Abstract
A method of recovering sublethally heat-injured bacteria was developed. The procedure (termed the agar underlay method) uses a nonselective agar underlaid with a selective medium. In a two-chambered petri dish, the Lutri plate (LP), a nonselective agar is inoculated with a population of sublethally heat-injured bacteria. After a 2-h repair incubation period, selective agar is added to the bottom chamber of the LP and incubated. By diffusing through the nonselective top agar, selective agents from the underlay medium impart selectivity to the system. By the agar underlay method, recovery rates of the heat-injured food-borne pathogens Escherichia coli O157:H7 and Salmonella typhimurium were not different (P > 0.05) from recovery rates determined with nonselective media. Sublethally heat-injured cells (60°C for 1.5 min in buffer or 80°C for 30 s on meat surfaces) grew and produced a typical colony morphology and color reaction when the agar underlay procedure was used with the appropriate respective selective agars. Unlike agar overlay methods for injury repair, the agar underlay procedure allows the typical selective-medium colony morphology to develop and allows colonies to be more easily picked for further characterization. Higher recovery rates of heat-injured fecal enterococci from bovine fecal samples and total coliforms from animal waste lagoons were obtained by the agar underlay method with selective agars than by direct plating on the respective selective media.
Microorganisms subjected to sublethal environmental stresses undergo metabolic injury, often manifested as the inability to form colonies on selective agars on which uninjured cells can survive and grow (17, 18). The differential in counts between selective and nonselective media is a means to determine the degree to which a microbial population is sublethally injured (2, 23). Bacteria undergo sublethal cellular injury from a variety of inimical processes, including acidification, heating, and freezing (2, 23). The aforementioned treatments are commonly used antimicrobial interventions for reducing bacterial contamination and pathogens, such as toxigenic Escherichia coli O157:H7 and Salmonella spp., on cattle, swine, and sheep carcasses (16, 21, 30). These interventions include organic acid sprays (generally lactic or acetic acids), hot water or steam treatments, and antimicrobial chemical applications, such as chlorine, chlorine dioxide, and trisodium phosphate (1, 3, 11–14, 26, 29, 32). While sufficient exposure to the aforementioned treatments can result in bactericidal effects, more often the pathogen population is reduced but not completely inactivated. Depending on the antimicrobial agent, after the initial microbial reduction from the antimicrobial treatment, either a residual antimicrobial effect, such as in the cases of lactic and acetic acid treatments (14), or only an immediate reduction with no residual bacteriostatic effect, as in the case of hot water or steam, can be observed. Following heat treatment, sublethally injured food-borne pathogens could assume added significance because they are potentially as dangerous as their uninjured counterparts (2, 22, 23). Heat-injured E. coli O157:H7 or Salmonella typhimurium cannot undergo repair and form colonies on selective media, such as Sorbitol MacConkey agar (SMAC) or xylose lysine decarboxylase agar (XLD), respectively, because the selective agents or dyes in these selective agars can inhibit the repair of heat-injured pathogens (20, 23, 25). Significant differences between SMAC and tryptic soy agar (TSA; nonselective medium) for recovery of injured microorganisms have been observed (1, 8, 23).
Several workers have reported identification methods and media for detecting sublethally injured food-borne pathogens in foods (5, 6, 15, 19, 23, 27, 31). These methods have limitations that include the difficulty of picking well-isolated colonies from agar overlays, as well as the disparity between the colony morphologies and color reactions of colonies formed under agar overlays and those formed on the agar surface. Simpler, more effective methods for recovery of injured food-borne pathogens are necessary. We report a new method, termed the agar underlay procedure, to culture sublethally injured bacteria, including E. coli O157:H7, Salmonella, fecal enterococci, and coliforms. The agar underlay method offers advantages over other injury recovery methods.
MATERIALS AND METHODS
Cultures and cell suspension.
E. coli O157:H7 (ATCC 35150 and ATCC 43890), S. typhimurium (ATCC 19585 and UNL 10636-97), and Listeria monocytogenes Scott A were obtained from the Roman L. Hruska U.S. Meat Animal Research Center (MARC) culture collection. All strains were maintained in 75% glycerol at −20°C. Each culture was propagated in tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.) at 37°C for 18 h before experiments.
Agar underlay method for recovery of heat-injured E. coli O157:H7 and S. typhimurium.
The Lutri (Starkville, Miss.) plate (LP) is a dual-chambered culture dish designed for the screening of natural samples for antibiotic activities (4, 7). It is composed of compartments A and B; agar is poured into compartment A. Following solidification, this agar surface can be inoculated. After sufficient growth time, the plastic divider between compartments A and B is removed. Selective agar is then poured into compartment B, forming an underlay of the second agar beneath the agar in layer A. We have used the LP to create a means of subjecting an inoculated sample to conditions of nonselective growth followed by indirect contact with selective medium to achieve selectivity. This method was termed the agar underlay method.
First, TSA (20 ml) was poured into compartment A. After solidification of the agar, injured microorganisms were spread plated directly on the TSA in compartment A. The plate was incubated at 37°C for 2 h for resuscitation of injured cells. After the 2-h preincubation resuscitation step, 40 ml of melted selective medium was poured into compartment B. The differential and selective agents from the compartment B underlay then diffused into the compartment A chamber.
Recovery of heat-injured E. coli O157:H7 and S. typhimurium from BPW.
Broth cultures in TSB were separately incubated at 37°C for 18 h and diluted in buffered peptone water (BPW) to obtain viable cell counts of ∼6.0 log CFU/ml. One hundred microliters of each culture suspension was added to each of three test tubes containing 5 ml of BPW, which had been preheated and maintained at 60°C. After inoculation, each tube was tightly sealed, immersed completely in a shaking water bath, and heated at 60°C for 1.5 min. After being heated, the tubes were cooled immediately in ice. Heat-injured cell suspensions were spread plated on TSA and selective media (SMAC [Difco Laboratories] for E. coli O157:H7 or XLD [Difco Laboratories] for S. typhimurium) and incubated at 37°C for 24 h. For the agar underlay method, another 100 μl of each diluted sample was spread plated on TSA in LP compartment A. The plate was incubated at 37°C for 2 h for resuscitation of heat-injured E. coli O157:H7 or S. typhimurium. After 2 h of incubation, the 40 ml of melted selective agar (SMAC or XLD) was poured into compartment B, allowed to solidify, and incubated at 37°C for 22 h. Each experiment was performed three times.
Recovery of heat-injured E. coli O157:H7 and S. typhimurium from beef trim.
E. coli O157:H7 (ATCC 43890) and S. typhimurium (ATCC 19585) were inoculated as a mixed culture. Each pathogen was transferred to 9.0 ml of fresh TSB and incubated at 37°C for 24 h. After incubation, 400 μl of each culture was transferred into a sterile 50-ml conical centrifuge tube containing 40 ml of TSB. This mixed culture was incubated an additional 18 h at 37°C. The cells were harvested by centrifugation (Beckman Instruments, Inc., Palo Alto, Calif.) at 2,000 × g for 20 min at 4°C and washed in BPW. Beef trim (cut meat) obtained from the MARC abattoir was trimmed to uniform sizes of ∼7.5 by 7.5 by 2 cm with an ethanol-flamed knife and sterilized by UV light as previously described (9, 10). Individual sections of lean trim were placed fascia side down into a sterile weigh boat (14 by 14 cm) and, with a commercial spray bottle, the serially diluted bacterial suspension (3.75 ml) was sprayed on the meat surface to reach a level of ca. 4 to 5 log CFU/cm2. The inoculated meat was incubated for 15 min at room temperature, after which two 5- by 5- by 0.5-cm sections were excised. One section was aseptically placed into a Sterefil Stomacher bag (Spiral Biotech Inc., Bethesda, Md.) containing 50 ml of BPW with 0.1% Tween 20. The samples were homogenized with a stomacher (Seward Medical, London, United Kingdom) for 2 min. The homogenates were serially diluted with BPW. One hundred microliters of the appropriate sample dilution was spread plated on SMAC or XLD. For the underlay repair method, another 100 μl was spread plated on TSA in compartment A of an LP and treated as described above. The underlay recovery plates were further incubated for 22 h at 37°C.
The other excised meat sample was subjected to a hot-water (80°C) immersion treatment in a 2-liter beaker for 30 s. Following heat treatment, the treated section was stomached and subjected to the agar underlay method as described above. These experiments were performed three times.
Recovery of heat-injured fecal enterococci from bovine fecal samples.
A mixture of equal masses of fresh bovine cattle feces (obtained from three animals at MARC) was made and diluted 1:2 in BPW. A sample of the diluted feces was heated at 55°C for 10 min in a glass screw cap test tube (16 by 125 mm). Both before and after treatment the numbers of fecal enterococci were enumerated on KF Streptococcus agar (Difco Laboratories) by a previously published agar overlay injury repair procedure with VRB agar (Difco Laboratories) overlaid with TSA (15) and by the agar underlay method. Each experiment was performed three times.
Recovery of coliform bacteria from animal waste lagoon water samples.
Water samples were obtained from three waste lagoons and within 30 min of sampling were serially diluted in BPW; dilutions were spread plated directly onto VRB agar or subjected to the agar underlay method with VRB agar as the selective agar. The samples were incubated at 37°C for 24 h.
Statistical analysis.
Analysis of variance was performed on cell numbers by the general linear model procedure of SAS (28). The mean populations of three replicates were recorded and converted into logarithm values (log10) to determine the significance of differences (P < 0.05) by Duncan’s multiple-range test.
RESULTS
Recovery of heat-injured E. coli O157:H7 or S. typhimurium from buffered peptone water by the agar underlay method.
As a preliminary experiment, we evaluated the resuscitation times for recovery of heat-injured microorganisms by the agar underlay method (data not shown). At hourly intervals, 40 ml of SMAC (45 to 48°C) was poured into compartment B of an LP to evaluate the resuscitation time of a sublethally injured inoculum in the nonselective compartment A medium. We concluded that 2 h of resuscitation time on TSA was optimal for the agar underlay method for the two pathogens tested. Previously, a period of 2 h in a nutritionally rich nonselective medium has been shown to lead to the recovery of most injured bacterial cells (2, 24).
The number of viable cells of E. coli O157:H7 (ATCC 35150 and 43890) detected by the agar underlay method was not significantly different from recovery and growth observed with nonselective TSA medium (P > 0.05) (Table 1). Both the agar underlay method and the nonselective method recovered higher numbers of heat-injured E. coli O157:H7 cells than direct plating on the selective SMAC agar (P < 0.05). Heat-injured S. typhimurium (ATCC 19585 and UNL 10636-97) resuscitated and grew to equal levels by the agar underlay method and with nonselective TSA (P > 0.05). The recoveries determined by plating on TSA and plating with the agar underlay method with XLD agar were significantly higher than with direct plating on XLD agar (P < 0.05) (Table 2).
TABLE 1.
Recovery of heat-injured E. coli O157:H7 with SMAC, directly and with the agar underlay technique, compared to plating on nonselective TSA
| Recovery method | Log CFU/mla
|
|
|---|---|---|
| E. coli O157:H7 (ATCC 35150) | E. coli O157:H7 (ATCC 43890) | |
| TSA | 2.85 ± 0.16a | 2.59 ± 0.18a |
| SMAC, direct | 1.76 ± 0.14b | 2.01 ± 0.06b |
| SMAC, agar underlay | 2.74 ± 0.20a | 2.49 ± 0.20a |
Data represent means ± standard deviations of three measurements after heat treatment. Values followed by different letters are statistically different (P < 0.05).
TABLE 2.
Recovery of heat-injured S. typhimurium with XLD agar, directly and with the agar underlay technique compared to plating on nonselective TSA
| Recovery method | Log CFU/mla
|
|
|---|---|---|
| S. typhimurium (ATCC 19585) | S. typhimurium (UNL 10636-97) | |
| TSA | 2.65 ± 0.27a | 2.49 ± 0.18a |
| XLD, direct | 1.89 ± 0.26b | 1.83 ± 0.20b |
| XLD, agar underlay | 2.65 ± 0.20a | 2.45 ± 0.19a |
Data represent means ± standard deviations of three measurements after heat treatment. Values followed by different letters are statistically different (P < 0.05).
Recovery of heat-injured E. coli O157:H7 and S. typhimurium from beef trim by the agar underlay method.
The uninjured E. coli O157:H7 ATCC 43890 and S. typhimurium ATCC 19585 inoculated onto beef trim and subsequently heat injured were enumerated by the agar underlay method and nonselective agar plating. Prior to heat treatment, the recovery of E. coli and S. typhimurium on selective media was not significantly different, whether determined by the agar underlay method or by direct plating on SMAC or XLD agar for E. coli O157:H7 and S. typhimurium, respectively (P > 0.05) (Table 3). Following heat treatment, the recovery of E. coli O157:H7 by the agar underlay method with SMAC was significantly higher than by direct plating on SMAC (P < 0.05). The recovery of heat-injured S. typhimurium from the beef trim samples by the agar underlay system with XLD agar as the selective medium was significantly higher than recovery from direct plating on XLD agar (P < 0.05). Using XLD as the selective agar in the agar underlay system retained colony morphology selectivity to differentiate S. typhimurium from a mixture containing E. coli (typical appearance on XLD was observed).
TABLE 3.
Recovery of heat-injured E. coli O157:H7 and S. typhimurium from inoculated beef trim with TSA, selective media, and the agar underlay method with selective mediaa
| Time | Log CFU/g
|
|||
|---|---|---|---|---|
|
E. coli O157:H7
|
S. typhimurium
|
|||
| SMAC, direct | SMAC, agar underlay | XLD, direct | XLD, agar underlay | |
| Before heat treatment | 4.23 ± 0.26a | 4.48 ± 0.39a | 4.41 ± 0.43a | 4.57 ± 0.25a |
| After heat treatment | 2.66 ± 0.36b | 3.64 ± 0.16c | 2.59 ± 0.52b | 3.41 ± 0.28c |
Data represent means ± standard deviations of three measurements before or after treatment (80°C for 30 s). Values followed by different letters are statistically different (P < 0.05).
Agar underlay recovery of heat-injured fecal enterococci from bovine feces and coliforms from animal waste lagoon water.
Since much of the reported injury repair research employs laboratory-cultured microorganisms, we sought to test the efficacy of the agar underlay procedure to resuscitate sublethally injured bacterial cells of the major bacterial fecal indicator groups from naturally contaminated sources: fecal enterococci in bovine feces and coliforms from animal waste lagoons. Before heat treatment, the recovery rates of fecal enterococci (Table 4) by direct plating on KF Streptococcus agar, the overlay method with KF Streptococcus agar, and the agar underlay method with KF Streptococcus agar were not significantly different (P < 0.05). Following sublethal heat injury, the numbers of fecal enterococci recovered by the agar overlay and agar underlay methods were statistically higher than those recovered by direct plating on KF Streptococcus agar (P < 0.05).
TABLE 4.
Recovery of heat-injured fecal enterococci from cow feces with KF Streptococcus selective agar, directly and with the agar underlay methoda
| Time | Log CFU/ml
|
||
|---|---|---|---|
| KF, direct | KF, overlay | KF, agar underlay | |
| Before heat treatment | 5.67 ± 0.09a | 5.84 ± 0.16a | 5.76 ± 0.11a |
| After heat treatment | 4.58 ± 0.18b | 5.20 ± 0.32c | 5.14 ± 0.29c |
Data represent means ± standard deviations of three measurements before or after treatment (80°C for 30 s). Values followed by different letters are statistically different (P < 0.05).
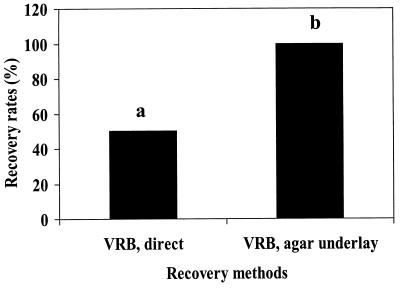
Hartman et al. (15) reported that coliform bacteria in freshwater lake samples can be injured by natural factors. Three different water samples were obtained from animal waste lagoons at MARC. The recovery of animal waste lagoon water coliforms by VRB direct plating was converted to percentages and compared to those of the agar underlay method with VRB (Fig. 1). The recovery rate of coliforms by VRB direct plating was lower than that of the VRB agar underlay method (P < 0.05). Only 50% of coliforms recovered by the VRB agar underlay method were recovered by direct plating on VRB.
FIG. 1.
Recovery of coliform bacteria from animal waste lagoons with VRB agar both directly and with the agar underlay method. Bars with different letters are statistically different (P < 0.05).
DISCUSSION
Several injury repair protocols have been published (5, 6, 15, 19, 23, 27, 31). Injury repair protocols are generally based on the principle that sublethally injured bacteria are more sensitive to selective agents (24, 25) and require a resuscitation step or growth period under nonselective conditions. Cole et al. (6) reported that substantial repair of injured cells can occur in a nonselective medium, such as TSB, within 1 h at 25°C. The disadvantage of this liquid method is that injured food-borne pathogens vary in the time required for repair, and therefore, uninjured and nontarget cells can multiply before the population of interest recovers. This liquid repair method would not be appropriate for direct enumeration. The thin agar overlay method for recovery of heat-injured food-borne pathogens is effective (15, 23) but has the following limitations: (i) picking isolated colonies that grow under the selective medium overlay is difficult and (ii) the temperature of the melted selective overlay agar (45 to 48°C) can further affect heat-injured target microorganisms being resuscitated on the nonselective agar.
As a preliminary experiment, we inoculated L. monocytogenes (Scott A) on an agar underlay plate with SMAC as the selective agar. L. monocytogenes was strongly inhibited by the SMAC agar underlay (data not shown). In addition, a mixture of non-O157 E. coli (known sorbitol positive) and E. coli O157:H7 (known sorbitol negative) was inoculated and cultured by the agar underlay procedure with SMAC as the selective agar. Colonies of sorbitol-positive cells (red colonies) and sorbitol negative cells (faint-pink colonies) were easily differentiated in the agar underlay system, whereas the agar overlay method precludes differentiation of E. coli (sorbitol positive) and E. coli O157:H7 (sorbitol negative) (data not shown). The agar underlay method eliminates any chance of further sublethal injury from heat contributed by molten agar, since the underlay is poured underneath the bottom surface of the inoculated agar (compartment A) and therefore the underlay cools quickly without increasing the temperature of the inoculated-agar surface.
Sage and Ingham (27) reported the use of hydrophobic grid membrane filtration to enumerate acid-injured E. coli O157:H7. The combination of hydrophobic grid membrane technology with the agar underlay method is just one possible configuration for using this versatile injury repair method. Recently, another injury recovery method, the thin agar layer method, was reported (19). The thin agar layer method covers a selective agar with a thin layer of a nonselective agar. Sublethally injured bacteria are then inoculated onto the top of the nonselective overlay. Although simple and rapid, the thin agar overlay method is limited by the rapid diffusion of selective agents into the nonselective agar overlay. The agar underlay method effects slower diffusion of selective agents from the selective agar underlay into the nonselective recovery agar than the thin agar overlay technique.
In conclusion, these data indicate that the agar underlay injury repair method is an efficient means for recovery and subsequent selective culture of sublethally heat-injured microorganisms, and the agar underlay technique offers several advantages over other injury repair techniques. We have not overlooked the potential for this method to be applied to other inimical processes that might result in sublethal cellular injury to bacteria, such as acid and freeze-thaw-induced sublethal injury. Even broader in scope might be the application of the agar underlay technique to other selective-agar-based microbial culture techniques which rely on growth media with selective agents that might be toxic to even a portion of uninjured target cells.
REFERENCES
- 1.Abdul-Raouf U M, Beuchat L R, Ammar M S. Survival and growth of Escherichia coli O157:H7 in ground roasted beef as affected by pH, acidulants, and temperature. Appl Environ Microbiol. 1993;59:2364–2368. doi: 10.1128/aem.59.8.2364-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews W H, Ray B. Importance and regulatory implication of the recovery of injured microorganisms from foods and water. In: Ray B, editor. Injured index and pathogenic bacteria: occurrence and detection in foods, water and feeds. Boca Raton, Fla: CRC Press Inc.; 1989. pp. 217–223. [Google Scholar]
- 3.Barkate M L, Acuff G R, Lucia L M, Hale D S. Hot water decontamination of beef carcasses for reduction of initial bacterial numbers. Meat Sci. 1993;35:397–401. doi: 10.1016/0309-1740(93)90044-I. [DOI] [PubMed] [Google Scholar]
- 4.Childers G W, Ricks G M, Nelson E T. Enhancement of opportunistic microorganisms due to pentachlorophenol in a controlled laboratory system. Dev Ind Microbiol. 1983;25:547–555. [Google Scholar]
- 5.Clavero M R S, Beuchat L R. Suitability of selective plating media for recovering heat- or freeze-stressed Escherichia coli O157:H7 from tryptic soy broth and ground beef. Appl Environ Microbiol. 1995;61:3268–3273. doi: 10.1128/aem.61.9.3268-3273.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole M B, Davies K W, Munro G, Holyoak C D, Kilsby D C. A vitalistic model to describe the thermal inactivation of Listeria monocytogenes. J Ind Microbiol. 1993;12:232–239. [Google Scholar]
- 7.Colwell F S, Speidel H K. Diffusion through a double-sided plate: development of a method to study alga-bacterium interactions. Appl Environ Microbiol. 1985;50:1357–1360. doi: 10.1128/aem.50.6.1357-1360.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conner D E, Hall G S. Efficacy of selected media for recovery of Escherichia coli O157:H7 from frozen chicken meat containing sodium chloride, sodium lactate or phosphate. Food Microbiol. 1994;11:337–344. [Google Scholar]
- 9.Cutter C N, Siragusa G R. Decontamination of beef carcass tissue with nisin using a pilot scale model carcass washer. Food Microbiol. 1994;11:481–489. doi: 10.4315/0362-028X-57.2.97. [DOI] [PubMed] [Google Scholar]
- 10.Cutter C N, Siragusa G R. Efficacy of organic acids against Escherichia coli O157:H7 attached to beef carcass tissue using a pilot scale model carcass washer. J Food Prot. 1994;57:97–103. doi: 10.4315/0362-028X-57.2.97. [DOI] [PubMed] [Google Scholar]
- 11.Davey K R, Smith M G. A laboratory evaluation of a novel hot water cabinet for the decontamination of sides of beef. Int J Food Sci Technol. 1989;24:305–316. [Google Scholar]
- 12.Dickson J S, Anderson M E. Control of Salmonella on beef tissue surfaces in a model system by pre- and post-evisceration washing and sanitizing, with and without spray chilling. J Food Prot. 1991;54:514–518. doi: 10.4315/0362-028X-54.7.514. [DOI] [PubMed] [Google Scholar]
- 13.Dickson J S, Siragusa G R. Survival of Salmonella typhimurium, Escherichia coli O157:H7, and Listeria monocytogenes during storage on beef sanitized with organic acids. J Food Safety. 1994;14:313–327. [Google Scholar]
- 14.Dorsa W J, Cutter C N, Siragusa G R, Koohmaraie M. Microbial decontamination of beef and sheep carcasses by steam, hot water spray washes, and a steam-vacuum sanitizer. J Food Prot. 1996;59:127–135. doi: 10.4315/0362-028X-59.2.127. [DOI] [PubMed] [Google Scholar]
- 15.Hartman P A, Hartman P S, Lanz W W. Violet Red Bile 2 Agar for stressed coliforms. Appl Microbiol. 1975;29:537–539. doi: 10.1128/am.29.4.537-539.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartung M. Occurrence of enteritis-causing salmonellae in food and in domestic animals in 1991. DTW Dtsch Tierartzl Wochenschr. 1993;100:259–261. [PubMed] [Google Scholar]
- 17.Hurst A. Bacterial injury: a review. Can J Microbiol. 1977;23:936–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- 18.Jay J M. Modern food microbiology. 3rd ed. New York, N.Y: Van Nostrand Reonhold; 1986. p. 100. [Google Scholar]
- 19.Kang, D. H., and D. Y. C. Fung. Thin agar layer method for recovery of heat-injured Listeria monocytogenes. J. Food Prot., in press. [DOI] [PubMed]
- 20.Kang D H, Fung D Y C. Development of a medium for differentiation between Escherichia coli and Escherichia coli O157:H7. J Food Prot. 1999;62:313–317. doi: 10.4315/0362-028x-62.4.313. [DOI] [PubMed] [Google Scholar]
- 21.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy J, Holbrook R, Stephens P J. An improved direct plate method for the enumeration of stressed Escherichia coli O157:H7 from food. J Food Prot. 1998;61:1093–1097. doi: 10.4315/0362-028x-61.9.1093. [DOI] [PubMed] [Google Scholar]
- 23.McCleer D R, Rowe M T. Development of a selective plating technique for the recovery of Escherichia coli O157:H7 after heat stress. Lett Appl Microbiol. 1995;21:252–256. doi: 10.1111/j.1472-765x.1995.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 24.Ray B. Impact of bacterial injury and repair in food microbiology; its past, present and future. J Food Prot. 1986;49:651–655. doi: 10.4315/0362-028X-49.8.651. [DOI] [PubMed] [Google Scholar]
- 25.Ray B, Adams D M. Repair and detection of injured microorganisms. In: Speck M L, editor. Compendium of methods for the microbiological examination of foods. Washington, D.C.: American Public Health Association; 1984. pp. 112–113. [Google Scholar]
- 26.Rochelle M, Clavero S, Beuchat L R. Survival of Escherichia coli O157:H7 in broth and processed salami as influenced by pH, water activity, and temperature and suitability of media for its recovery. Appl Environ Microbiol. 1996;62:2735–2740. doi: 10.1128/aem.62.8.2735-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sage J R, Ingham S C. Evaluating survival of Escherichia coli O157:H7 in frozen and thawed apple cider: potential use of a hydrophobic grid membrane filter-SD-39 agar method. J Food Prot. 1998;61:490–494. doi: 10.4315/0362-028x-61.4.490. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute. SAS users guide. 6th ed. Cary, N.C: SAS Institute; 1991. [Google Scholar]
- 29.Siragusa G R. The effectiveness of carcass decontamination systems for controlling the presence of pathogens on the surfaces of meat animal carcasses. J Food Safety. 1995;15:229–238. [Google Scholar]
- 30.Sockett P N, Cowden J M, Lebaigue S, Ross D, Adak G K, Evans H. Foodborne disease surveillance in England and Wales: 1989–1991. Commun Dis Rep Rev. 1993;3:R159–R173. [PubMed] [Google Scholar]
- 31.Speck M L, Ray B, Read R B., Jr Repair and enumeration of injured coliforms by a plating procedure. Appl Microbiol. 1975;29:549–550. doi: 10.1128/am.29.4.549-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao T, Doyle M P, Besser R. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]