Abstract
A 65‐year‐old woman reported orbital symptoms two days after her first dose and presented exacerbation of signs after the second dose of BNT162b2 mRNA vaccine. The temporal relationship between the COVID‐19 vaccination and orbital symptoms suggests a probable link between SARS‐CoV‐2 mRNA vaccine and this orbital inflammatory disease.
Keywords: ASIA, COVID‐19, orbital inflammatory disease, orbitopathy, SARS‐CoV‐2, side effects, vaccines
We report the first case of orbital inflammatory disease following mRNA SARS‐CoV‐2 vaccination.
1. INTRODUCTION
SARS‐CoV‐2 vaccination campaigns document a satisfactorily high protection profile against COVID‐19 infection. SARS‐CoV‐2 vaccines do have adverse effects that are generally well tolerated. Immune‐related reactions including induction or reactivation of autoimmune diseases have been reported such as Guillain–Barre syndrome, thrombocytopenic purpura, myocarditis, arthritis, systemic lupus erythematosus, rheumatoid arthritis, and autoimmune thyroid diseases (Graves’ disease, Hashimoto’s thyroiditis). 1 , 2 To our knowledge, no case of active thyroid eye disease or orbital inflammatory disease has been reported to date following the SARS‐CoV‐2 vaccination.
This study presents the first description of an orbital inflammatory disease following mRNA SARS‐CoV‐2 vaccination.
2. PATIENT
The patient is a 65‐year‐old Caucasian female and non‐smoker with a medical history of subtotal resection of the right lobe of the thyroid gland due to toxic adenoma in 2003, seropositive rheumatoid arthritis in 2014, and amnesic stroke in 2017. She was treated chronically with levothyroxine (50 micrograms/day).
Eye symptoms and clinical signs developed two days after the first dose of the BNT162b2 mRNA COVID‐19 vaccine (Pfizer Laboratory). She complained of tearing, eye irritation, conjunctival redness, peri‐orbital swelling with spontaneous hematoma of the lower right eyelid, visual changes with bilateral ocular proptosis and predominantly right exophthalmia. She reported a spontaneous improvement in ophthalmic signs and symptoms over the next two weeks, mainly regarding the left orbit. She received the second dose of BNT162b2 mRNA COVID‐19 vaccine 6 weeks later and noted exacerbated right‐sided exophthalmia.
An ophthalmic examination revealed inflammation of the four eyelids, bilateral conjunctival redness, chemosis, predominantly right proptosis and high intraocular pressure of 30 mmHg in the right eye and of 19 mmHg in the left eye. Topical treatment with brinzolamide and timolol eye drops was introduced with partial response before initiating latanoprost eye drops.
After 3 months, the patient attended a multidisciplinary thyroid‐eye outpatient clinic. Conjunctival redness and erythema of eyelids were observed mainly in the right eye (Figure 1A). On physical examination, Hertel exophthalmometry values were 25 mm in the right eye and 19 mm in the left eye. Palpebral fissures of 12 and 11 mm were observed in the right and left eye, respectively, but no diplopia. The clinical activity score (CAS) was 4/7, thus confirming active inflammation in the right orbit. 3 Visual acuity, ocular motility, and the standardized color vision test were all normal (Table 1).
FIGURE 1.
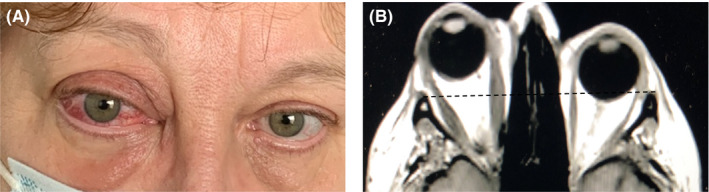
Picture (A) and IRM scan (B) of the female patient with an orbital inflammatory disease
TABLE 1.
Ophthalmic parameters after intravenous glucocorticoid infusions and right orbital decompression surgery
| Treatment | Hertel values (mm) | Palpebral fissure (mm) | Diplopia | CAS a | ||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | |||
| Baseline | 25 | 19 | 12 | 11 | – | 4/7 |
| After 2 i.v. infusions | 25 | 19 | 11 | 10 | – | 4/7 |
| After 6 i.v. infusions | 26 | 20 | 11 | 11 | – | 4/7 |
| After orbital surgery | 23 | 21 | 10 | 10 | – | 2/7 |
Clinical activity score
A normal‐sized left thyroid lobe was noted on cervical palpation with evidence of 3 hypoechoic nodules, each measuring less than one centimeter, on the ultrasound scan.
The blood biochemistry profile was unremarkable. The thyroid function test was normal (thyroid‐stimulating hormone: TSH = 0.83 mU/L, normal range 0.4–3.65 mU/L) with chronic levothyroxine treatment (50 μg/day). Anti‐thyroid peroxidase, anti‐thyroglobulin, and anti‐TSH receptor antibodies were negative. Computed tomography (CT) scans and magnetic resonance imaging (MRI) confirmed asymmetric exophthalmia with diffuse infiltration of the orbital fat and limited hypertrophy of the extraocular muscles in the right orbit (Figure 1B).
During the 4th month of follow‐up, the woman reported loss of visual acuity in the right eye, subsequently confirmed by ophthalmic assessment (right eye 20/30 and left eye 20/20). Examination of the fundus and optical coherence tomography were normal, thereby ruling out optic neuropathy. Serum TSH levels decreased (0.18 mU/L), and the patient presented palpitations. Levothyroxine treatment was withdrawn, and then, thyroid function test proved normal (0.56 mU/L).
Intravenous pulse therapy was initiated (500 mg methylprednisolone every two days), and a mild, transient improvement in inflammatory clinical signs and symptoms in the right eye was documented. Intravenous infusions of 500 mg methylprednisolone were then administered every week for 4 weeks. A transient improvement in pain and eyelid edema was noted after each infusion, albeit with no reduction in proptosis (26 mm) and no change in the clinical activity score (4/7) during the ophthalmic assessment performed after the first 6 intravenous infusions (Table 1). The patient subsequently received intravenous infusions of 250 mg methylprednisolone once a week for 4 weeks until orbital bone‐wall decompression of the right orbit was performed.
Pathological examination of the retro‐orbital tissue revealed adipose tissue with mature adipocytes and fibro‐vascular cloisons accompanied by discrete inflammation with edema. Immunohistochemical study highlighted the presence of CD3(+) T lymphocyte infiltration.
The ophthalmic assessment performed one month after surgery revealed the following: Hertel value 23 mm, intraocular pressure 17 mmHg and CAS 2/7 for the right eye with normal visual acuity and no diplopia (Table 1).
3. DISCUSSION
Graves’ hyperthyroidism is an autoimmune disease caused by stimulant anti‐TSH receptor antibodies with thyrotoxicosis typically accompanied by vascular goiter and occasional orbital and dermatological manifestations. Infectious agents are believed to be capable of triggering or exacerbating autoimmunity in genetically predisposed individuals, and the onset of autoimmune diseases has been reported after SARS‐CoV‐2 infection. A few patients were diagnosed with Graves’ hyperthyroidism following COVID‐19 infection either without clinically overt and active Graves’ orbitopathy 4 , 5 , 6 or with mild, inactive Graves’ orbitopathy. 7 Therefore, post‐COVID‐19 reports of Graves’ disease suggest that SARS‐CoV‐2 could play a role in triggering latent or new onset thyroid autoimmunity. On the contrary, the SARS‐CoV‐2 vaccination may also trigger autoimmune or inflammatory diseases in predisposed patients. Newly diagnosed or recurrent Graves’ disease has been reported following SARS‐CoV‐2 vaccination. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 No data on autoimmune or inflammatory orbitopathy have been documented in most patients, 8 , 9 , 14 , 15 an eyelid edema in one female patient 16 and a moderate to severe ophthalmopathy at 10 weeks of medical treatment were recently reported. 17 Most of the reported cases of Graves’ hyperthyroidism developed after mRNA vaccines although they were also triggered by adenoviral vector vaccines.
Despite the presence of SARS‐CoV‐2 coronavirus in conjunctival specimens, ocular and ophthalmic manifestations of COVID‐19 disease are relatively rare (<2%) and non‐specific (dry eye, conjunctival hyperemia, eye redness, photophobia, conjunctivitis, episcleritis, keratitis, chemosis, eyelid dermatitis, optic neuropathy, cranial nerve palsies). 18 , 19 However, the ocular and orbital adverse effects of SARS‐CoV‐2 vaccines notably include acute uveitis, multifocal choroiditis, central retinal or ophthalmic vein thrombosis, facial nerve or abducens nerve palsies, multiple evanescent white dot syndrome, Vogt–Koyanagi–Harada disease and graft rejections, thereby resembling the reported ocular manifestations of COVID‐19 per se. 20 , 21 , 22
The clear temporal relationship between the two doses of COVID‐19 vaccination and the onset of ophthalmic and orbital symptoms in our female patient suggest that the SARS‐CoV‐2 mRNA vaccine is probably associated with this orbital side effect. The clinical evaluation was consistent with the diagnosis of thyroid eye orbitopathy or orbital inflammatory disease. Orbital inflammatory disease can manifest with inflammation of any orbital structure (retro‐orbital fat, extraocular muscles). Both Graves’ orbitopathy and orbital inflammatory disease share the characteristics of proptosis, motility disturbances and often orbital pain. However, some features can be used to differentiate between Graves’ orbitopathy and orbital inflammatory disease. The presence of upper eyelid retraction, detection of anti‐thyroperoxidase, anti‐thyroglobulin and anti‐TSH receptor antibodies as well as enlargement of the bellies of extraocular muscles are considered pathognomonic of autoimmune thyroid eye disease. 23 The reported patient presented normal thyroid function test, absence of anti‐thyroid antibodies with diffuse infiltration of orbital fat and limited hypertrophy of extraocular muscles on radiological assessment in favor of orbital inflammatory disease.
The mechanisms of ocular and orbital side effects occurring after mRNA SARS‐CoV‐2 vaccine injections are a matter of discussion. An aberrant immune response induced by molecular mimicry and bystander activation may well be implicated, especially in predisposed individuals. The genetic similarity between the spike glycoprotein of SARS‐CoV‐2 coronavirus and human orbital proteins may trigger autoimmune diseases together with an inflammatory reaction in genetically predisposed individuals with autoimmune diseases (rheumatoid arthritis, Hashimoto’s thyroiditis) or specific HLA genotypes (mainly HLAB35). However, most vaccines contain adjuvants (lipid‐nanoparticles in mRNA vaccines) known to increase the innate and adaptive immune response. They can also trigger a pathogenic immune response possibly leading to the formation of autoantibodies and/or inflammation, and potentially leading to autoimmune/inflammatory diseases. 24 Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) may be related to the post‐vaccination immune response or to genetic predisposition. ASIA has been reported as early as 3–5 days post‐vaccine, and most commonly following the first dose, as observed in our patient. ASIA has been associated with several endocrine diseases (type 1 diabetes mellitus, premature ovarian failure and adrenal insufficiency). Furthermore, subacute thyroiditis, that met the criteria for ASIA syndrome, was described following papillomavirus, influenza, hepatitis B, and, more recently, COVID‐19 vaccination. 25 , 26 , 27 , 28 , 29 , 30 , 31 In the context of ASIA, most dysthyroid patients were tested negative for thyroid antibodies.
As for Graves’ orbitopathy, glucocorticoids represent first‐line therapy for orbital inflammatory disease due to their availability and efficacy in inducing a rapid response and disease remission. 32 Prolonged use of high‐dose corticosteroids is, however, limited by numerous adverse reactions. Intravenous glucocorticoids are better tolerated than oral corticosteroids. Based on the experience in patients with Graves’ orbitopathy, 3 , 33 our patient received a cumulative dose of 4 g of methylprednisolone in weekly infusions. The clinical efficacy of glucocorticoid treatment generally manifests as rapid remission, although incomplete response and recurrence in patients with glucocorticoid‐resistant forms of orbital inflammatory disease cannot be ruled out. There is no consensus regarding the treatment regimen for such patients, who either fail to respond, or who present relapses despite high doses and/or intravenous glucocorticoid therapy. Immunosuppressive or immunomodulatory therapy may be an option for selected patients, but orbital radiotherapy and decompressive surgery may prove to be alternative therapies for such patients. 34
To the best of our knowledge, this is the first report of orbital inflammatory disease in the context of autoimmune/inflammatory syndrome induced by adjuvants (ASIA) following mRNA SARS‐CoV‐2 vaccination. With booster vaccine doses, the rate of orbital side effects post‐SARS‐CoV‐2 vaccines may increase. Nevertheless, the benefits of SARS‐CoV‐2 vaccination far out‐weigh the potential risks of orbital inflammatory disease. Predisposed patients with existing or previous autoimmune diseases, such as rheumatoid arthritis or Hashimoto’s thyroiditis, may require post‐vaccine ophthalmic assessment and/or treatment. Finally, such orbital side effects following the COVID‐19 vaccination should be monitored and reported in order to gain a better understanding of the underlying mechanisms of autoimmune/inflammatory diseases in the context of SARS‐CoV‐2 vaccination.
AUTHOR CONTRIBUTIONS
All authors have been involved in medical and surgical care of the patient. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript was written by Philippe Caron, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript to be published, and agree to be accountable for all aspects of the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
CONSENT
Published with written consent of the patient.
ETHICAL APPROVAL
Informed and written consent was obtained from the patient, and the work conforms with the 1964 Declaration of Helsinki, Good Clinical practice Guideline.
ACKNOWLEDGEMENTS
None.
Grunenwald S, Lethellier G, Imbert P, Dekeister C, Caron P. Orbital inflammatory disease following mRNA SARS‐CoV‐2 vaccine. Clin Case Rep. 2022;10:e05926. doi: 10.1002/ccr3.5926
Funding information
This research did not receive any specific grant or support from any funding agency in the public, commercial, or not‐for‐profit sector
DATA AVAILABILITY STATEMENT
All authors confirm that all data and materials reported in this manuscript support their published claims and comply with field standards. The data that support the findings of the study are available on request from the corresponding author.
REFERENCES
- 1. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group (2021) Safety and efficacy of the ChAdOx1nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99‐111. doi: 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185:G43‐G67. doi: 10.1530/EJE-21-0479 [DOI] [PubMed] [Google Scholar]
- 4. Mateu‐Salat M, Urgell E, Chico A. SARS‐COV‐2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID‐19. J Endocrinol Invest. 2020;43:1527‐1528. doi: 10.1007/s40618-020-01366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiménez‐Blanco S, Pla‐Peris B, Marazuela M. COVID‐19: a cause of recurrent Graves’ hyperthyroidism? J Endocrinol Invest. 2021;44:387‐388. doi: 10.1007/s40618-020-01440-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris A, Al MM. Graves’ thyrotoxicosis following SARS‐CoV‐2 infection. AACE Clin Case Rep. 2021;7:14‐16. doi: 10.1016/j.aace.2020.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lanzolla G, Marcocci C, Marinò M. Graves’ disease and Graves’ orbitopathy following COVID‐19. J Endocrinol Invest. 2021;44:2011‐2012. doi: 10.1007/s40618-021-01576-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vera‐Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of Graves’ disease following SARS‐CoV‐2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31:1436‐1439. doi: 10.1089/thy.2021.0142 [DOI] [PubMed] [Google Scholar]
- 9. Zettinig G, Krebs M. Two further cases of Graves’ disease following SARS‐Cov‐2 vaccination. J Endocrinol Invest. 2021;3:1‐2. doi: 10.1007/s40618-021-01650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime‐boost immunization with inactivated and adenovirus‐vectored SARS‐CoV‐2 vaccine in a patient with Graves’ disease. Endocrine. 2021;74:226‐227. doi: 10.1007/s12020-021-02879-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sriphrapradang C, Shantavasinkul PC. Graves’ disease following SARS‐CoV‐2 vaccination. Endocrine. 2021;74:473‐474. doi: 10.1007/s12020-021-02902-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pujol A, Gómez LA, Gallegos C, et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA‐based SARS‐CoV2 vaccination: from Graves’ disease to silent thyroiditis. J Endocrinol Invest. 2021;1‐8. doi: 10.1007/s40618-021-01707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee KA, Kim YJ, Jin HY. Thyrotoxicosis after COVID‐19 vaccination: seven case reports and a literature review. Endocrine. 2021;74:470‐472. doi: 10.1007/s12020-021-02898-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of Graves’ Disease After SARS‐CoV‐2 mRNA Vaccination: A Case Report and Literature Review. Front Publ Health. 2021;9:778964. doi: 10.3389/fpubh.2021.778964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamouche W, El Soufi Y, Alzaraq S, Okafor BV, Zhang F, Paras C. A case report of new onset graves’ disease induced by SARS‐CoV‐2 infection or vaccine? J Clin Transl Endocrinol Case Rep. 2022;23:100104. doi: 10.1016/j.jecr.2021.100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pierman G, Delgrange E, Jonas C. Recurrence of Graves’ disease (a Th1‐type Cytokine Disease) following SARS‐CoV‐2 mRNA vaccine administration: a simple coincidence? Eur J Case Rep Intern Med. 2021;8:002807. doi: 10.12890/2021_002807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goblirsch TJ, Paulson AE, Tashko G, Mekonnen AJ. Graves’ disease following administration of second dose of SARS‐CoV‐2 vaccine. BMJ Case Rep. 2021;14:e246432. doi: 10.1136/bcr-2021-246432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eleiwa T, Abdelrahman SN, ElSheikh RH, Elhusseiny AM. Orbital inflammatory disease associated with COVID‐19 infection. J AAPOS. 2021;25:232‐234. doi: 10.1016/j.jaapos.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhong Y, Wang K, Zhu Y, et al. Ocular manifestations in COVID‐19 patients: a systematic review and meta‐analysis. Travel Med Infect Dis. 2021;44:102191. doi: 10.1016/j.tmaid.2021.102191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng XL, Betzler BK, Testi I, et al. Ocular adverse events after COVID‐19 vaccination. Ocul Immunol Inflamm. 2021;29(6):1216‐1224. doi: 10.1080/09273948.2021.1976221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Endo B, Bahamon S, Martínez‐Pulgarín DF. Central retinal vein occlusion after mRNA SARS‐CoV‐2 vaccination: a case report. Indian J Ophthalmol. 2021;69:2865‐2866. doi: 10.4103/ijo.IJO_1477_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eleiwa TK, Gaier ED, Haseeb A, ElSheikh RH, Sallam AB, Elhusseiny AM. Adverse ocular events following COVID‐19 vaccination. Inflamm Res. 2021;70:1005‐1009. doi: 10.1007/s00011-021-01506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bijlsma WR, Kalmann R. Idiopathic orbital inflammation and Graves ophthalmopathy. Arch Ophthalmol. 2010;128:131‐132. doi: 10.1001/archophthalmol.2009.324 [DOI] [PubMed] [Google Scholar]
- 24. Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34:101412. doi: 10.1016/j.beem.2020.101412 [DOI] [PubMed] [Google Scholar]
- 25. Ratnayake GM, Dworakowska D, Grossman AB. Can COVID‐19 immunisation cause subacute thyroiditis? Clin Endocrinol (Oxf). 2021. doi: 10.1111/cen.14555. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS‐CoV‐2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106:2600‐2605. doi: 10.1210/clinem/dgab373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schimmel J, Alba EL, Chen A, Russell M, Srinath R. Letter to the editor: thyroiditis and thyrotoxicosis after the SARS‐CoV‐2 mRNA vaccine. Thyroid. 2021;31:1440. doi: 10.1089/thy.2021.0184 [DOI] [PubMed] [Google Scholar]
- 28. Şahin Tekin M, Şaylısoy S, Yorulmaz G. Subacute thyroiditis following COVID‐19 vaccination in a 67‐year‐old male patient: a case report. Hum Vaccin Immunother. 2021;17(11):4090‐4092. doi: 10.1080/21645515.2021.1947102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS‐CoV‐2 vaccination. Front Med (Lausanne). 2021;8:737142. doi: 10.3389/fmed.2021.737142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oyibo SO. Subacute thyroiditis after receiving the adenovirus‐vectored vaccine for coronavirus disease (COVID‐19). Cureus. 2021;13:e16045. doi: 10.7759/cureus.16045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sözen M, Topaloğlu Ö, Çetinarslan B, et al. COVID‐19 mRNA vaccine may trigger subacute thyroiditis. Hum Vaccin Immunother. 2021;17(12):5120‐5125. doi: 10.1080/21645515.2021.2013083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mombaerts I, Bilyk JR, Rose GE, et al. Expert panel of the orbital society. consensus on diagnostic criteria of idiopathic orbital inflammation using a modified delphi approach. JAMA Ophthalmol. 2017;135(7):769‐776. doi: 10.1001/jamaophthalmol.2017.1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tramunt B, Imbert P, Grunenwald S, Boutault F, Caron P. Sight‐threatening Graves’ orbitopathy: Twenty years’ experience of a multidisciplinary thyroid‐eye outpatient clinic. Clin Endocrinol (Oxf). 2019;90:208‐213. doi: 10.1111/cen.13880 [DOI] [PubMed] [Google Scholar]
- 34. Yeşiltaş YS, Gündüz AK. Idiopathic orbital inflammation: review of literature and new advances. Middle East Afr J Ophthalmol. 2018;25:71‐80. doi: 10.4103/meajo [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors confirm that all data and materials reported in this manuscript support their published claims and comply with field standards. The data that support the findings of the study are available on request from the corresponding author.


