, results in potentially higher spatial information metrics as compared with electrophysiology, which may cause the identification of more place cells. Finally, compared with electrophysiology, calcium imaging provides additional characteristics (cell shape, size, and brightness) by which to separate adjacent or nearby cells. Because conflation of multiple cells in electrophysiology may disguise place fields and disrupt place field measurements (such as MI or bits per spike), the additional resolution afforded by calcium imaging may allow for more accurate place cell differentiation and identification.
Similar to electrophysiology and in contrast to IEG studies, calcium imaging is biased toward capturing cells active in an environment and not the total cellular population. Additionally, as discussed above, because of limits on single spike resolution, calcium imaging may be picking up even fewer cells with infrequent spiking than does electrophysiology. Thus, this bias of calcium imaging, and electrophysiology, toward detecting only active cells may yield a higher percentage of place cells than do IEG studies that estimate a total of 30–40% of cells to be active in any given environment (Table 1; Guzowski et al., 1999; Witharana et al., 2016).
The percentage of place cells in rat calcium imaging exceeds the percentage in mouse calcium imaging
Significantly, we found that a higher percentage of cells were place cells in our calcium imaging experiments in rats than those reported in mouse CA1 calcium imaging studies (Table 1). This may, in part, reflect the fact that electrophysiological studies indicate that place cells are less frequent in mouse than rat (Table 1), a finding that may be related to reports that mouse place cells are less spatially tuned, contain less spatial information, and have less stable place fields than those in rat (Hok et al., 2016; Mou et al., 2018). More striking, however, is the fact that whereas the percentage of place cells observed in the current study is only slightly higher than that observed in rat electrophysiological studies, examination of Table 1 indicates that calcium imaging experiments in mice typically yield values drastically lower than those observed in mouse electrophysiological studies (mouse calcium imaging experiments have found place cell percentages as low as 4%; Stefanini et al., 2020). These comparisons suggest that there is a much greater concordance between calcium imaging and electrophysiological studies in the rat than in the mouse. These discrepancies may reflect physiological differences between rats and mice that affect the type of activity captured in calcium imaging recordings. CA1 cells in mice are more excitable than those in rat (Routh et al., 2009; Hok et al., 2016), and prolonged GCaMP expression in mice has been shown to lead to cell hyperexcitability, seizures, and even cell death (Tian et al., 2009; Grienberger and Konnerth, 2012; Resendez et al., 2016; Steinmetz et al., 2017; Yang et al., 2018). The higher baseline excitability of mouse cells, combined with a further increase in cellular excitability after GCaMP expression may result in a lower threshold for cell firing and less defined place fields, less stable fields, and, therefore, a seemingly lower number of place cells compared with electrophysiology recordings (Hok et al., 2016). Because rats have a lower baseline excitability, they may be less prone to these GCaMP effects, although further slice electrophysiology work will need to be done to directly compare the effect of GCaMP on mouse and rat neurons.
We considered that differences in calcium event rate between our data and other studies might lead to the high percentage of place cells we saw in this work. On one end of the spectrum, Stefanini et al. (2020) found a particularly low percentage of place cells (∼4% of recorded cells in mouse CA1, using calcium imaging) and investigated whether this might be a consequence of collecting many cells with low calcium event rates. Their reported mean rate was 0.06 ± 0.04 events/s as recorded in mice in an open field, consistent with 0.05–0.5 events/s in other calcium imaging experiments in mouse. Even after eliminating cells with <0.1events/s (a 10× less stringent criteria than we used in Fig. 3a), they still only found that 10–20% of cells were place cells (Stefanini et al., 2020). Conversely, Ziv, who reported a high (for mouse) 20% of imaged CA1 mouse cells were place cells, reported a higher mean event rate, at ∼0.1–0.5 events/s (Ziv et al., 2013). Our results (0.090 ± 0.019 events/s; Fig. 2d,e) are on the lower end of those reported by Ziv et al. (2013), and potentially consistent with the fact that rat neurons are somewhat less excitable than those in mice (Routh et al., 2009; Hok et al., 2016). Additionally, our very slightly lower event rate could be the result of less “out of field” firing of place cells, which also tends to be more predominant in mice (Hok et al., 2016). However, our results are within the range of those reported in mouse imaging studies (Table 1), so differences in event rate are not a likely to be a major contributor to the place cell percentage we observed.
It is also possible that viral infection of different populations of cells may have contributed to the observed rat/mouse differences. For example, if mouse calcium imaging experiments were recording a larger proportion of interneurons than was the case in the present experiment, this might alter the proportion of place cells. However, in the numerous 1-photon calcium imaging experiments done of mouse HPC, we could find little documentation regarding the percentage of excitatory versus inhibitory neurons labeled (Ziv et al., 2013; Kinsky et al., 2018, 2020; Stefanini et al., 2020). Additionally, to our knowledge, little data on the location GCaMP expression in the mouse HPC are available, and, given the low total number of interneurons in the pyramidal cell layer, it seems unlikely that such an effect could be of sufficient magnitude to account for the observed species differences.
In addition to potentially allowing the recording of a higher number of place cells, the lower excitability of rat CA1 neurons, compared with mouse, also has other advantages. We saw no behavioral evidence of postoperative seizures in any of our implanted animals, and no behavioral or imaging evidence of seizures several months postviral injection and lens implementation. This is in contrast to calcium imaging experiments in mice, which frequently report that animals have to be removed from behavior or analysis because of the development of seizure activity (Steinmetz et al., 2017; Huang et al., 2021).
1p calcium imaging using GCAMP7c is movement responsive and minimally activated when the animal is still
Importantly, while calcium transients and events are correlated with action potentials, they are far from a 1:1 correspondence and may capture more or less information than electrophysiology (Huang et al., 2021). During our recording sessions, we saw clear evidence that calcium event rate was correlated with periods of movement (Fig. 3). In electrophysiology experiments, place cell spiking is generally linearly correlated with running speed (McNaughton et al., 1983; Huxter et al., 2003), and significant spiking occurs during ripples when the animal is not moving (Ylinen et al., 1995; Chrobak and Buzsáki, 1996; Csicsvari et al., 1999; Kudrimoti et al., 1999). Our finding greatly differs from the patterns of spiking seen in electrophysiology data, as we saw minimal calcium events during periods of no movement, with a pattern of logarithmic growth of the number of calcium events as the animal began moving and sped up (Fig. 3c). Because the vast majority of CA1 pyramidal cells are speed and/or ripple modulated, and we have imaged primarily pyramidal cells, this result is likely not explainable by the population of cells that express GCaMP (Huxter et al., 2003). Our result is consistent with calcium imaging experiments done in mice by Zhou et al. (2019), in which more calcium events were seen during movement in an environment where food reward could be obtained than during quiet wakefulness in a neutral box. In our experiment, periods of quiet wake were in the same environment (the track) as periods of movement, which shows that changes in calcium event frequency are related to movement and not to contextual associations. Zhou and colleagues posit that the high amount of calcium activity that occurs during movement, but not during sharp wave ripples in mouse, implicate calcium events in theta dependent processes, such as memory encoding and planning (Buzsáki, 1989; Hasselmo et al., 2002; Hasselmo, 2005; Shirvalkar et al., 2010; Colgin, 2013; Wikenheiser and Redish, 2015; Zhou et al., 2019; Wirtshafter and Wilson, 2021). It is possible, and consistent with slice data, that NMDA receptors, which permit calcium influx, are minimally involved in cell spiking during ripples, and the process is more heavily modulated by AMPA receptors, which do not permit calcium influx (Maier et al., 2003; Colgin et al., 2004). There is also evidence that activity during ripples can be visualized using 2-photon calcium imaging in mice (Malvache et al., 2016; Grosmark et al., 2021), although we could not find any experiments showing this activity using one-photon imaging. It is therefore unclear whether limits to imaging spiking activity during ripples is because of the imaging technique or to the use of rats. Further work will be needed to determine whether, in rat, frequent calcium events are also unique to movement, as opposed to sharp wave ripples.
Results show stable place cell identification and these cells have stable place fields over days
Our findings on cell activity over days were similar to and consistent with prior results reported in mice. Prior work has reported that ∼57% of cells are active in only one or two sessions (Ziv et al., 2013), we found a higher proportion (59.4 ± 5.4% of cells were visible in only one session, 19.9 ± 2.4 were visible in two; Fig. 6b), likely because we worked to optimize cell count over precisely consistent focal planes. We also reported that probability of a cell reoccurring decreased by 0.43% per day separating recording sessions. This result was exactly consistent with prior results in mouse that cell reoccurrence decreases from ∼25% for sessions 5 d apart to ∼15% for sessions 30 d apart (25-d difference × 0.43 = ∼10.8% change; Fig. 6e; Ziv et al., 2013). This potential cell turnover did not impact the accuracy of the representation of the environment, as decoding accuracy was consistent throughout sessions (Levy et al., 2021).
There are differing results regarding whether an environment has a single semi-stable map (Ziv et al., 2013; Mau et al., 2018; Kinsky et al., 2020), or there are multiple maps, involving the same cells, that code for a single environment (Sheintuch et al., 2020). In this study, we only saw evidence of the former, as the vast majority of sessions had cells with place fields correlated to previous sessions (Fig. 6f) and place field locations did not change as a function of the number of days between sessions (Fig. 6g). In other words, cell reoccurrence decreases across days, but apparently when a cell does reoccur, its place field remains largely the same.
In conclusion, as previously mentioned, similarities between rat and human physiology, as well as the increased capacity for complex tasks of learning and memory (Whishaw, 1995; Frick et al., 2000; Whishaw et al., 2001; Cressant et al., 2007; Rosenfeld and Ferguson, 2014; Hok et al., 2016), position rats as a well-suited model organism for studying hippocampal function, with several advantages over the mouse. Rats are advantageous over mice in that, for many diseases and disorders, including those with substantial hippocampal involvement, their physiology and behavior more closely mirrors that of humans (Ellenbroek and Youn, 2016). For instance, 5-HT serotonin receptors, which are prominent in the HPC and are implicated in a variety of disorders (including mood and anxiety disorders, psychiatric disorders such as schizophrenia, addiction, and ADHD), are distributed in rats, but not in mice, in a similar pattern as seen in humans (Hirst et al., 2003; Ellenbroek and Youn, 2016). Hippocampal neurogenesis, which is believed to play an important role in learning, memory, and the treatment of depression, is believed to follow a similar pattern in rats and humans, and is less pronounced in mice (Snyder et al., 2009). In addition, both pharmacologic and behavioral responses to addiction are more similar between rats and humans than mice and humans (Jupp et al., 2013; Parker et al., 2014; Ellenbroek and Youn, 2016).
Having the ability to do calcium imaging in rat CA1 will allow increased research into these disorders, as well as basic research in learning and memory. Because rats are substantially larger than mice, they can support larger lens implants and cameras, which may lead to the ability to record from thousands of hippocampal cells in a single session during complex tasks. The ability to record a large number of cells across days will allow research into cell firing changes in the HPC, which will provide insight into more advanced learning and disease mechanisms that are difficult to understand when only investigating mice. Additionally, the uniquely high percentage of recorded place cells (>80% of recorded cells) makes CA1 calcium imaging in rats an ideal method by which to study spatial navigation and learning over days, including mechanisms behind memory consolidation, spatial remapping, and planning over time.
Footnotes
This work was supported by the National Institute on Aging (NIA) Grant T32-AG020506/AG/NIA, the NIA Grant R37-AG008796/AG/NIA, and the National Institute of Neurological Disorders and Stroke Grant R01 NS113804/NS/NINDS. We thank all members of the Disterhoft lab, especially Matthew Oh, Craig Weiss, and Kent Park. We also thank the Miniscope team, in particular Daniel Aharoni and Federico Sangiuliano Jimka. We additionally thank Biafra Ahanonu for assistance with CIATAH, Amy Christensen for providing advice during imaging setup, Drew Ames for consultation on figures, and David Wirtshafter for discussion, editing, and his assistance and expertise with immunohistochemistry.
The authors declare no competing financial interests.
References
- Ahanonu B (2018) CIAtah: a software package for analyzing one- and two-photon calcium imaging datasets, v1.0.0 Edition. Zenodo. [Google Scholar]
- Aharoni D, Hoogland TM (2019) Circuit investigations with open-source miniaturized microscopes: past, present and future. Front Cell Neurosci 13:141. 10.3389/fncel.2019.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni D, Khakh BS, Silva AJ, Golshani P (2019) All the light that we can see: a new era in miniaturized microscopy. Nat Methods 16:11–13. 10.1038/s41592-018-0266-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aika Y, Ren JQ, Kosaka K, Kosaka T (1994) Quantitative analysis of GABA-like-immunoreactive and parvalbumin-containing neurons in the CA1 region of the rat hippocampus using a stereological method, the disector. Exp Brain Res 99:267–276. 10.1007/BF00239593 [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Ryals C (2016) Dissociable hippocampal and amygdalar D1-like receptor contribution to discriminated Pavlovian conditioned approach learning. Behav Brain Res 299:111–121. 10.1016/j.bbr.2015.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anner P, Passecker J, Klausberger T, Dorffner G (2020) Ca2+ imaging of neurons in freely moving rats with automatic post hoc histological identification. J Neurosci Methods 341:108765. 10.1016/j.jneumeth.2020.108765 [DOI] [PubMed] [Google Scholar]
- Avigan PD, Cammack K, Shapiro ML (2020) Flexible spatial learning requires both the dorsal and ventral hippocampus and their functional interactions with the prefrontal cortex. Hippocampus 30:733–744. 10.1002/hipo.23198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkvadze T, Pitkänen A (2012) Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma 29:789–812. 10.1089/neu.2011.1954 [DOI] [PubMed] [Google Scholar]
- Buerge T, Weiss T (2004) Handling and restraint. In: The laboratory mouse (handbook of experimental animals (Hedrich HJ, Bullock G, eds), pp 517–526. San Diego: Elsevier. [Google Scholar]
- Buzsáki G (1989) Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31:551–570. [DOI] [PubMed] [Google Scholar]
- Cai DJ, et al. (2016) A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534:115–118. 10.1038/nature17955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Richardson A, Huffman DM, Austad S (2020) Bring back the rat! J Gerontol A Biol Sci Med Sci 75:405–415. 10.1093/gerona/glz298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL (2001) Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res 119:111–130. 10.1016/s0166-4328(00)00363-6 [DOI] [PubMed] [Google Scholar]
- Chen LJ, Wang YJ, Chen JR, Tseng GF (2017) Hydrocephalus compacted cortex and hippocampus and altered their output neurons in association with spatial learning and memory deficits in rats. Brain Pathol 27:419–436. 10.1111/bpa.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499:295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsáki G (1996) High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci 16:3056–3066. 10.1523/JNEUROSCI.16-09-03056.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL (2013) Mechanisms and functions of theta rhythms. Annu Rev Neurosci 36:295–312. 10.1146/annurev-neuro-062012-170330 [DOI] [PubMed] [Google Scholar]
- Colgin LL, Kubota D, Jia Y, Rex CS, Lynch G (2004) Long-term potentiation is impaired in rat hippocampal slices that produce spontaneous sharp waves. J Physiol 558:953–961. 10.1113/jphysiol.2004.068080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Ahanonu B, Grewe BF, Wang D, Schnitzer MJ, Scherrer G (2019) An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363:276–281. 10.1126/science.aap8586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressant A, Besson M, Suarez S, Cormier A, Granon S (2007) Spatial learning in Long-Evans Hooded rats and C57BL/6J mice: different strategies for different performance. Behav Brain Res 177:22–29. 10.1016/j.bbr.2006.11.010 [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G (1999) Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci 19:RC20. 10.1523/JNEUROSCI.19-16-j0001.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW (2010) Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 13:1433–1440. 10.1038/nn.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA (2007) Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus 17:161–174. 10.1002/hipo.20253 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H (1999) The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23:209–226. 10.1016/s0896-6273(00)80773-4 [DOI] [PubMed] [Google Scholar]
- Ellenbroek B, Youn J (2016) Rodent models in neuroscience research: is it a rat race? Dis Model Mech 9:1079–1087. 10.1242/dmm.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Kao H-Y, Neymotin SA, Olypher A, Vayntrub Y, Lytton WW, Ludvig N (2008) Unmasking the CA1 ensemble place code by exposures to small and large environments: more place cells and multiple, irregularly arranged, and expanded place fields in the larger space. J Neurosci 28:11250–11262. 10.1523/JNEUROSCI.2862-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shapiro ML (2003) Prospective and retrospective memory coding in the hippocampus. Neuron 40:1227–1239. 10.1016/s0896-6273(03)00752-9 [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Kennedy PJ, Shapiro ML (2006) Episodic memory—from brain to mind. Hippocampus 16:691–703. 10.1002/hipo.20204 [DOI] [PubMed] [Google Scholar]
- Foster DJ, Knierim JJ (2012) Sequence learning and the role of the hippocampus in rodent navigation. Curr Opin Neurobiol 22:294–300. 10.1016/j.conb.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Morris RG, Dayan P (2000) A model of hippocampally dependent navigation, using the temporal difference learning rule. Hippocampus 10:1–16. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (2013) Paxinos and Franklin's the mouse brain in stereotaxic coordinates. Amsterdam: Elsevier. [Google Scholar]
- Frick KM, Stillner ET, Berger-Sweeney J (2000) Mice are not little rats: species differences in a one-day water maze task. Neuroreport 11:3461–3465. 10.1097/00001756-200011090-00013 [DOI] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A (2012) Imaging calcium in neurons. Neuron 73:862–885. 10.1016/j.neuron.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Grosmark AD, Sparks FT, Davis MJ, Losonczy A (2021) Reactivation predicts the consolidation of unbiased long-term cognitive maps. Nat Neurosci 24:1574–1585. 10.1038/s41593-021-00920-7 [DOI] [PubMed] [Google Scholar]
- Guo W, Zhang JJ, Newman JP, Wilson MA (2020) Latent learning drives sleep-dependent plasticity in distinct CA1 subpopulations. bioRxiv 2020.2002.2027.967794. [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci 2:1120–1124. 10.1038/16046 [DOI] [PubMed] [Google Scholar]
- Hainmueller T, Bartos M (2018) Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558:292–296. 10.1038/s41586-018-0191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel EJ, Grewe BF, Parker JG, Schnitzer MJ (2015) Cellular level brain imaging in behaving mammals: an engineering approach. Neuron 86:140–159. 10.1016/j.neuron.2015.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EE, Blair GJ, O'Dell TJ, Blair HT, Izquierdo A (2020) Chemogenetic modulation and single-photon calcium imaging in anterior cingulate cortex reveal a mechanism for effort-based decisions. J Neurosci 40:5628–5643. 10.1523/JNEUROSCI.2548-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME (2005) What is the function of hippocampal theta rhythm?–linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus 15:936–949. 10.1002/hipo.20116 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McClelland JL (1999) Neural models of memory. Curr Opin Neurobiol 9:184–188. 10.1016/s0959-4388(99)80025-7 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Hay J, Ilyn M, Gorchetchnikov A (2002) Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw 15:689–707. 10.1016/s0893-6080(02)00057-6 [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD (2003) Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol 64:1295–1308. 10.1124/mol.64.6.1295 [DOI] [PubMed] [Google Scholar]
- Hok V, Poucet B, Duvelle É, Save É, Sargolini F (2016) Spatial cognition in mice and rats: similarities and differences in brain and behavior. Wiley Interdiscip Rev Cogn Sci 7:406–421. 10.1002/wcs.1411 [DOI] [PubMed] [Google Scholar]
- Huang L, Ledochowitsch P, Knoblich U, Lecoq J, Murphy GJ, Reid RC, de Vries SE, Koch C, Zeng H, Buice MA, Waters J, Li L (2021) Relationship between simultaneously recorded spiking activity and fluorescence signal in GCaMP6 transgenic mice. Elife 10:e51675. 10.7554/eLife.51675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN (2009) Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol 215:243–252. 10.1016/j.expneurol.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN (2010) Regionally localized recurrent excitation in the dentate gyrus of a cortical contusion model of posttraumatic epilepsy. J Neurophysiol 103:1490–1500. 10.1152/jn.00957.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O'Keefe J (2003) Independent rate and temporal coding in hippocampal pyramidal cells. Nature 425:828–832. 10.1038/nature02058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone PM, Jacob HJ (2009) Rats! Dis Model Mech 2:206–210. 10.1242/dmm.002733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Everitt BJ, Robbins TW (2005) The hippocampus and appetitive Pavlovian conditioning: effects of excitotoxic hippocampal lesions on conditioned locomotor activity and autoshaping. Hippocampus 15:713–721. 10.1002/hipo.20094 [DOI] [PubMed] [Google Scholar]
- Jimenez JC, Berry JE, Lim SC, Ong SK, Kheirbek MA, Hen R (2020) Contextual fear memory retrieval by correlated ensembles of ventral CA1 neurons. Nat Commun 11:3492. 10.1038/s41467-020-17270-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn SA, Tonegawa S (2020) Memory engrams: recalling the past and imagining the future. Science 367:eaaw4325. 10.1126/science.aaw4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H, Bramian A, Soma S, Saito T, Saido TC, Igarashi KM (2020) Disrupted place cell remapping and impaired grid cells in a knockin model of Alzheimer's disease. Neuron 107:1095–1112.e6. 10.1016/j.neuron.2020.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Dalley JW (2013) Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Dis Model Mech 6:302–311. 10.1242/dmm.010934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky NR, Sullivan DW, Mau W, Hasselmo ME, Eichenbaum HB (2018) Hippocampal place fields maintain a coherent and flexible map across long timescales. Curr Biol 28:3578–3588.e6. 10.1016/j.cub.2018.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky NR, Mau W, Sullivan DW, Levy SJ, Ruesch EA, Hasselmo ME (2020) Trajectory-modulated hippocampal neurons persist throughout memory-guided navigation. Nat Commun 11:2443. 10.1038/s41467-020-16226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Martins GJ, Paixao VB, Zhou P, Paninski L, Costa RM (2017) The spatiotemporal organization of the striatum encodes action space. Neuron 95:1171–1180.e7. 10.1016/j.neuron.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL (1999) Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci 19:4090–4101. 10.1523/JNEUROSCI.19-10-04090.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI (2004) Distinct ensemble codes in hippocampal areas CA3 and CA1. Science 305:1295–1298. 10.1126/science.1100265 [DOI] [PubMed] [Google Scholar]
- Levy SJ, Kinsky NR, Mau W, Sullivan DW, Hasselmo ME (2021) Hippocampal spatial memory representations in mice are heterogeneously stable. Hippocampus 31:244–260. 10.1002/hipo.23272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes G, Bonacchi N, Frazão J, Neto JP, Atallah BV, Soares S, Moreira L, Matias S, Itskov PM, Correia PA, Medina RE, Calcaterra L, Dreosti E, Paton JJ, Kampff AR (2015) Bonsai: an event-based framework for processing and controlling data streams. Front Neuroinform 9:7. 10.3389/fninf.2015.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Nimmrich V, Draguhn A (2003) Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices. J Physiol 550:873–887. 10.1113/jphysiol.2003.044602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvache A, Reichinnek S, Villette V, Haimerl C, Cossart R (2016) Awake hippocampal reactivations project onto orthogonal neuronal assemblies. Science 353:1280–1283. 10.1126/science.aaf3319 [DOI] [PubMed] [Google Scholar]
- Markus EJ, Barnes CA, McNaughton BL, Gladden VL, Skaggs WE (1994) Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus 4:410–421. 10.1002/hipo.450040404 [DOI] [PubMed] [Google Scholar]
- Mau W, Sullivan DW, Kinsky NR, Hasselmo ME, Howard MW, Eichenbaum H (2018) The same hippocampal CA1 population simultaneously codes temporal information over multiple timescales. Curr Biol 28:1499–1508.e4. 10.1016/j.cub.2018.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL (2006) Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus 16:785–794. 10.1002/hipo.20202 [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF (1999) Hippocampal encoding of non-spatial trace conditioning. Hippocampus 9:385–396. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52:41–49. 10.1007/BF00237147 [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB (2006) Path integration and the neural basis of the 'cognitive map'. Nat Rev Neurosci 7:663–678. 10.1038/nrn1932 [DOI] [PubMed] [Google Scholar]
- Meshulam L, Gauthier JL, Brody CD, Tank DW, Bialek W (2017) Collective behavior of place and non-place neurons in the hippocampal network. Neuron 96:1178–1191.e4. 10.1016/j.neuron.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Moser M-B, McNaughton BL (2017) Spatial representation in the hippocampal formation: a history. Nat Neurosci 20:1448–1464. 10.1038/nn.4653 [DOI] [PubMed] [Google Scholar]
- Mou X, Cheng J, Yu YS, Kee SE, Ji D (2018) Comparing mouse and rat hippocampal place cell activities and firing sequences in the same environments. Front Cell Neurosci 12:332. 10.3389/fncel.2018.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR Jr, Deyo RA, Disterhoft JF (1990) Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 104:243–252. 10.1037/0735-7044.104.2.243 [DOI] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM (2009) Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161:441–450. 10.1016/j.neuroscience.2009.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109. 10.1016/0014-4886(76)90055-8 [DOI] [PubMed] [Google Scholar]
- Olypher AV, Lánský P, Muller RU, Fenton AA (2003) Quantifying location-specific information in the discharge of rat hippocampal place cells. J Neurosci Methods 127:123–135. 10.1016/s0165-0270(03)00123-7 [DOI] [PubMed] [Google Scholar]
- Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, Woods LCS, Palmer AA (2014) Rats are the smart choice: rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology 76:250–258. 10.1016/j.neuropharm.2013.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podor B, Hu Yl, Ohkura M, Nakai J, Croll R, Fine A (2015) Comparison of genetically encoded calcium indicators for monitoring action potentials in mammalian brain by two-photon excitation fluorescence microscopy. Neurophotonics 2:021014. 10.1117/1.NPh.2.2.021014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard A, Hornung R, Kramer PR (2021) Measuring calcium activity within individual neurons within the rat thalamus. MethodsX 8:101273. 10.1016/j.mex.2021.101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Jennings JH, Ung RL, Namboodiri VM, Zhou ZC, Otis JM, Nomura H, McHenry JA, Kosyk O, Stuber GD (2016) Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses. Nat Protoc 11:566–597. 10.1038/nprot.2016.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PD, Liaw HP, Lee AK (2014) Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science 345:814–817. 10.1126/science.1255635 [DOI] [PubMed] [Google Scholar]
- Rickgauer JP, Deisseroth K, Tank DW (2014) Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat Neurosci 17:1816–1824. 10.1038/nn.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Ferguson SA (2014) Barnes maze testing strategies with small and large rodent models. J Vis Exp (84):e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Redish AD, McNaughton BL, Barnes CA (2003) Hippocampal map realignment and spatial learning. Nat Neurosci 6:609–615. 10.1038/nn1053 [DOI] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Harris K, Chitwood RA (2009) Anatomical and electrophysiological comparison of CA1 pyramidal neurons of the rat and mouse. J Neurophysiol 102:2288–2302. 10.1152/jn.00082.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski LA, Lipa P, Barnes CA (2013) Tracking the course of hippocampal representations during learning: when is the map required? J Neurosci 33:3094–3106. 10.1523/JNEUROSCI.1348-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld G, Carta S, Rupprecht P, Ayaz A, Helmchen F (2021) In vivo calcium imaging of CA3 pyramidal neuron populations in adult mouse hippocampus. eNeuro 8:ENEURO.0023-21.2021. 10.1523/ENEURO.0023-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BB, Thiberge SY, Guo C, Tervo DGR, Brody CD, Karpova AY, Tank DW (2018) Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope. Neuron 100:1045–1058.e5. 10.1016/j.neuron.2018.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheintuch L, Rubin A, Brande-Eilat N, Geva N, Sadeh N, Pinchasof O, Ziv Y (2017) Tracking the same neurons across multiple days in Ca(2+) imaging data. Cell Rep 21:1102–1115. 10.1016/j.celrep.2017.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheintuch L, Geva N, Baumer H, Rechavi Y, Rubin A, Ziv Y (2020) Multiple maps of the same spatial context can stably coexist in the mouse hippocampus. Curr Biol 30:1467–1476.e6. 10.1016/j.cub.2020.02.018 [DOI] [PubMed] [Google Scholar]
- Shemesh OA, et al. (2020) Precision calcium imaging of dense neural populations via a cell-body-targeted calcium indicator. Neuron 107:470–486.e11. 10.1016/j.neuron.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim I, Ha Y, Chung JY, Lee HJ, Yang KH, Chang JW (2003) Association of learning and memory impairments with changes in the septohippocampal cholinergic system in rats with kaolin-induced hydrocephalus. Neurosurgery 53:416–425. 10.1227/01.neu.0000073989.07810.d8 [DOI] [PubMed] [Google Scholar]
- Shin JH, Song M, Paik SB, Jung MW (2020) Spatial organization of functional clusters representing reward and movement information in the striatal direct and indirect pathways. Proc Natl Acad Sci U S A 117:27004–27015. 10.1073/pnas.2010361117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirvalkar PR, Rapp PR, Shapiro ML (2010) Bidirectional changes to hippocampal theta–gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci U S A 107:7054–7059. 10.1073/pnas.0911184107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman T, et al. (2020) Breakdown of spatial coding and interneuron synchronization in epileptic mice. Nat Neurosci 23:229–238. 10.1038/s41593-019-0559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ (2017) Miniaturized two-photon microscope: seeing clearer and deeper into the brain. Light Sci Appl 6:e17104–e17104. 10.1038/lsa.2017.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ (2006) Hippocampal place cells, context, and episodic memory. Hippocampus 16:716–729. 10.1002/hipo.20208 [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA (2009) Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci 29:14484–14495. 10.1523/JNEUROSCI.1768-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza BC, Pavão R, Belchior H, Tort AB (2018) On information metrics for spatial coding. Neuroscience 375:62–73. 10.1016/j.neuroscience.2018.01.066 [DOI] [PubMed] [Google Scholar]
- Squire LR (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231. 10.1037/0033-295x.99.2.195 [DOI] [PubMed] [Google Scholar]
- Stefanini F, Kushnir L, Jimenez JC, Jennings JH, Woods NI, Stuber GD, Kheirbek MA, Hen R, Fusi S (2020) A distributed neural code in the dentate gyrus and in CA1. Neuron 107:703–716.e4. 10.1016/j.neuron.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, et al. (2017) Aberrant cortical activity in multiple GCaMP6-expressing transgenic mouse lines. eNeuro 4:ENEURO.0207-17.2017. 10.1523/ENEURO.0207-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L (2004) Brain maps: structure of the rat brain. Houston: Gulf Professional Publishing. [Google Scholar]
- Talbot ZN, Sparks FT, Dvorak D, Curran BM, Alarcon JM, Fenton AA (2018) Normal CA1 place fields but discoordinated network discharge in a Fmr1-null mouse model of fragile X syndrome. Neuron 97:684–697.e4. 10.1016/j.neuron.2017.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz P, Ruttimann UE, Unser M (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process 7:27–41. 10.1109/83.650848 [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL (2009) Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods 6:875–881. 10.1038/nmeth.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD (2013) Dismantling the Papez circuit for memory in rats. Elife 2:e00736. 10.7554/eLife.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Spanagel R (2014) The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol 48:313–320. 10.1016/j.alcohol.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Voigts J, Siegle JH, Pritchett DL, Moore CI (2013) The flexDrive: an ultra-light implant for optical control and highly parallel chronic recording of neuronal ensembles in freely moving mice. Front Syst Neurosci 7:8. 10.3389/fnsys.2013.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigts J, Newman JP, Wilson MA, Harnett MT (2020) An easy-to-assemble, robust, and lightweight drive implant for chronic tetrode recordings in freely moving animals. J Neural Eng 17:026044. 10.1088/1741-2552/ab77f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu Y, Li X, Zhang Z, Yang H, Zhang Y, Williams PR, Alwahab NSA, Kapur K, Yu B, Zhang Y, Chen M, Ding H, Gerfen CR, Wang KH, He Z (2017) Deconstruction of corticospinal circuits for goal-directed motor skills. Cell 171:440–455.e14. 10.1016/j.cell.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ (1995) A comparison of rats and mice in a swimming pool place task and matching to place task: some surprising differences. Physiol Behav 58:687–693. 10.1016/0031-9384(95)00110-5 [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Metz GA, Kolb B, Pellis SM (2001) Accelerated nervous system development contributes to behavioral efficiency in the laboratory mouse: a behavioral review and theoretical proposal. Dev Psychobiol 39:151–170. 10.1002/dev.1041 [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD (2015) Hippocampal theta sequences reflect current goals. Nat Neurosci 18:289–294. 10.1038/nn.3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Nitz DA (2007) Discrete place fields of hippocampal formation interneurons. J Neurophysiol 97:4152–4161. 10.1152/jn.01200.2006 [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1993) Dynamics of the hippocampal ensemble code for space. Science 261:1055–1058. 10.1126/science.8351520 [DOI] [PubMed] [Google Scholar]
- Wirtshafter HS, Wilson MA (2019) Locomotor and hippocampal processing converge in the lateral septum. Curr Biol 29:3177–3192. 10.1016/j.cub.2019.07.089 [DOI] [PubMed] [Google Scholar]
- Wirtshafter HS, Wilson MA (2020) Differences in reward biased spatial representations in the lateral septum and hippocampus. Elife 9:e55252. 10.7554/eLife.55252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtshafter HS, Wilson MA (2021) Lateral septum as a nexus for mood, motivation, and movement. Neurosci Biobehav Rev 126:544–559. 10.1016/j.neubiorev.2021.03.029 [DOI] [PubMed] [Google Scholar]
- Wirtshafter HS, Disterhoft JF (2022) Place cells are non-randomly clustered by field location in CA1 hippocampus. bioRxiv. doi: 10.1101/2022.03.15.484476. 10.1101/2022.03.15.484476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witharana WK, Cardiff J, Chawla MK, Xie JY, Alme CB, Eckert M, Lapointe V, Demchuk A, Maurer AP, Trivedi V, Sutherland RJ, Guzowski JF, Barnes CA, McNaughton BL (2016) Nonuniform allocation of hippocampal neurons to place fields across all hippocampal subfields. Hippocampus 26:1328–1344. 10.1002/hipo.22609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu N, He Y, Liu Y, Ge L, Zou L, Song S, Xiong W, Liu X (2018) Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP. Nat Commun 9:1504. 10.1038/s41467-018-03719-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G (1995) Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15:30–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Ginzburg I, McNaughton BL, Sejnowski TJ (1998) Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J Neurophysiol 79:1017–1044. 10.1152/jn.1998.79.2.1017 [DOI] [PubMed] [Google Scholar]
- Zhang X, Li B (2018) Population coding of valence in the basolateral amygdala. Nat Commun 9:5195. 10.1038/s41467-018-07679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Neville KR, Goldstein N, Kabu S, Kausar N, Ye R, Nguyen TT, Gelwan N, Hyman BT, Gomperts SN (2019) Cholinergic modulation of hippocampal calcium activity across the sleep-wake cycle. Elife 8:e39777. 10.7554/eLife.39777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Resendez SL, Rodriguez-Romaguera J, Jimenez JC, Neufeld SQ, Giovannucci A, Friedrich J, Pnevmatikakis EA, Stuber GD, Hen R, Kheirbek MA, Sabatini BL, Kass RE, Paninski L (2018) Efficient and accurate extraction of in vivo calcium signals from microendoscopic video data. Elife 7:e28728. 10.7554/eLife.28728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El Gamal A, Schnitzer MJ (2013) Long-term dynamics of CA1 hippocampal place codes. Nat Neurosci 16:264–266. 10.1038/nn.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]