Abstract
Intraarticular stem cell therapy has become increasingly used to treat knee osteoarthritis (KOA) with minimal high-quality evidence to support its use. This study aims to see how well intra-articular injections of mesenchymal stem cells (MSCs) worked and how safe they were for individuals with KOA. A total of 10 studies were extracted using PubMed, Cochrane Library, and PMC from 2017 to 2021 in the English language. An assessment of the risk of bias was applied via the Cochrane Collaborative Bias Risk Tool and Newcastle-Ottawa Quality. Changes in pain and functional outcomes in patients with KOA were measured by a Knee injury and Osteoarthritis Outcome Score (KOOS) scores, Visual Analogue Scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores at baseline, and follow-up evaluation criteria. The magnetic resonance imaging (MRI) was evaluated using the whole-organ magnetic resonance imaging score (WORMS) and cartilage volume changes. A total of six randomized controlled trials (RCTs), three prospective retrospective clinical trials, and one retrospective clinical trial included 723 patients. They were diagnosed with unilateral or bilateral KOA with Kellgren-Lawrence (KL) grade 1-4 KOA and followed up for six, 12, and 24 months. The experimental groups received multipotent MSCs, mesenchymal progenitor cells (MPCs), adipose tissue progenitor stem cells (AD-MPCs), adipose tissue mesenchymal stem cells (AD-MSCs), bone marrow mesenchymal stem cells (BM-MSCs), bone marrow aspiration (BMA), bone marrow aspiration concentration (BMAC), or micro fragmented adipose tissue (MFAT) while the controlled groups received normal saline (NS), hyaluronic acid (HA), placebo, or went through conservative management.
In conclusion, significant improvements were noticed in the MSCs groups via different outcome measuring tools like KOOS, VAS, WOMAC, and MRI. Furthermore, no significant adverse events (AEs) have been observed. Therefore, intra-articular injections of MSCs are effective and safe in relieving pain and improving motor function in individuals with KOA in the short term, contrary to earlier research findings.
Keywords: injection, intra-articular, mesenchymal stem cells, osteoarthritis, knee
Introduction and background
Osteoarthritis (OA) is the most common type of arthritis [1], and it is characterized by a progressive loss of articular cartilage, subchondral bone edema, sclerosis, synovitis, and marginal osteophyte formation. Pain, stiffness, and a restriction in joint movement are the most common symptoms whose severity varies. However, the condition gradually worsens over time and often results in significant functional impairment and reduced quality of life [2,3]. It was anticipated to become the fourth leading cause of disability by 2020 [1,4,5], posing a significant socioeconomic burden impacting developed countries' gross domestic product [1,6]. Knee osteoarthritis (KOA) accounts for 85 percent of the global burden of OA and affects 19% of adults over 45-year-old and 37% of people over 60. KOA produces significant pain and physical impairment, lowering the quality of life and ranking as the eleventh leading cause of global disability. The average annual total expense per KOA patient is over US$15 000, resulting in total healthcare expenditure of nearly US$34 billion. Given population aging and the rise in obesity, KOA healthcare expenses are expected to quadruple by 2040 [7]. It is necessary to develop sufficient medicines capable of slowing the progression of the disease and, as a result, preventing the loss of articular function and joint replacement. To provide more effective therapies, current conservative choices such as exercise and physiotherapy and weight loss with analgesics and naturally occurring substances should be integrated [1,8]. Developing effective conservative methods would be especially important for treating young people with early OA because their more active and physically demanding lifestyle negatively correlates with prosthetic implant survival [1,9].
The main treatment in the clinic is non-steroidal anti-inflammatory drugs (NSAIDs), which are recommended for all patients except those having surgical treatment in the American Academy of Orthopaedic Surgeons (AAOS) clinical practice recommendations for KOA treatment [10-12]. However, long-term usage of these treatments will cause major adverse reactions in patients, such as gastrointestinal ulcers, digestive system hemorrhage, and cardiovascular and cerebrovascular side effects, regardless of the toxicity of the drugs themselves [10,13]. Intra-articular injections of HA, platelet-rich plasma (PRP), or corticosteroids (CC) are also clinical possibilities, but their efficacy and the prevalence of side effects are still debated [10,14,15].
MSCs, be a possible treatment option for KOA [16-20]. MSCs, also called MPCs, secrete various cytokines that modulate an anti-inflammatory milieu in the OA joint, giving them immunomodulatory characteristics [18,21]. They may also have a unique ability to induce the growth of new cartilage-like cells in vitro [17,18,22], as improvements in cartilage morphology have been found in some situations [23-26]. These characteristics make them a suitable candidate for use in knee cartilage repair [27-32]. For OA treatment, orthobiologics injections containing MSCs as effector cells have recently been used. Because of their accessibility, bone marrow (BM) and adipose tissue (AD) have traditionally been the most used autologous tissue sources for orthopedic usage. In several studies, the use of autologous orthobiologics treatments in the treatment of OA is safe, with an extensive multicenter prospective analysis revealing no higher risk of neoplasia [33,34].
MSCs treatment looks to be safe based on published clinical study results. There were no significant side effects other than transitory fever in a comprehensive systematic review and meta-analysis of trials involving intravascular delivery of autologous or allogeneic expanded MSCs treatments (totaling over 1000 participants) [35,36]. A systematic evaluation of clinical trials involving intra-articular autologous expanded MSCs therapy that included 844 procedures. They had a mean follow-up of 21 months and found no link between infection, cancer, or death [35,37].
As a result, we undertook this study to examine all current high-quality information on the therapeutic efficacy and safety of MSCs in the treatment of KOA qualitatively and quantitatively. This is crucial, and the study's findings will give evidence and recommendations for the promotion and deployment of MSCs therapy in clinical practice.
Review
Method
We developed and implemented the study according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) system [38], the review's preferred reporting items.
Database
On December 30, 2021, we began our research using online libraries as a database. For our data gathering, we used PubMed, the Cochrane Library, and PMC.
Search Strategy
We included studies related to KOA, MSCs, and intra-articular injection. Our keywords and medical subject heading (MeSH) search strategies included knee, osteoarthritis, mesenchymal stem cells, intra-articular, and injection. The main MeSH terms used were: ("injections, intra articular"[MeSH Terms] OR ("injections"[All Fields] AND "intra articular"[All Fields]) OR "intra-articular injections"[All Fields] OR ("intra"[All Fields] AND "articular"[All Fields] AND "injection"[All Fields]) OR "intra articular injection"[All Fields]) AND ("mesenchymal stem cells"[MeSH Terms] OR ("mesenchymal"[All Fields] AND "stem"[All Fields] AND "cells"[All Fields]) OR "mesenchymal stem cells"[All Fields]) AND ("osteoarthritis, knee"[MeSH Terms] OR ("osteoarthritis"[All Fields] AND "knee"[All Fields]) OR "knee osteoarthritis"[All Fields] OR ("knee"[All Fields] AND "osteoarthritis"[All Fields])) and “Knee Osteoarthritis”, “Mesenchymal Stem Cells”, “Intra-articular Injections”. MeSH terms carried out a further supplementary search with free words. In addition, to prevent eliminating papers that satisfied the inclusion criteria, we searched retrieved studies that were cited.
Inclusion Criteria
We included RCTs and clinical trial studies conducted between 2017-and 2021, with complete free texts in the English language from all countries. Also, men and women aged 18 years or older with osteoarthritis in their knees and the severity of their osteoarthritis are shown in KL grade.
Exclusion Criteria
We excluded studies before the last five years, not in English, that included animals, HA, PRP, arthroscopy, ultrasound waves, and combination treatment in the intervention, other than knee joints like shoulder and hip.
Quality Assessment Tools
Two authors, S.S and S.V, independently assessed the study's overall quality and risk of bias by using the Cochrane Collaboration risk-of-bias tool for the RCTs and Newcastle Ottawa Scale (NOS) for the clinical trials. The Cochrane Collaboration risk-of-bias tool included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each included RCT was rated as having a low, unclear, or high risk of bias based on these factors. The following are the contents for the NOS, including selection, comparability, and outcome. According to these items, each included clinical trial was scored as good, fair, and poor quality.
Data Extraction
Two writers, S.S and S.V, worked independently to extract data using a standardized manner. Disagreements that arose during the procedure were resolved through debate between the two writers or contact with a third author, just as they were with the inclusion of literature into the study. The following were the contents of the data extraction form: the first author's name, the year of publication, the sample size, basic patient information (age, male-to-female ratio, body mass index (BMI)), osteoarthritis grading KL grade, donor source (autogenous/allogeneic), cell processing, culture, and harvesting, number of cells, immunophenotype, intervention, and control situation, follow-up, and outcome clinical effectiveness and safety were among the outcomes.
Results
Literature Search
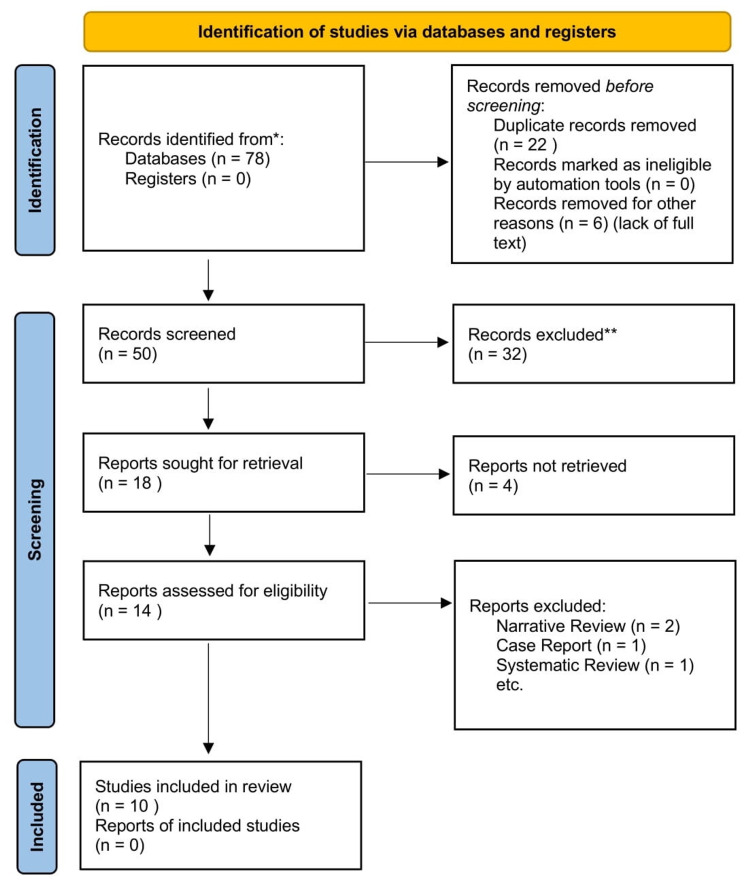
Using the literature search, we discovered 78 relevant papers. After eliminating duplicates and screening titles and abstracts, 50 articles were excluded. The remaining 18 articles were subjected to a full-text review, with eight being excluded, as shown in figure 1.
Figure 1. The literature screening process is strictly according to the inclusion/exclusion criteria. RCT and Clinical Trials.
Characteristics of the Included Studies
A total of six RCTs (577 participants) [2,7,17,18,32,35], including one study which had a pilot study, commenced in November and completed in June 2021, where recruitment commenced in January and August 2021 and will be finished by December 2024 [7]. Four clinical trial studies, including three prospective [16,23,32], and one retrospective [33] clinical trial, were included in this systematic review. Publication intervals for all 10 were from 2017 to 2021 [7]. All studies used autologous MSCs except two studies [2,7], which used allogeneic MSCs. Five studies [2,17,18,35,39], used AD-MSCs two studies [23,32], used BM-MSCs, one study [16], used BMA, one study [33], used both concentrations BMAC and MFATand one study [7], used multipotent MSCs. A placebo was utilized as a control group [2,39]. For one study, NS was used as the control group [7]; for one trial, HA was used as the control group [17], In one study's control group, cautious management was adopted [35], and five of the investigations [16,18,23,32,33], were uncontrolled. Furthermore, four trials [2,16,17,35] were monitored for a year, three trials [7,23,32] were monitored for 24 months, and two trials [33,39] were followed for six months after they were completed, and one study [18], had a 48-weeks follow-up period. Table 1 illustrates the features of the 10 articles that were featured.
Table 1. Features of the included studies.
BMI = body mass index, KL = Kellgren-Lawrence, RCT = randomized control trial, MSCs = mesenchymal stem cells, NS = normal saline, CD = cluster of differentiation, KOOS = knee injury and osteoarthritis outcome score, MOAKS = MRI osteoarthritis knee Score, AD-MPCs = adipose tissue mesenchymal progenitor cells, WOMAC = Western Ontario and McMaster universities osteoarthritis index, VAS = visual analogue scale, WORMS = whole-organ magnetic resonance imaging score, MRI = magnetic resonance imaging, HA = hyaluronic acid, AD- MSCs = adipose tissue mesenchymal stem cells, NPRS = numeric pain rating scale, BMAC = bone marrow aspiration concentration, MFAT = microfragmented adipose tissue
| Study (year) | Sample (M:F) | Age | BMI | Kl Grade | Type of Study | Intervention | Control | Donor | Immunophenotype | Dose (cell) (×106) | Outcome Measures | Follow up |
| Liu (2021 [7] | 440 (Not mentioned) | 40-90 | Not mentioned | 2-3 | Double-Blinded RCT | multipotent MSCs 220 | NS, 220 | Allogeneic | CD34 | 2.5×10 | KOOS, MOAKS, others | 24 months |
| Lu (2020)[18] | 22 (3:19) | 18-70 | 27.77 (±1.93), 26.69 (±2.63), 24.51 (± 2.49) | 2-3 | Double-Blinded RCT | AD-MPCs 22 | Not Controlled | Autologous | Positive marker (CD90, CD73, CD105) Negative (HLA-DR, CD14, CD45) | 1 × 10, 2 × 10, 5 × 10 | WOMAC, VAS, WORMS, MRI, others | 48 weeks |
| Lu (2019)[17] | 52 (6:46) | 18–70 | 24 | 1-3 | Double-Blinded RCT | AD-MPCs 26 | HA, 26 | Autologous | Positive: CD90,CD73, CD29, CD49 Negative: CD14,CD34,CD45, HLA-DR | 50 × 2 | WOMAC, VAS, MRI, others | 12 Months |
| Lee (2019)[39] | 24 (6:18) | 18-75 | 25.3 (± 4.9), 25.4 (± 3.0) | 2-4 | Double-Blinded RCT | AD-MSCs 12 | Placebo, 12 | Autologous | Positive: CD90,CD73 Negative: CD31, CD34, CD45 | 100 | KOOS, WOMAC, VAS, MRI, others | 6 months |
| Freitag (2019) [35] | 30 (16:14) | > 18 | 25.2 (±3.4), 31.6 (±5.9), 30.4 (±5.6) | 2-3 | Non-Blinded RCT | AD-MSCs 20 | Conservative Management, 10 | Autologous | Positive: CD90,CD73, CD105 Negative: CD14,CD19, CD34, CD45 | 100, 100 × 2 | KOOS, NPRS, WOMAC, others | 12 months |
| Kuah (2018) [2] | 20 (12:8) | 40–65 | 20-30 | 1-3 | Double-Blinded RCT | AD-MSCs 16 | Placebo, 4 | Allogeneic | Not mentioned | 3.9, 6.7 | VAS, WOMAC, MOAKS, MRI, others | 12 months |
| Al-Najar (2017) [32] | 13 (6:7) | 34–63 | Not mentioned | 2-3 | Prospective Clinical Trial | BM-MSCs 13 | Not Controlled | Autologous | Positive: CD90, CD105, CD73, CD44 Negative: CD34, CD45, CD11b, CD19, HLA-DR | 30.8, 30.4 | KOOS, MRI, others | 24 months |
| Chahal (2019) [23] | 12 (7:5) | 40-65 | Not mentioned | 3-4 | Prospective Clinical Trial | BM-MSCs 12 | Not Controlled | Autologous | Positive: CD90, CD105, CD73 Negative: CD45, CD34, CD19, CD14, HLA-DR | 1, 10, 50 | KOOS, WOMAC, WORMS, others | 24 months |
| Wells (2021) [16] | 10 (4:6) | 18–79 | Not mentioned | 1-2 | Prospective Clinical Trial | BMA-MSCs 11 | Not Controlled | Autologous | Positive: CD90, CD73, and CD105 Negative: CD19, CD34, CD45, CD11b, and HLA-DR | 9.9±1.2 / ml (without × 106) | KOOS, NRSP, others | 12 months |
| Mautner (2019) [33] | 76 (36:40) | 52-74 | Not mentioned | 1-4 | Retrospective Clinical Trial | BMAC 41, MFAT 35 | Not Controlled | Autologous | Not mentioned | BMAC 8 cc, MFAT 30 cc (without × 106) | KOOS, VAS, MRI, others | 6 months |
Risk of Bias Assessment
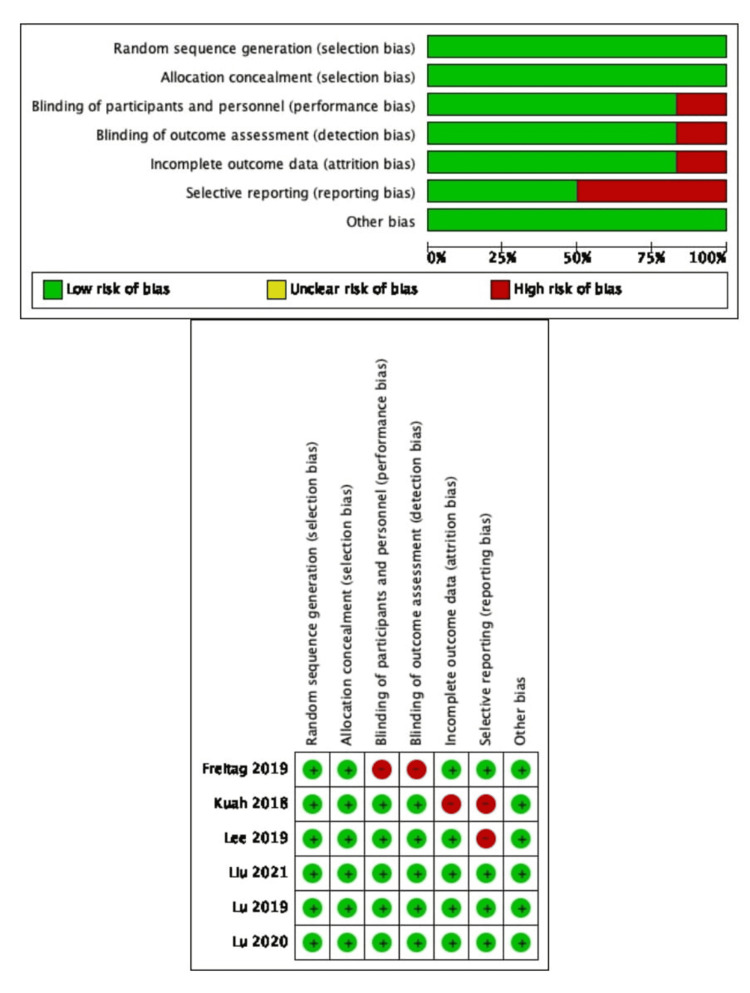
Figure 2 shows the results of the risk of bias evaluation for six studies [2,7,17,18,35,39], while table 2 shows the results of the NOS for four studies [16,23,32,33]. Lee et al. [39], although relevant images were drawn, we could not retrieve the original data and conduct the combined statistics; hence this study was classified as having a high risk of reporting bias. Freitag et al. and Kuah et al. incomplete data on overall WOMAC scores and subscales (pain, stiffness, and function) were also given, and one or more of these characteristics may have been missing. As a result, attrition bias was found to be considered a risk in these two investigations [2,35]. Freitag et al. performed BM or subcutaneous tissue extraction only in the intervention group. Even though moral restraint precluded the same measures from being used in the control group, this study was classified as having a high risk of detection and performance bias [35].
Table 2. Summary of the Newcastle Ottawa Scale for the included clinical trials studies.
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain
Figure 2. Summary of the risk of bias assessment for the RCT included studies.
Freitag (2019) [35], Kuah (2018) [2], Lee (2019) [39], Liu (2021) [7], Lu (2019) [17], and Lu (2020) [18]
Outcomes
Knee Injury and Osteoarthritis Outcome Score (KOOS): A total of seven studies [7,16,23,32,33,35,39] reported KOOS [40] at baseline and final follow-up in the intervention and the control groups, including 650 patients. Three studies [7,23,32] were followed up for 24 months, two studies [16,35] were followed up for 12 months, and two studies [33,39] were followed up for six months. Normalized KOOS was used to measure positive changes in all five primary areas, and all were significantly better at six, 12, and 24-months post first injection [32]. Significant improvements in Knee Injury and Osteoarthritis Outcome Score for Joint Replacement (KOOS-JR) scores were observed over time (F (4,12) =12.29, p<0.001) in a cohort. Following the procedure, clinical significance was accomplished at three, six, and 12-months following the procedure [16]. As evaluated by normalized KOOS, table 3 demonstrates the favorable changes in all five essential categories. All were much improved at six, 12, and 24 months after the first treatment [32]. Using all sample time points, the Sport Score and quality of life (QOL) score were nominally linked with an unadjusted p-value of 0.031 and 0.046, respectively [23].
Table 3. For 13 patients with KOA who were treated with BM-MSCs, a univariate analysis of normalized KOOS was performed.
KOA = knee osteoarthritis, BM-MSCs = bone marrow mesenchymal stem cells, KOOS = knee injury and osteoarthritis outcome score
| Normalized KOOS sections | Baseline (mean) | 6th-month follow-up (mean) | P-value | 1-year follow-up (mean) | P-value | 2 years follow-up (mean) | P-value |
| Symptoms | 67.300 | 912.308 | 0.000 | 89.9 | 0.000 | 88.7 | 0.000 |
| Pain | 62.585 | 890.538 | 0.000 | 89.7 | 0.000 | 89.4 | 0.000 |
| Daily life activity | 64.223 | 908.308 | 0.000 | 92.2 | 0.000 | 93 | 0.000 |
| Sport | 40.25 | 799.769 | 0.000 | 81.1 | 0.000 | 81.6 | 0.000 |
| Quality of life | 34.162 | 754.923 | 0.000 | 76.9 | 0.000 | 77.4 | 0.000 |
Magnetic Resonance Imaging (MRI) Evaluation
A total of eight studies reported MRI evaluation at baseline and follow-up in the groups, including 659 patients [2,7,17,18,23,32,33,39]. Three studies [7,23,32] were followed for a total of 24 months, for 12 months, two studies [2,17] were followed up on, and two studies [33,39] were followed up for six months after they were completed, and one study [18], had a 48-weeks follow-up period. The transformation of the central medial femorotibial compartment (cMFTC) cartilage thickness [41] for a 24-month was −0.32 mm (SD=0.40) for those who have narrowed medial tibiofemoral joint and maintained knee pain at baseline in comparison to the control neither of which radiographic nor pain development (−0.12mm, SD=0.28) [7,42]. 67 percent of patients had progressed cartilage degeneration within the control group, with another 56 percent having extended osteophyte formation. Only 30% of individuals saw additional cartilage loss in the one-injection group, whereas 50% experienced osteophyte development advancement at 12 months. In the two-injection group, 89 percent of participants had cartilage improvement or no progression in cartilage loss, indicating that OA had stabilized, as seen by 89 percent of subjects having no progression in osteophyte formation [35]. The size of the cartilage defect in the MSCs group did not change substantially on MRI at six months (p =.5803), but the size of the cartilage defect in the control group grew significantly (p =.0049). Furthermore, the change in cartilage defect following the injection was significantly different between the two groups (p =.0051) [39]. Using the WORMS technique, the low-dose group had a mean change from baseline of -0.36 and -0.86 in both the left and right knees at week 48. Furthermore, the mean changes in total cartilage volume, knee femur end cartilage volume and knee patellar cartilage volume in the low-dose group were 54.58, 38.63, and 39.69 mm³, respectively. The knee tibial end cartilage volume and knee cartilage volume in the medium-dose group improved by 243.32 and 34.44 mm³, respectively. Increases of -0.42 and 122.92 mm³ in the left knee WORMS and knee femur end cartilage volume were reported in the high-dose group [18].
Two bilateral intra-articular knee injections, three weeks apart (18-20 days), were used in this preclinical study with AlloJoin. Because the high prevalence of bilateral KOA in the treatment population was investigated [18,43,44]. MRI showed no significant change in cartilage thickness after six months. As indicated in Table 4, there was a considerable improvement in knee cartilage thickness in the femoral and tibia plates after 12 months [32]. Time 2 (T2) scores in the patella region increased by a negligible amount (p =.055 for a two-sided test, nonadjusted). T2 changes (from baseline to 12 months) did not differ across the one, 10, or 50 million BM-MSCs cohorts [23]. The 50 million BM-MSCs doses (effect estimate [B] = 1.828, p =.002) maintained synovitis at lower levels than the one million BM-MSCs dose, according to statistical analysis of the effects of dose adjusted for both time and baseline levels of synovitis [23]. We found a decrease in pro-inflammatory monocytes/macrophages in synovial fluid three months after MSCs infusion, suggesting a potential mechanism of action. We do not see statistical significance relative to baseline levels (p =.062) because of the small number of patients who presented synovial fluid at baseline and three months after MSC infusion (n = 5). However, this downregulation suggests a potential mechanism of action of MSCs in the arthritic joint [23].
Table 4. Changes in knee cartilage thickness after the first injection after 12 months.
Mean baseline in mm = 2.15; mean at 12 months in mm = 2.45
T = time, SD = standard deviation, SE = standard error, P-value = probability value
| Variable | Baseline (T1) | SD | SE | After 12 months of treatment (T2) | SD | SE | P-value (two-tailed) |
| Mean tibial plate thickness in mm | 2.15 | 0.67 | 0.076 | 2.38 | 0.63 | 0.072 | 0.000 |
| Mean femoral plate thickness in mm | 2.16 | 0.78 | 0.09 | 2.5 | 0.76 | 0.086 | 0.000 |
Visual Analogue Scale (VAS)
A total of five studies [2,17,18,33,39] reported VAS evaluation at baseline and follow-up in the groups, including 194 patients. Two studies [2,17] were followed up for 12 months, two studies [33,39] were followed up for six months, and one study [18] was followed up for 48 weeks. VAS≤32 [7], (P < .00001) [10], (p ≤ 0.005) in Progenza (PRG) combined group [2]. In the MSCs group exclusively, the VAS for knee discomfort dropped dramatically from 6.8 0.6 to 3.4 1.5 (p.001) [39]. Our VAS data confirmed clinical improvement with these cell injections, as seen by the study's reported VAS minimal clinical improvement differences (MCID) score of 30.0 mm [18,45,46].
Western Ontario, and McMaster Universities Osteoarthritis Index (WOMAC)
A total of six studies [2,17,18,23,35,39] reported WOMAC [47], evaluation at baseline, and follow-up in the groups, including 160 patients. Three studies [2,17,35] were tracked for 12 a year, one trial [23] was monitored for 24 months, one study [18] had a 48-weeks follow-up period, and for six months, one trial [39] was followed. (All P values were less than .05) [10]. Also, compared to the HA group, significantly more individuals had a 50% improvement in WOMAC, and after 12 months, the Re-Join® group had a 70% improvement rate, indicating that more patients were improving [17].
At six months after injection, a single injection of AD-MSCs resulted in a 55 percent reduction in the WOMAC total score, a 59 percent reduction in the WOMAC pain score, a 54 percent reduction in the WOMAC stiffness score, and a 54 percent reduction in the WOMAC physical function score [39]. According to a study in previous research [24,48-50], clinical outcomes improved six months following MSCs injection. The findings of this investigation support this. Furthermore, similar to earlier research [49,50], even six months following injection, the clinical outcomes were still good. This finding implies that with a single intra-articular MSCs injection, symptom alleviation can be sustained for up to six months [39]. Improvements in short form 36 (SF-36), -23.71 in WOMAC total, -17.14 in WOMAC-function, -2.29 in WOMAC stiffness, and -4.29 in WOMAC-pain were seen in the low-dose cohort. Improvements in left knee VAS were -2.25, right knee VAS was -2.13, WOMAC-total was -16.50, WOMAC-function was -11.88, WOMAC-stiffness was -1.71, and WOMAC-pain was -3.25 in the medium-dose cohort. The high-dose cohort observed statistically significant improvements in the left knee VAS of -1.36 and the right knee VAS of -2.07 [18]. The MCID averages for the WOMAC with KOA have been published [51]. The WOMAC functional score ranges between 9.1 to 19.9 mm, indicating that the WOMAC scores in this trial indicated considerable clinical improvement for the overall WOMAC functional (17.1) for both the left and right knees after 48 weeks for two of the doses [18,52-55].
Adverse Events (AEs)
A total of four studies [7,16,17,32] reported AEs evaluation at baseline and follow-up in the groups, including 550 patients. Two studies [7,32] were followed up for 24 months, and the others [16,17] were followed up for 12 months. Patient satisfaction was high (range: 8.1±2.1-8.8±1.9). All the patients said they would recommend the treatment to a friend, and 85 percent said they would do it again [16]. In the MSCs group, 10 (83%) patients experienced AEs, compared to seven (58%) individuals in the control group. No significant AEs or grade 4 or 5 AEs on the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) scale. All the grade 3 AEs on the NCI-CTCAE scale were arthralgia, which completely disappeared within three days [39,56]. In the low-, middle-and high-dose groups, the incidence of AEs was 71.42 percent (5/7), 87.50 percent (7/8), and 100 percent (7/7), correspondingly [18].
Discussions
We evaluated the clinical efficacy and safety of intra-articular injection of MSCs in this study by thoroughly analyzing six RCTs and four clinical trials. The study's first strength is its comprehensiveness, a compilation of all current high-quality studies. Second, we assessed the included studies' cell adherence, cell immunophenotype, and cell differentiation ability using the MSC criteria established by the Mesenchymal Stem Cell Committee of the International Society for Cell Therapy (ISCT), and discovered that half of them meet the minimum requirements [16,18,23,35,39], as shown in table 1. Third, it contains tight inclusion and exclusion rules. Concurrent therapy studies, such as HA and PRP were omitted. The addition of newly incorporated research of AT and BM sources, we believe, is what has led to the divergent results. This is one of the reasons we are so adamant about completing this research. Compared to the control group, the MSCs group showed a considerable increase in cartilage volume.
The selection of the appropriate donor source and the optimal dose has become an essential issue due to the extensive research into MSCstherapy. BM, AT, placenta, and umbilical cord are among the most popular donor sources for MSCs in clinical research. Initially, people preferred to cultivate and expand BM-MSCs. Later research discovered that AT was more accessible than BM, had a simpler isolation technique, a larger yield, and the same chondrogenic capacity [10,57,58].
A reduction in pain is connected to the ability of cells to release bioactive chemicals. These elements are hypothesized to change the inflammatory milieu in the joint from pro-inflammatory to anti-inflammatory. PRG includes a high concentration of these bioactive substances in the cell culture supernatant, unlike other cell therapies. PRG may decrease the progression of OA based on the favorable cartilage outcomes from preclinical and clinical investigations. Many studies have found that beneficial effects are primarily apparent in the lateral tibial region. Although OA affects the entire joint, it has been hypothesized that the medial tibiofemoral region is more severely damaged than the lateral tibiofemoral region. As a result, because the medial tibiofemoral region is later, there may be fewer opportunities to demonstrate progress [2].
MPCs tagged with fluorescent dye lasted locally in the joint for up to 10 weeks in preclinical rat studies before becoming undetectable [18,59]. Furthermore, the serious adverse events (SAEs) contradict all preclinical animal investigations that revealed no evidence of systemic exposure [18,59-61]. In addition, earlier research has shown that Re-Join® is beneficial in rabbit and sheep models of OA [17,60,61]. The repair of osteoarthritis in rabbits and goats appears to be mediated by paracrine effects involving the stimulation of endogenous repair systems [26,32]. In a systematic evaluation of MSCs therapies, Lalu et al. found no significant side effects [23,62,63]. Following the aspiration of BM, there were no systemic side effects observed, and there were no issues that were noted [23]. Therefore, no individuals dropped out of the study [2].
Our findings show that there are statistically significant improvements in pain and function [2,7,10,16-18,33,35]. The average percentage of patients who have passed the Patient-Acceptable Symptom State (PASS) [64] the threshold was 35% in the placebo cohort(ranging from 33.1 to 35.5) and 48% in the intervention cohorts (varying between 42.2% to 56.1%) [7,65,66]. There were also decreases in present, typical, best, and worst numerical rating scale (NRS) pain [67], scores statistically significant over time (F(4,12)=14.5, p<0.001; F(4,12)=17.5, p<0.001; F(4,12)=2.9, p=0.003; and F(4,12)=35.5, p<0.001, respectively) [16]. Also, NRS pain in both the single and two injection protocol treatment groups, when compared to baseline, within-group improvement was statistically significant (0.05) at all time intervals [35]. Therefore, we found that all statistical tests for pain and functional outcome measures (n = 21) had a mean power of 0.877 15 SD [35]. The NPRS improved by 69 percent from baseline to the last follow-up at 12 months in both therapy groups. In comparison, arthroscopic debridement resulted in a 14 percent improvement in pain scores after 12 months, while a prescribed exercise regimen resulted in a 12 percent improvement in pain scores [35,68,69]. The range of motion in the MSCs group improved considerably from 127.9 10.3 to 134.6 12.5 at six months after injection (p =.0299) [39]. When these established MCID values were applied at 48 weeks, there was a reduction in pain and an improvement in knee function; however, due to the small number of participants included in this pilot investigation, these findings should be regarded with caution [18].
In addition, they discovered a link between the number of cells injected and pain relief [33]. Furthermore, two RCTs were recently reported, revealing significant improvements in pain and function in KOA patients after injection of autologous AD-MSCs versus controls [33]. MSCs generated from autologous BM showed a significant increase in clinical ratings [33,39]. Because the researchers differ in study design, cell type, supplementary therapy, and rehabilitation methods, it is difficult to determine the true differences in intra-articular injections of BM-MSCs and AD-MSCs [39].
Data reveal that one or more outcomes, such as KOOS pain, have improved statistically significantly [23,32,35], symptoms, SF-36 [18], VAS [2,10,16,18,33,39], and QOL scores [17,23,33], as well as WOMAC stiffness [2,10,16-18,23,33,35,39]. NPRS improved [16,35], from baseline to final follow-up at 12 months, by a percentage of 69 percent previous clinical trials have shown that intra-articular MSCs treatment can slow the course of OA [35]. All symptoms decreased dramatically, resulting in a considerable improvement in the quality of life of these grade 2 to 4 KOA patients. There is also evidence of safety. However, more research is required. Another concern is that most research focuses on short-term safety rather than long-term results [32]. Starting three months after the procedure, KOOS-JR scores improved dramatically, with clinically meaningful improvements lasting 12 months [16]. Within 48 weeks of follow-up, MCID scores for SF-36 are approximately 10%, which this study's data has surpassed [18,53,70,71]. Both groups improved significantly in Emory Quality of Life (EQOL), VAS, and all KOOS indicators pre-and post-procedure (p < .001) [33]. During follow-up, the two treatment groups' EQOL ratings altered in similar ways (similar temporal patterns across time) (p =0.98, test for interaction between time on study and treatment group) [33].
We report putative chondroprotective benefits and decreased synovial inflammation, with the 50 million cell dosage potentially being more beneficial. However, when compared to the 50 million and/or 10 million BM-MSC dosages, serum carboxy-terminus of the three-quarter peptide from cleavage of C I and C II (C1, C2), urine type II collagen cleavage neoepitope (C2C), and C-telopeptide of type II collagen (CTX-II) all increased significantly, suggesting a chondroprotective MSCs dose effect, as previously described [23]. Furthermore, exploratory MRI analyses of average cartilage volumes and average WORMS from baseline at week 48 revealed no change in the medium-dose (2*107 cells) and high-dose (5*107 cells) groups but an improvement in the low-dose AlloJoin (1*107 cells) group [18]. Over radiography x-rays, MRI assessments offered a more accurate picture of articular cartilage deterioration and change in location of the menisci [18,72]. Because MOAKS [73] is a semi-quantitative metric, the MRI analysis is limited [18]. Furthermore, MOAKs analysis demonstrating effective stabilization despite continuous bone marrow lesions (BMLs)contrasts with previous research that has found a link between BMLs and OA progression [35].
Because Orozco et al. showed a consistent improvement in cartilage quality during a two-year follow-up period from the baseline, we expect cartilage improvement in our series over a longer follow-up time [39,48]. Our research also saw increased cartilage volume and quality [2,17,18,23,32,39]. Furthermore, an MRI examination at 48 weeks revealed no signs of ectopic bone development [18]. Intra-articular injections of Re-Join® were found to enhance cartilage volume, with a significant rise 12 months after injection, suggesting that this could be a viable therapeutic intervention and cartilage regeneration for OA patients [17].
We believe that the subsequent trials should be greater [23]. The following trials should, in our opinion, be larger [18] and also look at the MSCs dose and the MSCs source. The safety of allogeneic MSCs for KOA must be established [23,32,39]. The usage of allogenic MSCs can be standardized, the dose can be more precisely regulated, and cell variability may be minimized. We should also examine the efficacy of BM and AD-derived orthobiologics treatments to develop a reliable judgment on which is the better choice for treating KOA [33]. MSCs, we feel, has the potential to be a definitive treatment for KOA [32]. It is also critical to distinguish the findings of this study from those of previous studies that used more various cell-based products, such as stromal vascular fraction [35].
This research has several limitations. The results should be treated with care first and foremost. We did our utmost to avoid simultaneous surgical treatment affecting efficacy. Second, all the studies we looked at used intra-articular injections. MSCs implantation by open or arthroscopic surgery has been proven to be more conducive to cartilage repair in several studies. While MSCs transplantation on a scaffold may help rebuild the anterior cruciate ligament and meniscus [10]. Third, four of our studies [16,23,32,33], were not RCTs. Fourth, we included three studies [23,33,39] that included KL grade 4 KOA patients. We do not know if the disease can be slowed or even reversed at this point in the disease's progression, especially using autologous-derived MSCs. Furthermore, as the human body ages, MSCs' ability to self-renew and differentiate decreases; particularly, the potential of MSCs in individuals with OA is lower than that of healthy persons [10,17,23,33,35].
Conclusions
Our findings suggest that intra-articular injection of MSCs can reduce pain and enhance function in patients with KOA in a short period while also being relatively safe, even in the late stages of OA of the knee. Although there is inadequate evidence to suggest that MSCs may heal cartilage abnormalities at this time, we have reason to believe that they protect cartilage and slow down the deterioration of articular cartilage. These findings show that MSCs treatment has a bright future, but additional research and more homogeneous RCTs are needed to confirm it.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Injective mesenchymal stem cell-based treatments for knee osteoarthritis: from mechanisms of action to current clinical evidences. Lopa S, Colombini A, Moretti M, de Girolamo L. Knee Surg Sports Traumatol Arthrosc. 2019;27:2003–2020. doi: 10.1007/s00167-018-5118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. Kuah D, Sivell S, Longworth T, et al. J Transl Med. 2018;16:49. doi: 10.1186/s12967-018-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The impact of arthritis on pain and quality of life: an Australian survey. Hunter DJ, Riordan EA. Int J Rheum Dis. 2014;17:149–155. doi: 10.1111/1756-185X.12232. [DOI] [PubMed] [Google Scholar]
- 4.Osteoarthritis. Glyn-Jones S, Palmer A, Agricola R, et al. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 5.Burden of major musculoskeletal conditions. Woolf AD, Pfleger B. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2572542/ Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 6.Health economics in the field of osteoarthritis: an expert's consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Hiligsmann M, Cooper C, Arden N, et al. Semin Arthritis Rheum. 2013;43:303–313. doi: 10.1016/j.semarthrit.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Efficacy and cost-effectiveness of Stem Cell injections for symptomatic relief and strUctural improvement in people with Tibiofemoral knee OsteoaRthritis: protocol for a randomised placebo-controlled trial (the SCUlpTOR trial) Liu X, Robbins S, Wang X, et al. BMJ Open. 2021;11:0. doi: 10.1136/bmjopen-2021-056382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Current evidence for osteoarthritis treatments. Anandacoomarasamy A, March L. Ther Adv Musculoskelet Dis. 2010;2:17–28. doi: 10.1177/1759720X09359889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. Paxton EW, Namba RS, Maletis GB, et al. J Bone Joint Surg Am. 2010;92 Suppl 2:117–132. doi: 10.2106/JBJS.J.00807. [DOI] [PubMed] [Google Scholar]
- 10.Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Ma W, Liu C, Wang S, Xu H, Sun H, Fan X. Medicine (Baltimore) 2020;99:0. doi: 10.1097/MD.0000000000023343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Extracorporeal shockwave therapy for the treatment of knee osteoarthritis: a meta-analysis. Hsieh CK, Chang CJ, Liu ZW, Tai TW. Int Orthop. 2020;44:877–884. doi: 10.1007/s00264-020-04489-x. [DOI] [PubMed] [Google Scholar]
- 12.Stem cell injections in knee osteoarthritis: a systematic review of the literature. Pas HI, Winters M, Haisma HJ, Koenis MJ, Tol JL, Moen MH. Br J Sports Med. 2017;51:1125–1133. doi: 10.1136/bjsports-2016-096793. [DOI] [PubMed] [Google Scholar]
- 13.The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition. Jevsevar DS, Brown GA, Jones DL, et al. J Bone Joint Surg Am. 2013;95:1885–1886. doi: 10.2106/00004623-201310160-00010. [DOI] [PubMed] [Google Scholar]
- 14.Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. Harirforoosh S, Asghar W, Jamali F. J Pharm Pharm Sci. 2013;16:821–847. doi: 10.18433/j3vw2f. [DOI] [PubMed] [Google Scholar]
- 15.Individual nonsteroidal antiinflammatory drugs and other risk factors for upper gastrointestinal bleeding and perforation. Gutthann SP, García Rodríguez LA, Raiford DS. https://pubmed.ncbi.nlm.nih.gov/9116088/ Epidemiology. 1997;8:18–24. doi: 10.1097/00001648-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Cellular and clinical analyses of autologous bone marrow aspirate injectate for knee osteoarthritis: a pilot study. Wells K, Klein M, Hurwitz N, et al. PM&R. 2021;13:387–396. doi: 10.1002/pmrj.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Lu L, Dai C, Zhang Z, et al. Stem Cell Res Ther. 2019;10:143. doi: 10.1186/s13287-019-1248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intra-articular injections of allogeneic human adipose-derived mesenchymal progenitor cells in patients with symptomatic bilateral knee osteoarthritis: a phase I pilot study. Lu L, Dai C, Du H, et al. Regen Med. 2020;15:1625–1636. doi: 10.2217/rme-2019-0106. [DOI] [PubMed] [Google Scholar]
- 19.Intra-articular mesenchymal stem cell therapy for the human joint: a systematic review. McIntyre JA, Jones IA, Han B, Vangsness CT Jr. Am J Sports Med. 2018;46:3550–3563. doi: 10.1177/0363546517735844. [DOI] [PubMed] [Google Scholar]
- 20.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Global Burden of Disease Study 2013 Collaborators. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Manferdini C, Maumus M, Gabusi E, et al. Arthritis Rheum. 2013;65:1271–1281. doi: 10.1002/art.37908. [DOI] [PubMed] [Google Scholar]
- 22.Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Wang Y, Yuan M, Guo QY, Lu SB, Peng J. Cell Transplant. 2015;24:1661–1678. doi: 10.3727/096368914X683485. [DOI] [PubMed] [Google Scholar]
- 23.Bone marrow mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Chahal J, Gómez-Aristizábal A, Shestopaloff K, et al. Stem Cells Transl Med. 2019;8:746–757. doi: 10.1002/sctm.18-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Jo CH, Lee YG, Shin WH, et al. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 25.Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Stem Cells. 2017;35:256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
- 26.Stem cell therapy in a caprine model of osteoarthritis. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Arthritis Rheum. 2003;48:3464–3474. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 27.Mesenchymal stem cells for cartilage repair in osteoarthritis. Gupta PK, Das AK, Chullikana A, Majumdar AS. Stem Cell Res Ther. 2012;3:25. doi: 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Stem Cells. 2007;25:2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 29.Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Horie M, Choi H, Lee RH, et al. Osteoarthr Cartil. 2012;20:1197–1207. doi: 10.1016/j.joca.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Use of a chronic model of articular cartilage and meniscal injury for the assessment of long-term effects after autologous mesenchymal stromal cell treatment in sheep. Caminal M, Fonseca C, Peris D, et al. N Biotechnol. 2014;31:492–498. doi: 10.1016/j.nbt.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Stem Cells Transl Med. 2017;6:613–621. doi: 10.5966/sctm.2016-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. Al-Najar M, Khalil H, Al-Ajlouni J, et al. J Orthop Surg Res. 2017;12:190. doi: 10.1186/s13018-017-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Functional outcomes following microfragmented adipose tissue versus bone marrow aspirate concentrate injections for symptomatic knee osteoarthritis. Mautner K, Bowers R, Easley K, Fausel Z, Robinson R. Stem Cells Transl Med. 2019;8:1149–1156. doi: 10.1002/sctm.18-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A multi-center analysis of adverse events among two thousand, three hundred and seventy two adult patients undergoing adult autologous stem cell therapy for orthopaedic conditions. Centeno CJ, Al-Sayegh H, Freeman MD, Smith J, Murrell WD, Bubnov R. Int Orthop. 2016;40:1755–1765. doi: 10.1007/s00264-016-3162-y. [DOI] [PubMed] [Google Scholar]
- 35.Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Freitag J, Bates D, Wickham J, et al. Regen Med. 2019;14:213–230. doi: 10.2217/rme-2018-0161. [DOI] [PubMed] [Google Scholar]
- 36.Safety of cell therapy with mesenchymal stromal cells (MSCs): a systematic review. Lalu MM, McIntyre L, Pugliese C, Stewart DJ. The Annals of the American Thoracic Society Journal (ATS Journal) 2010;49:0. [Google Scholar]
- 37.Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Peeters CM, Leijs MJ, Reijman M, van Osch GJ, Bos PK. Osteoarthritis Cartilage. 2013;21:1465–1473. doi: 10.1016/j.joca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 38.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Page MJ, McKenzie JE, Bossuyt PM, et al. BMJ. 2021;372:0. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase iib, randomized, placebo-controlled clinical trial. Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Stem Cells Transl Med. 2019;8:504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The knee injury and osteoarthritis outcome score (KOOS): from joint injury to osteoarthritis. Roos EM, Lohmander LS. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Wirth W, Hellio Le Graverand MP, Wyman BT, et al. Osteoarthr Cartil. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brief report: cartilage thickness change as an imaging biomarker of knee osteoarthritis progression: data from the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Eckstein F, Collins JE, Nevitt MC, et al. Arthritis Rheumatol. 2015;67:3184–3189. doi: 10.1002/art.39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: the Beijing osteoarthritis study. Zhang Y, Xu L, Nevitt MC, Aliabadi P, Yu W, Qin M. Arthritis Rheumatol. 2001;44:2065–2071. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 44.The high prevalence of knee osteoarthritis in a rural Chinese population: the Wuchuan osteoarthritis study. Kang X, Fransen M, Zhang Y, et al. Arthritis Rheum. 2009;61:641–647. doi: 10.1002/art.24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Establishing minimum clinically important difference values for the Patient-Reported Outcomes Measurement Information System Physical Function, hip disability and osteoarthritis outcome score for joint reconstruction, and knee injury and osteoarthritis outcome score for joint reconstruction in orthopaedics. Hung M, Bounsanga J, Voss MW, Saltzman CL. World J Orthop. 2018;9:41–49. doi: 10.5312/wjo.v9.i3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.How much pain is significant? defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. Danoff JR, Goel R, Sutton R, Maltenfort MG, Austin MS. J Arthroplasty. 2018;33:0. doi: 10.1016/j.arth.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 47.Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. https://pubmed.ncbi.nlm.nih.gov/3068365/ J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 48.Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Orozco L, Munar A, Soler R, et al. Transplantation. 2013;95:1535–1541. doi: 10.1097/TP.0b013e318291a2da. [DOI] [PubMed] [Google Scholar]
- 49.Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Vega A, Martín-Ferrero MA, Del Canto F, et al. Transplantation. 2015;99:1681–1690. doi: 10.1097/TP.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 50.Treatment of knee osteoarthritis with autologous mesenchymal stem cells: two-year follow-up results. Orozco L, Munar A, Soler R, et al. Transplantation. 2014;97:0–8. doi: 10.1097/TP.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 51.Definition of nonresponse to analgesic treatment of arthritic pain: an analytical literature review of the smallest detectable difference, the minimal detectable change, and the minimal clinically important difference on the pain visual analog scale. Stauffer ME, Taylor SD, Watson DJ, Peloso PM, Morrison A. Int J Inflam. 2011;2011:231926. doi: 10.4061/2011/231926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. https://pubmed.ncbi.nlm.nih.gov/11093446/ J Rheumatol. 2000;27:2635–2641. [PubMed] [Google Scholar]
- 53.Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. Angst F, Aeschlimann A, Michel BA, Stucki G. https://pubmed.ncbi.nlm.nih.gov/11824949/ J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- 54.A systematic review of estimates of the minimal clinically important difference and patient acceptable symptom state of the Western Ontario and McMaster Universities Osteoarthritis Index in patients who underwent total hip and total knee replacement. MacKay C, Clements N, Wong R, Davis AM. Osteoarthritis Cartilage. 2019;27:1408–1419. doi: 10.1016/j.joca.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Tubach F, Ravaud P, Baron G, et al. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Basch E, Iasonos A, McDonough T, et al. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 57.Evaluating the current literature on treatments containing adipose-derived stem cells for osteoarthritis: a progress update. Ranmuthu CD, Ranmuthu CK, Khan WS. Curr Rheumatol Rep. 2018;20:67. doi: 10.1007/s11926-018-0776-7. [DOI] [PubMed] [Google Scholar]
- 58.Osteoarthritis and stem cell therapy in humans: a systematic review. Jevotovsky DS, Alfonso AR, Einhorn TA, Chiu ES. Osteoarthr Cartil. 2018;26:711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
- 59.In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Li M, Luo X, Lv X, et al. Stem Cell Res Ther. 2016;7:160. doi: 10.1186/s13287-016-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Feng C, Luo X, He N, et al. Tissue Eng Part A. 2018;24:219–233. doi: 10.1089/ten.TEA.2017.0039. [DOI] [PubMed] [Google Scholar]
- 61.Human adipose-derived mesenchymal progenitor cells engraft into rabbit articular cartilage. Wang W, He N, Feng C, et al. Int J Mol Sci. 2015;16:12076–12091. doi: 10.3390/ijms160612076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Pers YM, Rackwitz L, Ferreira R, et al. Stem Cells Transl Med. 2016;5:847–856. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. Lalu MM, McIntyre L, Pugliese C, et al. PLoS One. 2012;7:0. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evaluation of clinically relevant states in patient reported outcomes in knee and hip osteoarthritis: the patient acceptable symptom state. Tubach F, Ravaud P, Baron G, et al. Ann Rheum Dis. 2005;64:34–37. doi: 10.1136/ard.2004.023028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clinically relevant outcomes based on analysis of pooled data from 2 trials of duloxetine in patients with knee osteoarthritis. Hochberg MC, Wohlreich M, Gaynor P, Hanna S, Risser R. J Rheumatol. 2012;39:352–358. doi: 10.3899/jrheum.110307. [DOI] [PubMed] [Google Scholar]
- 66.Evaluation of the patient acceptable symptom state in a pooled analysis of two multicentre, randomised, double-blind, placebo-controlled studies evaluating lumiracoxib and celecoxib in patients with osteoarthritis. Dougados M, Moore A, Yu S, Gitton X. Arthritis Res Ther. 2007;9:0. doi: 10.1186/ar2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Dworkin RH, Turk DC, Farrar JT, et al. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 68.A controlled trial of arthroscopic surgery for osteoarthritis of the knee. Moseley JB, O'Malley K, Petersen NJ, et al. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 69.Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. Thomas KS, Muir KR, Doherty M, Jones AC, O'Reilly SC, Bassey EJ. BMJ. 2002;325:752. doi: 10.1136/bmj.325.7367.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Multidimensional minimal clinically important differences in knee osteoarthritis after comprehensive rehabilitation: a prospective evaluation from the Bad Zurzach Osteoarthritis Study. Angst F, Benz T, Lehmann S, Aeschlimann A, Angst J. RMD Open. 2018;4:0. doi: 10.1136/rmdopen-2018-000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.The minimal clinically important difference for Knee Society Clinical Rating System after total knee arthroplasty for primary osteoarthritis. Lee WC, Kwan YH, Chong HC, Yeo SJ. Knee Surg Sports Traumatol Arthrosc. 2017;25:3354–3359. doi: 10.1007/s00167-016-4208-9. [DOI] [PubMed] [Google Scholar]
- 72.MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. Nat Rev Rheumatol. 2013;9:236–251. doi: 10.1038/nrrheum.2012.223. [DOI] [PubMed] [Google Scholar]
- 73.How to define subregional osteoarthritis progression using semi-quantitative MRI osteoarthritis knee score (MOAKS) Runhaar J, Schiphof D, van Meer B, Reijman M, Bierma-Zeinstra SM, Oei EH. Osteoarthritis Cartilage. 2014;22:1533–1536. doi: 10.1016/j.joca.2014.06.022. [DOI] [PubMed] [Google Scholar]