ABSTRACT
Objectives:
The coronavirus disease 2019 (COVID-19) pandemic has drastically altered endoscopic practices. We initially reported the international impact of COVID-19 on pediatric endoscopic practice. This follow-up study aimed to assess changes 7 months following the initial survey to delineate practice change patterns as the pandemic evolved.
Methods:
Pediatric gastroenterologists who responded to the initial survey were re-surveyed seven months later using Research Electronic Data Capture (REDCap). The survey recorded information on changes in pediatric endoscopic practice patterns, including COVID-19 screening and testing processes and personal protective equipment (PPE) utilization. Additionally, endoscopists’ risk tolerance of COVID-19 transmission was evaluated.
Results:
Seventy-five unique institutions from 21 countries completed surveys from the 145 initial responses (51.7% response rate). Procedural volumes increased at most institutions (70.7%) and most were performing previously postponed cases (90.7%). Ninety-seven percent of institutions were performing pre-endoscopy screening with 78.7% testing all patients. Many institutions (34.7%) have performed procedures on COVID-19 positive patients. There was significantly less PPE reuse (P < 0.05) and fewer institutions recommending full PPE for all endoscopies (43.2% vs 59.2%, P = 0.013). Overall, pediatric endoscopists’ risk tolerance of COVID-19 transmission is low.
Conclusions:
This is the first survey to highlight the evolution of pediatric endoscopic practices related to the COVID-19 pandemic, underscoring the need for ongoing pandemic-related guidance for pediatric endoscopic practice.
Keywords: coronavirus disease 2019, gastrointestinal endoscopy, severe acute respiratory syndrome coronavirus 2
What Is Known
The practice of medicine continues to change as the coronavirus disease 2019 pandemic evolves
The impact of coronavirus disease 2019 on pediatric endoscopic practice was previously assessed at the beginning of the pandemic; however, its evolution has not been assessed.
What Is New
Procedural volumes have increased at most institutions with the majority now performing elective and emergent/urgent pediatric endoscopy procedures.
More institutions are performing pre-endoscopy severe acute respiratory syndrome coronavirus 2 testing with plans in place for performing endoscopy on coronavirus disease 2019-positive patients.
There is less reuse of personal protective equipment as availability has increased and fewer institutions are recommending full personal protective equipment for all endoscopies.
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has impacted the practice of medicine significantly. This is particularly prominent in procedural-focused specialties, such as gastroenterology (1).
Gastrointestinal endoscopy is an aerosol-generating procedure with the potential for viral transmission (2). The virus has also been found in feces, independent of the presence of diarrhea, for up to 126 days after symptom onset and may spread through fecal aerosol transmission (3). This increases the risk of COVID-19 exposure for both patients and healthcare professionals working in endoscopy given the close physical proximity between patients and personnel as well as the high-risk of exposure to bodily fluids during procedures. Compounding this, during the early months of the pandemic, there were critical supply shortages of personal protective equipment (PPE) that limited the capacity of hospitals to function optimally (4). As a result, many gastroenterology organizations published recommendations outlining best practices in performing endoscopy safely in the context of the COVID-19 pandemic to help mitigate the risk to patients, caregivers, and staff while preserving supplies of PPE (5,6).
Pediatric endoscopy has additional considerations that effect the risk of exposure to SARS-CoV-2, including differing epidemiology of gastrointestinal diseases in children (7), differences in anesthesia practice (8), and a higher incidence of mild or asymptomatic disease as compared with adults (9). We were the first to report on the impact of COVID-19 on pediatric endoscopy practice at the beginning of the pandemic (10). Our initial study demonstrated marked decreases in pediatric endoscopy volumes to less than 10% of normal at most institutions with significant variations in the use of pre-procedure testing and PPE precautions during endoscopy. As new evidence has emerged and vaccine availability has improved, it is important to understand the continued impact of the COVID-19 pandemic on pediatric endoscopy services. Therefore, we aimed to explore the evolution of the impact of COVID-19 7 months following an initial study on pediatric endoscopic practice worldwide.
METHODS
This survey-based descriptive study of pediatric endoscopy practices worldwide was conducted in November 2020. The study received ethical approval of the Baylor College of Medicine Institutional Review Board.
Participants
The target population for the study was pediatric gastroen-terologists who were members of European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) who had responded to an initial survey examining the impact of COVID-19 on pediatric endoscopic practice worldwide at the start of the pandemic in April 2020 (10).
Survey Instrument
The survey instrument consisted of 4 sections. The first collected institutional demographic information. The second and third sections evaluated changes in endoscopic practice related to the COVID-19 pandemic, and SARS-CoV-2 screening and testing practices, respectively. The fourth section probed changes in PPE practices. Additionally, to evaluate pediatric endoscopists’ tolerance of risk of COVID-19 transmission, a question modified from an American Gastroenterological Association (AGA) questionnaire was utilized (5). Respondents were asked to indicate the threshold for the risk of COVID transmission they were willing to assume as the endoscopist for an asymptomatic pediatric patient undergoing elective endoscopy. The answer choices included a risk of transmission of SARS-CoV-2 of 1 of 200, which corresponds to a willingness to perform endoscopy in a patient with no PPE and no pre-endoscopy testing, 1 of 500 (surgical mask, no pre-endoscopy testing), 1 of 2000 (N95 mask, no pre-endoscopy testing), 1 of 5000 (surgical mask, negative SARS-CoV-2 pre-endoscopy testing), 1 of 10,000, 1 of 20,000 (N95 mask, negative SARS-CoV-2 pre-endoscopy testing), and 1 of 40,000. The choices were based on the following assumptions: well-established tests are never 100% accurate; a local prevalence of asymptomatic COVID-19 of 1%; patients are screened using Center for Disease Control's symptom screening checklist; a baseline risk of transmission of 50% if an endoscopist performs endoscopy without PPE in a patient with COVID-19; and a reduction of risk of transmission with PPE to 20% with a surgical mask, and 5% with a N95 and face shield.
Data Collection
Endoscopists who responded to the initial survey in April 2020 were sent an email invitation containing a direct link to the follow-up electronic survey. To maximize response rates, data collection remained open for 30 days and up to 2 reminders were sent. Respondents were asked to speak with their Division leadership to ensure submission of accurate information. Survey data were collected and managed using Research Electronic Data Capture (REDCap Software V9.5.11 2020 Vanderbilt University).
Data Analysis
Data were analyzed using descriptive statistics with continuous variables summarized using means and standard deviations and categorical variables summarized using proportions. Wherever applicable, responses at the beginning of the pandemic were compared to responses 7 months later using Student t test. GraphPad Prism 8 (Prism Software San Diego, CA) was used for statistical analysis.
RESULTS
Surveys from 75 unique institutions worldwide were completed, representing 51.7% of the 145 initial responses. Respondents were from 21 nations with 45 (60%) from North America, 17 (22.7%) from Europe, and 13 (17.3%) from other regions. The majority (n = 53/75, 70.7%) reported an increased number of confirmed COVID-19 cases compared with April 2020. Institutional characteristics are detailed in Table 1, Supplemental Digital Content.
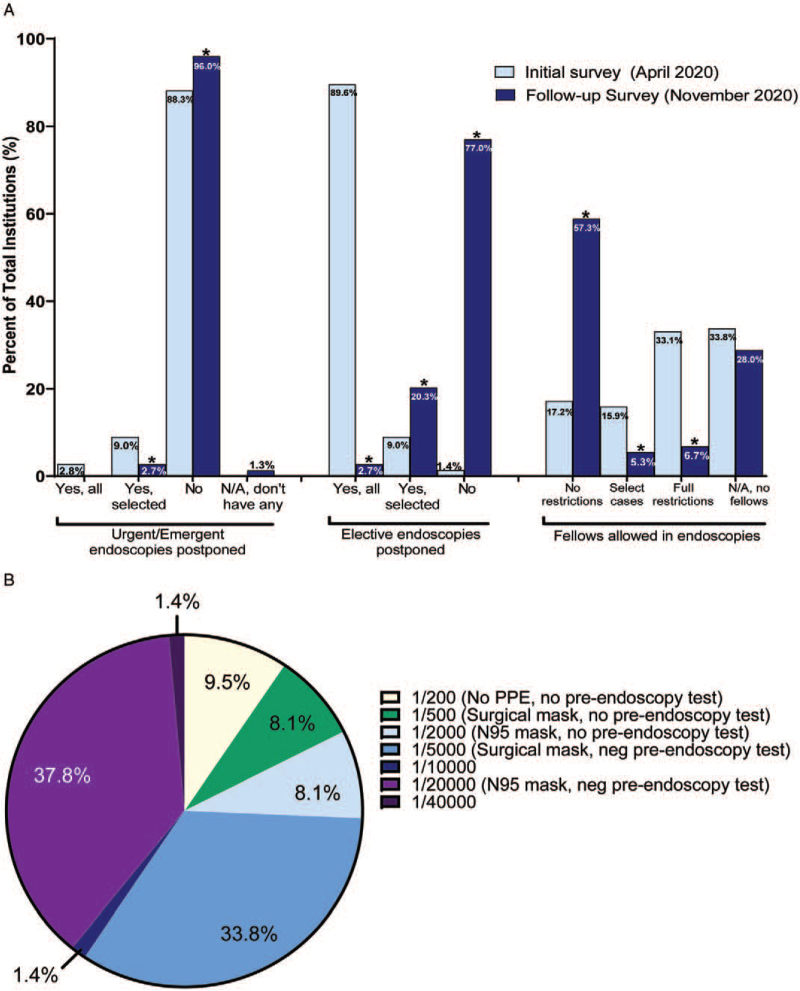
Overall, procedural volumes were increased at most institutions (n = 53/75, 70.7%) compared with April 2020 when pediatric endoscopy volumes had decreased to less than 10% of normal at most institutions. In the 7 days before the follow-up survey, most institutions (n = 26/63, 63.4%) had returned to 100% of their prepandemic procedural volumes. Emergent or urgent procedures were largely not postponed (96%, n = 72/75), and most institutions (77%, n = 57/74) were performing elective procedures as usual (Fig. 1A). Previously postponed procedures were also being performed again at most institutions (90.7%, n = 68/75). Methods used to reschedule these procedures included use of a triage algorithm (67.6%, n = 46/68), the previous order date or previous scheduled date (36.8%, n = 25/68), or other methods (13%, n = 9/68), suchas parental request, primary care physician input, or on a first-come first-serve basis. The individual deciding how procedures were rescheduled was the most responsible physician at 66.2% (n = 45/68) of institutions, endoscopy director at 22.1% (n = 15/ 68), endoscopy booking clerk/delegate at 20.6% (n = 14/68), and physician delegate at 4.4% (n = 4/68) of institutions. Fewer institutions reported a change in the location where endoscopy is performed (e.g., operating room, endoscopy suite), compared with previously (18.6% vs 32.6%). Additionally, fewer institutions limited the number of personnel in the endoscopy unit (36% vs 60.1%), with fewer restrictions on fellows (Fig. 1A). Figure 1A depicts the changes to endoscopy practices between the initial and follow-up surveys. When the responses were compared within the subset of 75 respondents who answered both surveys, the differences were still present.
FIGURE 1.
(A) Differences in endoscopie practices between the initial survey (April 2020) and follow-up survey (November 2020). Fewer urgent/ emergent and elective endoscopies were postponed at the time of the follow-up survey compared with the initial survey. Additionally, more fellows were allowed in endoscopies without restrictions on the follow-up survey compared with the initial survey. ∗P < 0.05. (B) Pediatric endoscopists risk threshold for performing endoscopy. Pediatric endoscopists were asked the following question: You have an asymptomatic pediatric patient undergoing elective endoscopy. Please indicate your threshold for the risk of COVID-19 that you are willing to assume as the endoscopist for this patient? Pre-endoscopy testing, whenever applicable, was negative. Assumptions are: (1) well-established tests are never 100% accurate; (2) low prevalence of asymptomatic COVID-19 patients in your area (1%). Patients are screened using CDC symptoms screening checklist. (3) On the basis of the best available evidence, the risk of COVID-19 infection is 50% if an endoscopist performs endoscopy with no PPE in a patient with COVID-19, 20% if wearing a surgical mask, and 5% if wearing N95 with face shield.
Pre-endoscopy screening questionnaires and SARS-CoV-2 testing also changed as the pandemic evolved. Forty-one percentage of institutions endorsed changes to screening questionnaires compared with April 2020. Of these institutions, all (n = 31) reported screening all patients before endoscopy, with 54.8% screening both before and on the day of endoscopy. This represents 97.3% (n = 73/75) of institutions performing pre-endoscopy screening. The most common symptoms and risk factors screened for were fever (n = 31/31, 100%), cough (n = 29/31, 93.5%), known COVID-19 exposure (n = 29/31, 93.5), shortness of breath (n = 27/31, 87.1%), loss of taste and/or smell (n = 26/31, 83.9%), and recent travel to a high-risk area (n = 22/31, 71.0%). More institutions (n = 67/75, 89.3% vs 78%) reported having protocols in place for a positive screen.
At the time of the follow-up survey, 78.7% (n = 59/75) of institutions reported testing all patients for SARS-CoV-2 preendoscopy. Testing was performed for the purposes of patient triage (n = 56/75, 74.7%), anesthesia risk determination (n = 20/75, 26.7%), and/or PPE determination for SARS-CoV-2 positive (n = 31/75, 41.3%) and/or negative (n = 13/75, 17.3%) patients.
Most institutions would proceed with urgent/emergent upper (n = 66/75, 88%) and lower (n = 64/75, 85.3%) endoscopies on SARS-CoV-2 positive patients. At the time of this follow-up survey, 34.7% (n = 26/75) ofinstitutions had performed procedures on SARS-CoV-2-positive patients. Practices varied for elective endoscopies on SARS-CoV-2-positive patients, with most rescheduling after a specific timeframe without retesting (n = 26/75, 34.6%) followed by rescheduling after a specific timeframe with routine preprocedural testing (n = 21/75, 28.0%), and rescheduling after 1 (n = 15/75, 20.0%) or 2 (n = 9/75, 12.0%) negative repeat SARS-CoV-2 tests.
Additionally, PPE practices have evolved, with 29% (n = 22/ 75) reporting changes to institutional PPE recommendations. Significantly fewer institutions reused surgical masks (27% vs 38.5%, P < 0.05) and N95 masks (55.4% vs 67.8%, P < 0.05) compared with April 2020. Table 1 compares PPE utilization practices between April and November 2020, with significantly fewer institutions recommending full PPE for all endoscopies (P = 0.013) and more institutions using full PPE only in cases of suspected or confirmed COVID-19 (P = 0.021).
TABLE 1.
Differences between personal protective equipment usage by pediatric endoscopic institutions during the coronavirus disease 2019 pandemic from April 2020 to November 2020
| Level of PPE used by type of endoscopic procedure being performed∗ | ||||
| Airborne, contact, and droplet | Contact, droplet | April 2020, N (%) | November 2020, N (%) | PPE use in April 2020 compared with November 2020, P value |
| All UE | n/a | 84 (59.2%) | 32 (43.2%) | 0.013 |
| All LE | ||||
| UE suspected/confirmed COVID-19 | UE low risk for COVID-19 | 30 (21.1%) | 25 (33.8%) | 0.021 |
| LE suspected/confirmed COVID-19 | LE low risk for COVID-19 | |||
| All UE | n/a | 10 (7.0%) | 3 (4.1%) | 0.190 |
| LE suspected/confirmed COVID-19 | LE low risk for COVID-19 | |||
| UE confirmed COVID-19 | UE low risk and suspected COVID-19 | 6 (4.2%) | 8 (10.8%) | 0.031 |
| LE confirmed COVID-19 | LE low risk and suspected COVID-19 | |||
| n/a | All UE | 5 (3.5%) | 3 (4.1%) | 0.422 |
| All LE | ||||
| No institutional guidance on personal protective equipment provided | 1 (0.7%) | 1 (1.4%) | 0.319 | |
| Other | 6 (4.23%) | 2 (2.7%) | 0.287 | |
COVID-19 = coronavirus disease 2019; LE = lower endoscopies; N/A = not applicable; UE = upper endoscopies.
The type of PPE endoscopists use for different endoscopic procedures is outlined. For example, the first row demonstrates that endoscopists utilize airborne, contact, and droplet precautions for all upper endoscopies and all lower endoscopies and there are no endoscopic procedures for which only contact and droplet precautions are used.
Eleven percentage (n = 8/73) of respondents stated that their own PPE practices were different from their institution's practices. The question modified from the AGA questionnaire assessing pediatric endoscopists’ tolerance of risk of COVID-19 transmission showed that most were risk adverse (n = 28/74, 37.8%) and desired a low risk of transmission (Fig. 1B).
DISCUSSION
Our study highlights the continuing impact of COVID-19 on pediatric endoscopic practice worldwide. Overall, as the pandemic evolved from April to November 2020, procedural volumes increased with resumption of previously cancelled or postponed elective procedures.
Most procedures are proceeding as they would before the pandemic, with fewer changes in the location in which endoscopy is performed and less limitations on the number of personnel and/or trainees. This is reflective of most regions being in phases of reopening, which has occurred despite an increasing number of COVID-19 cases and endoscopists being risk adverse. These changes may be related to improved PPE supply, improved pre-procedural screening questionnaires and protocols, and/or improved wide-scale pre-endoscopy SARS-CoV-2 testing. This is reflected in the number of patients undergoing pre-endoscopy SARS-CoV-2 testing. Previously, only 30.8% of institutions tested all patients, 24.5% tested some patients, and 44.8% tested none. This has dramatically increased to 78.7% of institutions testing all patients and 17.3% testing some patients. Additionally, the increased recognition of the potential unintended consequences of delayed and/or cancelled procedures has resulted in calls for the resumption of endoscopy services and corresponding guidance for adult endoscopy (11–15).
As compared with the start of the pandemic, fewer institutions reported utilizing full airborne, contact, and droplet precautions for all endoscopies. There were also more institutions using full PPE only in cases of suspected or confirmed COVID-19. These PPE practices changes may be an area of concern given the continued increase in COVID-19 cases globally (16), the emergence of new, more contagious, SARS-CoV-2 variants (17) and the endoscopy-related aero-solization risk (18). Full airborne, contact, and droplet precautions continue to currently be recommended for all pediatric endoscopies regardless of patient risk stratification (19). Alternative approaches to PPE have been suggested for adult endoscopy based on pre-endoscopy SARS-CoV-2 testing (5,20); however, the sensitivity and specificity of SARS-CoV-2 assays in the pediatric population remains unknown (19). Additionally, while adult and pediatric endoscopists have similar risk tolerance of COVID-19 transmission (5), and the AGA used their data to inform pre-endoscopy testing and PPE recommendations, further information on pediatric asymptomatic COVID-19 infection prevalence rates and testing parameters are needed before adjusting recommendations for pediatric endoscopy. Moreover, consensus guidelines have been developed to guide the recovery of adult endoscopy services in the postpandemic era (12), while similar recommendations do not exist in pediatrics and need to be established.
This study has several limitations. As with all self-reported surveys, our study was susceptible to respondent recall bias. The lower response rate also leaves room for nonresponse bias and may serve to limit the generalizability of the study findings. Additionally, generalizability worldwide may be limited as most respondents were from Europe and North America, and institutions in other countries may have different resources available to them for preprocedure COVID-19 testing and/or PPE precautions.
CONCLUSIONS
As the pandemic evolves, COVID-19 continues to drastically impact pediatric endoscopic practices worldwide. More procedures are being performed compared with the beginning of the pandemic when nonurgent endoscopies were largely halted, and PPE practices have continued to change with fewer institutions utilizing full airborne, contact, and droplet precautions. Further research is needed to evaluate practice change now that SARS-CoV-2 vaccines are approved in younger children and as access to vaccines improves worldwide and infections with SARS-CoV-2 variants increase. As we recover from the pandemic, a cohesive international approach to resuming pediatric endoscopy services safely is required to minimize SARS-CoV-2 transmission risk and avoid unintended harm from delayed procedures.
Supplementary Material
Footnotes
W.R. is supported by the Pfizer Global Medical Grant #59570215. C.M.W. holds an Early Researcher Award from the Ontario Ministry of Research and Innovation. The funders had no role in the design and conduct of the review, decision to publish and preparation, review, or approval of the manuscript.
The authors report no conflicts of interest.
Supplemental digital content is available for this article.
REFERENCES
- 1.Tam SS, Picoraro JA, Gupta SK, et al. International Pediatric Gastroenterology COVID-19 Alliance. Changes to pediatric gastroenterology practice during the COVID-19 pandemic and lessons learned: an international survey of division and group heads. Gastroenterology 2021; 161:1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Doremalen N, Bushmaker T, Morris D, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. NEngl J Med 2020; 382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021; 2:e13–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med 2020; 382:e41. [DOI] [PubMed] [Google Scholar]
- 5.Sultan S, Lim JK, Altayar O, et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology 2020; 159:739–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh CM, Fishman DS, Lerner DG. NASPGHAN Endoscopy, Procedures Committee,. Pediatric endoscopy in the era of coronavirus disease 2019: a North American Society for Pediatric Gastroenterology, Hepatology and Nutrition position paper. J Pediatr Gastroenterol Nutr 2020; 70:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010; 16:953–961. [DOI] [PubMed] [Google Scholar]
- 8.Lightdale JR, Acosta R, et al. ASGE Standards of Practice Committee,. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc 2014; 79:699–710. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. NEngl J Med 2020; 382:1633–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan W, Fishman DS, Lerner DG, et al. Changes in pediatric endoscopic practice during the COVID-19 pandemic: results from an international survey. Gastroenterology 2020; 159:1547–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayee B, Thoufeeq M, Rees CJ, et al. Safely restarting GI endoscopy in the era of COVID-19. Gut 2020; 69:2063–2070. [DOI] [PubMed] [Google Scholar]
- 12.Bhandari P, Subramaniam S, Bourke MJ, et al. Recovery of endoscopy services in the era of COVID-19: recommendations from an international Delphi consensus. Gut 2020; 69:1915–1924. [DOI] [PubMed] [Google Scholar]
- 13.Guda NM, Emura F, Reddy DN, et al. Recommendations for the operation of endoscopy centers in the setting of the COVID-19 pandemic - World Endoscopy Organization guidance document. Dig Endosc 2020; 32:844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menard C, Waschke K, Tse F, et al. COVID-19: framework for the resumption of endoscopic activities from the Canadian Association of Gastroenterology. J Can Assoc Gastroenterol 2020; 3:243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonelli G, Karstensen JG, Bhat P, et al. Endoscopy and COVID-19 Cascade Working Group, European Society of Gastrointestinal Endoscopy, World Endoscopy Organization and World Gastroenterology Organization. Resuming endoscopy during COVID-19 pandemic: ESGE, WEO and WGO Joint Cascade Guideline for Resource Limited Settings. Endosc Int Open 2021; 9:E543–E551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pei S, Yamana TK, Kandula S, et al. Burden and characteristics of COVID-19 in the United States during 2020. Nature 2021; 598:338–341. [DOI] [PubMed] [Google Scholar]
- 17.Tao K, Tzou PL, Nouhin J, et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet 2021; 22:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagami R, Nishikiori H, Sato T, et al. Aerosols produced by upper gastrointestinal endoscopy: a quantitative evaluation. Am J Gastroen-terol 2021; 116:202–205. [DOI] [PubMed] [Google Scholar]
- 19.Walsh CM, Fishman DS, Lerner DG. Response: Pediatric endoscopy during the COVID-19 pandemic: implications of universal preprocedural testing for PPE utilization. J Pediatr Gastroenterol Nutr 2021; 72:e26–e27. [DOI] [PubMed] [Google Scholar]
- 20.Hsu EK, Ambartsumyan L, Wahbeh GT, et al. Pediatric endoscopy during the COVID-19 pandemic: implications of universal preprocedural testing for PPE utilization. J Pediatr Gastroenterol Nutr 2021; 72:e26–e28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.