Abstract
The concept of the right temporal variant of frontotemporal dementia (rtvFTD) is still equivocal. The syndrome accompanying predominant right anterior temporal atrophy has previously been described as memory loss, prosopagnosia, getting lost and behavioural changes. Accurate detection is challenging, as the clinical syndrome might be confused with either behavioural variant FTD (bvFTD) or Alzheimer’s disease. Furthermore, based on neuroimaging features, the syndrome has been considered a right-sided variant of semantic variant primary progressive aphasia (svPPA). Therefore, we aimed to demarcate the clinical and neuropsychological characteristics of rtvFTD versus svPPA, bvFTD and Alzheimer’s disease. Moreover, we aimed to compare its neuroimaging profile against svPPA, which is associated with predominant left anterior temporal atrophy. Of 619 subjects with a clinical diagnosis of frontotemporal dementia or primary progressive aphasia, we included 70 subjects with a negative amyloid status in whom predominant right temporal lobar atrophy was identified based on blinded visual assessment of their initial brain MRI scans. Clinical symptoms were assessed retrospectively and compared with age- and sex-matched patients with svPPA (n = 70), bvFTD (n = 70) and Alzheimer’s disease (n = 70). Prosopagnosia, episodic memory impairment and behavioural changes such as disinhibition, apathy, compulsiveness and loss of empathy were the most common initial symptoms, whereas during the disease course, patients developed language problems such as word-finding difficulties and anomia. Distinctive symptoms of rtvFTD compared to the other groups included depression, somatic complaints, and motor/mental slowness. Aside from right temporal atrophy, the imaging pattern showed volume loss of the right ventral frontal area and the left temporal lobe, which represented a close mirror image of svPPA. Atrophy of the bilateral temporal poles and the fusiform gyrus were associated with prosopagnosia in rtvFTD. Our results highlight that rtvFTD has a unique clinical presentation. Since current diagnostic criteria do not cover specific symptoms of the rtvFTD, we propose a diagnostic tree to be used to define diagnostic criteria and call for an international validation.
Keywords: dementia, frontotemporal lobar degeneration, frontotemporal dementia, right temporal lobe atrophy, prosopagnosia
Whether the right temporal variant of frontotemporal dementia (rtvFTD) is truly a distinct entity is unclear. By examining clinical and neuroimaging profiles, Ulugut Erkoyun et al. provide evidence that rtvFTD is a unique neurodegenerative disorder and propose a framework to be used to define diagnostic criteria.
Introduction
Frontotemporal dementia (FTD) is a neurodegenerative disorder that predominantly affects the frontal and/or temporal lobes. Three different prototypic FTD syndromes have been described, being semantic dementia, progressive non-fluent aphasia (PNFA) and behavioural variant frontotemporal dementia (bvFTD) (Neary et al., 1998). In 2011, consensus clinical diagnostic criteria were revised and FTD was classified as behavioural variant (Rascovsky et al., 2011) whereas semantic dementia and PNFA were classified under the umbrella of primary progressive aphasia (PPA), including the semantic variant (svPPA), the non-fluent/agrammatic variant and the logopenic variant of PPA (Gorno-Tempini et al., 2011).
The typical neuroimaging pattern of bvFTD consists of frontal and/or temporal atrophy (Rascovsky et al., 2011), whereas bilateral anterior temporal atrophy is suggestive of svPPA with usually a greater amount of atrophy on the left side, and predominant left posterior frontal and insular atrophy is the neuroimaging pattern of the non-fluent variant of PPA (Gorno-Tempini et al., 2011).
On the other hand, a number of authors have mentioned a separate syndromic variant that predominantly affects the right temporal lobe (Thompson et al., 2003; Chan et al., 2009). The main clinical characteristics that have been associated with the right temporal variant of frontotemporal dementia (rtvFTD) are prosopagnosia, memory deficits, getting lost and profound behavioural changes such as disinhibition and obsessive personality (Thompson et al., 2003; Chan et al., 2009; Josephs et al., 2009; Everhart et al., 2015; Kamminga et al., 2015; Veronelli et al., 2017; Pozueta et al., 2019). Additional symptoms particularly linked to rtvFTD include hyper-religiosity, visual hallucinations and cross-modal sensory experiences (Chan et al., 2009).
Since the revision of consensus criteria for bvFTD (Rascovsky et al., 2011) and semantic dementia being considered a variant of PPA (Gorno-Tempini et al., 2011), the syndrome of rtvFTD has been relatively neglected in the literature. In the most recent diagnostic criteria (Gorno-Tempini et al., 2011), bilateral anterior temporal atrophy has been the ‘imaging supported diagnostic’ criterion for svPPA, and therefore rtvFTD has been classified as svPPA. On the other hand, an early amnestic presentation and behavioural changes may fulfil clinical diagnostic criteria for either bvFTD or Alzheimer’s disease (McKhann et al., 2011; Rascovsky et al., 2011). Reflective of all this, there is not even agreement on its name. Over the years, the syndrome has been termed as ‘right temporal lobe atrophy’, ‘right variant FTD’, ‘temporal variant FTD’ and ‘right temporal variant of FTD’ (Gainotti et al., 2003; Seeley et al., 2005; Joubert et al., 2006; Chan et al., 2009; Henry et al., 2014; Everhart et al., 2015), whereas those authors who consider rtvFTD as part of semantic dementia use terms such as ‘right variant of semantic dementia’, ‘right predominant semantic dementia’ or ‘right-lateralized semantic dementia’ (Thompson et al., 2003; Brambati et al., 2009; Kamminga et al., 2015; Kumfor et al., 2016; Snowden et al., 2018; Pozueta et al., 2019). However, in most available clinical and radiological studies, the number of patients has been limited (n = 6–20 patients) and none of them excluded subjects with underlying Alzheimer’s disease pathology based on CSF biomarker profile or amyloid PET (Thompson et al., 2003; Seeley et al., 2005; Brambati et al., 2009; Chan et al., 2009; Kumfor et al., 2016), except a single post-mortem study (Josephs et al., 2009).
To delineate the potentially unique clinical syndrome of rtvFTD, we set out to examine the clinical and neuropsychological profile of rtvFTD and compare it to svPPA, bvFTD, and Alzheimer’s disease. Additionally, we aimed to identify the neuroimaging pattern of rtvFTD in comparison with svPPA to establish whether these distinct clinical presentations also involve distinct anatomical underpinnings.
Materials and methods
Patient selection
Six hundred and nineteen patients with a clinical diagnosis of FTD and/or PPA whose amyloid status data were available, diagnosed between January 1998 and June 2018, were collected from the Amsterdam Dementia Cohort (van der Flier et al., 2014). All patients were diagnosed by a multidisciplinary team according to clinical diagnostic criteria (Neary et al., 1998; Gorno-Tempini et al., 2011; Rascovsky et al., 2011). Thirty-two patients who had a positive Alzheimer’s disease CSF profile (Tijms et al., 2018) and/or a positive amyloid-PET scan were excluded. Our inclusion criterion was having a predominant temporal lobar atrophy on the right side on the initial brain MRI (Supplementary Fig. 1). Therefore, three patients were excluded due to lack of brain MRI scans. All MRI scans had been visually assessed by experienced neuro-radiologists (F.B., M.W.) who were blinded to clinical and paraclinical details. Based on visual assessment (Rhodius-Meester et al., 2017), subjects were included in the study if temporal cortical atrophy and/or mesial temporal atrophy scores (Scheltens et al., 1992) were at least more than one grade higher on the right side than on the left side. This yielded a sample of 70 subjects with right predominant temporal lobe atrophy. Hereby, 11.3% of our FTD cohort were identified as rtvFTD. The remaining 514 patients showed predominant frontal or equal bilateral temporal or predominant left temporal atrophy and were therefore not included. To elucidate the potential rtvFTD subjects in the excluded groups (patients with positive Alzheimer’s disease CSF profile and/or PET scan and patients without MRI), all initial neuroimaging of excluded subjects was also assessed. However, none of the subjects had predominant right temporal lobe atrophy.
Four of the 70 rtvFTD subjects had a post-mortem pathological diagnosis showing frontotemporal lobar degeneration with tau pathology (FTLD-tau, n = 1, with a mutation in the tau gene), FTLD with TAR DNA binding protein 43 (n = 2) and FTLD with fused in sarcoma protein (n = 1). Additionally, one subject without a post-mortem examination was carrier of a pathogenic variant in the progranulin gene.
To compare the clinical characteristics of the diseases, age and gender-matched, biomarker-based svPPA (n = 70), bvFTD (n = 70) and Alzheimer’s disease patients (n = 70) diagnosed between January 1998 and June 2018 were selected from the Amsterdam Dementia Cohort (van der Flier et al., 2014), as control groups with an unbiased method (logistic regression model) (Hosmer, 2013).
Additionally, 70 age- and sex-matched (age: 62.9 ± 8.3, 34% female) healthy volunteers and subjective cognitive decline patients from the Amsterdam Dementia Database were added as a reference for cognitive tests.
For the radiological part of the study, we also selected 121 amyloid-β-negative cognitively normal subjects [age: 57.4 ± 8.9, 41% male, Mini-Mental State Examination (MMSE): 29.0 ± 0.8] from the Amsterdam Dementia Cohort. This group served as a reference in voxel-wise contrasts. Supplementary Fig. 2 displays the patient selection.
Clinical data collection and assessment
For clinical data analysis, in this retrospective study both qualitative and quantitative methods were used. The case notes written by senior neurologists Y.P. and P.S. were scrutinized and all described symptoms were extracted. Symptoms were subclassified as ‘initial symptoms’ (at the initial visit) and ‘later symptoms’ (at any stage of the disease, only rated when reported at follow-up). Similar symptoms were combined into one umbrella term by R.H. and Y.P., based on similar meaning and/or cognitive/behavioural domains (Supplementary material). Subsequently, 21 single symptoms were categorized in the following four groups; cognitive, language, behavioural, and other symptoms. All 21 symptoms were recorded as present or absent for each patient. As part of their functional assessment, the Clinical Dementia Rating Scale (CDR) was performed (Morris, 1993) in all patients. General cognitive functioning was measured using the MMSE (Folstein et al., 1975), whereas executive functioning was screened with the Frontal Assessment Battery (FAB) (Dubois et al., 2000). The patients’ behavioural and psychological status was assessed by the neuropsychiatric inventory (NPI) (Cummings et al., 1994).
Neuropsychological assessment
Neuropsychological examination was performed for diagnostic purposes at first presentation to the Alzheimer Centre Amsterdam. A standard test battery was administered to assess multiple cognitive domains such as episodic memory [visual association test (VAT) A (Lindeboom et al., 2002) and the Dutch version of the Rey Auditory Verbal Learning Test (RAVLT)], executive functions [Trail Making Test (TMT) B (Tombaugh, 2004) and digit span backward (Wechsler, 2008)], semantic memory [category fluency animals (Morris et al., 1989)], confrontation naming [VAT naming (Lindeboom et al., 2002)], attention [digit span forward (Wechsler, 2008) and TMT A (Tombaugh, 2004)] and visuospatial functions [Visual Objective and Space Perception (VOSP)–fragmented letters and VOSP–dot counting (Quental et al., 2013)]. Details of the clinical assessment and tests have been published previously (van der Flier et al., 2014; van der Flier and Scheltens, 2018).
All data for cognitive, psychological and functional assessment were collected retrospectively.
MRI acquisition and processing
MRI of the brain was acquired on a 1 T, 1.5 T or 3 T whole body magnetic resonance system (Siemens Magnetom Impact, Avanto and Sonata, GE Healthcare Signa HDXT, Discovery MR750, GE Medical Systems; Ingenuity TF PET/MR, Philips Medical Systems; Titan, Toshiba Medical Systems), using previously described protocols (Ten Kate et al., 2017; Groot et al., 2018). Eleven of 70 rtvFTD and 18 of 70 svPPA subjects did not have a suitable MRI available for voxel-based morphometry (VBM) analysis. MRI scans of the remaining 59 rtvFTD, 52 svPPA and 121 control subjects were collected and the structural 3D T1-weighted magnetic resonance images were segmented into grey matter, white matter and CSF volumes, which were summed to provide the total intracranial volume. Next, Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) was used to generate a study-specific template by aligning grey matter images nonl-inearly to a common space in SPM12 (Wellcome Trust Centre for Neuroimaging, Institute of Neurology at University College London). Native space grey matter images were then spatially normalized to the DARTEL template using individual flow fields. Modulation was applied to preserve the total amount of signal, and images were smoothed using an 8 mm full-width at half-maximum isotropic Gaussian kernel. Visual inspection was performed after each processing step and the images of eight rtvFTD patients and six svPPA patients were excluded based on these inspections. All images of the control group were suitable for analysis. Thus, the final selection included 51 rtvFTD patients, 46 svPPA patients and 121 cognitively normal participants and the normalized, smoothed and modulated images of these subjects were used in the VBM analyses. Additionally, the automated anatomical labelling (AAL) atlas was used to extract regional grey matter volumes across 62 regions, which were used in the region of interest analyses.
Statistical analysis
Analyses were conducted using SPSS Statistics, version 24.0 (IBM) and SPM12.
Differences in categorical variables between groups (rtvFTD, svPPA, bvFTD, and Alzheimer’s disease) were assessed with chi-square and continuous variables between groups were assessed with one-way ANOVA or Kruskal-Wallis variance analysis depending on the distribution of the variables based on normality test. Post hoc comparisons were corrected for multiple comparisons using the Bonferroni correction. The results were thresholded at a corrected P-value of <0.05.
The combination of clinical features that were considered characteristic of rtvFTD based on chart review was reported in a diagnostic tree of rtvFTD including the negative amyloid status and its radiological features. Sensitivity, specificity, positive and negative predictive values of the clinical syndrome were calculated with cross tables with 95% confidence intervals.
To identify patterns of neurodegeneration in each syndrome with respect to healthy controls we performed voxel-wise contrasts of grey matter volumes between groups (rtvFTD, svPPA) and controls using general linear models adjusted for age, sex, intracranial volume, and scanner field strength. In addition, to compare the atrophy pattern of rtvFTD and svPPA, an asymmetry index was calculated within regions of interest with the formula [AI (%) = 200 × (R − L)/(R + L)] (Ossenkoppele et al., 2016). Thus, negative outcomes indicate more atrophy in the right hemisphere, while positive values reflect left lateralized asymmetry.
Additionally, to identify the anatomical correlate of prosopagnosia, which was observed to be the most distinguishing symptom of rtvFTD, we compared the initial MRI scans of rtvFTD subjects with prosopagnosia (n = 37) and without prosopagnosia (n = 33) at the initial visit while adjusting for age, sex, intracranial volume, scanner field strength and whole-brain grey matter to intracranial volume ratios.
Ethical approval
The local Medical Ethics Committee approved a general protocol for using the clinical data for research purposes (Protocol No: 2016.061).
Data availability
Data are available on request from the corresponding author.
Results
Demographic data
Table 1 displays demographic data, symptom duration, follow-up duration and handedness per patient group. The rtvFTD group comprised 49 male and 21 female patients with a mean age of 64.7 years [standard deviation (SD) 8.4] and a mean symptom duration of 2.6 years (SD 1.6). Mean symptom duration and median follow-up duration did not differ significantly between diagnostic groups (P = 0.102, P = 0.666). Handedness varied among patients, but no statistical differences in the distribution of handedness per group were found (P = 0.074). To establish receptive language dominance in left-handed, ambidexter and handedness unknown subjects, we checked whether clinical symptoms showed concordance with the anatomic distribution of cortical atrophy and clinical presentation. All patients demonstrated the same pattern of hemispheric lateralization as the right-handers (Table 1).
Table 1.
Demographic data, symptom and follow-up duration, and handedness per group
| rtvFTD | svPPA | bvFTD | AD | P | |
|---|---|---|---|---|---|
| n | 70 | 70 | 70 | 70 | – |
| Gender, n female (%) | 21 (30) | 24 (34) | 25 (35) | 22 (31) | 0.885a |
| Age, years, mean ± SD | 64.7 ± 8.4 | 64.0 ± 7.6 | 63.6 ± 6.7 | 65.1 ± 7.6 | 0.470b |
| Handedness: left/right/ambidexterous/unknown | 6/57/1/6 | 1/55/0/14 | 7/51/3/9 | 8/52/0/10 | 0.074c |
| Symptom duration, years, mean ± SD | 2.6 ± 1.6 | 3.8 ± 1.4 | 4.4 ± 1.4 | 3.6 ± 4.6 | 0.102b |
| Follow-up period, years, median (min–max) | 2 (0–11) | 1 (1–8) | 2 (0–11) | 2 (1–7) | 0.666d |
Chi-square.
One-way ANOVA.
Fisher’s exact test.
Kruskal-Wallis non-parametric tests.
AD = Alzheimer’s disease; SD = standard deviation.
Core symptoms of right temporal variant FTD
Detailed initial and later symptoms per disease group are displayed in Table 2. It should be noted that multiple symptoms could be present simultaneously in one patient, hence the total number of symptoms exceeds the number of patients.
Table 2.
Clinical features of the diagnostic groups
| Symptoms a | Initial (% affected) |
Later (% affected) |
||||||
|---|---|---|---|---|---|---|---|---|
| rtvFTD | svPPA | bvFTD | AD | rtvFTD | svPPA | bvFTD | AD | |
| Cognitive | ||||||||
| Memory problems | 60 | 25 | 49 | 99 | 90 | 67 | 76 | 100 |
| Prosopagnosia | 54 | 21 | 4 | 0 | 70 | 29 | 13 | 0 |
| Executive dysfunction | 21 | 18 | 52 | 83 | 58 | 41 | 80 | 87 |
| Orientation problems | 6 | 17 | 27 | 66 | 34 | 26 | 36 | 74 |
| Getting lost | 7 | 4 | 12 | 16 | 20 | 6 | 17 | 26 |
| Visuo- spatial problems | 7 | 7 | 10 | 46 | 23 | 11 | 22 | 54 |
| Language | 48 | 100 | 43 | 79 | 82 | 100 | 62 | 89 |
| Word-finding difficulties | 31 | 72 | 30 | 79 | 61 | 79 | 47 | 89 |
| Single word comprehension deficit | 18 | 61 | 7 | 0 | 35 | 60 | 14 | 6 |
| Paraphasias | 14 | 51 | 3 | 13 | 19 | 64 | 14 | 21 |
| Naming difficulties | 28 | 85 | 21 | 23 | 51 | 87 | 30 | 30 |
| Behavioural | 95 | 65 | 100 | 42 | 97 | 90 | 100 | 75 |
| Disinhibition | 60 | 31 | 81 | 20 | 74 | 82 | 90 | 37 |
| Compulsive behaviour | 40 | 35 | 46 | 1 | 71 | 66 | 66 | 9 |
| Apathy or inertia | 55 | 41 | 75 | 40 | 91 | 61 | 85 | 52 |
| Loss of empathy and egocentrism | 50 | 14 | 55 | 3 | 65 | 47 | 64 | 20 |
| Hyper-orality and dietary changes | 22 | 8 | 50 | 14 | 68 | 37 | 61 | 18 |
| Other symptoms | ||||||||
| Motor / mental slowness | 27 | 15 | 17 | 27 | 70 | 25 | 37 | 34 |
| Hyper-religiosity | 1 | 1 | 0 | 0 | 4 | 4 | 0 | 0 |
| Depression | 27 | 15 | 4 | 36 | 44 | 23 | 11 | 44 |
| Delusions / hallucinations | 7 | 7 | 9 | 7 | 22 | 13 | 10 | 9 |
| Somatic complaints and aches | 15 | 8 | 20 | 14 | 40 | 27 | 27 | 27 |
| Feeling of anxiety/ panic | 11 | 11 | 11 | 28 | 38 | 25 | 18 | 34 |
Symptoms were collected based on the case notes written by senior neurologists. For further information see the Supplementary material.
AD = Alzheimer’s disease.
Episodic memory problems and prosopagnosia were two of the most common initial symptoms of rtvFTD with a prevalence of 60% and 54%, respectively, increasing to 90% and 70% during follow-up. Besides these symptoms, behavioural problems were almost universally present at the initial visit and included behavioural disinhibition (60%), apathy or inertia (55%), loss of empathy and egocentrism (50%), and compulsive behaviour (40%). The latter not only consisted of simple compulsive behaviour, such as clock watching, but also of ritualistic preoccupations, such as dressing each day of the week in a different colour, and repeatedly driving more than 1 h to the same shop, to buy objects at a minimal discount. Language problems such as word-finding difficulties (31%) and anomia (28%) were relatively less frequent at the first assessment. However, over the disease course, 82% of the cases developed language difficulties. Of note, the characteristic language symptoms of svPPA, such as single word comprehension deficits (18%) and paraphasias (14%), were recorded less frequently.
Main differences between diagnostic groups
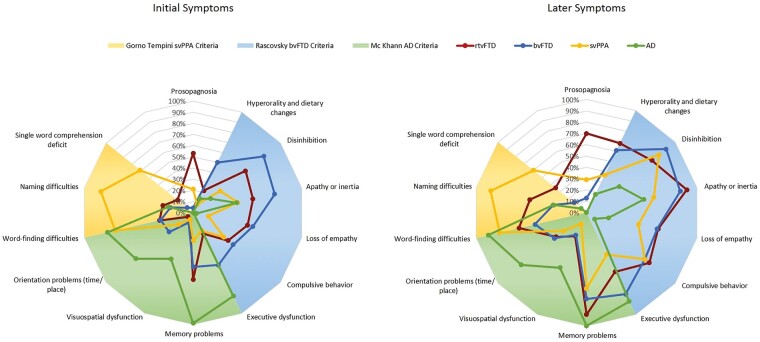
To compare the clinical profiles of rtvFTD, svPPA, bvFTD and Alzheimer’s disease, the prominent symptoms of the disease groups were displayed against the current diagnostic criteria for bvFTD (Rascovsky et al., 2011), svPPA (Gorno-Tempini et al., 2011) and Alzheimer’s disease (McKhann et al., 2011) on a descriptive spider graph (Fig. 1).
Figure 1.
Main differences among disease groups at first assessment (initial symptoms) and at any stage of the disease (later symptoms). The shadow graphs on the background were adapted from current diagnostic criteria (Gorno-Tempini et al., 2011; McKhann et al., 2011; Rascovsky et al., 2011). AD = Alzheimer’s disease.
As expected, the pattern of svPPA, bvFTD, and Alzheimer’s disease clinical symptoms were in line with their respective clinical criteria. Cases with rtvFTD were characterized by prosopagnosia, behavioural problems, language problems, and episodic memory problems, thereby combining unique features and common features with each of the comparative patient groups. During the disease course, the most prominent clinical features of rtvFTD were still not completely overlapping with one of the other groups, meaning that also during the disease course, rtvFTD kept its own clinical profile.
Prosopagnosia was the most unique symptom of rtvFTD. It was not seen in Alzheimer’s disease, and much less prevalent in svPPA and bvFTD. Memory problems were most commonly present in Alzheimer’s disease, but not unique, but were also present (to a lesser extent) in rtvFTD and bvFTD, and eventually also in svPPA. Even though all bvFTD patients exhibited behavioural changes at the initial presentation, both rtvFTD (95%) and svPPA (65%) groups initially exhibited behavioural changes as well. However, the characteristics of the behavioural problems were different in rtvFTD. Compulsiveness and apathy-inertia were the most prominent behavioural changes in svPPA, whereas rtvFTD patients exhibited various and more frequent behavioural symptoms such as disinhibition, loss of empathy, as well as compulsiveness and apathy-inertia initially. Although these behavioural problems were also prominent in bvFTD, over the disease course, behavioural symptoms of rtvFTD and bvFTD showed different progression patterns, where compulsive behaviour, apathy-inertia, and hyperorality and dietary changes evolved most prominently in rtvFTD. In contrast, patients with bvFTD demonstrated greater executive dysfunction than rtvFTD. In addition, depression was more common in rtvFTD (27% initial, 44% later) than bvFTD (4% initial, 11% later). Language disorder was the prominent feature of svPPA. Even though patients with rtvFTD demonstrated relatively less frequent language problems initially, at the following visits the majority of patients developed language dysfunction. The two most common language symptoms recorded at the initial visit were word-finding difficulty and anomia for rtvFTD whereas svPPA patients exhibited highly frequent language problems with a wide range of symptom distribution such as single word comprehension deficits, paraphasias, as well as word-finding difficulties and anomia. Visuospatial and orientation problems and getting lost were more common in Alzheimer’s disease than in the FTD groups in both the initial and later stages.
Even though motor/mental slowness was not common in rtvFTD at initial presentation, it became one of the distinguishing symptoms of rtvFTD during follow-up. Psychiatric features, such as depression, psychotic symptoms, and anxiety evolved during the course of rtvFTD at a higher frequency compared with the other disease groups. Somatic complaints and aches, for which no medical cause was found, were present in 40% of rtvFTD cases, compared to 27% in the other groups. In rtvFTD, these were also associated with beliefs that the body was containing valves or tubes that could be influenced from the outside. Hyper-religiosity was less common, but was uniquely observed in the rtvFTD and svPPA groups (Table 2).
Cognitive test scores and neuropsychiatric inventory
Dementia severity and neuropsychological test scores are shown per diagnostic group in Table 3. Because of a change in the test protocols used over the years, some patients’ data were not available. The numbers of data available patients are displayed in the figures and tables.
Table 3.
Cognitive test scores of the diagnostic groups
| HC |
rtvFTD |
svPPA |
bvFTD |
AD |
One-way ANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive domain | Test | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | P | Group differences |
| Disease severity | CDR | – | – | 49 | 0.6 ± 0.35 | 37 | 0.9 ± 0.63 | 54 | 0.8 ± 0.47 | 49 | 0.9 ± 0.43 | 0.051 | NS |
| Global cognition | MMSE | 70 | 28.9 ± 1.10 | 70 | 25.34 ± 3.23 | 59 | 21.08 ± 6.30 | 67 | 25.37 ± 3.87 | 67 | 20.22 ± 5.10 | <0.001 | HC > rtvFTD, bvFTD > svPPA, AD |
| Episodic memory | VAT-A | 70 | 11.61 ± 0.71 | 58 | 10.05 ± 2.64 | 46 | 8.37 ± 3.73 | 55 | 10.38 ± 2.52 | 57 | 5.19 ± 4.06 | <0.001 | HC, rtvFTD, bvFTD, svPPA > AD |
| RAVLT delayed recall | 70 | 8.89 ± 2.83 | 50 | 4.62 ± 3.34 | 28 | 2.86 ± 2.86 | 58 | 5.26 ± 3.33 | 40 | 1.85 ± 02.00 | <0.001 | HC > rtvFTD, bvFTD, svPPA > AD | |
| Executive functioning | FAB | 70 | 17.23 ± 1.13 | 48 | 15.02 ± 3.41 | 30 | 12.40 ± 3.74 | 52 | 12.96 ± 4.27 | 29 | 11.55 ± 3.56 | <0.001 | HC > rtvFTD>bvFTD, svPPA, AD |
| Digit span backward | 70 | 13.91 ± 2.79 | 59 | 8.37 ± 2.65 | 46 | 6.70 ± 2.57 | 58 | 7.50 ± 2.69 | 56 | 5.88 ± 2.53 | <0.001 | HC > rtvFTD, bvFTD > svPPA, AD | |
| TMT-B | 70 | 81.54 ± 34.21 | 54 | 121.63 ± 77.17 | 41 | 167.10 ± 97.36 | 51 | 138.33 ± 72.60 | 29 | 220.52 ± 155.29 | <0.001 | HC > rtvFTD, bvFTD, svPPA > AD | |
| Language | VAT Naming | 70 | 11.89 ± 1.11 | 60 | 9.98 ± 2.48 | 43 | 6.49 ± 3.80 | 55 | 11.53 ± 1.33 | 55 | 11.51 ± 0.76 | <0.001 | HC, bvFTD, AD > rtvFTD > svPPA |
| Animal fluency | 70 | 23.7 ± 5.72 | 60 | 14.30 ± 5.33 | 45 | 7.58 ± 5.53 | 57 | 14.88 ± 6.03 | 60 | 12.37 ± 5.01 | <0.001 | HC > rtvFTD, bvFTD, AD > svPPA | |
| Attention | Digit span forward | 70 | 15.2 ± 3.12 | 60 | 11.72 ± 2.91 | 48 | 10.21 ± 3.05 | 58 | 11.22 ± 2.93 | 57 | 10.70 ± 3.34 | 0.061 | NS |
| TMT-A | 70 | 48.7 ± 20.39 | 63 | 54.60 ± 31.42 | 49 | 61.55 ± 29.67 | 61 | 56.59 ± 31.95 | 52 | 103.54 ± 76.91 | <0.001 | HC, rtvFTD, bvFTD, svPPA > AD | |
| Visuospatial function | Fragmented letters (VOSP) | 70 | 19.3 ± 0.84 | 42 | 16.62 ± 4.83 | 23 | 17.39 ± 4.34 | 42 | 16.62 ± 4.83 | 24 | 15.46 ± 4.86 | 0.574 | NS |
| Dot counting (VOSP) | 70 | 9.8 ± 0.51 | 39 | 9.74 ± 1.14 | 20 | 9.55 ± 1.19 | 39 | 9.74 ± 1.14 | 22 | 8.55 ± 1.62 | 0.018 | HC, rtvFTD, bvFTD, svPPA > AD | |
Statistically significant values (P < 0.05) are presented in bold. Group differences are displayed in the next column. AD = Alzheimer’s disease; CDR = Clinical Dementia Rating; FAB = Frontal Assessment Battery; HC = healthy control; MMSE = Mini-Mental State Examination; NS = not significant; RAVLT = Dutch version of the Rey Auditory Verbal Learning Test; SD = standard deviaiton; TMT = Trail Making Test; VAT = Visual Association Test; VOSP = Visual Objective And Space Perception.
Dementia severity, as measured with the CDR, was lower in the rtvFTD group; however, no significant difference was detected between disease groups (P = 0.051). MMSE scores were higher in rtvFTD and bvFTD compared to svPPA and Alzheimer’s disease (P < 0.001). Alzheimer’s disease patients demonstrated greater memory impairment (VAT-A and RAVLT delayed recall P < 0.001), attention deficits (TMT-A P < 0.001, digit span forward P = 0.065) and visuospatial dysfunction (dot counting P = 0.020, fragmented letters P = 0.574) than other groups whereas language deficits were most profound in the svPPA group (VAT naming and animal fluency P < 0.001). Patients with rtvFTD exhibited similar performance to bvFTD generally, except on the naming test and FAB. The patients with rtvFTD demonstrated worse performance than bvFTD on the naming test (P < 0.001), whereas bvFTD patients exhibited greater executive dysfunction (FAB P = 0.001). As a result, rtvFTD patients exhibited a generally better performance on neuropsychological tests compared to the other diagnostic groups, except on the naming test (Table 3). On the other hand, patients with rtvFTD exhibited worse performance than cognitively normal subjects on global cognition, episodic memory, language and executive functions.
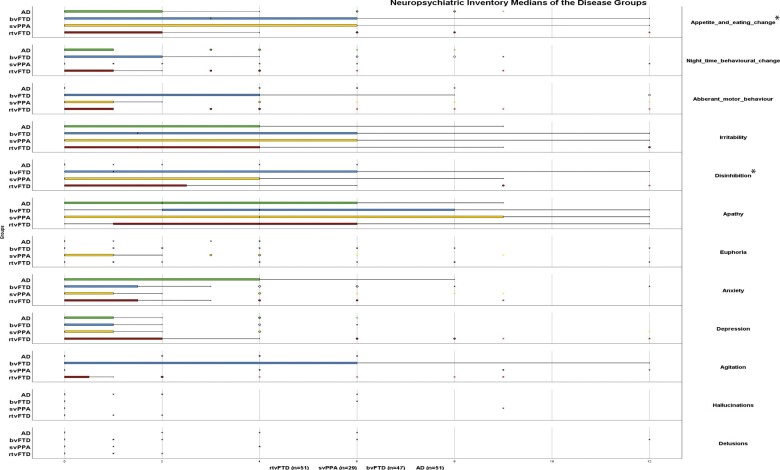
NPI results showed that neuropsychiatric symptoms were most severe in patients with bvFTD, as indicated by the overall NPI score and by the scores for aberrant motor behaviour, sleep time behaviour problems, changing eating habits, irritability, aggression and disinhibition. However, a statistically significant difference was observed only in the overall NPI score and the items related with disinhibition and changing eating habits (P < 0.05, bvFTD versus other diagnostic groups). Although bvFTD has the highest overall NPI score, the item related with depression was higher in rtvFTD; however, this difference was not statistically significant (P = 0.101) (Fig. 2).
Figure 2.
Neuropsychiatric inventory medians of the disease groups. AD = Alzheimer’s disease. Frequency × Severity scores were analysed. *P < 0.05, bvFTD versus other diagnostic groups.
Radiological characteristics of right temporal variant FTD and comparison with semantic variant PPA
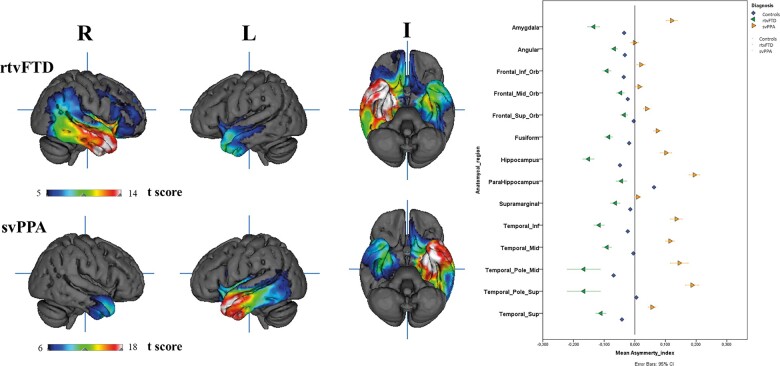
VBM analysis revealed that, compared with controls, patients with rtvFTD showed bilateral asymmetrical (right > left) grey matter volume loss in the anterior temporal lobes and in the right ventral frontal area. Right-sided grey matter loss was observed in the temporal poles, the superior, medial, and inferior temporal gyri, medial temporal lobe, insula, fusiform gyrus, angular gyrus, and supramarginal gyrus. The same regions were involved in the left temporal lobe, though to a lesser extent. Grey matter loss was also observed in the right inferior frontal gyrus, gyrus rectus, orbitofrontal cortex, with a greater degree of loss observed in the inferior orbitofrontal lobe. Patients with svPPA showed a mirrored pattern. Asymmetry index analysis showed that the frontal and temporal lobes were affected almost equally, but in opposite directions in rtvFTD and svPPA. Both in rtvFTD and svPPA, the temporal poles were the most affected areas (Fig. 3).
Figure 3.
3D T-maps of the rtvFTD and svPPA and the asymmetry index.
Clinico-radiological correlation of prosopagnosia in right temporal variant FTD
Mean symptom duration did not differ significantly between prosopagnosia present (3.4 ± 1.9 years) and absent (2.65 ± 1.5 years) groups (P = 0.445). Visual inspection of voxelwise contrasts between rtvFTD patients with and without prosopagnosia revealed that the patients with prosopagnosia showed more grey matter loss bilaterally in the temporal poles and anterior fusiform gyrus (P < 0.001, uncorrected). This association survived family-wise error correction (P < 0.05) in the left-anterior fusiform gyrus (Supplementary Fig. 3).
A diagnostic tree to identify right temporal variant FTD
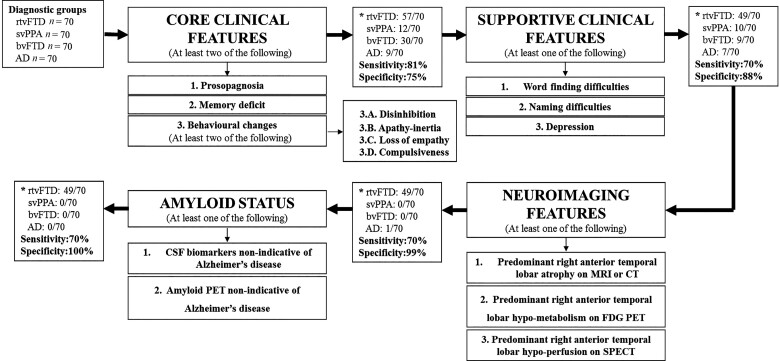
Based on the combination of the literature review and our data, we summarized the core and supportive symptoms of rtvFTD and prepared a diagnostic tree including clinical and radiological features of rtvFTD and amyloid status (Fig. 4). To validate the proposed algorithm, sensitivity and specificity analysis for rtvFTD was performed against the background of the non-rtvFTD syndromes of bvFTD, svPPA, and Alzheimer’s disease. The sensitivity value of the presence of two or more core symptoms (prosopagnosia, memory deficit, and behavioural changes) was 81% whereas the specificity value was relatively low (75%). The core symptoms distinguished rtvFTD from svPPA and Alzheimer’s disease while approximately half of the bvFTD subjects met the core symptoms. However, when we added the supportive symptoms such as language problems and depression, the specificity value increased to 88% at the cost of sensitivity. Moreover, when the neuroimaging and negative amyloid status were taken into account, we reached a specificity of 100% of the characteristics of rtvFTD (Fig. 4). Details of the cases and diagnostic symptoms are displayed in the Supplementary material.
Figure 4.
A diagnostic tree to identify rtvFTD. *Number of the subjects who met the proposed criteria. AD = Alzheimer’s disease.
Discussion
In this large systematic, retrospective study, we identified a uniquely large cohort of patients with rtvFTD based on brain atrophy pattern and set out to determine their clinical profile. Furthermore, we investigated overlapping and distinguishing clinical features of rtvFTD compared with svPPA, bvFTD, and Alzheimer’s disease. We also studied the imaging phenotype of rtvFTD in more detail using VBM analysis and compared it with svPPA, the radiological differential diagnosis of rtvFTD. Prosopagnosia, episodic memory impairment and behavioural problems such as disinhibition, apathy, loss of empathy and compulsiveness were the most prominent initial symptoms of rtvFTD, whereas language ability was relatively spared initially, unlike in svPPA. During the progressive disease course, language problems such as word finding difficulties and anomia became the main features of the disease. None of the current diagnostic criteria for bvFTD or svPPA fitted rtvFTD. VBM analysis revealed, apart from predominant right anterior temporal atrophy, involvement of the left temporal and the right ventral frontal areas. Notably, it exhibited a radiological mirror image of svPPA. Additionally, the temporal poles and the anterior fusiform gyrus—especially on the left-side—were associated with prosopagnosia in rtvFTD.
Prosopagnosia was the most unique symptom of rtvFTD. This result is consistent with expectations, as the relationship between prosopagnosia and right temporal lobe involvement has been described frequently (Gainotti et al., 2003; Joubert et al., 2003, 2006; Thompson et al., 2003; Gorno-Tempini et al., 2004b; Chan et al., 2009; Everhart et al., 2015). Thompson et al. (2003) reported prosopagnosia in 10 of 11 cases with a right > left temporal atrophy, whereas Chan et al. (2009) reported prosopagnosia in 60% (12 of 20 cases) of patients with rtvFTD. A possible explanation for this discrepancy is that impaired face recognition may not be mentioned as a specific problem by the patients and caregivers and specific tests for face recognition are usually not performed in general practice. Since it is not a clinical feature in one of the current diagnostic criteria for svPPA, bvFTD, and Alzheimer’s disease, it might also easily be neglected by physicians.
Over the past 20 years, the general view has been that episodic memory processing is relatively intact in FTD (Neary et al., 1998; Gorno-Tempini et al., 2011; Rascovsky et al., 2011). However, episodic memory deficit was one of the prominent presenting symptoms of rtvFTD, and its frequency increased up to 90% later on. Although Thompson et al. (2003) found memory problems in only 27.3% of the rtvFTD patients, episodic memory deficit has been highlighted as an initial symptom of rtvFTD in a number of clinical studies and case reports (Tyrrell et al., 1990; Joubert et al., 2003, 2006; Gorno-Tempini et al., 2004a; Chan et al., 2009; Josephs et al., 2009; Everhart et al., 2015). Since the presence of amnesia remains a diagnostic exclusion criterion for FTD (Neary et al., 1998; Gorno-Tempini et al., 2011; Rascovsky et al., 2011), the amnestic/prosopagnostic presentation of rtvFTD might easily be confused with Alzheimer’s disease in the early stages of the disease. It should be noted, however, that even though episodic memory deficit was one of the most common symptoms of rtvFTD, in line with previous studies (Pleizier et al., 2012), we found that they showed better performance on memory tests than Alzheimer’s disease patients, however worse than healthy control subjects (RAVLT P < 0.001). Whereas episodic memory processing in semantic dementia and bvFTD has been studied previously (Hornberger et al., 2010; Irish et al., 2016), the mechanism of episodic memory deficits in rtvFTD is still unknown.
Although disinhibition and apathy were the most common behavioural symptoms in both rtvFTD and bvFTD, in accordance with the findings of Kamminga et al. (2015), who compared clinical features between rtvFTD and bvFTD, we also found prominent language dysfunction and prosopagnosia in the rtvFTD group versus more severe executive dysfunction in bvFTD. Contrary to that study, revealing dietary changes as common in both disorders, in the present study these were initially less frequent in rtvFTD than in bvFTD. Compulsiveness was a distinct symptom observed frequently in both svPPA and rtvFTD. Another important result of our study was the loss of empathy, which was common in both rtvFTD and bvFTD, while it was relatively rare as a presenting feature in svPPA. This finding supports the argument that empathy is associated with the right frontotemporal areas (Rankin et al., 2006; Kamminga et al., 2015; Perry et al., 2017). One of the striking results of our study was that at both initial and later stages, depression was observed more commonly in rtvFTD, with higher depression scores on the NPI than bvFTD. In addition, in line with previous studies, somatic complaints were observed prominently in rtvFTD at the follow-up visits, as well as depression (Gainotti et al., 2003; Thompson et al., 2003; Chan et al., 2009; Everhart et al., 2015).
Overall, rtvFTD patients were more depressive, compulsive, somatic and they demonstrated pronounced deficits in face recognition and language, whereas patients with bvFTD exhibited disproportionate disinhibition, apathy and greater executive dysfunction. Nevertheless, the initial behavioural changes in rtvFTD can be a diagnostic issue, particularly in the early stages of the disease. Prosopagnosia and language problems distinguish rtvFTD from bvFTD and we suggest that the presence of predominant depression at the initial visit might also be helpful in differentiating the behavioural symptoms of rtvFTD and bvFTD.
Language disorder was one of the important features of rtvFTD. However, unlike svPPA, language problems in rtvFTD were not prominent in the early stages of the disease. Similar to other studies, the most common language problems were word-finding difficulties and anomia in rtvFTD (Thompson et al., 2003; Gorno-Tempini et al., 2004b; Seeley et al., 2005; Joubert et al., 2006; Josephs et al., 2009), whereas the characteristic svPPA symptoms, such as single-word comprehension deficits, were relatively infrequent in the rtvFTD versus the svPPA. The svPPA is traditionally seen as inherently tied to language and current diagnostic criteria have been updated from this perspective (Gorno-Tempini et al., 2011). Even though it has been acknowledged that language abilities are relatively spared in rtvFTD (Thompson et al., 2003; Seeley et al., 2005; Chan et al., 2009; Josephs et al., 2009; Everhart et al., 2015), the syndrome is still classified as the right-sided semantic variant of progressive aphasias based on its atrophy pattern (Gorno-Tempini et al., 2011). From a clinical perspective, this is incorrect, as language abilities can in fact be spared, in the context of prominent clinical features like behavioural abnormalities, memory and face recognition deficits.
Besides these core symptoms, hyper-religiosity (Edwards-Lee et al., 1997; Chan et al., 2009; Josephs et al., 2009; Everhart et al., 2015; Veronelli et al., 2017), getting lost (Chan et al., 2009; Josephs et al., 2009) and delusions (Chan et al., 2009) have been reported as symptoms associated with rtvFTD. Hyper-religiosity was a symptom reported by 4% of rtvFTD patients in our study. Even though this symptom has been described as almost pathognomonic in case reports (Edwards-Lee et al., 1997; Everhart et al., 2015; Veronelli et al., 2017), it has been reported in only ∼5–15% of the clinical studies (Thompson et al., 2003; Chan et al., 2009; Josephs et al., 2009) and it has also been observed in svPPA patients (Thompson et al., 2003). In our study, hyper-religiosity was observed in both rtvFTD and svPPA, whereas neither bvFTD nor Alzheimer’s disease patients presented it. Chan et al. (2009) reported that getting lost was observed in 65% of patients in contrast to the low frequency (18%) of our study. An explanation of this discrepancy could be the exclusion of patients with positive amyloid pathology. Regarding delusions and visual hallucinations, although their prevalence increased during the disease course of rtvFTD, it was not a distinct symptom of rtvFTD, as was suggested by Chan et al. (2009).
On the other hand, motor/mental slowness was a symptom in rtvFTD, which was not recorded to the same extent in svPPA, bvFTD and Alzheimer’s disease. Since clinical studies and case reports have often focused on initial symptoms, ‘slowness’ might not be mentioned as a symptom associated with rtvFTD in previous literature. However, a post-mortem-based study has revealed that over the disease course, 35% of the rtvFTD patients developed parkinsonism (Josephs et al., 2009). In addition, some studies have pointed out the relationship between rtvFTD and motor neuron disease as well as parkinsonism (Davion et al., 2007; Kobayashi et al., 2010; Coon et al., 2012; Lee et al., 2012; Josephs et al., 2013; Miki et al., 2019). Although some authors have suggested that rtvFTD and svPPA reflect the same pathophysiological process and converge clinically within 3 years from symptom onset (Seeley et al., 2005), one longitudinal study has revealed the divergent progression pattern of these two related syndromes (Kumfor et al., 2016). Our results also show that rtvFTD patients might exhibit a different progression pattern than svPPA. As symptom duration at presentation and follow-up duration were comparable in rtvFTD and svPPA, this finding cannot be attributed to a hypothesized later presentation of rtvFTD.
Radiological characteristics of right temporal variant FTD and comparison with semantic variant PPA
One of the key questions is whether these distinct clinical presentations have a distinct underlying atrophy pattern. To our knowledge, only three studies have assessed the atrophy pattern of rtvFTD systematically and the number of patients has been limited (n = 6–20) in these studies (Brambati et al., 2009; Chan et al., 2009; Kumfor et al., 2016). In line with those studies, predominant anterior temporal atrophy with a greater degree on the right side was the characteristic imaging pattern of rtvFTD. However, different from those studies we found that the ipsilateral ventral frontal areas were also affected in both rtvFTD and svPPA initially. On the other hand, one longitudinal study has found that atrophy in the later stages of rtvFTD can be observed in right orbitofrontal areas (Kumfor et al., 2016) whereas another study has argued that initial right anterior temporal atrophy is followed by subsequent involvement of the left temporal lobe to resemble patterns observed in svPPA (Brambati et al., 2009). Although our study is not a longitudinal study, our results for the rtvFTD group showed involvement of both contralateral temporal and ipsilateral ventromedial frontal areas, in particular the inferior orbitofrontal lobe areas, which were also observed to be affected in the svPPA group. Even if rtvFTD and svPPA display a radiological mirror image initially, our results show that even in later clinical stages they do not have the same manifestation. Future studies combining longitudinal clinical and neuroimaging findings will be essential to further understand the disease course and large pathological studies will shed light on the pathophysiological basis of these related syndromes.
Clinico-radiological correlation of prosopagnosia in right temporal variant FTD
There is a general agreement that right hemisphere damage is necessary for the occurrence of prosopagnosia (Gorno-Tempini et al., 1998; Snowden et al., 2004), but disagreement exists about the role of the left hemisphere (Meadows, 1974; Damasio et al., 1990; De Renzi et al., 1994). A recent prospective VBM study has shown that face identification is positively associated with right anterior fusiform gyrus volume in FTD (Omar et al., 2011). However, in that study, only one patient had the right predominant temporal lobe atrophy characteristic of rtvFTD (Omar et al., 2011). Another VBM analysis in semantic dementia has revealed that the right anterior temporal pole, the right fusiform gyrus and the right medial temporal lobe were associated with prosopagnosia in patients with semantic dementia (Josephs et al., 2008). Although our results are similar to those earlier findings, we observed that the left temporal lobe, in particular the temporal pole and the fusiform area, was also associated with prosopagnosia in rtvFTD.
Strengths and limitations
Our study differs from the previous studies in one key aspect; this is the first large clinical case-control study that excludes patients with amyloid pathology and presents a small sample size of patients with genetic/pathologically verified FTD. However, there are some limitations that need to be addressed. First, the study was performed retrospectively and although symptoms were recorded systematically in our specialized memory clinic, some symptoms might have gone unnoticed because they were not specifically asked for. This might particularly be the case for the more uncommon symptoms, such as hyper-religiosity. Second, the initial visit was not the same moment in every patients’ course of the disease. Some patients were referred from another hospital for a second opinion, whereas other patients had only been showing a few symptoms for a few months before the appointment. The other limitations were the lack of a specific cognitive test for face recognition, social cognition and missing data in cognitive tests and NPI ratings, due to change of test protocols in years. Lastly, as we performed a memory-clinic based study, all of the identified cases were symptomatic, and therefore, theoretically our sensitivity and specificity analysis of the clinical characteristics accompanying predominant right temporal atrophy might be an overestimation.
Clinical relevance
Neither the Gorno-Tempini diagnostic criteria for PPA (Gorno-Tempini et al., 2011), nor the Rascovsky diagnostic criteria for bvFTD (Rascovsky et al., 2011) cover the initial amnestic, prosopagnostic presentation of rtvFTD. RtvFTD is a unique progressive neurodegenerative disorder that has a distinctive cognitive, behavioural and language profile and a characteristic atrophy pattern. To cover specific symptoms of rtvFTD, we prepared a diagnostic tree including the main characteristics of rtvFTD and tested its distinguishing accuracy among the various patient groups. Even though combining core and supportive symptoms decreased the sensitivity value, accompanying language problems and depression distinguished rtvFTD from bvFTD and this yielded a specificity of 88% of clinical characteristics of rtvFTD. Furthermore, it should be underscored that neuroimaging characteristics of rtvFTD distinguished it from other FTD spectrums whereas negative amyloid status was crucial for differential diagnosis of Alzheimer’s disease. Therefore, the combination of amyloid status, clinical and radiological features yielded a 100% specificity. From a clinical point of view, the high specificity value implicates that when a patient presents with behavioural problems, the characteristic symptoms of rtvFTD, such as prosopagnosia, depression and language problems, should be examined. Following the clinical assessment, the right temporal lobe should be explored on neuroimaging, and diagnoses such as Alzheimer’s disease should be rejected unless their amyloid status is highly indicative for Alzheimer’s disease. We hope that our framework will serve as a roadmap to identify these patients in a clinical setting. In the near future, multicentre studies will be needed to define diagnostic criteria for rtvFTD and establish their accuracy in prospective cohorts.
Supplementary Material
Acknowledgements
Research of the Alzheimer Centre Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. The Alzheimer Centre Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds.
Funding
H.U.E. has received research support from the Turkish Neurological Society. F.B. is supported by the NIHR biomedical research centre at UCLH.
Competing interests
The authors report no competing interests.
Glossary
- bvFTD =
behavioural variant frontotemporal dementia
- NPI =
Neuropsychiatric Inventory
- PPA =
primary progressive aphasia
- rtvFTD =
right temporal variant frontotemporal dementia
- svPPA =
semantic variant primary progressive aphasia
- VBM =
voxel based morphometry
References
- Bang J, Spina S, Miller B.. Frontotemporal dementia. Lancet 2015; 386: 1672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging 2009; 30: 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain 2009; 132: 1287–98. [DOI] [PubMed] [Google Scholar]
- Coon EA, Whitwell JL, Parisi JE, Dickson DW, Josephs KA.. Right temporal variant frontotemporal dementia with motor neuron disease. J Clin Neurosci 2012; 19: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J.. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–14. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H.. Face agnosia and the neural substrates of memory. Annu Rev Neurosci 1990; 13: 89–109. [DOI] [PubMed] [Google Scholar]
- Davion S, Johnson N, Weintraub S, Mesulam MM, Engberg A, Mishra M, et al. Clinicopathologic correlation in PGRN mutations. Neurology 2007; 69: 1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E, Perani D, Carlesimo GA, Silveri MC, Fazio F.. Prosopagnosia can be associated with damage confined to the right hemisphere–an MRI and PET study and a review of the literature. Neuropsychologia 1994; 32: 893–902. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, Pillon B.. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000; 55: 1621–6. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain 1997; 120: 1027–40. [DOI] [PubMed] [Google Scholar]
- Everhart DE, Watson EM, Bickel KL, Stephenson AJ.. Right Temporal Lobe Atrophy: A Case That Initially Presented as Excessive Piety. Clin Neuropsychol 2015; 29: 1053–67. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR.. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Barbier A, Marra C.. Slowly progressive defect in recognition of familiar people in a patient with right anterior temporal atrophy. Brain 2003; 126: 792–803. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004. a; 55: 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, et al. The neural systems sustaining face and proper-name processing. Brain 1998; 121: 2103–18. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Rankin KP, Woolley JD, Rosen HJ, Phengrasamy L, Miller BL.. Cognitive and behavioral profile in a case of right anterior temporal lobe neurodegeneration. Cortex 2004. b; 40: 631–44. [DOI] [PubMed] [Google Scholar]
- Groot C, Sudre CH, Barkhof F, Teunissen CE, van Berckel BNM, Seo SW, et al. Clinical phenotype, atrophy, and small vessel disease in APOEepsilon2 carriers with Alzheimer disease. Neurology 2018; 91: e1851–e9. [DOI] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Ogar JM, Sidhu MS, Rankin KP, Cattaruzza T, et al. Neuropsychological, behavioral, and anatomical evolution in right temporal variant frontotemporal dementia: a longitudinal and post-mortem single case analysis. Neurocase 2014; 20: 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR.. How preserved is episodic memory in behavioral variant frontotemporal dementia?. Neurology 2010; 74: 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DL, Sturdivant RX, Applied logistic regression. Third ed. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- Irish M, Bunk S, Tu S, Kamminga J, Hodges JR, Hornberger M, et al. Preservation of episodic memory in semantic dementia: the importance of regions beyond the medial temporal lobes. Neuropsychologia 2016; 81: 50–60. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 2009; 73: 1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Murray ME, Parisi JE, Graff-Radford NR, Knopman DS, et al. Corticospinal tract degeneration associated with TDP-43 type C pathology and semantic dementia. Brain 2013; 136: 455–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Vemuri P, Senjem ML, Boeve BF, Knopman DS, et al. The anatomic correlate of prosopagnosia in semantic dementia. Neurology 2008; 71: 1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Ranjeva JP, Christophe M, Didic M, et al. The right temporal lobe variant of frontotemporal dementia: cognitive and neuroanatomical profile of three patients. J Neurol 2006; 253: 1447–58. [DOI] [PubMed] [Google Scholar]
- Joubert S, Felician O, Barbeau E, Sontheimer A, Barton JJ, Ceccaldi M, et al. Impaired configurational processing in a case of progressive prosopagnosia associated with predominant right temporal lobe atrophy. Brain 2003; 126: 2537–50. [DOI] [PubMed] [Google Scholar]
- Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M.. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry 2015; 86: 1082–8. [DOI] [PubMed] [Google Scholar]
- Kobayashi Z, Tsuchiya K, Arai T, Yokota O, Yoshida M, Shimomura Y, et al. Clinicopathological characteristics of FTLD-TDP showing corticospinal tract degeneration but lacking lower motor neuron loss. J Neurol Sci 2010; 298: 70–7. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Landin-Romero R, Devenney E, Hutchings R, Grasso R, Hodges JR, et al. On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain 2016; 139: 986–98. [DOI] [PubMed] [Google Scholar]
- Lee SE, Seeley WW, Poorzand P, Rademakers R, Karydas A, Stanley CM, et al. Clinical characterization of bvFTD due to FUS neuropathology. Neurocase 2012; 18: 305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C.. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry 2002; 73: 126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows JC. The anatomical basis of prosopagnosia. J Neurol Neurosurg Psychiatry 1974; 37: 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Ling H, Crampsie S, Mummery CJ, Rohrer JD, Jaunmuktane Z, et al. Corticospinal tract degeneration and temporal lobe atrophy in frontotemporal lobar degeneration TDP-43 type C pathology. Neuropathol Appl Neurobiol 2019; [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–4. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39: 1159–65. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–54. [DOI] [PubMed] [Google Scholar]
- Omar R, Rohrer JD, Hailstone JC, Warren JD.. Structural neuroanatomy of face processing in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 2011; 82: 1341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 2016; 139: 1551–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain 2017; 140: 3329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleizier CM, van der Vlies AE, Koedam E, Koene T, Barkhof F, van der Flier WM, et al. Episodic memory and the medial temporal lobe: not all it seems. Evidence from the temporal variants of frontotemporal dementia. J Neurol Neurosurg Psychiatry 2012; 83: 1145–8. [DOI] [PubMed] [Google Scholar]
- Pozueta A, Lage C, Garcia-Martinez M, Kazimierczak M, Bravo M, Lopez-Garcia S, et al. Cognitive and Behavioral Profiles of Left and Right Semantic Dementia: differential Diagnosis with Behavioral Variant Frontotemporal Dementia and Alzheimer's Disease. J Alzheimers Dis 2019; [DOI] [PubMed] [Google Scholar]
- Quental NB, Brucki SM, Bueno OF.. Visuospatial function in early Alzheimer's disease–the use of the Visual Object and Space Perception (VOSP) battery. PLoS One 2013; 8: e68398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, et al. Structural anatomy of empathy in neurodegenerative disease. Brain 2006; 129: 2945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodius-Meester HFM, Benedictus MR, Wattjes MP, Barkhof F, Scheltens P, Muller M, et al. MRI Visual Ratings of Brain Atrophy and White Matter Hyperintensities across the Spectrum of Cognitive Decline Are Differently Affected by Age and Diagnosis. Front Aging Neurosci 2017; 9: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992; 55: 967–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, et al. The natural history of temporal variant frontotemporal dementia. Neurology 2005; 64: 1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Harris JM, Thompson JC, Kobylecki C, Jones M, Richardson AM, et al. Semantic dementia and the left and right temporal lobes. Cortex 2018; 107: 188–203. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D.. Knowledge of famous faces and names in semantic dementia. Brain 2004; 127: 860–72. [DOI] [PubMed] [Google Scholar]
- Ten Kate M, Barkhof F, Visser PJ, Teunissen CE, Scheltens P, van der Flier WM, et al. Amyloid-independent atrophy patterns predict time to progression to dementia in mild cognitive impairment. Alzheimers Res Ther 2017; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR.. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 2003; 61: 1196–203. [DOI] [PubMed] [Google Scholar]
- Tijms BM, Willemse EAJ, Zwan MD, Mulder SD, Visser PJ, van Berckel BNM, et al. Unbiased Approach to Counteract Upward Drift in Cerebrospinal Fluid Amyloid-beta 1-42 Analysis Results. Clin Chem 2018; 64: 576–85. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–14. [DOI] [PubMed] [Google Scholar]
- Tyrrell PJ, Warrington EK, Frackowiak RS, Rossor MN.. Progressive degeneration of the right temporal lobe studied with positron emission tomography. J Neurol Neurosurg Psychiatry 1990; 53: 1046–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, et al. Optimizing patient care and research: the Amsterdam Dementia Cohort. Jad 2014; 41: 313–27. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Scheltens P.. Amsterdam Dementia Cohort: performing Research to Optimize Care. Jad 2018; 62: 1091–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronelli L, Makaretz SJ, Quimby M, Dickerson BC, Collins JA.. Geschwind Syndrome in frontotemporal lobar degeneration: neuroanatomical and neuropsychological features over 9 years. Cortex 2017; 94: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler. Wechsler adult intelligence scale–fourth edition (WAIS–IV). San Antonio, TX NCS Pearson; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request from the corresponding author.