Abstract
Continuous cultures in which a high-pressure chemostat was used were employed to study the growth responses of (i) deep-sea microbial populations with the naturally occurring carbon available in seawater and with limiting concentrations of supplemental organic substrates and (ii) pure cultures of copiotrophic barophilic and barotolerant deep-sea isolates in the presence of limiting carbon concentrations at various pressures, dilution rates, and temperatures. We found that the growth rates of natural populations could not be measured or were extremely low (e.g., a doubling time of 629 h), as determined from the difference between the dilution rate and the washout rate. A low concentration of supplemental carbon (0.33 mg/liter) resulted in positive growth responses in the natural population, which resulted in an increase in the number of cells and eventually a steady population of cells. We found that the growth responses to imposed growth pressure by barophilic and barotolerant pure-culture isolates that were previously isolated and characterized under high-nutrient-concentration conditions were maintained under the low-nutrient-concentration limiting conditions (0.33 to 3.33 mg of C per liter) characteristic of the deep-sea environment. Our results indicate that deep-sea microbes can respond to small changes in substrate availability. Also, barophilic microbes that are copiotrophic as determined by their isolation in the presence of high carbon concentrations and their preference for high carbon concentrations are versatile and are able to compete and grow as barophiles in the low-carbon-concentration oligotrophic deep-sea environment in which they normally exist.
Our knowledge concerning the physical conditions under which microbes can grow has increased significantly in recent years. A lower temperature limit of −7.5°C was reported early in marine microbiology studies (4), and the upper temperature limit was recently reported to be 113°C (5). In most of the deep sea, microorganisms grow at 2 to 3°C and hundreds of bars of hydrostatic pressure. At nearly 11,000 m, the Challenger Deep is the deepest known oceanic site, and the microbes that are active there must be able to function at pressures greater than 100 MPa. Viable microbes have been isolated from this trench and other trenches of similar depths and studied (25, 44, 49). While the growth temperatures of these organisms define them primarily as psychrophiles, their pressure optima characterize them as barotolerant, barophilic, or obligately barophilic strains. Recently, Yayanos and coworkers isolated barophilic and obligately barophilic strains (46, 47), and in a recent review (45) Yayanos refers to these organisms as piezophiles and hyperpiezophiles rather than barophiles and obligate barophiles. At this time, the maximum growth pressure is believed to be around 1,150 × 105 Pa for an obligate barophile (11), but it may be even higher (up to 1,400 × 105 Pa), as reported by ZoBell (50). The ways in which genetic expression in barophilic microbes is regulated by pressure have just recently become an active area of research (23, 28, 30). Since ZoBell’s early work which showed that barophilic bacteria occur in deep-sea samples and in most of the recent isolation and characterization studies of barophilic isolates (11, 19, 22, 25, 26, 31, 47), the growth media employed have generally contained very high concentrations of organic carbon (i.e., copiotrophic conditions). This is not characteristic of the oligotrophic nutrient conditions which these microbes generally experience in most of their natural habitats (i.e., oligotrophic conditions).
The major genera of cultivated barophiles include the genera Shewanella, Photobacterium, Colwellia, and Moritella, as well as a new unidentified group (7), and all of these organisms can be classified as copiotrophs. True oligotrophs are often present as ultramicrobacteria in seawater (1, 37). They are able to grow under very nutrient-limited conditions because they have a high specific affinity for substrates (6) and the ability to utilize a mixture of limiting substrates (38). To date, none of these organisms have been characterized as barophilic. The deep sea is generally described as an oligotrophic environment that contains 0.03 to 0.2 mM dissolved organic carbon (12, 29), and the flux of particulate organic carbon is particularly important in determining the residence time and turnover of carbon (35). There are deep-sea niches, such as in animal gut tracts, in which microbes may be periodically exposed to higher substrate concentrations (9, 42).
Limited work has been done on the growth responses of barophiles to reduced substrate levels, and in the studies that have been done the workers have used batch culture approaches (8, 20). If the deep-sea barophiles that have been isolated and studied to date are obligately copiotrophic with respect to carbon sources, then their in situ growth activities may well vary with their level of nutrition (44). They may not respond as barophiles at habitat pressures in the deep sea, where growth substrates are likely limiting, and in fact there may be an evolutionary distinction between oligotrophic and copiotrophic barophiles (8).
To address the question of how natural deep-sea populations, as well as barophilic pure cultures, respond to constant growth-limiting concentrations of organic carbon under elevated hydrostatic pressure conditions, we performed the series of experiments described below. In this study, we used a high-pressure chemostat (21) and continuous culture techniques to examine (i) the growth responses of natural deep-sea populations whose growth-limiting substrate was not known but whose growth rates could be determined from the difference between the dilution rate and the washout rate (17) and (ii) the growth responses of pure cultures of deep-sea psychrophilic barotolerant and barophilic microbes to pressure and dilution rate changes in the presence of limiting carbon concentrations. While we could obtain time-independent steady states in the presence of limiting substrate concentrations with the chemostat, we had to consider the effects of threshold substrate concentrations that affected minimum population levels (16), the fact that steady states were influenced by the dilution rate in the presence of various substrate concentrations (15), and the fact that cell growth and cell removal are not closely linked in the natural environment (i.e., deep sea), as they are in a chemostat (18).
MATERIALS AND METHODS
Organisms.
Deep natural seawater (NSW) was collected from the North Atlantic Ocean (37°23′N, 68°49′W) at depths of 4,400 to 4,500 m by using Niskin bottles rinsed with 70% alcohol and sterile distilled water. Some of this seawater was immediately transferred to sterile chilled bottles and stored at 105 Pa and 3°C. Other 2-liter aliquots were filtered (pore size, 0.2 μm; sterile Nuclepore) at 3°C. The filters with retained cells were placed in sterile stoppered Nalgene tubes containing 10 ml of seawater obtained from the sampling depth and pressurized to the in situ collection pressure; these samples were stored at 3°C until they were used. When a natural-population experiment was initiated in the laboratory, a sterile chilled chemostat vessel was filled with cold NSW (500 ml) obtained from the collection depth. A Nalgene tube was decompressed, and the contents (10 ml) along with the Nuclepore filter were added to the vessel. The contents of the chemostat were then stirred for 15 min, the filter was removed, and samples were taken to determine zero-time direct counts. The chemostat was then fully assembled (21), and the experiment was initiated by starting the flow of NSW and pressurizing the chemostat.
The following pure cultures of psychrophilic organisms were studied with the chemostat. Barophilic isolate F1 from 4,900 m, barophilic isolate 27AB from 5,100 m, and barotolerant isolate K-4 from 4,000 m were originally isolated on Difco 2216 marine medium or on medium containing 0.1% peptone and 0.1% yeast extract in chilled slush (0.4%) agar. The growth characteristics of these organisms, including their maximum growth rates in batch culture, have been determined previously (19). F1 has been identified as a Shewanella sp. (7). Slightly barotolerant strain 82 was isolated from relatively shallow water (depth, 2,600 m) on defined sodium glutamate (0.5 g/liter) medium (41). It had a 10-h doubling time in this medium supplemented with vitamins (3). Isolate O-96-2 was obtained from a deep-sea natural population (depth, 4,500 m) which had been enriched in the chemostat at 450 × 105 Pa and 3°C by using 1.0 mg of yeast extract per liter. It was isolated from a decompressed chemostat sample on 2216 marine agar at 105 Pa and grew well in full-strength and 10% 2216 marine broth. The doubling times at 3°C were determined to be 6.9 h at 105 Pa and 4.0 h at 300 × 105 Pa in batch cultures grown on medium containing 100 mg of yeast extract per liter. Isolate O-96-12 was obtained from a sample collected at a depth of 4,500 m which was immediately enriched aboard a ship at 105 Pa; the strain was isolated conventionally at 3°C by using oligotrophic AGL medium (see below) containing 1.0 mg of C per liter. A stock culture of this isolate was maintained on this medium before the isolate was tested in the chemostat. While isolate O-96-12 was never exposed to more than 1 mg of C per liter in AGL medium during the enrichment and isolation procedure, aliquots of the stock culture were found to grow quite well on 25× AGL medium, as well as on full-strength 2216 marine agar. The best estimate of the maximum growth rate of this organism at 3°C was determined by using 10× and 100× AGL medium; this estimate resulted in a doubling time of approximately 20 h at 105 Pa.
Media.
NSW that was collected at a depth of 4,500 m was used in the chemostat reservoir for natural-population studies and was either filter sterilized twice (pore size, 0.2 μm; Nuclepore filters) or autoclaved, and it was supplemented with 1.0 g of (NH4)2SO4 per liter, 0.015 g of KH2PO4 per liter, and 5.0 ml of a vitamin mixture (3) per liter to ensure that growth was not limited by these nutrients and vitamins. Artificial seawater (ASW) containing the same supplements was used as the chemostat reservoir medium (21) when we studied pure cultures; this ensured that growth was limited by the organic carbon source (21). Filter-sterilized yeast extract (1 or 10 mg/liter) or sodium glutamate (1 or 100 mg/liter) was added to the ASW in the reservoir after autoclaving in order to obtain the desired final concentration of organic carbon. Yeast extract contains 33% carbon (13), and sodium glutamate contains 35% carbon; therefore, when we used 1 mg of yeast extract per liter and 1 mg of sodium glutamate per liter, the limiting carbon concentrations were 0.33 and 0.35 mg/liter, respectively. Oligotrophic AGL medium consisted of ASW to which we added (per liter) 1 mg of glucose, 1 mg of sodium acetate, and 1 mg of sodium lactate, equivalent to a total C concentration of 1 mg/liter. When solid AGL medium was used for enrichments cultures, Noble agar was prewashed with 95% ethanol–acetone and then repeatedly rinsed with distilled water to increase its purity before use.
The pure cultures used to inoculate the chemostat were pregrown at 3°C in batch cultures in ASW supplemented with either 0.1 or 0.01 g of organic substrate (e.g., yeast extract or sodium glutamate) per liter and then added to the vessel containing ASW during assembly at a dilution which resulted in a carbon concentration which was the same as that used in the experiment. Difco 2216 marine agar was used to determine pure-culture viable counts for chemostat subsamples of isolates F1, 27AB, K-4, and O-96-2, and sodium glutamate medium (41) was used for barotolerant isolate 82. When the viable O-96-12 cells were counted, AGL agar containing a 25-fold-greater organic compound content was used in addition to Difco 2216 marine agar. The pH values of all media were 7.2 to 7.3.
Chemostat operation, subsampling, and counting.
The chemostat was sterilized, assembled, and operated at pressures ranging from 105 to 450 × 105 Pa as previously described (21). At time intervals subsamples were removed from the pressurized chemostat by using a chilled sterile subsampler apparatus. Epifluorescence direct counts (14) were obtained in the natural-population studies. For the pure-culture studies both viable and direct counts were obtained. Standard deviations were determined for the pure-culture direct counts. The growth rates (μ) of pressurized natural populations, obtained by using NSW that was not supplemented with organic carbon, were calculated from the difference between the dilution rate (D) and the washout rate (A), as described by Jannasch (17):
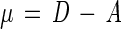 |
1 |
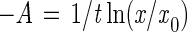 |
2 |
where x0 is the number of cells per milliliter at zero time, x is the number of cells per milliliter at the sampling time, and t is the time (in hours).
In pure-culture studies, at least 5 retention times and generally 8 retention times were allowed after the chemostat flow rate or pressure was changed to allow a new steady-state population to become established. The subsampling instruments, as well as the seawater used for dilution and the growth media, were prechilled and kept cold during use to ensure that the psychrophilic isolates were not exposed to high temperatures that could injure them. The dilution rates used for the pure-culture studies were imposed growth rates that were equivalent to 60 or 90% of the 105-Pa maximum growth rates of the organisms. These growth rates were determined in batch culture experiments in which we used the same growth substrates (e.g., 2216 marine broth, yeast extract medium, sodium glutamate medium).
RESULTS
Natural populations.
We performed the following experiments to determine the growth responses of natural deep-sea populations at the in situ pressure when only the naturally available dissolved organic carbon was present, as well as how the organisms responded to limited nutrient supplementation.
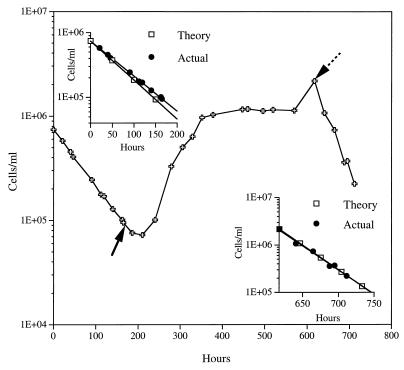
A NSW population obtained at a depth of 4,500 m was grown at a dilution rate of 0.02 h−1 (retention time, 50 h) and a pressure of 450 × 105 Pa (Fig. 1). Under these conditions, the population grew at a rate which was much less than the imposed growth rate but somewhat greater than the theoretical washout rate. This growth rate was determined (by using exponential curve fitting and equation 1) from the difference between the theoretical washout rate (growth rate, 0) and the actual washout rate (growth rate, >0) of cells for the first 160 h (Fig. 1, upper inset), which resulted in a generation time of 629 h (growth rate, 0.00159 h−1). Starting at 160 h, the reservoir seawater was supplemented with 1.0 mg of yeast extract per liter (0.33 mg of C per liter), and the pressurized chemostat was continued at a dilution rate of 0.02 h−1. A positive growth response occurred over the next 6 retention times, and a steady population of cells containing just more than 106 cells/ml became established. It was from a subsample taken at this point that isolate OC-96-2 was isolated. When the population was allowed to continue to grow as a pressurized batch culture from 560 to 617 h, about another doubling of cells occurred; during this time essentially all of the yeast extract carbon was consumed. At this point (Fig. 1, dotted arrow), a new reservoir containing NSW without yeast extract was connected, and a higher dilution rate (0.035 h−1) (retention time, 29 h) was imposed. At this higher dilution rate there was no measurable difference between the actual washout rate and the theoretical washout rate (Fig. 1, lower inset), which indicated that the growth rate (or cell division) of the existing population on any naturally occurring carbon had been equaled or exceeded by the imposed dilution rate.
FIG. 1.
Direct cell counts for a deep-sea NSW sample grown at 450 × 105 Pa and 3°C at a dilution rate of 0.02 h−1 (retention time, 50 h) before and after the introduction at 160 h (solid arrow) of 1.0 mg of yeast extract per liter to the seawater reservoir. Growth under these conditions was continued until 560 h, and then the flow was stopped and the pressurized population was held as a batch culture until 617 h (dotted arrow). This was followed by a return to a NSW flow with no yeast extract supplement at a dilution rate of 0.035 h−1 (retention time, 29 h). (Insets) Exponential curve fits for cell numbers at actual and theoretical washout rates in NSW that was not supplemented.
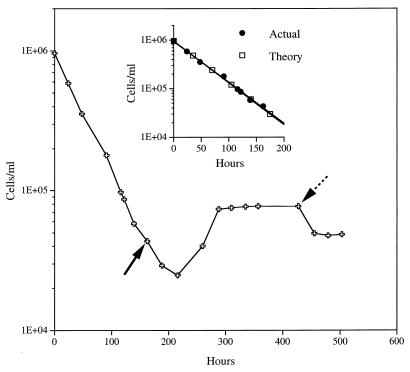
This experiment was repeated at a pressure of 450 × 105 Pa with a new sample at a dilution rate (0.028 h−1) (retention time, 35 h) that was between the dilution rates used in the experiment described above. The actual and theoretical washout rates were again equivalent (dilution rate = washout rate) for the first 160 h, indicating that there was no measurable growth (Fig. 2, inset) of the population on the naturally occurring substrate in the NSW. The size of the population declined to 4.0 × 104 cells/ml, and then the contents of the seawater reservoir were supplemented with 10 mg of glucose per liter. Growth occurred after this carbon source was added (Fig. 2). The glucose concentration in the chemostat vessel increased asymptotically relative to the input concentration (e.g., 6.3, 8.6, and 9.5 mg/liter after 1, 2, and 3 retention times, respectively). The responding population reached a steady state soon after 4 retention times following glucose addition and remained at this level from 288 to 427 h; then the chemostat was decompressed to a pressure of 105 Pa (Fig. 2, dotted arrow), and incubation was continued at the imposed dilution rate. There was a noticeable decrease in the steady-state population of cells at 105 Pa compared with the population that had established at 450 × 105 Pa, indicating that there was some barophilic enrichment of the population exhibiting better growth at the elevated pressure, which was lost when the chemostat was decompressed.
FIG. 2.
Direct cell counts for a deep-sea NSW sample grown at 450 × 105 Pa and 3°C at a dilution rate of 0.028 h−1 (retention time, 35 h) before and after the introduction at 160 h (solid arrow) of 10 mg of glucose per liter to the seawater reservoir. Growth under these conditions was continued until 427 h (dotted arrow), and then the chemostat was decompressed to 105 Pa with the flow continuing at the same dilution rate. (Inset) Exponential curve fit for cell numbers at the actual and theoretical washout rates in NSW that was not supplemented.
Pure cultures.
We performed a series of experiments in which we utilized barophilic and barotolerant deep-sea isolates whose previously determined growth characteristics identified them as copiotrophs. The goal of these experiments was to determine if the organisms maintained their phenotypic growth responses to pressure over a range of growth pressures in the presence of the growth-limiting carbon concentrations characteristic of their deep-sea habitat.
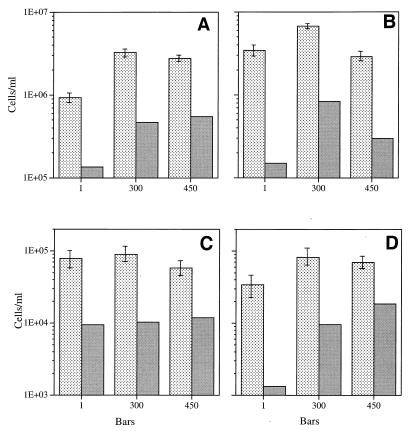
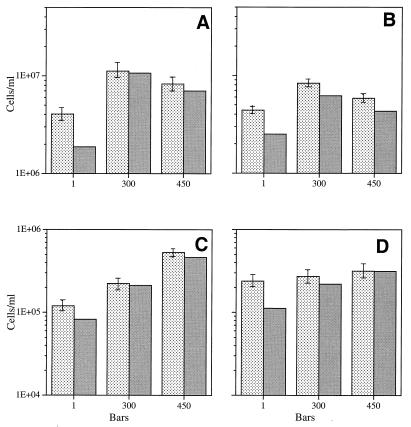
The most extensive pressure comparison at different growth rates, temperatures, and limiting carbon concentrations was performed with barophilic strain F1. The data obtained at 3°C (Fig. 3) show that at different growth rates and substrate concentrations this isolate maintained its overall barophilic character; the steady-state populations at elevated pressures were larger than the populations at 105 Pa. This was determined by both direct counting and viable counting. Similar results were obtained at 8°C (Fig. 4), which is the optimum temperature for F1. With one exception (when optimum growth occurred at 450 × 105 Pa [Fig. 4A]), optimum growth occurred at 300 × 105 Pa, which has been determined previously to be the optimum growth pressure for this organism under high-substrate-concentration batch culture conditions. When the number of viable cells was expressed as a percentage of the direct count, the value was usually higher for elevated-pressure samples than for 105-Pa samples. The percentage of viable cells was relatively low overall compared to the direct cell count (i.e., about 25% of the maximum value).
FIG. 3.
Steady-state direct counts (mean ± standard deviation) and viable cell counts for barophilic isolate F1 grown at 3°C at different pressures in the presence of different yeast extract concentrations at different growth rates. (A) Culture grown in the presence of 10 mg of yeast extract per liter at a growth rate of 0.044 h−1 (60% of the maximum growth rate). (B) Culture grown in the presence of 10 mg of yeast extract per liter at a growth rate of 0.066 h−1 (90% of the maximum growth rate). (C) Culture grown in the presence of 1 mg of yeast extract per liter at a growth rate of 0.044 h−1 (60% of the maximum growth rate). (D) Culture grown in the presence of 1 mg of yeast extract per liter at a growth rate of 0.066 h−1 (90% of the maximum growth rate). Stippled bars, direct counts; shaded bars, viable counts. One bar is equal to 105 Pa.
FIG. 4.
Steady state-direct counts (mean ± standard deviation) and viable cell counts for barophilic isolate F1 grown at 8°C at different pressures in the presence of different yeast extract concentrations at different growth rates. (A) Culture grown in the presence of 10 mg of yeast extract per liter at a growth rate of 0.044 h−1 (60% of the maximum growth rate). (B) Culture grown in the presence of 10 mg of yeast extract per liter at a growth rate of 0.066 h−1 (90% of the maximum growth rate). (C) Culture grown in the presence of 1 mg of yeast extract per liter at a growth rate of 0.044 h−1 (60% of the maximum growth rate). (D) Culture grown in the presence of 1 mg of yeast extract per liter at a growth rate of 0.066 h−1 (90% of the maximum growth rate). Stippled bars, direct counts; shaded bars, viable counts. One bar is equal to 105 Pa.
Table 1 shows the percentage of the direct count cells that were viable for each of the organisms and culture conditions tested. With the exception of isolate K-4, the percentages ranged from around 10% to almost 100%. We cannot offer an explanation for the variability in the percentages of viability obtained for strains other than (i) the possibility that pressure-grown cells were susceptible to decompression, (ii) the possibility that the organisms were grown nonoptimally in the case of the cultures grown at 105 Pa, and (iii) the fact that the carbon concentration in the plating medium was much higher than the carbon concentration in the chemostat growth medium.
TABLE 1.
Numbers of viable cells, expressed as percentages of the total direct counts obtained for barophilic and barotolerant psychrophiles at steady states in the chemostat at different growth pressures
| Organism | Figure | % of total direct counts ata:
|
|||||
|---|---|---|---|---|---|---|---|
| 105 Pa | 150 × 105 Pa | 200 × 105 Pa | 300 × 105 Pa | 400 × 105 Pa | 450 × 105 Pa | ||
| F1 | 3A | 14 | 10 | 20 | |||
| F1 | 3B | 5 | 12 | 10 | |||
| F1 | 3C | 12 | 12 | 20 | |||
| F1 | 3D | 4 | 12 | 27 | |||
| F1 | 4A | 4 | 20 | 15 | |||
| F1 | 4B | 14 | 14 | 20 | |||
| F1 | 4C | 7 | 20 | 20 | |||
| F1 | 4D | 8 | 14 | 25 | |||
| 27AB | 5A | 46 | 96 | 85 | |||
| 27AB | 5B | 57 | 75 | 74 | |||
| 27AB | 5C | 68 | 95 | 87 | |||
| 27AB | 5D | 47 | 81 | 99 | |||
| O-96-2 | 6A | 32 | 66 | 12 | |||
| O-96-2 | 6B | 77 | 78 | 49 | |||
| O-96-12 | 7 | 12, 14b | 8, 6b | 18, 14b | |||
| K-4 | 8A | 10 | <1 | <1 | |||
| K-4 | 8B | <1 | <1 | <1 | |||
| 82 | 9 | 99 | 96 | 99 | |||
Values are rounded off to the nearest whole number.
The first value is the value obtained in 2216 marine medium, and the second value is the value obtained in 25× AGL medium.
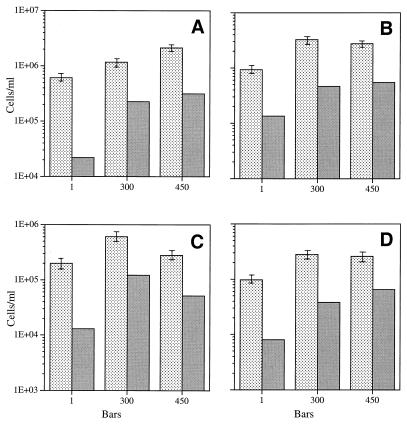
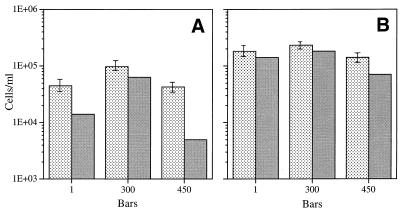
Barophilic isolate 27AB, which previously has been reported to grow optimally at 300 × 105 Pa on 2216 marine broth medium, retained this pressure optimum when it was grown in the chemostat at 60 and 90% of its maximum rate growth in the presence of 10 mg of yeast extract per liter (Fig. 5A and B). When yeast extract was added at a lower limiting concentration, 1.0 mg/liter, the maximum steady-state population densities occurred at an even higher pressure, 450 × 105 Pa (Fig. 5C and D). As was the case with F1, the steady-state direct count for isolate 27AB decreased somewhat more than 10-fold when the organism was grown in the presence of a 10-fold-lower limiting substrate concentration (i.e., 1 mg/liter instead of 10 mg/liter). Again, when the number of viable cells was expressed as a percentage of the direct count, the value was always higher for elevated-pressure samples. When the number of viable cells was expressed as a percentage of the direct count, the value was much higher for isolate 27AB than for isolate F1 overall, approaching 100% in some cases (Table 1).
FIG. 5.
Steady-state direct counts (mean ± standard deviation) and viable cell counts for barophilic isolate 27AB grown at 3°C at different pressures in the presence of different yeast extract concentrations at different growth rates. (A) Culture grown in the presence of 10 mg of yeast extract per liter at a growth rate of 0.06 h−1 (60% of the maximum growth rate). (B) Culture grown in the presence of 10 mg of yeast extract per liter at a growth rate of 0.09 h−1 (90% of the maximum growth rate). (C) Culture grown in the presence of 1 mg of yeast extract per liter at a growth rate of 0.06 h−1 (60% of the maximum growth rate). (D) Culture grown in the presence of 1 mg of yeast extract per liter at a growth rate of 0.09 h−1 (90% of the maximum growth rate). Stippled bars, direct counts; shaded bars, viable counts. One bar is equal to 105 Pa.
The next two isolates which we tested originated from seawater samples collected at a depth of 4,400 m. Isolate O-96-2, which was isolated from a pressurized chemostat natural population after enrichment with 1 mg of yeast extract per liter at 450 × 105 Pa (Fig. 1, at 560 h), was expected to be highly barotolerant or perhaps even barophilic. As shown in Fig. 6, O-96-2 exhibited barophilic properties at both 60 and 90% of its maximum growth rate in the presence of a limiting concentration of yeast extract (10 mg/liter). The optimum pressure was 300 × 105 Pa, and only a slight reduction in growth was observed at 450 × 105 Pa. The largest steady-state cell populations established at a dilution rate equal to 90% (growth rate, 0.09 h−1) of the maximum growth rate compared to 60% values; this indicated that the maximum growth efficiency occurred at the higher growth rate. This organism exhibited a relatively high level of viability, and the maximum percentage of direct counts occurred at 300 × 105 Pa at both growth rates (Table 1).
FIG. 6.
Steady-state direct counts (mean ± standard deviation) and viable cell counts for barophilic isolate O-96-2 grown at 3°C in the presence of 10 mg of yeast extract per liter at different pressures and different growth rates. (A) Culture grown at a growth rate of 0.06 h−1 (60% of the maximum growth rate). (B) Culture grown at a growth rate of 0.09 h−1 (90% of the maximum growth rate). Stippled bars, direct counts; shaded bars, viable counts. One bar is equal to 105 Pa.
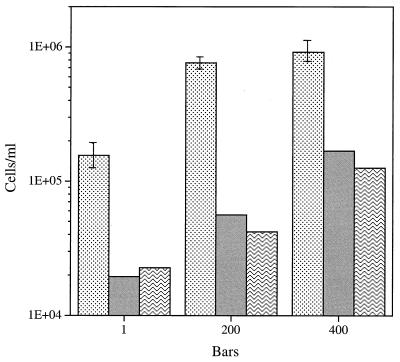
The second isolate from this seawater source, isolate O-96-12, was enriched and isolated in a conventional fashion in the presence of a very low concentration of defined carbon substrates (i.e., oligotrophic conditions), as described above. Based on a doubling time of 20 h, this isolate was grown in the chemostat at 90% of the maximum growth rate. O-96-12 exhibited barophilic growth in the presence of 1 mg of C per liter as larger steady-state populations became established at both 200 × 105 and 400 × 105 Pa than at 105 Pa (Fig. 7). When the viable counts for samples grown at 105 Pa and for decompressed subsamples were expressed as percentages of the total direct counts, the values were low but similar (6 to 18%) (Table 1), whether the organisms were grown on 25× AGL medium or on 2216 marine agar.
FIG. 7.
Steady-state direct counts (mean ± standard deviation) and viable cell counts for barophilic isolate O-96-12 grown at 3°C in the presence of 1 mg of C per liter (AGL medium) at a growth rate of 0.031 h−1 (90% of the maximum growth rate) at different pressures. Stippled bars, direct counts; shaded bars, viable counts on 2216 marine agar; wave pattern bars, viable counts on 25× AGL medium. One bar is equal to 105 Pa.
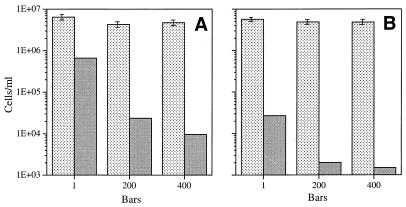
Having determined that all of the previously tested strains responded in a barophilic fashion to limiting concentrations of substrate, we decided to test two organisms which were known from previous batch culture studies to be highly or slightly barotolerant when substrate was not limiting. Highly barotolerant strain K-4 was grown in the chemostat in the presence of 1.0 mg of glutamate per liter. In batch culture studies, this isolate has been shown to have growth rates that are quite similar at pressures ranging from 105 to 300 × 105 Pa; at 400 × 105 Pa the growth rate is about 20% lower (19). As shown in Fig. 8, the steady-state populations of K-4 at 105 Pa were only slightly larger than the steady-state populations at 200 × 105 and 400 × 105 Pa. This was the case at growth rates that were 60 and 90% of the maximum growth rate; thus, K-4 kept its highly barotolerant growth characteristics under carbon-limiting conditions. The cell densities established by this organism in the presence of a low level of available carbon indicate that anabolism was extremely efficient. The plate count viability of K-4 was generally very low compared to the total count, particularly when samples were removed from growth under pressure, which resulted in values less than 1% of the direct count (Table 1).
FIG. 8.
Steady-state direct counts (mean ± standard deviation) and viable cell counts for barotolerant isolate K-4 grown at 3°C in the presence of 1 mg of sodium glutamate per liter at different pressures and growth rates. (A) Culture grown at a growth rate of 0.017 h−1 (60% of the maximum growth rate). (B) Culture grown at a growth rate of 0.028 h−1 (90% of the maximum growth rate). Stippled bars, direct counts; shaded bars, viable counts. One bar is equal to 105 Pa.
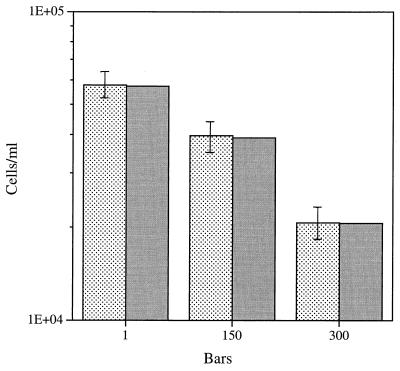
The final pure-culture isolate tested was psychrophilic isolate 82, which previously was shown to be only somewhat barotolerant in batch culture experiments (41). When isolate 82 was grown in the chemostat at either 60 or 90% of the maximum growth rate in the presence of a limiting concentration of sodium glutamate (10 mg/liter), the starter population washed out of the chemostat at elevated pressures, perhaps due to a minimum required reservoir substrate concentration (16). Therefore, the sodium glutamate concentration was increased to 100 mg/liter, and the growth rate used was 60% of the maximum growth rate. As shown in Fig. 9, isolate 82 grew best at a pressure of 105 Pa, and the size of the steady-state population decreased as the pressure was increased to 150 × 105 and 300 × 105 Pa. In contrast to the generally low viability of the barophilic or highly barotolerant isolates described above (except isolate 27AB), the number of viable isolate 82 cells was close to 100% of the direct count in all cases (Table 1).
FIG. 9.
Steady-state direct counts (mean ± standard deviation) and viable cell counts for barotolerant isolate 82 grown at 3°C in the presence of 100 mg of sodium glutamate per liter at a growth rate of .037 h−1 (60% of the maximum growth rate) at different pressures. Stippled bars, direct counts; shaded bars, viable counts. One bar is equal to 105 Pa.
DISCUSSION
Since the deep sea comprises such a large part of the earth’s biosphere, it is important to understand how the microbes in the deep sea function under the in situ environmental conditions. Since in the deep sea microorganisms are exposed to growth-limiting concentrations of essential nutrients, particularly carbon (12, 29), chemostats are well suited for metabolic studies of microorganisms grown under low-nutrient-concentration conditions (40). Using this approach, we found that the growth rate of deep-sea populations in the presence of natural concentrations of available carbon could not be measured or was extremely low, but a low concentration of supplemental carbon (0.33 mg/liter) resulted in positive growth responses in the natural population; there was an increase in the number of cells, and eventually a steady population of cells became established. Our other very important finding was that the growth responses of barophilic and barotolerant deep-sea isolates, which were isolated previously and were characterized under copiotrophic high-nutrient-concentration conditions, were still maintained under the low-nutrient-concentration limiting conditions characteristic of the natural oligotrophic deep-sea environment.
An advantage of using continuous-culture approaches with a chemostat is that relatively large, constant populations of cells growing in the presence of low concentrations of a limiting nutrient can be maintained; thus, the continuously changing conditions characteristic of a batch culture are eliminated (40). It must be emphasized, however, that in a natural system, such as the deep sea, the supply of nutrients and cell removal are not closely linked, as they are in a chemostat (18), and therefore, the deep sea does not actually exhibit steady-state conditions. Chemostats, however, have been used to obtain steady states of mixed populations obtained from surface seawater (16, 18) in which natural enrichment occurs for the organism growing the fastest under the conditions used. As long as the dilution rate is lower than the maximum growth rate, the successful species outcompetes all of the other species, and a pure-culture steady state is approached. As shown in Fig. 1 and 2, it was evident that, based on the small amount of naturally occurring carbon in seawater, the microbial populations present were not able to grow at the in situ pressure at a rate equal to or even approaching the imposed dilution rates. With respect to the available carbon, there may be another limitation, as much of this carbon has been shown to be relatively recalcitrant to microbial oxidation (2). However, in the case of the lowest imposed dilution rate (Fig. 1) (0.2 h−1) (retention time, 50 h), a growth rate of 0.00159 h−1 (generation time, 629 h) was calculated from the difference between the theoretical and actual washout rates for cells in the population.
In both experiments (Fig. 1 and 2) it was possible to enrich for a steady-state population of cells by using very low concentrations of supplemental organic carbon (1 mg of yeast extract per liter [0.33 mg of C per liter; 0.027 mM C] or 10 mg of glucose per liter [4 mg of C per liter; 0.33 mM C]) at the same imposed dilution rates as those used with unamended NSW. In both cases the populations grew, and a steady cell number (growth rate = dilution rate) was obtained at the in situ pressure and temperature; however, in neither case should the population be considered a steady-state population of a single pure culture.
Members of potentially dormant (39) or extremely slowly growing deep-sea populations may be very barotolerant or for the most part barophilic (44, 48). As our experiments were conducted at the in situ pressure (450 × 105 Pa) measured where the natural populations were collected, we could have expected enrichment for pressure-tolerant or barophilic cells. This was in fact observed with barophilic isolate O-96-2, which was enriched in the chemostat in the presence of 1 mg of yeast extract per liter at a dilution rate of 0.02 h−1. These experiments showed that resident deep-sea populations of microbes that exist naturally under severely nutrient-limited conditions are able to respond to small increases in the available carbon concentration and grow competitively and, depending on their growth characteristics, can become the predominant component of a population. We could speculate that deep-sea barophilic heterotrophs are quite versatile and capable of responding to very low levels of available carbon, as shown in this study. They may also grow well at the high carbon concentrations which traditionally have been used for enrichment and isolation of pure cultures of deep-sea microbes, including the copiotrophic barophilic isolates used in this study (e.g., isolates F1 and 27AB).
New barophilic and obligately barophilic bacteria have been isolated and described recently (25, 33, 34, 36), and the phylogenetic diversity of these new bacteria is quite broad (7). While the presence of psychrophilic and barophilic bacteria is well established, it should be pointed out that many isolates are classified as barophilic on the basis of growth experiments performed at 10 to 15°C (24, 26, 33, 34). When many of the same isolates are grown at 4°C, a temperature characteristic of their natural habitat, or even at temperatures up to 10°C, they do not respond as barophiles but exhibit only barotolerant properties. This implies that many deep-sea bacteria do not exist in situ under conditions which allow them to act as barophiles, and it follows that deep-sea bacteria that actually grow as barophiles (44) under natural conditions may not be ubiquitous. With respect to the present study, a question posed by Yayanos (44), whether the pressure tolerance of these microbes varies with nutrition, is important. This question is important not only with respect to the mode of nutrition (e.g., autotrophy, heterotrophy, and oligotrophy) but also with respect to the concentration of the growth-limiting substrate.
While the copiotrophic barophiles that have been described do appear to be adapted for life in their extreme environment (25, 32, 43), it remains to be determined whether this phenotypic pressure adaptation is retained under carbon-limiting conditions. These conditions may dramatically shift the metabolism/incorporation ratio, as previously shown for nonbarophilic psychrophiles, and may make them less efficient under oligotrophic conditions (41). The numerical predominance of oligotrophic marine bacteria over more copiotrophic heterotrophs has been shown to occur when carbohydrates are supplied at concentrations less than 0.5 mg/liter (1). However, invariably, isolated obligately oligotrophic bacteria have been found to adapt and become facultative oligotrophs capable of growth in the presence of a higher concentration of substrate (37). Conversely, decreasing the substrate concentration (to the limits that can be tested in batch cultures) has been shown to induce a more efficient barophilic response in certain copiotrophic deep-sea psychrophiles (8, 20).
The deep sea contains a very limited supply of utilizable organic carbon, and the functioning of barophilic microbes is linked to the saturation constant (Ks) and to the efficiency of substrate uptake, as well as to the ability to grow at high pressures and at low temperatures. In the present study we obtained evidence showing that there are not two distinct classes of barophilic microbes with respect to the carbon concentration requirement (i.e., microbes that are oligotrophic and microbes that are copiotrophic taking advantage of different growth niches in the deep sea and not being able to adapt to growth in characteristically different nutrient niches). As previously shown, barophilic bacteria can exist as actively metabolizing cells in association with gut tracts of abyssal animals (9) or with nutrient-rich particulates (10). However, in most of the deep-sea biosphere the free-living cells in the water and in the surface sediments exist under severe, carbon-limiting conditions. Our data show that the moderately barophilic, highly barotolerant, and weakly barotolerant organisms which were isolated and characterized in batch cultures under high-nutrient-concentration (copiotrophic) conditions can adapt to and grow in the presence of a wide range of substrate concentrations, including oligotrophic levels. This conclusion is supported by the fact that the Ks values for a number of copiotrophic marine bacteria are the same order of magnitude as the Ks value for the upper limit for oligotrophic growth (27), which makes the copiotrophs competitive with the oligotrophs under nutrient conditions characteristic of the deep sea. In the present study, each of the isolates maintained its pressure optimum (barophilic or barotolerant) under nutrient-limiting conditions in the chemostat; thus, these organisms are quite versatile competitors in their low-temperature, high-pressure, nutrient-limited, deep-sea milieu. This conclusion may be extended in reverse based on the data obtained for oligotrophic isolate O-96-12, which exhibited a barophilic growth pattern under carbon-limiting conditions but also grew well in batch cultures in the presence of high concentrations of carbon, such as the concentration in 2216 marine broth. Such versatility is critical for deep-sea bacteria, which must maintain metabolic activity as they encounter changing substrate concentrations.
ACKNOWLEDGMENTS
This work was supported by grant OCE-9415371 from the National Science Foundation.
We acknowledge the expert engineering contribution of Kenneth Doherty, who designed the high-pressure chemostat.
Footnotes
Contribution no. 10019 of the Woods Hole Oceanographic Institution. This paper is dedicated to Holger W. Jannasch, who was our longtime mentor, coworker, and friend.
REFERENCES
- 1.Akagi Y, Taga N, Simidu U. Isolation and distribution of oligotrophic marine bacteria. Can J Microbiol. 1977;23:981–987. doi: 10.1139/m77-146. [DOI] [PubMed] [Google Scholar]
- 2.Barber R T. Dissolved organic carbon from deep waters resists microbial oxidation. Nature. 1968;220:274–275. doi: 10.1038/220274a0. [DOI] [PubMed] [Google Scholar]
- 3.Bazylinski D A, Wirsen C O, Jannasch H W. Microbial utilization of naturally occurring hydrocarbons at the Guaymas Basin hydrothermal vent site. Appl Environ Microbiol. 1989;55:2832–2836. doi: 10.1128/aem.55.11.2832-2836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedford R H. Marine bacteria of the northern Pacific Ocean: the temperature range of growth. Contrib Can Biol Fish. 1933;7:431–438. [Google Scholar]
- 5.Blochl E, Rachel R, Burggraf S, Hafenbradl D, Jannasch H W, Stetter K O. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113°C. Extremophiles. 1997;1:14–21. doi: 10.1007/s007920050010. [DOI] [PubMed] [Google Scholar]
- 6.Button D K. Biochemical basis for whole-cell uptake kinetics: specific affinity, oligotrophic capacity, and the meaning of the Michaelis constant. Appl Environ Microbiol. 1991;57:881–891. doi: 10.1128/aem.57.7.2033-2038.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delong E F, Franks D G, Yayanos A A. Evolutionary relationships of cultivated psychrophilic and barophilic deep-sea bacteria. Appl Environ Microbiol. 1997;63:2105–2108. doi: 10.1128/aem.63.5.2105-2108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deming J W. Ecological strategies of barophilic bacteria in the deep ocean. Microbiol Sci. 1986;3:205–211. [PubMed] [Google Scholar]
- 9.Deming J W, Colwell R R. Barophilic bacteria associated with digestive tracts of abyssal holothurians. Appl Environ Microbiol. 1982;44:1222–1230. doi: 10.1128/aem.44.5.1222-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deming J W, Colwell R R. Observations of barophilic microbial activity in samples of sediment and intercepted particulates from the Demerara abyssal plain. Appl Environ Microbiol. 1985;50:1002–1006. doi: 10.1128/aem.50.4.1002-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deming J W, Somers L K, Straube W L, Swartz D G, MacDonell M T. Isolation of an obligately barophilic bacterium and description of a new genus, Colwellia gen. nov. Syst Appl Microbiol. 1988;10:152–160. [Google Scholar]
- 12.Druffel E R M, Williams P M, Susuki Y. Concentrations and radiocarbon signatures of dissolved organic matter in the Pacific Ocean. Geophys Res Lett. 1989;16:991–994. [Google Scholar]
- 13.Hoaki T, Wirsen C O, Hanzawa S, Maruyama T, Jannasch H W. Amino acid requirements of two hyperthermophilic archaeal isolates from deep sea vents: Desulfurococcus strain SY and Pyrococcus strain GB-D. Appl Environ Microbiol. 1993;59:610–613. doi: 10.1128/aem.59.2.610-613.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jannasch H W. Starter populations as determined under steady state conditions. Biotechnol Bioeng. 1965;7:279–283. [Google Scholar]
- 16.Jannasch H W. Growth of marine bacteria at limiting concentrations of organic carbon in seawater. Limnol Oceanogr. 1967;12:264–271. [Google Scholar]
- 17.Jannasch H W. Estimations of bacterial growth rates in natural waters. J Bacteriol. 1969;99:156–160. doi: 10.1128/jb.99.1.156-160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jannasch H W. Steady state and the chemostat in ecology. Limnol Oceanogr. 1974;19:716–720. [Google Scholar]
- 19.Jannasch H W, Wirsen C O. Variability of pressure adaptation in deep sea bacteria. Arch Microbiol. 1984;139:281–288. [Google Scholar]
- 20.Jannasch H W, Wirsen C O. Growth response to hydrostatic pressure in marine psychrophilic and oligocarbophilic (oligotrophic) bacteria. J Mar Biotechnol. 1995;3:73–75. [Google Scholar]
- 21.Jannasch H W, Wirsen C O, Doherty K W. A pressurized chemostat for the study of marine barophilic and oligotrophic bacteria. Appl Environ Microbiol. 1996;62:1593–1596. doi: 10.1128/aem.62.5.1593-1596.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jannasch H W, Wirsen C O, Taylor C D. Deep sea bacteria: isolation in the absence of decompression. Science. 1982;216:1315–1317. doi: 10.1126/science.216.4552.1315. [DOI] [PubMed] [Google Scholar]
- 23.Kato C, Bartlett D H. The molecular biology of barophilic bacteria. Extremophiles. 1997;1:111–116. doi: 10.1007/s007920050023. [DOI] [PubMed] [Google Scholar]
- 24.Kato C, Inoue A, Horikoshi K. Isolating and characterizing deep-sea marine microorganisms. Trends Biotechnol. 1996;14:6–12. doi: 10.1016/0167-7799(96)80907-3. [DOI] [PubMed] [Google Scholar]
- 25.Kato C, Li L, Nakamura Y, Tamaoka J, Horikoshi K. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl Environ Microbiol. 1998;64:1510–1513. doi: 10.1128/aem.64.4.1510-1513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato C, Sato T, Horikoshi K. Isolation and properties of barophilic and barotolerant bacteria from deep-sea mud samples. Biodivers Conserv. 1995;4:1–9. [Google Scholar]
- 27.Kjelleberg S, Hermansson M. Short term responses to energy fluctuation by marine heterotrophic bacteria. In: Sleigh M A, editor. Microbes in the sea. New York, N.Y: John Wiley & Sons; 1987. pp. 203–219. [Google Scholar]
- 28.Li L N, Kato C, Nogi Y, Horikoshi K. Distribution of the pressure-regulated operons in deep-sea bacteria. FEMS Microbiol Lett. 1998;159:159–166. doi: 10.1111/j.1574-6968.1998.tb12855.x. [DOI] [PubMed] [Google Scholar]
- 29.Menzel D W, Ryther J H. Distribution and cycling of organic carbon in the oceans. Inst Mar Sci Univ Alaska Publ. 1970;1:31–54. [Google Scholar]
- 30.Nakasone K, Ikegame A, Kato C, Usami R, Horikoski K. Mechanisms of gene expression controlled by pressure in deep-sea microorganisms. Extremophiles. 1998;2:149–154. doi: 10.1007/s007920050054. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama A, Yano Y, Yoshida K. New method for isolating barophiles from intestinal contents of deep-sea fishes retrieved from the abyssal zone. Appl Environ Microbiol. 1994;60:4210–4212. doi: 10.1128/aem.60.11.4210-4212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogi Y, Kato C. Taxonomic studies of extremely barophilic bacteria isolated from the Mariana Trench and description of Moritella yayanosii sp. nov., a new barophilic bacterial isolate. Extremophiles. 1999;3:71–77. doi: 10.1007/s007920050101. [DOI] [PubMed] [Google Scholar]
- 33.Nogi Y, Kato C, Horikoshi K. Moritella japonica sp. nov., a novel barophilic bacterium isolated from a Japan Trench sediment. J Gen Appl Microbiol. 1998;44:289–295. doi: 10.2323/jgam.44.289. [DOI] [PubMed] [Google Scholar]
- 34.Nogi Y, Masui N, Kato C. Photobacterium profundum sp. nov., a new, moderately barophilic bacterial species isolated from a deep-sea sediment. Extremophiles. 1998;2:1–7. doi: 10.1007/s007920050036. [DOI] [PubMed] [Google Scholar]
- 35.Rowe G T, Sibuet M, Deming J, Tietjen J, Khripounoff A. Organic carbon turnover in deep-sea benthos. Prog Oceanogr. 1990;24:141–160. [Google Scholar]
- 36.Sakiyama T, Ohwada K. Isolation and growth characteristics of deep-sea barophilic bacteria from the Japan Trench. Fish Sci. 1997;63:228–232. [Google Scholar]
- 37.Schut F, de Vries E J, Gottschal J C, Robertson B R, Harder W, Prins R A, Button D K. Isolation of typical marine bacteria by dilution culture: growth maintenance and characteristics of isolates under laboratory conditions. Appl Environ Microbiol. 1993;59:2150–2160. doi: 10.1128/aem.59.7.2150-2160.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schut F, Jansen M, Gomes T, Gottschal J C, Harder W, Prins R A. Substrate uptake and utilization by a marine ultramicrobacterium. Microbiology. 1995;141:351–361. doi: 10.1099/13500872-141-2-351. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson L H. A case for bacterial dormancy in aquatic systems. Microb Ecol. 1978;4:127–133. doi: 10.1007/BF02014283. [DOI] [PubMed] [Google Scholar]
- 40.Veldkamp H, Kuenen J G. The chemostat as a model system for ecological studies. Bull Ecol Res Comm. 1973;17:347–355. [Google Scholar]
- 41.Wirsen C O, Jannasch H W. Activity of marine psychrophilic bacteria at elevated hydrostatic pressures and low temperatures. Mar Biol. 1975;31:201–208. [Google Scholar]
- 42.Wirsen C O, Jannasch H W. In situ studies on deep sea amphipods and their intestinal microflora. Mar Biol. 1983;78:69–73. [Google Scholar]
- 43.Wirsen C O, Jannasch H W, Wakeham S G, Canuel E A. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr Microbiol. 1987;14:319–322. [Google Scholar]
- 44.Yayanos A A. Evolutional and ecological implications of the properties of deep-sea barophilic bacteria. Proc Natl Acad Sci USA. 1986;83:9542–9546. doi: 10.1073/pnas.83.24.9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yayanos A A. Microbiology to 10,500 meters in the deep sea. Annu Rev Microbiol. 1995;49:777–805. doi: 10.1146/annurev.mi.49.100195.004021. [DOI] [PubMed] [Google Scholar]
- 46.Yayanos A A, Dietz A S, Van Boxtel R. Isolation of a deep sea barophilic bacterium and some of its growth characteristics. Science. 1979;205:808–810. doi: 10.1126/science.205.4408.808. [DOI] [PubMed] [Google Scholar]
- 47.Yayanos A A, Dietz A S, Van Boxtel R. Obligately barophilic bacterium from the Mariana Trench. Proc Natl Acad Sci USA. 1981;78:5212–5215. doi: 10.1073/pnas.78.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yayanos A A, Dietz A S, Van Boxtel R. Dependence of reproduction rate on pressure as a hallmark of deep-sea bacteria. Appl Environ Microbiol. 1982;44:1356–1361. doi: 10.1128/aem.44.6.1356-1361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ZoBell C E, Morita R Y. Barophilic bacteria in some deep sea sediments. J Bacteriol. 1957;73:563–568. doi: 10.1128/jb.73.4.563-568.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ZoBell C E. Hydrostatic pressure as a factor affecting the activities of marine microbes. In: Miyake Y, Koyama T, editors. Recent researches in the fields of hydrosphere, atmosphere and nuclear geochemistry. Tokyo, Japan: Maruzen Co.; 1964. pp. 83–116. [Google Scholar]