Correction to: Journal of Molecular Medicine https://doi.org/10.1007/s00109-021–02124-9
In the published original paper, Fig. 1 is incomplete. Only Fig. 1a is shown. This erratum corrects Fig. 1 and adds the missing panel 1 b-f to the article.
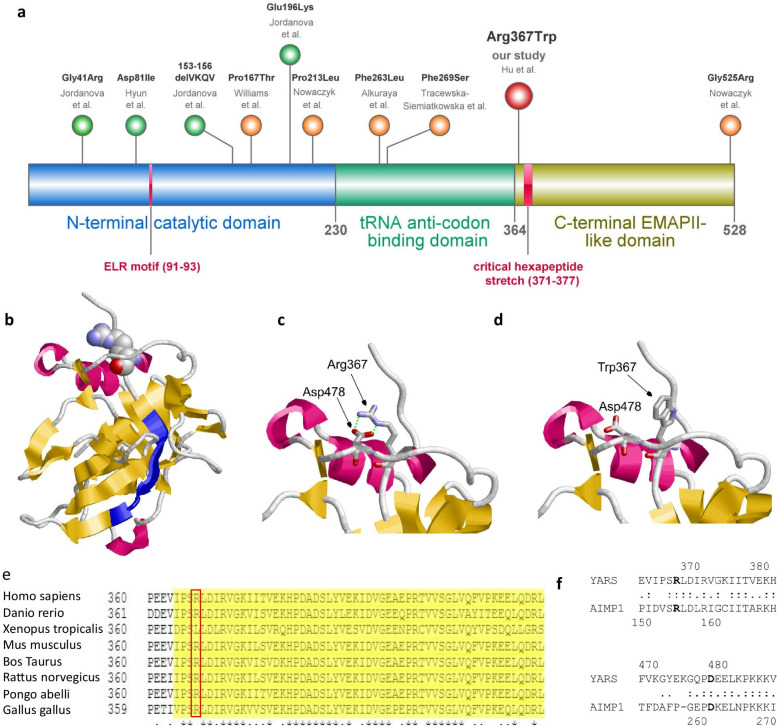
Fig. 1.
(a) Organization of the three domains of human TyrRS and biallelic variants reported in the literature (orange dots). Three domains: (i) The catalytic N-terminal domain is essential for aminoacylation of tRNA. As a monomeric fragment, the N-terminal domain has cytokine activity and the ELR motif is critical for this activity. (ii) tRNA anti-codon binding domain (iii) The C-terminal EMAP-II-like domain that was shown to be dispensable for aminoacylation. The heptapeptide sequence within this domain is critical for the cytokine activity. The homozygous variants p.(Pro167Thr) and p.(Pro213Leu) reside in the catalytic domain, harboring to the critical ELR motif, while most of the other variants reside outside this domain (orange dots). All five heterozygous mutations causing Charcot-Marie-Tooth neuropathy reside in the catalytic domain (green dots). (b) Structure of the C-terminal domain of TyrRS shown as backbone representation indicating the elements of secondary structure. Arg367 is shown in space-filled presentation and colored according to the atom types. The heptapeptide sequence stretch critical for the cytokine activity (residues 371–377) is shown in blue. (c) Structural role of Arg367 in TyrRS: In the wildtype, Arg367 forms a salt-bridge to Asp478 (indicated by green dotted lines). (d) In the p.(Arg367Trp) mutant, the bulky uncharged tryptophan cannot form an electrostatic interaction resulting in domain destabilization. (c + d) Arg367/Asp478 are shown in stick presentation. (e) Multi-species amino acid alignment indicates that Arg367 is highly conserved in mammals and down to zebrafish (danio rerio), but not in the western clawed frog (xenopus tropicalis) [1] (f) Amino acid alignment of the C-terminal EMAP-II-like domain of TyrRS compared to AIMP1. The corresponding residues Arg367 and Asp478 are conserved in both domains. TyrRS, tyrosine tRNA synthetase. AIMP1, Aminoacyl tRNA Synthetase Complex Interacting Multifunctional Protein 1
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UniProt C. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]