Abstract
Dietary supplementation with NAD+ precursors or ketone esters has been shown to improve mitochondrial function in preclinical models of heart failure with either reduced or preserved ejection fraction. Both supplementation approaches hold promise but are in the early stages of development as clinical therapies for heart failure.
Despite substantial advances in pharmacological treatments for patients with heart failure (HF), the morbidity and mortality associated with the condition remain high, with a prevalence of HF of approximately 6.2 million patients and a HF-related mortality of 380,000 people per year in the USA alone. Therefore, there is considerable interest in identifying novel therapeutics for HF, including therapies targeting the mitochondrial dysfunction that occurs in both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF)1. This Comment focuses on the latest progress in using nutritional supplements to target mitochondrial dysfunction and bioenergetics in HF in two categories: NAD+ precursors2-4 and ketone esters5,6. These targets might have potential as novel therapies for both HFrEF2,5 and HFpEF3-5. Moreover, both approaches might have beneficial effects on reducing the inflammation that is characteristic of human HF2,6. FIGURE 1 illustrates potential mechanisms through which targeting NAD+ pathways1,7 or ketone bodies8,9 might benefit patients with HF.
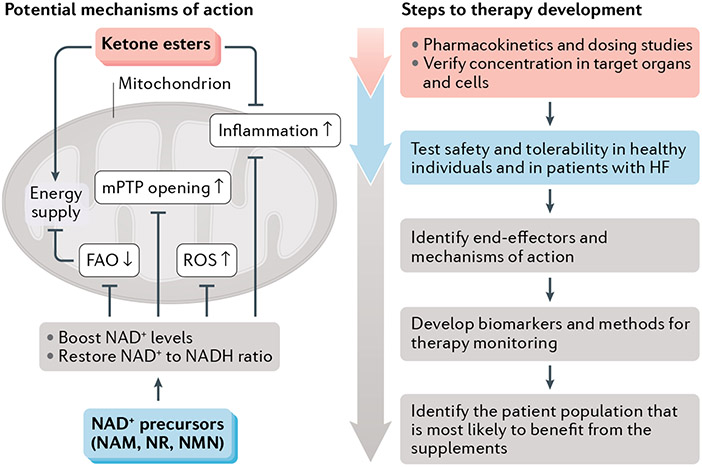
Fig. 1 ∣. Potential mechanisms of dietary supplements to boost mitochondrial metabolism in HF.
Summary of the potential mechanisms at the level of the mitochondrion for the benefit of supplementation with NAD+ precursors or ketone esters in heart failure (HF). At present, each therapy is at an early stage in the continuum of evidence necessary to establish clinical benefit in patients with HF. To advance these or any other mitochondria-targeted therapies into patient care, the development of biomarkers for the clinical assessment of mitochondrial function will be crucial. Identification of patients with HF who are most likely to benefit from a specific dietary supplement is also important. FAO, fatty acid oxidation; mPTP, mitochondrial permeability transition pore; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside; ROS, reactive oxygen species.
NAD+ precursors
Compared with healthy individuals, myocardial NAD+ levels are decreased in patients with HF1,7. NAD+ depletion has protean cellular effects1,7, including impaired activity of sirtuin deacetylase, increased oxidative stress and apoptosis, and promoting a pro-inflammatory macrophage phenotype7. In preclinical models of HFrEF, raising NAD+ levels improves cardiac function and delays HF development1,2,7. Multiple mechanisms for this benefit have been proposed, mostly centring on mitochondrial function (FIG. 1). Studies published in 2021 suggest that NAD+ is also depleted in the myocardium of patients with HFpEF3,4. Supplementation with the NAD+ precursors nicotinamide3 or nicotinamide riboside4 improves diastolic dysfunction in preclinical models of HFpEF, as does the activation of nicotinamide phosphoribosyl transferase, which is a key enzyme in the NAD+ salvage pathway4. Therefore, mitochondrial dysfunction is common to both HFrEF and HFpEF, and its targeting with NAD+ precursors holds promise for the treatment of both types of HF.
The NAD+ precursors nicotinamide mononucleotide, nicotinamide riboside and nicotinamide are orally bioavailable. Nicotinamide riboside dosing at up to 2 g per day is well tolerated in healthy volunteers1, and its safety and tolerability are being evaluated in patients with HFrEF (NCT03423342). A small, non-randomized study in four patients with ACC stage D HFrEF reported in 2020 that treatment for 5–9 days with nicotinamide riboside increased whole-blood NAD+ levels, improved mitochondrial respiration and decreased NLRP3, IL1B, IL6 and IL18 expression in peripheral blood mononuclear cells compared with baseline levels2. Although these findings are promising, several additional steps are required before conducting larger trials powered for efficacy (FIG. 1). First, biomarkers of cardiac NAD+ metabolism and NAD+ response to supplementation are required. In addition, mechanistic insights into NAD+ metabolism in HF are necessary to determine whether all or only subpopulations of patients with HF would benefit from an NAD+-boosting strategy.
Ketones and ketosis
In HF, cardiac metabolism shifts away from fatty acid oxidation to glycolysis and increased utilization of ketone bodies8,9. Rather than being maladaptive, this shift to the utilization of ketones such as β-hydroxybutyrate (BHB) seems to be beneficial, raising the possibility of supplying ketones as an alternative fuel for the failing heart10. In humans, circulating levels of ketone bodies can be raised through a number of dietary manipulations (such as prolonged fasting or diets that are very low in carbohydrate: ‘ketogenic’ diets) or through the use of nutritional supplements (such as 1,3-butanediol, medium-chain triglycerides, ketone salts or ketone esters)8,9.
A study published in 2021 evaluated supplementation with a ketone ester in mouse and rat models of HFrEF5. In a mouse model of myocardial infarction, administration of a synthesized ketone ester mixed in the chow diet blunted the decline in left ventricular ejection fraction5, although the effect might have been limited by the inability of the supplemented diet to sustain ketosis outside of the animals’ nocturnal feeding period. In a rat model of myocardial infarction, supplementation with a commercially available ketone ester (β-hydroxybutyrate-(R)-1,3-butanediol monoester, DeltaG) sustained ketosis for 24 h and attenuated both the decline in ejection fraction and pathological left ventricular remodelling5. Furthermore, myocardial ATP levels were improved with ketone ester supplementation5, suggesting that increasing circulating ketone levels does provide an auxiliary fuel source for the failing heart when fatty acid oxidation is impaired (FIG. 1).
Another study used a different mouse model of HFpEF to assess the effects of administration of either ketone esters or the sodium–glucose cotransporter 2 (SGLT2) inhibitor empagliflozin6, which is known to induce a mild ketosis. Ketone ester supplementation reduced inflammation and protected against mitochondrial dysfunction in the myocardium, thereby breaking the circuit of mitochondrial dysfunction and inflammation in the development of HFpEF. Interestingly, in this study, the benefit of ketone ester supplementation was independent of ketone body oxidation, because promoting ketone body utilization as a source of fuel in cardiomyocytes eliminated the protective effect of the supplement6. This finding suggests that the mechanisms for the benefit of ketone ester supplementation might be distinct in HFpEF versus HFrEF. Moreover, although ketone ester supplementation induced greater ketosis than SGLT2 inhibition, both strategies were similarly effective in increasing ketone body levels, improving mitochondrial respiration in the myocardium, and decreasing circulating IL-1β and IL-18 levels6. Importantly, ketone ester supplementation and SGLT2 inhibition also were similarly effective in improving diastolic relaxation and increasing treadmill exercise time in these mice6.
Although ketone ester supplementation has been studied in a variety of other clinical contexts8,9, clinical study of supplementation with ketone esters in patients with HF is limited to a short-term study in ten patients with HFrEF (cited in REFS8,9). In that study, sodium–BHB infusion was associated with increased cardiac output and decreased systemic vascular resistance. Gastrointestinal distress might be an obstacle to oral ketone ester supplementation. Published studies used either BHB infusion (in patients) or ketone ester gavage (in animal models). In 2021, topline results were announced for the EMPEROR-Preserved trial on empagliflozin in patients with HFpEF. The company reported a significant reduction in the primary composite end point of the rate of cardiovascular death or hospitalization for HF. We await peer-reviewed confirmation of these results. Whether ketone ester supplementation would provide any additional benefit in patients with HF over that obtained with SGLT2 inhibitors remains to be determined.
In conclusion, NAD+ precursors might have therapeutic potential in patients with HF but more studies are needed to develop biomarkers of response to treatment and to identify clinical populations of patients with HF who are most likely to benefit from this approach (FIG. 1). Ketone ester supplementation might be an alternative or an addition to SGLT2 inhibitors in patients with HF, if an oral regime can be developed and the risk of euglycaemic ketoacidosis can be avoided.
Acknowledgements
The authors receive grant support from the NIH: R01 HL126209 and HL144937 to K.D.O. and R.T.; and HL110349, HL142628 and HL149695 to R.T.
Footnotes
Competing interests
K.D.O. and R.T. are listed as co-inventors on a patent application submitted by the University of Washington, USA, regarding targeting NAD+ metabolism to treat inflammation in heart failure. R.T. is a member of the Scientific Advisory Board of Cytokinetics, USA.
References
- 1.Tian R et al. Unlocking the secrets of mitochondria in the cardiovascular system: path to a cure in heart failure — a report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation 140, 1205–1216 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou B et al. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Invest 130, 6054–6063 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdellatif M et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl Med 13, eabd7064 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong D et al. NAD+ repletion reverses heart failure with preserved ejection fraction. Circ. Res 128, 1629–1641 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yurista SR et al. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ. Heart Fail 14, e007684 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpHF. Circ. Res 128, 232–245 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Zhou B & Tian R Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Invest 128, 3716–3726 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selvaraj S, Kelly DP & Margulies KB Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation 141, 1800–1812 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurista SR et al. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol 77, 1660–1669 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Horton JL et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 4, e124079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]