Abstract
The European Commission has determined that cannabidiol (CBD) can be considered as a novel food (NF), and currently, 19 applications are under assessment at EFSA. While assessing these, it has become clear that there are knowledge gaps that need to be addressed before a conclusion on the safety of CBD can be reached. Consequently, EFSA has issued this statement, summarising the state of knowledge on the safety of CBD consumption and highlighting areas where more data are needed. Literature searches for both animal and human studies have been conducted to identify safety concerns. Many human studies have been carried out with Epidyolex®, a CBD drug authorised to treat refractory epilepsies. In the context of medical conditions, adverse effects are tolerated if the benefit outweighs the adverse effect. This is, however, not acceptable when considering CBD as a NF. Furthermore, most of the human data referred to in the CBD applications investigated the efficacy of Epidyolex (or CBD) at therapeutic doses. No NOAEL could be identified from these studies. Given the complexity and importance of CBD receptors and pathways, interactions need to be taken into account when considering CBD as a NF. The effects on drug metabolism need to be clarified. Toxicokinetics in different matrices, the half‐life and accumulation need to be examined. The effect of CBD on liver, gastrointestinal tract, endocrine system, nervous system and on psychological function needs to be clarified. Studies in animals show significant reproductive toxicity, and the extent to which this occurs in humans generally and in women of child‐bearing age specifically needs to be assessed. Considering the significant uncertainties and data gaps, the Panel concludes that the safety of CBD as a NF cannot currently be established.
Keywords: Cannabidiol, Novel Food, safety, data gaps
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Regulation (EU) 2015/2283 1 lays down the applicable rules for the placing of novel foods (NFs) on the market within the European Union (EU). According to the regulation, in order to ensure the harmonised scientific assessment of NFs in the EU, such assessments should be carried out by EFSA. In performing its scientific assessment EFSA should assess, inter alia, all the characteristics of the NF that may pose a safety risk to human health and consider possible effects on vulnerable groups of the population. EFSA received to date five mandates for the assessment of synthetic cannabidiol (CBD) as NF and 15 additional applications on CBD extracted from hemp that are under validity check. 2 In addition, the EC is receiving further NF applications related mainly to CBD extracted from hemp.
Extraction of CBD from hemp can be performed through several extraction procedures resulting in very different profiles of extracted cannabinoids [CBD, tetrahydrocannabinol (THC) and other cannabinoids] depending on the source material (e.g., variety and part of the plants) and extraction procedure employed (e.g., solvents and technique employed). The extraction procedures may then potentially result in a variety of chemical mixtures composed of biologically active substances.
Owing to the scientific and technical complexity to (i) fully characterise the toxicological profile of CBD as individual substance, (ii) thoroughly assess the potentially adverse effects associated to CBD consumption reported in the literature, (iii) assess the impact of the reported potential of CBD to interfere with drug metabolism, and (iv) assess the long‐term effects in humans from chronic consumption of CBD as food, which are at present not thoroughly investigated, the EFSA NDA Panel considers that a comprehensive assessment of all the information available in the scientific literature on CBD as pure substance beyond the information provided on the current NF applications should be undertaken. The outcome of this comprehensive assessment will further inform the EFSA NDA Panel on the evaluation of the safety of ongoing and upcoming NF applications on CBD. The outcomes of this activity should then be transparently communicated to the EFSA stakeholders.
To this end, the NDA Panel is asked to prepare a statement to present and discuss the data gaps identified in the available scientific literature with regards to the safety of CBD as a NF.
1.1.2. Terms of Reference
The NDA Panel is requested by EFSA to prepare a statement on the safety of CBD as NF.
The statement shall present and discuss the data gaps identified in the available scientific literature with regards to the safety of CBD as NF.
1.2. Scope of the statement
The scope of the current document is to:
identify the hazards of CBD used as food supplement and/or food ingredient;
provide an overview of the uncertainties and data gaps that need to be addressed before the safety assessment of applications for CBD as a NF can be concluded.
2. Data and methodologies
2.1. Data
Literature searches (in Web of Science, Scopus, SciFinder, PubMed) including both animal and human studies have been conducted to identify the safety concerns for CBD as a NF. The NDA Panel is conducting a systematic review on human studies reporting the use of CBD (with purity > 95%). In this statement, the NDA Panel has summarised data and has identified areas where data are either considered missing or inadequate to draw conclusions on the safety of CBD as a NF.
3. Regulatory aspects
CBD is a substance that can be obtained from Cannabis sativa L. plants and which can also be synthesised chemically. In November 2020, in case C‐663/18, the Court of Justice of the European Union concluded that CBD should not be considered as a narcotic drug within the meaning of the 1961 Single Convention on Narcotic Drugs. As a consequence, the European Commission considered that CBD could be qualified as a food, provided that the other conditions regarding the definition of food 3 are also met. CBD, which was not demonstrated to be used for human consumption to a significant degree prior to 15 May 1997, must thereby be considered a NF. This ruling applies to both extracted and chemically synthesised CBD.
As of mid‐March 2022, the European Commission has received more than 150 applications for CBD as NF and 19 are currently under assessment by EFSA. Most of the applications are for CBD extracted from hemp plants, but there are also several applications with chemically synthesised CBD.
While assessing the data in these submissions, it became clear that there are significant data gaps that need to be addressed before a conclusion on the safety of CBD as a NF can be reached. Consequently, EFSA has issued this statement, summarising the state of knowledge of the safety of CBD consumption, and highlighting areas where more data are needed.
3.1. Status of CBD authorisation and assessment
At present, the only authorised CBD product on the European Union market is Epidyolex® (or Epidiolex® outside the EU), a prescription medicine containing highly purified CBD. The active component in Epidyolex® is a CBD extract from Cannabis sativa with a purity of ≥ 98%. It has been favourably assessed by the European Medicines Agency (EMA) 4 and authorised by the European Commission 5 as an adjunctive therapy for seizures associated with Lennox–Gastaut syndrome (LGS), Dravet syndrome (intractable childhood epilepsy) or tuberous sclerosis complex (TSC) for patients 2 years of age and older. Epidyolex® was designated as an ‘orphan medicine.’ 6
It should be noted that, as prescribed by Directive 2001/83/EC 7 , the opinions of EMA are based on balancing the desired therapeutic effects or ‘benefits’ of a medicine against its undesired effects or ‘risks’ and ‘The Agency can recommend the authorisation of a medicine whose benefits are judged to be greater than its risks’ 8 , even if adverse effects have been reported to be causally related to the administration of the drug concerned. In contrast, according to Regulation (EC) No 178/2002 and Regulation (EU) 2015/2283, foods, including NFs, must be safe. Consequently, the opinions of EFSA are based solely on the analysis of the health risks and must conclude on that basis alone. To conclude on the safety of a NF, the NDA Panel follows the EFSA guidance on the preparation and presentation of an application for authorisation of a NF (EFSA NDA Panel, 2021). As of today, CBD has not been authorised under Regulation (EU) 2015/2283.
Other non‐EU authorities have already expressed concerns about data gaps and limited information on CBD. For example, the Food and Drug Administration in the USA (FDA) has stated in 2020 that the safety profile for products containing cannabis or cannabis‐derived compounds, including CBD, is still limited and has extended indefinitely a ‘public hearing to obtain scientific data and information about the safety, manufacturing, product quality, marketing, labelling, and sale of products containing cannabis‐derived compounds’. 9 In this document, FDA also highlighted the need for additional data to address uncertainties and data gaps related to CBD.
In 2020, the Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT) (the independent scientific committee that provides advice to the UK Food Standards Agency) published a ‘Position paper on the potential risk of CBD in CBD food products’. 10 COT evaluated particularly the data available for Epidyolex® and established a ‘pragmatic upper level of intake above which there would be clear concerns about safety, until further data are available’ at 1 mg CBD/kg body weight (bw) per day. It should be highlighted, however, that the COT stated that available data ‘were insufficient to undertake a provisional risk assessment as it was not possible to determine a reliable point of departure such as a NOAEL’ and that the advice provided ‘does not mean that these levels are definitely safe, but that there is evidence that adverse health effects could occur at intakes above this level’.
3.2. Target population with CBD as food supplement and food ingredient
Different approaches to assess exposure apply, depending on the proposed uses of CBD, i.e., whether it is intended to be used as food supplement or as food ingredient to be added to foods.
If CBD is intended to be used as an ingredient for food supplements, applicants need to demonstrate the safety of CBD only for the intended target population, and labelling measures could be proposed by applicants to limit the maximum daily dose, to specify other restrictions or precautions regarding the consumption of CBD.
In relation to CBD as food ingredient, Article 5(6) of the Commission Implementing Regulation (EU) 2017/2469 11 states that ‘where it cannot be excluded that a novel food intended for a particular group of the population would be also consumed by other groups of the population the safety data provided shall also cover those groups’.
The underlying reason for the requirement to provide safety data for all population groups is that labelling measures are considered by EU risk managers in the NF regulation not to be an appropriate tool to prevent consumption of a NF by non‐target population groups (e.g., children, and pregnant or lactating women), when the NF is used as an ingredient added to foods other than food supplements or added to foods that fall under the scope of Regulation (EU) No 609/2013 12 , i.e., foods intended for infants and young children, foods for special medical purposes and total diet replacement for weight control. Both risk managers and EFSA consider that foods, such as non‐alcoholic beverages, bars, bakery wares, dairy products, etc., could be consumed by all age groups of the general population including children, and pregnant and lactating women regardless of whether or not an applicant intends to market such foods with added CBD to only a certain age group, e.g., adults. Labelling measures alone are not adequate to address this problem. Consequently, the safety of CBD must be demonstrated for all groups of the general population if CBD is intended to be added to such foods.
4. Limitations and uncertainties in scientific literature
Several toxicological studies on CBD have been conducted in vivo (in mice, rats, dogs and monkeys) as well as a range of in vitro studies. The Panel noted that a major issue in interpreting many of these studies is that they were conducted with different preparations or extracts, containing varying concentrations of CBD and other cannabinoids. Furthermore, the content of other components from the extraction and enrichment process and their identity are rarely described. Consequently, there is some degree of uncertainty as to whether the effects reported in studies conducted with products with low CBD content can be attributed exclusively to CBD. A consequence of conducting studies with CBD extracts rather than the pure compound is that it is difficult to identify a CBD‐specific reference point (point of departure) for adversity.
In humans, many studies have involved patients that required concomitant use of other medications. Furthermore, most of the human data referred to in the CBD applications are taken from studies examining the efficacy of Epidyolex® at therapeutic doses at which adverse effects were sometimes observed. Therefore, no NOAEL could be identified from these studies.
4.1. Molecular targets of CBD
CBD can trigger a variety of biological effects by interacting with different molecular targets (Turner et al., 2017; de Almeida and Devi, 2020; Peng et al., 2022), as illustrated in Figure 1. These are widely distributed in the body, including brain, gut, heart, muscle, bone, adipose tissue and endocrine system, resulting in a variety of biological effects (Turner et al., 2017; de Almeida and Devi, 2020; Peng et al., 2022).
Figure 1.
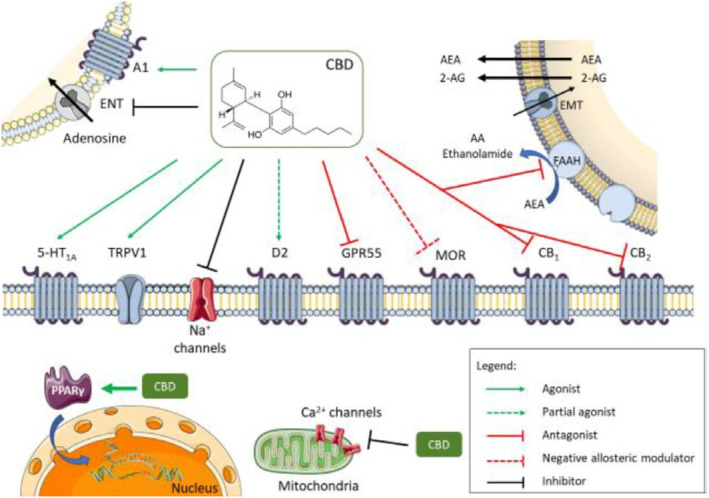
Diversity of molecular targets and signalling pathways for CBD
- Source: de Almeida and Devi (2020). Abbreviations: 2‐AG, 2‐arachidonoylglycerol; 5‐HT1A, 5‐hydroxytryptamine 1A receptor; A1, adenosine 1; AA, arachidonic acid; AEA, anandamide; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; D2, dopamine receptor 2; EMT, endocannabinoid membrane transporter; ENT, equilibrative nucleoside transporter; FAAH, fatty acid amide hydrolase; GPR55, G‐protein receptor 55; MOR μ opioid receptor; PPARγ, peroxisome proliferator‐activated receptor gamma; TRPV1, transient receptor potential vanilloid 1.
CBD acts as an antagonist for CB1 and CB2 cannabinoid receptors (Pertwee et al., 2002; Thomas et al., 2007; Peng et al., 2022). CB1 receptors are mainly distributed in the central nervous system, and they are particularly abundant in brain areas associated with motor control, emotional responses, motivated behaviour and energy homoeostasis (Freund et al., 2003; Cristino et al., 2020). CB1 receptors are also expressed in heart, liver, pancreas, muscles, adipose tissue and reproductive system. Conversely, CB2 receptors are mainly present in peripheral nerve terminals and immune cells, although evidence has shown that they are expressed in the brain as well (Di Marzo et al., 2004).
In addition to its action on cannabinoid receptors, CBD was found to inhibit the activity of fatty acid amide hydrolase (FAAH) (Bisogno et al., 2001), a major enzyme involved in anandamide (AEA) hydrolysis. As AEA is the main endogenous CB1 receptor agonist, this suggests an indirect effect of CBD on cannabinoid receptors due to increase in endogenous AEA concentration. This could explain some of the cannabinoid‐mediated effects attributed to CBD, even though it has been otherwise shown to be also a direct cannabinoid receptor antagonist.
G‐protein‐coupled receptor 55 (GPR55) has been proposed to be a third cannabinoid receptor responsible for some effects attributed to cannabinoids that do not seem to be mediated through CB1 or CB2 receptors (Ross, 2009). The GPR55 is a G‐protein‐coupled receptor with high expression in the immune and nervous systems and in other tissues, such as the bone. CBD appears to function as a GPR55 antagonist (Ross, 2009; Whyte et al., 2009). The antagonistic effect of CBD on GPR55 may cause the overexpression of endo‐cannabinoids and interleukin 10 (IL10) (Sunda and Arowolo, 2020).
Furthermore, CBD exerts agonistic activity at the serotonin receptor 5‐HT1A (Russo et al., 2005) and acts as an allosteric inhibitor at 5‐HT3A receptors (Yang et al., 2010) widely distributed in the body. CBD induces various 5‐HT1A‐mediated physiological responses (Turner et al., 2017; de Almeida and Devi, 2020). These effects have been confirmed by in vivo studies. For instance, the ability of CBD to affect emotional states in mice were blocked by a 5‐HT1A receptor antagonist (Hartmann et al., 2019). In addition, 5‐HT1A receptor blockade prevented CBD modulation of spatial memory in mice (Magen et al., 2010). Moreover, CBD behaves as a partial agonist of D2 dopamine receptors, which may account for its antipsychotic effects (Seeman, 2016).
Alongside THC, CBD was also shown to inhibit adenosine reuptake by acting as competitive inhibitor at the adenosine transporter on EOC‐20 microglia cells (Carrier et al., 2006); this increases the endogenous adenosine content available for adenosine receptor activation. Interference with adenosine signalling has been shown in both in vitro and in vivo studies and it has been suggested to contribute to the modulatory effects of CBD in inflammatory processes (Ribeiro et al., 2012), immune responses (Turner et al., 2017), cardiovascular tone (Gonca and Darici, 2015) and cognitive states (Magen et al., 2009).
Investigations by Kathmann and colleagues showed that both THC and CBD behave as negative allosteric modulators of μ and δ opioid receptors (Kathmann et al., 2006), with CBD reducing Opioid Receptor Mu 1 (Oprm1) gene expression (Viudez‐Martínez et al., 2018).
Another proposed molecular target for CBD is the transient receptor potential vanilloid 1 (TRPV1) receptor (also known as VR1 receptor), which is involved in pain perception and regulation of various physiological functions, such as the release of inflammatory mediators in the body, gastrointestinal motility function and temperature regulation (Du et al., 2019). A study by Bisogno and colleagues (Bisogno et al., 2001) showed that CBD can displace capsaicin from the TRPV1 receptor and increase intracellular Ca2+ levels to the same extent as the full agonist capsaicin in heterologous cells overexpressing TRVR1, suggesting that it functions as an agonist of this receptor. The interaction of CBD with TRPV1 receptors has been confirmed by in vivo studies (Turner et al., 2017). In addition to TRPV‐regulated Ca2+ channels, CBD has also been shown to engage Na+ and Ca2+ channels (Ali et al., 2015; Ghovanloo et al., 2018). CBD also influences membrane fluidity and Na+ channel conductance (Gaston and Szaflarski, 2018).
Bakas and colleagues also identified CBD as a positive allosteric modulator of γ‐aminobutyric acid type A (GABAA) receptors (Bakas et al., 2017), which could account for its anti‐seizure, anxiolytic and analgesic effects. CBD potentiates glycine currents (Ahrens et al., 2009) by acting as a positive allosteric modulator, possibly at α1 glycine receptors.
Finally, CBD has been shown to have agonistic activities at peroxisome proliferator‐activated receptor gamma (PPARγ) receptors (Jadoon et al., 2016; Iannotti and Vitale, 2021) that are involved in glucose metabolism and insulin signalling in skeletal muscle and liver.
In summary, CBD interacts with several receptors and signalling pathways both in vitro and in vivo, resulting in a wide range of biological effects. It is essential that these receptor interactions are taken into consideration when evaluating the safety of CBD as a NF, taking into account the differences in study design (model, assays, concentrations) and interspecies differences in receptor distribution.
4.2. Absorption, distribution, metabolism and excretion (ADME)
Due to its poor aqueous solubility and higher lipid solubility, the absorption of CBD from the gastrointestinal tract is variable, albeit low, leading to a toxicokinetic profile that is equally variable. Overall, bioavailability from oral administration was estimated to be on average 6% due to significant presystemic metabolism (Hawksworth and McArdle, 2004; Chayasirisobhon, 2020). The bioavailability of CBD can be increased up to five times by a high‐fat meal (Taylor et al., 2018) or the concomitant consumption of vegetal oil (typically, hemp oil) (Patrician et al., 2019). Furthermore, CBD powder may form nanoparticles, which require appropriate assessment (further addressed in Section 4.9). Limited conversion of CBD to Δ9‐THC in the presence of acid has been suggested to occur in in vitro models (Merrick et al., 2016; Bonn‐Miller et al., 2017). However, there is no evidence that this transformation occurs in humans in vivo (Nahler et al., 2017; Lachenmeier et al., 2019; Golombek et al., 2020).
After oral CBD administration in humans, the increase in plasma concentration of CBD and its main metabolites is proportional to the dose, but variable. The time to the maximum plasma concentration (tmax) is reported as ~ 2–5 h (Atsmon et al., 2018). Maximum concentrations of CBD in plasma (Cmax) following an oral dose vary considerably between individuals. For example, following a single dose of 600 mg CBD, Cmax values varied from 1.6 to 271 mg/mL plasma (Haney et al., 2016). Oral clearance of CBD is reported as high (10–15 L/h/kg) and CBD is rapidly distributed into body tissues. The apparent volume of distribution is large (~ 20–40 × 103 L; Taylor et al., 2018). CBD may preferentially accumulate in adipose tissues due to its high lipophilicity. CBD is reported to cross the blood–brain barrier in rodents, and the brain may also be a site of accumulation (Calapai et al., 2020).
Effective half‐life estimates for CBD in humans in plasma have mostly been reported to range from 10 to 24 h, although higher values have been noted, depending on experimental conditions (Consroe et al., 1991). Twice‐daily administration of CBD doses for 2–6 days is necessary before the steady‐state concentration of the substance is reached in humans (Wheless et al., 2019). It should be noted that the concentration of CBD in plasma increases with time in mice and rats that had been given daily doses of 50 mg/kg body weight (bw) per day (EMA, 2019). Whether the same occurs in humans is not certain, although some data do support the hypothesis (Taylor et al., 2018). Whether the uptake process saturates or not is uncertain. In one study, the increase in plasma CBD concentration with increasing dose (range from 20 to 40 mg/kg bw per day calculated for an average of 70 kg body weight) did not show proportionality (Taylor et al., 2018). In contrast, in a large study including adults and children, concentrations of CBD in plasma were linearly related to intake (range from 5 to 50 mg/kg bw per day; Szaflarski et al., 2019). The simultaneous consumption of CBD and food with high fat content could lead to significantly increased systemic exposure to CBD both in animal models (Zgair et al., 2016) and in humans (Birnbaum et al., 2019).
CBD is extensively metabolised in the liver (please refer to the review by Ujváry and Hanuš, 2016). The primary route is hydroxylation to 7‐OH‐CBD, which is then metabolised further, resulting in several metabolites, including 7‐COOH‐CBD, which are excreted in faeces and urine. 6‐OH‐CBD is a relatively minor circulating metabolite after oral administration of CBD. Glucuronidation of CBD at the phenolic oxygen is a major Phase II biotransformation in humans, but hydroxylated metabolites of CBD may also be substrates (Hawksworth and McArdle, 2004). More detail is included in Section 4.4, CBD interaction with drug metabolism.
CBD and its metabolites are excreted through faeces and urine. Following oral administration of a capsule (gelcap) of 100 mg CBD, the maximum concentration of CBD itself and its metabolites, 7‐OH‐CBD and 7‐COOH‐CBD, is found at ~ 6 h post‐administration in urine (Sholler et al., 2021). Excretion levels and rates are affected by many parameters, including route and time of ingestion, independent of whether CBD is taken with or without food and the form of food used, e.g., whether as a syrup or an oral capsule (Sholler et al., 2021).
4.2.1. Data gaps
Several studies have shown that the matrix used to deliver the CBD, and food consumed at the same time, can have a marked effect on bioavailability. This needs to be considered when proposing CBD as a food ingredient, and the matrix used when delivering CBD as a supplement.
Furthermore, the kinetic behaviour of CBD in humans following long‐term exposure is not fully understood. The possibility that the long‐term accumulation observed in rats could also occur in humans is of concern and represents a data gap.
4.3. Liver
4.3.1. Animal studies
Two 90‐day studies conducted on rats using different enriched CBD extracts (25% CBD, Marx et al., 2018; 6.24% CBD, Dziwenka et al., 2020) were available to the Panel. In addition, limited information from a 26‐week study with rats with the drug Epidyolex® (≥ 98% CBD) is available from the EMA (EMA, 2019). These studies revealed the effects on the liver of the extract‐exposed animals, with increases in absolute and relative liver weights in both males and females. This correlated with centrilobular hypertrophy. In the study by Marx et al. (2018), dose‐dependent increases of alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT) and bilirubin were also found. In the EMA summary data, increases in ALT and ALP were detected, while no changes in transaminases were detected in the study by Dziwenka et al. (2020) with the extract with low CBD content. The LOAELs ranged from 12 to 90 mg/kg bw equivalent of CBD in the different studies.
The increase in relative liver weight and the corresponding histopathological findings were confirmed in a 10‐day study on mice. Using an enriched CBD extract (57.9%), there was an increase in liver weight, starting at doses of 61.5 mg/kg bw per day of CBD and reaching statistical significance at 184.5 mg/kg bw per day of CBD (Ewing et al., 2019). Similarly, a 90‐day study with Epidyolex® in mice (EMA, 2019, limited information) gave increases in liver weight and histopathological changes, starting at the lowest dose of 100 mg/kg body weight per day. In the Epidyolex® study, increases in ALT were also reported. The study by Ewing et al. (2019) showed increases in ALT, aspartate aminotransferase (AST) and total bilirubin at higher dose levels and that expression of more than 50 genes involved in hepatotoxicity were affected. Several cytochrome P450 isoforms and glucuronosyl transferases showed marked mRNA increases.
In a 39‐week study with dogs (EMA, 2019), liver weight was increased, with accompanying hepatocyte hypertrophy, at the lowest dose investigated of 10 mg/kg bw per day of Epidyolex®. In a 28‐day dog study with CBD (˃ 95% purity, in MCT oil, 0, 1, 2, 4, 12 mg/kg bw), doses above 2 mg/kg bw per day caused dose‐dependent increases in ALP, statistically significant at 12 mg/kg bw (Vaughn et al., 2020). No changes were found for ALT, AST, GGT and bilirubin and pathology was not assessed.
In a study on rhesus monkeys exposed to oral doses of 99% pure CBD of 0, 30, 100, or 300 mg CBD/kg bw per day for 90 days (Rosenkrantz et al., 1981), an increase in relative liver weight occurred in all groups; this increase was found to be reversible in a separate 30‐day recovery animal (n = 1).
Overall, the toxicity studies with rats, mice, dogs and rhesus monkeys with CBD extracts of different purity and durations up to 39 weeks consistently showed increases in absolute and relative liver weights, hypertrophy of liver cells and increases in ALT, AST, ALP, GGT or bilirubin, but the pattern was different in different species and with different CBD preparations. The lowest LOAEL of 10 mg/kg bw – which was the lowest dose investigated – was reported in a 39‐week study with dogs with highly purified CBD.
4.3.2. Human data
In a study on the liver toxicity of Epidyolex®, 16 healthy participants (18–60 years) received 1,500 mg Epidyolex® per day (750 mg in the morning and evening, corresponding to ~ 20 mg/kg bw per day) for 27 days, including a phase‐in‐period of 11 days (Watkins et al., 2021). ALT, ALP, AST, GGT and bilirubin in serum were monitored during the study. On days 1 and 26 of the study, the participants received in addition 200 mg of caffeine to investigate the effect on CYP1A2 (Thai et al., 2021; please refer to Section 4.4).
In seven of the 16 participants, ALT was above the upper limit of normal (ULN). In five of these participants, the increase was fivefold above the ULN. All elevations were observed within 2–4 weeks after the start of CBD exposure. Data provided for the five participants with ALT above fivefold ULN also showed increases in GGT, AST and ALP, while bilirubin was unchanged. There was no correlation between transaminase elevations and baseline characteristics, CYP2C19 genotype, or CBD plasma concentrations. Watkins et al. (2021) discussed that the ULNs for ALT (68 IU/L for men and women) were approximately twofold compared with those in an international consensus document (33 IU/L for men, 25 IU/L for women; Kwo et al., 2017). Applying these consensus ULNs, 69% of the participants had elevated ALT levels instead of 44%.
Increases in ALT were found in another study with healthy volunteers, treated with 750 mg Epidyolex® twice per day for 4 weeks (Taylor et al., 2020).
Liver toxicity was also detected at lower dose levels in health‐care workers in a randomised controlled trial on treatment of emotional exhaustion and burnout during the COVID‐19 pandemic. In total, 120 healthy male and female adults (24–60 years), not using any medication that may have had potential interactions with CBD, were randomised to receive either CBD, 300 mg daily (99.6% purity, 150 mg twice per day, corresponding to 4.3 mg/kg bw per day), plus standard care (n = 61) or standard care alone (control arm; n = 59) for 4 weeks. Four participants (6.8%) had elevated liver enzymes (˃ 3‐fold higher than ULN) that led to discontinuation of treatment in one participant. The authors reported that in none of these participants, the increase of total bilirubin was more than twofold. No increases were seen in the controls (Crippa et al., 2021).
In addition, several studies are available that investigated ALT, AST, ALP, GGT and bilirubin in patients with epilepsy treated with CBD.
Serum concentrations of liver enzymes were studied in a series of double‐blind, placebo‐controlled, randomised clinical trials with patients with epilepsy due to Dravet syndrome (Devinsky et al., 2017, 2018b; Miller et al., 2020), Lennox–Gastaut syndrome (Devinsky et al., 2018a; Thiele et al., 2018) or patients with TSC (Thiele et al., 2021). The studies comprised mainly children and adolescents, (2–18 years of age in Devinsky et al., 2017, 2018b and Miller et al., 2020; 2–55 years of age in Devinsky et al., 2018a and Thiele et al., 2018; 1–65 years of age in Thiele et al., 2021). Patients (n = 59–86 per group) received (including a period of 1–2 weeks of dose escalation) 20 mg/kg bw per day for 14 weeks of placebo or Epidyolex® divided in two equal doses of 10 mg/kg bw (Devinsky et al., 2017; Thiele et al., 2018), 10 and 20 mg/kg bw per day (Devinsky et al., 2018a; Miller et al., 2020), 5, 10 or 20 mg/kg bw per day (Devinsky et al., 2018b) or 25 and 50 mg/kg bw per day (Thiele et al., 2021). During the time of the study, all patients took antiepileptic medication (clobazam and valproate) in addition to Epidyolex®.
ALT or AST increases of more than threefold the ULN (normal range not provided) were reported in all studies at all dose levels (lowest dose being 10 mg/kg bw per day) that, in several cases, resulted in patients being withdrawn from the study. The increases showed a clear dose–response relationship in frequency and severity, while no or minimal increases were observed in the placebo groups. The frequency of increases was higher in patients with concomitant treatment with valproate compared with other drugs. Elevations in transaminase levels were reversible, either spontaneously during treatment, after reduction of concomitant valproate dose or after cessation of CBD treatment. In two of these studies, in addition, increased GGT levels were detected in patients at higher frequency than in controls (Thiele et al., 2018, 2021).
In the publications, the ULN was not specified for the changes in liver enzyme levels. In addition, patients were only recorded in case of a threefold increase, while minor changes are not reported.
Increases in transaminases at similar dose levels were also found in open‐label extensions of these trials as well as other open‐label studies with patients with epilepsy with durations of up to 2 years (Devinsky et al., 2016, 2019; Szaflarski et al., 2018; Sands et al., 2019; Thiele et al., 2019; D’Onofrio et al., 2020; Iannone et al., 2021).
All studies with doses of 10 mg/kg bw per day and higher included a phase‐in period of 2 weeks to accustom the patients to CBD. D’Onofrio et al. (2020) used a longer phase‐in period of 1 month for reaching 10 mg/kg bw per day, and then gradually increased to 20 mg/kg bw per day over the next 5 months. They found a lower incidence of cases with increased transaminases than that observed in other studies.
Two studies gave low doses of CBD in placebo‐controlled trials in patients with Crohn’s disease (20 patients, 20 mg highly purified CBD per day in olive oil, 8 weeks; Naftali et al., 2017) or non‐insulin‐treated type 2 diabetes (13 patients, 200 mg CBD per day for 13 weeks, twice per day in fasted state, no information on CBD source; Jadoon et al., 2016). It was stated that no effects were seen on liver function/biochemistry, but no further information was provided to substantiate these results.
Overall, studies with healthy volunteers showed increases in ALT, AST, ALP and GGT at doses of Epidyolex® of 20 mg/kg bw per day. However, increases in ALT and AST were also seen in a study with 4.3 mg/kg bw per day. In patients with epilepsy that were concomitantly treated also with other antiepileptic medication, increased liver enzymes were reported in the same dose range.
4.3.3. Data gaps
There is clear evidence for liver toxicity of CBD, demonstrated by liver hypertrophy in laboratory animals and increases in liver enzymes in experimental animals and in human studies. No NOAEL can be derived from these studies.
Therefore, studies are necessary both in humans and in experimental animals that enable the identification of a point of departure (i.e., NOAEL).
4.4. CBD interaction with drug metabolism
The extensive metabolism of CBD by cytochromes P450 (CYPs) has been shown to affect the metabolism of several drugs.
4.4.1. Phase I metabolic pathways
Table 1 describes the in vitro inhibition of several human CYPs in human liver microsomes by CBD (Bansal et al., 2020).
Table 1.
In vitro inhibition of selected CYP (human) by CBD
| CYP | KI (μM) | KI,u (μM)* |
|---|---|---|
| CYP1A2 | 3.76 ± 1.44 | 0.45 ± 0.17 |
| CYP2C9 | 1.43 ± 0.28 | 0.17 ± 0.03 |
| CYP2C19 | 2.58 ± 0.46 | 0.30 ± 0.06 |
| CYP2D6 | 7.88 ± 4.14 | 0.95 ± 0.50 |
| CYP3A | 3.16 ± 0.96 | 0.38 ± 0.11 |
*: KI value corrected for the non‐specific binding of CBD due to its high lipophilicity.
The study by Bansal et al. (2020) also examined the time‐dependent inactivation of CYP1A2, 2C19 and CYP3A by CBD, with inactivation efficiencies (kinact/KI,u) of 0.70 ± 0.34, 0.11 ± 0.06 and 0.14 ± 0.04 min–1 μM–1, respectively. A combined (reversible inhibition and time‐dependent inactivation) mechanistic static model populated with these data was used by these authors to predict a moderate to strong pharmacokinetic interaction risk between orally administered CBD and drugs extensively metabolised by CYP1A2/2C9/2C19/2D6/3A (Bansal et al., 2020).
In addition, polymorphisms of CYP2C19 and, to a lesser extent, CYP3A4 are not rare (~ 20%) and should be considered an additional source of variability and concern in the presence of CBD and other substrates of these enzymes.
In addition to CYP inhibition, the administration of CBD in mice has been reported to result in the induction of several hepatic CYPs. A study by Bornheim et al. (1994) documented a dose‐ and time‐dependent increase in both CYP2B, 3A and 2C content and activity as well as in mRNA levels after daily administration of CBD (120 mg/kg bw for 4 days, intraperitoneal). A similar observation was made by Ewing et al. (2019) who showed a dose‐dependent increase in several CYP mRNA levels at 24 h in the livers of mice administered CBD by gavage. In that study, the induction occurred at doses of CBD that were also hepatotoxic based on the increase in serum AST and ALT levels at 24 h. The human relevance of the induction of CYPs observed in mice is not clear.
4.4.2. Phase II metabolic pathways
Data suggest that CBD may have inhibitory effects at clinically relevant doses on UDP‐glucuronosyltransferases (UGT)1A9 and UGT2B7 (Mazur et al., 2009; Millar et al., 2018). The clinical impact of this activity has not been assessed but, recently, a levothyroxine–CBD interaction was reported (Cáceres Guido et al., 2021) indicating a possible interaction of CBD with thyroid hormone concentrations via UGT. The latter suggests that CBD may interfere with thyroid hormone metabolism (please also refer to Section 4.6.1).
4.4.3. Drug transport
CBD and its 7‐OH‐CBD metabolite have no predicted activity on drug transporters (Brown and Winterstein, 2019). However, 7‐COOH‐CBD, a main metabolite of CBD (please refer to Section 4.2) is a substrate for P‐glycoprotein and has been reported to weakly inhibit this transporter as well as OAT1, breast cancer resistance protein (BCRP), OATP1B1, OATP1B3, OAT3 and a bile salt export pump (Brown and Winterstein, 2019; EMA, 2019).
4.4.4. CBD–drug pharmacokinetic interactions
Most of the data on the interaction of CBD with other drugs pertain to antiepileptic drugs. The study by Ben‐Menachem et al. (2020) showed that CBD administration (patients, Epidiolex®, at a dose of 20 mg/kg bw per day from days 12 to 26, concomitant with stiripentol, following a 10‐day dose‐escalation period) led to a small increase in stiripentol plasma levels (17% increase in maximum observed plasma concentration [Cmax]; 30% increase in area under the concentration–time curve over the dosing interval [AUC(tau)]). Co‐administration of cannabidiol with valproate or its metabolite, 4‐ene‐VPA, did not affect the pharmacokinetics in adult patients with epilepsy. The clinical relevance of the increase in stiripentol exposure is unclear.
One study (Geffrey et al., 2015) tested in children with epilepsy the in vivo drug–drug interaction potential between CBD (20–25 mg/kg bw per day) and co‐administered clobazam. Clobazam is metabolised extensively by CYP3A4, CYP2C19 and CYP2B6, and may also be a competitive inhibitor on these isoforms. CBD metabolism was affected (increases in Cmax of 73% and AUC of 47% for CBD and 7‐OH‐CBD). Similarly, clobazam concentrations increased on average by 60% and its active metabolite norclobazam was increased by three‐ to fivefold. These findings were confirmed in other in vivo studies with additional observed increases in topiramate, rufinamide, zonisamide and eslicarbazepine for which incremental doses of CBD (5–50 mg/kg bw per day) were used (Gaston et al., 2017). A similar observation was made with tacrolimus (Leino et al., 2019). In contrast, in a study on healthy volunteers (Morrison et al., 2019), concomitant CBD (750 mg twice daily) had little effect on clobazam exposure (Cmax and AUC) but N‐desmethylclobazam exposure increased (Cmax and AUC, 3.4‐fold), stiripentol exposure increased slightly (Cmax, 1.3‐fold; AUC, 1.6‐fold), while no clinically relevant effect on valproate exposure was observed.
In another study (Thai et al., 2021), healthy subjects were given increasing doses of CBD from 250 mg once daily to 750 mg twice daily between days 3 and 11 and 750 mg CBD twice daily between days 12 and 27. On day 26, subjects received a single 200 mg caffeine dose with their CBD morning dose. When caffeine was administered with steady‐state CBD, caffeine exposure increased by 15% for Cmax and 95% for AUC0‐∞, tmax increased from 1.5 to 3.0 h, and t½ increased from 5.4 to 10.9 h compared with caffeine administered with placebo, which suggest that CBD inhibits caffeine degradation. Under the same conditions, para‐xanthine exposure decreased by 22% for Cmax and increased by 18% for AUC0‐∞, tmax increased from 8.0 to 14.0 h, and t½ increased from 7.2 to 13.7 h.
A phase II trial conducted in epileptic patients reported no interaction between CBD (20 mg/kg bw per day) and clobazam but interaction with its major active metabolite N‐desmethylclobazam (VanLandingham et al., 2020).
4.4.5. Data gaps
Most studies have focused on interactions between CBD and neurological drugs used to treat epilepsy, and data on potential interactions with other drugs are lacking. It should be noted that interaction between other drugs and CBD, because of common metabolic pathways, would also impact on the kinetics of CBD. This concern needs to be addressed.
Both animal and human data presented here and in Section 4.3 (Liver) show that CBD has extensive effects on liver function. The extent to which these liver effects might modify the metabolism of drugs is not known, and it is an important data gap, especially given the wide range of CYP enzymes with which CBD interacts and the possibility of enzymes induction. Additionally, the CBD concentrations at which these interactions manifest is not clear.
4.5. Gastrointestinal tract
The most common and consistently reported gastrointestinal effect associated with the oral intake of CBD in human studies is diarrhoea, while the occurrence of vomiting, nausea, gastrointestinal discomfort or constipation is less frequently observed.
Five randomised control trials with an oral exposure to highly purified plant‐derived CBD for 2 weeks up to 16 weeks (Devinsky et al., 2018a; Thiele et al., 2018, 2021; Ben‐Menachem et al., 2020; Miller et al., 2020) were carried out with patients (aged between 1 and 65 years) suffering from epilepsy or seizures associated with either TSC, Lennox–Gastaut syndrome or Dravet syndrome who concomitantly received multiple antiepileptic medications. The total CBD doses provided in these studies varied between 10 and 50 mg/kg bw per day and were taken in two separate doses (morning and evening). The results of these studies pointed to a dose‐dependent increase in the occurrence of diarrhoea, starting at intakes of 10 mg/kg bw per day and going up to as high as 57% at doses of 50 mg/kg bw per day.
Five randomised control trials reporting on gastrointestinal effects in conjunction with CBD administration were conducted in apparently healthy adults (Taylor et al., 2018; Hurd et al., 2019; Perkins et al., 2020; Crippa et al., 2021; Arout et al., 2022). The test substance in these studies was purified plant‐derived CBD, except for the study by Arout et al. (2022) in which a non‐natural isomer, (+)CBD, was used. The randomised control trial by Taylor et al. (2018) consisted of three study arms: (i) at single doses of either placebo or 1,500, 3,000, 4,500 or 6,000 mg CBD, occurrence of diarrhoea was 25%, 0%, 83.3%, 50% and 66.7%, respectively; (ii) at doses of placebo, 750 or 1,500 mg/day, given in two doses for 6 days and as a single dose on day 7, occurrence of diarrhoea was 0%, 44% and 88.9%, respectively; (iii) at a single dose of 1,500 mg in either fed or fasted state, occurrence of diarrhoea was 25% in the fasted state and 0% in the fed state. In the randomised control trials by Hurd et al. (2019), in subjects given either placebo or single doses of 400 or 800 mg CBD (corresponding to ~ 5 and 10 mg/kg bw) on 3 consecutive days, diarrhoea was reported by 0%, 7.1% and 23% of the respective groups. In the three other randomised control trials, diarrhoea was not observed at acute CBD doses of 5 and 10 mg/kg bw per day (Perkins et al., 2020) and of 200, 400 and 800 mg/day (Arout et al., 2022) or at a CBD intake of 300 mg per day (divided in two doses) over 4 weeks (Crippa et al., 2021).
Several open‐label trials also reported the occurrence of diarrhoea as the most common and consistently observed gastrointestinal effect associated with oral intake of CBD, albeit none of them included placebo or control groups. Most of these studies were again on patients suffering from epilepsy of various aetiologies (age range 0.4–62 years), whereby purified plant‐derived CBD or, in one case, also synthetic (+)CBD was given as adjunctive therapy to multiple antiepileptic medication (Devinsky et al., 2016, 2019; Klotz et al., 2019; Laux et al., 2019; Sands et al., 2019; D'Onofrio et al., 2020; Gaston et al., 2021; Iannone et al., 2021; Patel et al., 2021). These studies included between 26 and 607 patients, who ingested doses of 14 up to 32 mg/kg bw per day over periods varying between 12 weeks up to 2 years. The reported occurrence of diarrhoea ranged between 4.8% and 38.3%. In one open‐label clinical trial by Watkins et al. (2021) in 16 healthy adult subjects receiving CBD (Epidiolex®) over 27 days in escalating doses up to 1,500 mg in two daily doses, diarrhoea was reported by 50% of the participants.
4.5.1. Data gaps
Evidence suggests that CBD may affect gastrointestinal function by triggering diarrhoea in humans. There is a lack of studies specifically designed to investigate this diarrhoea‐inducing effect during acute and longer‐term exposure to CBD in healthy human population groups. Furthermore, there is a lack of understanding the mechanism by which CBD may exert this diarrhoea‐triggering effect.
4.6. Neurological, psychiatric and psychologic effects
Mechanistic studies, many of which have been performed using in vitro systems, indicate that CBD may interact with multiple molecular targets and influence some different signalling pathways (Figure 1).
Numerous potential targets of CBD (please also refer to Section 4.1), including CB1 or CB2 receptors, the FAAH, GABAA receptors, 5HT1A receptors or D2 receptors, are expressed in the nervous system. The extent of the CBD effect(s) will depend on the interplay between the target receptors, the concentration and duration of the dose, whether diseases or medical conditions are present and the concomitant use of other drugs.
In controlled clinical trials that were reviewed in the context of authorisation of Epidiolex®/Epidyolex® as an adjunctive antiepileptic drug in combination with at least one other antiepileptic drug for the treatment of seizures in patients, i.e., with Lennox–Gastaut or Dravet syndrome, treatment‐related adverse events were reported that occurred at a higher incidence in individuals who had received Epidiolex®/Epidyolex® compared with subjects that had received a placebo (FDA, 2018; EMA, 2019). Trials involved oral doses of 5, 10 or 20 mg/kg bw per day.
For adverse events affecting the nervous system, the summary review for Epidiolex® of the FDA Center for Drug Evaluation and Research reported somnolence, sedation and lethargy in different studies (occurring in 27–40% of treated patients compared with 11% of placebo controls), but also ataxia, abnormal motor coordination (in 1.3–20% of treated patients compared with 0% of controls), aggression, anger (up to 4.6% in treated patients as opposed to 0.4% of controls) or insomnia and sleep disorders (up to ~ 10% in treated patients as opposed to 4.4% in controls). Some of these adverse events (somnolence, sedation, lethargy, ataxia, abnormal coordination) were observed already at a dose of 5 mg/kg bw per day. The review stated that most of the cases of discontinuation of treatment in the trials were related to increases in liver transaminase levels and to manifestations of somnolence and sedation (FDA, 2018).
Similar adverse events were also described in the EMA Public Assessment Report for Epidyolex® (EMA, 2019). In controlled studies involving treatment over several weeks, neurological effects such as somnolence (24.3% of all treated individuals vs. 9.6% of controls) and sedation (4.6% vs. 0.7%) were observed. In addition, psychiatric disorders that occurred mainly within the first 2 weeks of treatment were reported, i.e., irritability (5.5% of treated individuals vs. 1.7% of controls), aggression (3.9% vs. 1%), agitation or abnormal behaviour, but without hallucinations or psychosis (EMA, 2019).
The Panel notes that most of the data on adverse events for Epidiolex®/Epidyolex® were obtained in trials with patients who were also being treated with other medications, e.g., other antiepileptic drugs.
Other placebo‐controlled human studies were retrieved from the open literature. However, many of these trials had focused on the efficacy of CBD in view of potential pharmacological applications rather than on safety aspects and/or also often involved patients with underlying neurological conditions that required the concomitant use of other medications.
Some of the studies, however, in which adverse neurological or psychological events of CBD alone were assessed, were conducted with healthy volunteers. For example, a placebo‐controlled study by Taylor et al. (2018), in which healthy male or female volunteers (nine per dose group) received CBD for 7 days, with two doses of 750 or 1,500 mg CBD per day up to day 6, and a single dose on day 7, reported adverse events that included headaches (occurring in ~ 44% of treated individuals compared with 0% of placebo controls), somnolence (in ~ 44% of subjects receiving 1,500 mg CBD twice daily compared with ~ 33% of placebo controls) and insomnia (~ 22% of individuals treated with 1,500 mg CBD twice daily compared with 0% of placebo controls).
In a trial with volunteers who had been selected for high paranoid traits, anxiety scores were determined after subjecting participants to an anxiety‐inducing virtual reality situation. Prior intake of a single dose of 600 mg CBD as Epidiolex® was associated with a further increase in anxiety scores, although this did not reach statistical significance (Hundal et al., 2018). By contrast, a single dose of 600 mg CBD appeared to reduce anxiety in patients with social anxiety disorder during simulation of a public speaking test (Bergamaschi et al., 2011). In a study in which healthy participants received CBD (400 mg) or placebo at consecutive sessions, CBD administration was associated with a significant increase in mental sedation and a decrease in subjective anxiety (determined 60 and 75 min after CBD intake) compared with baseline and to placebo treatment of the same individuals (Crippa et al., 2004). In healthy subjects who had been requested to give a public speech, significantly lower anxiety scores were observed after (but not during) the speech in participants who had received 300 mg CBD compared with those who had received a placebo (Zuardi et al., 2017). The relevance of this finding is unclear, as a significant effect was not observed for subjects treated with 100 or 900 mg CBD. In a further study in which healthy volunteers received a single dose of either THC, CBD or placebo in consecutive sessions separated by an interval of 1 month, treatment with 600 mg CBD did not lead to significant differences in physiological or mental (including anxiety) test parameters compared with placebo (Martín‐Santos et al., 2012).
4.6.1. Data gaps
The Panel notes that most human studies from the open literature concerning CBD were designed to investigate the efficacy of pharmacological use of CBD preparations rather than on safety‐related aspects. In studies with healthy volunteers, only short‐term effects after single administration of CBD preparations were investigated in most cases. Often only one dose level was tested, and dose–response relationships for neurological effects of CBD could therefore not be established.
Taking into account the interaction of CBD with multiple molecular targets that are also involved in regulation of neurophysiological processes, the Panel considers that the paucity of information on potential long‐term effects of CBD in healthy individuals and the limited information on dose–response relationships reflect major knowledge gaps.
4.7. Endocrine system
4.7.1. Thyroid
The EMA assessment report on the safety of Epidyolex® (EMA, 2019) indicates that data on triiodothyronine (T3), thyroxine (T4) and thyroid‐stimulating hormone (TSH) were provided as draft results within a 26‐week oral toxicity study in the rat. Dose‐dependent decreases in T4 and increases in TSH were noted mostly in male rats and in individual female rats. Such changes were associated with changes in thyroid weight and with thyroid follicular cell hypertrophy in both sexes. Similarly, subchronic oral exposure to CBD in the rhesus monkey resulted in significant decreases in relative thyroid weight [dose not given] (Rosenkrantz et al., 1981).
4.7.2. Hypothalamic–pituitary–gonadal axis
In oral in vivo toxicity studies in sexually mature murine and simian models, CBD displayed potential to affect gonadotropin [including luteinising hormone (LH) and follicle‐stimulating hormone (FSH)] and sex hormone (including, testosterone, oestradiol and progesterone) levels, in both males and females, at doses starting from 30 mg/kg per day (Rosenkrantz and Esber, 1980; Carvalho et al., 2018a, 2018b). A series of single dose (50 mg/kg bw CBD) gestational and perinatal exposure studies in mice also produced these changes at adult age in males (Dalterio et al., 1984a,b, 1986; Dalterio and deRooij, 1986).
Alterations of testosterone production were substantiated by in vitro data on rat testicular microsomes, which demonstrate inhibition of the steroidogenic enzyme progesterone 17‐α hydroxylase. Similar studies in rat liver microsomes suggested that CBD exposure could also alter hepatic metabolism of sex steroids (as reviewed in Carvalho et al., 2020).
Although the specific mechanisms of action at play still need to be elucidated, two main hypotheses have been proposed: (i) a direct action of CBD on the endocannabinoid signalling pathway(s), which is present in both the testes and the liver, and (ii) an interaction with CYP‐450 isoenzymes, which are involved in both hepatic hydroxylation of testosterone and steroidogenesis.
For the safety assessment of Epidyolex® (EMA, 2019), no data on hormonal levels were available.
A mode of action of CBD on the endocrine system and its relevance to humans remain unclear. However, some effects of hormonal dysregulation (please refer to Section 4.8 Reproductive system) were observed across the studies carried out with Epidyolex®, leading EMA risk assessors to conclude that ‘Monitoring for potential hormonal disturbance via clinical and pharmacovigilance activities should be initiated and the final study report with characterisation of potential risk due to hormonal disturbances should be submitted as a post‐authorisation measure’.
4.7.3. Data gaps
Evidence suggests that oral exposure to CBD affects the endocrine system. The Panel notes an important knowledge gap in animal studies on the potential endocrine effects of exposure to CBD, in particular in females. Furthermore, given the effects on IL10 expression (please refer to Section 4.1 Molecular targets of CBD), other aspects of immune function should be examined.
4.8. Reproductive system
4.8.1. Developmental toxicity
Developmental toxicity studies were carried out in both the rabbit and the rat within the assessment of Epidyolex® (EMA, 2019). In the rat, an in vivo oral embryo–foetal developmental toxicity study resulted in complete litter loss for two out of 20 females and an increased number of foetuses presenting with a supernumerary liver lobe after exposure to 250 mg/kg bw per day CBD from gestational day (GD) 6 to GD17. In the rabbit, foetal weights were reduced compared with control animals after exposure to 125 mg/kg bw per day CBD from GD7 to GD19. Numerous foetal variations at 125 mg/kg bw per day – including unossified metacarpal, bulging eyes and non‐erupted incisors – were observed in treated animals, but deemed in the EMA assessment report to be secondary to reduced foetal weights.
4.8.2. Reproductive tract toxicity
4.8.2.1. Reproductive organ weights
Oral exposure to CBD (obtained by National Institute of Drug Abuse) in murine and simian models has been associated with alterations of reproductive organ weights starting from 30 mg/kg bw per day onward. In sexually mature males specifically, literature data demonstrate changes in testicular weight following subchronic exposure to CBD (Rosenkrantz et al., 1981; Patra and Wadsworth, 1991).
Single‐dose gestational or perinatal exposure to 50 mg/kg bw per day CBD resulted in similar alterations in male offspring, with reported increases or decreases in testicular and/or seminal vesicle weights in adulthood (Dalterio et al., 1984a,b; Dalterio and deRooij, 1986; Nahas et al., 1999). Similarly, exposure to Epidyolex® throughout gestation and lactation in rats led to a dose‐related increase in the number of male offspring with small testes, starting at 75 mg/kg bw per day (EMA, 2019).
In females, a single study in adult rhesus monkeys showed effects on relative ovarian weight after a 90‐day exposure to doses ≥ 30 mg/kg bw per day CBD (Rosenkrantz et al., 1981).
4.8.2.2. Histopathology
Histopathological analysis of testes following exposure to CBD in animal studies during adolescence or adulthood revealed a range of effects pertaining to an impairment of spermatogenesis, including significant changes in spermatogenetic stages, decreased number of germ cells and lower mitotic index at doses starting from 15 mg/kg bw per day (Rosenkrantz et al., 1981; Patra and Wadsworth, 1991; Carvalho et al., 2018a). Such changes were accompanied by significant histomorphometric changes, including alteration of seminiferous tubule perimeter and epithelium height. In females, a 26‐week oral toxicity study in the rat revealed ovarian interstitial cell hyperplasia following exposure to Epidyolex® [dose not given] (EMA, 2019).
Similarly, early acute postnatal exposure to CBD revealed a focal degeneration of spermatocytes and spermatids, as well as decreases in the number of elongated spermatids in the offspring’s testes following exposure to 50 mg/kg bw per day (Dalterio and deRooij, 1986; Nahas et al., 1999).
4.8.3. Fertility
4.8.3.1. Sperm quality
The impairment of spermatogenesis following exposure to doses ≥ 15 mg/kg bw per day CBD during at least one cycle of spermatogenesis in sexually mature murine and simian models was confirmed through associations with decreased sperm quality, including decreases in epididymal sperm counts and increases in sperm morphological abnormalities (Rosenkrantz et al., 1981; Patra and Wadsworth, 1991; Carvalho et al., 2018a).
4.8.3.2. Fertility measures
Literature data indicate that subchronic oral exposure to CBD in adult male mice resulted in statistically significant decrease in fertility rate, impregnation rates, and number of litters, as well as increases in prenatal loss starting at 30 mg/kg bw per day (Dalterio et al., 1982; Carvalho et al., 2018b). In mice, an acute exposure to 50 mg/kg bw per day CBD at birth resulted in a decreased rate of successful impregnations when mated at adult age, with a decrease in the number of live pups in pregnancies produced by exposed males (Dalterio and deRooij, 1986).
Similarly, several rats exposed to doses of Epidyolex® ranging from 75 to 250 mg/kg bw per day throughout gestation and lactation presented with small testes (please refer to Section 4.8.2.1. Reproductive organ weights), a third of which were unable to successfully impregnate females (EMA, 2019).
4.8.3.3. Sexual behaviour
In a single study in male mice, subchronic exposure to 15 or 30 mg/kg bw per day CBD at adult age resulted in changes in sexual behaviour parameters such as latency to first mount before and after first ejaculation, or latency for first intromission post ejaculation (Carvalho et al., 2018b).
4.8.4. Data gaps
Evidence suggests that CBD affects the reproductive system. The Panel notes an important knowledge gap in animal studies on the potential reprotoxic and teratogenic effects of exposure to CBD, in particular in females and in relation to lower doses.
4.9. Presence of small particles, including nanoparticles, or production of CBD as nanomaterial and CBD nanoformulation
Synthesised CBD but also CBD derived by extraction from Cannabis sativa are produced in powder form and have lipophilic characteristics with low water solubility.
In order to assess the presence of a fraction of small particles in CBD as a NF, experimental data are needed according to EFSA’s ‘Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles’ (EFSA Scientific Committee, 2021b).
If the presence of small particles is confirmed, the toxicological assessment needs to be performed according to Section 4 of that same guidance (EFSA Scientific Committee, 2021b).
If the manufacturing or formulation of the NF foresees the use of nanotechnology resulting in the production of a nanomaterial or a nanoformulated form, the EFSA ‘Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health’ applies (EFSA Scientific Committee, 2021a).
In particular, any reduction of the particle size, modification of the surface chemistry of CBD particles or processes promoting the formation of nanoemulsions to achieve a better water solubility and/or improved absorption, trigger the need for an assessment according to the above‐mentioned guidance (EFSA Scientific Committee, 2021a).
4.9.1. Data gaps
In order to properly assess the safety of CBD, the presence of small and/or nanoparticles has to be ascertained according to relevant EFSA guidance (EFSA Scientific Committee, 2021b). If such presence is established, safety studies have to conform with the approaches detailed therein (EFSA Scientific Committee, 2021a).
4.10. Genotoxicity
The assessment of the potential for genotoxicity is a basic component of any risk assessment, including that of NFs. According to the respective EFSA guidance document (EFSA Scientific Committee, 2011), the assessment of genotoxicity should follow a stepwise approach, starting with a basic battery of in vitro tests, which generally include a bacterial reverse mutation assay to assess mutagenicity and an in vitro micronucleus test to assess clastogenicity and aneugenicity. Following positive result in vitro, an in vivo follow‐up test should be performed that covers the same endpoint that was found to be positive in vitro (EFSA Scientific Committee, 2011). It should be noted that, if insoluble nanoparticles and/or small particles are detected in the test substance, the approach for genotoxicity and toxicity assessment should follow the EFSA ‘Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health’ (EFSA Scientific Committee, 2021a). In particular, with respect to mutagenicity testing of insoluble nanoparticles, a mammalian cell model should be used instead of bacterial models. Additionally, as mentioned in Section 4, the Panel noted that a major issue in interpreting many of the genotoxicity studies available in the public domain is that they were conducted with different preparations of CBD containing various concentrations of CBD and other constituents.
Different preparations containing CBD have been evaluated for potential genotoxicity in several in vitro and in vivo tests. Mutagenicity of different CBD preparations was negative in bacterial reverse mutation assays (Marx et al., 2018; Dziwenka et al., 2020). In vitro micronucleus assay using highly purified CBD and assessing structural and numerical chromosomal aberrations has been reported to be positive (Russo et al., 2019) in human HepG2 cells. However, the test did not investigate whether the positive outcome of CBD was due to an induction of aneugenicity or clastogenicity. Aneugens can be distinguished from clastogens in mammalian cell micronucleus tests by combining in vitro micronucleus tests with kinetochore staining or fluorescence in situ hybridisation (FISH). Russo et al. (2019) also reported positive in vitro single‐cell gel electrophoresis (SCGE) studies in human cells of different origin.
In vitro chromosomal aberration tests on cannabis extracts with less than 25% CBD are reported in the literature, in which structural chromosomal aberrations were negative, without providing data on numerical aberrations (Marx et al., 2018). Follow‐up studies in vivo using a mammalian erythrocytes micronucleus test or a comet assay in bone marrow or liver, respectively, as potential target tissues, showed negative results (EMA, 2019), while Zimmerman and Raj (1980) reported induction of micronuclei in bone marrow after intraperitoneal exposure. These tests are not sufficient to rule out concern for potential clastogenic or aneugenic effects at the first site of contact.
4.10.1. Data gaps
Considering the above, the Panel identified data gaps regarding the potential genotoxicity of CBD for all three genetic endpoints (gene mutation, structural and numerical chromosomal alterations), which should be assessed based on the characteristics of the NF.
5. Conclusions
The Panel has identified several hazards related to CBD intake and pointed out deficiencies in both the experimental animal and human data. The data gaps and uncertainties identified in this statement need to be addressed by the applicants to provide the basis for the safety assessment of CBD as a NF. The Panel concludes that the safety of CBD as a NF cannot currently be established.
Abbreviations
- 2‐AG
2 arachidonoylglycerol
- 5‐HT1A
5‐hydroxytryptamine 1A receptor
- A1
Adenosine 1
- AA
Arachidonic acid
- ADME
Absorption, distribution, metabolism and excretion
- AEA
Anandamide
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BCRP
Breast cancer resistance protein
- Bw
Body weight
- CB
Cannabinoid receptors
- CBD
Cannabidiol
- COT
Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment
- CYPs
Cytochromes P450
- D2
Dopamine receptor 2
- EMA
European Medicines Agency
- EMT
Endocannabinoid membrane transporter
- ENT
Equilibrative nucleoside transporter
- FAAH
Fatty Acid Amide Hydrolase
- FDA
Food and Drug Administration
- FISH
Fluorescence in situ hybridisation
- FSH
Follicle stimulating hormone
- GABAA
Gamma‐aminobutyric acid type A receptor
- GD
Gestational day
- GGT
Gamma‐glutamyl transferase
- GPR55
G‐protein‐coupled receptor 55
- IL10
Interleukin 10
- LGS
Lennox–Gastaut syndrome
- LH
Luteinising hormone
- LOAEL
Lowest observed adverse effect level
- MCT
Medium‐chain triglycerides
- NDA panel
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NF
Novel food
- NOAEL
No observed adverse effect level
- MOR
Mu Opioid receptor
- OAT
Organic anion transporter
- OATP
Organic anion transporting polypeptides
- Oprm1
Opioid Receptor Mu 1
- PPARγ
Peroxisome proliferator‐activated receptor gamma
- SCGE
Single-cell gel electrophoresis
- T3
Triiodothyronine
- T4
Thyroxine
- THC
Tetrahydrocannabinol
- TRPV1
Transient receptor potential vanilloid 1
- TSC
Tuberous Sclerosis Complex
- TSH
Thyroid‐stimulating hormone
- UK
United Kingdom
- UDP
Uridine 5'‐diphosphate
- UGT
UDP‐glucuronosyltransferases
- ULN
Upper limit of normal
- USA
United States of America
- VR 1
Vanilloid receptor 1
Supporting information
Plain language summary
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Prieto Maradona M, Schlatter JR, Trezza V, van Loveren H, Albert O, Dumas C, Germini A, Gelbmann W, Kass G, Kouloura E, Noriega Fernandez E, Rossi A and Knutsen HK, 2022. Statement on safety of cannabidiol as a novel food: data gaps and uncertainties. EFSA Journal 2022;20(6):7322, 25 pp. 10.2903/j.efsa.2022.7322
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00735
Panel members: Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to thank the following for the support provided to this scientific output: Roman Svejstil.
Adopted: 26 April 2022
Plain language summary: A plain language summary of this statement is available under Supporting Information. It aims to enhance transparency and inform interested parties on EFSA's work on the topic using simplified language. Those interested in the more technical analysis should consult the full statement.
Notes
Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001.
At time of adoption, 14 applications on CBD extracted from hemp have been validated and are presently under risk assessment in EFSA.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1.
Directive 2001/83/EC of the European Parliament and of The Council of 6 November 2001 on the community code relating to medicinal products for human use.
Federal Register Vol. 85, No. 48, p. 14206.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64‐71.
Regulation (EC) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009.
References
- Ahrens J, Demir R, Leuwer M, de la Roche J, Krampfl K, Foadi N, Karst M and Haeseler G, 2009. The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha‐1 and alpha‐1‐beta glycine receptor function. Pharmacology, 83, 217–222. 10.1159/000201556 [DOI] [PubMed] [Google Scholar]
- Ali RM, Al Kury LT, Yang KH, Qureshi A, Rajesh M, Galadari S, Shuba YM, Howarth FC and Oz M, 2015. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell Calcium, 57, 290–299. 10.1016/j.ceca.2015.02.001 [DOI] [PubMed] [Google Scholar]
- de Almeida DL and Devi LA, 2020. Diversity of molecular targets and signaling pathways for CBD. Pharmacology Research Perspectives, 8, e00682. 10.1002/prp2.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arout CA, Haney M, Herrmann ES, Bedi G and Cooper ZD, 2022. A placebo‐controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. British Journal of Clinical Pharmacology, 88, 347–355. 10.1111/bcp.14973 [DOI] [PubMed] [Google Scholar]
- Atsmon J, Heffetz D, Deutsch L, Deutsch F and Sacks H, 2018. Single‐dose pharmacokinetics of oral cannabidiol following administration of PTL101: a new formulation based on gelatin matrix pellets technology. Clinical Pharmacology in Drug Development, 7, 751–758. 10.1002/cpdd.408 [DOI] [PubMed] [Google Scholar]
- Bakas T, van Nieuwenhuijzen PS, Devenish SO, McGregor IS, Arnold JC and Chebib M, 2017. The direct actions of cannabidiol and 2‐arachidonoyl glycerol at GABAA receptors. Pharmacological Research, 119, 358–370. 10.1016/j.phrs.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Bansal S, Maharao N, Paine MF and Unadkat JD, 2020. Predicting the potential for cannabinoids to precipitate pharmacokinetic drug interactions via reversible inhibition or inactivation of major cytochromes P450. Drug Metabolism and Disposition, 48, 1008–1017. 10.1124/dmd.120.000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Menachem E, Gunning B, Arenas Cabrera CM, VanLandingham K, Crockett J, Critchley D, Wray L, Tayo B, Morrison G and Toledo M, 2020. A phase II randomized trial to explore the potential for pharmacokinetic drug–drug interactions with stiripentol or valproate when combined with cannabidiol in patients with epilepsy. CNS Drugs, 34, 661–672. 10.1007/s40263-020-00726-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schröder N, Nardi AE, Martín‐Santos R, Hallak JE, Zuardi AW and Crippa JA, 2011. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment‐naive social phobia patients. Neuropsychopharmacology, 36, 1219–1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, Gramling‐Aden M and Leppik IE, 2019. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia, 60, 1586–1592. 10.1111/epi.16093 [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R and Di Marzo V, 2001. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology, 134, 845–852. 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn‐Miller MO, Banks SL and Sebree T, 2017. Conversion of Cannabidiol Following Oral Administration: authors' Response to Grotenhermen et al 10.1089/can.2016.0036. Cannabis and Cannabinoid Research, 2, 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornheim LM, Everhart ET, Li J and Correia MA, 1994. Induction and genetic regulation of mouse hepatic cytochrome P450 by cannabidiol. Biochemical Pharmacology, 48, 161–171. 10.1016/0006-2952(94)90236-4 [DOI] [PubMed] [Google Scholar]
- Brown JD and Winterstein AG, 2019. Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) Use. Journal of Clinical Medicine, 8. 10.3390/jcm8070989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres Guido P, Riva N, Caraballo R, Reyes G, Huaman M, Gutierrez R, Agostini S, Fabiana Delaven S, Pérez Montilla CA, García Bournissen F and Schaiquevich P, 2021. Pharmacokinetics of cannabidiol in children with refractory epileptic encephalopathy. Epilepsia, 62, e7–e12. 10.1111/epi.16781 [DOI] [PubMed] [Google Scholar]
- Calapai F, Cardia L, Sorbara EE, Navarra M, Gangemi S, Calapai G and Mannucci C, 2020. Cannabinoids, blood–brain barrier, and brain disposition. Pharmaceutics, 12. 10.3390/pharmaceutics12030265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA and Hillard CJ, 2006. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proceeding of the National Academy of Sciences of the United States of America, 103, 7895–7900. 10.1073/pnas.0511232103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RK, Andersen ML and Mazaro‐Costa R, 2020. The effects of cannabidiol on male reproductive system: a literature review. Journal of Applied Toxicology, 40, 132–150. 10.1002/jat.3831 [DOI] [PubMed] [Google Scholar]
- Carvalho RK, Santos ML, Souza MR, Rocha TL, Guimarães FS, Anselmo‐Franci JA and Mazaro‐Costa R, 2018a. Chronic exposure to cannabidiol induces reproductive toxicity in male Swiss mice. Journal of Applied Toxicology, 38, 1215–1223. 10.1002/jat.3631 [DOI] [PubMed] [Google Scholar]
- Carvalho RK, Souza MR, Santos ML, Guimarães FS, Pobbe RLH, Andersen ML and Mazaro‐Costa R, 2018b. Chronic cannabidiol exposure promotes functional impairment in sexual behavior and fertility of male mice. Reproductive Toxicology, 81, 34–40. 10.1016/j.reprotox.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Chayasirisobhon S, 2020. Mechanisms of action and pharmacokinetics of cannabis. Permanente Journal, 25, 1–3. 10.7812/TPP/19.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K and Schram K, 1991. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacology, Biochemistry and Behavior, 40, 701–708. 10.1016/0091-3057(91)90386-g [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert‐Ana L, Guarnieri R, Ferrari L, Azevedo‐Marques PM, Hallak JE, McGuire PK and Filho Busatto G, 2004. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology, 29, 417–426. 10.1038/sj.npp.1300340 [DOI] [PubMed] [Google Scholar]
- Crippa JAS, Zuardi AW, Guimarães FS, Campos AC, de Lima Osório F, Loureiro SR, dos Santos RG, Souza JDS, Ushirohira JM, Pacheco JC, Ferreira RR, Mancini Costa KC, Scomparin DS, Scarante FF, Pires‐Dos‐Santos I, Mechoulam R, Kapczinski F, Fonseca BAL, Esposito DLA, Pereira‐Lima K, Sen S, Andraus MH, Hallak JEC, Litcanov DC, Rodrigues L, Alves TF and Coutinho BM, 2021. Efficacy and safety of cannabidiol plus standard care vs standard care alone for the treatment of emotional exhaustion and burnout among frontline health care workers during the COVID‐19 pandemic: a randomized clinical trial. JAMA Network Open, 4, e2120603. 10.1001/jamanetworkopen.2021.20603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, Bisogno T and Di Marzo V, 2020. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews. Neurology, 16, 9–29. 10.1038/s41582-019-0284-z [DOI] [PubMed] [Google Scholar]
- Dalterio S, Badr F, Bartke A and Mayfield D, 1982. Cannabinoids in male mice: effects on fertility and spermatogenesis. Science, 216, 315–316. 10.1126/science.6801767 [DOI] [PubMed] [Google Scholar]
- Dalterio SL and deRooij DG, 1986. Maternal cannabinoid exposure. Effects on spermatogenesis in male offspring. International Journal of Andrology, 9, 250–258. 10.1111/j.1365-2605.1986.tb00888.x [DOI] [PubMed] [Google Scholar]
- Dalterio SL, Michael SD and Thomford PJ, 1986. Perinatal cannabinoid exposure: demasculinization in male mice. Neurobehavioral Toxicology and Teratology, 8, 391–397. [PubMed] [Google Scholar]
- Dalterio S, Steger R, Mayfield D and Bartke A, 1984a. Early cannabinoid exposure influences neuroendocrine and reproductive functions in male mice: I. Prenatal exposure. Pharmacology, Biochemistry and Behavior, 20, 107–113. 10.1016/0091-3057(84)90110-2 [DOI] [PubMed] [Google Scholar]
- Dalterio S, Steger R, Mayfield D and Bartke A, 1984b. Early cannabinoid exposure influences neuroendocrine and reproductive functions in mice: II. Postnatal effects. Pharmacology, Biochemistry and Behavior, 20, 115–123. 10.1016/0091-3057(84)90111-4 [DOI] [PubMed] [Google Scholar]
- Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, Miller I, Flamini R, Wilfong A, Filloux F, Wong M, Tilton N, Bruno P, Bluvstein J, Hedlund J, Kamens R, Maclean J, Nangia S, Singhal NS, Wilson CA, Patel A and Cilio MR, 2016. Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurology, 15, 270–278. 10.1016/S1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA and Wright S and Cannabidiol in Dravet Syndrome Study Group, 2017. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. New England Journal of Medicine, 376, 2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE, Zuberi SM and GWPCARE3 Study Group, 2018a. Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. New England Journal of Medicine, 378, 1888–1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, Greenwood S, Morrison G, Sommerville K and GWPCARE1 Part A Study Group, 2018b. Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology, 90, e1204–e1211. 10.1212/WNL.0000000000005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S and Roberts C, 2019. Long‐term cannabidiol treatment in patients with Dravet syndrome: an open‐label extension trial. Epilepsia, 60, 294–302. 10.1111/epi.14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M and De Petrocellis L, 2004. The endocannabinoid system and its therapeutic exploitation. Nature Reviews Drug Discovery, 3, 771–784. 10.1038/nrd1495 [DOI] [PubMed] [Google Scholar]
- D'Onofrio G, Kuchenbuch M, Hachon‐Le Camus C, Desnous B, Staath V, Napuri S, Ville D, Pedespan JM, Lépine A, Cances C, de Saint‐Martin A, Teng T, Chemaly N, Milh M, Villeneuve N and Nabbout R, 2020. Slow titration of cannabidiol add‐on in drug‐resistant epilepsies can improve safety with maintained efficacy in an open‐label study. Frontiers in Neurology, 11, 829. 10.3389/fneur.2020.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q, Liao Q, Chen C, Yang X, Xie R and Xu J, 2019. The role of transient receptor potential vanilloid 1 in common diseases of the digestive tract and the cardiovascular and respiratory system. Frontiers in Physiology, 10, 1064. 10.3389/fphys.2019.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziwenka M, Coppock R, Alexander M, Palumbo E, Ramirez C and Lermer S, 2020. Safety assessment of a hemp extract using genotoxicity and oral repeat‐dose toxicity studies in Sprague‐Dawley rats. Toxicology Reports, 7, 376–385. 10.1016/j.toxrep.2020.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel , Turck D, Bresson JL, Burlingame B, Dean T, Fairweather‐Tait S, Heinonen M, Hirsch‐Ernst KI, Mangelsdorf I, McArdle HJ, Naska A, Neuhauser‐Berthold M, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjodin A, Stern M, Tome D, Vinceti M, Willatts P, Engel KH, Marchelli R, Poting A, Poulsen M, Salminen S, Schlatter J, Arcella D, Gelbmann W, de Sesmaisons‐Lecarre A, Verhagen H and van Loveren H, 2021. Guidance on the preparation and submission of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283 (Revision 1)(2). EFSA Journal 2021;19(2):e06555, 45 pp. 10.2903/j.efsa.2021.6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2012;9(3):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernández‐Jerez A, Bennekou SH, Koutsoumanis K, Lambré C, Machera K, Naegeli H, Nielsen S, Schlatter J, Schrenk D, Silano (deceased) V, Turck D, Younes M, Castenmiller J, Chaudhry Q, Cubadda F, Franz R, Gott D, Mast J, Mortensen A, Oomen AG, Weigel S, Barthelemy E, Rincon A, Tarazona J and Schoonjans R, 2021a. Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health. EFSA Journal 2021;19(8):6768, 32 pp. 10.2903/j.efsa.2021.6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Bragard C, Halldorsson T, Hernández‐Jerez A, Bennekou SH, Koutsoumanis K, Lambré C, Machera K, Naegeli H, Nielsen S, Schlatter J, Schrenk D, Silano (deceased) V, Turck D, Younes M, Castenmiller J, Chaudhry Q, Cubadda F, Franz R, Gott D, Mast J, Mortensen A, Oomen AG, Weigel S, Barthelemy E, Rincon A, Tarazona J and Schoonjans R, 2021b. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 2021;19(8):6769, 45 pp. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (European Medicine Agency) , 2019. Assessment report, Epidyolex. EMA/458106/2019. Available online: https://www.ema.europa.eu/en/documents/assessment‐report/epidyolex‐epar‐public‐assessment‐report_en.pdf
- Ewing LE, Skinner CM, Quick CM, Kennon‐McGill S, McGill MR, Walker LA, ElSohly MA, Gurley BJ and Koturbash I, 2019. Hepatotoxicity of a cannabidiol‐rich cannabis extract in the mouse model. Molecules, 24, 1694. 10.3390/molecules24091694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration) , 2018. Summary review, 210365Orig1s000. Reference ID, 4282210. Availabel online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000SumR.pdf
- Freund TF, Katona I and Piomelli D, 2003. Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews, 83, 1017–1066. 10.1152/physrev.00004.2003 [DOI] [PubMed] [Google Scholar]
- Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP and Program UC, 2017. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia, 58, 1586–1592. 10.1111/epi.13852 [DOI] [PubMed] [Google Scholar]
- Gaston TE, Ampah SB, Martina Bebin E, Grayson LP, Cutter GR, Hernando K, Szaflarski JP and Program UABCBD, 2021. Long‐term safety and efficacy of highly purified cannabidiol for treatment refractory epilepsy. Epilepsy and Behavior, 117, 107862. 10.1016/j.yebeh.2021.107862 [DOI] [PubMed] [Google Scholar]
- Gaston TE and Szaflarski JP, 2018. Cannabis for the treatment of epilepsy: an update. Current Neurology and Neuroscience Reports, 18, 73. 10.1007/s11910-018-0882-y [DOI] [PubMed] [Google Scholar]
- Geffrey AL, Pollack SF, Bruno PL and Thiele EA, 2015. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia, 56, 1246–1251. 10.1111/epi.13060 [DOI] [PubMed] [Google Scholar]
- Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC and Goodchild SJ, 2018. Inhibitory effects of cannabidiol on voltage‐dependent sodium currents. Journal of Biological Chemistry, 293, 16546–16558. 10.1074/jbc.RA118.004929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek P, Müller M, Barthlott I, Sproll C and Lachenmeier DW, 2020. Conversion of cannabidiol (CBD) into psychotropic cannabinoids including tetrahydrocannabinol (THC), a controversy in the scientific literature. Toxics, 8, 41. 10.3390/toxics8020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonca E and Darici F, 2015. The effect of cannabidiol on ischemia/reperfusion‐induced ventricular arrhythmias: the role of adenosine A1 receptors. Journal of Cardiovascular Pharmacology and Therapeutics, 20, 76–83. 10.1177/1074248414532013 [DOI] [PubMed] [Google Scholar]
- Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, Gray KM, McRae‐Clark A, Lofwall MR, Sparenborg S and Walsh SL, 2016. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology, 41, 1974–1982. 10.1038/npp.2015.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Lisboa SF, Sonego AB, Coutinho D, Gomes FV and Guimarães FS, 2019. Cannabidiol attenuates aggressive behavior induced by social isolation in mice: involvement of 5‐HT1A and CB1 receptors. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 94, 109637. 10.1016/j.pnpbp.2019.109637 [DOI] [PubMed] [Google Scholar]
- Hawksworth G and McArdle K, 2004. Metabolism and pharmacokinetics of cannabinoids. In: Whittle BA and Robson PJ (eds.). Guy GW, The medicinal uses of cannabis and cannabinoids. Pharmaceutical Press, London, UK. pp. 205–228. [Google Scholar]
- Hundal H, Lister R, Evans N, Antley A, Englund A, Murray RM, Freeman D and Morrison PD, 2018. The effects of cannabidiol on persecutory ideation and anxiety in a high trait paranoid group. Journal of Psychopharmacology, 32, 276–282. 10.1177/0269881117737400 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Spriggs S, Alishayev J, Winkel G, Gurgov K, Kudrich C, Oprescu AM and Salsitz E, 2019. Cannabidiol for the reduction of cue‐induced craving and anxiety in drug‐abstinent individuals with heroin use disorder: a double‐blind randomized placebo‐controlled trial. American Journal of Psychiatry, 176, 911–922. 10.1176/appi.ajp.2019.18101191 [DOI] [PubMed] [Google Scholar]
- Iannone LF, Arena G, Battaglia D, Bisulli F, Bonanni P, Boni A, Canevini MP, Cantalupo G, Cesaroni E, Contin M, Coppola A, Cordelli DM, Cricchiuti G, De Giorgis V, De Leva MF, De Rinaldis M, d'Orsi G, Elia M, Galimberti CA, Morano A, Granata T, Guerrini R, Lodi MAM, La Neve A, Marchese F, Masnada S, Michelucci R, Nosadini M, Pilolli N, Pruna D, Ragona F, Rosati A, Santucci M, Spalice A, Pietrafusa N, Striano P, Tartara E, Tassi L, Papa A, Zucca C, Russo E and Mecarelli O, 2021. Results From an Italian expanded access program on cannabidiol treatment in highly refractory Dravet syndrome and Lennox‐Gastaut syndrome. Frontiers in Neurology, 12, 673135. 10.3389/fneur.2021.673135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti FA and Vitale RM, 2021. The endocannabinoid system and PPARs: focus on their signalling crosstalk, action and transcriptional regulation. Cells, 10. 10.3390/cells10030586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, O'Sullivan SE and Tan GD, 2016. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, parallel group pilot study. Diabetes Care, 39, 1777–1786. 10.2337/dc16-0650 [DOI] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C and Schlicker E, 2006. Cannabidiol is an allosteric modulator at mu‐ and delta‐opioid receptors. Naunyn‐Schmiedeberg’s Archives of Pharmacology, 372, 354–361. 10.1007/s00210-006-0033-x [DOI] [PubMed] [Google Scholar]
- Klotz KA, Grob D, Hirsch M, Metternich B, Schulze‐Bonhage A and Jacobs J, 2019. Efficacy and tolerance of synthetic cannabidiol for treatment of drug resistant epilepsy. Frontiers in Neurology, 10, 1313. 10.3389/fneur.2019.01313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwo PY, Cohen SM and Lim JK, 2017. ACG Clinical guideline: evaluation of abnormal liver chemistries. American Journal of Gastroenterology, 112, 18–35. 10.1038/ajg.2016.517 [DOI] [PubMed] [Google Scholar]
- Lachenmeier DW, Habel S, Fischer B, Herbi F, Zerbe Y, Bock V, Rajcic de Rezende T, Walch SG and Sproll C, 2019. Are adverse effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination? F1000Research, 8, 1394. 10.12688/f1000research.19931.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, Miller I, Nichol K, Park Y, Segal E, Seltzer L, Szaflarski JP, Thiele EA, Weinstock A and CBD EAP study group, 2019. Long‐term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox‐Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Research, 154, 13–20. 10.1016/j.eplepsyres.2019.03.015 [DOI] [PubMed] [Google Scholar]
- Leino AD, Emoto C, Fukuda T, Privitera M, Vinks AA and Alloway RR, 2019. Evidence of a clinically significant drug–drug interaction between cannabidiol and tacrolimus. American Journal of Transplantation, 19, 2944–2948. 10.1111/ajt.15398 [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R and Berry EM, 2009. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. Journal of Hepatology, 51, 528–534. 10.1016/j.jhep.2009.04.021 [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R and Berry EM, 2010. Cannabidiol ameliorates cognitive and motor impairments in bile‐duct ligated mice via 5‐HT1A receptor activation. British Journal of Pharmacology, 159, 950–957. 10.1111/j.1476-5381.2009.00589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farré M, Zuardi AW and McGuire PK, 2012. Acute effects of a single, oral dose of d9‐tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Current Pharmaceutical Design, 18, 4966–4979. 10.2174/138161212802884780 [DOI] [PubMed] [Google Scholar]
- Marx TK, Reddeman R, Clewell AE, Endres JR, Béres E, Vértesi A, Glávits R, Hirka G and Szakonyiné IP, 2018. An assessment of the genotoxicity and subchronic toxicity of a supercritical fluid extract of the aerial parts of hemp. Journal of Toxicology, 2018, 8143582. 10.1155/2018/8143582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus‐Zawada A, Finel M, Miller GP, Radomińska‐Pandya A and Moran JH, 2009. Characterization of human hepatic and extrahepatic UDP‐glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metabolism and Disposition, 37, 1496–1504. 10.1124/dmd.109.026898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick J, Lane B, Sebree T, Yaksh T, O'Neill C and Banks SL, 2016. Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis Cannabinoid Research, 1, 102–112. 10.1089/can.2015.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SA, Stone NL, Yates AS and O'Sullivan SE, 2018. A systematic review on the pharmacokinetics of cannabidiol in humans. Frontier in Pharmacology, 9, 1365. 10.3389/fphar.2018.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I, Scheffer IE, Gunning B, Sanchez‐Carpintero R, Gil‐Nagel A, Perry MS, Saneto RP, Checketts D, Dunayevich E, Knappertz V and GWPCARE2 Study Group, 2020. Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: a randomized clinical trial. JAMA Neurology, 77, 613–621. 10.1001/jamaneurol.2020.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison G, Crockett J, Blakey G and Sommerville K, 2019. A phase 1, open‐label, pharmacokinetic trial to investigate possible drug–drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clinical Pharmacology in Drug Development, 8, 1009–1031. 10.1002/cpdd.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, Laish I, Benjaminov F and Konikoff FM, 2017. Low‐dose cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Digestive Diseases and Science, 62, 1615–1620. 10.1007/s10620-017-4540-z [DOI] [PubMed] [Google Scholar]
- Nahas GG, Sutin KM, Harvey DJ and Agurell S, 1999. Marihuana and Medicine. Humana Press. [Google Scholar]
- Nahler G, Grotenhermen F, Zuardi AW and Crippa JAS, 2017. A conversion of oral cannabidiol to delta9‐tetrahydrocannabinol seems not to occur in humans. Cannabis and Cannabinoid Research, 2, 81–86. 10.1089/can.2017.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AD, Mazurkiewicz‐Belłdzinńska M, Chin RF, Gil‐Nagel A, Gunning B, Halford JJ, Mitchell W, Scott Perry M, Thiele EA, Weinstock A, Dunayevich E, Checketts D and Devinsky O, 2021. Long‐term safety and efficacy of add‐on cannabidiol in patients with Lennox‐Gastaut syndrome: results of a long‐term open‐label extension trial. Epilepsia, 62, 2228–2239. 10.1111/epi.17000 [DOI] [PubMed] [Google Scholar]
- Patra PB and Wadsworth RM, 1991. Quantitative evaluation of spermatogenesis in mice following chronic exposure to cannabinoids. Andrologia, 23, 151–156. 10.1111/j.1439-0272.1991.tb02520.x [DOI] [PubMed] [Google Scholar]
- Patrician A, Versic‐Bratincevic M, Mijacika T, Banic I, Marendic M, Sutlović D, Dujić Ž and Ainslie PN, 2019. Examination of a new delivery approach for oral cannabidiol in healthy subjects: a randomized, double‐blinded, placebo‐controlled pharmacokinetics study. Advances in Therapy, 36, 3196–3210. 10.1007/s12325-019-01074-6 [DOI] [PubMed] [Google Scholar]
- Peng J, Fan M, An C, Ni F, Huang W and Luo J, 2022. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic and Clinical Pharmacology and Toxicology, 130, 439–456. 10.1111/bcpt.13710 [DOI] [PubMed] [Google Scholar]
- Perkins D, Butler J, Ong K, Nguyen TH, Cox S, Francis B, McIntosh M and Lilley B, 2020. A phase 1, randomised, placebo‐controlled, dose escalation study to investigate the safety, tolerability and pharmacokinetics of cannabidiol in fed healthy volunteers. European Journal of Drug Metabolism and Pharmacokinetics, 45, 575–586. 10.1007/s13318-020-00624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Ross RA, Craib SJ and Thomas A, 2002. (−)‐Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. European Journal of Pharmacology, 456, 99–106. 10.1016/s0014-2999(02)02624-9 [DOI] [PubMed] [Google Scholar]
- Ribeiro A, Ferraz‐de‐Paula V, Pinheiro ML, Vitoretti LB, Mariano‐Souza DP, Quinteiro‐Filho WM, Akamine AT, Almeida VI, Quevedo J, Dal‐Pizzol F, Hallak JE, Zuardi AW, Crippa JA and Palermo‐Neto J, 2012. Cannabidiol, a non‐psychotropic plant‐derived cannabinoid, decreases inflammation in a murine model of acute lung injury: role for the adenosine A(2A) receptor. European Journal of Pharmacology, 678, 78–85. 10.1016/j.ejphar.2011.12.043 [DOI] [PubMed] [Google Scholar]
- Rosenkrantz H and Esber HJ, 1980. Cannabinoid‐induced hormone changes in monkeys and rats. Journal of Toxicology and Environmental Health, 6, 297–313. 10.1080/15287398009529853 [DOI] [PubMed] [Google Scholar]
- Rosenkrantz H, Fleischman RW and Grant RJ, 1981. Toxicity of short‐term administration of cannabinoids to rhesus monkeys. Toxicology Applied Pharmacology, 58, 118–131. 10.1016/0041-008x(81)90122-8 [DOI] [PubMed] [Google Scholar]
- Ross RA, 2009. The enigmatic pharmacology of GPR55. Trends in Pharmacological Sciences, 30, 156–163. 10.1016/j.tips.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B and Parker KK, 2005. Agonistic properties of cannabidiol at 5‐HT1a receptors. Neurochemical Research, 30, 1037–1043. 10.1007/s11064-005-6978-1 [DOI] [PubMed] [Google Scholar]
- Russo C, Ferk F, Mišík M, Ropek N, Nersesyan A, Mejri D, Holzmann K, Lavorgna M, Isidori M and Knasmüller S, 2019. Low doses of widely consumed cannabinoids (cannabidiol and cannabidivarin) cause DNA damage and chromosomal aberrations in human‐derived cells. Archives of Toxicology, 93, 179–188. 10.1007/s00204-018-2322-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands TT, Rahdari S, Oldham MS, Caminha Nunes E, Tilton N and Cilio MR, 2019. Long‐term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. CNS Drugs, 33, 47–60. 10.1007/s40263-018-058-2 [DOI] [PubMed] [Google Scholar]
- Seeman P, 2016. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Translational Psychiatry, 6, e920. 10.1038/tp.2016.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholler DJ, Spindle TR, Cone EJ, Goffi E, Kuntz D, Mitchell JM, Winecker RE, Bigelow GE, Flegel RR and Vandrey R, 2021. Urinary pharmacokinetic profile of cannabidiol (CBD), delta9‐tetrahydrocannabinol (THC), and their metabolites following oral and vaporized CBD and vaporized CBD‐dominant cannabis administration. Journal of Analytical Toxicology, 10.1093/jat/bkab059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda F and Arowolo A, 2020. A molecular basis for the anti‐inflammatory and anti‐fibrosis properties of cannabidiol. FASEB Journal, 34, 14083–14092. 10.1096/fj.202000975R [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, Kankirawatana P, Liu Y, Singh R, Standaert DG, Thomas AE, Ver Hoef LW and Program UABCBD, 2018. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open‐label add‐on prospective study. Epilepsy and Behavior, 87, 131–136. 10.1016/j.yebeh.2018.07.020 [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Hernando K, Bebin EM, Gaston TE, Grayson LE, Ampah SB and Moreadith R, 2019. Higher cannabidiol plasma levels are associated with better seizure response following treatment with a pharmaceutical grade cannabidiol. Epilepsy and Behavior, 95, 131–136. 10.1016/j.yebeh.2019.03.042 [DOI] [PubMed] [Google Scholar]
- Taylor L, Gidal B, Blakey G, Tayo B and Morrison G, 2018. A phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs, 32, 1053–1067. 10.1007/s40263-018-0578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Crockett J, Tayo B, Checketts D and Sommerville K, 2020. Abrupt withdrawal of cannabidiol (CBD): a randomized trial. Epilepsy and Behavior, 104, 106938. 10.1016/j.yebeh.2020.106938 [DOI] [PubMed] [Google Scholar]
- Thai C, Tayo B and Critchley D, 2021. A phase 1 open‐label, fixed‐sequence pharmacokinetic drug interaction trial to investigate the effect of cannabidiol on the CYP1A2 Probe caffeine in healthy subjects. Clinical Pharmacology in Drug Development, 10, 1279–1289. 10.1002/cpdd.950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele EA, Marsh ED, French JA, Mazurkiewicz‐Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C and GWPCARE4 Study Group, 2018. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet, 391, 1085–1096. 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- Thiele E, Marsh E, Mazurkiewicz‐Beldzinska M, Halford JJ, Gunning B, Devinsky O, Checketts D and Roberts C, 2019. Cannabidiol in patients with Lennox‐Gastaut syndrome: interim analysis of an open‐label extension study. Epilepsia, 60, 419–428. 10.1111/epi.14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, O'Callaghan FJ, Wong M, Sahebkar F, Checketts D, Knappertz V and GWPCARE6 Study Group, 2021. Add‐on cannabidiol treatment for drug‐resistant seizures in tuberous sclerosis complex: a placebo‐controlled randomized clinical trial. JAMA Neurology, 78, 285–292. 10.1001/jamaneurol.2020.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA and Pertwee RG, 2007. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro . British Journal of Pharmacology, 150, 613–623. 10.1038/sj.bjp.0707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SE, Williams CM, Iversen L and Whalley BJ, 2017. Molecular pharmacology of phytocannabinoids. Progress in Chemistry of Organic and Natural Products, 103, 61–101. 10.1007/978-3-319-45541-9_3 [DOI] [PubMed] [Google Scholar]
- Ujváry I and Hanuš L, 2016. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Research, 1, 90–101. 10.1089/can.2015.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLandingham KE, Crockett J, Taylor L and Morrison G, 2020. A phase 2, double‐blind, placebo‐controlled trial to investigate potential drug–drug interactions between cannabidiol and clobazam. Journal of Clinical Pharmacology, 60 , 1304–1313. 10.1002/jcph.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn D, Kulpa J and Paulionis L, 2020. Preliminary investigation of the safety of escalating cannabinoid doses in healthy dogs. Frontiers in Veterinary Science, 7, 51. 10.3389/fvets.2020.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez‐Martínez A, García‐Gutiérrez MS, Navarrón CM, Morales‐Calero MI, Navarrete F, Torres‐Suárez AI and Manzanares J, 2018. Cannabidiol reduces ethanol consumption, motivation and relapse in mice. Addict Biology, 23, 154–164. 10.1111/adb.12495 [DOI] [PubMed] [Google Scholar]
- Watkins PB, Church RJ, Li J and Knappertz V, 2021. Cannabidiol and abnormal liver chemistries in healthy adults: results of a phase I clinical trial. Clinical Pharmacology and Therapeutics, 109, 1224–1231. 10.1002/cpt.2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheless JW, Dlugos D, Miller I, Oh DA, Parikh N, Phillips S, Renfroe JB, Roberts CM, Saeed I, Sparagana SP, Yu J, Cilio MR and INS011‐14‐029 Study Investigators, 2019. Pharmacokinetics and tolerability of multiple doses of pharmaceutical‐grade synthetic cannabidiol in pediatric patients with treatment‐resistant epilepsy. CNS Drugs, 33 pp. 593–604. 10.1007/s40263-019-00624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte LS, Ryberg E, Sims NA, Ridge SA, Mackie K, Greasley PJ, Ross RA and Rogers MJ, 2009. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo . Proceedings of the National Academy of Science of the United States of America, 106, 16511–16516. 10.1073/pnas.0902743106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang KH, Galadari S, Isaev D, Petroianu G, Shippenberg TS and Oz M, 2010. The nonpsychoactive cannabinoid cannabidiol inhibits 5‐hydroxytryptamine3A receptor‐mediated currents in Xenopus laevis oocytes. Journal of Pharmacology and Experimental Therapy, 333, 547–554. 10.1124/jpet.109.162594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgair A, Wong JC, Lee JB, Mistry J, Sivak O, Wasan KM, Hennig IM, Barrett DA, Constantinescu CS, Fischer PM and Gershkovich P, 2016. Dietary fats and pharmaceutical lipid excipients increase systemic exposure to orally administered cannabis and cannabis‐based medicines. American Journal of Translational Research, 8, 3448–3459. [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AM and Raj AY, 1980. Influence of cannabinoids on somatic cells in vivo . Pharmacology, 21, 277–287. 10.1159/000137442 [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JEC, Guimarães FS and Crippa JAS, 2017. Inverted U‐shaped dose‐response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Frontiers in Pharmacology, 8, 259. 10.3389/fphar.2017.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plain language summary


