Abstract
Background
Mutations in the KMT2C gene can cause Kleefstra syndrome-2 (KLEFS2).
Case
In this study, we analyzed the clinical, genetic testing, and 10-year follow-up data of a child with KLEFS2 treated at the Child Healthcare Department, Children's Hospital of Nanjing Medical University, Nanjing. The case of KLEFS2 presented feeding difficulty and developmental delay, both intervened by nutritional support and family rehabilitation. Obvious attention deficit hyperactivity disorder (ADHD) occurred in preschool and school-age children and was managed by behavioral and pharmaceutical interventions.
Conclusion
Features of KLEFS2 include feeding difficulty and developmental delays in an early age, as well as ADHD in preschool and school age. Satisfactory outcomes are not achieved in early nutritional support for correcting malnutrition and pharmaceutical intervention for relieving ADHD, but both measures can counter developmental delay.
Keywords: Kleefstra syndrome-2, KMT2C, children, ADHD, follow-up
Introduction
Kleefstra syndrome (KLEFS) is a rare autosomal dominant disease characterized by abnormal facial appearance, language and motor delays, intellectual disability, and childhood hypotonia, and some patients may present epilepsy, gastroesophageal reflux disease, and sleep disorders (1, 2). KLEFS is classified into Kleefstra syndrome-1 (KLEFS1, OMIM: 610253) caused by terminal deletions of chromosome 9q34.3 or mutations of the Euchromatic Histone Lysine Methyltransferase 1 (EHMT1) gene (3, 4) and Kleefstra syndrome-2 (KLEFS2, OMIM: 617786) caused by mutations of the KMT2C gene (5). KLEFS1 was initially described in 1999. At present, there are more than 100 cases of KLEFS1 worldwide. Only 7 cases of KLEFS2 have been reported so far, but all lack long-term follow-up data. In the present case report, we reviewed the clinical manifestations and follow-up data of a case of KLEFS2 from 9 months to 10 years of age and analyzed the pathogenicity of frameshift mutations of the KMT2C gene, aiming to elucidate its therapeutic strategy, clinical outcome, and prognosis.
Clinical Data
Baseline Characteristics
This male infant was the second full-term birth delivered through cesarean section, with a birth weight of 2.5 kg and a height of 50 cm. His parents were not consanguineously married and the mother's pregnancy history was normal. All his parents and sibling were healthy. At the age of 9 months, the patient was taken to the Child Healthcare Department several times for feeding difficulty, comprehensive developmental delay, anemia, and eczema. From the age of 5 years and
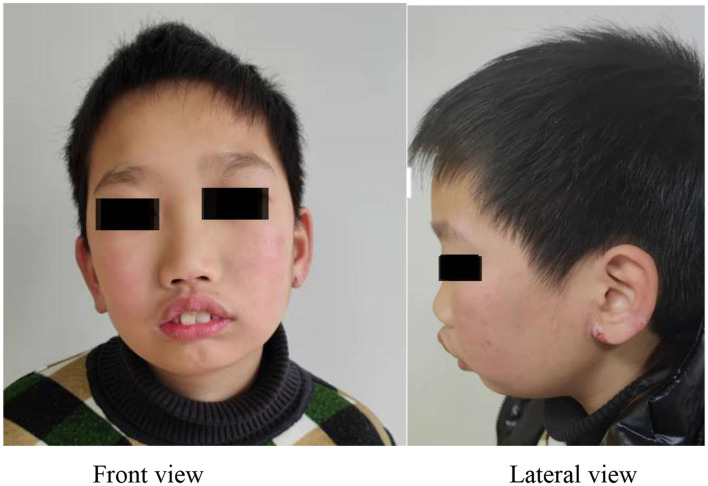
5 months, the patient was treated in our center for obvious attention deficit hyperactivity disorder (ADHD) symptoms. Physical examinations showed distinctive facial features, such as bushy brows, mandibular retrusion, and ear eczema (Figure 1). Cardiopulmonary palpation was normal, and subcutaneous fat in the abdomen was rich. The spine was located in the midline without scoliosis. Muscle tension and strength of extremities were normal. The body skin was dry.
Figure 1.
Facial features of the patient, such as bushy brows, mandibular retrusion, and ear eczema.
Treatment
Physical Development Assessment
At the age of 9 months, the patient presented at the Child Healthcare Department for feeding difficulty, comprehensive developmental delay, anemia, and eczema. Interventions with an amino acid-based high-energy formula and a high-energy high-protein diet exerted a positive effect on emaciation and developmental delay, but not on ear eczema. At the age of 7 years and 3 months, the patient was diagnosed with ADHD with weight loss and was treated with methylphenidate (CONCERTA®). The patient was presented to Nanjing Brain Hospital at the age of 9 years for ADHD again. After starting aripiprazole medication, his body weight increased rapidly (Figure 2; Table 1).
Figure 2.
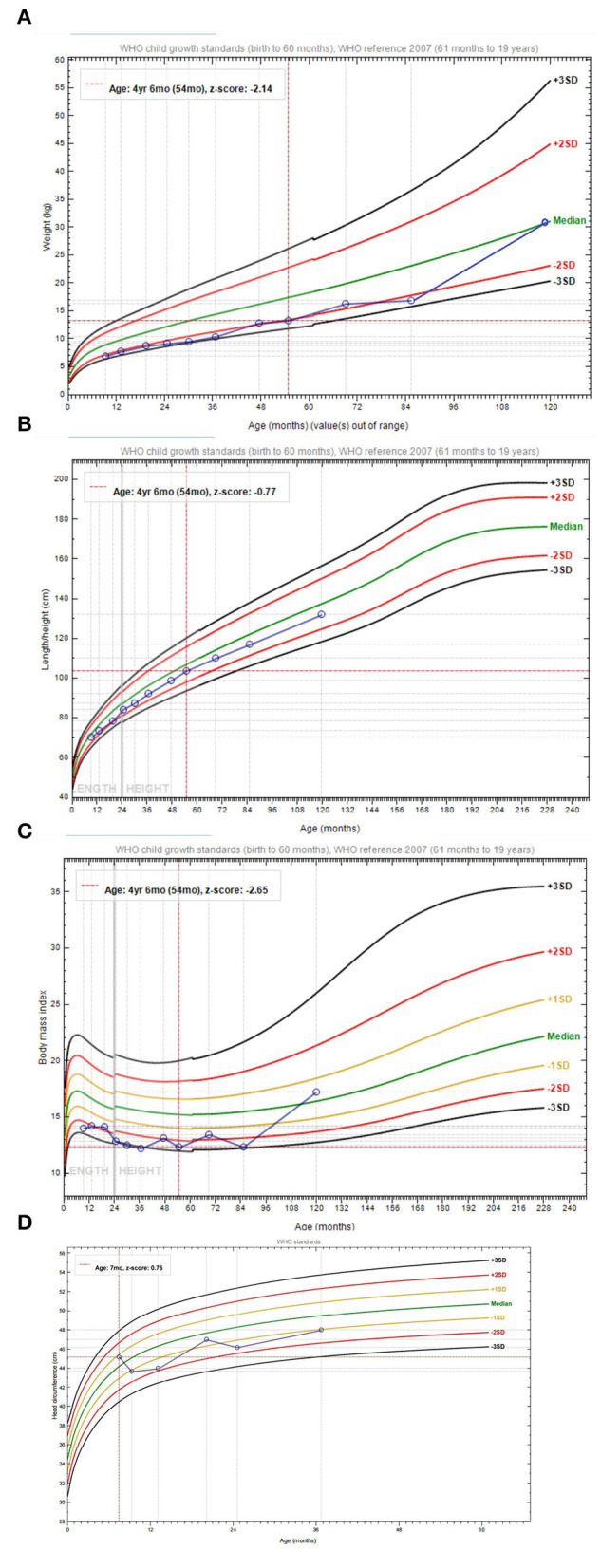
The growth curve. (A) The body weight curve (0–10 years). (B) The height curve (0–10 years). (C) Body mass index curve (0–10 years). (D) Head circumference curve (0–3 years).
Table 1.
Follow-up data of the patient with Kleefstra syndrome-2 (KLEFS2) from 9 months to 10 years.
| Event | Treatment/testtime | Interventions/test items | Outcomes |
|---|---|---|---|
| Difficult feeding, developmental delay and eczema | 9 months to 6 years |
Amino acid-based high-energy formula and high-energy high-protein diet | Emaciation and developmental delay were inconspicuously alleviated; Eczema still existed. |
| 6 to 8 years |
High-energy high-protein diet | Emaciation, developmental delay and eczema were not alleviated. | |
| Anemia | 9 months to 1 year |
Ferrotherapy for 3 months | Anemia was not obviously alleviated. Mild β-thalassemia was diagnosed. |
| Comprehensive developmental delay | 1.58 years | Children's Developmental Center of China (CDCC) | MDI: 63, PDI <50 |
| 3.25 years | Peabody Picture Vocabulary Test (PPVT) | IQ:80 points | |
| 4.75 years | Wechsler Preschool and Primary Scale of Intelligence (WPPSI-II) | FIQ:90 points | |
| 7.1 years | Wechsler Preschool and Primary Scale of Intelligence (WPPSI-II) | FIQ:97 points | |
| 10.5 years | Wechsler Intelligence Scale for Children (WISC-IV Chinese version) | FIQ:87 points | |
| Attention deficit | 1.58 years | Neglected by parents. | |
| 5.42 years | Parents were trained about interventions and parenting skills | Attention deficit was improved. | |
| 7.1 to 7.25 years |
20 mg/d atomoxetine hydrochloride | Irascibility and impulse behaviors. | |
| 7.25 to 8.1 years | 18 mg/d atomoxetine hydrochloride | Hyperactivity symptoms were improved, and attention deficit was not obviously improved. Loss of body weight of 2 kg within 10 months. |
|
| 9 to 10 years |
1.25 mg/d aripiprazole | Leaving a seat in the classroom was corrected, and impulsive behaviors were improved. Body weight increased by 14 kg per year, especially the gain of abdominal fat. |
|
| 10.42 years | 18 mg/d methylphenidate hydrochloride | Impulsive behaviors were improved. The body weight was followed up. | |
| Kleefstra syndrome-2 | 10.42 years | Being followed up | |
MDI, mental development index; PDI, physical development index; IQ, verbal intelligence quotient; FIQ, full-scale intelligence quotient.
Developmental Assessment
Developmental assessment of this patient showed a comprehensive developmental delay at an early age, which accelerated later (Table 1).
Behavioral Assessment
The patient poorly cooperated and was easily distracted during the behavioral assessment and interactive process, which were not considered seriously by his parents. His hyperactivity and attention deficit were reported by his kindergarten teachers at the age of 5 years and 5 months, and he was finally diagnosed with ADHD. Psychiatrists interviewed the child and his parents and made the clinical diagnosis using the diagnostic criteria of ADHD in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). When we trained his parents to perform behavioral interventions and parenting skills, the hyperactivity symptoms of the patient were alleviated. However, his ADHD symptoms aggravated at the age of 6 years, and atomoxetine hydrochloride was given at the age of 7 years and 1 month, with an initial dose of 0.5 mg/kg/day and a target dose of 1.2 mg/kg/day. After a 2-month medication, ADHD symptoms were not alleviated, and the patient developed irascibility and impulsive behaviors. Methylphenidate at the dose of 18 mg/day was given at the age of 7 years and 3 months, and it significantly alleviated hyperactivity symptoms. Inattention symptoms, however, were not remarkably improved. The patient's appetite was low and his body weight decreased by 2 kg during the 10 months of methylphenidate medication, which was therefore withdrawn by his parents intentionally. At the age of 8 years and 1 month, the patient showed serious violations against classroom rules, and he was again presented to the Nanjing Brain Hospital at the age of 9 years for ADHD. Medication of 1.25 mg/day of aripiprazole could keep him seated in the classroom at the early stage but could not remarkably correct attention deficit and hyperactivity symptoms. His body weight increased by 14 kg after 1 year of aripiprazole medication, especially the high gain in abdominal fat. Considering the well-controlled ADHD symptoms, aripiprazole was withdrawn by his parents. However, his parents did not perform any behavioral interventions during the 5 months after withdrawal, and the patient could not stay seated in the classroom again or even ran away from home. At the age of 10 years and 5 months, the patient was represented to our center for medical management. Medication of 18 mg/day methylphenidate significantly alleviated his behavioral disorders in the classroom and corrected other hyperactive and impulsive behaviors to some extent (Table 1).
Assessment of Autism Spectrum Disorder
An assessment of autism spectrum disorder (ASD) was performed in the present case at the age of 10 years and 6 months. The Autism Behavior Checklist (ABC) score of 26 points and the Childhood Autism Rating Scale (CARS) score of 23 points both indicated the absence of ASD.
Other Examinations
Both the blood routine test and the thyroid function test at the age of 9 months were normal. A computed tomography (CT) scan of the brain showed no dilation of bilateral lateral ventricles, the low position of the cerebellar tonsil, enlargement of cisterna magna, and small sella turcica.
Genetic Testing
At the age of 9 months, a 3-month ferrotherapy was given to this patient for his low level of hemoglobin, but the clinical outcome was unsatisfactory. The next-generation sequencing (NGS) using the GenCap Platform revealed mutations of c.316-197C>T in the HBB gene, confirming the diagnosis of mild β-thalassemia. The mutation site was subsequently identified to be inherited from his father. Then, at the age of 10 years, the whole-exome sequencing (MyGenostics, Beijing, China) revealed NM_170606 (KMT2C): c.9284delC (p.P3095Lfs*2), suggesting a frameshift mutation. Pedigree verification identified it as a de novo mutation. According to the evidence-based criteria of the American College of Medical Genetics and Genomics (ACMG), the NM_170606 (KMT2C): c.9284delC (p.P3095Lfs*2) was a pathogenic frameshift mutation (PVS1 + PS1 + PM2_Supporting), which may result in a loss of function (LOF) effect (PVS1). It was a de novo mutation (PS1), which was not detected in his parents. In addition, this novel mutation has not yet been reported in any database (PM2_Supporting).
Discussion
Kleefstra syndrome, caused by the haploinsufficiency of EHMT1, is characterized by intellectual disability, ASD, characteristic facial dysmorphisms, and other variable clinical features. In addition to EHMT1 mutations, de novo variants have been reported in four additional genes (MBD5, SMARCB1, NR1I3, and KMT2C). Although KLEFS1 cases have been reported, the number of KLEFS2 cases are rare and related long-term follow-up data are scant. This study reported a case of KLEFS2, providing a complete narrative of clinical features, genetic testing results, and long-term treatment and follow-up.
Koemans et al. (5–8) summarized the common features of seven patients with KLEFS2 aged 7–31 years, such as mild to severe intellectual disability, language/motor development delay, and behavioral disorders (e.g., autism spectrum disorder, ADHD, sleep problems, self-injurious behavior, and aggressive behavior). To note, three patients with KLEFS2 developed hypotonia and two developed epilepsy in childhood. Scoliosis, short stature, microcephaly, recurrent respiratory infections, phenylketonuria, hypospadias, dry skin, and hoarseness were reported in these cases as well. In the present report, the patient also developed language/motor development delay, ADHD, and dry skin. In addition, obvious feeding difficulty, persistent weight loss, and repeated eczema were not reported in previous cases. Notably, his physical development was improved to the normal level at the age of 3 years, but his intellectual development remained retarded.
In the present case, the nutritional intervention did not reverse weight loss after birth due to feeding difficulty and emaciation. Through reviewing his disease course and genetic testing data, we suggest that feeding difficulty and emaciation may be early-stage manifestations of KLEFS2, and patients with KLEFS2 may not benefit from nutritional interventions. Providing aripiprazole medication significantly increased body weight, especially the gain of abdominal fat. Nevertheless, this medication did not remarkably increase the height. Considering the close link between abdominal fat and visceral fat, the risk of long-term metabolic syndrome in KLEFS2 children should be well estimated. During the follow-up period, the liver function and the blood lipid profile of this case were normal, but the potential influence of KLEFS2 on metabolic diseases should be further evaluated.
Both KLEFS1 and KLEFS2 are caused by genetic defects involving histone methyltransferases. KMT2C, pathogenic gene of KLEFS2, is located on human chromosome 7q36. It encodes a histone methyltransferase that regulates gene transcription by altering chromatin structure. KMT2C induces transcriptional activation via mediating H3K4me1 and H3K4me3. A previous study has shown that the neuropathological mechanism of EHMT1 (KLEFS1) mutations in Drosophila is similar to those of KMT2C (KLEFS2). There is a molecular convergence between the KMT2C/D homolog TRR and the EHMT1 homolog G9a; this convergence plays a decisive role in gene regulatory networks responsible for normal neuronal development and function, especially for complex brain functions, such as learning and memory (5). In addition, four of the five potential target genes of TRR, such as REG-2, Acer, PCB, and Arc-1, are directly or indirectly implicated in the formation of memory. Among them, REG-2 and Acer can regulate circadian rhythm (9, 10). Arc is an immediate-early gene that is activated in neurons associated with learning and memory (11). Memory is a basic element of intelligence. In the present case report, the frameshift mutation in the KMT2C gene may cause an LOF effect, thus impairing his learning and memory. In addition, the intelligence of this case might be underestimated due to attention deficit, a condition arising from comprehensive developmental delay. Notably, the intelligence of this case gradually returned to normal with aging, suggesting that patients with KLEFS2 may be exempted from intellectual disability after a long term.
In this case, severe ADHD symptoms significantly influenced his sociability, and he was unable to study attentively in the classroom. Low-dose atomoxetine hydrochloride, methylphenidate hydrochloride, and aripiprazole did not remarkably alleviate ADHD symptoms, but induced adverse events, such as irascibility, appetite loss, and body weight fluctuation. It is suggested that patients with KLEFS2 may not be sensitive to ADHD medications. Impulsive behaviors and attention deficit, in this case, were significantly improved at the age of 5 years through behavioral interventions performed by his parents but aggravated again after these interventions were interrupted. Therefore, behavioral interventions should be regularly given for a long period until the patient with KLEFS2 grows up, which is challenging and requires support from the family and the society.
Dry skin has been reported in KLEFS2 cases. In addition to dry skin, the present case also suffered persistent ear eczema and an increased eosinophil count, which have never been reported in previous cases. In the present case, electroencephalography findings were normal and epileptic seizures were not detected. The patient should be further followed-up for other manifestations, such as epileptic seizure, hypotonia, and scoliosis.
During the 10-year follow-up from 9 months to 10 years in this case, his pathological growth was difficult to be intervened with and his severe ADHD symptoms significantly disrupted his development. For those who only presented developmental delay without specific facial features, KLEFS2 was easily misdiagnosed because genetic testing needs time. Therefore, genetic testing should be timely carried out for pediatric patients with early-stage feeding difficulties, weight loss, repeated eczema, distinctive facial features, and ADHD symptoms insensitive to medications.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Children's Hospital of Nanjing Medical University. Written informed consent was obtained from the participant's legal guardian for the publication of this case report. Written informed consent was obtained from the participant's legal guardian for the publication of any potentially identifiable images or data included in this article.
Author Contributions
RL designed the study and helped in writing the manuscript. DW performed the experiments, interpreted data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Open Fund Program of Jiangsu Population Association (JSPA2019019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors gratefully acknowledge the contributions of the doctors and nurses in the Children's Hospital of Nanjing and the participation of the child and his parents.
References
- 1.Kleefstra T, Smidt M, Banning MJ, Oudakker AR, Van Esch H, de Brouwer AP, et al. Disruption of the gene Euchromatin Histone Methyl Transferase1 (Eu-HMTase1) is associated with the 9q34 subtelomeric deletion syndrome. J Med Genet. (2005) 42:299–306. 10.1136/jmg.2004.028464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight SJ, Regan R, Nicod A, Horsley SW, Kearney L, Homfray T, et al. Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet. (1999) 354:1676–81. 10.1016/S0140-6736(99)03070-6 [DOI] [PubMed] [Google Scholar]
- 3.Willemsen MH, Vulto-van Silfhout AT, Nillesen WM, Wissink-Lindhout WM, van Bokhoven H, Philip N, et al. Update on Kleefstra Syndrome. Mol Syndromol. (2012) 2:202–12. 10.1159/000335648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleefstra T, Kramer JM, Neveling K, Willemsen MH, Koemans TS, Vissers LE, et al. Disruption of an EHMT1-associated chromatin-modification module causes intellectual disability. Am J Hum Genet. (2012) 91:73–82. 10.1016/j.ajhg.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koemans TS, Kleefstra T, Chubak MC, Stone MH, Reijnders MRF, de Munnik S, et al. Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet. (2017) 13:e1006864. 10.1371/journal.pgen.1006864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoch K, Tan QK, Stong N, Deak KL, McConkie-Rosell A, McDonald MT, et al. Alternative transcripts in variant interpretation: the potential for missed diagnoses and misdiagnoses. Genet Med. (2020) 22:1269–75. 10.1038/s41436-020-0781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavery WJ, Barski A, Wiley S, Schorry EK, Lindsley AW. KMT2C/D COMPASS complex-associated diseases [KCDCOM-ADs]: an emerging class of congenital regulopathies. Clin Epigenetics. (2020) 12:10. 10.1186/s13148-019-0802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwaibold EM, Smogavec M, Hobbiebrunken E, Winter L, Zoll B, Burfeind P, et al. Intragenic duplication of EHMT1 gene results in Kleefstra syndrome. Mol Cytogenet. (2014) 7:74. 10.1186/s13039-014-0074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carhan A, Tang K, Shirras CA, Shirras AD, Isaac RE. Loss of Angiotensin-converting enzyme-related (ACER) peptidase disrupts night-time sleep in adult Drosophila melanogaster. J Exp Biol. (2011) 214:680–6. 10.1242/jeb.049353 [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder RN, Bae H, Palazzolo MJ, Krasnow MA. Extent and character of circadian gene expression in Drosophila melanogaster: identification of twenty oscillating mRNAs in the fly head. Curr Biol. (1995) 5:1424–36. 10.1016/S0960-9822(95)00280-6 [DOI] [PubMed] [Google Scholar]
- 11.Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Arc mRNA induction in striatal efferent neurons associated with response learning. Eur J Neurosci. (2007) 26:228–41. 10.1111/j.1460-9568.2007.05630.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.