Abstract
The specific conditions in the batter of raw fermented sausages may reduce the efficiency of bacteriocin-producing starter cultures. In this work, using in vitro fermentation, we found that sodium chloride and sodium nitrite interfere with the growth of Lactobacillus sakei CTC 494, an organism which produces the antilisterial bacteriocin sakacin K. Because sakacin K production follows primary metabolite kinetics, a decrease in cell formation resulted in a decrease in sakacin K production as well. Sodium chloride dramatically influenced bacteriocin production by decreasing both biomass production and specific bacteriocin production. Sodium nitrite, however, had no effect on specific bacteriocin production and decreased bacteriocin production only because of its effect on cell growth. Moreover, sodium nitrite enhanced the toxic effect of lactic acid on bacterial growth.
Fermentation of meats is an ancient and excellent preservation technique which results in stable and safe end products. However, misfermentation due to the growth of spoilage bacteria still occurs, and the presence of emerging food-borne pathogens is raising new concerns. One of the concerns is the presence of Listeria monocytogenes, which has been recovered frequently from raw fermented sausages (17, 20, 27, 30). This bacterium is able to survive both the fermentation and drying stages of the sausage-manufacturing process (16) because of its ability to survive acidic conditions (10) and its tolerance to considerable amounts of sodium chloride and nitrite (17, 26). In particular, sausages that are subjected to very short ripening periods (e.g., German mettwurst) could be hazardous. The use of bacteriocinogenic starter cultures and/or cocultures may offer a promising solution which minimizes the risks associated with the growth of undesirable bacteria. Bacteriocins are proteins or peptides that exhibit antibacterial activity against species that are closely related to the organisms that produce them (6). Bacteriocins produced by some lactic acid bacterial strains have been shown to exhibit activity against several spoilage bacteria and food-borne pathogens, including Staphylococcus aureus, Enterococcus faecalis, Clostridium botulinum, and Listeria monocytogenes (23). However, the possibility of using these bacteria is still being questioned since the amount of available bacteriocin in the meat environment appears to be lower than expected. This is due to the specific conditions that occur in the sausage matrix (mainly limited diffusion of the bacteriocin through the product, inactivation by proteases, and adsorption to fat and meat particles) (13, 28). The importance of a decrease in bacteriocin bioavailability resulting from the presence of additives, such as salt and curing agents (nitrite and nitrate), may have been neglected. Adding salt (2.5 to 3.0%, wt/wt) to raw sausage is essential; salt decreases water activity (aw) and contributes to flavor and microbial selection (20). Adding nitrate and nitrite to sausage batter is common. Nitrite is added to produce color, to prevent lipid rancidity, to inhibit the growth of salmonellae and clostridia (20), and to obtain the typical cured flavor (2). Nitrate is basically a source of nitrite and has only an indirect effect (3). The concentration of sodium nitrite may vary from 20 to 200 ppm depending on the type of sausage and on the legislation (19). Lactobacillus sakei CTC 494, an isolate from Spanish fermented sausage, has been shown to produce the antilisterial bacteriocin sakacin K (14). Through in vitro experiments it has been demonstrated that the temperatures and pH values encountered during the manufacture of fermented sausages are favorable for sakacin K production by L. sakei CTC 494 (21). In this paper we describe the results of a kinetic investigation of the effects of sodium chloride and sodium nitrite on production of sakacin K by L. sakei CTC 494 during in vitro fermentations; we performed this investigation in order to evaluate bacteriocin bioavailability during sausage manufacturing.
MATERIALS AND METHODS
Microorganisms and media.
L. sakei CTC 494 was the organism used to produce the antilisterial bacteriocin sakacin K (14). Listeria innocua LMG 13568 was used as an indicator organism to estimate sakacin K activity. Both strains were maintained as previously described (21). The MRS broth (4) used for the fermentation experiments was prepared from single ingredients.
Fermentation experiments.
We performed a series of in vitro fermentation experiments with MRS broth containing different concentrations of sodium chloride and sodium nitrite in order to examine the effects of these compounds on both cell growth and the production of sakacin K by L. sakei CTC 494. These fermentation experiments were carried out in a 15-liter laboratory fermentor (BiostatC; B. Braun Biotech International, Melsungen, Germany) that contained 10 liters of MRS broth. The vessel was sterilized in situ at 121°C for 20 min. Glucose (2%, wt/vol) was sterilized separately and added aseptically to the fermentor. The inoculum (1%, vol/vol) was prepared and agitation (50 rpm), temperature (25°C), and pH (5.5) were controlled on-line as previously described (21). The increase in volume and the amount of Na+ added due to the addition of 10 M NaOH for pH control were considered negligible. NaCl and NaNO2 (the latter was sterilized separately by microfiltration [Sartolab-p20; Sartorius, Göttingen, Germany]) were added to the broth at different concentrations (1, 2, 4, 6, and 8% [wt/vol] and 0.01, 0.02, and 0.04% [wt/vol], respectively), and an additional fermentation experiment was performed without salt and nitrite. Moreover, to examine the effect of reduced aw, two fermentation experiments were performed without salt and sodium nitrite but with 9.9 and 21.1% (wt/vol) glycerol (sterilized separately). In order to validate the mathematical model, two additional experiments were carried out; in one experiment 5% NaCl and 0.02% NaNO2 were added, and in the other 2% NaCl and 0.03% NaNO2 were added.
Assays.
Samples were aseptically withdrawn from the fermentor in order to determine the concentration of cell dry mass (CDM), the level of soluble bacteriocin activity, the amount of lactic acid produced, and the residual glucose concentration. Values were determined as described previously (7, 8, 21). Briefly, the concentration of CDM was determined by membrane filtration, the amount of lactic acid produced and the residual glucose concentration were determined by high-pressure liquid chromatography, and the level of bacteriocin activity was determined by a critical dilution method by using L. innocua LMG 13568 as the indicator organism. The standard deviations for the CDM and high-pressure liquid chromatography measurements were 0.11 and 0.04 g liter−1, respectively. To avoid large errors in bacteriocin activity values, the same person made all observations. This resulted in good reproducibility of the activity titers. We checked that the presence of salt or nitrite in the supernatant did not prevent growth of the Listeria indicator strain used, so that inhibition zones could be attributed to sakacin K activity alone. Furthermore, we demonstrated that adding 8% salt to sakacin K-containing supernatant did not affect inhibition of the Listeria indicator strain compared to results obtained with salt-free supernatant (data not shown).
Modeling.
Growth behavior, acidification, and bacteriocin production by L. sakei CTC 494 were modeled by using elementary mathematics. The following set of equations was used to model the fermentation data (21):
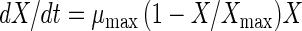 |
1 |
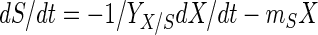 |
2 |
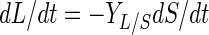 |
3 |
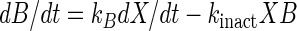 |
4 |
where t is time (in hours), X is the biomass concentration (in grams of CDM per liter), S is the residual sugar concentration (in grams of glucose per liter), L is the amount of lactic acid produced (in grams of lactic acid per liter), and B is the bacteriocin activity (in arbitrary units per liter). The parameters used in these equations are the maximum specific growth rate (μmax) (per hour), the maximum biomass concentration (Xmax) (in grams of CDM per liter), the cell yield coefficient (YX/S) (in grams of CDM per gram of glucose), the maintenance coefficient (mS) (in grams of glucose per gram of CDM per hour), the yield coefficient for lactic acid production (YL/S) (in grams of lactic acid per gram of glucose), the specific bacteriocin production coefficient (kB) (in arbitrary units per gram of CDM), and the specific bacteriocin inactivation rate (kinact) (in liters per gram of CDM per hour). The equations were integrated by using the Euler integration technique with Microsoft Excel, version 7.0, and all of the parameters were estimated by adjusting them until a good visual fit was obtained. Previously, estimating the various parameters of the model for growth behavior and bacteriocin production by L. sakei CTC 494 was shown to be a statistically reliable method (21). Where appropriate, the quadratic correlation coefficient (r2) and the standard deviation (ς) are mentioned below. The mathematical description of the combined effect of salt and nitrite on the parameters of the model was based on the hurdle theory and the γ concept (32) (see below).
RESULTS
Influence of sodium chloride.
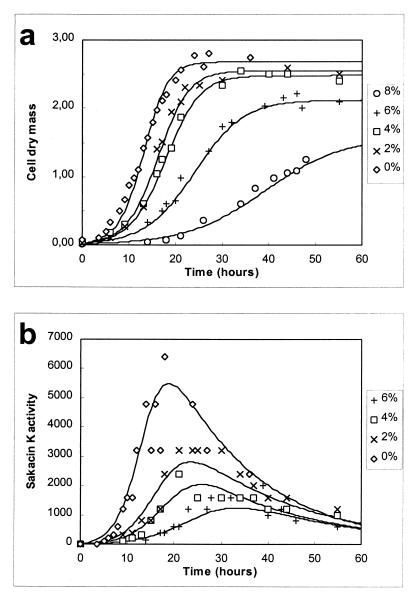
Cell growth and sakacin K activity in the presence of different salt concentrations were measured over time (Fig. 1), as were consumption of glucose and production of lactic acid (data not shown). The data were modeled with equations 1 to 4. The parameters μmax, Xmax, YX/S, mS, YL/S, kB, and kinact were estimated for each fermentation experiment.
FIG. 1.
Modeling of cell growth (in grams of CDM per liter) (a) and bacteriocin activity (in arbitrary units [×103] per liter) (b) of L. sakei CTC 494 grown in MRS broth at 25°C and pH 5.5 in the presence of different concentrations of NaCl.
L. sakei CTC 494 appeared to be quite salt tolerant. However, as the salt concentration increased, the cells grew more slowly and biomass production became less efficient. Xmax in the presence of 8% NaCl was only 60% of the concentration which was obtained when no salt was added. Also, sakacin K activity decreased drastically in the presence of increasing salt concentrations and could not be detected in the presence of 8% sodium chloride. At 30°C and pH 6.5 addition of 2% NaCl decreased Bmax from 800 to 1,000 arbitrary units ml−1 to 100 arbitrary units ml−1 (data not shown).
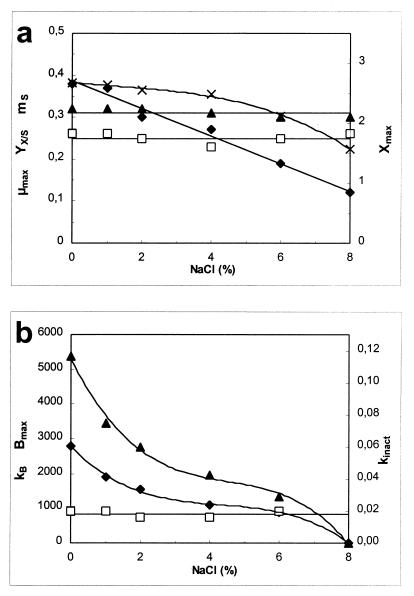
Figure 2 shows the effects of different salt concentrations on the parameters used in the model. μmax decreased linearly as the salt concentration increased, while mS and YX/S did not change significantly. Slower cell growth resulted in a decrease in Xmax. Bacteriocin activity decreased because of decreased cell growth and lower specific bacteriocin production when the salt concentration increased.
FIG. 2.
Influence of the concentration of NaCl on the parameters of the model. (a) Biomass production parameters, including YX/S (in grams of CDM per gram of glucose) (▴), mS (in grams of glucose per gram of CDM per hour) (□), μmax (per hour) (⧫), and Xmax (in grams of CDM per liter) (×). (b) Bacteriocin production parameters, including Bmax (in arbitrary units [×103] per liter) (▴), kB (in arbitrary units [×103] per gram of CDM (⧫), and kinact (in liters per gram of CDM per hour) (□). L. sakei CTC 494 was grown at 25°C and pH 5.5. Lines were drawn according to the model.
In the presence of 0 to 8% NaCl, the responses of the parameters to salt exhibited the following simple mathematical relationships: μmax = (1 − [% NaCl]/11.810) × (μmax)NaCl=0% (r2 = 0.981); Xmax = (1 − 0.0009[% NaCl]3 + 0.0040[% NaCl]2 − 0.0210[% NaCl]) × (Xmax)NaCl=0% (r2 = 0.997); YX/S = (YX/S)NaCl=0% (ς = 0.01); mS = (mS)NaCl=0% (ς = 0.01); YL/S = (YL/S)NaCl=0% (ς = 0.01); kB = (1 − 0.0060[% NaCl]3 + 0.078[% NaCl]2 − 0.3643[% NaCl]) × (kb)NaCl=0% (r2 = 0.998); and kinact = (kinact)NaCl=0% (ς = 0.004).
The parameters of the model for when the NaCl concentration was zero were obtained by regression of the experimental data for μmax, Xmax, and kB and by calculating the mean values for YX/S, mS, YL/S, and kinact. For 25°C and pH 5.5, conditions that may be encountered during sausage fermentation, the values were as follows: (μmax)NaCl=0% = 0.39 h−1; (Xmax)NaCl=0% = 2.68 g of CDM liter−1; (YX/S)NaCl=0% = 0.31 g of CDM per g of glucose; (mS)NaCl=0% = 0.25 g of glucose per g of CDM per h; (YL/S)NaCl=0% = 0.99 g of lactic acid per g of glucose; (kB)NaCl=0% = 2,780 arbitrary units per g of CDM; and (kinact)NaCl=0% = 0.018 liter per g of CDM per h.
Influence of aw.
To investigate whether the inhibitory effect of sodium chloride on cell growth and bacteriocin production in L. sakei CTC 494 was due to a reduction in the aw or to other (ionic) effects, two additional fermentation experiments were performed with glycerol as an agent which decreased the aw (Table 1). The presence of glycerol in the growth medium had similar effects on the growth rate and specific bacteriocin production, as was the case for salt. Based on extrapolation of data from previous studies (1, 22), addition of 9.9 and 21.1% glycerol to the basal growth medium should have resulted in the same decreases in the aw as approximately 4 and 8% sodium chloride, respectively. In this study, the effects of 9.9 and 21.1% glycerol were roughly comparable to the putative effects of 3.0 and 6.5% sodium chloride, respectively. The fact that our values were slightly lower than the values expected may be explained by differences in the growth medium, experimental error, or unknown effects.
TABLE 1.
Effect of glycerol on the μmax, Xmax, YX/S, mS, YL/S, kB, and kinact for L. sakei CTC 494 grown in MRS broth at 25°C and pH 5.5 in the absence of salt and nitrite
| Glycerol concn (%) | μmax (h−1) | Xmax (g of CDM liter−1) | YX/S (g of CDM g of glucose−1) | mS (g of glucose h−1 g of CDM−1) | YL/S (g of lactic acid g of glucose−1) | kB (103 arbitrary units g of CDM−1) | kinact (liter g of CDM−1 h−1) |
|---|---|---|---|---|---|---|---|
| 0 | 0.38 | 2.68 | 0.32 | 0.26 | 1.00 | 2,800 | 0.020 |
| 9.9 | 0.29 | 2.45 | 0.31 | 0.25 | 1.00 | 1,200 | 0.020 |
| 21.1 | 0.17 | 1.85 | 0.33 | 0.23 | 0.99 | 650 | 0.020 |
Influence of sodium nitrite.
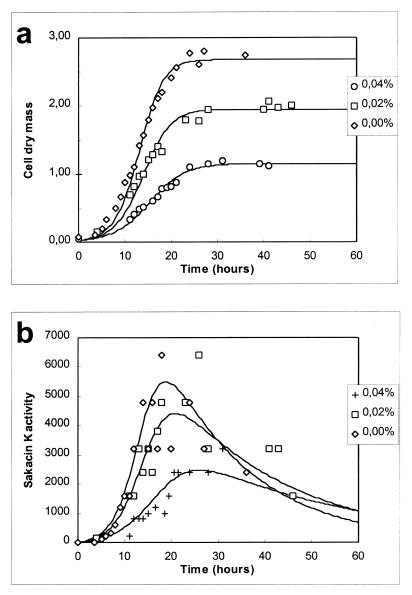
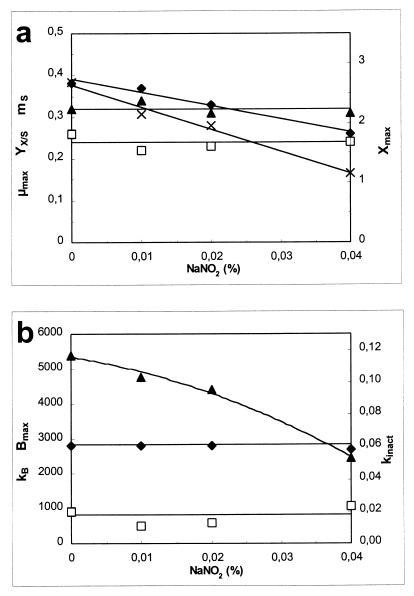
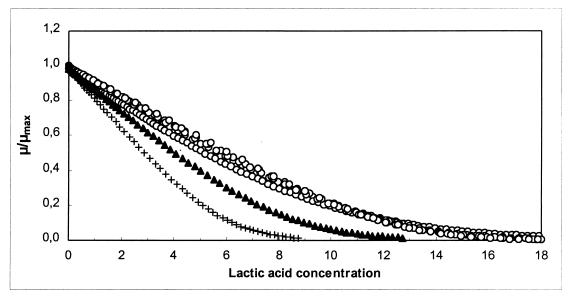
As Fig. 3 shows, addition of nitrite decreased the growth rate, the maximum cell yield, and the bacteriocin activity. It appears from Fig. 4 that the decrease in sakacin K activity was due to the decrease in cell growth alone, because specific bacteriocin production and the inactivation rate remained constant. Sakacin K production is growth related, and a lower growth rate results in formation of less biomass and, thus in lower sakacin K production (cf. equation 4). The presence of sodium nitrite affected only biomass production (μmax, Xmax); there were no effects on mS, YX/S, kB, and kinact, or the effects were insignificant (Fig. 4). Xmax (1.22 g of CDM liter−1) was very low when 0.04% NaNO2 was used, and cell growth ceased earlier than expected. A similar decrease in μmax when salt was added instead of nitrite almost doubled Xmax (Fig. 2 and 4). This is surprising since YX/S and mS were not affected. Production of lactic acid contributes to inhibition of L. sakei CTC 494 growth. At a certain concentration of lactic acid, before all of the sugar is consumed, the specific growth rate (i.e., μmax[1 − X/Xmax]) decreases to zero. This concentration of lactic acid (approximately 17 g liter−1 at pH 5.5 and 25°C) was not significantly influenced by the addition of sodium chloride, but remarkably, it was decreased to approximately 9 g liter−1 when 0.04% sodium nitrite was added (Fig. 5). Somehow, the presence of nitrite seemed to enhance the toxic effect of lactic acid.
FIG. 3.
Modeling of cell growth (in grams of CDM per liter) (a) and bacteriocin activity (in arbitrary units [×103] per liter) (b) of L. sakei CTC 494 cultivated in MRS broth at 25°C and pH 5.5 in the presence of different concentrations of NaNO2.
FIG. 4.
Influence of the concentration of NaNO2 on the parameters of the model. (a) Biomass production parameters, including YX/S (in grams of CDM per gram of glucose) (▴), mS (in grams of glucose per gram of CDM per hour) (□), μmax (per hour) (⧫), and Xmax (in grams of CDM per liter) (×). (b) Bacteriocin production parameters, including Bmax (in arbitrary units [×103] per liter) (▴), kB (in arbitrary units [×103] per gram of CDM (⧫), and kinact (in liters per gram of CDM per hour) (□). L. sakei CTC 494 was cultivated at 25°C and pH 5.5. Lines were drawn according to the model.
FIG. 5.
Inhibitory effect (specific growth rate/μmax) of lactic acid (in grams per liter) on the growth of L. sakei CTC 494 in MRS broth at 25°C and pH 5.5 in the absence of nitrite with different concentrations (0 to 8%) of sodium chloride (○) and in the absence of salt with 0.02% (▴) or 0.04% (+) sodium nitrite.
In the presence of 0 to 0.04% NaNO2, the responses of the parameters to sodium nitrite exhibited the following simple mathematical relationships: μmax = (1 − [% NaNO2]/0.124) × (μmax)NaNO2=0.00% (r2 = 0.971); Xmax = (1 − [% NaNO2]/0.072) × (Xmax)NaNO2=0.00% (r2 = 0.985); YX/S = (YX/S)NaNO2=0.00% (ς = 0.01); mS = (mS)NaNO2=0.00% (ς = 0.02); YL/S = (YL/S)NaNO2=0.00% (ς = 0.01); kB = (kB)NaNO2=0.00% (ς = 50); and kinact = (kinact)NaNO2=0.00% (ς = 0.006).
The parameters of the model for when the NaNO2 concentration was zero were obtained by regression of the experimental data for μmax and Xmax and by calculating the mean values for YX/S, mS, YL/S, kB, and kinact. For 25°C and pH 5.5, conditions that may be encountered during sausage fermentation, the values were as follows: (μmax)NaNO2=0.00% = 0.39 h−1, (Xmax)NaNO2=0.00% = 2.61 g of CDM liter−1, (YX/S)NaNO2=0.00% = 0.32 g of CDM per g of glucose; (mS)NaNO2=0.00% = 0.24 g of glucose per g of CDM per h; (YL/S)NaNO2=0.00% = 0.99 g of lactic acid per g of glucose; (kB)NaNO2=0.00% = 2,780 arbitrary units per g of CDM; and (kinact)NaNO2=0.00% = 0.018 liter per g of CDM per h. These values are not significantly different from the values obtained in the NaCl experiments.
Combined effect of sodium chloride and sodium nitrite.
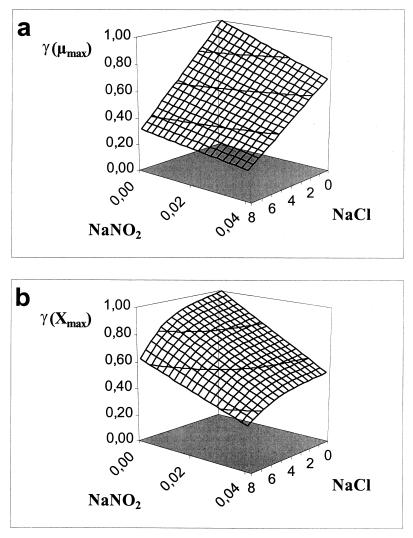
Adding salt and/or curing agents has consequences for both growth of and bacteriocin production by L. sakei CTC 494. The combined action of salt and nitrite influences μmax and Xmax (Fig. 6). kB is influenced only by the salt concentration. The decreases in μmax, Xmax, and kB due to the presence of salt at concentrations up to 8% and/or nitrite at concentrations up to 0.04% can be described with the following equations:
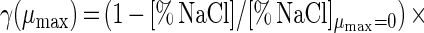 |
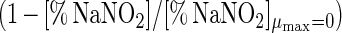 |
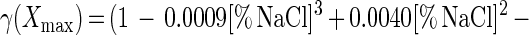 |
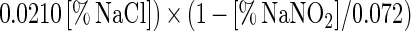 |
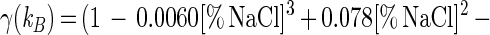 |
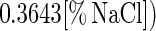 |
where γ(μmax), γ(Xmax), and γ(kB) are inhibition coefficients and [% NaCl]μmax=0 and [% NaNO2]μmax=0 are 11.8 and 0.12%, respectively. The inhibition coefficients for the parameters μmax, Xmax, and kB are equal to the actual values of these parameters in the presence of salt and nitrite divided by the optimal values. The γ concept (32) presupposes different factors do not have combined effects. The other parameters of the model (mS, YX/S, YL/S, kB, and kinact) are not affected by salt or nitrite addition. The overall validity of the model was assessed by performing two additional fermentation experiments. The experimental and predicted values of the various parameters for both fermentation experiments are compared in Table 2. It turned out that the predictive capacity of the model was satisfactory.
FIG. 6.
Combined inhibitory effects of sodium chloride and sodium nitrite on μmax (per hour) (a) and Xmax (in grams of CDM per liter) (b).
TABLE 2.
Predicted and experimental values for μmax, Xmax, YX/S, mS, YL/S, kB, and kinact for L. sakei CTC 494 grown in MRS broth at 25°C and pH 5.5 in the presence of different concentrations of NaCl and NaNO2
| NaCl concn (%) | NaNO2 concn (%) | μmax (h−1)
|
Xmax (g of CDM liter−1)
|
YX/S (g of CDM g of glucose−1)
|
mS (g of glucose h−1 g of CDM−1)
|
YL/S (g of lactic acid g of glucose−1)
|
kB (103 arbitrary units g of CDM−1)
|
kinact (liter g of CDM−1 h−1)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predicted | Exptl | Predicted | Exptl | Predicted | Exptl | Predicted | Exptl | Predicted | Exptl | Predicted | Exptl | Predicted | Exptl | ||
| 2 | 0.03 | 0.25 | 0.26 | 1.47 | 1.40 | 0.31 | 0.29 | 0.25 | 0.24 | 0.99 | 1.00 | 1,490 | 1,500 | 0.018 | 0.018 |
| 5 | 0.02 | 0.19 | 0.20 | 1.66 | 1.60 | 0.31 | 0.30 | 0.25 | 0.27 | 0.99 | 0.99 | 1,050 | 1,000 | 0.018 | 0.020 |
DISCUSSION
The use of bacteriocin-producing starter cultures for raw sausage fermentation may contribute to more uniform and safer products. However, the bacteriocin activity levels in a meat matrix are less than the expected activity levels. This is due to the specific conditions in the food environment. For this reason it is necessary to select for strains that are adapted to the meat environment. L. sakei CTC 494, an isolate obtained from Spanish fermented sausage and an organism that produces the antilisterial bacteriocin sakacin K (14), was found to be able to exhibit maximum bacteriocin activity levels in the temperature and pH ranges which are typical for the fermentation stage of raw fermented sausages (21). However, how other factors, such as the presence of salt and curing agents, influence bacteriocin titers was unclear. Previously, the effect of salt on production and/or activity of bacteriocins produced by lactic acid bacteria has been reported to be beneficial (12, 29) or harmful (8, 24). In this study, using a mathematical model, we investigated how sodium chloride and sodium nitrite interfere with the kinetics of bacteriocin production by L. sakei CTC 494 during in vitro fermentation.
Due to its water binding and ionic characteristics, salt affects the metabolism of a starter culture. The growth of lactic acid bacteria is sometimes enhanced in the presence of low concentrations of sodium chloride (1 to 2%, wt/vol), but growth is clearly inhibited in the presence of NaCl concentrations greater than 3% (wt/vol) (11, 18, 25, 31). Homofermentative lactic acid bacteria are more resistant to sodium chloride than heterofermentative lactic acid bacteria are, and strains resembling L. sakei have been shown to be more resistant than strains resembling Lactobacillus curvatus (18) or Lactobacillus pentosus (9). Increases in the salt concentration decrease the growth rate of L. sakei CTC 494 linearly. Indeed, the growth rate often decreases linearly at aw values below the optimum aw (22, 25). Moreover, sodium chloride negatively affects the production of sakacin K by L. sakei CTC 494. Production of sakacin K decreases because the amount of biomass formed decreases (bacteriocin production generally exhibits primary metabolic kinetics [5, 7, 8, 21]) and because specific bacteriocin production decreases. It has been suggested that the decrease in bacteriocin production in the presence of salt is due to interference of sodium chloride molecules with binding of the induction factor, which is essential for bacteriocin production, to its receptor (24). In the case of L. sakei CTC 494, however, it appears that the water binding effect of salt molecules is the major factor responsible for the decrease in specific bacteriocin production since using glycerol as an agent to decrease aw instead of salt has a comparable effect. Hence, because salt decreases aw, the presence of relatively high salt concentrations in sausage batter may be one of the predominant factors that reduce the efficacy of bacteriocin-producing starter cultures or cocultures. During the fermentation stage, a salt concentration of 4 to 6% in the water phase of the sausage batter does indeed decrease sakacin K production considerably. However, the activity probably is sufficient to have a significant antilisterial effect in the sausage environment, as demonstrated by Hugas et al. (14). Moreover, the results of in situ experiments suggest that nitrite and pepper have a synergistic effect on the antilisterial activity of L. sakei CTC 494 in sausage (15).
Nitrite is known mainly for its antimicrobial activity against sporeformers; it has a limited effect on the growth of lactic acid bacteria at concentrations less than 200 mg liter−1 (9, 18, 31), but at 400 mg liter−1 inhibition is more pronounced (18). It has been shown that biomass formation and bacteriocin production by L. sakei CTC 494 decrease as the concentration of sodium nitrite increases. Nitrite has no effect on specific sakacin K production but decreases the bacteriocin titer indirectly because of its effect on cell growth. Since sakacin K production is growth related, formation of a small amount of biomass results in a low sakacin K yield.
Remarkably, whereas addition of salt does not have a significant effect on the concentration of lactic acid at which the specific growth rate becomes zero (approximately 17 g of lactic acid liter−1), addition of 0.04% sodium nitrite decreases this concentration to approximately 9 g liter−1. Nitrite seems to enhance the toxic effect of lactic acid, as if the ability of the cells to protect themselves against this molecule were diminished, for instance, as a result of intracellular accumulation. It has been mentioned previously that nitrite might interfere with active transport mechanisms (3). This would explain the surprisingly low Xmax which is obtained when 0.04% sodium nitrite is used.
In this paper, we present a mathematical model that describes the combined effects of sodium chloride and sodium nitrite on growth and bacteriocin production in L. sakei CTC 494. The predictive power of the model was confirmed by successful validation of its equations. The model accounts for a broad range of sodium chloride and sodium nitrite concentrations (0 to 8 and 0.00 to 0.04%, respectively) in MRS broth at 25°C and pH 5.5, conditions that are encountered during sausage fermentation. The predictive capacity of the model may be extended to other temperatures and pH values if the equations are combined with a previously described temperature-pH model (21). However, the accuracy of such a combined model approach needs to be evaluated.
In this work, we examined the effects of sodium chloride and sodium nitrite on bacteriocin production by L. sakei CTC 494, a potential starter culture for sausage fermentation. Whereas nitrite affected bacteriocin production only slightly because it decreased cell growth, salt had a more drastic effect because it decreased both cell growth and specific bacteriocin production. Addition of salt may be one of the major causes of the reduced efficacy of bacteriocin-producing starter cultures in food environments. Work is in progress to examine the roles of other compounds in sausage batter, such as spices, proteins, and fat particles.
ACKNOWLEDGMENTS
This work was supported by the Research Council of Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders, the European Community (grant FAIR-CT97-3227), and the Institute for the Encouragement of Scientific and Technological Research in the Industry.
L. sakei CTC 494 was kindly provided by M. Hugas (Institut de Recerca i Tecnologia Agroalimentáries, Centre de Tecnologia de la Carn, Monells, Spain).
REFERENCES
- 1.Chandler R E, McMeekin T A. Modelling the growth response of Staphylococcus xylosus to changes in temperature and glycerol concentration/water activity. J Appl Bacteriol. 1989;66:543–548. doi: 10.1111/j.1365-2672.1989.tb04576.x. [DOI] [PubMed] [Google Scholar]
- 2.Dainty R, Blom H. Flavour chemistry of fermented sausages. In: Campbell-Platt G, Cook P E, editors. Fermented meats. London, United Kingdom: Blackie Academic & Professional; 1995. pp. 176–193. [Google Scholar]
- 3.Davidson P M. Chemical preservatives and natural antimicrobial compounds. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C.: ASM Press; 1997. pp. 520–556. [Google Scholar]
- 4.De Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 5.De Vuyst L, Vandamme E J. Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. J Gen Microbiol. 1992;138:571–578. doi: 10.1099/00221287-138-3-571. [DOI] [PubMed] [Google Scholar]
- 6.De Vuyst L, Vandamme E J. Antimicrobial potential of lactic acid bacteria. In: De Vuyst L, Vandamme E J, editors. Bacteriocins of lactic acid bacteria: microbiology, genetics and applications. London, United Kingdom: Blackie Academic & Professional; 1994. pp. 91–142. [Google Scholar]
- 7.De Vuyst L, Callewaert R, Pot B. Characterisation and antagonistic activity of Lactobacillus amylovorus DCE 471 and large scale isolation of its bacteriocin amylovorin L471. Syst Appl Microbiol. 1996;19:9–20. [Google Scholar]
- 8.De Vuyst L, Callewaert R, Crabbé K. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology. 1996;142:817–827. doi: 10.1099/00221287-142-4-817. [DOI] [PubMed] [Google Scholar]
- 9.Doßmann M U, Vogel R F, Hammes W P. Mathematical description of the growth of Lactobacillus sake and Lactobacillus pentosus under conditions prevailing in fermented sausages. Appl Microbiol Biotechnol. 1996;46:334–339. doi: 10.1007/BF00166226. [DOI] [PubMed] [Google Scholar]
- 10.Gahan C G M, O’Driscoll B, Hill C. Acid adaptation of Listeria monocytogenes can enhance survival in acidic foods and during milk fermentation. Appl Environ Microbiol. 1996;62:3128–3132. doi: 10.1128/aem.62.9.3128-3132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gänzle M G, Ehmann M, Hammes W P. Modeling of growth of Lactobacillus sanfranciscensis and Candida milleri in response to process parameters of sourdough fermentation. Appl Environ Microbiol. 1998;64:2616–2623. doi: 10.1128/aem.64.7.2616-2623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gänzle M G, Hertel C, Hammes W P. Modelling the effect of pH, NaCl, and nitrite concentrations on the antimicrobial activity of sakacin P against Listeria ivanovii DSM 20750. Fleischwirtschaft. 1996;76:409–412. [Google Scholar]
- 13.Holzapfel W H, Geisen R, Schillinger U. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int J Food Microbiol. 1995;24:343–362. doi: 10.1016/0168-1605(94)00036-6. [DOI] [PubMed] [Google Scholar]
- 14.Hugas M, Garriga M, Aymerich M T, Monfort J M. Inhibition of Listeria in dry fermented sausages by the bacteriocinogenic Lactobacillus sake CTC 494. J Appl Bacteriol. 1995;79:322–330. [Google Scholar]
- 15.Hugas M, Garriga M, Pascual M, Aymerich M T, Monfort J M. Congress Proceedings of the 44th International Congress of Meat Science and Technology. Madrid, Spain: Estrategias Alimentarias; 1998. Influence of technological ingredients on the inhibition of Listeria monocytogenes by a bacteriocinogenic starter culture in dry fermented sausages, abstr. no. A39; pp. 338–339. [Google Scholar]
- 16.Johnson J L, Doyle M P, Cassens R G, Schoeni J L. Fate of Listeria monocytogenes in tissues of experimentally infected cattle and in hard salami. Appl Environ Microbiol. 1988;54:497–501. doi: 10.1128/aem.54.2.497-501.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson J L, Doyle M P, Cassens R G. Listeria monocytogenes and other Listeria spp. in meat and meat products. J Food Prot. 1990;53:81–91. doi: 10.4315/0362-028X-53.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Korkeala H, Alanko T, Tiusanen T. Effect of sodium nitrite and sodium chloride on growth of lactic acid bacteria. Acta Vet Scand. 1992;33:27–32. doi: 10.1186/BF03546933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kröckel L. Bacterial fermentation of meats. In: Campbell-Platt G, Cook P E, editors. Fermented meats. London, United Kingdom: Blackie Academic & Professional; 1995. pp. 69–109. [Google Scholar]
- 20.Leistner L. Stable and safe fermented sausages world-wide. In: Campbell-Platt G, Cook P E, editors. Fermented meats. London, United Kingdom: Blackie Academic & Professional; 1995. pp. 160–175. [Google Scholar]
- 21.Leroy F, De Vuyst L. Temperature and pH conditions that prevail during the fermentation of sausages are optimal for the production of the antilisterial bacteriocin sakacin K. Appl Environ Microbiol. 1999;65:974–981. doi: 10.1128/aem.65.3.974-981.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMeekin T A, Chandler R E, Doe P E, Garland C D, Olley J, Putro S, Ratkowsky D A. Model for combined effect of temperature and salt concentration/water activity on the growth rate of Staphylococcus xylosus. J Appl Bacteriol. 1987;62:543–550. doi: 10.1111/j.1365-2672.1987.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 23.Nettles C G, Barefoot S F. Biochemical and genetic characteristics of bacteriocins of food-associated lactic acid bacteria. J Food Prot. 1993;56:338–356. doi: 10.4315/0362-028X-56.4.338. [DOI] [PubMed] [Google Scholar]
- 24.Nilsen T, Nes I F, Holo H. An exported inducer peptide regulates bacteriocin production in Enterococcus faecium CTC 492. J Bacteriol. 1998;180:1848–1854. doi: 10.1128/jb.180.7.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passos F V, Fleming H P, Ollis D F, Hassan H M, Felder R M. Modeling the specific growth rate of Lactobacillus plantarum in cucumber extract. Appl Microbiol Biotechnol. 1993;40:143–150. [Google Scholar]
- 26.Razavilar V, Genigeorgis C. Prediction of Listeria spp. growth as affected by various levels of chemicals, pH, temperature and storage time in a model broth. Int J Food Microbiol. 1998;40:149–157. doi: 10.1016/s0168-1605(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 27.Steinhauserova I, Smola J, Stegnerova H. Congress Proceedings of the 44th International Congress of Meat Science and Technology. Madrid, Spain: Estrategias Alimentarias; 1998. Occurrence of Listeria monocytogenes in meat products and identification by applied PCR method, abstr. no. B39; pp. 562–563. [Google Scholar]
- 28.Stiles M E, Hastings J W. Bacteriocin production by lactic acid bacteria: potential for use in meat preservation. Trends Food Sci Technol. 1991;2:247–251. [Google Scholar]
- 29.Uguen P, Hamelin J, Le Pennec J P, Blanco C. Influence of osmolarity and the presence of an osmoprotectant on Lactococcus lactis growth and bacteriocin production. Appl Environ Microbiol. 1999;65:291–293. doi: 10.1128/aem.65.1.291-293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varabioff Y. Incidence of Listeria in smallgoods. Lett Appl Microbiol. 1992;14:167–169. [Google Scholar]
- 31.Vignolo G M, de Kairuz M N, de Ruiz Holgado A A P, Oliver G. Influence of growth conditions on the production of lactocin 705, a bacteriocin produced by Lactobacillus casei CRL 705. J Appl Bacteriol. 1995;78:5–10. [Google Scholar]
- 32.Wijtzes T, Kant-Muermans M L T, De Waard A, De Wit J C, Zwietering M H. Proceedings of the Lactic ’97 Conference. Villers-Bocage, France: Adria Normandie; 1997. Validation of a combined temperature, water activity, acidity model for bacterial growth of Lactobacillus curvatus; pp. 115–126. [Google Scholar]