Abstract
We determined the variations in the surface physicochemical properties of Listeria monocytogenes Scott A cells that occurred under various environmental conditions. The surface charges, the hydrophobicities, and the electron donor and acceptor characteristics of L. monocytogenes Scott A cells were compared after the organism was grown in different growth media and at different temperatures; to do this, we used microelectrophoresis and the microbial adhesion to solvents method. Supplementing the growth media with glucose or lactic acid affected the electrical, hydrophobic, and electron donor and acceptor properties of the cells, whereas the growth temperature (37, 20, 15, or 8°C) primarily affected the electrical and electron donor and acceptor properties. The nonlinear effects of the growth temperature on the physicochemical properties of the cells were similar for cells cultivated in two different growth media, but bacteria cultivated in Trypticase soy broth supplemented with 6 g of yeast extract per liter (TSYE) were slightly more hydrophobic than cells cultivated in brain heart infusion medium (P < 0.05). Adhesion experiments conducted with L. monocytogenes Scott A cells cultivated in TSYE at 37, 20, 15, and 8°C and then suspended in a sodium chloride solution (1.5 × 10−1 or 1.5 × 10−3 M NaCl) confirmed that the cell surface charge and the electron donor and acceptor properties of the cells had an influence on their attachment to stainless steel.
Listeria monocytogenes is a gram-positive human pathogen that is responsible for serious infections in immunocompromised individuals and pregnant women (17). L. monocytogenes has been implicated in several food-borne disease outbreaks. This organism is found not only in raw food but also on working surfaces in food-processing plants (40, 41). Microorganisms attached to a surface are an important potential source of contamination for any food material coming into contact with that surface (27). Bacterial attachment to an inert surface results from complex physicochemical interactions among the cell, the surface, and the liquid phase (24), which are caused by the cell surface charge (15), the hydrophobicity (45), and electron acceptor and donor properties (47).
Many workers have described the effects of various environmental parameters on L. monocytogenes growth (1, 7, 13), survival (31, 32, 34), pathogenicity (14, 44), and adhesion (2, 24). The most frequently studied parameters are growth temperature (12, 39), acidification of the growth media with organic acids (20, 43), and changes in the water activity of the growth medium caused by adding NaCl (29, 33). It has also been shown that results may be depend on the growth medium (16, 21, 25). For L. monocytogenes growth, the following two media have been used most frequently previously: brain heart infusion (BHI) broth (4, 30) and Trypticase soy broth supplemented with 6 g of yeast extract per liter (TSYE) (5, 22, 26).
Limited data concerning the effects of environmental and cultivation parameters on the physicochemical properties of L. monocytogenes have been published previously. Hence, the aim of this study was to determine the surface electrical properties, hydrophobicities, and electron donor and acceptor properties of L. monocytogenes Scott A cells under different growth conditions.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and preparation of microbial suspension.
L. monocytogenes Scott A (a human isolate obtained from the 1983 Massachusetts milk outbreak [18]) was provided by Bongrain Research and Development Centre (SOREDAB, La Boissière Ecole, France) and was stored at −80°C.
The following four growth media were used for experiments carried out at 37°C: BHI medium (Oxoid, Dardilly, France), TSYE (Biomérieux, Marcy l’Etoile, France), TSYE supplemented with 7.5 g of glucose per liter (Prolabo, Fontenay-sous-Bois, France), and TSYE supplemented with 1 N lactic acid (Prolabo) to obtain a pH of 6.0. The effects of growth temperatures (37, 20, 15, and 8°C) were studied by using TSYE and BHI medium. For all of the experiments, frozen cells were subcultured twice in the same medium and at the same growth temperature as the final culture. For the final culture, 1 ml of the second subculture was inoculated into 200 ml of fresh medium, and the preparation was incubated at the appropriate temperature until the stationary stage was reached. The stationary stage was reached after 15, 24, 30, and 192 h for cells grown at 37, 20, 15, and 8°C, respectively.
Cells were harvested by centrifugation for 10 min at 7,000 × g at 4°C and were washed twice with and resuspended in the relevant suspending liquid (1.5 × 10−1 or 1.5 × 10−3 M NaCl).
MATS.
Microbial adhesion to solvents (MATS) is based on comparing microbial cell affinity to a polar solvent and microbial cell affinity to a nonpolar solvent (8). The polar solvent can be an electron acceptor or an electron donor, but both solvents must have similar van der Waals surface tension components. The following pairs of solvents, as described by Bellon-Fontaine et al. (8), were used: chloroform, an electron acceptor solvent, and hexadecane, a nonpolar solvent; and ethyl acetate, a strong electron donor solvent, and decane, a nonpolar solvent. Due to the surface tension properties of these solvents, differences between the results obtained with chloroform and hexadecane and the results obtained with ethyl acetate and decane indicated that there were electron donor-electron acceptor interactions at the bacterial cell surface and revealed hydrophobic and hydrophilic properties. A microbial suspension containing approximately 108 CFU in 2.4 ml of 1.5 × 10−1 M NaCl was vortexed for 60 s with 0.4 ml of a solvent. This high concentration of electrolyte was used to avoid charge interference by a masking cell charge, because some solvent droplets, especially hexadecane, become negatively charged in aqueous suspensions (19). The mixture was allowed to stand for 15 min to ensure that the two phases were completely separated before a sample (1 ml) was carefully removed from the aqueous phase and the optical density at 400 nm was determined. The percentage of cells present in each solvent was subsequently calculated by using the equation: % Affinity = 100 × [1 − (A/A0)], where A0 is the optical density at 400 nm of the bacterial suspension before mixing and A is the absorbance after mixing. Each experiment was performed in triplicate by using three independently prepared cultures.
Electrophoretic mobility.
To measure electrophoretic mobility, bacteria were suspended in 1.5 × 10−3 M sodium chloride at a concentration of 107 CFU · ml−1. The pH of the suspension was adjusted to pH 2 to 7 by adding nitric acid (HNO3) or potassium hydroxide (KOH). Electrophoretic mobility was measured by using a 50-V electric field and a Laser Zetameter (Zêtaphoremètre II; Société d’Étude Physico-Chimiques, Limours, France). The results were based on an automated video analysis of about 200 particles for each measurement. Each experiment was performed in duplicate by using two independently prepared cultures. The typical standard deviation for the electrophoretic mobility mean was 0.25 μm/V/cm/s.
Microbial adhesion to AISI 304 stainless steel. (i) Solid surface and cleaning treatment.
The solid support selected for this study was AISI 304 stainless steel (Goodfellow, Cambridge Science Park, United Kingdom). Before physicochemical characterization and adhesion assays were begun, the steel was cut into rectangular chips (3 by 1 cm) and cleaned by soaking for 10 min at 50°C in a 2% (vol/vol) solution of the commercial surfactant RBS 35 (Société des Traitements Chimiques de Surface, Lambersart, France), rinsing for 10 min in Milli-Q water (Millipore, Saint-Quentin en Yvelines, France) at 50°C, and rinsing five times in 500 ml of Milli-Q water at room temperature.
(ii) Determination of the physicochemical properties of the solid surface.
The Lifshitz-van der Waals (γLW), electron donor (γ−), and electron acceptor (γ+) surface tension components of stainless steel (S) were determined by measuring contact angles by using the approach proposed by van Oss et al. (46). In this approach, in which spreading pressure is ignored, the contact angles (θ), measured with three pure liquids (L), can be expressed as:
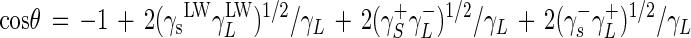 |
The three pure liquids used were Milli-Q water (Millipore), formamide (Sigma, Saint-Quentin Fallanier, France), and diiodomethane (Sigma).
(iii) Adhesion experiments.
Adhesion assays were performed by using microbial cells cultured at 37, 20, 15, or 8°C in TSYE and resuspended in 1.5 × 10−1 or 1.5 × 10−3 M sodium chloride as described below. Thirty milliliters of a bacterial suspension containing approximately 108 CFU · ml−1 was incubated in a petri dish (diameter, 10 cm) containing 3-cm2 stainless steel chips for 3 h at the temperature at which the bacteria were grown (37, 20, 15, or 8°C). The stainless steel chips were then rinsed to remove the nonadhering bacteria by pouring 30 ml of Milli-Q water onto the chips three times. The stainless steel chips were immersed in a test tube containing 10 ml of sterile 1.5 × 10−1 or 1.5 × 10−3 M NaCl. Bacterial cells were detached from the inert support by using a sonication bath (Ultrasonik) for 2 min at 40 kHz and 35°C. CFUs were counted by using the serial dilution technique and the bacterial suspension obtained after sonication. Counts were determined on TSYE agar (Biomérieux), and the preparations were incubated for 24 h at 37°C. Each experiment was performed in triplicate by using two independently grown cultures. We assessed the viability of the bacteria in the two suspending liquids and at the four temperatures analyzed by counting suspended cells on TSYE agar at the beginning and end of the adhesion period.
Statistical analysis.
A principal-component analysis was performed by using STATITCF software (Institut Technique des Céréales et des Fourrages, Paris, France), and a two-way analysis of variance was performed with Statgraphics 6.0 (Manugistics, Rockville, Md.).
RESULTS
Influence of growth conditions (medium and temperature) on L. monocytogenes Scott A surface physicochemical properties. (i) Influence of growth medium on cell surface physicochemical properties.
The MATS results obtained for L. monocytogenes Scott A grown at 37°C in BHI, TSYE, TSYE supplemented with glucose, and TSYE supplemented with lactic acid are shown in Table 1. Regardless of the medium used, the affinity of L. monocytogenes Scott A was always higher with chloroform (an electron acceptor solvent) than with hexadecane (a nonpolar solvent). The differences in bacterial affinity between these two solvents were due to Lewis acid-base interactions (i.e., electron donor-electron acceptor interactions resulting from the electron donor nature of the bacteria). The electron donor nature was maximized for cells cultivated in BHI medium or TSYE. Likewise, bacterial affinity was lower with ethyl acetate (a strongly electron-donating solvent) than with decane, indicating that the electron-accepting nature of L. monocytogenes Scott A grown in either medium was weak. Affinity to ethyl acetate was maximized with cells cultivated in the glucose-supplemented medium; this culture was also the culture with the lowest pH in the stationary phase (pH 4.1 and 4.7 for culture media supplemented with glucose and lactic acid, respectively; pH 5.1 for cells grown in TSYE).
TABLE 1.
Affinities of L. monocytogenes Scott A cells for the four solvents used in the MATS analysis after growth in either BHI medium or TSYE at 37, 20, 15, and 8°C and after growth at 37°C in TSYE supplemented with glucose or lactic acida
| Medium | Temp (°C) | % Affinity for:
|
|||
|---|---|---|---|---|---|
| Chloroform | Hexadecane | Ethyl acetate | Decane | ||
| TSYE + glucose | 37 | 90 ± 1 | 70 ± 6 | 56 ± 3 | 71 ± 9 |
| TSYE + lactic acid | 37 | 96 ± 1 | 85 ± 7 | 33 ± 3 | 96 ± 8 |
| TSYE | 37 | 91 ± 7 | 46 ± 11 | 17 ± 9 | 51 ± 11 |
| 20 | 94 ± 6 | 48 ± 10 | 17 ± 2 | 64 ± 6 | |
| 15 | 91 ± 3 | 48 ± 10 | 7 ± 3 | 66 ± 7 | |
| 8 | 94 ± 2 | 53 ± 8 | 14 ± 4 | 67 ± 11 | |
| BHI | 37 | 89 ± 3 | 41 ± 11 | 24 ± 5 | 44 ± 10 |
| 20 | 92 ± 3 | 48 ± 7 | 13 ± 5 | 47 ± 5 | |
| 15 | 92 ± 4 | 39 ± 11 | 13 ± 2 | 64 ± 3 | |
| 8 | 93 ± 4 | 44 ± 4 | 17 ± 1 | 55 ± 6 | |
Affinity values are means ± standard deviations (n = 9).
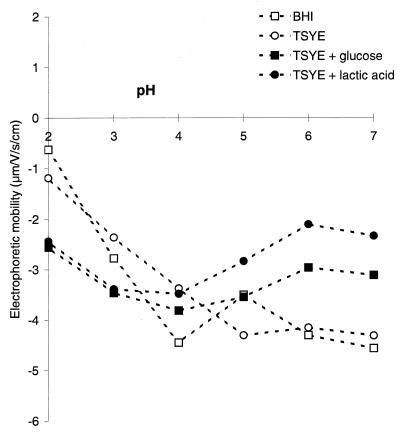
Figure 1 shows the electrophoretic mobilities of L. monocytogenes Scott A at pH 2 to 7 when it was grown in BHI medium, TSYE, TSYE supplemented with glucose, or TSYE supplemented with lactic acid. All of the cells were highly negatively charged, and so an isoelectric point could not be determined in the pH range studied. Conversely, cells grown in TSYE or BHI medium had very similar electrical characteristics (high mobility at a neutral pH and low mobility at an acidic pH) compared with cells cultivated in medium supplemented with glucose or lactic acid.
FIG. 1.
Electrophoretic mobilities of L. monocytogenes Scott A cells suspended in 1.5 × 10−3 M NaCl at pH 2 to 7 after growth at 37°C in BHI, TSYE, TSYE supplemented with glucose, or TSYE supplemented with lactic acid.
(ii) Influence of growth temperature on cell surface physicochemical properties.
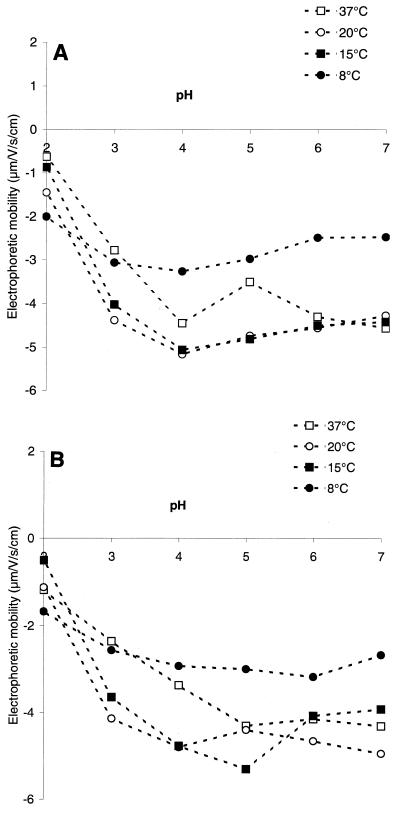
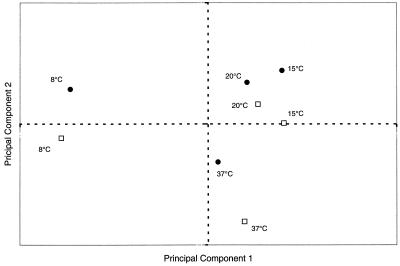
Table 1 shows the affinities with the four solvents used in the MATS method of the bacteria grown in TSYE or BHI medium at 37, 20, 15, and 8°C. Cells cultivated in TSYE exhibited slightly greater affinity with nonpolar solvents than cells cultivated in BHI medium exhibited (P < 0.05), and the affinity with ethyl acetate was greatest at 37°C and least at 15°C (P < 0.05). Moreover, the growth temperature had a significant effect (P < 0.05) on the electrophoretic mobility of the bacteria, as shown in Fig. 2. Our results indicated that the nonlinear effects of the growth temperature on the electrophoretic mobility of microbial cells were the same for both growth media. Except for the most acidic pH (pH 2.0), electrophoretic mobility was greatest for cells grown at 15 or 20°C and lowest for cells cultivated at 8°C. The electrophoretic mobility of the bacteria was between −0.5 and −2 μm/s/V/cm at pH 2.0. The isoelectric point of microbial cells could not be determined at any of the growth temperatures used in this study. Data were analyzed by using the data compression step of principal-component analysis, which removes the redundancy in an original data set so that only the first few principal-component scores are needed to describe most of the information in the original data set (11). The results of a principal-component analysis of the combined physicochemical properties of L. monocytogenes Scott A grown in TSYE or BHI medium at all temperatures are shown in Fig. 3. The first principal-component score, which accounts for 43% of the total variation in the original data set, is positively correlated with a high negative cell wall charge. This principal-component score discriminates among the samples by relating to the growth temperature. The second principal-component score represents 29% of the total variation in the original data set, and it is positively correlated with cell affinity for nonpolar solvents (hexadecane and decane) and negatively correlated with cell affinity for ethyl acetate. This principal-component score mainly distinguishes between cells grown in BHI and cells grown in TSYE.
FIG. 2.
Electrophoretic mobilities of L. monocytogenes Scott A cells suspended in 1.5 × 10−3 M NaCl at pH 2 to 7 after growth at four temperatures in BHI medium (A) or TSYE (B).
FIG. 3.
Principal-component analysis of combined physicochemical properties (affinity for the solvents used with the MATS method and electrophoretic mobility of bacteria) of L. monocytogenes Scott A grown at different temperatures in TSYE (●) and BHI medium (□). The first principal component (principal component 1) was positively correlated with a high negative charge of bacterial cells. The second principal component (principal component 2) was positively correlated with bacterial cell affinity for nonpolar solvents and negatively correlated with cell affinity for ethyl acetate.
Adhesion of L. monocytogenes Scott A grown in TSYE at different temperatures.
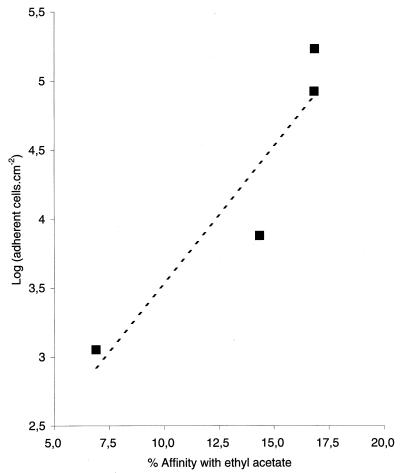
The numbers of culturable bacteria adhering to stainless steel under the eight conditions studied here are shown in Table 2. At the four growth temperatures, bacterial cells bound more effectively to the stainless steel when they were suspended in 1.5 × 10−1 M NaCl than when they were suspended in 1.5 × 10−3 M NaCl (P < 0.05). Furthermore, adhesion was greatest for bacterial cells cultivated at 37°C and lowest for cells cultivated at 15°C (P < 0.05), and the number of attached bacteria decreased between 20 and 15°C. A graph of the affinity of cells for ethyl acetate as determined by the MATS method versus the number of adherent cells in the presence of 1.5 × 10−3 M NaCl at the four growth temperatures used is shown in Fig. 4. The linear coefficients of correlation between bacterial affinity for ethyl acetate and bacterial adhesion to stainless steel were 0.93 and 0.80 for adhesion assays performed in the presence of 1.5 × 10−3 and 1.5 × 10−1 M NaCl, respectively.
TABLE 2.
Numbers of adherent L. monocytogenes Scott A cells on stainless steel chips as determined by adhesion assays performed in the presence of 1.5 × 10−1 or 1.5 × 10−3 M NaCl after growth in TSYE at four different temperaturesa
| NaCl concn (M) | Log no. of adherent cells after growth at:
|
|||
|---|---|---|---|---|
| 37°C | 20°C | 15°C | 8°C | |
| 1.5 × 10−1 | 6.4 ± 1.2 | 5.6 ± 0.9 | 4.1 ± 0.8 | 4.4 ± 0.2 |
| 1.5 × 10−3 | 5.2 ± 1.0 | 4.9 ± 0.9 | 3.0 ± 0.3 | 3.9 ± 0.6 |
The values are means ± standard deviations (n = 6).
FIG. 4.
Percentage of bacteria bound to ethyl acetate, a solvent used with the MATS method, versus the number of cells that adhered to stainless steel in the presence of 1.5 × 10−3 M NaCl.
Differences in adherence were not due to a loss of cell viability during the adhesion experiments, because after 3 h of incubation at 37, 20, 15, or 8°C in the presence of either 1.5 × 10−1 or 1.5 × 10−3 M NaCl, the viable cell counts were not affected (P < 0.05).
The Lifshnitz-van der Waals, electron donor, and electron acceptor components of the surface free energy of the stainless steel chips, as determined by contact angle measurements, were 39, 13, and 0.3 mJ · m−2, respectively.
DISCUSSION
The aim of this study was to investigate the influence of growth conditions (medium and temperature) on the physicochemical surface properties of L. monocytogenes Scott A. Microbial cell surfaces were found to be hydrophilic when bacteria were grown at 37°C in BHI medium or TSYE. However, the hydrophilicity decreased when microbial cells were cultivated in TSYE supplemented with either glucose or lactic acid. These findings are consistent with results obtained by Mafu et al. (28), who used the hydrophobic interaction chromatography technique and found that the hydrophobicity of L. monocytogenes Scott A increased as the pH decreased.
Furthermore, bacterial cells cultivated in BHI medium and bacterial cells cultivated in TSYE exhibited the same physicochemical differences when they were cultivated at different temperatures, and cells grown in media supplemented with glucose or lactic acid exhibited greater affinity with the electron donor solvent (ethyl acetate), which indicated that more electron acceptor groups were present on the cell wall. The presence of bound acidic compounds on the cell walls of the bacteria could explain this phenomenon. The low pH of a culture grown in a glucose-enriched medium in the stationary phase was undoubtedly related to the metabolism of glucose to lactic or acetic acid (38).
Under all of the growth conditions studied, L. monocytogenes cells had a high negative charge, and the electrophoretic mobilities at pH 7 were greater than the value (−2.07 μm/V/cm/s) obtained for the same strain by Mafu et al. (27). However, the storage conditions (4°C), growth medium (Trypticase soy broth supplemented with 1% yeast extract), and suspending medium (sodium phosphate buffer) were different in the study of Mafu et al.
Bacterial charge is attributed to cell wall constituents (36) (e.g., phosphate, carboxylate groups, and proteins). Progressive reductions in the electrophoretic mobility of cells as the pH decreases are due to gradual increases in the protonation of several chemical groups. Our failure to determine the isoelectric points in the pH range which we used (pH 2 to 7) indicates that compounds with very low pKa were present on the cell surfaces. Considering the composition of the L. monocytogenes cell wall (42), the characteristics described above might be related to the low pKa (pKa < 2.1) of the phosphate groups in phosphodiester bridges (R-O-HPO2-O-R/R-O-PO2−-O-R) of cell wall teichoic acids (37).
The electrophoretic mobility of cells cultivated in either one of the supplemented media seemed to be less pH dependent, and the decrease in mobility was more subtle when the pH of the microbial suspension was reduced below pH 4. Supplementing the growth medium with glucose or lactic acid also reduced the electrophoretic mobility of the bacteria at pH values above 4 through direct neutralization of the negatively charged groups present on cell walls linked to a decrease in the pH of the medium.
The growth temperature had a significant nonlinear effect on the electrophoretic mobility of L. monocytogenes Scott A cells grown in either TSYE or BHI medium. In particular, changes in electrophoretic mobility were observed between 15 and 8°C; at these temperatures the values decreased dramatically and were less pH dependent, and almost no changes were observed after the microbial suspension was acidified. This difference in activity at low pH values could have been due to differences in the nature of the negative charge of the cells. The rapid reduction in electrophoretic mobility around pH 4 may indicate that protein- or peptidoglycan-associated COOH/COO− (4 < pKa < 5) was present on the cell walls (37). Hence, cells grown at 8°C may have had fewer carboxyl groups than cells grown at the other temperatures. Differences observed at this growth temperature may also have been related to a glycolipid that was isolated from L. monocytogenes only after growth at a low temperature (23).
The high negative charge of bacterial cells at 15 and 20°C may have been linked to the presence of temperature-dependent production of flagella by L. monocytogenes. Many flagella were observed on cells grown at 20°C, whereas at 37°C very few flagella were observed (35). The rich protein (and protein-associated COOH/COO−) content of flagella could explain the specific electric properties of cells cultivated at 15 or 20°C. The differences in physicochemical properties of cells grown in different media and at different temperatures may also have reflected the synthesis of acclimation proteins (6) due to acidic or thermal stresses during bacterial growth, which could have led to changes in cell wall composition.
Unlike van der Waals and Lewis acid-base interactions, electrostatic interactions are inhibited in a high-ionic-strength suspending liquid. The greatest adhesion of microbial cells in a high-ionic-strength suspending liquid (1.5 × 10−1 M NaCl) indicated that there was electrostatic repulsion between the negatively charged bacteria and the stainless steel. These results are consistent with previously published data which indicated that the isoelectric point of stainless steel was around pH 4 and varied slightly depending on the surface finish and the cleaning treatments (9). The L. monocytogenes Scott A adhesion data correlated best with cell affinity for ethyl acetate, which indicates the importance of Lewis acid-base interactions for cell adhesion to stainless steel. This correlation is consistent with the physicochemical characteristics of ethyl acetate and stainless steel, both of which have strong electron donor surface properties and weak electron acceptor characteristics. Hence, our data suggest that both electrical and Lewis acid-base interactions are involved in L. monocytogenes Scott A adhesion to stainless steel. The influence of lactic acid in the growth medium on the adhesion of L. monocytogenes to stainless steel has been described previously (10). Variations in cell hydrophobicity caused by lactic acid led to significant variations in the adherent bacteria, implying that van der Waals interactions play a role in cell adhesion. Therefore, the growth conditions can influence the various components of microbial cell surface physicochemical properties (electrostatic, Lewis acid-base, and van der Waals interactions) and, consequently, the adhesion of cells to surfaces. Recently, Andersson et al. found that there is a relationship among Bacillus cereus spore adhesion to epithelial cells, surface hydrophobicity, and the virulence of strains (3). Adhesion assays performed with different surfaces that are representative of plants or epithelial cells and with L. monocytogenes strains whose pathogenicity is known are needed to improve our understanding of the relationships between physicochemical properties of microbial cells and pathogenicity.
ACKNOWLEDGMENTS
This work was supported by the UNIR (Ultrapropre Nutrition Industrie Recherche) program, which involves food companies, the French Ministry of Agriculture, and the French Ministry of Research.
We thank Michael Dever and Dipak Sarker for revising the English in the manuscript.
REFERENCES
- 1.Ahamad N, Marth E H. Behavior of Listeria monocytogenes at 7, 13, 21, and 35°C in tryptose broth acidified with acetic, citric, or lactic acid. J Food Prot. 1989;52:688–695. doi: 10.4315/0362-028X-52.10.688. [DOI] [PubMed] [Google Scholar]
- 2.Al-Makhlafi H. Influence of preadsorbed milk proteins on adhesion of Listeria monocytogenes to hydrophobic and hydrophilic silica surfaces. Appl Environ Microbiol. 1994;60:3560–3565. doi: 10.1128/aem.60.10.3560-3565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson A, Granum P E, Rönner U. The adhesion of Bacillus cereus spores to epithelial cells might be an additional virulence mechanism. Int J Food Microbiol. 1998;39:93–99. doi: 10.1016/s0168-1605(97)00121-9. [DOI] [PubMed] [Google Scholar]
- 4.Baloga A O, Harlander S K. Comparison of methods for discrimination between strains of Listeria monocytogenes from epidemiological surveys. Appl Environ Microbiol. 1991;57:2324–2331. doi: 10.1128/aem.57.8.2324-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa W B, Cabedo L, Wederquist H J, Sofos J N, Schmidt G R. Growth variation among species and strains of Listeria in culture broth. J Food Prot. 1994;57:765–775. doi: 10.4315/0362-028X-57.9.765. [DOI] [PubMed] [Google Scholar]
- 6.Bayles D O, Annous B A, Wilkinson B J. Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl Environ Microbiol. 1996;62:1116–1119. doi: 10.1128/aem.62.3.1116-1119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begot C, Lebert I, Lebert A. Variability of the response of 66 Listeria monocytogenes and Listeria innocua strains to different growth conditions. Food Microbiol. 1997;14:403–412. [Google Scholar]
- 8.Bellon-Fontaine M N, Rault J, van Oss C J. Microbial adhesion to solvents: a novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surfaces B Biointerfaces. 1996;7:47–53. [Google Scholar]
- 9.Boulangé-Petermann L, Doren A, Baroux B, Bellon-Fontaine M N. Zeta potential measurements on passive metals. J Colloid Interface Sci. 1995;171:179–186. [Google Scholar]
- 10.Briandet R, Leriche V, Carpentier B, Bellon-Fontaine M N. Effects of the growth conditions on the surface hydrophobicity of Listeria monocytogenes cells and their adhesion to stainless steel. J Food Prot. 1999;62:994–998. doi: 10.4315/0362-028x-62.9.994. [DOI] [PubMed] [Google Scholar]
- 11.Briandet R, Kemsley E K, Wilson R H. Approaches to adulteration detection in instant coffees using infrared spectroscopy and chemometrics. J Sci Food Agric. 1996;71:359–366. [Google Scholar]
- 12.Buchanan R L, Stahl H G, Whiting R C. Effects and interactions of temperature, pH, atmosphere, sodium chloride, and sodium nitrite on the growth of Listeria monocytogenes. J Food Prot. 1989;52:844–851. doi: 10.4315/0362-028X-52.12.844. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan R L, Phillips J G. Response surface model for predicting the effects of temperature, pH, sodium chloride content, sodium nitrite concentration and atmosphere on the growth of Listeria monocytogenes. J Food Prot. 1990;53:370–376. doi: 10.4315/0362-028X-53.5.370. [DOI] [PubMed] [Google Scholar]
- 14.Buncic S, Avery S M. Relationship between variations in pathogenicity and lag phase at 37°C of Listeria monocytogenes previously stored at 4°C. Lett Appl Microbiol. 1996;23:18–22. doi: 10.1111/j.1472-765x.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 15.Dickson J S, Koohmaraie M. Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl Environ Microbiol. 1989;55:832–836. doi: 10.1128/aem.55.4.832-836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy G, Sheridan J J. The effect of temperature, pH and medium in a surface adhesion immunofluorescent technique for detection of Listeria monocytogenes. J Appl Microbiol. 1997;83:95–101. doi: 10.1111/j.1361-5072.1997.00195.x. [DOI] [PubMed] [Google Scholar]
- 17.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming J M, Cochi S L, MacDonald K L, Brondum J, Hayes P S, Plikaytis B D, Holmes M B, Audurier A, Broome C V, Reingold A L. Pasteurized milk as a vehicle of infection in an outbreak of listeriolisis. N Engl J Med. 1985;312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 19.Geertsema-Doornbush G I, Van der Mei H C, Bussher H J. Microbial cell surface hydrophobicity: the involvement of electrostatic interactions in microbial adhesion to hydrocarbons (MATH) J Microbiol Methods. 1993;18:61–68. [Google Scholar]
- 20.George S M, Lund B M, Brocklehurst T F. The effect of pH and temperature on initiation of growth of Listeria monocytogenes. Lett Appl Microbiol. 1988;6:153–156. [Google Scholar]
- 21.Horska E, Pokorny J, Labajova M. Changes of surface charge and hydrophobicity of the outer bacterial membrane depending on the cultivation medium. Biologia (Bratislava) 1993;48:343–347. [Google Scholar]
- 22.Ita P S, Hutkins R W. Intracellular pH and survival of Listeria monocytogenes Scott A in tryptic soy broth containing acetic, lactic, citric, and hydrochloric acids. J Food Prot. 1991;54:15–19. doi: 10.4315/0362-028X-54.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Jones C E, Shama G, Jones D, Roberts I S, Andrew P W. Physiological and biochemical studies on psychrotolerance in Listeria monocytogenes. J Appl Microbiol. 1997;83:31–35. doi: 10.1046/j.1365-2672.1997.d01-391.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim K Y, Frank J F. Effect of growth nutrients on attachment of Listeria monocytogenes to stainless steel. J Food Prot. 1994;57:720–726. doi: 10.4315/0362-028X-57.8.720. [DOI] [PubMed] [Google Scholar]
- 25.Kim K Y, Frank J F. Effect of nutrients on biofilm formation by Listeria monocytogenes on stainless steel. J Food Prot. 1995;58:24–28. doi: 10.4315/0362-028X-58.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Kouassi Y, Shelef L A. Metabolic activities of Listeria monocytogenes in the presence of sodium propionate, acetate, lactate and citrate. J Appl Bacteriol. 1996;81:147–153. doi: 10.1111/j.1365-2672.1996.tb04492.x. [DOI] [PubMed] [Google Scholar]
- 27.Mafu A A, Roy D, Goulet J, Magny P. Attachment of Listeria monocytogenes to stainless steel, glass, polypropylene, and rubber surfaces after short contact times. J Food Prot. 1990;53:742–746. doi: 10.4315/0362-028X-53.9.742. [DOI] [PubMed] [Google Scholar]
- 28.Mafu A A, Roy D, Goulet J, Savoie L. Characterization of physicochemical forces involved in adhesion of Listeria monocytogenes to surfaces. Appl Environ Microbiol. 1991;57:1969–1973. doi: 10.1128/aem.57.7.1969-1973.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClure P J, Beaumont A L, Sutherland J P, Roberts T A. Predictive modelling of growth of Listeria monocytogenes. The effects on growth of NaCl, pH, storage temperature and NaNO2. Int J Food Microbiol. 1996;34:221–232. doi: 10.1016/s0168-1605(96)01193-2. [DOI] [PubMed] [Google Scholar]
- 30.McLauchlin J, Tidley A M, Taylor A G. The use of monoclonal antibodies against Listeria monocytogenes in a direct immunofluorescence technique for the rapid presumptive identification and direct demonstration of Listeria in food. Acta Microbiol Hung. 1989;36:467–471. [PubMed] [Google Scholar]
- 31.Oh D-H, Marshall D L. Influence of temperature, pH, and glycerol monolaurate on growth and survival of Listeria monocytogenes. J Food Prot. 1993;56:744–749. doi: 10.4315/0362-028X-56.9.744. [DOI] [PubMed] [Google Scholar]
- 32.Palumbo S A, Williams A C. Effect of temperature, relative humidity, and suspending menstrue on the resistance of Listeria monocytogenes to drying. J Food Prot. 1990;53:377–381. doi: 10.4315/0362-028X-53.5.377. [DOI] [PubMed] [Google Scholar]
- 33.Papageorgiou D K, Marth E H. Behaviour of Listeria monocytogenes at 4 and 22°C in whey and skim milk containing 6 or 12% sodium chloride. J Food Prot. 1989;52:625–630. doi: 10.4315/0362-028X-52.9.625. [DOI] [PubMed] [Google Scholar]
- 34.Patchett R A, Watson N, Fernandez P S, Kroll R G. The effect of temperature and growth rate on the susceptibility of Listeria monocytogenes to environmental stress conditions. Appl Microbiol. 1996;22:121–124. doi: 10.1111/j.1472-765x.1996.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 35.Peel M, Donachie W, Shaw A. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and Western blotting. J Gen Microbiol. 1988;134:2171–2178. doi: 10.1099/00221287-134-8-2171. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier C, Bouley C, Cayvela C, Boutier S, Bourlioux P, Bellon-Fontaine M N. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl Environ Microbiol. 1997;63:1725–1731. doi: 10.1128/aem.63.5.1725-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rijnaarts H H M, Norbe W, Lyklema J, Zehnder A J B. The isoelectric point of bacteria as an indicator for the presence of cell surface polymers that inhibit adhesion. Colloids Surf B Biointerfaces. 1995;4:191–197. [Google Scholar]
- 38.Romick T L, Fleming H P. Acetoin production as an indicator of growth and metabolic inhibition of Listeria monocytogenes. J Appl Microbiol. 1998;84:18–24. doi: 10.1046/j.1365-2672.1997.00302.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosenow E M, Marth E H. Growth of Listeria monocytogenes in skim, whole and chocolate milk, and in whipping cream during incubation at 4, 8, 13, 21 and 35°C. J Food Prot. 1987;50:452–459. doi: 10.4315/0362-028X-50.6.452. [DOI] [PubMed] [Google Scholar]
- 40.Roy B, Ackermann H W, Pandian S, Picard G, Goulet G. Biological inactivation of adhering Listeria monocytogenes by listeria phages and a quaternary ammonium compound. Appl Environ Microbiol. 1993;59:2914–2917. doi: 10.1128/aem.59.9.2914-2917.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasahara K C, Zottola E A. Biofilm formation by Listeria monocytogenes utilizes a primary colonizing microorganism in flowing systems. J Food Prot. 1993;56:1022–1028. doi: 10.4315/0362-028X-56.12.1022. [DOI] [PubMed] [Google Scholar]
- 42.Seeliger H P R, Jones D. Listeria. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1235–1244. [Google Scholar]
- 43.Sorrells K M, Enigl D C, Hatfield J R. Effect of pH, acidulant, time, and temperature on the growth and survival of Listeria monocytogenes. J Food Prot. 1989;52:571–573. doi: 10.4315/0362-028X-52.8.571. [DOI] [PubMed] [Google Scholar]
- 44.Stephens J C, Roberts I S, Jones D, Andrew P W. Effect of growth temperature on virulence of strains of Listeria monocytogenes in the mouse: evidence for a dose dependence. J Appl Bacteriol. 1991;70:239–244. doi: 10.1111/j.1365-2672.1991.tb02931.x. [DOI] [PubMed] [Google Scholar]
- 45.van Loosdrecht M C M, Lyklema J, Norde W, Schraa G, Zehnder A J B. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987;53:1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Oss C J, Chaudhury M K, Good R J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev. 1988;88:927–941. [Google Scholar]
- 47.van Oss C J. Acid-base interfacial interactions in aqueous media. Colloids Surf A Physicochem Eng Aspects. 1993;78:1–49. [Google Scholar]