Abstract
BACKGROUND:
The intrinsic properties of pelvic soft tissues in women who do and do not sustain birth injuries are likely divergent. However, little is known about this. Rat pelvic floor muscles undergo protective pregnancy-induced structural adaptations-sarcomerogenesis and increase in intramuscular collagen content-that protect against birth injury.
OBJECTIVE:
We aimed to test the following hypotheses: (1) the increased mechanical load of a gravid uterus drives antepartum adaptations; (2) load-induced changes are sufficient to protect pelvic muscles from birth injury.
STUDY DESIGN:
The independent effects of load uncoupled from the hormonal milieu of pregnancy were tested in 3- to 4-month-old Sprague-Dawley rats randomly divided into the following 4 groups, with N of 5 to 14 per group: (1) load−/pregnancy hormones− (controls), (2) load+/pregnancy hormones−, (3) reduced load/pregnancy hormones+, and (4) load+/pregnancy hormones+. Mechanical load of a gravid uterus was simulated by weighing uterine horns with beads similar to fetal rat size and weight. A reduced load was achieved by unilateral pregnancy after unilateral uterine horn ligation. To assess the acute and chronic phases required for sarcomerogenesis, the rats were sacrificed at 4 hours or 21 days after bead loading. The coccygeus, iliocaudalis, pubocaudalis, and nonpelvic tibialis anterior musles were harvested for myofiber and sarcomere length measurements. The intramuscular collagen content was assessed using a hydroxyproline assay. An additional 20 load+/pregnancy hormones− rats underwent vaginal distention to determine whether the load-induced changes are sufficient to protect from mechanical muscle injury in response to parturition-associated strains of various magnitude. The data, compared using 2-way repeated measures analysis of variance followed by pairwise comparisons, are presented as mean±standard error of mean.
RESULTS:
An acute increase in load resulted in significant pelvic floor muscle stretch, accompanied by an acute increase in sarcomere length compared with nonloaded control muscles (coccygeus: 2.69±0.03 vs 2.30±0.06 μm, respectively, P<.001; pubocaudalis: 2.71±0.04 vs 2.25±0.03 μm, respectively, P<.0001; and iliocaudalis: 2.80±0.06 vs 2.35±0.04 μm, respectively, P<.0001). After 21 days of sustained load, the sarcomeres returned to operational length in all pelvic muscles (P>.05). However, the myofibers remained significantly longer in the load+/pregnancy hormones− than the load−/pregnancy hormones− in coccygeus (13.33±0.94 vs 9.97±0.26 mm, respectively, P<.0001) and pubocaudalis (21.20±0.52 vs 19.52±0.34 mm, respectively, P<.04) and not different from load+/pregnancy hormones+ (12.82±0.30 and 22.53±0.32 mm, respectively, P>.1), indicating that sustained load-induced sarcomerogenesis in these muscles. The intramuscular collagen content in the load+/pregnancy hormones− group was significantly greater relative to the controls in coccygeus (6.55±0.85 vs 3.11 ±0.47 μg/mg, respectively, P<.001) and pubocaudalis (5.93±0.79 vs 3.46±0.52 μg/mg, respectively, P<.05) and not different from load+/pregnancy hormones+ (7.45±0.65 and 6.05±0.62 μg/mg, respectively, P>.5). The iliocaudalis required both mechanical and endocrine cues for sarcomerogenesis. The tibialis anterior was not affected by mechanical or endocrine alterations. Despite an equivalent extent of adaptations, load-induced changes were only partially protective against sarcomere hyperelongation.
CONCLUSION:
Load induces plasticity of the intrinsic pelvic floor muscle components, which renders protection against mechanical birth injury. The protective effect, which varies between the individual muscles and strain magnitudes, is further augmented by the presence of pregnancy hormones. Maximizing the impact of mechanical load on the pelvic floor muscles during pregnancy, such as with specialized pelvic floor muscle stretching regimens, is a potentially actionable target for augmenting pregnancy-induced adaptations to decrease birth injury in women who may otherwise have incomplete antepartum muscle adaptations.
Keywords: adaptations, birth injury, pelvic floor muscles, pregnancy, rat, sarcomerogenesis
Introduction
Pelvic floor disorders (PFDs), including pelvic organ prolapse and urinary and fecal incontinence, are highly prevalent conditions that adversely impact the quality of life of women. Dysfunction of the pelvic floor muscles (PFMs), which include 3 paired muscles: (1) coccygeus, (2) iliococcygeus and (3) pubovisceralis, with the latter two comprising levator ani. levator ani has been implicated as a key risk factor in the pathogenesis of PFDs.1,2 Vaginal childbirth is an inciting event for pelvic floor dysfunction in many women, in part because parturition results in the elongation of PFMs up to 300% of their resting muscle length.3 These dramatic strains substantially exceed the 60% elongation that has been shown to result in reproducible injury in limb skeletal muscles.3-5 Curiously, for reasons yet unknown, a large proportion of vaginally parous women do not develop pelvic floor dysfunction despite similar obstetrical variables to women who do have such dysfunction postpartum.6
PFMs are skeletal muscles composed of myofibers that, in turn, are made of basic muscle contractile units called sarcomeres. The skeletal muscles exhibit plasticity in response to alterations in physiological cues,7 including the dynamic assembly of the sarcomere units, which is known as sarcomerogenesis. In the limbs, when muscles are subjected to increased mechanical load, the sarcomeres acutely elongate in response to muscle stretching.8 If the muscle stretch is sustained, the sarcomeres are added in series to restore the operational sarcomere length, a process that takes at least 2 weeks, which results in increased fiber length and facilitates optimal in vivo muscle function.5,9,10 Another important structural component of skeletal muscles is the intramuscular connective tissue network that surrounds the contractile myofibers. The intramuscular extracellular matrix (ECM), primarily composed of collagens, provides support to the myofibers and bears most of the muscle’s passive load. Both in vitro and in vivo studies suggest that mechanical loading induces ECM remodeling in the limb muscles via growth-factormediated cell-signaling pathways, presumably to ensure the mechanical stability of the muscle fibers. However, this process is not well understood.11,12
Significant technical and ethical constraints preclude direct studies of the human PFMs, especially in pregnant women. Consequently, we utilize a validated rat model, as the rat PFM anatomy and architecture are well suited for the studies of the human PFMs.13,14 Moreover, despite being quadrupeds, rats spend much of their time in an upright posture, making them an appropriate model in which to study the effects of the gravid uterus on the pelvic floor.15,16 In response to the physiological cues associated with pregnancy, the rat vagina and supportive tissues exhibit significant plasticity, which facilitates the ability of the vagina to withstand parturition.17-19 We have previously shown that the rat PFMs also undergo adaptations during pregnancy, specifically myofiber elongation via sarcomerogenesis.20 This allows PFMs to maintain the operational sarcomere length and preserves the muscle force generation capacity necessary to support the increased load of the pregnant uterus. In addition, sarcomerogenesis appears to protect against PFM injury during parturition because of an increased ability to withstand muscle strain without pathologic sarcomere hyperelongation and the associated myofibrillar disruption.21 Pregnancy-induced alterations also take place in PFMs’ ECM. We found that in contrast to other pelvic tissues, intramuscular collagen content significantly increases in PFMs in pregnancy.22 This increase in the ECM collagen content is presumed to stabilize elongated PFM fibers and to further protect them from overstretching during parturition. However, the mechanisms leading to sarcomerogenesis and ECM remodeling during pregnancy remain unknown.
In the current study, using the rat preclinical model validated for the investigations of human PFMs,13,14 we aimed to: (1a) determine the acute effect of increased mechanical load on PFMs, (1b) decipher the relative contributions of the chronic mechanical load and pregnancy hormonal milieu on the pregnancy-induced adaptations of PFMs; and (2) assess whether load-induced alterations modulate PFMs’ response to parturition-associated strains. We hypothesized that an acute increase in the mechanical load imposed on PFMs would result in muscle and sarcomere stretch. We opined that this acute change in the sarcomere length (Ls), combined with a sustained increased load, would be sufficient to induce sarcomerogenesis and increased ECM collagen content, observed in PFMs during pregnancy. Finally, we hypothesized that load-induced sarcomerogenesis and increased ECM collagen content are sufficient to protect PFMs from intrapartum sarcomere hyperelongation, the major cause of mechanical muscle injury, in the absence of other physiological changes of pregnancy.
Materials and Methods
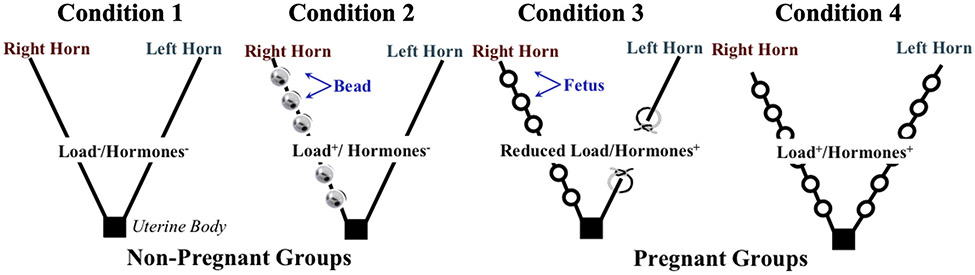
To delve into the potential mechanisms that govern protective PFM adaptations, we started by uncoupling the endocrine and mechanical effects of pregnancy on the muscle structural parameters. To segregate mechanical load from the hormonal cues, we exposed PFMs to the following 4 sets of conditions: (1) load−/pregnancy hormones− (nonpregnant control); (2) load+/pregnancy hormones− (nonpregnant loaded); (3) reduced load/pregnancy hormones+ (unilateral pregnancy); and (4) load+/pregnancy hormones+ (pregnant) (Figure 1). Group 3 was chosen as a model of reduced load/pregnancy hormones+ because it is not possible to recapitulate the full array of complex hormonal alterations that occur in pregnancy without exposing PFMs to at least some increased load, as the hormonal milieu is naturally induced by the conceptuses themselves.
FIGURE 1. A schematic diagram of the experimental approach.
To determine the independent and combined roles of mechanical load and hormonal milieu of pregnancy in driving pregnancy-induced adaptations in the rat PFMs, structural muscle parameters were compared between PFMs subjected to 4 sets of conditions: (1) load−/pregnancy hormones−; (2) load+/pregnancy hormones−; (3) reduced load/pregnancy hormones+; and (4) load+/pregnancy hormones+. For conditions 2 and 3, the side of the experimentally manipulated horn (right or left) was selected randomly for each rat.
PFM, pelvic floor muscles.
The University of California San Diego Institutional Animal Care and Use Committee (IACUC) approved all the study procedures. Three- and 4-month-old Sprague-Dawley rats (Envigo, Indianapolis, IN) were used in the following series of experiments. The rats were housed in groups of 2 to 3 per cage according to the IACUC standards and allowed ad lib access to food and water.
The load+/hormone+ group comprised timed primigravid rats. The number of fetuses was, for the most part, evenly distributed between the 2 uterine horns, with a median of 12.5 fetuses (10—15) carried by each dame. The average fetal weight at the time of animal sacrifice on gestational day 21 was 4.01±0.2 g.
Nonpregnant rat model of mechanical loading of the pelvic floor muscles
To simulate the increased load imposed on PFMs during pregnancy without the concomitant effects of hormonal alterations, we developed a novel nonpregnant rat model with weight-loaded uterine horns (Figure 1). Three-monthold nulligravid rats (N=14) were anesthetized with isofluorane and were administered a preoperative subcutaneous injection of buprenorphine sustained release at a dosage of 1.0 mg/kg. The abdominal fur was removed with a depilatory cream (Nair Hair Remover, Ewing, NJ), the skin was sterilized with 4% chlorhexidine gluconate solution (Hibiclens, Norcross, GA), and the rats were sterilely draped. A midline laparotomy was performed using standard aseptic techniques. One of the 2 uterine horns, chosen at random, was exteriorized, and 6 3-gm sterile stainless-steel beads (Bullet Weights, Alda, NE), each similar in size and weight to a late pregnant rat fetus, were attached to the selected uterine horn. The number of stainless-steel beads was chosen because 6 fetuses is the median number of conceptuses in each uterine horn during spontaneous rat pregnancy.19 To attach each bead to the uterus, a 3-0 silk suture on a 3/8 circle 26 mm cutting needle (Oasis, Mettawa, IL) was used to penetrate the full-thickness uterine horn on the antimesenteric border. The suture was then passed through the hole in the bead. The bead was secured to the horn with several knots. This was repeated with each bead so that the beads were evenly spaced along the uterine horn. The weight-loaded uterine horn was returned into the peritoneal cavity. The fascia was closed with a 3-0 polyglactin 910 suture (coated VICRYL; Ethicon, Somerville, NJ) in a continuous running fashion, and the skin was closed with the same suture material in a continuous subcuticular fashion. Animals received an immediate postoperative subcutaneous intraincisional injection of 0.25% bupivacaine at a dosage of 0.4 mL/kg. The animals were euthanized either 4 h postloading (N=4) to assess the acute effect of mechanical load on PFMs, or 21 days postloading (N=10), to simulate sustained load until late gestation. One rat died during the postoperative period, and therefore N=9 for the sustained load group. The purpose of examining the acute effect of the load was to establish that in our novel load+/pregnancy hormones− model, the weighted uterus acutely induced PFM sarcomere elongation, which is the major impetus for sarcomerogenesis in skeletal muscles.9
In our initial perturbation of the model, we hoped to capitalize on the bihorn rat uterine anatomy, with contralateral PFMs within 1 animal representing 2 sets of conditions: load−/pregnancy hormones− (side of nonloaded horn, control) and load+/pregnancy hormones− (side with horn loaded with beads, nonpregnant loaded). Interestingly, fiber length comparisons between contralateral PFMs revealed that the sustained load affected both sides, with no significant differences in fiber lengths identified between the sides for the PFM pairs examined (P>.5) (Supplemental Table). Thus, we selected rats with unilaterally weighed uterine horns to represent the load+/pregnancy hormones− condition, with a separate group of nonpregnant unperturbed rats used to represent load−/pregnancy hormones− condition. In addition, the fiber length in nonpregnant rats with a single loaded uterine horn for 21 days did not differ from that in load+/hormones+ (ie, late pregnant) group (Table 1). This is likely because beads equal in weight and size to fetal rats in the second half of gestational period were attached for the entire duration. Therefore, to avoid the risk of exceeding adaptations observed in pregnancy, we proceeded with unilateral loading in the load+/pregnancy hormones− group.
TABLE 1.
Normalized fiber length (Lfn, millimeters) of the pelvic floor muscles and tibialis anterior in the presence of load and/or pregnancy hormones presented as mean±standard error of mean
| Muscle | Load−/Hormones− (N=10) | Load+/Hormones− (N=9) | P valuea | Reduced Load/Hormones− (N=9) | P valuea | Load+/Hormones+ (N=5) | P valuea |
|---|---|---|---|---|---|---|---|
| Coccygeus | 9.97±0.26 | 13.33±0.94 | <.0001 | 12.06±0.44 | .0075 | 12.82±0.30 | .0018 |
| Pubocaudalis | 19.52±0.34 | 21.20±0.52 | .0406 | 20.80±0.59 | .1567 | 22.53±0.33 | .0009 |
| Iliocaudalis | 19.45±0.42 | 21.00±0.46 | .0646 | 20.74±0.49 | .1540 | 21.45±0.83 | .0407 |
| Tibialis anterior | 20.07±0.39 | 20.58±0.44 | .8076 | 21.44±0.51 | .1202 | 21.21 ±0.39 | .3770 |
| P valueb | ||||||
|---|---|---|---|---|---|---|
| Muscle | Load−/Hormones− vs Load+/Hormones+ |
Load−/Hormones− vs Load+/Hormones− |
Load−/Hormones− vs Reduced Load/Hormones+ |
Load+/Hormones+ vs Load+/Hormones− |
Load+/Hormones+ vs Reduced Load/Hormones+ |
Load+/Hormones− vs Reduced Load/Hormones+ |
| Coccygeus | .003 | <.0001 | .01 | .93 | .79 | .27 |
| Pubocaudalis | .002 | .07 | .24 | .38 | .16 | .94 |
| Iliocaudalis | .07 | .11 | .24 | .95 | .82 | .98 |
| Tibialis anterior | .50 | .88 | .19 | .87 | .99 | .61 |
The P values were derived from 2-way analysis of variance followed by Dunnett multiple comparisons test, with the significance levels set to 5%
P values were derived from 2-way analysis of variance followed by Tukey multiple comparisons test, with the significance levels set to 5%.
Pregnant rat model of reduced loading of the pelvic floor muscles
To create the physiological state of pregnancy with a reduced mechanical load, 3-month-old nulligravid rats (N=10) were subjected to a unilateral horn ligation on the right or the left side, with the side selected at random. The selected uterine horn was exteriorized through the laparotomy incision, as described above, and 2 silk sutures (Ethicon, Somerville, NJ) were placed approximately 1.5 cm apart and tied down. The intervening portion of the horn was excised (Figure 1), and the uterine horn was returned to the peritoneal cavity. The incisions were closed as described above. After 5 days of recovery, the rats were mated and examined daily. The day the vaginal plug was observed was designated as gestational day 1. The animals were euthanized on gestational day 21 (late pregnant) and before parturition. All 10 rats conceived successfully on mating. However, 1 delivered before the planned date of euthanasia and was excluded from the analysis, thus N=9 for this group.
The effect of sustained mechanical load on the pelvic floor muscles’ response to parturition-associated strains
To determine whether sustained exposure to an increased load in the absence of a pregnancy hormonal milieu impacts PFM response to parturition-associated strains, 3-month-old nulligravid rats (N=20) underwent mechanical loading, as described above. The animals were housed for 21 days. On day 21 after loading, the rats underwent an established vaginal balloon distension procedure.21 Two distension volumes representing physiological (3 mL, well-approximates fetal rat size) and supraphysiologic (5 mL, approximately 67% larger than fetal rat size) strains were tested (N=10/volume).21 The animals were euthanized after 2 h of vaginal distension.
Muscle architectural parameters
The rat coccygeus and the 2 components of levator ani (pubocaudalis and iliocaudalis),23 and the tibialis anterior that served as a nonpelvic control muscle were fixed in 10% formaldehyde for 3—5 days after euthanasia. The muscles were fixed in situ attached to the skeleton to preserve in vivo muscle architecture. The muscle length (Lm) was measured in situ using electronic digital calipers, after which bilateral PFMs and tibialis anterior were harvested. Three small fiber bundles (10—20 fibers) were microdissected from each muscle for fiber length (Lf) measurements. Lf was measured using electronic digital calipers, and the 3 measurements were averaged. This value was used for the calculation of normalized fiber length.20 The myofibers were then microdissected from each fiber bundle, and at least 3 high-throughput Ls measurements were performed on each fiber by laser diffraction using validated methods.20,24
Intramuscular extracellular matrix assessment
Hydroxyproline, a major component of collagen, was measured to determine the intramuscular ECM collagen content using a validated protocol.22,25 Samples were procured from the midbelly of PFMs and tibialis anterior (3 samples/each muscle), weighed, and hydrolyzed in 6 N hydrochloric acid at 110°C for 24 hours. The experimental samples were placed into the 96-well plates in duplicate along with the standards and incubated with a chloramine-T solution, followed by the addition of a p-dimethylaminobenzaldehyde solution. We used spectrophotometry at 550 nm to determine hydroxyproline concentration, normalized to the wet weight of the sample, and converted to collagen using the constant of 7.46, the number of hydroxyproline residues per collagen molecule.
Statistical analysis
Structural parameters of each individual PFM subjected to variable conditions, illustrated in Figure 1, and tibialis anterior were compared using 2-way repeated measures analysis of variance (factors: load/hormonal status × muscle). Sample size was explored for the key variables of interest (normalized fiber length [Lfn],20 sarcomere length [Ls]). We set type I error to be α=0.05, and power (1-β) = 0.80. On the basis of Cohen d effect size of 3.2, power calculation (G*Power) yielded N=4 animals per group.26 We increased the sample size in the animals subjected to surgical procedure (load+/hormones− and reduced load/hormones+) to account for potential attrition because of postoperative complications. Given a large effect size for collagen content in our previous studies,20 this sample size was sufficient for this outcome of interest. For Ls changes in response to vaginal distention, N=4 to 5 rats per group per volume was needed for coccygeus and pubocaudalis, given the large effect size, and N=10 per group per volume was needed for iliocaudalis that experiences less strain.21 Post hoc pairwise comparisons, when appropriate, were conducted with tests adjusted for multiple comparisons. Ls and Lfn were compared between muscles and between experimental groups using Tukey multiple comparison test. In addition, PFM Lfn was compared between each experimental group and controls (load−/hormones−) using Dunnet test. All data were checked for normality to satisfy the assumptions of the parametric tests. All analyses were performed with GraphPad Prism 9.1.1 (GraphPad Software, Inc, San Diego, CA).
Results
The acute effect of increased mechanical load on the pelvic floor muscles’ myofibers
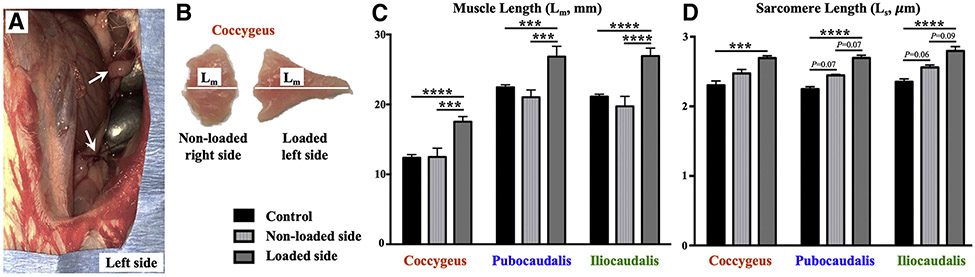
The increase in the mechanical load, induced by uterine bead loading (Figure 2, A), resulted in immediate PFM stretch, evidenced by significantly increased muscle length (Lm) of each PFM on the loaded side compared to the contralateral nonloaded side and unperturbed PFMs in nonpregnant animals (Figure 2, B and C). In the acute setting, an increase in sarcomere length (Ls) on the loaded side was similarly observed (Figure 2, D). Although we did not directly measure the load imposed on the PFMs by the beads, these results strongly suggest that increased load was imposed on PFMs by the weighted uterine horn and led to an acute whole muscle stretch and paralleling sarcomere elongation, indicated by increased Ls. In appendicular muscles, increase in Ls serves as a strong impetus for sarcomerogenesis in the face of continued exposure to mechanical load.3 Thus, we next proceeded to assess whether load-induced sarcomerogenesis takes place in PFMs in the nonpregnant model subjected to the sustained load, such as that observed in pregnancy.
FIGURE 2. Acute response of the PFMs myofibers to mechanical load.
A, Left uterine horn in nonpregnant rat, loaded with 3-g stainless-steel beads (arrows), each similar in size and weight to a late pregnant rat fetus and visible through a laparotomy incision. B, Bilateral coccygeus muscles with significantly increased muscle length (Lm) of the left muscle (loaded side) relative to the right muscle. C, Acute changes in the muscle lengths (in millimeters) of the PFMs subjected to an increased load relative to nonloaded contralateral PFMs and unperturbed controls. N=4 per group. D, Acute changes in the sarcomere lengths (in micrometers) of the PFMs subjected to increased load, relative to nonloaded contralateral PFMs and unperturbed controls. N=4 per group.
Data are presented as mean±standard error of mean. Single asterisk represents P<.05; double asterisks represent P<.01; triple asterisks represent P<.001; four asterisks represent P<.0001 derived from repeated measures of 2-way analysis of variance followed by pairwise comparisons with the Tukey test.
PFM, pelvic floor muscles.
The effect of sustained mechanical load, uncoupled from the endocrine milieu of pregnancy, on the pelvic floor muscles’ structural parameters
In skeletal muscles, fiber length (Lf) can change secondary to either (1) sarcomere elongation/contraction or (2) adaptive assembly/disassembly of the sarcomeres. Thus, we first examined Ls of PFMs and tibialis anterior. There was no difference in Ls between any of the experimental conditions (load−/pregnancy hormones−, load+/pregnancy hormones−, reduced load/pregnancy hormones+, and load+/pregnancy hormones+) for all muscles examined (Table 2). These results mean that any fiber elongation is the result of adaptive sarcomerogenesis because of sustained load rather than persistent sarcomere stretch observed in the acute phase.
TABLE 2.
Sarcomere length (in micrometers) of the pelvic floor muscles and tibialis anterior in the presence of load and/or pregnancy hormones presented as mean±standard error of mean
| Muscle | Load−/Hormones− (N=10) | Load+/Hormones− (N=9) | Reduced Load/Hormones− (N=9) | Load+/Hormones+ (N=5) |
|---|---|---|---|---|
| Coccygeus | 2.38±0.05 | 2.36±0.05 | 2.38±0.03 | 2.26±0.04 |
| Pubocaudalis | 2.36±0.03 | 2.32±0.05 | 2.39±0.04 | 2.27±0.03 |
| Iliocaudalis | 2.46±0.03 | 2.36±0.04 | 2.46±0.03 | 2.40±0.05 |
| Tibialis anterior | 2.60±0.02 | 2.60±0.02 | 2.64±0.02 | 2.54±0.01 |
| P valuea | ||||||
|---|---|---|---|---|---|---|
| Muscle | Load−/Hormones− vs Load+/Hormones+ |
Load−/Hormones− vs Load+/Hormones− |
Load−/Hormones− vs Reduced Load/Hormones+ |
Load+/Hormones+ vs Load +/Hormones− |
Load+/Hormones+ vs Reduced Load/Hormones+ |
Load+/Hormones− vs Reduced Load/Hormones+ |
| Coccygeus | .06 | .98 | .99 | .17 | .09 | .99 |
| Pubocaudalis | .21 | .72 | .95 | .82 | .07 | .42 |
| Iliocaudalis | .66 | .19 | .99 | .81 | .63 | .18 |
| Tibialis anterior | .66 | .99 | .87 | .63 | .24 | .90 |
P values were derived from two-way analysis of variance followed by Tukey multiple comparisons test, with the significance levels set to 5%.
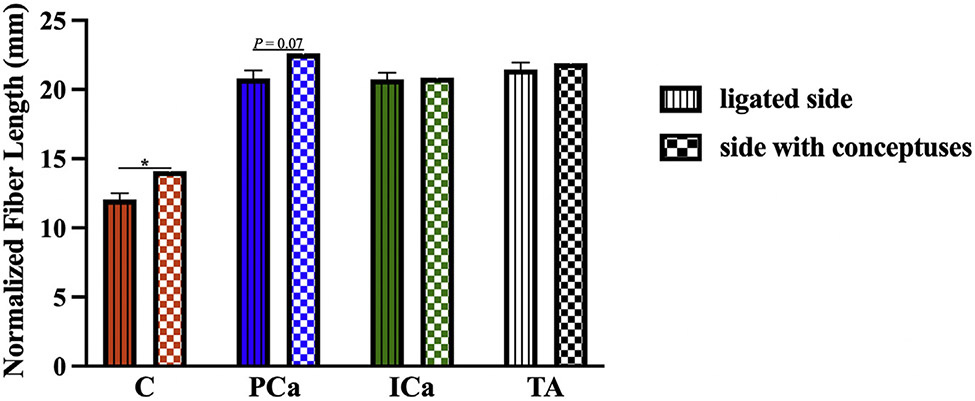
We measured the length of the muscle fibers (Lf). To control for any potential differences between specimens at the time of fixation we calculated normalized fiber length (Lfn). that takes into account Ls within each specimen, using previously established methods (Lfn=Sn×Lso, where Sn is sarcomere number [Sn=Lf/Ls] and Lso is species-specific optimal Ls [2.4 μm in rat]).20 For the reduced load/pregnancy hormones+ group, we compared PFM Lfn between the sides with and without conceptuses. Differences between the contralateral sides were observed in coccygeus and pubocaudalis, with Lfn on the side ipsilateral to the uterine horn with conceptuses significantly exceeding that on the side with ligated uterine horn in coccygeus (P=.03) and approaching statistical significance in pubocaudalis (P=.07) (Figure 3). There were no differences in Lfn between the right vs left iliocaudalis or the right vs left tibialis anterior muscles in the reduced load/pregnancy hormones+ group, P>.9. We, therefore, used the values from the side ipsilateral to the ligated uterine horn for comparisons across the experimental groups.
FIGURE 3. Comparison of PFM normalized fiber lengths between the sides with vs without conceptuses.
The data are presented as mean±standard error of mean. Some standard errors of mean values were too small to be visible as error bars. Single asterisk represents P<.05 derived from paired Student t test.
C, coccygeus; ICa, iliocaudalis; PCa, pubocaudalis; TA, tibialis anterior.
The following results are presented relative to the load−/pregnancy hormones− control group, unless stated otherwise (Table 1). Coccygeus demonstrated addition of sarcomeres in series in response to muscle and sarcomere stretch induced by the increased load uncoupled from pregnancy hormones, and in response to reduced load in the presence of pregnancy hormones. This is evident from the increased Lfn in both the load+/pregnancy hormones− (P<.0001) and reduced load/pregnancy hormones+ groups (P=.01). Moreover, coccygeus Lfn in these groups did not differ from that observed in the load+/pregnancy hormones+ group (P>.5). In contrast, pubocaudalis Lfn increased significantly in the load+/pregnancy hormones− (P<.05), but not in the reduced load/pregnancy hormones+ (P>.1). For iliocaudalis, substantial sarcomerogenesis occurred in response to nonreduced load and pregnancy hormones together, as evident by significant increase in Lfn only in the load+/pregnancy hormones+ group (P<.05). In contrast to PFMs, tibialis anterior was not affected by either the increased load, pregnancy hormones, or the combination of these physiological cues (P>.1).
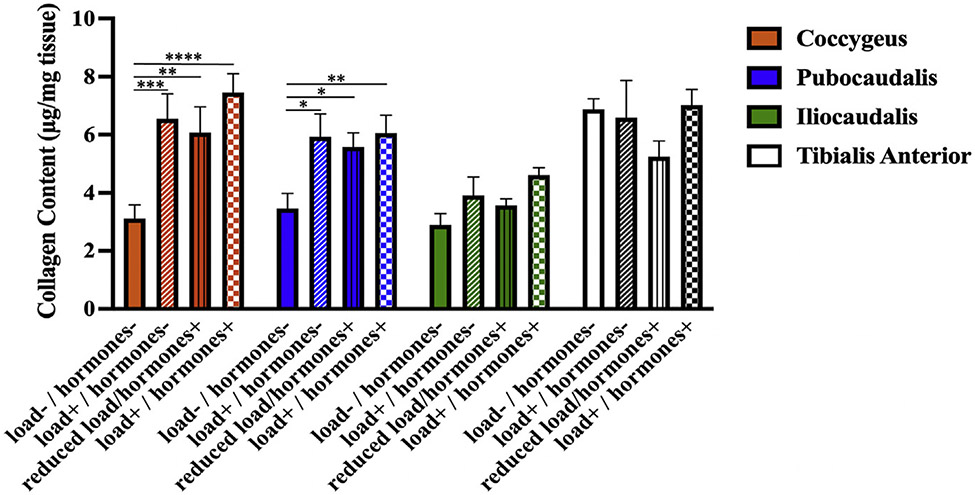
Next, we compared the ECM collagen content of PFMs and tibialis anterior subjected to the same experimental conditions. The intramuscular collagen content of coccygeus and pubocaudalis was significantly greater in the load+/pregnancy hormones− group and the reduced load/pregnancy hormones+ group than in the load−/pregnancy hormones− group, (P<.05) (Figure 4). Moreover, the collagen content of coccygeus and pubocaudalis in these groups did not differ from that observed in the load+/pregnancy hormones+ group (P>.5). These data suggest that load or pregnancy hormones can induce the ECM remodeling in these PFMs, with no additive effect of the 2 cues observed with respect to increase in the intramuscular collagen content. In the iliocaudalis and tibialis anterior, there were no differences in collagen content between any of the experimental groups (P>.2).
FIGURE 4. Comparison of the intramuscular extracellular matrix collagen content between experimental groups.
The changes in the intramuscular collagen content (in micrograms per milligram of muscle tissue) in response to the independent and combinatorial effect of mechanical load and pregnancy hormone milieu as determined by the measurement of intramuscular hydroxyproline content.
The data are presented as mean±standard error of mean. The p values were derived from repeated measures of the 2-way analysis of variance followed by pairwise comparisons with Dunnett and Tukey tests. Single asterisk represents P<.05; double asterisks represent P<.01; triple asterisks represent P<.001; four asterisks represent P<.0001.
The effect of sustained mechanical load, uncoupled from the endocrine milieu of pregnancy, on the pelvic floor musles’ response to parturition-related strains
We have previously shown that pregnancy-induced adaptations protect PFMs against birth injury, as indicated by the absence of sarcomere hyperelongation, a major cause of mechanical muscle injury, in response to parturition-related strains.21 To determine whether sarcomerogenesis of coccygeus and pubocaudalis induced by increased load was similarly protective against parturition-related strains, we compared the impact of vaginal distention of various magnitudes between 3 experimental conditions (load−/pregnancy hormones−, load+/pregnancy hormones−, and load+/pregnancy hormones+). The data from historic controls were used for the load−/pregnancy hormones− and load+/pregnancy hormones+ conditions,21 given confirmed reproducibility of our vaginal distention model.27
Response to physiological parturition-related strains
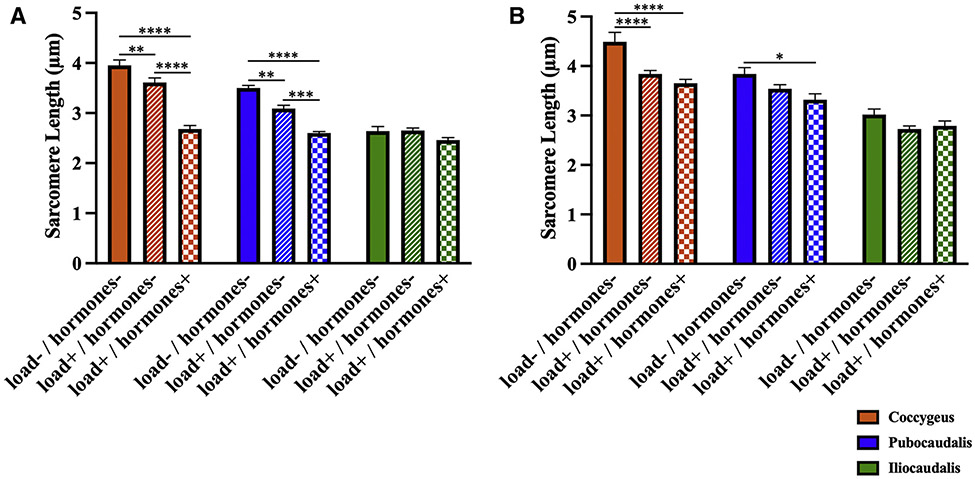
In response to vaginal distension with the 3 mL balloon volume (physiological strain) (Figure 5, A), Ls of coccygeus and pubocaudalis in the load+/hormones− group were substantially shorter than Ls in the load−/hormones− group (P<.01). However, sarcomere elongation was still significantly greater than that in the load+/hormones+ group (P<.001). Taken together, these data indicate that adaptations induced by an increased load in the absence of hormonally-driven alterations confer an intermediate protective effect against mechanical muscle injury caused by parturition-associated strains. As expected, there were no differences in iliocaudalis Ls between the groups (P>.2), because this PFM experienced lesser strains during vaginal balloon distention.21
FIGURE 5. Response to parturition-related strains.
A, The sarcomere length measurements (in micrometers) of the rat PFMs at 3 mL (physiological) strain via vaginal distension in the load−/hormones−, load+/hormones−, and load+/hormones+ groups. B, Sarcomere length measurements (in micrometers) of the rat PFMs at 5 mL (supraphysiologic) strain via vaginal distension in the load−/hormones−, load+/hormones−, and load+/hormones+ groups.
The data are presented as mean±standard error of mean. The P values were derived from repeated 2-way analysis of variance followed by pairwise comparisons with the Tukey test. Single asterisk represents P<.05; double asterisks represent P<.01; triple asterisks represent P<.001; four asterisks represent P<.0001.
PFM, pelvic floor muscles.
Response to supraphysiologic parturition-related strains
Next, we determined whether mechanical load provided protection against supraphysiologic strains (Figure 5, B). Like the response to vaginal distention with 3 mL volume, coccygeus Ls in the load+/hormones− group was significantly shorter than the load−/pregnancy hormones− group. However, as opposed to what was observed with the 3 mL strain, Ls in the load+/hormones− group did not differ from Ls in the load+/hormones+ group (P>.5). With respect to pubocaudalis, Ls in load+/hormones− group did not differ from that in either load−/pregnancy hormones− (P>.1) or the load+/hormones+ (P>.2) group. This confirms that our model reproduced adaptations that are protective against physiological strains, and that the protective effect of these adaptations diminishes when mechanical insult associated with parturition is excessive. Iliocaudalis Ls did not differ between the groups (P>.1).
Comment
Principal findings
The plasticity of the individual components of the rat PFM complex in response to mechanical and endocrine cues is variable. Out of all PFMs, coccygeus is the most susceptible to either stimulus with sustained exposure, with sarcomerogenesis observed in all experimental conditions tested (load+/pregnancy hormones−, reduced load/pregnancy hormones+, load+/pregnancy hormones+) compared with the unperturbed controls (load−/pregnancy hormones−). In pubocaudalis, fiber length increased in response to load alone and in combination with pregnancy hormones. Fiber length of iliocaudalis increased only in response to the combinatorial effect of mechanical and hormonal cues. These results indicate that coccygeus responds to either mechanical or endocrine stimulus. With respect to pubocaudalis, loading is sufficient to induce sarcomerogenesis and mechanical cues are likely the dominant driver of this adaptation in this portion of the rat levator ani muscle.
The key role of mechanical load in the plasticity of the contractile component of these muscles is further supported by our findings in the unilaterally pregnant (reduced load/hormones+) group. In coccygeus, Lfn on the side ipsilateral to the uterine horn with conceptuses was significantly greater than on the side without conceptuses, and we also observed a Lfn difference between sides in pubocaudalis that approached statistical significance. Importantly, the extent of PFM elongation by sarcomerogenesis in coccygeus and pubocaudalis in the load+/hormones− group was equivalent to that observed in the unperturbed pregnant (load+/hormones+) rats. On the contrary, neither load alone nor hormonal stimulation with reduced load are sufficient to induce sarcomerogenesis of iliocaudalis, which required both mechanical and endocrine cues. In contrast to PFMs and consistent with our previous findings,20 the hind limb tibialis anterior muscle was not affected by either the increased mechanical load imposed by the weighted uterine horns, pregnancy hormones, or the combination of these physiological cues, suggesting that PFMs are uniquely and differentially susceptible to these perturbations.
Although specific cellular mechanisms that lead to PFM plasticity in response to mechanical and endocrine cues have not been deciphered to date, the differential response of the individual components of the PFM complex is most likely because of the variable magnitude of mechanical load imposed on each muscle. Previously, we have demonstrated a divergent response to pregnancy of the iliocaudalis and pubocaudalis portions of the rat levator ani.22 These differences could be because of the more rostra-caudal orientation of iliocaudalis. The above is also the most likely reason for the smaller parturition-related strains experienced by the iliocaudalis compared with pubocaudalis and coccygeus, as evident from significantly less sarcomere hyperelongation in response to vaginal distention and spontaneous vaginal delivery.21 However, it is also possible that the plasticity of the individual components of the PFM complex differs. To address the latter, we are currently examining the impact of pregnancy on the functional state of the resident muscle stem cells in each muscle.
Like the response of the contractile myofibers, intramuscular ECM remodeling induced by mechanical load in the presence or absence of pregnant hormonal milieu varied across individual PFMs. The intramuscular collagen content of coccygeus and pubocaudalis increased in response to either load or hormonal stimuli. Like with PFM elongation by sarcomerogenesis, the increase in the ECM collagen content of coccygeus and pubocaudalis in the load+/hormones− group was equivalent to that observed in the pregnant rats (load+/hormones+). We did not observe an increase in the collagen content in iliocaudalis or tibialis anterior in any of the experimental conditions compared with the load−/hormones− controls. These results indicate that, as with sarcomerogenesis, ECM remodeling of coccygeus and pubocaudalis is induced by either mechanical or endocrine stimulus. Additional studies are needed to understand the relationship between collagen content (quantity), collagen arrangement/fibril alignment,28,29 and sarcomere length in PFMs. In the future, we aim to ascertain whether the physiological cues associated with pregnancy affect collagen fibril size and arrangement.
The importance of pregnancy-induced adaptations in the pelvic soft tissues mainly lies in their protective function against maternal birth injury. To this effect, we examined the response of chronically loaded PFMs to parturition-associated strains of various magnitudes. We found that adaptations of coccygeus and pubocaudalis, resultant from increased load, conferred protective effect against mechanical muscle injury relative to the response of the control muscles (load−/hormones−). However, this protective effect was smaller than that afforded by the adaptative changes of PFMs exposed to load and pregnant hormonal milieu. With respect to the supraphysiological strains, adaptations of coccygeus induced by load alone are sufficient to protect against mechanical muscle injury, on the basis of our finding that sarcomere length in the load+/hormones− group was significantly less than the hyperelongated sarcomeres in the unperturbed load−/hormones− group and not different from the load+/hormones+ group. Load-induced adaptations of pubocaudalis were inadequate to confer protection against sarcomere hyperelongation when this muscle experienced a higher magnitude strain.
Results in the context of what is known
Put together, these results support our hypothesis that initial sarcomere elongation because of an acute increase in mechanical load is associated with sarcomerogenesis of PFMs under sustained load, ultimately leading to return of sarcomeres to their operational length, which is necessary for optimal in vivo muscle function. Previous investigations in various animal models have demonstrated a similar phenomenon in limb muscles-muscles placed under chronic stretch elongate via sarcomerogenesis.30,31 Overall, increased mechanical load appears to play a key role in driving pregnancy-induced adaptations in the rat PFMs. In our study, load-induced sarcomerogenesis and increase in the intramuscular collagen content in the nonpregnant model varied by muscle. Alterations of coccygeus were induced either with load or pregnancy hormones, while iliocaudalis was not altered with modulation of load or pregnancy hormones, and pubocaudalis demonstrating responsiveness intermediate to coccygeus and iliocaudalis. Importantly, PFM plasticity in response to increased load afforded protection against PFM mechanical birth injury, with degree of protection varying between PFMs and strain magnitude. Considered together, this points toward a differential sensitivity of the individual pelvic skeletal muscles to the physiological cues associated with pregnancy. In addition, our findings suggest that the combinatorial effect of the endocrine and mechanical signals plays an important role in the PFM response to parturition-related strains.
Clinical implications
Our findings suggest that modulation of the PFM stretch induced by mechanical load during pregnancy, such as with specific pelvic floor training regimens, may be a potential therapeutic intervention for augmenting the protective antepartum PFM plasticity and preventing muscle injury during vaginal delivery.
Research implications
In the current study, we begin to elucidate the multifactorial mechanisms that govern PFM plasticity during pregnancy. Determining the key drivers of the protective adaptations of PFMs in the preclinical model is essential for promoting our understanding of the potential PFM plasticity in pregnant women and its role in modulating one’s predisposition to birth injury. To date, the causes underlying differential pelvic soft tissue damage during parturition and the subsequent development of PFDs in vaginally parous women remain unknown, and no effective strategies exist for the prevention of maternal birth injury.
Strengths and limitations
The strengths of the current work include the use of the rat model, which is specifically validated for the study of the human PFMs13,14; the development of the novel nonpregnant rat model of PFM mechanical loading and the first evaluation of the role of mechanical load and related muscle stretch, in the presence and absence of the endocrine cues of pregnancy, in PFM plasticity.
The limitations of our study are inherent to the use of experimental models to simulate the human condition. However, direct PFM tissue studies are not possible in asymptomatic living women. Thus, precise tissue-level experiments in the animal model are a necessary step in the continuum of these clinically relevant studies. Moreover, given a reduction in the placentally-derived factors in rats with a smaller number of gestations, the hormonal milieu in the reduced load/pregnancy hormones+ may be different compared with animals with a larger number of conceptuses. Unfortunately, to date there is no known way to induce the complete spectrum of endocrine changes that occur in pregnancy without the pregnancy itself, which constitutes a load on PFMs. Furthermore, we were unable to steadily increase the mechanical load over time as it occurs in a normal gestation. Instead, PFMs were exposed to the same load for the entire span of 21 days. Therefore, the effects of mechanical load in pregnancy could be overestimated. However, the extent of sarcomerogenesis and increase in PFM ECM collagen content were comparable in our nonpregnant loaded model and pregnant animals. In addition, because we based our a priori sample size calculation on differences between the nonpregnant (load−/hormones−) and pregnant (load+/hormones+) controls, we performed post hoc power analysis and found that we only had 48% power to detect a difference in pubocaudalis Lfn between reduced load/hormones+ and nonpregnant controls. Finally, although collagen is the major component of the intramuscular ECM, an investigation of the response of other ECM components such as elastin to physiological cues associated with pregnancy is an important area for future research.
Conclusions
Load induces plasticity of the intrinsic pelvic floor muscle components that renders protection against mechanical birth injury. The protective effect, which varies between individual muscles and magnitude of strain imposed on the muscles, can be further augmented by the presence of pregnancy hormones. Maximizing impact of mechanical load on PFMs during pregnancy, such as with specialized pelvic floor muscle stretching regimens, is a potentially actionable target for augmenting pregnancy-induced adaptations to decrease birth injury in women who may otherwise have incomplete antepartum muscle adaptations.
Supplementary Material
AJOG at a Glance.
Why was this study conducted?
To determine the role of mechanical load uncoupled from the hormonal milieu of pregnancy in driving protective pregnancy-induced adaptations previously discovered in the rat pelvic floor muscles.
Key findings
Mechanical load, in the absence of pregnancy hormones, induces sarcomerogenesis and extracellular matrix remodeling in rat pelvic floor muscles. Load-induced adaptations are partially protective against mechanical pelvic floor muscle injury consequent to parturition-associated strains.
What does this add to what is known?
The effect of sustained increased mechanical load uncoupled from the hormonal milieu of pregnancy on pelvic floor muscle plasticity has not been previously studied.
Modulating the pelvic floor muscles’ stretch antepartum, such as with specialized pelvic floor physical therapy regimens, could be a promising approach for the augmentation of protective muscle adaptations in women.
Acknowledgments
We gratefully acknowledge our funding source—National Institutes of Health grant R01 HD092515, from the Eunice Kennedy Shriver National Institute of Child Health and Human Development—that supported this project.
Footnotes
M.A. is a member of the Medical Advisory Board, Renovia, Inc. The remaining authors report no conflict of interest.
Portions of this work were presented at the annual scientific meeting of the American Urogynecologic Society/International Urogynecology Association, Nashville, TN, September 24—29, 2019 and at the annual scientific meeting of the American Urogynecologic Society, Pelvic Floor Disorders Week, held virtually, October 8—10, 2020.
References
- 1.Dietz HP. Pelvic organ prolapse - a review. Aust Fam Physician 2015;44:446–52. [PubMed] [Google Scholar]
- 2.Minassian VA, Bazi T, Stewart WF. Clinical epidemiological insights into urinary incontinence. Int Urogynecol J 2017;28:687–96. [DOI] [PubMed] [Google Scholar]
- 3.Hoyte L, Damaser MS, Warfield SK, et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am J Obstet Gynecol 2008;199:198.e1–5. [DOI] [PubMed] [Google Scholar]
- 4.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol 2004;103:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks SV, Zerba E, Faulkner JA. Injury to muscle fibres after single stretches of passive and maximally stimulated muscles in mice. J Physiol 1995;488:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallock JL, Handa VL. The epidemiology of pelvic floor disorders and childbirth: an update. Obstet Gynecol Clin North Am 2016;43:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 2000;23:1647–66. [DOI] [PubMed] [Google Scholar]
- 8.Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J 1990;57:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peviani SM, Guzzoni V, Pinheiro-Dardis CM, et al. Regulation of extracellular matrix elements and sarcomerogenesis in response to different periods of passive stretching in the soleus muscle of rats. Sci Rep 2018;8:9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Deyne PG, Hayatsu K, Meyer R, Paley D, Herzenberg JE. Muscle regeneration and fiber-type transformation during distraction osteogenesis. J Orthop Res 1999;17:560–70. [DOI] [PubMed] [Google Scholar]
- 11.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 2004;84:649–98. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda T, Kondo S, Homma T, Harris RC. Regulation of extracellular matrix by mechanical stress in rat glomerular mesangial cells. J Clin Invest 1996;98:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alperin M, Tuttle LJ, Conner BR, et al. Comparison of pelvic muscle architecture between humans and commonly used laboratory species. Int Urogynecol J 2014;25:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AM, Cook MS, Esparza MC, Slayden OD, Alperin M. Architectural assessment of rhesus macaque pelvic floor muscles: comparison for use as a human model. Int Urogynecol J 2017;28:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geisler HC, Westerga J, Gramsbergen A. Development of posture in the rat. Acta Neurobiol Exp (Wars) 1993;53:517–23. [PubMed] [Google Scholar]
- 16.Bailey AS, Adler F, Min Lai S, Asher MA. A comparison between bipedal and quadrupedal rats: do bipedal rats actually assume an upright posture? Spine (Phila Pa 1976) 2001;26:E308–13. [DOI] [PubMed] [Google Scholar]
- 17.Alperin M, Feola A, Duerr R, Moalli P, Abramowitch S. Pregnancy- and delivery-induced biomechanical changes in rat vagina persist postpartum. Int Urogynecol J 2010;21:1169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol 2007;109:136–43. [DOI] [PubMed] [Google Scholar]
- 19.Daucher JA, Clark KA, Stolz DB, Meyn LA, Moalli PA. Adaptations of the rat vagina in pregnancy to accommodate delivery. Obstet Gynecol 2007;109:128–35. [DOI] [PubMed] [Google Scholar]
- 20.Alperin M, Lawley DM, Esparza MC, Lieber RL. Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. Am J Obstet Gynecol 2015;213:191.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catanzarite T, Bremner S, Barlow CL, Bou-Malham L, O’Connor S, Alperin M. Pelvic muscles’ mechanical response to strains in the absence and presence of pregnancy-induced adaptations in a rat model. Am J Obstet Gynecol 2018;218:512.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alperin M, Kaddis T, Pichika R, Esparza MC, Lieber RL. Pregnancy-induced adaptations in intramuscular extracellular matrix of rat pelvic floor muscles. Am J Obstet Gynecol 2016;215:210.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremer RE, Barber MD, Coates KW, Dolber PC, Thor KB. Innervation of the levator ani and coccygeus muscles of the female rat. Anat Rec A Discov Mol Cell Evol Biol 2003;275:1031–41. [DOI] [PubMed] [Google Scholar]
- 24.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys J 1984;45:1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards CA, O’Brien WD Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta 1980;104:161–7. [DOI] [PubMed] [Google Scholar]
- 26.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 27.Duran P, Sesillo FB, Burnett L, et al. Proregenerative extracellular matrix hydrogel prevents and mitigates pathological alterations of pelvic muscles following birth injury. bioRxiv. Preprint posted online May 28, 2021. 10.1101/2021.05.28.446170 [DOI] [Google Scholar]
- 28.Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil 2004;25:159–66. [DOI] [PubMed] [Google Scholar]
- 29.Trotter JA, Purslow PP. Functional morphology of the endomysium in series fibered muscles. J Morphol 1992;212:109–22. [DOI] [PubMed] [Google Scholar]
- 30.Lieber RL, Roberts TJ, Blemker SS, Lee SSM, Herzog W. Skeletal muscle mechanics, energetics and plasticity. J Neuroeng Rehabil 2017;14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat’s soleus muscle due to immobilization at different lengths by plaster casts. J Physiol 1972;224:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.