Abstract
Bipolar Disorder (BD) is a mental disorder characterized by periods of depression and abnormally elevated moods. Recent studies proposed the existence of a correlation between inflammation, disease severity and response to antipsychotic therapy. The present study is aimed to investigate if treatment with second-generation antipsychotic, in monotherapy, influences the inflammatory process in BD patients. In 50 hospitalized BD patients who started monotherapy with second-generation antipsychotic, we investigated, after six-week of treatment, both clinical outcomes and change in inflammatory markers such as C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR). We observed a significant improvement of clinical symptoms (measured through MADRAS, YMRS, CGI and BPRS scales) in all treated patients. Moreover, we found that at the time of enrolment BD patients showed higher CRP levels compared to reference value, and that after 6 weeks of antipsychotic treatment CRP (but not ERS) plasma levels were significantly reduced returning to reference levels. The present exploratory study indicates that monotherapy with antipsychotic drugs reduces, not only BD symptoms, but also an inflammatory marker such as PCR. The evaluation of relationship between antipsychotic treatment and patients inflammatory conditions could be usefulness in clinical practice, both providing a marker to drug response, and permitting the identification of new targets in BD therapy.
Keywords: antipsychotic drugs, inflammation, bipolar disorder, CRP, ERS
Introduction
Bipolar Disorder (BD) is a psychiatric illness characterized by two or more episodes in “which the patient’s mood and activity levels are significantly disturbed” (ICD10, international classification of diseases). BD affects about 2% of the world’s population, with subthreshold forms of the disorder affecting another 2%.1 Starting in the 1970s, lithium has become the standard treatment for BD but despite his effectiveness, his use in clinical practice is diminished in favor of other agents such as second-generation antipsychotics.2 Atypical antipsychotics are indeed the first line medication used to treat BD, both for acute and chronic phases.3 However, even with treatment, about 37% of patients relapse into depression or mania within 1 year, and 60% within 2 years.4 Data of literature propose that the heterogeneity in antipsychotic treatment response may be due to the multifactorial nature of BD that include genetic, epigenetic and environmental factors.5–6 Several studies deepened the involvement of immune system in psychiatric diseases. For example, it has been shown that there is an increased risk for schizophrenia after a hospitalization for infection.7 In this regard it has been hypnotized that infectious agents could cause psychosis both via a direct noxious effect on neurons and brain structures and via an indirect effect mediated by activation of the immune system evoked by the infectious agent.8,9 The increasing knowledge about the neurobiology of BD suggest a central role of inflammatory mechanisms in the etiopathogenesis, as well as in therapy response in this, and other psychiatric disease such as depression.10–14
So far, several inflammatory markers, such as monocytosis, high-inflammatory gene expression, raised glucocorticoids levels, receptorβ/glucocorticoid receptor ratio and levels of pro- and anti-inflammatory cytokines, were looked in psychotic patients.15 In particular, for BD, recent studies have shown a correlation between inflammatory markers, such as reactive C-protein (CRP) and Erythrocyte Sedimentation Rate (ESR) and severity of this disease.16–17 Additional evidences about a central role of inflammation in BD and depression comes from data showing that during antidepressive and antipsychotic treatment, beside to a general improvement of the patient’s clinical condition, is observed a reduction of some inflammatory markers, such as IL-6 and IL-10.18–19 These clinical data are supported by preclinical studies showing how antipsychotic agents such as olanzapine, clozapine, quetiapine and its metabolite norquetapine, modulate the activation of the immune system and the inflammatory reaction through still largely unknown mechanisms.20–22
So far, however the effects of antipsychotic treatment on inflammatory process in BD patients it is not fully defined, and, in any case, these results are provided from studies in subjects undergoing poly-therapy. For this reason, the aim of present study is to investigate if treatment with a single second-generation of antipsychotic, together with clinical improving, could influences the inflammatory process in BD patients. To this end, we investigated, in a cohort of hospitalized BD patient’s, the effects of six weeks treatment with antipsychotics in monotherapy on clinical response and on inflammatory markers including CRP and ERS.
Methods
Patients Enrolment
We enrolled, from January 2016 to March 2017, a group of 50 hospitalized drug naïve patients that started therapy with atypical antipsychotic drugs as oral monotherapy. All patients were enrolled consecutively at Cantonal Psychiatric Clinic—Mendrisio (CH), and BD diagnosis was performed by the ICD10 classification (depressive episodes, manic episodes, mixed state WHO, 2010) according to the correspondent diagnosis of DSM 5. Patient’s therapy adherence was guaranteed by hospitalization and administration of drugs by qualified personnel.
The study has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans subjects and according to the Swiss guidelines of clinical management of these kind of subjects (Human Research Act, HRA) and approved by the local ethic committee (Cantonal Psychiatric Clinic—Mendrisio, CH) on November 20, 2015.
Inclusions criteria were: age over 18 years, diagnosis of BD according to ICD10 and confirmed through the Structured Clinical Interview SCID-P for mood disorders; indications at pharmacological treatment with second generations antipsychotic in monotherapy, and the possibility for all the subjects enrolled to give written informed consent before enrolment. Exclusions criteria were: intellectual disability, schizophrenia and other psychotic symptoms such as delirium and hallucinations, combined pharmacotherapy with mood stabilizers or typical and atypical antipsychotics, comorbidities requiring continuous pharmacological treatments, presence of inflammatory diseases, pregnancy, lack of informed consent.
All patients were visited three times: at moment of the inclusion in the study (T0) and after three (T1) and six (T2) weeks of treatment. At each visit, all subjects were evaluated for clinical conditions by using clinical psychopathological scales: I) depressive symptoms by hetero-administration Montgomery-Asberg Depression Scale (MADRS), this is a ten-item diagnostic questionnaire which used to measure the severity of depressive episodes in patients with mood disorders. The overall score ranges from 0 to 60. Higher MADRS score indicates more severe depression;23 II) mania symptoms by Young Mania Rating Scale (YMRS), this is the most used scale for maniac symptoms. 55% reduction in the scoring is generally used as a cut off for the response to a therapy;24 III) severity of illness by Clinical Global Impression (CGI) this scale has two components—the CGI-Severity (CGI-S), which rates illness severity is rated on a 1 (normal) to 7 (severely ill) scale and the CGI-Improvement (CGI-I), which evaluate change from the initiation (baseline) of treatment and ranges from 1 (very much improved) to 7 (very much worsen)25 and IV) psychiatric symptoms by Brief Psychiatric Rating Scale (BPRS) this scale explores 24 psychopathological domain by mean of guide questions; for each domain the clinician has to attribute a score from 1 (normal ) to 7 (most severely ill).26
Blood Sample Collection and Inflammatory Markers Evaluation
At each visit time (T0, T1 and T2), in the morning, after a fasting night, between 8:00 and 9:00 AM, a blood sample was taken (by use of heparinized vacuum tubes) and used for the analysis of complete blood formula including total count of white blood cells (WBC), lymphocytes (Ly) and neutrophils (Ne), and the inflammatory markers high-sensitivity CRP (hs-CRP), and ERS. For each patients several biochemical parameters such as creatinine, thyroid-stimulating hormone, glycaemia and blood formula, was also collected.
hs-CRP plasma levels was measured by an enzyme immunoassay (Abbott Diagnostics, Abbott Park, Illinois), the detection limit of the assay was 0.2 mg/liter. ERS expressed in millimeter/hour, was measured using non-haemolysed EDTA-anticoagulated whole blood that was analysed following the Westergren method (StaRRsed Auto Compact VERA109900, Mechatronics BV, Hoorn, the Netherlands). The analysis was performed by the Cantonal Psychiatric hospital clinical analysis laboratory—Mendrisio (CH).
Statistical Analysis
Data are presented as mean ± standard deviation (SD) or median with interquartile range. Data analysis was performed with the Friedmann’s global test. Wilcoxon’s test was used to compare analysed parameters at the different visits. Since additional comparisons have been carried out for every parameter, Bonferroni’s correction was added to the initial data analysis.
The clinical effects of antipsychotic drugs and the modifications in levels of inflammatory markers are respectively expressed as variation (Δ) between psychopathological scale score and plasma levels of the markers measured (comparison: T1–T0; T2–T0). Correlation analysis between selected variables was performed by linear regression analysis. Calculations were performed using commercial software (GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, California, USA, www.graphpad.com).
Results
Patients Characteristics and Clinical Response to Antipsychotic Drugs
Patients clinical characteristics and drug treatment were reported in Table 1. As shown, the enrolled patients include 31 female and 19 males. 38 of the enrolled subjects were smokers and 16 alcohol consumers. In Table 2 are reported the biochemical parameters (creatinine, thyroid-stimulating hormone, glycaemia and blood formula) and blood cells count (red blood cells, white blood cells, lymphocytes, neutrophils and ratio neutrophils/lymphocytes) measured at T0 (enrolment) and after 3 (T1) or 6 (T2) weeks of antipsychotic treatment. As shown, no significant changes occur during therapy for all the above mentioned parameters.
Table 1. Epidemiological Characteristics of the Patients Included in the Study and their Antipsychotic Treatment.
| Number of Patients (n) | 50 |
| Age (years; mean ± SD) | 41.3 ±14.6 |
| Gender (F/M) | 31/19 |
| Smokers (F/M) | 23/15 |
| Alcohol (F/M) | 8/8 |
| Drug Treatment | |
| Olanzapine (n) | 11 |
| Asenapine (n) | 5 |
| Quetiapine (n) | 14 |
| Risperidon (n) | 10 |
| Aripiprazole (n) | 10 |
Table 2. Biochemical Parameters and Blood Cells Count in BP Patient at Time of Enrolment (T0) and After 3 (T1) and 6 (T2) Weeks of Treatments.
| T0 | T1 | T2 | ||||
| Biochemical Parameters (mean ± SD) | ||||||
| Creatinine (mg/dl) | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.0 ± 1.0 | |||
| TSH (mUI/ml) | 2.0 ± 0.9 | 2.1 ± 1.3 | 2.0 ± 0.9 | |||
| Glycemia (mg/dl) | 78.6 ± 8.9 | 80.5 ± 8.5 | 82.4 ± 8.5 | |||
| Bold Cell Count (mean ± SD) | ||||||
| RBC (106 cell/ml) | 5.1 ± 0.3 | 5.1 ± 0.4 | 5.1 ± 0.3 | |||
| WBC (106 cell/ml) | 8.9 ± 1.2 | 8.8 ± 1.3 | 8.6 ± 1.2 | |||
| Ly (106 cell/ml) | 1.8 ± 0.4 | 1,7 ± 0.4 | 1.7 ± 0.4 | |||
| Ne (106 cell/ml) | 6.0 ± 1.1 | 5.9 ± 1.1 | 5.8 ± 1.1 | |||
| RNL | 3.6 ± 1.3 | 3.6 ± 1.3 | 3.7 ± 1.4 | |||
All the data are presented as mean ± SD.
TSH, thyroid-stimulating hormone; RBC, red blood cells; WBC, white blood cells; Ly, lymphocytes; Ne, neutrophils; RNL, ratio neutrophils lymphocytes.
We observed a significant improvement of clinical symptoms in all enrolled patients. In particular, for MADRAS and YMRS scale, we found a significant score reduction after 3 weeks of treatment without any significant further reduction, whereas for CGI and BPRS we found a significant score reductions after 3 weeks with further significant improvement after 6 weeks (Table 3). Finally, we did not find significant differences in treatment response between different antipsychotic used, each of them in monotherapy during the whole period (data not shown).
Table 3. Psychopathological Scale Score at Time of Enrolment (T0) and After 3 (T1) and 6 (T2) Weeks of Treatments.
| T0 | T1 | T2 | |
| MADRAS | 9.6 ± 10.8 | 5.4 ± 6.9* | 3.0 ± 3.8*** |
| YMRS | 14.2 ± 13.3 | 7.4 ± 10.9** | 4.1 ± 8.1*** |
| CGI | 3.7 ± 1.2 | 2.2 ± 1.1*** | 1.5 ± 0.8***## |
| BPRS | 83.6 ± 27.2 | 67.8 ± 19.5** | 56.8 ± 16.7***# |
All the data are presented as mean ± SD. *P < 0.05; **P < 0.01 vs T0, ***P < 0.001 vs T0, #P < 0.05 vs T1, ##P < 0.01 vs T1.
Effects of Antipsychotic Treatment on Inflammatory Parameters
Compared to laboratory reference values (0–3 mg/L), at time of enrollment, patients show higher CRP levels (14.6 ± 10.0 mg/L). After 3 weeks of antipsychotic treatment CRP values was significantly reduced, and the reduction was maintained after 6 weeks of antipsychotic treatment, (Figure 1, right panel).
Figure 1.
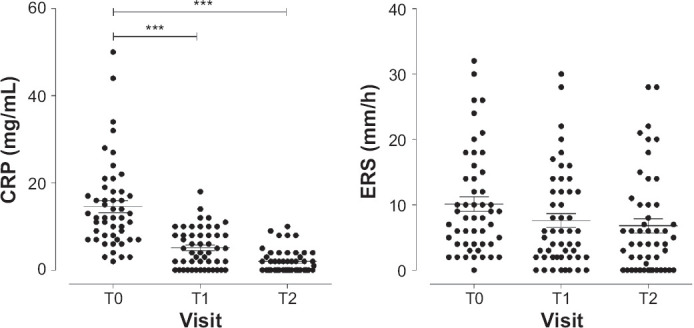
CRP (right panel) and ERS (left panel) Plasma Levels in BD Patients Treated with Antipsychotic Drugs at Time of Enrolment (T0) and After 3 (T1) and 6 (T2) Weeks of Treatments
Data were reported as mean with interquartile range. ***P < 0.001 vs T0.
On the contrary the ERS values were in normal range at time of enrollment (laboratory reference: 10.1 ± 7.9 mm/h; patient values: 10.2 ± 8.0 mm/h) and were unaffected by drug treatment (Figure 1, left panel).
Stratifying patients by gender, smoke habitude and alcohol consuming, we did not find any significant differences nor for CRP neither ERS values (data not shown).
Correlations Between Antipsychotic Clinical Response and Inflammatory Markers
Our results shown a significant correlation between improvement of clinical response (expressed as reduction in psychopathological BPRS and YMRS scales score—Δ = T2–T0), and CRP (expressed as reduction of CRP plasma levels—Δ = T2–T0) after treatment for 6 weeks (Figure 2). On the contrary, we did not found a significant relationship between CRP and clinical response evaluated by MADRS or CGI psychopathological scales. Finally, no correlations between ERS and antipsychotic treatment was found (data not shown).
Figure 2.
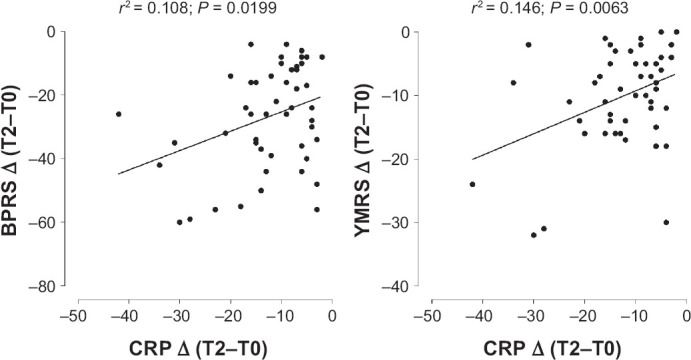
Correlation Between Response to Antipsychotic Treatment, Evaluated by BPRS (right panel) and YMRS (left panel) Psychopathological Scale, and CRP Plasma Levels Variations (Δ = T2–T0)
Clinical response were expressed as variation (Δ = T2–T0) psychopathological scale score. The data are presented as regression line of the correlation (solid) together with 95% of confidence interval (CI; dotted).
Discussion
The main result of the present study is that monotherapy with atypical antipsychotic drugs, not only improves clinical symptoms, but also reduces the inflammatory marker CRP in BD patients, thus suggesting that inflammation play a crucial role in this disease and that antipsychotic drugs may improve clinical outcome trough a modulation of inflammatory pathways.
We did not found differences in treatment response between different antipsychotic and this is in agreement with previously reported results suggesting that different antipsychotics lead to a similar response in BD treatment.27 However, if clinical effects were similar the response onset time was different. In particular, for MADRAS and YMRS scale, we found a significant score reduction after 3 weeks of treatment without any significant further reduction, whereas for CGI and BPRS we found a significant score reductions after 3 weeks with further significant improvement after 6 weeks of treatment. These data are in line with other study on efficacy for atypical antipsychotics in the treatment of acute BD,28–30 even if, our BD patients were all in monotherapy, while in clinical practice, BD patients are usually treated with a combination antipsychotic drugs.
BD has an inflammatory background and evidences from several studies suggest a central role for immuno-inflammatory mechanisms in etiopathogenesis of this illness.11–13 In this study, we found higher CRP plasmatic levels compared to standard references values before starting antipsychotic therapy. This is in agreement with previous study by Dickerson that found CRP levels higher in 229 bipolar patients compared to 280 control subjects.17 Interesting, we found that 3 weeks of antipsychotic treatment significantly reduced CRP plasma levels, with further reduction after 6 weeks of treatment. Moreover, we found a direct correlation between reductions in YMRS and PBRS score (but not with MADRS or CGI) and reductions in CRP plasma levels. A working hypothesis to explain this discrepancy could be that inflammatory conditions is more strictly related with positive symptoms, that were better evaluated by PBRS and YMRS scale rather that negative one. Of course, this consideration must be confirmed by specifically studies aimed to clarify the role of inflammatory conditions in patients with psychosis associated with positive symptoms.
Although some studies suggest that psychiatric diseases are related to high levels of inflammatory markers,15 nothing is reported in literature about the mechanism by which this conditions influence therapy response in bipolar patients. For this reason only speculative hypothesis, relying on indirect evidence, can be presented for explain about the role of inflammation in PB. For example, it has been shown that some genes implicated in psychiatric disease, such as schizophrenia, could be correlated with genes encoding for proteins involved in inflammation process, such as some cytokines and tumour necrosis factor alpha.31 This observation allow us to speculated that gene(s) associated with BD could be in linkage disequilibrium with gene(s) involved in immune response and inflammation process thus influencing CRP production.
In any cases, the correlation between clinical response to antipsychotic drug and CRP plasma levels could be an interesting start point in identification of prognostic markers for response to therapy in BD. Moreover, a potentially very interesting aspect, currently still just speculative, is given by possible direct inhibitory effects that antipsychotics could have on inflammatory process that, in turn, could improve, in an independent way, BD clinical symptoms.
We are conscious that the present study presents some limits, in particular the small number of enrolled patients and the lack of healthy subjects control group. However, in this regard, it is important to note that we have selected only patients drug naïve hospitalized and in monotherapy, so avoiding the possible confounding factor due to treatment with more drugs, very common in this disease. Moreover, the enrollment of hospitalized patients gives additional insurance on compliance with therapy. We are confident that this exploratory study will be confirmed in a study including a greater number of subjects. In any case, the present results allow to identify, in patients treated with antipsychotic agents in monotherapy, a correlation between drug treatment response and improvement of inflammatory parameters, thus increasing the understanding of antipsychotics role in modulation of inflammatory processes in BD. The evaluation of the relationship between response to antipsychotic treatment and patients inflammatory conditions could be usefulness in clinical practice, both by providing a possible marker to drug response, and permitting the identification of new targets and new therapies in BD treatment.
Footnotes
Funding
This study was performed during the PhD program of MG and part of the study was present in their PhD thesis. The study was supported by local grant of the hospital (Cantonal Psychiatric Clinic—Mendrisio (CH)) and by funds available for PhD student from the University of Insubria (fondi di dottorato a.a 2016–2019) to MG. The study was nonprofit.
Conflict of Interest Disclosures
The authors declare that they have no competing financial interests.
Authorship Contributions
Study conception and design: MG, FM and MC. Acquisition of data: MG, MF. Data handling and results: MG, MF, MC, FM. Clinical protocol and data analysis and interpretation: CC and MG.
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and declare to have confidence in the integrity of the contributions of their co-authors.
References
- 1.Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, Viana MC, Andrade LH, Hu C, Karam EG, Ladea M, Medina-Mora ME, Ono Y, Posada-Villa J, Sagar R, Wells JE, Zarkov Z. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry . 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagani R, Gasparini A, Ielmini M, Caselli I, Poloni N, Ferrari M, Marino F, Callegari C. Twenty years of Lithium pharmacogentics: A systematic review. Psychiatry Res . 2019;278:42–50. doi: 10.1016/j.psychres.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. APA practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry . 2002;159(4 Suppl):1–50. [PubMed] [Google Scholar]
- 4.Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet . 2013;381:1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolla E, Bortolaso P, Ferrari M, Poloni N, Callegari C, Marino F, Lecchini S, Vender S, Cosentino M. Are CYP1A2*1F and *1C associated with clozapine tolerability? a preliminary study. Psychiatry Res . 2011;189(3):483. doi: 10.1016/j.psychres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Salagre E, Dodd S, Aedo A, Rosa A, Amoretti S, Pinzon J, Reinares M, Berk M, Kapczinski FP, Vieta E, Grande I. Toward Precision Psychiatry in Bipolar Disorder: Staging 2.0. Front Psychiatry . 2018;29(9):641. doi: 10.3389/fpsyt.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry . 2008;13(5):470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- 8.Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psyciatry . 2011;9(35):760–768. doi: 10.1016/j.pnpbp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Petito CK, Torres-Muñoz JE, Zielger F, McCarthy M. Brain CD8+ and cytotoxic T lymphocytes are associated with, and may be specific for, human immunodeficiency virus type 1 encephalitis in patients with acquired immunodeficiency syndrome. J Neurovirol . 2006;12(4):272–283. doi: 10.1080/13550280600879204. [DOI] [PubMed] [Google Scholar]
- 10.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol . 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry . 2009;70(8):1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 12.Halaris A, Leonard BE. Inflammation in Psychiatry. Mod Trends Pharmacopsychiatry . 2013;28:VII–VIII. [PubMed] [Google Scholar]
- 13.Altamura AC, Buoli M, Pozzoli S. Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci . 2014;68(1):21–36. doi: 10.1111/pcn.12089. [DOI] [PubMed] [Google Scholar]
- 14.Liu HC, Yang YY, Chou YM, Chen KP, Shen WW, Leu SJ. Immunologic variables in acute mania of bipolar disorder. J Neuroimmunol . 2004;150(1–2):116–122. doi: 10.1016/j.jneuroim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Bergink V, Gibney SM, Drexhage HA. Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry . 2014;75(4):324–331. doi: 10.1016/j.biopsych.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment. Psychopharmacology (Berl) . 2015;233(9):1575–1589. doi: 10.1007/s00213-015-4044-5. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson F, Katsafanas E, Schweinfurth LA, Savage CL, Stallings C, Origoni A, Khushalani S, Lillehoj E, Yolken R. Immune alterations in acute bipolar depression. Acta Psychiatr Scand . 2015;132(3):204–210. doi: 10.1111/acps.12451. [DOI] [PubMed] [Google Scholar]
- 18.Haring L, Koido K, Vasar V, Leping V, Zilmer K, Zilmer M, Vasar E. Antipsychotic treatment reduces psychotic symptoms and markers of low-grade inflammation in first episode psychosis patients. Schizophr Res . 2015;169(1–3):22–9. doi: 10.1016/j.schres.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun . 2012;26(7):1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Bang J, Chang HW, Kim JY, Park KU, Kim SH, Lee KJ, Cho CH, Hwang I, Park SD, Ha E, Jung SW. Anti-inflammatory effect of quetiapine on collagen—induced arthritis of mouse. Eur J Pharmacol . 2012;678:55–60. doi: 10.1016/j.ejphar.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Jaehne EJ, Corrigan F, Toben C, Jawahar MC, Baune BT. The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol Biochem Behav . 2015;135:136–144. doi: 10.1016/j.pbb.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Egea E, Miller B, Garcia-Rizo C, Bernardo M, Kirkpatrick B. Metabolic effects of olanzapine in patients with newly diagnosed psychosis. J Clin Psychopharmacol . 2011;31(2):154–159. doi: 10.1097/JCP.0b013e31820fcea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montgomery SM. 1979 Depressive symptoms in acute schizophrenia. Prog Neuropsychopharmacol . 2011;3(4):429–433. doi: 10.1016/0364-7722(79)90058-4. [DOI] [PubMed] [Google Scholar]
- 24.Lukasiewicz M, Gerard S, Besnard A, Falissard B, Perrin E, Sapin H, Tohen M, Reed C, Azorin JM. Emblem Study Group. Young Mania Rating Scale: how to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res . 2013;22(1):46–58. doi: 10.1002/mpr.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) . 2007;4(7):28–37. [PMC free article] [PubMed] [Google Scholar]
- 26.Gorham DR, Overall JE. Dimensions of change in psychiatric symptomatology. Dis Nerv Syst . 1961;22:576–80. [PubMed] [Google Scholar]
- 27.Ertugrul A, Meltzer HY. Antipsychotic drugs in bipolar disorder. Int J Neuropsychopharmacol . 2003;6(3):277–284. doi: 10.1017/S1461145703003560. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre RS, Konarski JZ. Tolerability profiles of atypical antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry . 2005;66(Suppl 3):28–36. [PubMed] [Google Scholar]
- 29.Vieta E, Panicali F, Goetz I, Reed C, Comes M, Tohen M. EMBLEM Advisory Board. 2008. Olanzapine monotherapy and olanzapine combination therapy in the treatment of mania: 12-week results from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM) observational study. J Affect Disord . 2008;106(1–2):63–72. doi: 10.1016/j.jad.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Cruz N, Sanchez-Moreno J, Torres F, Goikolea JM, Valentí M, Vieta E. Efficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: a meta-analysis. Int J Neuropsychopharmacol . 2010;13(1):5–14. doi: 10.1017/S1461145709990344. [DOI] [PubMed] [Google Scholar]
- 31.Carter CJ. Schizophrenia susceptibility genes directly implicated in the life cycles of pathogens: cytomegalovirus, influenza, herpes simplex, rubella, and Toxoplasma gondii. Schizophr Bull . 2009;35(6):1163–1182. doi: 10.1093/schbul/sbn054. [DOI] [PMC free article] [PubMed] [Google Scholar]


