Abstract
Background:
Single-dose HPV vaccination, if efficacious, would be tremendously advantageous; simplifying implementation and decreasing costs.
Methods:
We performed a randomized, multi-center, double-blind, controlled trial of single-dose nonavalent (HPV 16/18/31/33/45/52/58/6/11) or bivalent (HPV 16/18) HPV vaccination compared to meningococcal vaccination among Kenyan women aged 15-20 years. Enrollment and six monthly cervical swabs and a month three vaginal swab were tested for HPV DNA. Enrollment sera were tested for HPV antibodies. The modified intent-to-treat (mITT) cohort comprised participants who tested HPV antibody negative at enrollment and HPV DNA negative at enrollment and month three. The primary outcome was incident persistent vaccine-type HPV infection by month 18.
Results:
Between December 2018 and June 2021, 2,275 women were randomly assigned and followed; 758 received the nonavalent HPV vaccine, 760 the bivalent HPV vaccine, and 757 the meningococcal vaccine; retention was 98%. Thirty-eight incident persistent infections were detected in the HPV 16/18 mITT cohort: one each among participants assigned to the bivalent and nonavalent groups and 36 among those assigned to the meningococcal group; nonavalent Vaccine Efficacy (VE) was 97.5% (95%CI 81.7-99.7%, p=<0.0001), and bivalent VE was 97.5% (95%CI 81.6-99.7%, p=<0.0001). Thirty-three incident persistent infections were detected in the HPV 16/18/31/33/45/52/58 mITT cohort: four in the nonavalent group and 29 in the meningococcal group; nonavalent VE for HPV 16/18/31/33/45/52/58 was 88.9% (95%CI 68.5-96.1%, p<0.0001). The rate of SAEs was 4.5-5.2% by group.
Conclusions:
Over the 18 month time-frame we studied, single-dose bivalent and nonavalent HPV vaccines were each highly effective in preventing incident persistent oncogenic HPV infection, similar to multidose regimens.
Introduction
Almost 90% of the more than 600,000 new cervical cancer cases and 340,000 cervical cancer deaths in 2020 occurred in low- and middle-income countries (LMICs).1 Vaccination to prevent human papillomavirus (HPV) infection, the primary cause of cervical cancer, is a key intervention in the World Health Organization’s (WHO) Global Cervical Cancer Elimination Strategy, which calls for vaccination of 90% of girls.2,3 HPV vaccines, licensed as 2-3 intramuscular injections over the course of 6-12 months, reduce an individual’s risk of acquiring persistent oncogenic HPV infection by >90%.4,5,6 At the population level, increasing vaccine coverage increases effectiveness; vaccination of multi-age adolescent cohorts (9-14 years) with catch-up vaccination (to age 26 years) doubles the prevention of HPV-associated precancerous lesions.7 However, HPV vaccine coverage remains low;8 in 2019, the global coverage for HPV vaccination was 15% among adolescent girls9.
In LMICs, low vaccine coverage is due, in part, to the cost and logistics of reaching girls with standard multi-dose vaccine schedule; single-dose vaccination could halve vaccination costs, potentially increase coverage, and simplify the logistics compared to multidose administration. Currently, four HPV vaccines are licensed, all targeting high-risk (oncogenic) HPV types that cause 70% of cancers (HPV 16/18) and two also targeting low-risk HPV types that cause genital warts (HPV 6/11); the bivalent vaccine (Cervarix® and Cecolin®) prevents HPV 16/18 infection, the quadrivalent vaccine (Gardasil®) prevents HPV 16/18/6/11, and the nonavalent vaccine (Gardasil-9®) prevents HPV 16/18/31/33/45/52/58/6/11 infection (including five additional high-risk HPV types).10
Observational studies suggest that single-dose HPV vaccine effectiveness is equivalent to a two- or three-dose regimen: however, vaccination guidelines recommend multidose strategies and questions persist regarding single-dose efficacy.11-14 In Kenya, multidose HPV vaccination is offered to 9-14-year-old girls through the national immunization program from October 2019; however, due to supply constraints, HPV vaccination was offered to 10-year-old girls only. To date, an estimated 10% of 10 year old girls have received their first HPV vaccine dose and 3% have received the second dose.15 Catch-up vaccination for adolescent girls and young women 15 years of age and older is not provided, with cervical cancer screening offered to older women. Testing the efficacy of single-dose HPV vaccination among young women age 15 years and older, within the context of cytological screening for dysplastic lesions in a clinical trial, was determined to be ethical as vaccination for this age group in Kenya and many LMICs is not currently supported through national programs or global immunization bodies. Specifically, we evaluated zero versus single-dose HPV vaccination against the backdrop of substantial disparities in cervical cancer incidence.16 Also, a superiority design was chosen, compared to a non-inferiority design, as the smaller sample size and shorter time line would support robust, feasible, and timely evidence. Here we report the findings of an efficacy trial of single-dose bivalent and nonavalent HPV vaccination among young women in Kenya.
Methods
Trial design and Oversight
This randomized, multi-center, double-blind, parallel, three-arm controlled, superiority trial tested the efficacy of single-dose bivalent (HPV 16/18) and single-dose nonavalent (HPV 16/18/31/33/45/52/58/6/11) HPV vaccination, as described in the published protocol paper.17 Kenya’s HPV National Immunization Program, launched in October 2019, offers two doses of the quadrivalent HPV vaccine to 9-10-year-old girls and is not provided through the National Immunization Program for persons aged 15 years and older; meningococcal vaccination is used during outbreaks.18 Meningococcal vaccination was chosen as the comparator because meningococcal antibodies offer potential clinical benefits and do not impact HPV outcomes. Participants were randomized to 1) immediate nonavalent HPV vaccination and delayed (36 months after enrollment) meningococcal vaccination, 2) immediate bivalent HPV vaccination and delayed meningococcal vaccination, or 3) immediate meningococcal vaccination and delayed HPV vaccination. The primary analysis was planned for month 18, with the final analysis at month 36 evaluating durability (not reported herein). At this time the trial is ongoing. After the 18 month results presented herein, we are continuing follow-up in a blinded cross-over design to evaluate vaccine durability.
The Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU) and the University of Washington (UW) Institutional Review Board (IRB) approved the study. The study was registered with ClinicalTrials.gov (NCT03675256).
Participants
Participants were recruited through community outreach. Participants were eligible for randomization if they were able to provide informed consent, age 15 to 20 years old, female sex assigned at birth, sexually active reporting one to five lifetime partners, and resident within the study area. Study exclusion criteria were a positive HIV diagnostic test, history of HPV vaccination, allergies to vaccine components or latex, current pregnancy, hysterectomy, or history of immunosuppressive conditions.
Setting
The study was conducted at three KEMRI clinical sites in Thika, Nairobi, and Kisumu. All participants, and their parents/guardians in the case of minors, provided informed consent, which included counseling about randomization, risks and benefits of participation, study procedures, and their rights as research participants.
Screening and enrollment
Potential participants completed eligibility screening with a provider including a detailed medical history, collection of external genital (labial/vulvar/perineal) and cervical swabs for HPV deoxyribonucleic acid (DNA) testing, and serum for HPV antibody testing. Participants received cytological cervical cancer screening at enrollment. Sexual and reproductive health services (contraception, sexually transmitted infection diagnosis and treatment, and HIV pre-exposure prophylaxis) were offered at enrollment and every visit. All questionnaires were conducted using electronic case report forms (DF/Net Research, Inc. ©, Seattle, WA, US).
Randomization and Vaccination
Randomization was stratified by site, using a fixed block size of 15, and a 1:1:1 allocation. Study staff, participants, investigators, clinic staff, lab technicians, and other study team members did not have access to the randomization codes, except for the unblinded statistical analysts and unblinded pharmacists at each site. Blinded study assignment was implemented via http://www.randomize.net (Ottawa, ON, Canada). An unblinded pharmacist entered the participant identification number on randomize.net, obtained the next sequential intervention assignment, recorded the participant identification number and randomization identifier on an electronic case report form, drew up the vaccine in a masked syringe, and administered the vaccination.
Study follow-up procedures
Participants were seen at months three, six, and then every six months for 18 months after enrollment. Providers administered clinical questionnaires and collected a cervical swab at each six-month visit. Participants self-collected vaginal swabs using validated instructions at month three; self-collected swabs, which have similar accuracy compared to provider collected cervical swabs,19 were available at subsequent follow-up visits by participant choice or to comply with COVID-19 research restrictions.
Laboratory methods
HPV DNA genotyping was conducted using the Anyplex II HPV28 assay (Seegene, Seoul, South Korea), a multiplexed type-specific real-time polymerase chain reaction (PCR) based assay20,21 at the UW East Africa STI Laboratory, Mombasa, Kenya with standard proficiency testing.22 For HPV-positive samples, a low (+), intermediate (++), or high (+++) positivity was indicated; + or greater were considered positive. All runs included negative and positive controls, and the housekeeping human gene, β-globin, as an internal control. Runs were performed with CFX96 Real-time PCR System (BioRad, Hercules, California).
Serum specimens were shipped to the University of Washington, Seattle, WA, US, and tested at the Galloway Lab, Fred Hutchinson Cancer Research Center. HPV IgG antibodies were detected using a multiplex Luminex assay.23,24 The mean pre-established fluorescent intensity (MFI) seropositivity cutoffs for HPV 16/18/31/33/45/52/58 were used (Table S14).
Sexually transmitted infections (Neisseria gonorrhoeae, Chlamydia trachomatis, or Trichomonas vaginalis) were assessed by nucleic acid amplification testing (APTIMA; Hologic/GenProbe, San Diego, CA) at the University of Washington-University of Nairobi East Africa STI Laboratory; HSV-2 was evaluated by the Focus ELISA and bacterial vaginosis was evaluated using the Nugent Score.
Outcomes and assessment
The primary trial endpoint was incident persistent cervical HPV infection among participants who tested HPV DNA negative (external genital and cervical swabs) at enrollment and month three (self-collected vaginal swab) and HPV antibody negative at enrollment (the modified intent-to-treat (mITT) cohort). For inclusion in the HPV 16/18 mITT cohort, participants were HPV 16/18 naïve. Similarly, for the HPV 16/18/31/33/45/52/58 mITT cohort, participants were HPV 16/18/31/33/45/52/58 naïve. Persistent HPV, a surrogate marker for cervical dysplasia/precancer, was defined as high-risk vaccine type specific HPV (i.e., HPV 16/18 for the bivalent vaccine and HPV 16/18/31/33/45/52/58 for the nonavalent vaccine) detected at two consecutive time points no less than four months apart after month three and up to and including month 18 (same HPV type at both time points) for the primary analysis.5 Participants without swabs post-month 3 did not contribute follow-up time in the primary analysis. Participants in the bivalent vaccine group were not included in the HPV 16/18/31/33/45/52/58 analysis as the study was not powered to detected cross-protection. Cervical swabs were tested for the primary endpoint; vaginal swabs were substituted if necessary. Sensitivity analysis was planned on the following subset: participants who tested HPV DNA negative at enrollment, month three, and month six and antibody negative at enrollment (extended sensitivity cohort) to match the analysis cohort for HPV vaccine licensure trials. The extended sensitivity cohort analysis used all available data, including visits after the pre-specified month 18 data cut. Safety was assessed through adverse event reporting following the United States National Institute of Allergy and Infectious Diseases guidelines.25
Statistical analysis
Sample size calculations assumed that 52% of participants would meet requirements for inclusion in the mITT cohort based on the observed prevalence of HPV infection in similar settings.26 The sample size calculations also assumed a combined persistent HPV 16/18/31/33/45/52/58 annual incidence of 5%, single-dose vaccine efficacy of 75%, and loss-to-follow-up of 10% with a fixed follow-up time of 12 months. Assuming a proportional hazards model (seqDesign in R) with 80% power to detect 75% efficacy, a sample size of 2250 participants was planned.
We used Cox proportional hazards (PH) models stratified by site to estimate the hazard ratios (HRs) of the interventions versus control for the primary and sensitivity analyses. Models for the sensitivity analyses used crude incidence rate ratios instead of Cox when no events were observed in a group. Follow-up was calculated as days since the month three visit for the primary analysis, and days since month six for the extended sensitivity analysis. Participants who did not reach the efficacy endpoints were censored at the time of the last negative test at or before the month 18 visit. Vaccine efficacy was expressed as a 1 minus the hazard ratio (or relative risk). The log-rank test stratified by site was used to calculate the p-value. Cumulative incidence Kaplan-Meier curves of time to infection were calculated by intervention group. Efficacy analyses were performed on the month 18 mITT cohorts. In post-hoc analysis, we evaluated the absolute difference in cumulative incidence of HPV from the Kaplan-Meier curves at month 18. We calculated the cumulative incidence of chlamydia and gonorrhea during follow-up by assigned group.
Safety was assessed among all participants; the three groups were compared using Fisher’s exact test. We performed all analyses using SAS software, version 9.2 (SAS Institute, North Carolina, US) and double coded in R (version 4.1).
An independent Data Safety and Monitoring Board (DSMB) was constituted to review study progress, participant safety, and the primary outcome; the DSMB met annually.
Results
Participants
Between December 20th, 2018, and November 15th, 2019, 3,090 participants were screened for study eligibility and 2,275 (74%) were enrolled. Of those ineligible, 132 (16%) had a positive pregnancy test, 51 (6%) declined study procedures, 34 (4%) had a positive rapid HIV test, and 172 (21%) met other exclusion criteria. Enrolled participants were randomized (Figure 1): 758 to the nonavalent HPV vaccine group, 760 to the bivalent HPV vaccine group, and 757 to the meningococcal vaccine group. At enrollment, 57% of participants (n=1,301) were age 15 to 17 years, and 61% (n=1,392) had one lifetime sexual partner with comparable baseline characteristics between the groups (Table S1). The group was representative of the population who would be eligible for HPV vaccination in this manner should such a decision be made - see Table S22 in the supplemental appendix.
Figure 1: Randomized trial profile.
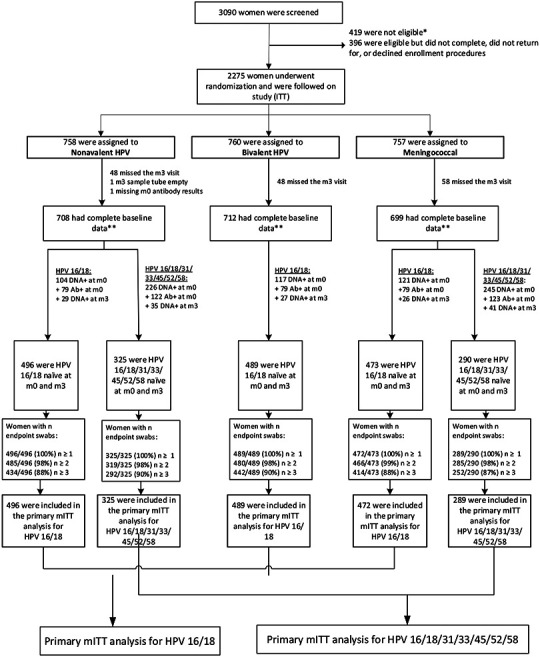
*of the 419 people who were ineligible for randomization, 132 (16%) had a positive pregnancy test, 51 (6%) were not willing to follow study procedures or be randomized, 34 (4%) had a positive rapid HIV diagnostic test, and 172 (21%) met other exclusion criteria.
**Complete baseline data includes HPV antibody results at month 0 and HPV DNA results at month 0 and month 3.
For HPV 16/18, participants who tested HPV 16/18 antibody or HPV 16/18 DNA positive at enrollment or HPV DNA positive month three (n=661), or had missing antibody results (n=1) or a missing month three swab (n=155) were excluded. Among the 1,458 participants meeting criteria for the primary HPV 16/18 mITT analysis, 496 were in the nonavalent, 489 in the bivalent, and 473 in the meningococcal group. For HPV 16/18/31/33/45/52/58, participants who tested HPV 16/18/31/33/45/52/58 antibody or HPV 16/18/31/33/45/52/58 DNA positive at enrollment or HPV DNA positive at month three (n=792) or had missing antibody results (n=1) or a missing month three swab (n=106) were excluded. Of the 615 participants eligible for the primary HPV 16/18/31/33/45/52/58 analysis, 325 were in the nonavalent and 290 in the meningococcal vaccine group. One participant in the meningococcal vaccine group did not have at least one post-month three endpoint swab. The median age was 17 years for the HPV 16/18 and HPV 16/18/31/33/45/52/58 mITT cohorts ; and, overall, the baseline characteristics by study groups were comparable (Tables 1 and S27).
Table 1:
Baseline characteristics: modified intention-to-treat (mITT) Cohort
| HPV 16/18 mITT | HPV 16/18/31/33/45/52/58 mITT | |||||
|---|---|---|---|---|---|---|
| Nonavalent HPV | Bivalent HPV | Meningococcal | Nonavalent HPV | Meningococcal | ||
| Characteristic | Category | |||||
| Total | 496 | 489 | 473 | 325 | 290 | |
| Age group (years) | 15-17 | 299 (60.3%) | 278 (56.9%) | 278 (58.8%) | 197 (60.6%) | 168 (57.9%) |
| 18-20 | 197 (39.7%) | 211 (43.1%) | 195 (41.2%) | 128 (39.4%) | 122 (42.1%) | |
| Marital status | Never married | 478 (96.4%) | 462 (94.5%) | 446 (94.3%) | 315 (96.9%) | 269 (92.8%) |
| Married | 14 (2.8%) | 24 (4.9%) | 20 (4.2%) | 7 (2.2%) | 15 (5.2%) | |
| Previously Married | 3 (0.6%) | 3 (0.6%) | 7 (1.5%) | 2 (0.6%) | 6 (2.1%) | |
| Other | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | |
| Education (highest level) | No schooling | 1 (0.2%) | 2 (0.4%) | 1 (0.2%) | 1 (0.3%) | 1 (0.3%) |
| Primary school, some or complete | 40 (8.1%) | 30 (6.1%) | 36 (7.6%) | 27 (8.3%) | 27 (9.3%) | |
| Secondary school, some or complete | 359 (72.4%) | 368 (75.3%) | 355 (75.1%) | 241 (74.2%) | 220 (75.9%) | |
| Post-secondary school | 96 (19.4%) | 89 (18.2%) | 81 (17.1%) | 56 (17.2%) | 42 (14.5%) | |
| Earns an income of her own | No | 437 (88.1%) | 417 (85.3%) | 417 (88.2%) | 284 (87.4%) | 248 (85.5%) |
| Yes | 59 (11.9%) | 72 (14.7%) | 56 (11.8%) | 41 (12.6%) | 42 (14.5%) | |
| Has a current main or steady sexual partner | No | 144 (29.0%) | 152 (31.1%) | 145 (30.7%) | 98 (30.2%) | 95 (32.8%) |
| Yes | 352 (71.0%) | 337 (68.9%) | 328 (69.3%) | 227 (69.8%) | 195 (67.2%) | |
| Age when first had vaginal intercourse (years) | <15 | 123 (24.8%) | 116 (23.7%) | 103 (21.8%) | 80 (24.6%) | 65 (22.4%) |
| 15-17 | 265 (53.4%) | 274 (56.0%) | 282 (59.6%) | 185 (56.9%) | 173 (59.7%) | |
| >18 | 96 (19.4%) | 93 (19.0%) | 79 (16.7%) | 54 (16.6%) | 46 (15.9%) | |
| Don't remember | 12 (2.4%) | 6 (1.2%) | 9 (1.9%) | 6 (1.8%) | 6 (2.1%) | |
| Lifetime number of sex partners | 1 | 322 (64.9%) | 332 (67.9%) | 289 (61.1%) | 217 (66.8%) | 184 (63.4%) |
| 2 | 121 (24.4%) | 100 (20.4%) | 113 (23.9%) | 78 (24.0%) | 65 (22.4%) | |
| >3 | 53 (10.7%) | 57 (11.7%) | 71 (15.0%) | 30 (9.2%) | 41 (14.1%) | |
| Condom use with last vaginal sex | No | 153 (30.8%) | 155 (31.7%) | 140 (29.6%) | 98 (30.2%) | 78 (26.9%) |
| Yes | 237 (47.8%) | 235 (48.1%) | 238 (50.3%) | 156 (48.0%) | 144 (49.7%) | |
| No sex in past year | 106 (21.4%) | 99 (20.2%) | 95 (20.1%) | 71 (21.8%) | 68 (23.4%) | |
The baseline characteristics of the intention-to-treat population are shown in Table S1.
One hundred percent of participants received their assigned vaccine, without administration error. By the month 18 visit, retention for assessment of the primary endpoints was 98% for two swabs and 94% for three swabs; 94% of swabs were cervical swabs and 6% of swabs were self-collected vaginal swabs (Tables S5-8, S13). The cumulative incidence of chlamydia, gonorrhea, and persistent non-vaccine HPV types was comparable across the three study groups (Tables S16 and S26).
Primary outcome
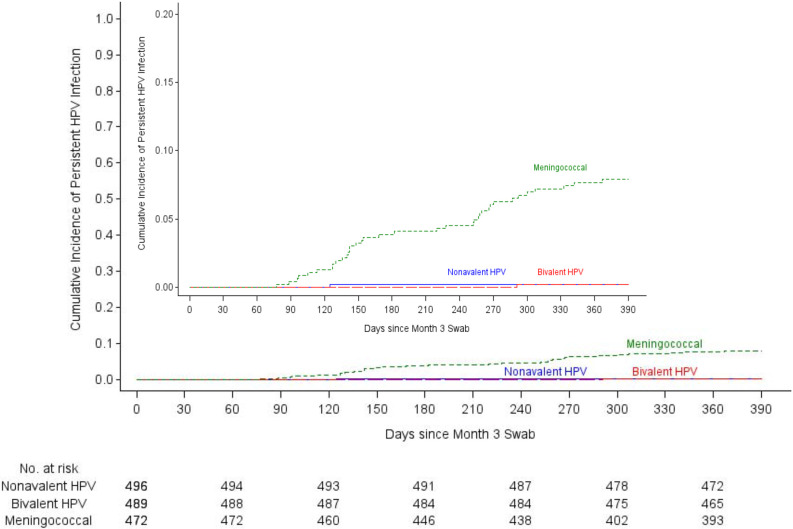
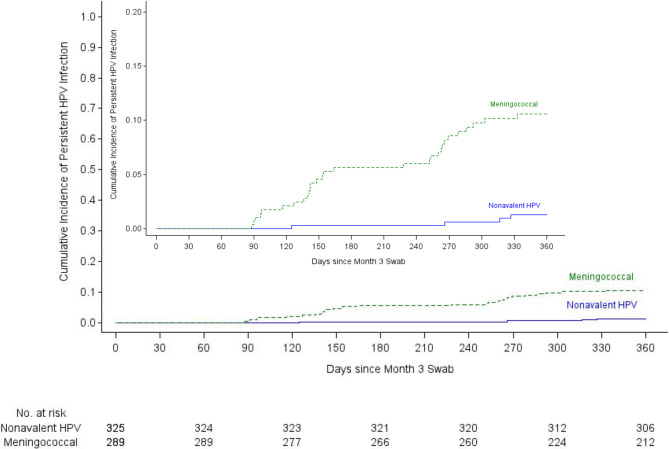
A total of 38 incident persistent infections were detected in the HPV 16/18 mITT cohort: one each among participants assigned to the bivalent and nonavalent vaccine groups and 36 among those assigned to the meningococcal vaccine group (Table 2a). The incidence of persistent HPV 16/18 was 0.17/100 woman-years in the bivalent and nonavalent vaccine groups, compared to 6.83/100 woman-years in the meningococcal vaccine control group. Bivalent Vaccine Efficacy (VE) was 97.5% (95% CI 81.7-99.7%, p=<0.0001) and nonavalent VE was 97.5% (95% CI 81.6-99.7%, p=<0.0001) (Figure 2a). Thirty-three incident persistent infections were detected in the HPV 16/18/31/33/45/52/58 mITT cohort: four in the nonavalent vaccine group and 29 in the meningococcal vaccine group (Table 2b). The incidence of persistent HPV 16/18/31/33/45/52/58 was 1.03/100 woman-years in the nonavalent vaccine group compared to 9.42/100 woman-years in the meningococcal group. Nonavalent VE for HPV 16/18/31/33/45/52/58 was 88.9% (95% CI 68.5-96.1%, p<0.0001) (Figure 2b).
Table 2a:
Incidence of persistent HPV 16/18 and Vaccine Efficacy by Month 18 (mITT Cohort)
| 95% Confidence Interval* |
Statistical Comparisons*** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm | Enrolled (n) |
HPV 16/18 naïve^ (mITT) (n) |
Incident persistent HPV 16/18 (n) |
Woman-years of Follow- up** |
Incidence of persistent HPV 16/18 per 100 Woman-years |
Lower Bound |
Upper Bound |
Comparison | Vaccine Efficacy |
95% CI | P-value (Log-rank) |
| Nonavalent HPV | 758 | 496 | 1 | 596.27 | 0.17 | 0.00 | 0.93 | Nonavalent v. Meningococcal | 97.5% | (81.7%, 99.7%) | <.0001 |
| Bivalent HPV | 760 | 489 | 1 | 589.38 | 0.17 | 0.00 | 0.95 | Bivalent v. Meningococcal | 97.5% | (81.6%, 99.7%) | <.0001 |
| Meningococcal | 757 | 473 | 36 | 527.35 | 6.83 | 4.78 | 9.45 | ||||
Exact 95% confidence interval for incidence rate computed using the Poisson distribution.
Follow-up time begins at 3 months and includes only women HPV 16/18 DNA-negative at month 0 and month 3, and antibody-negative at month 0.
Hazard ratios with 95% confidence intervals are estimated using a single Cox proportional hazards regression model with a three-way class variable for vaccine arm. The model is stratified by site, with Efron method for handling ties, and vaccine arm was the only covariate. Vaccine efficacy and 95% CI computed from the hazard ratio as 100*(1-HR). P-value (log-rank) computed for each comparison using the log-rank test.
HPV 16/18 naïve participants are those who tested negative for HPV 16/18 antibodies at enrollment and negative for HPV 16/18 DNA at enrollment and month three.
Figure 2a:
Kaplan-Meier curves for the primary, HPV 16/18 modified intention-to-treat analysis
Table 2b:
Incidence of persistent HPV 16/18/31/33/45/52/58 and Vaccine Efficacy by Month 18 (mITT Cohort)
| 95%
Confidence Interval* |
Statistical Comparisons*** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arm | Enrolled (n) |
HPV 16/18/31/ 33/45/52/ 58 naïve^ (mITT) (n) |
Incident persistent HPV 16/18/31/3 3/45/52/58 (n) |
Woman- years of Follow- up** |
Incidence
of persistent HPV 16/18/31/33/4 5/52/58 per 100 Woman- years |
Lower Bound |
Upper Bound |
Comparison | Vaccine Efficacy |
95% CI | P-value (Log- rank) |
| Nonavalent HPV | 758 | 325 | 4 | 389.18 | 1.03 | 0.28 | 2.63 | Nonavalent v. Meningococcal | 88.9% | (68.5%, 96.1%) | <.0001 |
| Meningococcal | 757 | 290 | 29 | 307.81 | 9.42 | 6.31 | 13.53 | ||||
Exact 95% confidence interval for incidence rate computed using the Poisson distribution.
Follow-up time amongst women HPV 16/18/31/33/45/52/58 DNA-negative at month 0 and month 3, and antibody-negative at month 0.
Hazard ratios with 95% confidence intervals are estimated using a single Cox proportional hazards regression model with a three-way class variable for vaccine arm. The model is stratified by site, with Efron method for handling ties, and vaccine arm was the only covariate. Vaccine efficacy and 95% CI computed from the hazard ratio as 100*(1-HR). P-value (log-rank) computed for each comparison using the log-rank test.
HPV 16/18/31/33/45/52/58 naïve participants are those who tested negative for HPV 16/18/31/33/45/52/58 antibodies at enrollment and negative for HPV 16/18/31/33/45/52/58 DNA at enrollment and month three.
In the extended sensitivity analysis, there were a total of 16 incident persistent infections in the HPV 16/18 mITT cohort: 0 each among participants assigned to the bivalent and nonavalent vaccine groups and 16 among those assigned to the meningococcal vaccine group (Table S9). HPV 16/18 incidence was 0 per 100 women-years in the nonavalent and bivalent vaccine groups and 3.9 per 100 women years in the meningococcal control group; nonavalent VE was 100% (p<0.0001) and bivalent VE was 100% (p<0.0001) (Table S9). In the extended sensitivity analysis, there were a total of 15 incident persistent infections in the HPV 16/18/31/33/45/52/58 mITT cohort: one among participants assigned to the nonavalent group and 14 among those assigned to the meningococcal group; nonavalent VE was 95.0% (95% CI 62.1-99.4%, p=<0.0001) (Table S10). Vaccine efficacy results were similar in the sensitivity analysis including participants with HPV antibodies at enrollment (Tables S23 and S24)
In post-hoc analysis, using only provider collected endpoint cervical swabs and excluding self-collected vaginal swabs, the results for the primary analysis were not different: the VE was 97.3% (95% CI 80.0-99.6 %) for each of the bivalent and nonavalent vaccines in the HPV 16/18 mITT cohort. Nonavalent vaccine efficacy was 91.4% (95% CI 71.8-97.4%) in the HPV 16/18/31/33/45/52/58 mITT cohort (Tables S11-12).
In post-hoc analysis, the absolute reduction in the HPV 16/18 mITT cohort for cumulative incident persistent HPV 16/18 infection was −7.7% (95% CI −10.4 – −5.0%) for both the bivalent and nonavalent vaccines; an absolute incidence of 0.2% (95% CI 0.0 – 0.6%) in the bivalent and nonavalent vaccine groups compared to 7.9% (95% CI 5.4 – 10.4%) in the meningococcal group. For the HPV 16/18/31/33/45/52/58 mITT cohort, the absolute reduction in persistent HPV 16/18/31/33/45/52/58 infection was −9.3% (95% CI −13.6 – −5.1%) for the nonavalent vaccine; an absolute incidence of 1.3% (95% CI 0.0 – 2.5%) in the nonavalent vaccine group compared to 10.6% (95% CI 6.9 – 14.2%) in the meningococcal group.
Safety
There were 112 participants who experienced serious adverse events (SAEs), which included 57 participants with pregnancy-related SAEs, 46 with infections or inflammatory conditions (of which 31 were malaria), seven injuries, and five mental health illnesses. Overall, the frequency was similar between groups (Table 3). There was one death in the study as a result of a septic abortion and systemic sepsis. SAEs were assessed as not related to the study vaccines. Five participants had abnormal cytology at enrollment, which were all followed until the lesions resolved or the participant received treatment. Social harms were reported by 0.09% of participants (n=2) and included lack of social support from friends and family for trial participation.
Table 3:
Participants experiencing adverse events (ITT)
| Randomized Arm | ||||
|---|---|---|---|---|
| Nonavalent HPV | Bivalent HPV | Meningococcal | All | |
| Enrolled, n | 758 | 760 | 757 | 2275 |
| Any SAE, n(%) | 34 (4.5%) | 39 (5.1%) | 39 (5.2%) | 112 (4.9%) |
| Any pregnancy related, n (%) | 24 (3.2%) | 19 (2.5%) | 14 (1.8%) | 57 (2.5%) |
| Any infection/inflammation, n (%) | 9 (1.2%) | 16 (2.1%) | 21 (2.8%) | 46 (2.0%) |
| Any injury, n (%) | 0 (0.0%) | 3 (0.4%) | 4 (0.5%) | 7 (0.3%) |
| Any mental health, n (%) | 2 (0.3%) | 1 (0.1%) | 2 (0.3%) | 5 (0.2%) |
NOTE: Participants may have more than one event across, but not within, event type categories. SAE: Serious adverse event
Discussion
Over the 18 months of this trial, the efficacy of single-dose bivalent or nonavalent HPV vaccine was very high among Kenyan adolescent girls and young women, demonstrating high levels of protection against vaccine-specific oncogenic HPV infection. Protection against HPV 16/18 infection was 97.5% for both vaccines; together with observed high reductions in the absolute cumulative incidence this suggests, should the protection have a durable effect, the potential for public health impact in the context of disparities in outcomes for cervical cancer cases and deaths (Table S22). Saliently, we were able to exclude single-dose HPV 16/18 vaccine efficacy less than 81%, the lower limit of the confident interval for both vaccines. Overall, the rate of HPV infection in this population of African adolescent girls and young women was high – 9.42 per 100 woman-years in the control group, approximately a third higher than in previous trials, highlighting the need for effective, scalable vaccine programs that can achieve high coverage and reduce this high incidence of HPV infection and potential cervical cancer.4,27 The high level of efficacy builds on observational data11,12 and provides, should the effect be sustained, evidence for single-dose HPV vaccination to prevent persistent HPV infections, which could increase vaccine access and coverage, offering a cost-effective strategy for cervical cancer prevention.28
Strengths of the study include the randomized, double-blind, controlled design, high retention, measurement of cervical HPV DNA as the outcome, determination of persistent HPV DNA, and the head-to-head comparison of the licensed bivalent and nonavalent HPV vaccines in protection against persistent infection with oncogenic HPV types included in the vaccines. In addition, the trial successfully enrolled persons exposed to HPV infection who were successfully retained in all randomized groups, allowing rapid assessment of single-dose efficacy.
We acknowledge that the study has limitations. First, the duration of follow-up is 18 months and the durability of single-dose vaccine efficacy remains to be demonstrated. However, observational data for single-dose HPV vaccination supports efficacy over a decade.11 Following these results, participants will receive blinded cross-over29 vaccination, ensuring all receive HPV vaccination, with an additional 18 months follow-up to evaluate single-dose durability, and access to the second dose following guidelines. The blinded cross-over design will allow us to calculate the durability of the vaccine efficacy demonstrated to date. Second, the proportion of randomized participants who were naive to HPV 16/18/31/33/45/52/58 was lower than expected (~40%) potentially decreasing the study power; however, incidence was higher than assumed and the efficacy result is statistically significant. Third, 6% of primary endpoint swabs were self-collected, and 94% were provider collected. Ideally, collection would be identical; however, the correlation between self-collected vaginal and provider collected cervical swabs is high19 and there was no difference in the results when self-collected swabs were excluded. An additional concern is whether antibody levels were declining over the observation period such that the high efficacy initially observed would be sustained. However, in a study conducted in India over ten years duration, antibody levels at plateau were such that vaccine efficacy is high (>95%)11 suggesting that even higher antibody levels could only demonstrate a small further increase in vaccine efficacy. In addition, the plateau level for single-dose HPV vaccination is reached by month 12.30 Lastly, while the GST-ELISA multiplex assay used to exclude participants with HPV antibodies at enrollment demonstrated overall agreement of 89% with the gold standard secreted alkaline phosphatase pseudovirion based neutralization assay,31 misclassification of participants as antibody naïve would not be different by study group. Further in sensitivity analysis including participants with HPV antibodies at baseline, overall vaccine efficacy was in keeping with the primary findings (Tables S23 and S24).
Cervical cancer is the fourth most common cancer among women globally, the second most frequent in sub-Saharan Africa and primarily affects women between ages 30-49 years and is the leading cause of cancer deaths in sub-Saharan Africa.32,33 Cervical cancer is almost entirely preventable through HPV vaccination. If the effects of single-dose HPV vaccination are durable, as we have reason to believe they will be, this approach could serve to close the gap between the WHO’s goal of 90% HPV vaccination coverage by 2030 and the 15% of girls globally currently vaccinated,9,34 alleviate vaccine supply constraints,35 and provide global policy makers with options to allocate existing HPV vaccine supply.
Supplementary Material
Figure 2b:
Kaplan-Meier curves for the primary, HPV 16/18/31/33/45/52/58 modified intention-to-treat analysis
Acknowledgements
We thank the adolescent girls and young women who participated in this study for their motivation and dedication; and the parents of minor participants for their support. We are grateful to the members of the trial’s Data and Safety Monitoring Board (Drs. Helen Weiss (Chair), Lynette Denny, Dorothy Mbori-Ngacha, Saidi Kapiga, Fred Were, and Fred Sawe), community advisory boards at each trial location, and overseeing ethics review committees for their expertise and guidance. We thank Dr. Peter Dull and Ms. Carolyn Wendell from the Bill & Melinda Gates Foundation for their attentive oversight. We are grateful to the KEN SHE Study Team for their dedication and perseverance. The article is dedicated to Kowselia Ramaswami Ramiah, Sarah Kanyi Mugo, Reginalda Auma Onono, Edwina Muga, Mary Nduta, and all our mothers.
Funding:
The KEN SHE Study was funded by the Bill & Melinda Gates Foundation (OPP1188693); and the University of Washington King K. Holmes Endowed Professorship in STDs and AIDS. LFP was supported by the University of Washington T32 Fellowship (5T32CA009515-34). The content is solely the responsibility of the authors and does not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated or the KEN SHE Study funders.
Footnotes
Trial registration: ClinicalTrials.gov number NCT03675256
Publisher's Disclaimer: This is an Author Accepted Manuscript, which is the version after external peer review and before publication in NEJM’s Evidence. The publisher’s version of record, which includes all Evidence editing and enhancements, is available at https://evidence.nejm.org/doi/full/10.1056/EVIDoa2100056.
Data sharing
Data cannot be shared publicly because this study was conducted with approval from the Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU), which requires that data from studies (including de-identified data) are released only after SERU has provided written approval for additional analyses. A complete de-identified dataset sufficient to reproduce the study findings will be made available upon written request after approval from SERU. To request these data, please contact the KEN SHE Scientific Committee at icrc@uw.edu.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2021. [DOI] [PubMed] [Google Scholar]
- 2.A Global Strategy for elimination of cervical cancer. 2020. (Accessed 09/20/2020, 2020, at https://www.who.int/activities/a-global-strategy-for-elimination-of-cervical-cancer.)
- 3.Australia set to eliminate cervical cancer by 2035. 2018. (Accessed April 29, 2019, at https://www.cancer.org.au/news/media-releases/australia-set-to-eliminate-cervical-cancer-by-2035.html.)
- 4.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. The New England journal of medicine 2015;372:711–23. [DOI] [PubMed] [Google Scholar]
- 5.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. The New England journal of medicine 2002;347:1645–51. [DOI] [PubMed] [Google Scholar]
- 6.La Torre G, de Waure C, Chiaradia G, Mannocci A, Ricciardi W. HPV vaccine efficacy in preventing persistent cervical HPV infection: a systematic review and meta-analysis. Vaccine 2007;25:8352–8. [DOI] [PubMed] [Google Scholar]
- 7.Drolet M, Benard E, Perez N, Brisson M, H. P. V. Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet (London, England) 2019;394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global HPV Vaccine Introduction 2021. (Accessed 10/23/2021, at https://www.path.org/resources/global-hpv-vaccine-introduction-overview/.)
- 9.Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Preventive medicine 2021;144:106399. [DOI] [PubMed] [Google Scholar]
- 10.UNICEF. Human Papillomavirus Vaccine: Supply and Demand Update 2020.
- 11.Basu P, Malvi SG, Joshi S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreimer AR, Sampson JN, Porras C, et al. Evaluation of Durability of a Single Dose of the Bivalent HPV Vaccine: The CVT Trial. Journal of the National Cancer Institute 2020;112:1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol 2015;16:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safaeian M, Sampson JN, Pan Y, et al. Durability of Protection Afforded by Fewer Doses of the HPV16/18 Vaccine: The CVT Trial. Journal of the National Cancer Institute 2018;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New vaccine, new cohort, and COVID-19 interruptions: Kenya’s HPV Vaccine Introduction (and JSI’s experiences). 2021. (Accessed Jan. 30 2022, 2022, at https://publications.jsi.com/JSIInternet/Inc/Common/_download_pub.cfm?id=24146&lid=3.)
- 16.Shadab R, Lavery JV, McFadden SM, Elharake JA, Malik F, Omer SB. Key ethical considerations to guide the adjudication of a single-dose HPV vaccine schedule. Human vaccines & immunotherapeutics 2021:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnabas RV, Brown ER, Onono M, et al. Single-dose HPV vaccination efficacy among adolescent girls and young women in Kenya (the KEN SHE Study): study protocol for a randomized controlled trial. Trials 2021;22:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meningococcal Disease. 2021. at https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/meningococcal-disease.)
- 19.Polman NJ, Ebisch RMF, Heideman DAM, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol 2019;20:229–38. [DOI] [PubMed] [Google Scholar]
- 20.Jung S, Lee B, Lee KN, Kim Y, Oh EJ. Clinical Validation of Anyplex II HPV HR Detection Test for Cervical Cancer Screening in Korea. Arch Pathol Lab Med 2016;140:276–80. [DOI] [PubMed] [Google Scholar]
- 21.Hesselink AT, Berkhof J, van der Salm ML, et al. Clinical validation of the HPV-risk assay, a novel real-time PCR assay for detection of high-risk human papillomavirus DNA by targeting the E7 region. Journal of clinical microbiology 2014;52:890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eklund C, Forslund O, Wallin KL, Dillner J. Continuing global improvement in human papillomavirus DNA genotyping services: The 2013 and 2014 HPV LabNet international proficiency studies. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 2018;101:74–85. [DOI] [PubMed] [Google Scholar]
- 23.Rowhani-Rahbar A, Carter JJ, Hawes SE, et al. Antibody responses in oral fluid after administration of prophylactic human papillomavirus vaccines. The Journal of infectious diseases 2009;200:1452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ-purified glutathione s-transferase fusion proteins. Clin Chem 2005;51:1845–53. [DOI] [PubMed] [Google Scholar]
- 25.DAIDS Adverse Event Grading Tables. 2018. (Accessed Feb 2021, 2021, at https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables.)
- 26.Watson-Jones D, Baisley K, Brown J, et al. High prevalence and incidence of human papillomavirus in a cohort of healthy young African female subjects. Sexually transmitted infections 2013;89:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet (London, England) 2004;364:1757–65. [DOI] [PubMed] [Google Scholar]
- 28.Prem K, Choi YH, Benard E, et al. Global impact and cost-effectiveness of one-dose versus two-dose human papillomavirus vaccination schedules: a comparative modelling analysis. medRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Follmann D, Fintzi J, Fay MP, et al. A Deferred-Vaccination Design to Assess Durability of COVID-19 Vaccine Effect After the Placebo Group Is Vaccinated. Ann Intern Med 2021;174:1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson-Jones D, Changalucha J, Whitworth H, et al. Month 24 immunogenecity and safety of 1, 2 and 3 doses of Gardasil-9® AND Cervarix® in Tanzanian girls aged 9-14Y: the DoRIS randomized trial IPVC Toronto, Canada, and Virtual 2021. [Google Scholar]
- 31.Robbins HA, Li Y, Porras C, et al. Glutathione S-transferase L1 multiplex serology as a measure of cumulative infection with human papillomavirus. BMC infectious diseases 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 33.Cervical Cancer. 2018. (Accessed March 20, 2019, at https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/.)
- 34.Monitoring and Surveillance of HPV Vaccination Programmes. 2020. (Accessed 09/20/2020, 2020, at https://www.who.int/immunization/hpv/monitor/en/.)
- 35.Human papillomavirus vaccine support. 2020. (Accessed 09/14/2020, 2020, at https://www.gavi.org/types-support/vaccine-support/human-papillomavirus.)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared publicly because this study was conducted with approval from the Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU), which requires that data from studies (including de-identified data) are released only after SERU has provided written approval for additional analyses. A complete de-identified dataset sufficient to reproduce the study findings will be made available upon written request after approval from SERU. To request these data, please contact the KEN SHE Scientific Committee at icrc@uw.edu.