Abstract
Pain management is challenging for patients with sickle cell disease (SCD) who present in vaso-occlusive crisis (VOC). Opioid therapy is highly effective, nevertheless undesirable side effects can hinder their effectiveness.
Regional anesthesia with deposition of perineural anesthetic offers nociceptive blockade, local vasodilatation and reduces the inflammatory response. Among pediatric patients, continuous peripheral nerve block (CPNB) for perioperative adjunctive analgesia is safe. Herein, we describe the trajectory of a cohort of pediatric SCD patients with opioid-refractory upper extremity VOC following placement of CPNBs for analgesia; highlighting reduced opioid consumption, improved pain scores and decreased length of hospitalization.
Keywords: sickle cell disease, regional anesthesia, pain management
Introduction
Sickle cell disease (SCD) comprises a group of hemoglobinopathies hallmarked by chronic hemolysis and recurrent vaso-occlusive crises (VOC), resulting in multi-organ dysfunction and early mortality. Disease-modifying therapies, such as hydroxyurea, have been shown to decrease VOC frequency. Admittedly, acute pain management remains particularly challenging.[1,2]
Opioids are a well-established therapy for VOC pain. However, the opioid-related side effects of sedation and respiratory depression can precipitate hypoxemia and atelectasis, worsening the underlying SCD pathophysiology. To minimize these risks, adjunctive therapies have been deployed, including: lidocaine, non-steroidal anti-inflammatories and muscle relaxants.[3–8]
Peripheral and central nerve blockade provides optimal analgesia for acute and chronic pain. Within the pediatric population, regional anesthesia (RA) utilizing continuous peripheral nerve block (CPNB) is safe and efficacious for perioperative and acute pain management.[9] Thoracic epidural analgesia has been successfully used for SCD-related pain management in acute chest syndrome.[10,11] However, the use of CPNB for extremity VOCs in this patient population has only been described in a few case reports.[12,13] This is the first case series to highlight uniform CPNB pain management for three patients with upper extremity VOCs.
Case Description
Case 1
A 16-year-old with HbSS was admitted for severe bilateral upper extremity VOC. Her right arm pain resolved within 48-hours, albeit sedation with oxygen requirement, pruritus and constipation ensued. On hospital day-3, she developed sharp pain radiating from the left shoulder to the elbow. This pain was exacerbated with motion and unresponsive to opioid titration; sub-anesthetic ketamine was initiated. Diagnostic radiography demonstrated avascular necrosis (AVN) of the left humeral head. Deep venous thrombosis was ruled out. The RA pain service was consulted on day-5 and offered a supraclavicular CPNB. With minimal sedation (ketamine 60mg, midazolam 2mg) under direct visualization with a high-frequency linear ultrasound transducer, a 22-G-echogenic needle was inserted in-plane postero-lateral to the subclavian artery. Ropivacaine (40mg) was deposited perineurally to the brachial plexus, followed by placement of a CPNB catheter. The CPNB was maintained for two days with a ropivacaine infusion (0.1mg/kg/hr). She was successfully weaned to an oral opioid regimen within 12-hours post-block and required no opioids by 24-hours. Ketamine infusion was discontinued on day-6. She was discharged on day-7. There was no evidence of CPNB complication. Post-hospitalization clinic follow-up at twelve days was notable for sustained pain resolution with limited oral opioid breakthrough requirement.
Case 2
A 13-year-old with HbSS and humeral AVN was admitted with back and shoulder VOC. His left shoulder pain persisted despite opioid titration. Moreover, he developed opioid-induced constipation and sedation with supplemental oxygen requirement. On hospital day-7, the RA service performed an interscalene CPNB under minimal sedation (ketamine 60mg, midazolam 2mg), as previously described. Ropivacaine (16mg) with dexmedetomidine (4mcg) was deposited perineurally. Thereafter, a ropivacaine infusion was initiated (0.1mg/kg/hr). He was weaned to an oral opioid regimen over the ensuing 16-hours and required no opioids 24-hours post-CPNB. The catheter was discontinued without complication following two days of treatment. He was discharged on day-10 and did not return for post-hospitalization outpatient clinic follow-up.
Case 3
An 11-year-old with HbS/β−0-thalassemia presented with severe neck, back and bilateral upper extremity VOC. Sub-anesthetic ketamine was initiated on hospital day-1. His pain improved however, he developed ketamine-associated hallucinations. Ketamine was discontinued on day-5 and he transitioned to oral opioids. On day-7, his right upper extremity acutely worsened requiring reinstitution of parental opioids. Imaging demonstrated AVN of the right humeral head. Under minimal sedation (ketamine 70mg, midazolam 2mg, dexmedetomidine 12mcg), the RA pain service placed an interscalene CPNB on day-10. Following a bolus dose of ropivacaine (50mg), he was maintained on a ropivacaine infusion (0.1mg/kg/hr). Parenteral and oral opioids were discontinued within 16-hours of CPNB placement. The CPNB was maintained for 48-hours and removed without complication. He was discharged on day-12. Post-hospitalization clinic follow-up at 1 week was notable for sustained pain resolution without home opioid requirement.
Methods
In this retrospective case series, we investigated patients hospitalized with VOC at Texas Children’s Hospital between September-December 2020. Informed consent was obtained from the parent(s) for this scientific contribution.
Results
All patients underwent CPNB placement for persistent localized pain despite multimodal therapy (Table 1). Pain scores and opioid consumption decreased within 24 hours of CPNB placement (Figure 1). Opioid reduction within the first 24 hours post-block ranged from 47.5% to 79.6%. Similarly, numeric pain scores decreased from 7–9/10 pre-block to 0–1/10 post-block. All patients were discharged within 48 hours of initiation of CPNB treatment.
Table 1:
Patient demographics and clinical course
| Indication | Block and Catheter | %Change in opioid use# | Catheter duration | Opioid related adverse events | Multimodal adjuncts for pain management | Admission duration | ||
|---|---|---|---|---|---|---|---|---|
|
Case 1
16y F with HbSS (wt: 60kg) |
Left upper extremity pain (AVN of left humeral head) | Left Supraclavicular nerve block catheter | −78.7% | 2 days | Constipation Sedation pruritis |
Ketorolac Ketamine Acetaminophen Lidocaine patch |
6 days | |
|
Case 2
13y M with HbSS (wt: 59kg) |
Left shoulder pain (history of AVN) | Left interscalene nerve block catheter | −47.5% | 2 days | Constipation Sedation |
Ketorolac/Ibuprofen Acetaminophen Lidocaine patch Methocarbamol |
10 days | |
|
Case 3 11y M with HbS/β0 (wt: 59kg) |
Right upper extremity pain (AVN of right humeral head) | Right interscalene nerve block catheter | −79.6% | 2 days | Constipation | Ketorolac/Ibuprofen Ketamine Acetaminophen Lidocaine Patch Methocarbamol Gabapentin Diclofenac gel |
12 days |
AVN = avascular necrosis.
percent change in opioid use, comparing 24 hours prior to block and 24 hours after block. (%change)= [(pre-block use)-(post-block use)]/(pre-block use)*100%
FIGURE 1. Hospital Opioid Consumption pre-CPNB and post-CPNB.
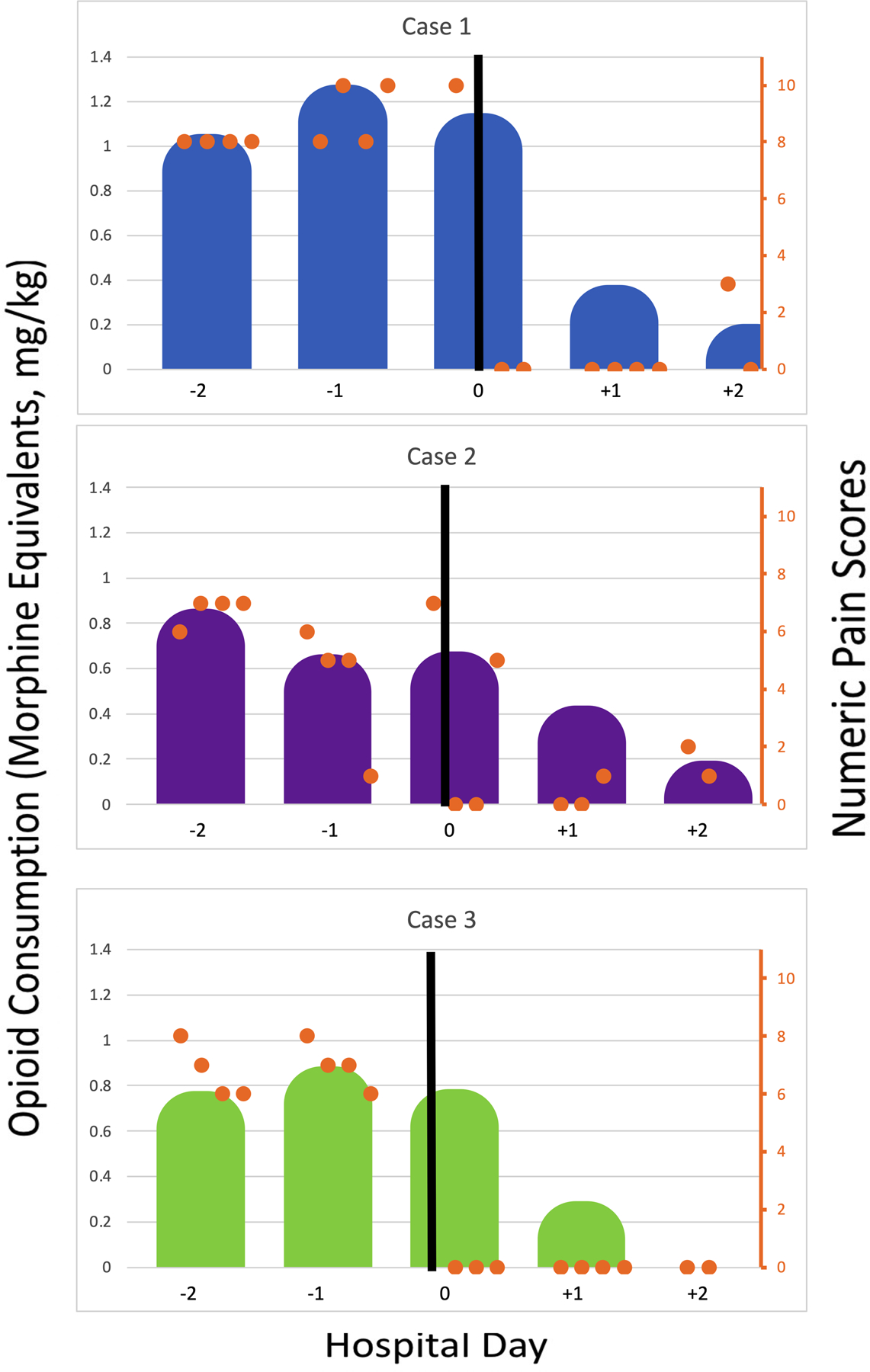
Day 0 is considered the day of CPNB. The black line represents CPNB. Shaded areas (Blue- case 1, Purple- case 2, Green- case 3) represent daily opioid consumption in morphine equivalents, mg/kg. Orange dots represent patient reported pain scores at 8am, 12pm, 4pm and 8pm. CPNB = Continuous peripheral nerve block.
Discussion
Acute VOC pain is the leading cause of hospital utilization for patients with SCD.[14] Pain management is challenging due to limited treatment modalities.[15,16] Pharmacotherapy for VOC targets peripheral and central nociceptive and inflammatory-mediated pathways. Opioid therapies are judicious, as they potently inhibit central and peripheral µ-receptors but they carry numerous undesirable side effects, most notably sedation, respiratory depression, increased risk of acute chest syndrome (ACS), constipation and opioid-induced hyperalgesia.[17,18] Incorporating RA proved instrumental in our subset of patients with VOC and humeral head AVN, resulting in decreased opioid requirement, markedly improved pain, and reduced length of hospitalization.
Among surgical patients, RA blunts autonomic nociception and the inflammatory response when compared to opioids, thereby reducing C-reactive protein and leukocyte counts.[6,19–20] Additionally, RA promotes vasodilation to improve local tissue oxygenation, which has been demonstrated in a prospective analysis using near-infrared spectroscopy.[21]
Retrospective case reports have shown beneficial outcomes with reduced opioid requirement, decreased length of hospitalization and improved patient satisfaction for RA in isolated limb VOC.[12,13] While there are no randomized clinical trials investigating the longitudinal efficacy and safety of RA in VOC, the available data suggests a role for RA exists. The 2020 American Society of Hematology (ASH) guidelines for management of acute and chronic pain in SCD provided recommendations for interdisciplinary and multimodal approaches for the treatment of pain.[22] With this goal in mind, ASH highlights RA for both adults and children presenting with acute, focal opioid-refractory SCD pain.
As with all clinical decision-making, an individualized approach is required when considering the addition of RA to the VOC pain regimen. Children and adolescents with isolated limb VOC are ideal candidates for RA. Appropriately dosed local anesthetics that facilitate sensory blockade with preservation of motor function and physical activity through an indwelling nerve block catheter or single injection can significantly reduce opioid consumption and support patient rehabilitation, as in our cohort. Procedural related risks including infection, hematoma and neurological injury such as peripheral neuropathy exist; albeit the reported incidence is extremely low.[23,24] General anesthesia increases the risk of SCD-related complications such as hypoxia, atelectasis, hypercarbia, acidosis and worsening VOC. However, ultrasound guidance and local anesthetics allow for CPNB to be performed with monitored anesthesia care or mild sedation, thereby reducing the risks associated with general anesthesia.[25] Procedural sedation with dexmedetomidine and sub-anesthetic ketamine preserves respiratory function and airway reflexes, mitigating the deleterious effects of general anesthesia. Collaborative pre-operative assessment is important, particularly for patients requiring sedation, and may require preparation with pre-procedure blood transfusion.[26]
Among our subset of pediatric patients, CPNB for limb VOC achieved dramatic reductions in pain scores and opioid utilization following 24-hours of therapy, and hospital discharge within 48-hours. While it remains unknown if RA addresses the underlying pathophysiology of VOC, our continued patient benefit at 1–2 week follow-up was promising. Future investigations examining the mechanisms of RA in VOC, the utility of RA as a primary modality for VOC and AVN-mediated pain, and the long-term safety and efficacy outcomes are warranted.
Key Points:
Regional anesthesia is an adjunct for pain management in sickle cell disease patients with localized vaso-occlusive crisis (VOC)
A hematology and regional anesthesiology collaborative can streamline VOC patients who will benefit most from peripheral nerve blocks
Acknowledgements:
VNT receives research funding from NIH K23-HL148548–01A1. The authors would like to acknowledge each patient featured in this publication as well as their families.
Abbreviations
- ACS
acute chest syndrome
- ASH
American Society of Hematology
- AVN
avascular necrosis
- CPNB
continuous peripheral nerve block
- NMDA
N-methyl-D-aspartate
- RA
regional anesthesia
- SCD
sickle cell disease
- VOC
vaso-occlusive crisis
Footnotes
Conflicts of Interest: TF has served as a consultant for Novartis, Global Blood Therapeutics, Emmaus Medical, Inc., and Bluebird Bio. VNT has served as a consultant for Novartis Pharmaceuticals, Global Blood Therapeutics, Forma Therapeutics, and Perkin Elmer. All other authors declare no conflicts of interest and or financial disclosures.
Bibliography
- 1.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995:332(20):1317–1322. [DOI] [PubMed] [Google Scholar]
- 2.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med 2016. [DOI] [PMC free article] [PubMed]
- 3.Edwards L, Edwards CV, Tolba R. 2020. May 11. Transforming Acute Pain Management in Sickle Cell Disease: Where Are We Now? ASRA Accessed 2020 May 11.
- 4.Uprety D, Baber A, Foy M. Ketamine infusion for sickle cell pain crisis refractory to opioids: a case report and review of literature. Ann Hematol 2014:93(5):769–771. [DOI] [PubMed] [Google Scholar]
- 5.Puri L, Morgan KJ, Anghelescu DL. Ketamine and lidocaine infusions decrease opioid consumption during vaso-occlusive crisis in adolescents with sickle cell disease. Curr Opin Support Palliat Care 2019:13(4):402–407. [DOI] [PubMed] [Google Scholar]
- 6.Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 2000:93(3):858–875. [DOI] [PubMed] [Google Scholar]
- 7.Than NN, Soe HHK, Palaniappan SK, et al. Magnesium for treating sickle cell disease. The Cochrane database of systematic reviews 2017:4:CD011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brousseau DC, Scott JP, Hillery CA, et al. The effect of magnesium on length of stay for pediatric sickle cell pain crisis. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine 2004:11(9):968–972. [DOI] [PubMed] [Google Scholar]
- 9.Walker BJ, Long JB, Sathyamoorthy M, et al. Complications in Pediatric Regional Anesthesia: An Analysis of More than 100,000 Blocks from the Pediatric Regional Anesthesia Network. Anesthesiology 2018:129(4):721–732. [DOI] [PubMed] [Google Scholar]
- 10.Yaster M, Tobin JR, Billett C, et al. Epidural analgesia in the management of severe vaso-occlusive sickle cell crisis. Pediatrics 1994:93(2):310–315. [PubMed] [Google Scholar]
- 11.New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. Journal of pediatric hematology/oncology 2014:36(6):e398–402. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt KE, Pranav H, Henry T, et al. Pericapsular nerve group blockade for sickle cell disease vaso-occlusive crisis. J Clin Anesth 2020:66:109932. [DOI] [PubMed] [Google Scholar]
- 13.Weber G, Liao S, Burns MA. Sciatic (Popliteal Fossa) Catheter for Pediatric Pain Management of Sickle Cell Crisis: A Case Report. A A Case Rep 2017:9(10):297–299. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf HR, Atrash HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med 2010:38(4 Suppl):S536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bou-Maroun LM, Meta F, Hanba CJ, et al. An analysis of inpatient pediatric sickle cell disease: Incidence, costs, and outcomes. Pediatric blood & cancer 2018:65(1). [DOI] [PubMed] [Google Scholar]
- 16.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA 2010:303(13):1288–1294. [DOI] [PubMed] [Google Scholar]
- 17.Buchanan ID, Woodward M, Reed GW. Opioid selection during sickle cell pain crisis and its impact on the development of acute chest syndrome. Pediatric blood & cancer 2005:45(5):716–724. [DOI] [PubMed] [Google Scholar]
- 18.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood 2012:120(18):3647–3656. [DOI] [PubMed] [Google Scholar]
- 19.Lirk P, Hollmann MW, Strichartz G. The Science of Local Anesthesia: Basic Research, Clinical Application, and Future Directions. Anesthesia and analgesia 2018:126(4):1381–1392. [DOI] [PubMed] [Google Scholar]
- 20.Bagry H, de la Cuadra Fontaine JC, Asenjo JF, et al. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg Anesth Pain Med 2008:33(1):17–23. [DOI] [PubMed] [Google Scholar]
- 21.Tighe PJ, Elliott CE, Lucas SD, et al. Noninvasive tissue oxygen saturation determined by near-infrared spectroscopy following peripheral nerve block. Acta Anaesthesiol Scand 2011:55(10):1239–1246. [DOI] [PubMed] [Google Scholar]
- 22.Brandow AM, Carroll CP, Creary S, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv 2020:4(12):2656–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neal JM, Barrington MJ, Brull R, et al. The Second ASRA Practice Advisory on Neurologic Complications Associated With Regional Anesthesia and Pain Medicine: Executive Summary 2015. Reg Anesth Pain Med 2015:40(5):401–430. [DOI] [PubMed] [Google Scholar]
- 24.Giabicani M, Compere V, Fourdrinier V, et al. Is sickle cell disease a possible risk factor for peripheral neuropathy after popliteal sciatic nerve block? Br J Anaesth 2013:111(3):508–510. [DOI] [PubMed] [Google Scholar]
- 25.Belmont AP, Nossair F, Brambilla D, et al. Safety of deep sedation in young children with sickle cell disease: a retrospective cohort study. The Journal of pediatrics 2015:166(5):1226–1232. [DOI] [PubMed] [Google Scholar]
- 26.Koshy M, Weiner SJ, Miller ST, et al. Surgery and anesthesia in sickle cell disease. Cooperative Study of Sickle Cell Diseases. Blood 1995:86(10):3676–368 [PubMed] [Google Scholar]


