Background:
Renal denervation (RDN) lowers blood pressure (BP), but BP response is variable in individual patients. We investigated whether measures of pulsatile hemodynamics, obtained during 24-hour ambulatory BP monitoring, predict BP drop following RDN.
Methods:
From the randomized, sham-controlled SPYRAL HTN-OFF MED Pivotal trial, we performed a post hoc analysis of BP waveforms from 111 RDN patients and 111 sham controls, obtained with a brachial cuff-based sphygmomanometer. Waveforms were acquired during ambulatory BP monitoring at diastolic BP level and processed with validated ARCSolver algorithms to derive hemodynamic parameters (augmentation index; augmentation pressure; backward and forward wave amplitude; estimated aortic pulse wave velocity). We investigated the relationship between averaged 24-hour values at baseline and the change in 24-hour BP at 3 months in RDN patients, corrected for observed trends in the sham group.
Results:
There was a consistent inverse relationship between baseline augmentation index/augmentation pressure/backward wave amplitude/forward wave amplitude/estimated aortic pulse wave velocity and BP response to RDN: the decrease in 24-hour systolic BP/diastolic BP was 7.8/5.9 (augmentation index), 8.0/6.3 (augmentation pressure), 6.7/5.4 (backward wave amplitude), 5.7/4.7 (forward wave amplitude), and 7.8/5.2 (estimated aortic pulse wave velocity) mm Hg greater for patients below versus above the respective median value (P<0.001 for all comparisons, respectively). Taking augmentation index/augmentation pressure/backward wave amplitude/forward wave amplitude/estimated aortic pulse wave velocity into account, a favorable BP response following RDN, defined as a drop in 24-hour systolic blood pressure of ≥5 mm Hg, could be predicted with an area under the curve of 0.70/0.74/0.70/0.65/0.62 (P<0.001 for all, respectively).
Conclusions:
These results suggest that pulsatile hemodynamics, obtained during 24-hour ambulatory BP monitoring, may predict BP response to RDN.
Keywords: area under the curve, blood pressure, denervation, heart rate, sphygmomanometer
Novelty and Relevance.
What Is New?
Measures of pulsatile hemodynamics are associated with blood pressure response after RF-based renal denervation. Specifically, 24-hour systolic blood pressure drop at 3 months following renal denervation was up to 8 mm Hg greater with baseline augmentation index/pressure augmentation/backward wave amplitude/forward wave amplitude/estimated aortic pulse wave velocity below the median, as compared with above the median.
What Is Relevant?
These measures can be derived from automated analysis of 24-hour blood pressure waveforms with commercially available 24-hour blood pressure monitors and predict a favourable blood pressure response to renal denervation with area under the curves in the range of 0.70 to 0.74 (for measures of wave reflections).
Clinical/Pathophysiological Implications
Automated measurement of pulsatile hemodynamics, particularly wave reflections, obtained during the routine workup before renal denervation may help selecting patients with a favourable blood pressure response to renal denervation. Additional studies are needed to confirm the findings and to expand the results to patients treated with antihypertensive drugs.
A series of well-conducted, randomized, sham-controlled clinical trials has established the blood pressure (BP) lowering effect of catheter-based renal denervation (RDN).1–3 However, mirroring the situation with antihypertensive drugs,4 the magnitude of the individual BP drop following RDN is highly variable.1–3 A number of factors may influence the short- and long-term treatment effect, for example, the variable contribution of the sympathetic nervous system to BP elevation, genetic background, comorbidities, or accompanying antihypertensive treatments. Therefore, a simple, noninvasive predictor of BP response to RDN remains a major unmet need. Multiple indices of increased sympathetic nervous system activity or reduced arterial stiffness have been proposed, although the only consistent finding has been the association between a higher baseline BP and a larger BP drop following the intervention, which is rather nonspecific for both device and pharmaceutical therapies.
Nighttime systolic BP measured by 24-hour ambulatory BP monitoring (ABPM) and its variability have been shown to predict BP response following ultrasound-based RDN, albeit with low sensitivity.5 Beyond BP measured at the brachial artery, other hemodynamic measures may play a role in identifying appropriate candidates for RDN. Often summarized as pulsatile hemodynamics, arterial stiffness (pulse wave velocity [PWV]), wave reflections, and central (aortic) hemodynamics can be quantified noninvasively, easily, and reproducibly.6,7 Arterial wave reflections predict cardiovascular events,8–11 independent of brachial BP, and may be more sensitive than brachial BP to detect antihypertensive drug-induced hemodynamic changes.12,13 Recently, assessment of pulsatile hemodynamics with dedicated brachial cuffs, suitable for 24-hour ABPM, became commercially available.14,15 We hypothesized that hemodynamic measures, particularly wave reflections, obtained during regular 24-hour ABPM in the workup before RDN, may predict BP response to RDN.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Medtronic at sandeep.brar@medtronic.com.
Study Design and Randomization
SPYRAL HTN-OFF MED Pivotal16 was a multicenter, single-blind, randomized, sham-controlled trial conducted at 44 sites in Australia, Austria, Canada, Germany, Greece, Ireland, Japan, the United Kingdom, and the United States. Adult patients (age 20–80 years) with office systolic BP (SBP) ≥150 mm Hg and <180 mm Hg, office diastolic BP (DBP) ≥90 mm Hg, and an average 24-hour ambulatory SBP ≥140 mm Hg and <170 mm Hg were enrolled in the trial. Twenty-four-hour ABPM was considered valid if at least 21 day-time readings and 12 night-time readings had been recorded and was performed at baseline and at 3 months. The trials complied with the Declaration of Helsinki, all local ethics committees approved the research protocols, and all patients provided written informed consent. T. Weber, B. Hametner, C.M. Mayer, and S. Wassertheurer had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Full details of the randomization strategy have been described previously.16 Briefly, patients were randomized 1:1 to RDN or sham procedure. Before randomization, patients were required to be off all antihypertensive medications. Tandem high performance liquid chromatography and mass spectroscopy of urine and plasma by an independent laboratory were used to evaluate and confirm absence of antihypertensive medications.17 Office BP measurements were obtained via automatic BP monitor (Omron, Omron Healthcare, Inc, Lake Forest, IL).
Procedures
Ablation treatment with the Symplicity Spyral multielectrode catheter (Medtronic, Galway, Ireland) and the Symplicity G3 (Medtronic, Minneapolis, MN) generator was performed using a standardized approach of targeting all accessible renal arterial vessels, including branch vessels and accessory arteries with a diameter >3 mm to <8 mm.1,16 The sham procedure consisted of a renal angiogram only. Patients remained off antihypertensive medications until the primary end point at 3 months, unless there were safety concerns related to uncontrolled hypertension.
Measurement of Pulsatile Hemodynamics
Twenty-four-hour ABPM, providing brachial SBP and DBP, as well as heart rate (HR), was performed in all study participants with an identical automated brachial cuff-based oscillometric device (Mobil-O-Graph PWA, IEM, Stolberg, Germany), following published recommendations.18 The device has been validated in adults for 24-hour HR,19 for brachial BP measurement according to recommendations of the British Hypertension Society20 and the European Society of Hypertension,21 for 24-hour brachial ABPM22 against a widely used device, and has received clearance from the US Food and Drug Administration and bears the Conformité Européenne mark.
The ARCSolver algorithm for assessment of aortic SBP with the device has been published and validated invasively against high-fidelity pressure measurements15 and fluid-filled catheter-based measurements.23 Briefly, immediately after the conventional brachial oscillometric BP measurement, pulse waves are recorded, using the brachial cuff, at DBP level for ≈10 seconds, using a high-fidelity pressure sensor (MPX5050, Freescale Inc., Tempe, AZ). The sensor is connected to a 12-bit A/D converter by means of an active analogue band bass filter. After digitalization, a 3-step quality-control algorithm is applied.15 The recorded brachial pulse wave is calibrated with measured brachial BP. Thereafter, an aortic pulse waveform is generated by means of a generalized transfer function. Modulus and phase characteristics of the transfer function have been published.24 Parameters associated with wave reflection, derived by mathematical analysis directly from pressure curves (pulse waveform analysis), are augmented pressure (the increase in BP following the inflection point of the BP curve) and augmentation index (AIx; the ratio pressure augmentation [AP]/cPP). As AIx is inversely related to HR, a normalization for HR 75/min can be used (AIx75). A complimentary method to quantify wave reflections is Wave Separation Analysis,25 a method using simultaneous calculation of pressure and flow waves, yielding estimates of the amplitudes of antegrade and reflected (forward: Pf; backward: Pb) waves—Figure 1. Finally, an estimate of aortic PWV (ePWV), based on age, SBP and waveform characteristics, is determined.26 A more detailed description is given in the Supplemental Material. Both sets of parameters, measured with the Mobil-O-Graph PWA device, have been validated against accepted gold standards.10,24 The reproducibility and the feasibility of ambulatory hemodynamic measurements with the device have also been confirmed.27 To allow a standardized procedure, all analyses were performed centralized using ARCSolver version DLL 1.7.2.
Figure 1.
Quantification of wave reflections from brachial cuff-based waveforms. Upper: Example of high-quality pressure waveforms, obtained with a brachial cuff. Lower: Using pulse waveform analysis, an inflection point is identified, and pressure augmentation (AP) is determined; augmentation index (AIx) is calculated as AP/central pulse pressure (PP). Using combined analysis of pressure and flow signals (derived from validated flow models), wave separation is performed, yielding amplitudes of forward (Pf) and backward (Pb) waves.
Statistical Analysis
All valid ambulatory BP measurements were included in the analysis based on the snapshot dated January 2020. Only study participants with available full pressure waveform data from 24-hour ABPM could be included.
Enrollment in the SPYRAL trial was not stratified per baseline pulsatile hemodynamics. Baseline continuous variables were summarized as mean±SD and compared using t tests or Welch test, as appropriate. Categorical variables were summarized as counts and percentages and compared between groups using χ2 or Fisher exact tests for categorical variables. For all parameters, 24-hour averages per patient were calculated. Average 24-hour BP changes from baseline to 3 months in each RDN and sham patient were compared, using paired t tests (within the group) and unpaired t tests (RDN versus sham).
The aim of this post hoc analysis of the SPYRAL HTN-OFF MED Pivotal trial was to investigate, whether pulsatile hemodynamics, in particular measures of wave reflections (AIx, AIx75, AP, Pb), can predict changes in 24-hour BP from baseline to 3 months after RDN. Due to the sham-controlled design of the studies, the BP changes due to the sham effect could be taken into account, which gave us the opportunity to investigate true effects of the intervention.
Linear regression was used to investigate the relationship between a baseline parameter and BP changes at 3 months in the sham group. When the baseline parameter values are replaced by the residuals from the regression line and the mean value of the parameter is added, the trends in the sham group can be removed (Figure S1). To account for this effect obtained from the sham group and the RDN group, the same procedure, that is, with the beta coefficient obtained from the Sham group, was applied to the data from the RDN group. By this, the linear trends between BP change and baseline parameters, as obtained in the sham group, were removed from the RDN group. This correction was performed for each parameter of interest separately. This approach was taken to allow the following analysis of prediction for corrected parameter values for multivariable models (combination of parameters as predictors, eg, AIx and HR).
Receiver-operating characteristics curve analysis was performed to assess the predictive value of the parameters of interest for a clinically relevant BP response at 3 months, defined by a reduction of average 24-hour SBP of at least 5 mm Hg.28 To investigate the additive/independent value of measures of pulsatile hemodynamics for BP responder status, logistic regression models (enter method) were constructed, including age, SBP, body height, and HR (with the exception of the model including AIx75) and one pulsatile hemodynamics parameter.
Statistical significance was assumed at a 5% level. Statistical analyses were performed using Matlab R2019b (The MathWorks, Inc, Natick, MA) and MedCalc Statistical Software 19.2 (MedCalc Software Ltd, Ostend, Belgium).
Results
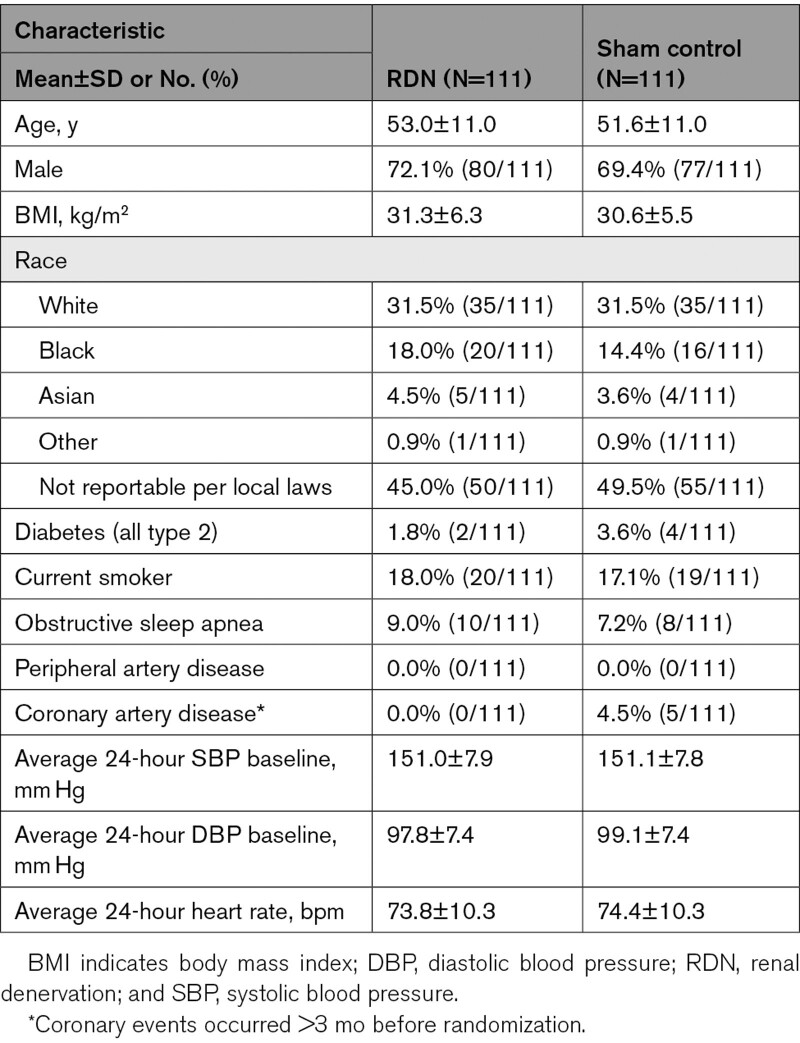
The final study sample consisted of 222 SPYRAL HTN-OFF MED pivotal patients (111 RDN, 111 sham), out of 166 RDN and 165 sham patients in the original publication.16 There were a few slight differences in baseline characteristics between patients included and not included (as full data were not available) in the current analysis, but importantly no differences between baseline SBP and DBP (Table S1).
Baseline characteristics were well balanced between RDN and sham patients (Table 1), as in the original publication.16 Average 24-hour SBP/DBP at baseline was 151 (SD 7.9)/98 (SD) mm Hg in RDN patients and 151 (SD 7.8)/99 (SD) mm Hg in sham controls, exactly matching these values in the original publication.16 Likewise, changes in average 24-hour SBP/DBP at 3 months were – 4.5 (SD 10.6)/−3.6 (SD 6.6) mm Hg (RDN patients) and −0.6 (SD 9.0)/−0.7 (SD 5.4) mm Hg (sham controls) in our sample, and −4.7 (SD 10.4)/−3.7 (SD 6.6) mm Hg (RDN patients) and −0.6 (SD 8.6)/−0.8 (SD 5.3) mm Hg (sham controls) in the original publication.16
Table 1.
Baseline Data of the Study Population
Average 24-hour measures of pulsatile hemodynamics (AIx, AIx75, AP, Pb, Pf, ePWV) at baseline were not different between RDN and sham controls groups (Table 2). AIx75 decreased significantly from baseline to 3 months in both groups. There were no significant differences in changes of measures of antegrade and reflected waves from baseline to 3 months between RDN and sham controls, only ePWV decreased slightly more in RDN patients (Table 2).
Table 2.
Average 24-Hour Measures of Wave Reflection at Baseline and Change at 3 mo Follow Up, Stratified by Treatment Group
Predictors of BP Response
Without adjustments, average 24-hour SBP at baseline was directly related to BP changes at 3 months (Figure S2) in the RDN and sham group, illustrating the law of the initial value in RDN patients and regression to the mean in sham controls. Of note, contrasting effects were seen for baseline HR and AIx in the RDN and sham group: higher baseline HR was associated with a greater BP response at 3 months in the RDN group, but not in the sham group (Figure S2). In addition, a lower AIx was associated with a greater change in average 24-hour SBP at 3 months in the RDN group, with an opposite trend in the sham group. Results for the remainder measures of wave reflections and antegrade wave as well as ePWV (AIx75, AP, Pb, Pf, ePWV) were consistent (Figure S2).
After corrections for the sham group (illustrated in Figure S1), baseline average 24-hour SBP in the RDN group was no longer associated with BP changes at 3 months (Figure S3). In contrast, sham group corrected average 24-hour HR at baseline was directly associated with BP changes following RDN, with SBP/DBP drop following RDN being 6.5/5.0 mm Hg greater in the group with baseline HR above as compared with below the median, respectively. All measures of wave reflections showed a similar association with RDN-related BP changes at 3 months: after correction for the BP changes in the sham group, average 24-hour SBP/DBP reduction in RDN patients was 7.8/5.9, 4.2/2.3, 8.0/6.3, and 6.7/5.4 mm Hg greater for the respective baseline values of AIx, AIx75, AP, and Pb below the median, as compared with above the median (Figure 2A through 2D, Table S2). Likewise, after correction for the BP changes in the sham group, average 24-hour SBP/DBP reduction at 3 months in RDN patients was 5.7/4.7 and 7.8/5.2 mm Hg greater for the respective baseline values of Pf (a measure of forward wave) and ePWV below the median, as compared with above the median (Figure 2E and 2F, Table S2).
Figure 2.
Changes in 24-hour systolic blood pressure (SBP) and diastolic blood pressure (DBP) at 3 mo in renal denervation (RDN) patients, corrected for changes in the sham group. Changes of average 24-hour SBP and DBP from baseline to 3 mo, stratified by baseline average 24-hour values (below and equal the respective median value—blue columns—vs above the respective median value—orange columns) in RDN patients, corrected for changes in the sham group. P values below the bars are from a pairwise comparison between baseline and 3 mo; P values above the bars comparing changes from baseline to 3 mo for ≤ median vs > median of corrected baseline value (unpaired t test); data in parentheses are 95% CIs. Upper: Augmentation Index (Aix; A), heart-rate 75 adjusted AIx (AIx75; B), pressure augmentation (AP; C). Lower: Backward wave amplitude (Pb; D), forward wave amplitude (Pf; E), estimated pulse wave velocity (ePWV; F).
Analysis stratified by age and sex showed similar trends in men and women, and young (<54 years) and older (≥54 years) participants (Figures S4 and S5).
Additional analysis, excluding the small number of patients in whom antihypertensive medications were detected at baseline (n=10 for RDN; n=9 for Sham), were consistent with the main findings (data not shown).
In receiver-operating characteristics analysis, sham- group corrected baseline average 24-hour HR, AIx, AIx75, AP, Pb, Pf, and ePWV were all significant predictors of a clinically relevant BP change following RDN (Table 3). The largest area under the curve (AUC) was obtained for AP (0.74 [CI, 0.64–0.84], P<0.0001), with AUCs in the same range for AIx75, AP and Pb, but also Pf and ePWV, respectively. In contrast, AUC for HR was 0.62 (CI, 0.52–0.71; P=0.02). The difference between AUCs for SBP and HR were statistically not different, whereas the AUCs for all wave reflection parameters were statistically different (larger) than the AUCs for HR (Table S3). The combination of HR with one of the parameters related to wave reflection led to minor improvements of AUCs (Table S4).
Table 3.
Receiver-Operating Curve Analysis Investigating the Predictive Value for Waveform Parameters and Heart Rate to Predict a Clinically Meaningful Blood Pressure Response to Renal Denervation, Defined as a Reduction in Average 24-Hour Systolic Blood Pressure From Baseline to 3 mo of at Least 5 mm Hg
In logistic regression models, including HR, age, body height, and baseline SBP, all measures of pulsatile hemodynamics were inversely and independently associated with BP responder status at 3 months (Table S5).
Discussion
Accurate noninvasive, reproducible, easy-to-obtain predictors of future BP reduction following RDN have not yet been identified. Against the background of a randomized, single-blinded, sham controlled clinical trial, we observed that measures of pulsatile hemodynamics, mainly pressure wave reflections, obtained during regular 24-hour ABPM, were associated with greater BP response 3 months after RDN in patients with uncontrolled hypertension in the absence of antihypertensive drug therapy.29
Wave reflections are thought to arise at sites of impedance change or mismatch along the arterial tree, such as points of branching, change in lumen diameter (taper) and structural properties.6 Multiple small reflections, originating from distributed reflection sites, are transmitted back toward the heart and merge and summate into a single net reflected wave.6 Wave reflections cannot be detected from conventional (auscultatory or oscillometric) BP measurement, but may be quantified from pressure waveforms alone, yielding AIx, AIx75 and AP, or through combined analysis with flow waveforms, yielding Pb. As a more sensitive measure of BP, wave reflections provide closer insights into hypertension-associated organ damage,10 cardiac function30 and the risk of cardiovascular events,8–10 as compared with brachial BP. Compared with brachial BP, measures of wave reflections are more sensitive to monitor antihypertensive drug-induced hemodynamic changes,31 as well as changes of hypertension-mediated organ damage.12,13 RDN has previously been shown to attenuate wave reflections in patients with resistant hypertension.32,33 Changes in AIx75 following RDN, however, were not related to changes in muscle sympathetic nerve activity.32
Analyses of increased RDN response have focused primarily on indices of increased sympathetic nervous system activity (or in association with an increased activity of the renin-angiotensin-system34) or lower arterial stiffness at baseline. The hypothesis that patients with a stiffer arterial system might not respond as well to RDN was initially based on observational trials showing apparently worse treatment response in patients with isolated systolic hypertension (defined as office DBP<90 mm Hg), a condition characterized by increased arterial stiffness.35 These findings were supported by observational results, suggesting that increased arterial stiffness, measured invasively as aortic PWV, may be associated with less BP lowering following RDN.36 Thus, patients with office DBP <90 mm Hg were excluded from the SPYRAL HTN-OFF MED Pilot1 and Pivotal16 trials. Wave reflections, in particular, when quantified with pulse waveform analysis, are related to arterial stiffness through the timing of the arrival of the reflected wave. Our results therefore confirm and extend these previous findings.
However, pulse waveform analysis-derived measures of wave reflections also depend on age, sex, body height, HR and left ventricular function.6,37–40 A previous analysis of the association between HR and BP response to RDN in the SPYRAL HTN OFF MED pilot trial41 demonstrated that RDN reduces 24-hour ambulatory HR (plus HR in numerous time windows) and that higher baseline 24-hour HR, likely a sign of higher sympathetic nervous system activity, predicted greater BP reductions following RDN.42 Taking the inverse relationship between HR and measures of wave reflections43 into consideration, our results are again consistent. Furthermore, receiver-operating characteristics analysis showed that the predictive value for RDN-induced BP response provided by measures of wave reflections outperformed HR alone, and is indeed additive when the less HR-dependent WSA-based measure Pb is used. The other determinants of a lower AIx/AP, that is, younger age and male sex, appear to play only a minor role, as evidenced by our stratified analysis. Also, severely impaired systolic function, another determinant of a low AIx/AP,37,38 was not present in the patients studied.
We would like to stress one methodological aspect of our study: previously, potential predictors of BP response to RDN have been addressed often in treatment cohorts36,44 only, that is, without a control group. Even if such analysis provides important insights, including the much-needed real-world evidence from large registries,45 interpretation of the results can be challenging. Statistical phenomena such as regression to the mean can occur, nicely depicted in the sham group of our study (Figure S1). The only consistent predictor of a greater BP response following RDN so far was a higher baseline BP.46 This finding is evident in the RDN group of our study as well. However, due to the study design, we were able to correct our results for BP changes in the sham group. This led to complete disappearance of the law of the initial value in RDN patients. In other words, sham-corrected baseline BP was no longer predictive of BP changes following RDN. In contrast, the predictive value of measures of wave reflections and, to a lesser degree, measures/estimates of forward wave and aortic stiffness, was strengthened, suggesting that the latter is a true biological mechanism.
Study Limitations
The current study is a post hoc analysis from a prospective, randomized sham-controlled clinical trial, including the majority, but not all of the original study participants. Next, as patient inclusion in the trial was not stratified by measures of pulsatile hemodynamics, causality cannot be inferred. However, assessment of wave reflections, forward wave and arterial stiffness was blinded, and independent from the treatment assigned. Moreover, taking advantage of the study design, the results in RDN patients could be compared with and adjusted for the sham-group results. Next, no adjustment for multiple statistical testing was performed due to the explorative design of the study. Finally, the predictive value of pulsatile hemodynamics for RDN-induced BP reduction in patients treated with antihypertensive drugs and in patients with ISH needs to be tested in the future.
Perspectives
Measures of pulsatile hemodynamics (wave reflections—AIx, AIx75, AP, Pb; forward wave—Pf; aortic stiffness—ePWV) obtained from routine 24-hour ABPM were associated with BP response to radiofrequency RDN at 3 months. More accurate identification of patients most likely to respond to RDN therapy may require a combination of 2 or more measurements, for instance pulsatile hemodynamics and neurohormonal indices.34
Article Information
Sources of Funding
S. Wassertheurer and C.C. Mayer are inventors (not holders) of a patent, which is partly used in the ARCSolver algorithms. AIT (S. Wassertheurer, B. Hametner, C.C. Mayer) received funding for the analyses of the data. The SPYRAL HTN-OFF MED pivotal trial was sponsored by Medtronic. Investigator initiated study, funding by Medtronic PLC, Santa Rosa, CA.
Disclosures
T. Weber received lecture fees from Medtronic and scientific support from IEM GmbH. R. Townsend has received consultant fees from Medtronic, Axio, and Regeneron. F. Mahfoud and M. Böhm are supported by Deutsche Forschungsgemeinschaft (SFB TRR219). F. Mahfoud has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical. M. Böhm reports personal fees from Abbott, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, Servier and Vifor during the conduct of the study. K. Kario has received scientific support and speaker honoraria from Daichi-Sankyo, Sanwa Chemical, Boehringer Ingelheim, Omron Healthcare, A&D Inc, Fukudadenshi, Medtronic, and ReCor Medical. M. Fahy, V. DeBruin, N. Peterson, and M. Negoita are employees of Medtronic. M. Weber has received consulting fees from Medtronic, ReCor, and Ablative Solutions. D.E. Kandzari has received institutional research/grant support from Medtronic Cardio- Vascular and Ablative Solutions and has received personal consulting honoraria from Medtronic CardioVascular. R.E. Schmieder has received consultant fees from Medtronic and Recor and has received grant support from Medtronic, Recor, and Ablative Solutions. K.P. Tsioufis has received honoraria for advisory boards and lectures from Medtronic, Servier, Bayer, Menarini, Novartis, AstraZeneca, Boehringer, Pfizer, Pythagoras, Sanofi, and Amgen. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ABPM
- ambulatory blood pressure monitoring
- AIx
- augmentation index
- AIx75
- heart-rate corrected augmentation index
- AP
- pressure augmentation
- AUC
- area under the curve
- BP
- blood pressure
- DBP
- diastolic blood pressure
- ePWV
- estimated aortic pulse wave velocity
- HR
- heart rate
- Pb
- backward wave amplitude
- Pf
- forward wave amplitude
- PWV
- pulse wave velocity
- RDN
- renal denervation
- SBP
- systolic blood pressure
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.18641.
For Sources of Funding and Disclosures, see page 1513.
References
- 1.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, et al. ; SPYRAL HTN-OFF MED trial investigators*. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X [DOI] [PubMed] [Google Scholar]
- 2.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, et al. ; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 3.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, et al. ; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. doi: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 4.Paz MA, de-La-Sierra A, Sáez M, Barceló MA, Rodríguez JJ, Castro S, Lagarón C, Garrido JM, Vera P, Coll-de-Tuero G. Treatment efficacy of anti-hypertensive drugs in monotherapy or combination: ATOM systematic review and meta-analysis of randomized clinical trials according to PRISMA statement. Medicine (Baltimore). 2016;95:e4071. doi: 10.1097/MD.0000000000004071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gosse P, Cremer A, Kirtane AJ, Lobo MD, Saxena M, Daemen J, Wang Y, Stegbauer J, Weber MA, Abraham J, et al. Ambulatory blood pressure monitoring to predict response to renal denervation: a post hoc analysis of the RADIANCE-HTN SOLO study. Hypertension. 2021;77:529–536. doi: 10.1161/HYPERTENSIONAHA.120.16292 [DOI] [PubMed] [Google Scholar]
- 6.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, et al. ; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American heart association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension. 2010;56:563–570. doi: 10.1161/HYPERTENSIONAHA.110.157339 [DOI] [PubMed] [Google Scholar]
- 8.Weber T, Auer J, O’rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2663. doi: 10.1093/eurheartj/ehi504 [DOI] [PubMed] [Google Scholar]
- 9.Li WF, Huang YQ, Feng YQ. Association between central haemodynamics and risk of all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J Hum Hypertens. 2019;33:531–541. doi: 10.1038/s41371-019-0187-x [DOI] [PubMed] [Google Scholar]
- 10.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571 [DOI] [PubMed] [Google Scholar]
- 11.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Luca N, Asmar RG, London GM, O’Rourke MF, Safar ME; REASON Project Investigators. Selective reduction of cardiac mass and central blood pressure on low-dose combination perindopril/indapamide in hypertensive subjects. J Hypertens. 2004;22:1623–1630. doi: 10.1097/01.hjh.0000125448.28861.fc [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto J, Imai Y, O’Rourke MF. Monitoring of antihypertensive therapy for reduction in left ventricular mass. Am J Hypertens. 2007;20:1229–1233. doi: 10.1016/j.amjhyper.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Weber T, Wassertheurer S, Schmidt-Trucksäss A, Rodilla E, Ablasser C, Jankowski P, Lorenza Muiesan M, Giannattasio C, Mang C, Wilkinson I, et al. Relationship between 24-hour ambulatory central systolic blood pressure and left ventricular mass: a prospective multicenter study. Hypertension. 2017;70:1157–1164. doi: 10.1161/HYPERTENSIONAHA.117.09917 [DOI] [PubMed] [Google Scholar]
- 15.Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, Kropf J, Eber B. Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension. 2011;58:825–832. doi: 10.1161/HYPERTENSIONAHA.111.176313 [DOI] [PubMed] [Google Scholar]
- 16.Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. ; SPYRAL HTN-OFF MED Pivotal Investigators. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 17.Helfer AG, Michely JA, Weber AA, Meyer MR, Maurer HH. Orbitrap technology for comprehensive metabolite-based liquid chromatographic-high resolution-tandem mass spectrometric urine drug screening - exemplified for cardiovascular drugs. Anal Chim Acta. 2015;891:221–233. doi: 10.1016/j.aca.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 18.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, et al. ; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366. doi: 10.1097/HJH.0000000000000221 [DOI] [PubMed] [Google Scholar]
- 19.Lauder L, Scholz SS, Ewen S, Lettner C, Ukena C, Böhm M, Mahfoud F. Accuracy of pulse rate derived from 24-h ambulatory blood pressure monitoring compared with heart rate from 24-h Holter-ECG. J Hypertens. 2020;38:2387–2392. doi: 10.1097/HJH.0000000000002566 [DOI] [PubMed] [Google Scholar]
- 20.Jones CR, Taylor K, Chowienczyk P, Poston L, Shennan AH. A validation of the Mobil O Graph (version 12) ambulatory blood pressure monitor. Blood Press Monit. 2000;5:233–238. doi: 10.1097/00126097-200008000-00007 [DOI] [PubMed] [Google Scholar]
- 21.Franssen PM, Imholz BP. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 2010;15:229–231. doi: 10.1097/mbp.0b013e328339be38 [DOI] [PubMed] [Google Scholar]
- 22.Sarafidis PA, Lazaridis AA, Imprialos KP, Georgianos PI, Avranas KA, Protogerou AD, Doumas MN, Athyros VG, Karagiannis AI. A comparison study of brachial blood pressure recorded with Spacelabs 90217A and Mobil-O-Graph NG devices under static and ambulatory conditions. J Hum Hypertens. 2016;30:742–749. doi: 10.1038/jhh.2016.11 [DOI] [PubMed] [Google Scholar]
- 23.Gotzmann M, Hogeweg M, Seibert FS, Rohn BJ, Bergbauer M, Babel N, Bauer F, Mügge A, Westhoff TH. Accuracy of fully automated oscillometric central aortic blood pressure measurement techniques. J Hypertens. 2020;38:235–242. doi: 10.1097/HJH.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 24.Wassertheurer S, Kropf J, Weber T, van der Giet M, Baulmann J, Ammer M, Hametner B, Mayer CC, Eber B, Magometschnigg D. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24:498–504. doi: 10.1038/jhh.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648 [DOI] [PubMed] [Google Scholar]
- 26.Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens. 2015;33:1023–1031. doi: 10.1097/HJH.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 27.Protogerou AD, Argyris A, Nasothimiou E, Vrachatis D, Papaioannou TG, Tzamouranis D, Blacher J, Safar ME, Sfikakis P, Stergiou GS. Feasibility and reproducibility of noninvasive 24-h ambulatory aortic blood pressure monitoring with a brachial cuff-based oscillometric device. Am J Hypertens. 2012;25:876–882. doi: 10.1038/ajh.2012.63 [DOI] [PubMed] [Google Scholar]
- 28.Mahfoud F, Böhm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, Tsioufis K, Andersson B, Blankestijn PJ, Burnier M, et al. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J. 2015;36:2219–2227. doi: 10.1093/eurheartj/ehv192 [DOI] [PubMed] [Google Scholar]
- 29.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. doi: 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 30.Weber T, Chirinos JA. Pulsatile arterial haemodynamics in heart failure. Eur Heart J. 2018;39:3847–3854. doi: 10.1093/eurheartj/ehy346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang XJ, O’Rourke MF, Jin WQ, Liu LS, Li CW, Tai PC, Zhang XC, Liu SZ. Quantification of glyceryl trinitrate effect through analysis of the synthesised ascending aortic pressure waveform. Heart. 2002;88:143–148. doi: 10.1136/heart.88.2.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hering D, Lambert EA, Marusic P, Ika-Sari C, Walton AS, Krum H, Sobotka PA, Mahfoud F, Böhm M, Lambert GW, et al. Renal nerve ablation reduces augmentation index in patients with resistant hypertension. J Hypertens. 2013;31:1893–1900. doi: 10.1097/HJH.0b013e3283622e58 [DOI] [PubMed] [Google Scholar]
- 33.Brandt MC, Reda S, Mahfoud F, Lenski M, Böhm M, Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012;60:1956–1965. doi: 10.1016/j.jacc.2012.08.959 [DOI] [PubMed] [Google Scholar]
- 34.Mahfoud F, Townsend RR, Kandzari DE, Kario K, Schmieder RE, Tsioufis K, Pocock S, David S, Patel K, Rao A, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77:2909–2919. doi: 10.1016/j.jacc.2021.04.044 [DOI] [PubMed] [Google Scholar]
- 35.Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, et al. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38:93–100. doi: 10.1093/eurheartj/ehw325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fengler K, Rommel KP, Hoellriegel R, Blazek S, Besler C, Desch S, Schuler G, Linke A, Lurz P. Pulse wave velocity predicts response to renal denervation in isolated systolic hypertension. J Am Heart Assoc. 2017;6:e005879. doi: 10.1161/JAHA.117.005879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tartière JM, Logeart D, Safar ME, Cohen-Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens. 2006;20:213–219. doi: 10.1038/sj.jhh.1001965 [DOI] [PubMed] [Google Scholar]
- 38.Parragh S, Hametner B, Bachler M, Weber T, Eber B, Wassertheurer S. Non-invasive wave reflection quantification in patients with reduced ejection fraction. Physiol Meas. 2015;36:179–190. doi: 10.1088/0967-3334/36/2/179 [DOI] [PubMed] [Google Scholar]
- 39.Parragh S, Hametner B, Bachler M, Kellermair J, Eber B, Wassertheurer S, Weber T. Determinants and covariates of central pressures and wave reflections in systolic heart failure. Int J Cardiol. 2015;190:308–314. doi: 10.1016/j.ijcard.2015.04.183 [DOI] [PubMed] [Google Scholar]
- 40.Hughes AD, Park C, Davies J, Francis D, McG Thom SA, Mayet J, Parker KH. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One. 2013;8:e59371. doi: 10.1371/journal.pone.0059371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Böhm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, Weber MA, Hoshide S, Patel M, Tyson CC, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–751. doi: 10.1093/eurheartj/ehy871 [DOI] [PubMed] [Google Scholar]
- 42.Böhm M, Tsioufis K, Kandzari DE, Kario K, Weber MA, Schmieder RE, Townsend RR, Kulenthiran S, Ukena C, Pocock S, et al. Effect of heart rate on the outcome of renal denervation in patients with uncontrolled hypertension. J Am Coll Cardiol. 2021;78:1028–1038. doi: 10.1016/j.jacc.2021.06.044 [DOI] [PubMed] [Google Scholar]
- 43.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(pt 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuern CS, Eick C, Rizas KD, Bauer S, Langer H, Gawaz M, Bauer A. Impaired cardiac baroreflex sensitivity predicts response to renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol. 2013;62:2124–2130. doi: 10.1016/j.jacc.2013.07.046 [DOI] [PubMed] [Google Scholar]
- 45.Mahfoud F, Mancia G, Schmieder R, Narkiewicz K, Ruilope L, Schlaich M, Whitbourn R, Zirlik A, Zeller T, Stawowy P, et al. Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol. 2020;75:2879–2888. doi: 10.1016/j.jacc.2020.04.036 [DOI] [PubMed] [Google Scholar]
- 46.Mahfoud F, Azizi M, Ewen S, Pathak A, Ukena C, Blankestijn PJ, Böhm M, Burnier M, Chatellier G, Durand Zaleski I, et al. Proceedings from the 3rd European clinical consensus conference for clinical trials in device-based hypertension therapies. Eur Heart J. 2020;41:1588–1599. doi: 10.1093/eurheartj/ehaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.