Abstract
Background
To address the problem of resource limitation, biomarkers having a potential for mortality prediction are urgently required. This study was designed to evaluate whether hemogram‐derived ratios could predict in‐hospital deaths in COVID‐19 patients.
Materials and Methods
This multicenter retrospective study included hospitalized COVID‐19 patients from four COVID‐19 dedicated hospitals in Sylhet, Bangladesh. Data on clinical characteristics, laboratory parameters, and survival outcomes were analyzed. Logistic regression models were fitted to identify the predictors of in‐hospital death.
Results
Out of 442 patients, 55 (12.44%) suffered in‐hospital death. The proportion of male was higher in nonsurvivor group (61.8%). The mean age was higher in nonsurvivors (69 ± 13 vs. 59 ± 14 years, p < 0.001). Compared to survivors, nonsurvivors exhibited higher frequency of comorbidities, such as chronic kidney disease (34.5% vs. 15.2%, p ≤ 0.001), chronic obstructive pulmonary disease (23.6% vs. 10.6%, p = 0.011), ischemic heart disease (41.8% vs. 19.4%, p < 0.001), and diabetes mellitus (76.4% vs. 61.8%, p = 0.05). Leukocytosis and lymphocytopenia were more prevalent in nonsurvivors (p < 0.05). Neutrophil‐to‐lymphocyte ratio (NLR), derived NLR (d‐NLR), and neutrophil‐to‐platelet ratio (NPR) were significantly higher in nonsurvivors (p < 0.05). After adjusting for potential covariates, NLR (odds ratio [OR] 1.05; 95% confidence interval [CI] 1.009‐1.08), d‐NLR (OR 1.08; 95% CI 1.006‐1.14), and NPR (OR 1.20; 95% CI 1.09‐1.32) have been found to be significant predictors of mortality in hospitalized COVID‐19 patients. The optimal cut‐off points for NLR, d‐NLR, and NPR for prediction of in‐hospital mortality for COVID‐19 patients were 7.57, 5.52 and 3.87, respectively.
Conclusion
Initial assessment of NLR, d‐NLR, and NPR values at hospital admission is of good prognostic value for predicting mortality of patients with COVID‐19.
Keywords: COVID‐19, mortality, predictors
1. INTRODUCTION
The ongoing COVID‐19 pandemic is threatening the global health system. Countries around the world reported 6.07 million deaths from severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) until mid‐March 2022. 1 However, preliminary estimates suggest an excess death of 1.2 million than officially reported in 2020. The underlying reason behind this discrepancy may be the lack of a proper national vital statistics system and lack of uniformity in test strategy as well as in defining COVID‐19 death. 2
Case fatality rate (CFR) is an important indicator to understand the severity and epidemiological features of infectious diseases. Reported CFR for COVID‐19 varies depending on geographic areas. For example, Central Europe and North America had a higher fatality than East Asia due to COVID‐19. 3 , 4 Among the SAARC countries, the reported CFR is highest in Afghanistan, followed by Pakistan, India, and then Bangladesh. The crude CFR in Bangladesh is about 1.458%. 5
For better utilization of the existing healthcare resources during the ongoing COVID‐19 pandemic, identification of the prognostic markers is of paramount importance. Several systematic reviews and meta‐analyses found higher age, pre‐existing comorbidities like diabetes, chronic obstructive pulmonary disease (COPD), hypertension, renal disease, or cardiovascular disease are important predictors of mortality. 6 , 7 , 8 Among the hematological parameters, higher baseline total white blood cell count (WBC), thrombocytopenia, C‐reactive protein (CRP), lactate‐dehydrogenase (LDH), creatine kinase (CK), dd‐dimer, and lower absolute lymphocyte count (ALC) were all associated with higher mortality rate. 7 , 9 However, prognostic parameters like procalcitonin, hs‐CRP, interleukin (IL)‐6, and LDH are costly and not widely available, particularly in low and middle‐income countries. To mitigate this economic and logistic constraint, we need to focus more on exploring rapid, inexpensive, and readily available prognostic tools. Hemogram‐derived ratios can play a very effective role in this regard. Hemogram‐derived ratios like neutrophil (NEU)‐to‐lymphocyte (LYM) ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) have been found to have significant prognostic value in predicting severe disease as well as mortality in COVID‐19. 10 , 11 In this study, we evaluated whether parameters derived from a routine blood test, at the time of hospital admission, can be valuable predictors of mortality in hospitalized COVID‐19 patients.
2. MATERIALS AND METHODS
2.1. Data collection
Data were extracted from the hospital record of patients who had been admitted with a diagnosis of COVID‐19 in four hospitals of Sylhet, Bangladesh (a major city in north‐eastern Bangladesh) during the COVID‐19 pandemic, between October 2020 and January 2021. Clinical, demographic, and laboratory data from all adult patients were recorded at the time of hospital admission. The blood samples were sent soon after hospital admission, preferably within 1 h. Cell count was done by fully automated analyzer SYSMEX‐XT2000i (Made in Japan).
Inclusion criteria: Patients above the age of 18 years, patients who were polymerase chain reaction (PCR) positive for SARS‐CoV‐2, and PCR negative patients who had typical clinical and radiographic findings of COVID‐19.
Exclusion criteria: Patients below the age of 18 years and patients without typical symptoms or absence of radiographic findings compatible with COVID‐19 pneumonia.
2.2. Definition
In‐hospital death refers to those patients who died at least 24 h after hospital admission due to COVID‐19.
COVID‐19 death was defined as certified by WHO, which states “A death due to COVID‐19 is defined as a death resulting from a clinically compatible illness, in a probable or confirmed COVID‐19 case, unless there is a clear alternative cause of death that cannot be related to COVID disease.”
Count of white blood cells (×109 cells/L), neutrophil (×109 cells/L), lymphocytes (×109 cells/L), and platelets (×1011 cells/L) were used to calculate the hemogram‐derived ratios. NLR is the ratio between neutrophils and lymphocytes, d‐NLR is derived NLR and calculated as d‐NLR = ANC/(WBC − ANC), NPR is the ratio between neutrophils and platelets, PLR is the ratio between platelets and lymphocytes, and systemic immune‐inflammation index (SII) is determined by multiplying the neutrophil and platelet counts and then divided by the lymphocyte count.
2.3. Study variables
The outcome variable was in‐hospital death (nonsurvivors and survivors); a binary variable. Clinical data included were age, sex, clinical features, presence of comorbidities like hypertension, chronic kidney disease (CKD), COPD, diabetes mellitus (DM), ischemic heart disease (IHD), and cerebrovascular accident (CVA), peripheral capillary oxygen saturation (SpO2) at admission and length of hospital stay (in days). Laboratory parameters included complete blood count (CBC), D‐dimer, S. Ferritin, and random blood sugar (RBS).
2.4. Statistical analysis
We used descriptive statistics to describe the data. Shapiro‐Wilk test was used to assess the normality of continuous variables. We presented continuous measurements by the mean and standard deviation (SD) for data that followed a normal distribution, and by the median and interquartile range (IQR) for data that were skewed. The mean difference between two groups (survivor vs. nonsurvivor) in a continuous variable was assessed using two independent sample mean tests (t‐test) for the normally distributed data and using nonparametric Mann–Whitney U test for the non‐normally distributed data. Categorical variables were presented using frequencies and percentages. The χ 2 test of independence was used to determine the association (difference) among categorical variables.
Multiple logistic regression models were used to identify the predictors of mortality. The variables that were significant at 10% level (cut‐off point p < 0.1) in the univariate or bivariate analysis were included in the multivariate analysis. We measured the correlations between the various hemogram‐derived indices and excluded the hemogram‐derived ratios that were responsible for multicollinearity (r > 0.8) from the multivariate analysis. We also excluded the hemogram‐derived ratios having a variance inflation factor of >5 to make sure that there was no multicollinearity in the multivariate logistic regression models. As there were high correlations among some of the hemogram‐derived ratios and as all the ratios are important, we fitted three separate multiple logistic regression models to avoid multicollinearity (Table A1). Model findings were presented using odds ratio (OR) and 95% confidence interval (CI). A p < 0.05 was considered statistically significant. We used Youden Index to determine the optimal cut‐off values for hemogram‐derived ratios for predicting in‐hospital mortality. Analysis was performed using R software. This study is reported following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) 12 statements.
3. RESULTS
3.1. Clinical characteristics and laboratory parameters of patients
The final analysis included 442 patients. Clinical characteristics of all patients are summarized (overall and by survivor status) and shown in Table 1. The proportion of patients who ended up with in‐hospital death was 12.44%. The mean age of study subjects was 60.2 ± 13.7 years. The mean age of the non‐survivor group was higher than the survivor group (69± 13 vs. 59 ± 14 years). Male comprises two‐thirds of the study sample (65.84%).
Table 1.
Clinical characteristics of patients, overall and by survivor status
| Univariable analysis | ||||||
|---|---|---|---|---|---|---|
| Variables | Total | Nonsurvivor (n= 55) | Survivor (n= 387) | p value | OR (95% CI) | p value |
| Age, mean (±SD) | 60 ± 14 | 69 ± 13 | 59 ± 14 | <0.001 | 1.06 (1.03−1.08) | 0.001 |
| Male | 291 (65.84%) | 34 (61.8%) | 257 (66.4%) | 0.63 | 0.81 (0.46–1.48) | 0.502 |
| Female | 151 (34.16%) | 21 (38.2%) | 130 (33.6%) | 0.603 | ||
| Comorbidity | ||||||
| Hypertension | 311 (70.36%) | 41 (74.5%) | 270 (69.8%) | 0.57 | 1.26 (0.68–2.49) | 0.469 |
| DM | 281 (63.57%) | 42 (76.4%) | 239 (61.8%) | 0.05 | 2.00 (1.06–3.99) | 0.038 |
| IHD | 98 (22.17%) | 23 (41.8%) | 75 (19.4%) | <.001 | 2.99 (1.64–5.39) | 0.0002 |
| CKD | 78 (17.65%) | 19 (34.5%) | 59 (15.2%) | <.001 | 2.93 (1.55–5.41) | 0.0006 |
| COPD | 54 (12.22%) | 13 (23.6%) | 41 (10.6%) | 0.011 | 2.61 (1.25−5.16) | 0.007 |
| CVA | 20 (4.52%) | 5 (9.1%) | 15 (3.9%) | 0.163 | 2.48 (0.78–6.71) | NA |
| Clinical feature | ||||||
| Fever | 399 (90.27%) | 52 (94.5%) | 353 (91.2%) | 0.566 | 1.67 (0.57–7.10) | 0.409 |
| Cough | 322 (72.85%) | 36 (65.5%) | 286 (73.9%) | 0.248 | 0.66 (0.37–1.23) | 0.19 |
| SOB | 294 (66.52%) | 42 (76.4%) | 252 (65.1%) | 0.133 | 1.73 (0.92–3.45) | 0.101 |
| Fatigability | 246 (55.66%) | 33 (60%) | 213 (55%) | 0.584 | 1.23 (0.69–2.20) | 0.489 |
| Loss of smell | 87 (19.68%) | 9 (16.4%) | 78 (20.2%) | 0.631 | 0.77 (0.34–1.58) | 0.509 |
| Diarrhea | 71 (16.06%) | 11 (20%) | 60 (15.5%) | 0.513 | 1.36 (0.63–2.70) | 0.397 |
| Sore throat | 47 (10.63%) | 13 (23.6%) | 34 (8.8%) | 0.002 | 3.21 (1.53–6.45) | 0.00137 |
| Anorexia | 13 (2.94%) | 2 (3.6%) | 11 (2.8%) | 1 | 1.28 (0.19–4.97) | 0.74 |
| Chest pain | 9 (2.04%) | 0 (0%) | 9 (2.3%) | 0.527 | NA | NA |
| Vomiting | 4 (0.90%) | 0 (0%) | 4 (1%) | 1 | NA | NA |
| Headache | 3 (0.68%) | 1 (1.8%) | 2 (0.5%) | 0.824 | 3.56 (0.16–37.82) | 0.303 |
| Admission SpO2 | 92 (88–95) | 84 (73–93) | 93 (89–96) | <0.001 | 0.91 (0.88–0.93) | 0.001 |
| LOS | 8.7 ± 4.5 | 10.9 ± 7.3) | 8.3 ± 3.8) | 0.013 | 1.10(1.04–1.16) | 0.0003 |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA cerebrovascular disease; DM, diabetes mellitus; IHD Ischemic heart disease; LOS, length of stay; OR, odds ratio; SpO2, peripheral capillary oxygen; SOB, shortness of breath saturation.
Compared to survivors, nonsurvivors had higher prevalence of comorbidities like CKD (34.5% vs. 15.2%, [OR]: 2.93; 95% CI: 1.55–5.41; p = <0.001), COPD (23.6% vs. 10.6%, [OR]: 2.61; 95% CI: 1.25−5.16; p = 0.011), IHD (41.8% vs. 19.4%, [OR]: 2.99; 95% CI: 1.64–5.39; p < 0.001), DM (76.4% vs. 61.8%, [OR]: 2.00; 95% CI: 1.06–3.99; p = 0.05) and hypertension (74.5% vs. 69.8%, [OR]: 1.26; 95% CI: 0.68–2.49; p = 0.57).
Nonsurvivors had significantly lower SpO2 at admission than survivors (median; 84 vs. 93; p < 0.001). Length of hospital stay was significantly higher in nonsurvivors (10.9± 7.3 vs. 8.3 ± 3.8, p = 0.013).
Regarding laboratory results (Table 2), median WBC count (10.8 vs. 8.1; p = 0.003) and neutrophil count (8.8 vs. 6.1; p < 0.001) were significantly higher while platelet (209 vs. 230; p = 0.05) and lymphocyte (1.18 vs. 1.4; p = 0.122) count was lower in nonsurvivors. Leukocytosis (52.7% vs. 32%; p = 0.004) and lymphocytopenia (72.7% vs. 51.9%; p = 0.006) were significantly higher in nonsurvivors. Nonsurvivors have higher median value of d‐dimer (900 vs. 567), ferritin (507 vs. 328), and RBS (12 vs. 9.4).
Table 2.
Lab findings on admission
| Univariable analysis | ||||||
|---|---|---|---|---|---|---|
| Variables | Normal range | Nonsurvivor | Survivor | p value | OR (95% CI) | p value |
| TC WBC (×109/L) | 4–10 | 10.8 (6.80–14) | 8.1 (6–11.3) | 0.003 | 2.48 (1.34–4.58) | 0.003 |
| >10 | 29 (52.7%) | 124 (32%) | 0.004 | 2.39 (1.35–4.25) | 0.00273 | |
| 4–10 | 26 (47.3%) | 252 (65.1%) | 0.014 | |||
| <4 | 0.0% | 11 (2.8%) | 0.422 | |||
| Neutrophil (×109/L) | 2.0–7.0 | 8.8 (4.84–12.59) | 6.1 (4.15–9.37) | <.001 | 2.47 (1.47–4.14) | 0.0006 |
| Lymphocyte (×109/L) | 0.8–4.5 | 1.18 (0.75‐1.8) | 1.4 (1.02–2.04) | 0.122 | 0.61 (0.38–0.97) | 0.0393 |
| <0.8 | 40 (72.7%) | 201 (51.9%) | 0.006 | 2.46 (1.35–4.74) | 0.004 | |
| 0.8–4.5 | 15 (27.3%) | 186 (48.1%) | 0.006 | |||
| >4.5 | 0 (0%) | 4 (1%) | 1 | |||
| Platelet (×109/L) | 150–350 | 209 (154–254) | 230 (180–300) | 0.05 | 0.48 (0.24–0.96) | 0.039 |
| <150 | 10 (18.2%) | 39 (10.1%) | 0.118 | 1.98 (0.88−4.11) | 0.07 | |
| 150–350 | 37 (67.3%) | 283 (73.1%) | 0.455 | |||
| >350 | 8 (14.5%) | 65 (16.8%) | 0.821 | |||
| d‐dimer (ng/L) | 0–500 | 900 (420–1411) | 567 (300–1230) | 0.12 | 1.26 (0.98–1.62) | 0.0626 |
| S. Ferritin | 20–300 | 507 (181–981.32) | 328 (169–748) | 0.21 | 1.27 (0.98–1.65) | 0.06 |
| RBS | 4.4–7.2 | 12 (8.9–14.7) | 9.4 (7.6–13) | 0.005 | 2.63 (1.32–5.22) | 0.005 |
Note: Statistically significant values are highlighted in bold.
Abbreviations: CI, confidence interval; OR, odds ratio; RBS, random blood sugar; TC WBC, total count of white blood cells.
Difference in hemogram‐derived ratios between survivors and non‐survivors are shown in Table 3. Median value of NLR (7.08 vs. 4.05; p = 0.005), d‐NLR (4.88 vs. 3.34; p = 0.001) and NPR (3.59 vs. 2.47; p = 0.003) were significantly higher in nonsurvivor while, though not statistically significant, median value of PLR (1.96 vs. 1.57; p = 0.87) and SII (14.11 vs. 9.37; p = 0.05) were also higher in nonsurvivors.
Table 3.
Hematological ratios predicting mortality
| Univariable analysis | |||||
|---|---|---|---|---|---|
| Variables | Nonsurvivors | Survivors | p value | OR (95% CI) | p value |
| NLR | 7.08 (3.85–11.12) | 4.05 (2.48–7.08) | 0.005 | 2.02 (1.42–2.88) | 0.001 |
| d‐NLR | 4.88 (2.70–10.11) | 3.34 (2.03–5.66) | 0.001 | 2.30 (1.55–3.42) | 0.001 |
| NPR | 3.59 (2.42–5.61) | 2.47 (1.78–3.82) | 0.003 | 2.77 (1.76–4.35) | 0.001 |
| PLR | 1.96 (1.12–2.77) | 1.57 (1.06–2.43) | 0.879 | 1.10 (0.74–1.62) | 0.623 |
| SII | 14.11 (7.63–25.50) | 9.37 (5–17.52) | 0.058 | 1.42 (1.06–1.89) | 0.0156 |
Abbreviations: CI, confidence interval; d‐NLR, derived neutrophil‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; NPR, neutrophil‐to‐platelet ratio; OR, odds ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index.
Independent mortality prediction ability is shown for each hemogram‐derived ratio (receiver operating characteristic [ROC] curves are shown in Figure 1). The result of multivariable regression models that assessed the prognostic capability of different hemogram‐derived ratios is shown in Table 4. Except for PLR and SII, all other ratios (NLR, d‐NLR, and NPR) remain as significant predictors for mortality.
Figure 1.
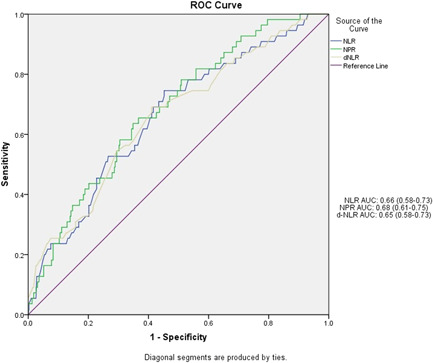
Receiver operating characteristic (ROC) curve for the different hemogram‐derived ratios and their respective area under the curves (AUCs)
Table 4.
Multivariable adjusted model for mortality prediction
| Model | NLR OR (95% CI) p value | d‐NLR | NPR OR (95% CI) p value | SII OR (95% CI) p value |
|---|---|---|---|---|
| Model A | ‐ | 1.08 (1.006–1.14) 0.033 | 1.17 (1.06–1.29) 0.002 | ‐ |
| Model B | ‐ | ‐ | 1.20 (1.09–1.32) <0.001 | 1.004 (0.99–1.01) 0.505 |
| Model C | 1.05 (1.009‐1.08) 0.014 | ‐ | ‐ | ‐ |
Note: Model A: Age, DM, CKD, COPD, RBS, D‐dimer, Ferritin, NPR, d‐NLR. Statistically significant values are highlighted in bold.
Model B: Age, DM, CKD, COPD, RBS, d‐dimer, Ferritin, NPR, SII.
Model C: Age, DM, CKD, COPD, RBS, d‐dimer, Ferritin, NLR.
Abbreviations: CI, confidence interval; d‐NLR, derived neutrophil‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; NPR, neutrophil‐to‐platelet ratio; OR, odds ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index.
3.2. ROC curve to determine optimal cut‐off values of the hematological ratios
We analyzed the optimal cut‐off values (Table 5) of NLR, d‐NLR, NPR, PLR, and SII, calculated by the ROC analysis and presented in Figure 1. Areas under the curve (AUC) of NLR, d‐NLR, NPR, PLR, and SII were 0.66 (0.58–0.73), 0.65 (0.58–0.73), 0.68 (0.61–0.75), 0.55 (0.46–0.63), and 0.60 (0.52–0.67) respectively. The optimal cut‐off values were NLR (7.57), d‐NLR (5.52), NPR (3.87), PLR (2.26), and SII (19.68). PLR had the highest sensitivity (0.78), followed by d‐NLR (0.67) and then NLR and NPR (both 0.65). SII (0.75) has the highest specificity, followed by NLR and the NPR (both 0.63).
Table 5.
Optimal cut‐off points for hemogram‐derived ratios for prediction of mortality
| Variable | Cut off | Sensitivity | Specificity |
|---|---|---|---|
| NLR | 7.57 | 0.65 | 0.63 |
| d‐NLR | 5.52 | 0.67 | 0.59 |
| NPR | 3.87 | 0.65 | 0.63 |
| PLR | 2.26 | 0.78 | 0.44 |
| SII | 19.68 | 0.43 | 0.75 |
Abbreviations: d‐NLR, derived neutrophil‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; NPR, neutrophil‐to‐platelet ratio; PLR, platelet‐to‐lymphocyte ratio; SII, systemic immune‐inflammation index.
4. DISCUSSION
4.1. Statement of principal findings
This study analyzed the data on clinical characteristics and laboratory parameters of hospitalized COVID‐19 patients with a particular focus on the predictive ability of hemogram‐derived ratios on mortality. Non‐survivors are of higher age and they had a higher prevalence of comorbidities. Length of hospital stay was more for non‐survivors. At admission, nonsurvivors had a more severe degree of hypoxia than survivors. Leukocytosis and lymphocytopenia were more frequent in patients who died. The level of d‐dimer, Ferritin, and RBS were higher in nonsurvivors. Adjusted multivariable models demonstrated that NLR, d‐NLR, and NPR are significant predictors for mortality. The AUC was highest for NPR.
4.2. Strengths and limitations
As this study included patients of four large hospitals of Sylhet city, it can be taken as representative of the wider population. Another strength of this study lies in the utilization of basic hematological parameters, which are widely available and can be measured even in a peripheral health center. However, Due to the retrospective nature of the present study, it has got some limitations. The questionnaire we used here was not a validated one. Data that could have the potential to influence the disease course like the presence of obesity, malignancy, smoking status, lab parameters like CRP, LDH, ALT, and troponin I were not available in the hospital records. So, their effects on the final outcome are overlooked here. Additionally, information on treatment before hospital admission was lacking in hospital records which could have modified the value of laboratory parameters. Besides, this study focuses on one region, a divisional city, not the whole of a country, that is why other studies involving large geographic areas need to be conducted to check the generalizability of these findings.
4.3. Interpretation in the context of the wider literature
With a new upsurge of COVID‐19 cases by emerging variants of coronavirus in the face of resource constraints, early prediction of mortality in COVID‐19 is an important tool in the triage process and resource allocation. Existing scoring systems such as the Acute Physiology and Chronic Health Evaluation (APACHE) II score and COVID‐GRAM are good prognostic clinical tools in COVID‐19 patients. 13 , 14 However, both scoring systems require laboratory parameters such as arterial pH and fraction of inspired oxygen (FiO₂) in the APACHE II and lactate dehydrogenase in the COVID‐GRAM, which is not available in resource‐limited healthcare settings. Therefore, tools that are cost‐effective and widely available with a potential for predicting the prognosis of COVID‐19 patients are needed.
Presentation of COVID‐19 is heterogeneous, ranging from asymptomatic infection to life‐threatening critical illness requiring intensive care support. Several inflammatory markers have been evaluated as predictors of death among hospitalized patients with COVID‐19. 15 , 16 , 17 , 18 Blood levels of different cytokines and inflammatory markers have been shown to predict the critical illness of COVID‐19 disease, 19 but these are not readily available outside of tertiary‐care medical centers. In this study, we investigated the predictive role of hematological parameters that are easily available at a low cost. Here we observed that NLR, d‐NLR, and NPR can successfully predict mortality at the time of hospital admission.
Advanced age and the presence of comorbidities are considered independent predictors of in‐hospital death in COVID‐19. 20 , 21 , 22 In this current study, we adjusted age, comorbidities like DM, CKD, COPD, and IHD to reduce the influence of confounding factors.
Sex differences in mortality have been widely reported in COVID‐19. Previous coronavirus outbreaks (SARS, MERS) demonstrated a higher risk of disease progression and high case fatality in males. 23 , 24 , 25 Male sex is also found to be associated with increased risk of severe disease, ICU admission, and mortality in COVID‐19. 26 , 27 , 28 Several hypothesis or explanation has been put forward to explain this increased vulnerability of male. Sexual dimorphism plays a crucial role in the regulation of immune respnses, both innate and adaptive immune systems. 29 Female sex hormones Estradiol may have a protective effect against the development of hyperinflammation while male sex hormone testosterone has an immunosuppressive effect. The difference in the expression of ACE‐2 receptor, which facilitates entry of SARS‐CoV2 virus inside the cell and their regulation may also contribute to this sex bias. Besides sex‐based differences in comorbidity also may play a role. 26 However, our study did not find the male sex as a significant predictor of mortality. This could be attributed to our demographically distinct population or racial difference. Few studies also reported findings similar to ours. 30 , 31
NLR represents the relationship between two arms (innate and adaptive) of the immune system during different stages of diseases. 32 Higher NLR is a poor prognostic factor in infectious diseases, malignancy, cardiac disease, and autoimmune diseases. 33 , 34 , 35 , 36 In the present study, elevated NLR was a significant prognostic biomarker in COVID‐19 patients. Our study finding is consistent with previous studies done to assess the relationship between NLR and the prognosis of COVID‐19. 37 , 38 This may be due to the following reason. The inflammatory reactions could stimulate the production of neutrophils, which then migrate to the immune organs. Afterward, neutrophil releases vast amounts of reactive oxygen species that lead to cell damage. As a result, antibody‐dependent cellular cytotoxicity may kill the virus directly. Besides, neutrophil production can be also triggered by factors released from the virus, such as IL‐6 and IL‐8, tumor necrosis factor‐α, and interferon‐γ. 39 In contrast, systemic inflammation promotes apoptosis of lymphocytes. 40 Lymphopenia also may be due to direct infection of bone marrow and lymphatic organ by SARS‐CoV‐2. 41 , 42 Furthermore, due to low immune function, these patients are at risk of co‐infection with bacteria, which could also explain the rise in the neutrophil count. 43 This study found that the associations between NLR and COVID‐19 mortality are independent of age and underlying diseases.
Previous studies 44 , 45 showed that derived NLR (d‐NLR) has a similar prognostic value to the NLR in cancer patients. The utility of d‐NLR has also been investigated in COVID‐19 and found to be a predictor of poor outcomes. 10 , 38 This study also found d‐NLR as a prognostic marker.
NPR was found to be a marker of disease activity in Ulcerative colitis 46 and predictors of survival in a variety of cancers. 47 Our study suggests NPR can be used as a prognostic marker in COVID‐19, which is consistent with other studies. 48 , 49
The PLR reflects the interaction between platelet count and lymphocyte count, which represents aggregation, as well as inflammatory pathways. Like NLR, PLR has been demonstrated to be predictive of worse overall survival in cancer patients 50 and correlated with disease severity in patients with Rheumatoid arthritis. 51 Several systematic reviews and meta‐analyses found the role of PLR as a prognostic marker in COVID‐19. 52 , 53 However, in our study, PLR was not found to be a significant prognostic factor of mortality in multivariable analysis, which is consistent with previous studies. 54 , 55
Although first reported in hepatocellular carcinoma, the prognostic value of SII has also been evaluated in other solid malignancies. 56 , 57 SII measured at admission can predict in‐hospital mortality in COVID‐19. 58 , 59 This current study found that SII can predict mortality in univariable analysis but not in multivariable analysis, which agrees with the study by Xue et al. 60
To date, no cut‐off value of the hematological ratio has been defined as optimal in COVID‐19. The cut‐off values of hemogram‐derived ratios for the prediction of mortality in this study are 7.57, 5.52, 3.87, 2.26, and 19.68, respectively, for NLR. d‐NLR, NPR, PLR, and SII. There is a wide variation in the cut‐off value of these parameters in published studies. 61 , 62 , 63 The underlying reason may be due to differences in demographic characteristics of the study population 44 , 64 , 65 and differences in the technique applied to select the cut‐off value. We used Youden Index to determine the optimal cut‐off values. In line with our study, many other studies, including systematic review and meta‐analysis 11 , 66 reported optimal cut‐off values for NLR with moderate sensitivity and specificity. For example, Yildiz et al. 61 estimated that the optimal cut‐off value of NLR was 6.4, with a sensitivity of 63% and specificity of 64%. Similarly, Cheng et al. 67 estimated an optimal cut‐off value of NLR of 7.9, which corresponded to a sensitivity of 65% and Tahtasakal et al. 68 reported NLR cut‐off >3.69 with a sensitivity of 78.68 and specificity of 66.08.
4.4. Implications for policy, practice, and future research
The unpredictable course of COVID‐19 disease, ranging from mild self‐limiting illness to cytokine storms, multiorgan failure, and death has been posing a great challenge to health care workers because which particular patients will develop the progressive disease is difficult to predict at admission. Therefore, there is an urgent need to identify early prognostic biomarkers which are reliable and widely available at low cost. With these objectives in mind, this study evaluated the usefulness of hemogram‐derived ratios, which can be calculated from the routine hematological test, and assessed their role as predictors of mortality in COVID‐19.
We hope, the findings of this study will guide the clinician in risk‐stratifying the patients upon admission and ensure better management with the best use of available resources. Considering the evolving nature of COVID‐19, research directed at identifying early predictors of poor outcomes of COVID‐19 needs to be continued.
5. CONCLUSION
Risk‐stratifying the patients based on prognostic markers can play a significant role in tackling the challenges of the ongoing COVID‐19 pandemic. By allocating medical resources more rationally, this strategy will reduce the pressure on the already exhausted health system, alleviate the shortage of resources, and ultimately will contribute to lowering of public health burden. This study revealed that hemogram‐derived ratios have a good predictive value on the mortality of COVID‐19 patients. This cost‐effective tool can help the clinician in early triaging of severe patients and to apply appropriate management in time.
AUTHOR CONTRIBUTIONS
MD Asaduzzaman: Data curation, formal analysis, methodology, writing –original draft, writing – review and editing. Mohammad Romel Bhuia: Methodology, validation, writing—review and editing. ZHM Nazmul Alam: Data curation, project administration, supervision. Mohammad Zabed Jillul Bari: Data curation, project administration, resources, supervision. Tasnim Ferdousi: Data curation, project administration, writing—review and editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was conducted following the Declaration of Helsinki. We obtained ethical approval from the Ethical committee of Sylhet Women's Medical College, Sylhet, Bangladesh, and the committee waived the need for consent.
TRANSPARENCY STATEMENT
The corresponding author confirms that the “manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained”.
ACKNOWLEDGMENTS
Special thanks to the authorities of Mount Adora Hospital, Sylhet Shahid Shamsuddin Ahmed District Hospital, Sylhet Women's Medical College Hospital, and North‐East Medical College Hospital, Sylhet, Bangladesh from where we collected data. We acknowledge the contributions of Dr. Md. Moyeen Uddin, Prof. Dr. Md. Ismail Patwary, Prof. Dr. Shishir R Chakraborty, Prof. Dr. Md. Shafiqul Bari and Dr. M M Jahangir Alam for inspiring us to do this study. Our deep respect and endless gratitude to those patients whose data we have worked with.
APPENDIX A.
Table A1
Table A1.
Model 1
| Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wald test | 95% confidence interval (odds ratio scale) | ||||||||
| Estimate | Standard error | Odds ratio | z | Wald statistic | df | p | Lower bound | Upper bound | |
| (Intercept) | −7.373 | 1.034 | 6.277e‐4 | −7.130 | 50.842 | 1 | <0.001 | 0.000 | 0.005 |
| Age | 0.051 | 0.013 | 1.053 | 3.934 | 15.475 | 1 | <0.001 | 1.026 | 1.080 |
| DM (yes) | 0.245 | 0.403 | 1.278 | 0.608 | 0.370 | 1 | 0.543 | 0.580 | 2.818 |
| CKD (yes) | 0.721 | 0.373 | 2.056 | 1.934 | 3.740 | 1 | 0.053 | 0.990 | 4.267 |
| COPD (yes) | 0.595 | 0.433 | 1.813 | 1.374 | 1.889 | 1 | 0.169 | 0.776 | 4.234 |
| RBS | 0.057 | 0.031 | 1.059 | 1.872 | 3.503 | 1 | 0.061 | 0.997 | 1.125 |
| d‐dimer | −0.000 | 0.000 | 1.000 | −1.060 | 1.124 | 1 | 0.289 | 1.000 | 1.000 |
| Ferritin | 0.000 | 0.000 | 1.000 | 0.717 | 0.514 | 1 | 0.474 | 1.000 | 1.001 |
| NPR | 0.157 | 0.050 | 1.170 | 3.157 | 9.965 | 1 | 0.002 | 1.061 | 1.291 |
| d‐NLR | 0.072 | 0.034 | 1.075 | 2.127 | 4.526 | 1 | 0.033 | 1.006 | 1.148 |
| Multicollinearity diagnostics | ||
|---|---|---|
| Tolerance | VIF | |
| Age | 0.901 | 1.110 |
| DM | 0.796 | 1.256 |
| CKD | 0.871 | 1.148 |
| COPD | 0.861 | 1.162 |
| RBS | 0.850 | 1.177 |
| d‐dimer | 0.850 | 1.176 |
| Ferritin | 0.927 | 1.079 |
| NPR | 0.834 | 1.200 |
| d‐NLR | 0.887 | 1.127 |
Note: Prognosis level “1” coded as class 1.
Abbreviations: COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DM, diabetes mellitus; NPR, neutrophil‐to‐platelet ratio; RBS, random blood sugar; VIF, variance inflation factor.
APPENDIX B.
Table B1
Table B1.
Model 2
| Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wald rest | 95% confidence interval (odds ratio scale) | ||||||||
| Estimate | Standard error | Odds ratio | z | Wald statistic | df | p | Lower bound | Upper bound | |
| (Intercept) | −7.422 | 1.036 | 5.978e‐4 | −7.163 | 51.316 | 1 | <.001 | 0.000 | 0.005 |
| Age | 0.055 | 0.013 | 1.056 | 4.219 | 17.798 | 1 | <.001 | 1.030 | 1.083 |
| DM (yes) | 0.338 | 0.402 | 1.403 | 0.842 | 0.710 | 1 | 0.400 | 0.638 | 3.082 |
| CKD (yes) | 0.715 | 0.369 | 2.045 | 1.940 | 3.763 | 1 | 0.052 | 0.993 | 4.213 |
| COPD (yes) | 0.711 | 0.425 | 2.035 | 1.671 | 2.791 | 1 | 0.095 | 0.884 | 4.686 |
| RBS | 0.055 | 0.030 | 1.057 | 1.816 | 3.297 | 1 | 0.069 | 0.996 | 1.121 |
| d‐dimer | −0.000 | 0.000 | 1.000 | −1.115 | 1.244 | 1 | 0.265 | 1.000 | 1.000 |
| Ferritin | 0.000 | 0.000 | 1.000 | 0.827 | 0.684 | 1 | 0.408 | 1.000 | 1.001 |
| NPR | 0.184 | 0.050 | 1.202 | 3.712 | 13.781 | 1 | <0.001 | 1.091 | 1.325 |
| SII | 0.004 | 0.006 | 1.004 | 0.666 | 0.444 | 1 | 0.505 | 0.993 | 1.015 |
| Multicollinearity diagnostics | ||
|---|---|---|
| Tolerance | VIF | |
| Age | 0.903 | 1.108 |
| DM | 0.800 | 1.250 |
| CKD | 0.862 | 1.159 |
| COPD | 0.855 | 1.169 |
| RBS | 0.857 | 1.167 |
| d‐dimer | 0.829 | 1.207 |
| Ferritin | 0.911 | 1.098 |
| NPR | 0.860 | 1.163 |
| SII | 0.922 | 1.084 |
Note. Prognosis level “1” coded as class 1.
Abbreviations: COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DM, diabetes mellitus; NPR, neutrophil‐to‐platelet ratio; RBS, random blood sugar; VIF, variance inflation factor.
APPENDIX C.
Table C1
Table C1.
Model 3
| Coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wald test | 95% confidence interval (odds ratio scale) | ||||||||
| Estimate | Standard error | Odds ratio | z | Wald statistic | df | p | Lower bound | Upper bound | |
| (Intercept) | −6.666 | 0.970 | 0.001 | −6.869 | 47.182 | 1 | <0.001 | 0.000 | 0.009 |
| Age | 0.049 | 0.013 | 1.050 | 3.855 | 14.863 | 1 | <0.001 | 1.024 | 1.077 |
| DM (Yes) | 0.213 | 0.387 | 1.238 | 0.550 | 0.303 | 1 | 0.582 | 0.579 | 2.644 |
| CKD (Yes) | 0.582 | 0.366 | 1.790 | 1.592 | 2.533 | 1 | 0.111 | 0.874 | 3.664 |
| COPD (Yes) | 0.771 | 0.420 | 2.161 | 1.834 | 3.363 | 1 | 0.067 | 0.948 | 4.926 |
| RBS | 0.062 | 0.030 | 1.064 | 2.093 | 4.381 | 1 | 0.036 | 1.004 | 1.128 |
| d‐dimer | −0.000 | 0.000 | 1.000 | −0.544 | 0.296 | 1 | 0.586 | 1.000 | 1.000 |
| Ferritin | 0.000 | 0.000 | 1.000 | 0.923 | 0.852 | 1 | 0.356 | 1.000 | 1.001 |
| NLR | 0.047 | 0.019 | 1.048 | 2.449 | 5.997 | 1 | 0.014 | 1.009 | 1.089 |
| Multicollinearity diagnostics | ||
|---|---|---|
| Tolerance | VIF | |
| Age | 0.926 | 1.080 |
| DM | 0.826 | 1.211 |
| CKD | 0.875 | 1.143 |
| COPD | 0.893 | 1.120 |
| RBS | 0.862 | 1.160 |
| d‐dimer | 0.889 | 1.125 |
| Ferritin | 0.935 | 1.070 |
| NLR | 0.914 | 1.094 |
Note. Prognosis level “1” coded as class 1.
Abbreviations: COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; DM, diabetes mellitus; NPR, neutrophil‐to‐platelet ratio; RBS, random blood sugar; VIF, variance inflation factor.
Asaduzzaman MD, Romel Bhuia M, Nazmul Alam ZHM, Zabed Jillul Bari M, Ferdousi T. Significance of hemogram‐derived ratios for predicting in‐hospital mortality in COVID‐19: a multicenter study. Health Sci Rep. 2022;5:e663. 10.1002/hsr2.663
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are not publicly available because of having no permission of the hospitals from where data were collected.
REFERENCES
- 1. COVID live—Coronavirus statistics—worldometer [Internet] . 2022. 2022. Accessed March 16, 2022. https://www.worldometers.info/coronavirus/
- 2. The true death toll of COVID‐19: estimating global excess mortality . n.d. Accessed March 26, 2022. https://www.who.int/data/stories/the-true-death-toll-of-covid-19-estimating-global-excess-mortality
- 3. Yamamoto N, Bauer G. Apparent difference in fatalities between Central Europe and East Asia due to SARS‐COV‐2 and COVID‐19: four hypotheses for possible explanation. Med Hypotheses. 2020;144:110160. 10.1016/j.mehy.2020.110160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahammed T, Anjum A, Rahman MM, Haider N, Kock R, Uddin MJ. Estimation of novel coronavirus (COVID‐19) reproduction number and case fatality rate: a systematic review and meta‐analysis. Health Science Reports. 2021;4(2):e274. 10.1002/hsr2.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah MRT, Ahammed T, Anjum A, Chowdhury AA, Suchana AJ. Finding the real COVID‐19 case‐fatality rates for SAARC countries. Biosafety and Health. 2021;3(3):164‐171. 10.1016/j.bsheal.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID‐19 patients: a systematic review and meta‐analysis. Intervirology. 2020;64(1):1‐12. 10.1159/000512592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi C, Wang L, Ye J, et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta‐analysis. BMC Infect Dis. 2021;21(1):663. 10.1186/s12879-021-06369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dessie ZG, Zewotir T. Mortality‐related risk factors of COVID‐19: a systematic review and meta‐analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855. 10.1186/s12879-021-06536-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiss S, Gede N, Hegyi P, et al. Early changes in laboratory parameters are predictors of mortality and ICU admission in patients with COVID‐19: a systematic review and meta‐analysis. Med Microbiol Immunol. 2021;210(1):33‐47. 10.1007/s00430-020-00696-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Citu C, Gorun F, Motoc A, et al. The predictive role of NLR, d‐NLR, MLR, and SIRI in COVID‐19 mortality. Diagnostics. 2022;12(1):122. 10.3390/diagnostics12010122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simadibrata DM, Calvin J, Wijaya AD, Ibrahim NAA. Neutrophil‐to‐lymphocyte ratio on admission to predict the severity and mortality of COVID‐19 patients: a meta‐analysis. Am J Emerg Med. 2021;42:60‐69. 10.1016/j.ajem.2021.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453‐1457. 10.1016/s0140-6736(07)61602-x [DOI] [PubMed] [Google Scholar]
- 13. Zou X, Li S, Fang M, et al. Acute Physiology and Chronic Health Evaluation II score as a predictor of hospital mortality in patients of Coronavirus disease 2019. Crit Care Med. 2020;48(8):e657‐e665. 10.1097/CCM.0000000000004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang W, Liang H, Ou L, Chen B, Chen A, Li C, China Medical Treatment Expert Group for COVID‐19 . Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180(8):1081‐1089. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haimovich AD, Ravindra NG, Stoytchev S, et al. Development and validation of the quick COVID‐19 Severity Index: a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76(4):442‐453. 10.1016/j.annemergmed.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang W, Liang H, Ou L, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID‐19. JAMA Intern Med. 2020;180(8):1081‐1089. 10.1001/jamainternmed.2020.2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torres‐Macho J, Ryan P, Valencia J, et al. The PANDEMYC Score. An easily applicable and interpretable model for predicting mortality associated with COVID‐19. J Clin Med. 2020;9(10):3066. 10.3390/jcm9103066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Altschul D, Unda S, Benton J, et al. A novel severity score to predict inpatient mortality in COVID‐19 patients. Sci Rep. 2020;10(1):16726. 10.1038/s41598-020-73962-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keddie S, Ziff O, Chou M, et al. Laboratory biomarkers associated with COVID‐19 severity and management. Clin Immunol. 2020;221:108614. 10.1016/j.clim.2020.108614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vafadar Moradi E, Teimouri A, Rezaee R, et al. Increased age, neutrophil‐to‐lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID‐19 mortality. Am J Emerg Med. 2021;40:11‐14. 10.1016/j.ajem.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dong G, Du Z, Zhu J, et al. The clinical characteristics and prognosis of COVID‐19 patients with comorbidities: a retrospective analysis of the infection peak in Wuhan. Ann Transl Med. 2021;9(4):280. 10.21037/atm-20-4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho S, Yoon S, Lee H. Impact of comorbidity burden on mortality in patients with COVID‐19 using the Korean health insurance database. Sci Rep. 2021;11(1):6375. 10.1038/s41598-021-85813-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229‐231. 10.1093/aje/kwh056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leong HN. SARS in Singapore‐predictors of disease severity. Ann Acad Med Singap.2006;35:326‐331. [PubMed] [Google Scholar]
- 25. Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El‐Sheemy MA. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417‐423. 10.2147/IJGM.S67061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jun T, Nirenberg S, Weinberger T, et al. Analysis of sex‐specific risk factors and clinical outcomes in COVID‐19. Commun Med. 2021;1(1):3. 10.1038/s43856-021-00006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim L, Garg S, O'Halloran A, et al. Risk factors for intensive care unit admission and in‐hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID‐19)‐Associated Hospitalization Surveillance Network (COVID‐NET). Clin Infect Dis. 2021;72(9):e206‐e214. 10.1093/cid/ciaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID‐19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116(14):2197‐2206. 10.1093/cvr/cvaa284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imam Z, Odish F, Gill I, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID‐19 patients in Michigan, United States. J Intern Med. 2020;288(4):469‐476. 10.1111/joim.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alizadehsani R, Alizadeh Sani Z, Behjati M, et al. Risk factors prediction, clinical outcomes, and mortality in COVID‐19 patients. J Med Virol. 2021;93(4):2307‐2320. 10.1002/jmv.26699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Faria SS, Fernandes PC Jr., Jr , Silva MJ, et al. The neutrophil‐to‐lymphocyte ratio: a narrative review. Ecancer. 2016;10:10. 10.3332/ecancer.2016.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naess A, Nilssen S, Mo R, Eide G, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2016;45(3):299‐307. 10.1007/s15010-016-0972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short‐ and long‐term mortality after Non–ST‐elevation myocardial infarction. Am J Cardiol. 2010;106(4):470‐476. 10.1016/j.amjcard.2010.03.062 [DOI] [PubMed] [Google Scholar]
- 35. Guthrie G, Charles K, Roxburgh C, Horgan P, McMillan D, Clarke S. The systemic inflammation‐based neutrophil–lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218‐230. 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 36. Ha YJ, Hur J, Go DJ, et al. Baseline peripheral blood neutrophil‐to‐lymphocyte ratio could predict survival in patients with adult polymyositis and dermatomyositis: a retrospective observational study. PLoS One. 2018;13(1):e0190411. 10.1371/journal.pone.0190411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ulloque‐Badaracco J, Ivan Salas‐Tello W, Al‐kassab‐Córdova A, et al. Prognostic value of neutrophil‐to‐lymphocyte ratio in COVID‐19 patients: a systematic review and meta‐analysis. Int J Clin Pract. 2021;75:14596. 10.1111/ijcp.14596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prasetya I, Cucunawangsih C, Lorens J, Sungono V, El‐Khobar K, Wijaya R. Prognostic value of inflammatory markers in patients with COVID‐19 in Indonesia. Clin Epidemiology Glob Health. 2021;11:100803. 10.1016/j.cegh.2021.100803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang A‐P, Liu J‐P, Tao W‐Q, Li H‐M. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol. 2020;84:106504. 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529‐539. 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tabary M, Khanmohammadi S, Araghi F, Dadkhahfar S, Tavangar S. Pathologic features of COVID‐19: a concise review. Pathol Res Pract. 2020;216(9):153097. 10.1016/j.prp.2020.153097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li M, Li L, Zhang Y, Wang X. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. 10.1186/s40249-020-00662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Y, Du X, Chen J, et al. Neutrophil‐to‐lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID‐19. J Infect. 2020;81(1):e6‐e12. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu L, Zou S, Wang C, Tan X, Yu M. Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc Disord. 2019;19(1):125. 10.1186/s12872-019-1110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Proctor M, McMillan D, Morrison D, Fletcher C, Horgan P, Clarke S. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107(4):695‐699. 10.1038/bjc.2012.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto‐Furusho J, Mendieta‐Escalante E. Diagnostic utility of the neutrophil‐platelet ratio as a novel marker of activity in patients with ulcerative colitis. PLoS One. 2020;15(4):e0231988. 10.1371/journal.pone.0231988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watt D, Proctor M, Park J, Horgan P, McMillan D. The Neutrophil‐Platelet Score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS One. 2015;10(11):e0142159. 10.1371/journal.pone.0142159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. López‐Escobar A, Madurga R, Castellano JM, et al. Risk Score for Predicting In‐Hospital Mortality in COVID‐19 (RIM Score). Diagnostics. 2021;11(4):596. 10.3390/diagnostics11040596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. López‐Escobar A, Madurga R, Castellano JM, et al. Hemogram as marker of in‐hospital mortality in COVID‐19. J Investig Med. 2021;69(5):962‐969. 10.1136/jim-2021-001810 [DOI] [PubMed] [Google Scholar]
- 50. Krenn‐Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet‐to‐lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524‐2530. 10.1038/bjc.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uslu A, Küçük A, Şahin A, et al. Two new inflammatory markers associated with Disease Activity Score‐28 in patients with rheumatoid arthritis: neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio. Int J Rheum Dis. 2015;18(7):731‐735. 10.1111/1756-185x.12582 [DOI] [PubMed] [Google Scholar]
- 52. Sarkar S, Kannan S, Khanna P, Singh AK. Role of platelet‐to‐lymphocyte count ratio (PLR), as a prognostic indicator in COVID‐19: a systematic review and meta‐analysis. J Med Virol. 2022;94(1):211‐221. 10.1002/jmv.27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simadibrata DM, Pandhita BAW, Ananta ME, Tango T. Platelet‐to‐lymphocyte ratio, a novel biomarker to predict the severity of COVID‐19 patients: a systematic review and meta‐analysis. J Intensive Care Soc. 2022;23(1):20‐26. 10.1177/1751143720969587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eslamijouybari M, Heydari K, Maleki I, et al. Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios in COVID‐19 patients and control group and relationship with disease prognosis. Caspian J Intern Med. 2020;11(Suppl 1):531‐535. 10.22088/cjim.11.0.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Li X, Shang Y, et al. Ratios of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte predict all‐cause mortality in inpatients with coronavirus disease 2019 (COVID‐19): a retrospective cohort study in a single medical centre. Epidemiol Infect. 2020;148:e211. 10.1017/S0950268820002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune‐inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(10):2077‐2086. 10.1007/s00432-017-2451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tong Y, Tan J, Zhou X, Song Y, Song Y. Systemic immune‐inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non‐small cell lung cancer. J Transl Med. 2017;15(1):221. 10.1186/s12967-017-1326-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in‐hospital mortality in COVID‐19 patients. Molecules. 2020;25(23):5725. 10.3390/molecules25235725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Doganci S, Ince ME, Ors N, et al. A new COVID‐19 prediction scoring model for in‐hospital mortality: experiences from Turkey, single center retrospective cohort analysis. Eur Rev Med Pharmacol Sci. 2020;24(19):10247‐10257. 10.26355/eurrev_202010_23249 [DOI] [PubMed] [Google Scholar]
- 60. Xue G, Gan X, Wu Z, et al. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID‐19. Int Immunopharmacol. 2020;89(Pt A):107065. 10.1016/j.intimp.2020.107065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yildiz H, Castanares‐Zapatero D, Pierman G, et al. Validation of neutrophil‐to‐lymphocyte ratio cut‐off value associated with high in‐hospital mortality in COVID‐19 patients. Int J Gen Med. 2021;14:5111‐5117. 10.2147/IJGM.S326666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aly MM, Meshref TS, Abdelhameid MA, et al. Can hematological ratios predict outcome of COVID‐19 patients? A multicentric study. J Blood Med. 2021;12:505‐515. 10.2147/JBM.S316681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan X, Li F, Wang X, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross‐sectional study. J Med Virol. 2020;92(11):2573‐2581. 10.1002/jmv.26061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Azab B, Camacho‐Rivera M, Taioli E. Average values and racial differences of neutrophil lymphocyte ratio among a nationally representative sample of United States subjects. PLoS One. 2014;9(11):e112361. 10.1371/journal.pone.0112361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil‐lymphocyte ratio, lymphocyte‐monocyte ratio, platelet‐lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine. 2018;97(26):e11138. 10.1097/md.0000000000011138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li X, Liu C, Mao Z, et al. Predictive values of neutrophil‐to‐lymphocyte ratio on disease severity and mortality in COVID‐19 patients: a systematic review and meta‐analysis. Critical Care (London, England). 2020;24(1):647. 10.1186/s13054-020-03374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou J, Huang L, Chen J, et al. Clinical features predicting mortality risk in older patients with COVID‐19. Curr Med Res Opin. 2020;36(11):1753‐1759. 10.1080/03007995.2020.1825365 [DOI] [PubMed] [Google Scholar]
- 68. Tahtasakal CA, Oncul A, Sevgi DY, et al. Could we predict the prognosis of the COVID‐19 disease? J Med Virol. 2021;93(4):2420‐2430. 10.1002/jmv.26751 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available because of having no permission of the hospitals from where data were collected.


