ABSTRACT
Objective
Maternal health and wellness during pregnancy are associated with long-term health outcomes in children. The current study examined whether infants of women who participated in a mindfulness-based intervention during pregnancy that reduced levels of stress and depression, increased physical activity, and improved glucose tolerance differed on biobehavioral markers of psychopathological and physical health risk compared with infants of women who did not.
Methods
Participants were 135 mother-infant dyads drawn from a racially and ethnically diverse, low-income sample experiencing high stress. The women participated in an intervention trial during pregnancy that involved assignment to either mindfulness-based intervention or treatment-as-usual (TAU). Infants of women from both groups were assessed at 6 months of age on sympathetic (preejection period), parasympathetic (respiratory sinus arrhythmia), and observed behavioral (negativity and object engagement) reactivity and regulation during the still face paradigm. Linear mixed-effects and generalized linear mixed-effects models were used to examine treatment group differences in infant outcomes.
Results
Relative to those in the intervention group, infants in the TAU group showed a delay in sympathetic activation and subsequent recovery across the still face paradigm. In addition, infants in the intervention group engaged in higher proportions of self-regulatory behavior during the paradigm, compared with the TAU group. No significant effect of intervention was found for parasympathetic response or for behavioral negativity during the still face paradigm.
Conclusions
Findings provide evidence that maternal participation in a short-term, group mindfulness-based intervention during pregnancy is associated with the early development of salutary profiles of biobehavioral reactivity and regulation in their infants. Because these systems are relevant for psychopathology and physical health, prenatal behavioral interventions may benefit two generations.
Key words/Abbreviations: intervention, mindfulness, prenatal programming, stress reactivity, infant, ANS = autonomic nervous system, OR = odds ratio, PEP = preejection period, PNS = parasympathetic nervous system, RSA = respiratory sinus arrhythmia, SNS = sympathetic nervous system, TAU = treatment-as-usual
INTRODUCTION
The developmental origins of health and disease (DOHaD) framework posits that maternal-placental-fetal interactions underlie long-term risk for many complex, common, and economically burdensome health problems (1), such as cardiovascular disease, diabetes, and an array of psychiatric disorders (2,3). Early DOHaD science revealed a range of maternal health and behavior factors during pregnancy, including nutrition, smoking, and alcohol consumption, that can program offspring health and development (4). More recently, maternal distress has received empirical attention as an intrauterine environment factor that impacts offspring developmental trajectories (5). Across these various types of maternal factors that can adversely program offspring disease risk, putative mechanisms involve endocrine, immune/inflammatory, and metabolic processes (1), although additional, multilevel (e.g., behavior, physiology) research is needed to fully understand the various pathways. There is also a notable need for inquiry into protective factors that buffer the deleterious effects of prenatal distress on poor offspring health, as not all exposed individuals develop psychiatric conditions or health problems. Such empirical gaps hinder the identification of potential avenues of prevention and intervention.
Recent summaries of this literature highlight the need for research that couples examination of how maternal distress and health behaviors affect child health with tests of prenatal intervention programs (6). Such a two-pronged approach affords the ability to make inferences about causality and identify factors promoting maternal and child health. The current study heeds this call by examining stress reactivity and regulation, established early phenotypes for health outcomes, in infants of women who received a mindfulness-based stress reduction and health promotion intervention during their pregnancy, relative to a matched control group.
It is biologically plausible that stress response systems, specifically the autonomic nervous system (ANS) and hypothalamic-pituitary-adrenal axis, contribute to developmental origins of child health (7). These systems develop rapidly in gestation and are vulnerable to established teratogens such as tobacco and opiates (8,9). Animal models (10) and emerging human studies (11,12) have provided evidence that maternal distress also affects fetal development of stress response systems. Postnatally, a common ANS response to a significant stressor (ANS “reactivity”) involves withdrawal (decrease) of the parasympathetic nervous system (PNS) and activation (increase) of the sympathetic nervous system (SNS). A response that includes the hyperreactivity or hyporeactivity of these systems to a stressor during infancy may have long-term consequences for physical and mental health (13).
Most examinations of prenatal programming of offspring stress response systems focus on the PNS and the hypothalamic-pituitary-adrenal axis, and findings generally show atypical stress system functioning after prenatal distress exposure. For example, exposure to high levels of prenatal distress has been associated with greater parasympathetic reactivity (PNS withdrawal) and weaker parasympathetic recovery (return to pre-stress level through increases in PNS activation) after a stressor in infancy (11,14). Among the emerging work examining sympathetic response in early childhood, prenatal distress has been related to dampened sympathetic reactivity to a stressor (12,15). These physiological findings are corroborated by studies of observed behavior that show infants exposed to higher levels of maternal prenatal distress exhibit a slower rate of behavioral recovery (16) and higher levels of negative reactivity (17) and fearfulness (18). Thus, young children’s autonomic stress responses have a demonstrated association with prenatal distress exposure, but because of the early stage of this body of work, more empirical inquiry is needed. Particularly, next steps should involve examination of autonomic stress response in early developmental stages (e.g., infancy), use of multiple indices of stress response (e.g., PNS, SNS, behavior), and identification of protective factors along the pathway.
These next steps are critical, considering the link between atypical ANS function (hyperresponsiveness, hyporesponsiveness, delayed recovery) and childhood mental and physical health problems. In cross-sectional examination, lower sympathetic reactivity has been associated with externalizing problems in childhood, such as antisocial behaviors and substance use (19,20). In addition, sympathetic hyperreactivity, parasympathetic hyporeactivity, and delayed parasympathetic recovery have been implicated in childhood internalizing problems, particularly anxiety (21–23). These relations may have early developmental origins, as atypical autonomic function in infancy is concurrently related to temperamental risk factors for psychopathology such as increased fearfulness (24) and negative affectivity (25). An additional body of work highlights the role of early childhood ANS moderating the association between the environment and child developmental outcomes, emphasizing that those with greater reactivity seem to be most susceptible to effects from both risky and promotive environments (26,27). Lastly, the importance of identifying early life factors associated with the development of ANS function is underscored by findings linking ANS dysfunction with physical health concerns, such as obesity and elevated systolic blood pressure in childhood (28,29), and cardiovascular disease, cancer, and obesity in adulthood (13,30,31).
The current study leverages a quasi-experimental design comparing an intervention demonstrated to improve mental and physical health with treatment-as-usual (TAU) (32). This approach allows for prospective examination of the effects of a protective, resilience-enhancing intervention on infant stress reactivity and recovery. In addition, the majority of evidence in this area has been drawn from outside of the United States (12,20,33), conducted with predominantly advantaged, White samples. Research on low-income, racially and ethnically diverse populations experiencing substantial exposure to stressors is needed to advance our understanding of risk and protective mechanisms underlying child physical and mental health outcomes and to design equitable solutions.
Prenatal mindfulness interventions, which aim to cultivate moment-to-moment, nonjudgmental awareness of the present moment (34), have been shown to have a wide range of mental health benefits during pregnancy and postpartum, such as reductions in depression (32,35), perceived stress (32,36), anxiety and negative affect (34), and pregnancy-specific anxiety (37). Emerging evidence has also shown benefits of mindfulness interventions on physical health, including improved glucose control (32,38) and reduced pain (36). Furthermore, findings from emerging experimental, correlational, and longitudinal investigations spanning pregnancy and early childhood support the possibility of two generations of benefit for prenatal interventions. First, real-time changes in fetal heart rate, heart rate variability, and motor activity have been observed in response to experimental inductions of maternal relaxation and stress (39). In addition, correlational approaches have found that higher levels of mother’s self-reported use of mindfulness during pregnancy are associated with maternal report of lower levels of infant self-regulation problems and negative affectivity (40). Thus, it seems likely that infant stress physiology and temperament are associated with maternal prenatal mood and behavior. Direct examination of ANS reactivity and regulation and observed infant behavior after a prenatal mindfulness-based intervention would fill the gaps in knowledge about early disease risk.
The aim of the current study was to examine whether infants of women who participated in a mindfulness-based intervention that significantly reduced levels of stress and depression, increased physical activity, and improved glucose tolerance during pregnancy (32) differed on biobehavioral markers of psychopathology and health risk compared with infants of women who received TAU. Our approach addresses critical gaps in this area through examination of prospective effects of maternal intervention during pregnancy on infant functioning, objective measurement of infant stress reactivity and regulation, both physiologically and behaviorally, and recruitment of a low-income, racially and ethnically diverse sample of families.
METHODS
Participants
Participants were mother-infant dyads in which the mothers (N = 215) were recruited during pregnancy to participate in an intervention study testing the effects of an 8-week mindfulness-based group program on stress, depression, healthy eating, and gestational weight gain (ClinicalTrials.gov identifier NCT01307683). Pregnant individuals were recruited from hospital-based clinics, community health centers, Supplemental Nutrition Assistance Program, and Women, Infants, and Children offices, organizations providing services to pregnant women, and through online advertisements (e.g., Craigslist). Inclusion criteria included English-speaking women with singleton pregnancies, aged 18 to 45 years, with a self-reported prepregnancy body mass index between 25 and 41 kg/m2, and with a household income less than 500% of the federal poverty level. Women had to be 12 to 19 weeks’ gestation at the start of the intervention, and those assigned to the intervention had to be able to attend eight weekly 2-hour classes at set times. Exclusion criteria included inability to complete forms in English, needle phobia or fainting response, substance abuse, medical conditions that might affect gestational weight gain (including known diabetes, HIV, hypertension, and eating disorders), polycystic ovarian syndrome treated with metformin, a regular meditation practice (20 or more minutes two times or more a week), recent weight loss (>5% within 6 months), chronic use of corticosteroids, or a history of gastric bypass surgery.
A subset of participants in the intervention study (n = 162) agreed to enroll in a subsequent study examining the effects of prenatal factors on offspring behavioral, physiological, and anthropometric development (see Ref. (11) for details on the sample and protocol). Of these dyads, 137 completed the standard protocol of the 6-month in-person visits, although 2 were excluded from analyses because the infant was beyond the intended age of visit, resulting in 135 mother-infant dyads with usable longitudinal data for the current study. In addition, because of delays in governmental processing of funding for equipment, ANS data were collected on 66 infants; of those, 1 was excluded for providing only one epoch of ANS data. Thus 65 infants were included in the subset of ANS analyses. The participants who enrolled in the current study did not differ from participants in the original intervention study on baseline (preintervention) self-reported depression, perceived stress, maternal age, race, or ethnicity (p > .6 for all).
Infants were approximately 6 months of age (mean [standard deviation {SD}] = 6.5 [0.60] months, range = 5.8–8.9 months; 49% female) at the time of the assessment. The sample represents an ethnically/racially diverse population, with 36% Black, 17% White, 1% Asian, 1% American Indian or Alaska Native, 46% mixed race or other, and 40% Hispanic. The majority (68%) of mothers were married/partnered, overweight/obese (body mass index: mean [SD] = 31.3 [4.99] kg/m2; range = 23–53 kg/m2), and multiparous (53%). Family annual income ranged from $0 to $86,000 (median = $18,000), with about half falling below the federal poverty guideline (41). The intervention group did not differ from the TAU group on key demographic variables at baseline, including maternal age, household income, or partnership status (Table 1). The groups did differ on gestational age at study enrollment, which was covaried in analyses, consistent with prior intervention tests in this study (32).
TABLE 1.
Demographics by Intervention and Treatment-as-Usual Group
| Characteristic | Intervention (n = 71)a | TAU (n = 64)a | pb |
|---|---|---|---|
| Maternal age, y | 27.9 (5.3) | 28.2 (6.3) | .77 |
| Gestational weeks at enrollment | 15.1 (2.9) | 19.7 (4.2) | <.001 |
| Maternal prepregnancy BMI, kg/m2 | 30.8 (4.5) | 31.7 (5.5) | .31 |
| Multiparous | 38 (54%) | 33 (52%) | .96 |
| Household income, $ | 16,800 [9000–35,000] | 18,500 [10,000–34,250] | .60 |
| Missing | 4 | 2 | |
| Family poverty | 104 [62–230] | 101 [54–196] | .47 |
| Missing | 4 | 2 | |
| Maternal gestational diabetes | 5 (7.0%) | 8 (13%) | .38 |
| Missing | 0 | 1 | |
| Maternal hypertension | 13 (18%) | 6 (9.5%) | .21 |
| Missing | 0 | 1 | |
| Maternal prenatal smoking | .26 | ||
| Current smoker | 2 (3.0%) | 5 (7.9%) | |
| Former smoker or never smoked | 65 (97%) | 58 (92%) | |
| Missing | 4 | 1 | |
| Child gestational age at birth, d | 279 [274–282] | 278 [274–282] | .86 |
| Child preterm birth | .67 | ||
| GA < 37 wk | 2 (2.8%) | 3 (4.7%) | |
| GA 37+ wk | 69 (97%) | 61 (95%) | |
| Breastfeeding | 64 (91%) | 59 (92%) | 1.00 |
| Missing | 1 | 0 | |
| Maternal depression (PHQ-9): prenatal | 7.2 (5.0) | 7.3 (4.8) | .93 |
| Missing | 3 | 1 | |
| Maternal depression (PHQ-9): postnatal | 3.8 (4.0) | 5.2 (4.0) | .061 |
| Missing | 9 | 4 | |
| Maternal perceived stress (PSS): prenatal | 19.0 (5.8) | 18.4 (5.9) | .55 |
| Missing | 2 | 1 | |
| Maternal perceived stress (PSS): postnatal | 15 (7) | 16 (7) | .46 |
| Missing | 10 | 4 | |
| PSI total (PSI-SF) | 62 (18) | 63 (17) | .82 |
| Missing | 12 | 11 | |
| Child ethnicity (Latinx/Hispanic) | 32 (45%) | 22 (34%) | .28 |
| Child age, mo | 6.56 (0.60) | 6.42 (0.59) | .17 |
| Child biological sex | .68 | ||
| Female | 33 (46%) | 33 (52%) | |
| Male | 38 (54%) | 31 (48%) | |
| Maternal partnership status | .92 | ||
| Married, in committed relationship, or engaged | 49 (69%) | 42 (67%) | |
| Single, separated, or divorced | 22 (31%) | 21 (33%) | |
| Missing | 0 | 1 |
TAU = treatment-as-usual; GA = gestational age; PHQ = Patient Health Questionnaire-9; PSS = Perceived Stress Scale; PSI-SF = Parenting Stress Index—Short Form; IQR = interquartile range.
aMean (SD), n (%), median [IQR].
bWelch two-sample t test, Pearson χ2 test, Fisher exact test.
Procedures
Intervention
Mindful Moms Training was adapted from three interventions: The Mindful Motherhood Training (34), Supporting Health by Integrating Nutrition and Exercise (42), and Mindfulness-Based Eating Awareness Training (43). In eight weekly, 2-hour, group sessions with 8 to 14 participants each, the intervention aimed to reduce stress and prevent excessive gestational weight gain using three experiential training components: a) nutrition and eating behavior, didactics on what to eat, how much to eat, and how and when to eat; b) mindful eating, discussion of hunger and satiety cues, taste satisfaction, and food choices; c) mindfulness for stress reduction, formal (e.g., sitting meditation, mindful movement) and informal (e.g., mindfulness of daily activities) practices to promote nonreactivity to and nonjudgmental acceptance of experiences. See Refs. (32,44,45) for more information on Mindful Moms Training.
At study enrollment, women were nonrandomly assigned to either Mindful Moms Training or TAU. Random assignment, although ideal, was not feasible for the current study, given the intervention requirement to enroll about 10 women at the same stage of pregnancy in each group. Therefore, gestational age and ability to attend the scheduled group classes affected randomization; women unable to attend intervention classes because of their schedules, or women with gestational age of 20 to 23 weeks who otherwise met the eligibility criteria, were assigned to the comparison group. Women in the comparison group proceeded with TAU. Notably, this assignment protocol resulted in balanced groups across demographics, other than the one difference in gestational weeks. No restrictions were placed on the mental health care that intervention or TAU participants received during the study period, and participants with elevated depressive symptom severity scores were provided a list of local mental health providers.
Initial examinations showed that participants in Mindful Moms Training reported pre-post intervention increases in mindfulness practice, acceptance of negative emotions, and emotion regulation (44). Primary outcome analyses from the intervention efficacy trial revealed that Mindful Moms Training was associated with significantly greater preintervention to postintervention improvements in several of the treatment targets. Specifically, women in the intervention group demonstrated significant declines in self-reported depressive symptom severity and perceived stress, increases in physical activity, and improved glucose control, compared with TAU (32). Furthermore, longitudinal examinations of depressive symptoms revealed lasting intervention effects of Mindful Moms Training on depression through 18 months postpartum (46).
For the present analysis, we compared the intervention group to the TAU group on a variety of health and behavior characteristics (Table 1). Consistent with prior publications in terms of balance of key factors at baseline (32), we found that women who participated in the intervention did not differ from women in TAU on these variables, including baseline stress and depression (p = .55 and p = .93, respectively).
Mother-Infant Research Visit
The 6-month mother-infant visits were completed in-person, at a research office (25%; n = 34) or in participants’ homes (75%; n = 101), depending on family preference. All assessors were unaware of treatment group assignment of the mothers. All procedures were reviewed and approved by the University of California, San Francisco Institutional Review Board. Each mother provided written, informed consent for herself and her infant.
Double Still Face Paradigm
Infants were exposed to the still face paradigm, a widely used experimental paradigm for evaluating infant physiological, behavioral, and emotional regulation (47) for approximately 10 minutes. A variation of the standard still face, the “double still face,” was used here, as it has been demonstrated to produce enhanced infant stress responses (47). This approach involves five 2-minute episodes: a) play (baseline), b) still face 1, c) reunion 1, d) still face 2, and e) reunion 2. During the play episode, mothers were instructed to interact with their seated infant as they usually would. During the still face episodes, mothers were instructed to stop playing with, touching, or responding to the infant and to hold a neutral facial expression. During the reunion episodes, mothers were instructed to resume interacting and playing with the then often-distressed child.
Measures
Autonomic Nervous System
ANS activity was collected continuously using BioNex hardware and BioLab acquisition software version 3.0 (Mindware Technologies, Ltd., www.mindwaretech.com) via spot electrodes placed on the infants at least 5 minutes before the first play period, which precedes the still face task. See Refs. (11,48) for the full ANS collection and scoring methods protocol.
Parasympathetic activity was assessed with respiratory sinus arrhythmia (RSA; the naturally occurring variation in heart rate as a function of respiration). The RSA index was calculated using the interbeat intervals detected from electrocardiogram readings, respiration rates detected from impedance waveforms (e.g., dZ/dt), and a bandwidth range of 0.15 to 1.04 Hz (49); decreases in RSA reflect withdrawal of the PNS. Sympathetic activity was assessed using preejection period (PEP), a systolic time interval representing the elapsed duration from the beginning of electrical stimulation until the ejection of blood from the left ventricle (50). PEP was measured in milliseconds as the time interval between the onset of ventricular depolarization (Q point on the electrocardiogram wave) and the onset of left ventricular ejection (B point on the dZ/dt wave); decreases in PEP (shortening) reflect activation of the SNS. The Mindware setting for the B-point calculation was 55% dZ/dt time + 4 ms. RSA and PEP data were filtered, extracted, and scored in 30-second epochs using Mindware software. Cleaning procedures involved examining for artifacts, deleting individual data files if more than 25% of the 30-second epochs were unscorable, and checking outliers (>3 SD) by task and summary scores. After data cleaning, participants provided a mean of 15.2 epochs of PEP data (SD = 5.8, median = 20) and 15.3 epochs of RSA data (SD = 5.8, median = 20).
Observed Infant Behavior
During the assessments, two cameras were strategically placed to record both mothers’ and infants’ responses during the still face paradigm for later behavioral coding. Videos of the infant’s responses were objectively coded by two trained research assistants, uninformed of treatment group, using a behavioral coding scheme adapted from Tronick’s Infant and Caregiver Engagement Phases (51). Codes were mutually exclusive and combined information from the infant’s face, direction of gaze, vocalizations, and body movements across the duration of the task. Three infant phases from the Infant and Caregiver Engagement Phases were used. Negative behavior was derived from the standardized composite of two codes: negative protest, characterized by active fussing, facial expressions of anger, or pulling away, and negative withdrawal, characterized by disengagement from the caregiver, sad facial expressions, whimpering/fussy vocalizations, or listless demeanor. Our measure of self-regulation, object engagement, was characterized by looking at proximal or distal objects with neutral, interested, or positive affect. Coders characterized behavior based on the aforementioned standards on a second-by-second scale across the entire task, with high interrater reliability (κ = 0.86). Time-standardized aggregate scores were computed for negative behavior and object engagement, representing the percent of time spent in that state during nonoverlapping 120-second episodes across the paradigm.
Data Analytic Plan
Obtaining up to 20 observations from participant episodes during the double still face (four 30-second epochs for each of up to five episodes), we used linear mixed-effects models to describe changes in ANS regulation (PEP, RSA) and examine whether differences in autonomic response patterns and behavior were moderated by maternal intervention group. Also, we examined changes in infant behavior (negativity, self-regulation) using generalized linear mixed-effects models and logit link function to model behavior observed as the percent of time during each of the five episodes. For each of the four outcomes, model 1 examined change over episodes of the still face task (main effects only), and model 2 examined treatment group effects (main effects and interaction effects).
In model 1 for each outcome, play is treated as a baseline intercept, and changes during each subsequent episode are examined. Each model 1 included the still face episode-type as sample fixed effect and allowed for random intercept, which allows for examination of episode-to-episode change. Fixed effects provide intercept as overall sample level during baseline episode and coefficient of change-from-baseline for each subsequent still face episode.
In model 2 for each outcome, we examined whether infant physiological activation or behavior during the still face task was moderated by maternal assignment to the treatment group (0 = TAU, 1 = intervention). Gestational weeks at enrollment (centered to the TAU group mean) was included as a covariate to account for this one postassignment group difference. Main fixed effects provide intercept as the TAU group level during baseline and coefficients of change for the TAU group. Interaction effects provide baseline difference between the TAU and intervention groups and change-from-baseline difference between the TAU and intervention groups for each still face episode. For ANS regulation, follow-up tests were performed to examine pairwise changes for sequential episode-to-episode changes within the repeated-measures framework (decreases in PEP and RSA indicate reactivity or activation of the stress response, whereas increases indicate a calming response [recovery postchallenge]). Infant behavior data modeled as log odds were transformed back to their original proportion response scale for interpretation. Odds ratios (ORs) were used to compare the TAU and intervention groups.
All ANS regulation multilevel models were fit to available participant data (3–20 repeated measurements of 30 seconds each) including a fixed effect for episode change and random intercept and slope and an autoregressive (lag 1) correlation error structure for subject-dependent epoch measurements using the nlme package in R (52). Infant behavior models were fit to observation data as proportion (no. of seconds displayed/no. of seconds total), with up to five episodes per participant, including a random intercept using the glmmTMB package in R (53). Incomplete or partial data were treated using standard missing-at-random structures. Overall level and interaction model statistical significance were evaluated at α = .05; p values for pairwise differences were adjusted using false discovery rate for 10 tests.
RESULTS
ANS Regulation
Results for ANS activity are shown in Table 2 and Figures 1 and 2. For PEP model 1 (main effects only), significant shortening (SNS activation) was observed after the still face 1 episode (B = −1.61, p < .001) and persisted through the final episode (reunion 2; B = −1.02, p < .05). For PEP model 2 (main effects + interactions), a significant treatment group interaction showed children of intervention participants demonstrated shortening earlier during the still face 1 episode, relative to children of TAU participants (B = −1.39, p < .05). Follow-up pairwise contrasts (Figure 1) showed that the TAU group in general followed the pattern of PEP shortening after still face 1 that persisted to task completion. Furthermore, the significant play-to-reunion 2 contrast for the TAU group, but not the intervention group, suggests PEP recovery only for the intervention group.
TABLE 2.
Regression Model Results
| Infant Outcome | PEP | RSA | Object Engagement | Negativity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model Type | |||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||
| Intercept/Play | B | 75.872 *** | 75.452 *** | 4.245 *** | 4.352 *** | −0.282 * | −0.554 ** | −5.757 *** | −5.000 *** |
| SE | 0.66 | 0.81 | 0.13 | 0.15 | 0.12 | 0.17 | 0.33 | 0.48 | |
| SF 1 | B | −0.181 | 0.260 | −0.231 | −0.222 | 0.361 *** | 0.296 *** | 3.203 *** | 3.079 *** |
| SE | 0.27 | 0.33 | 0.12 | 0.15 | 0.03 | 0.04 | 0.06 | 0.08 | |
| Reunion 1 | B | −1.161 *** | −1.066 ** | −0.345 * | −0.398 * | −0.473 *** | −0.753 *** | 2.840 *** | 2.693 *** |
| SE | 0.31 | 0.37 | 0.15 | 0.18 | 0.03 | 0.04 | 0.07 | 0.08 | |
| SF 2 | B | −1.102 ** | −1.161 * | −0.722 *** | −0.905 *** | −0.173 *** | −0.446 *** | 5.286 *** | 5.132 *** |
| SE | 0.40 | 0.48 | 0.18 | 0.22 | 0.03 | 0.04 | 0.07 | 0.09 | |
| Reunion 2 | B | −1.021 * | −1.220 * | −0.278 | −0.295 | −1.073 *** | −1.357 *** | 4.766 *** | 4.439 *** |
| SE | 0.42 | 0.51 | 0.15 | 0.18 | 0.03 | 0.05 | 0.07 | 0.09 | |
| Group | B | — | −0.194 | — | −0.441 | — | 0.420 | — | −1.474 |
| SE | — | 1.66 | — | 0.31 | — | 0.28 | — | 0.79 | |
| GW (covariate) | B | — | −0.294 | — | −0.026 | — | −0.018 | — | 0.011 |
| SE | — | 0.19 | — | 0.04 | — | 0.03 | — | 0.09 | |
| SF1 by group | B | — | −1.391 * | — | −0.042 | — | 0.115 * | — | 0.328 ** |
| SE | — | 0.58 | — | 0.27 | — | 0.05 | — | 0.12 | |
| Reunion 1 by group | B | — | −0.242 | — | 0.173 | — | 0.510 *** | — | 0.403 ** |
| SE | — | 0.67 | — | −0.32 | — | 0.06 | — | 0.14 | |
| SF2 by group | B | — | 0.208 | — | 0.460 | — | 0.493 *** | — | 0.417 ** |
| SE | — | 0.86 | — | 0.39 | — | 0.06 | — | 0.14 | |
| Reunion 2 by group | B | — | 0.653 | — | 0.059 | — | 0.496 *** | — | 0.784 *** |
| SE | — | 0.91 | — | 0.32 | — | 0.07 | — | 0.14 | |
Model 1: main effects only; model 2: main effects and interaction effects. Group: 0 = treatment-as-usual, 1 = intervention/Mindful Moms Training. For model 1 columns, B coefficients provide intercept as overall sample level during baseline episode and coefficient of change-from-baseline for each subsequent still face episode. For model 2 columns, interaction effects provide baseline difference between treatment-as-usual and intervention groups and intervention group difference-in-difference for each still face episode.
PEP = preejection period; RSA = respiratory sinus arrhythmia; SF = still face; SE = standard error; GW = gestational weeks at enrollment, centered around treatment-as-usual group mean.
* p < .05.
** p < .01.
*** p < .001.
FIGURE 1.
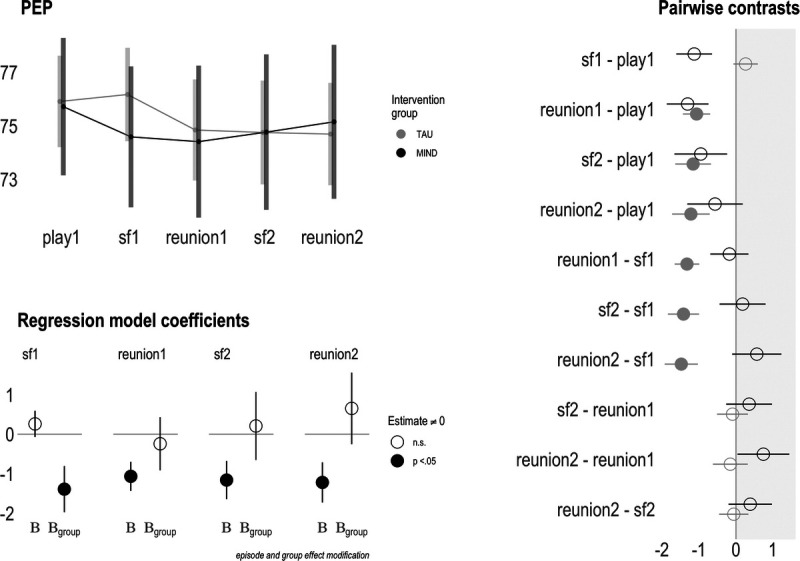
Maternal prenatal intervention effects on sympathetic nervous system activity at 6 months of age. A, Upper left panel shows group-level PEP means across the still face paradigm. B, Bottom left panel is a pictorial representation of PEP model 2 (main effects + interaction), where “B” shows the estimates for the control group (rows 2–5 of Table 2), and “Bgroup” shows the estimates for the intervention group (rows 8–11 of Table 2). Filled-in circles represent model coefficient significantly different (p < .05) from zero. C, Rightmost panel depicts episode-level contrasts between the intervention and control groups. Filled-in circles represent model coefficient significantly different (p < .05) from zero. PEP = preejection period. Color version of this figure is available online only with this article at www.psychosomaticmedicine.org.
FIGURE 2.
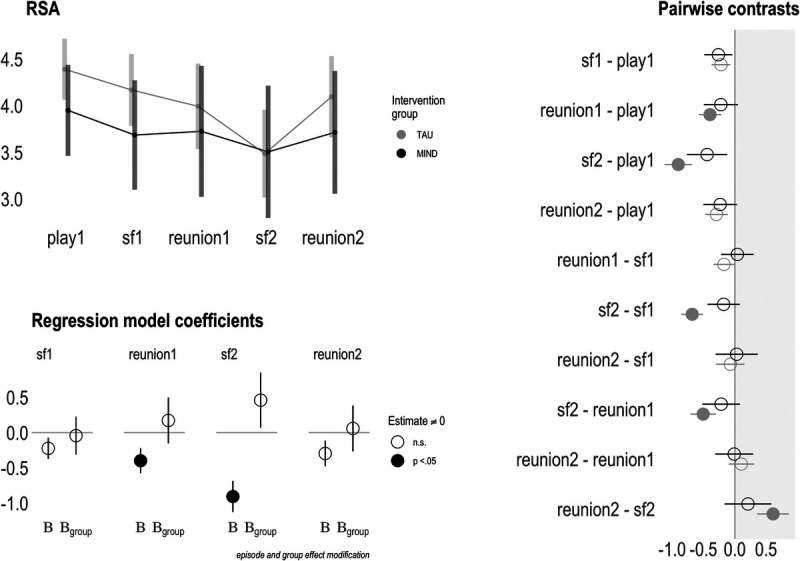
Maternal prenatal intervention effects on parasympathetic nervous system activity at 6 months of age. A, Upper left panel shows group-level RSA means across the still face paradigm. B, Bottom left panel is a pictorial representation of RSA model 2 (main effects + interaction), where “B” shows the estimates for the control group (rows 2–5 of Table 2), and “Bgroup” shows the estimates for the intervention group (rows 8–11 of Table 2). Filled-in circles represent model coefficient significantly different (p < .05) from zero. C, Rightmost panel depicts episode-level contrasts between the intervention and control groups. Filled-in circles represent model coefficient significantly different (p < .05) from zero. RSA = respiratory sinus arrhythmia. Color version of this figure is available online only with this article at www.psychosomaticmedicine.org.
For RSA model 1, suppression was observed linearly from baseline to still face 1 (B = −0.23, p = .06) to reunion 1 (B = −0.35, p < .05), with the greatest RSA withdrawal during the still face 2 episode (B = −0.72, p < .001), and recovery at reunion 2 (B = −0.28, p = .06). In RSA model 2, effect modification of this pattern by treatment group was not significant. Follow-up pairwise comparisons (Figure 2) show that the change pattern in RSA is more demonstrable in the TAU group, with some episode differences within the intervention group being smaller, although these became nonsignificant after false discovery rate adjustment.
Infant Behavior
Results for coded behavior are shown in Table 2 and Figure 3. In negativity model 1, both TAU and intervention group infants showed significant increases in negative behavior from baseline, most notably during still face 2 and reunion 2 episodes (B values = 2.84–5.29, p values < .001). For model 2, although significant interactions indicated differences in group changes from baseline, these were not very meaningful because of the low prevalence of observed negative behavior during baseline. Comparison of negative behavior during each episode slice revealed ORs between 1.99 and 4.37 (p values = .06–.38), with the TAU group displaying marginally higher rates of negative behavior across all episodes.
FIGURE 3.
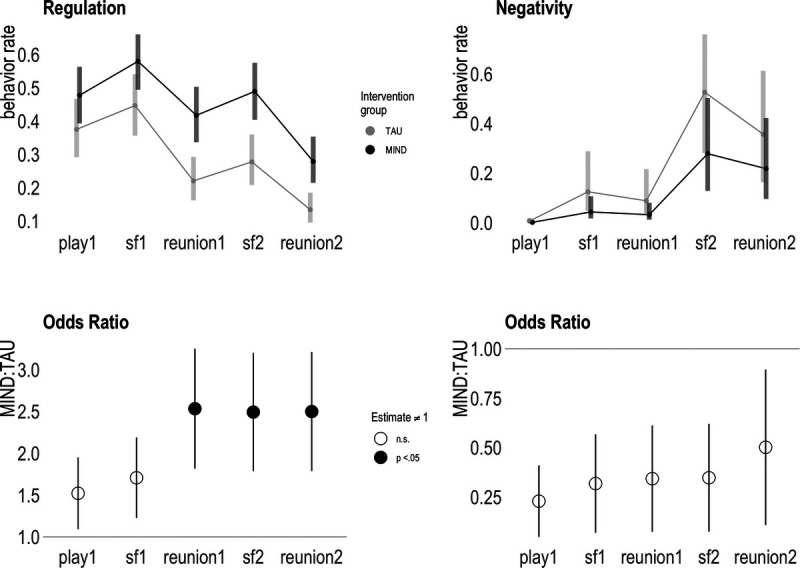
Maternal prenatal intervention effects on observed infant behavior at 6 months of age. A, Upper panels show group-level means for observed self-regulation (object engagement) and negativity across the still face paradigm. B, Bottom panels depict model 2 (main effects + interaction) odds ratios for both behavioral domains. Odds ratios greater than 1 indicate greater levels in the intervention group, whereas odds ratios less than 1 indicate greater levels in the control group. Filled-in circles represent model coefficient significantly different (p < .05) from zero. TAU = treatment-as-usual. Color version of this figure is available online only with this article at www.psychosomaticmedicine.org.
For self-regulation model 1, both groups demonstrated an overall significant decrease from baseline, with relatively more self-regulation observed during both still face episodes compared with reunion episodes. Model 2 revealed that intervention group infants demonstrated more self-regulatory behavior after still face 1 and a relatively smaller decrease in self-regulatory behavior from baseline. Specifically, the intervention group displayed significantly more self-regulatory behavior during reunion 1, still face 2, and reunion 2 (ORs = 2.49–2.53, p values < .01) compared with TAU. Both groups demonstrated a significant decline in self-regulation from baseline play to reunion 2. Comparing the two groups, the intervention group pattern suggests more persistent level of regulatory behavior between play and still face 2 (OR = 1.05, p = 1.00), whereas the TAU group pattern suggests a decline (OR = 0.64, p < .01).
DISCUSSION
We tested whether improving maternal health and well-being during pregnancy might promote salutary infant stress responses. Maternal participation in an evidence-based prenatal mindfulness intervention, which led to reduced perceptions of stress and depression, increased physical activity, and improved glucose tolerance relative to a TAU group (32), was associated with differing patterns of physiological and behavioral reactivity and regulation in their 6-month-old infants. Specifically, infants of women who participated in a prenatal mindfulness-based intervention exhibited patterns of SNS activation and regulation after a stressor that are consistent with lower risk for psychopathology and health problems (54,55). In contrast, infants of TAU mothers showed more delayed SNS activation and a lack of recovery. In addition, intervention group infants engaged in higher proportions of self-regulatory behavior during the stressful task, compared with TAU group infants. No significant effect of intervention was found for either infant parasympathetic response or observed negative behavior.
Overall, the current findings contribute to the literature through novel use of an intervention study design with longitudinal follow-up in an understudied population of low-income, racially and ethnically diverse women experiencing substantial levels of prenatal distress. The vast majority of prior work demonstrating prenatal DOHaD is correlational, which limits causal inference. Furthermore, most DOHaD studies have involved advantaged, White, non-US samples. Thus, the population studied here represents those at potentially greatest risk for intergenerational transmission of adversity effects and therefore warrants close study and pursuit of supportive solutions. In addition, the current findings provide evidence for the prenatal period as a particularly sensitive developmental window for both the mother and fetus, as maternal intervention during pregnancy improved maternal wellness and affected offspring’s physiology and behavior, early precursors for mental and physical health across the life span (56). More importantly, the results shift the focus from the role of poor maternal health in this programming to the role of improving wellness during pregnancy to optimize health outcomes for children.
Current behavioral findings are consistent with prior correlational work on prenatal stress and infant behavior (16,18,57), and a prior finding that mothers’ self-reported use of mindfulness during pregnancy is associated with maternal report of lower levels of infant self-regulation problems and negative affectivity (40). Furthermore, a previous randomized controlled trial using a meditation intervention during pregnancy found that infants of treatment group mothers received higher maternal-report ratings of approach behaviors and positive affect, compared with infants of control group mothers (58). Given the use of objective, observed measurement of infant behavior, the results in the current study add strong support to this area of inquiry that has relied on parent report. Furthermore, sample demographics of the present study are unique in this area in terms of income and associated exposures to stress.
Although the feasibility and reliability of collecting SNS data from infants have been established (59,60), very few studies have examined maternal health and behaviors during the prenatal period and infant sympathetic response (see, for exception, Refs. (12,15)). Alkon et al. (15) examined whether maternal prenatal adversities were related to infant ANS reactivity trajectories from 6 months to 5 years of age, in a predominantly low-income, Spanish-speaking sample. They found that children of mothers who experienced socioeconomic adversity during pregnancy showed dampened sympathetic reactivity to laboratory stressors over early childhood, whereas children of mothers who experienced no socioeconomic adversity during pregnancy showed heightened SNS sensitivity to stressors as they grew older. Because the current findings focus solely on 6-month sympathetic reactivity and regulation, they represent the first snapshot of the trajectories found by Alkon and colleagues; the intervention group children may continue to show reactivity and regulation, whereas the TAU group children may be expected to shift from delayed responsivity to dampened responsivity. Further longitudinal investigation is needed to determine the effects of prenatal stress on the developmental trajectories of sympathetic reactivity and regulation.
The current results indicated that infants of the TAU group, compared with the intervention group, showed delayed sympathetic activation and lack of recovery during the intended recovery period. Specifically, TAU group infants did not, on average, exhibit sympathetic reactivity during the first still face episode (the stressor). Instead, sympathetic reactivity emerged during the first reunion episode—an expected time for recovery. A return to baseline levels of sympathetic activity was not captured among TAU group infants within our measurement time frame of 10 minutes. This lack of observed PEP recovery is consistent with a prior study in which infants exposed to higher levels of early adversity (e.g., prenatal substance exposure, parent mental illness, family financial stress) showed increasing PEP activation during the recovery phase of the still face task (12). Conversely, similar to intervention group infants in the current study, infants with no exposure to these early adversities demonstrated both reactivity to the stressor and recovery during the reunion phase. In addition, it is possible that the observed group differences in PEP reactivity and regulation reflect postnatal parenting differences between the groups, as infants’ stress responses to the still face task are likely influenced by their history of prior interactions with the caregiver (61). For example, elevated or extended stress reactions to the still face paradigm have been correlated with exposure to insensitive caregiving (60). Furthermore, attenuated sympathetic response has been generally associated with externalizing problems and cardiovascular risk factors (e.g., obesity, high blood pressure) in children and adolescents (19,20,28,29), although risk is best predicted by considering the interactions between reactivity and a child’s social contexts, such as family, school, and community environments (27).
Analyses did not reveal significant treatment group differences in parasympathetic reactivity and regulation. This null finding suggests that the PNS, relative to the SNS, may be less sensitive to programming by prenatal intervention, or the effects may be more nuanced involving untested moderated associations. The PNS plays a broader role in homeostasis and social engagement and regulation (62), whereas the SNS is more specific to threat response. As previously published, the sample studied here experienced, on average, 2.6 types of major stressful life events during pregnancy (11); thus, the prenatal intervention may have been particularly salient for SNS development. More investigation is needed to clarify the relation between prenatal stress and parasympathetic function, with careful consideration for the level of stress exposure across samples.
Several mechanisms may underlie the associations between intervention participation and the infant outcomes found here. Notably, groups did not differ in gestational age at birth, breastfeeding, or maternal parenting stress, suggesting that those are unlikely mediators in this study. As previous work reported treatment group differences in postnatal depression in this sample (46), preliminary descriptive analyses were conducted to explore the potential role of postnatal depression in the observed relations. Its weak, nonsignificant associations with the infant outcomes suggest that maternal postnatal depression does not mediate the intervention associations reported here. Although these specific possible mediators were ruled out in this sample, the DOHaD framework indicates that endocrinological, metabolic, and inflammatory processes may have been impacted by the intervention. It is possible that group differences in experiences of stress and depression led to differing group levels of circulating maternal stress hormones, such as cortisol and corticotropin-releasing hormone (63,64), and subsequent differences in the development of infant sympathetic function and behavior. In addition, because gestational diabetes has long-term impacts on fetal heart development (65), glucose tolerance improvements associated with the intervention may also underlie the observed autonomic group differences. Lastly, participation in the intervention may have affected maternal parenting behaviors. Previous work has demonstrated an association between mindfulness and more attuned and responsive parenting behaviors (66); thus, it is possible that the still face task involving maternal behavior was experienced differently by infants across groups. Examining this range of prenatal and postnatal, and biological and behavioral, potential mechanisms in future research is important for illuminating the pathways underlying DOHaD processes.
These results suggest that effects of prenatal interventions are not limited to maternal wellness, but also extend to child development. Notably, the intervention in the present study was a short-term (8 weeks), group program, and participants were predominantly low-income women, a group that typically endures barriers to care due to insurance, access, costs, and stigma (67,68). The current intervention sample achieved excellent retention, attendance, and reporting of home practice outside of the class setting, and participants reported high satisfaction with the program’s content and logistics (44). It may be helpful to note, however, that many resources were used to recruit women early in pregnancy and help them attend a weekly meeting. This and similar pregnancy interventions may be more easily administered and successfully implemented if they are offered as part of group prenatal care, coordinating with medical visits to reduce travel burden. The current results suggest that such investments during pregnancy will facilitate maternal and child well-being in low-income populations.
The current findings should be interpreted in the context of several limitations. First, the maternal sample in this study comprised overweight and obese women, which may limit the generalizability of our findings. Considering the high prevalence of overweight and obesity among American women of childbearing age, particularly among women of color (69), the current sample may be more representative of this domain than typical research samples. Next, because of challenges of cohort-based recruitment and a short recruitment window (between 8 and 12 weeks’ gestational age), a comparison group was used rather than a fully randomized design. However, the two groups did not differ on key sociodemographic factors and demonstrated no baseline differences in stressful life events, perceived stress, or depressive symptoms. The sole significant difference between the groups was gestational weeks at study enrollment, which was covaried in all analyses here and was not associated with the infant outcomes tested. An additional limitation is modest sample size, particularly for the ANS outcomes. Although the analytic approaches used leveraged a multilevel design to increase statistical power to detect significant effects, larger samples are likely needed to optimally examine mediators and moderators of associations found.
Several methodological strengths should also be considered. In addition to the previously noted strength of providing much-needed data on diverse, low-income samples experiencing high stress, this study’s measurement of infant reactivity and regulation occurred at multiple levels: physiology and behavior. Our study leveraged a criterion standard measure of SNS activity, PEP, which is difficult to collect from infants because of procedural complexity and movement artifact. Thus, the current study provides some of the first data in the field for infant PEP activity (12,15), although a few prior studies have used other SNS indices such as skin conductance (70) and T-wave amplitude (71). In addition, to capture infant behavior, the current study used an intensive second-by-second coding scheme, which bolsters previous findings using parent reports of infant behavior that are subject to reporter bias. Lastly, the use of a quasi-experimental intervention design, as opposed to the more common cross-sectional/correlational design, enhances confidence in our interpretation that participation in the prenatal intervention led to differential outcomes for children.
Taken together, our findings provide evidence that participation in a short-term mindfulness-based intervention group during pregnancy was associated with the development of physiological and behavioral systems that manage stress and confer risk for mental and physical health problems in the offspring. These findings highlight the promise of prenatal intervention and the need for work illuminating mechanisms of change to enhance the efficacy and efficaciousness of such programs for the benefit of mothers and their children and the health of communities (72).
Acknowledgments
The authors acknowledge Hokhmah Joyallen, Sharifa Karen Krongold, and Shahara Godfrey for their intervention delivery and community engagement; Vanessa Tearnan, Marialma Gonzales-Cruz, Yurivia Cervantes, and Amy Engler for their assistance in collecting the data; Zoe Caron for leading the behavioral coding team; Michelle Stephens for her assistance with scoring the autonomic nervous system data; and Kim Coleman-Phox for leading the MAMAS data collection team that included the effort of Holly Wing, Gwen Valencia-Moscoso, Amber Benson, Samantha Schilf, and Danielle Emmet. Last but not least, we are especially thankful to the families for their generous participation in this research.
Source of Funding and Conflicts of Interest: No conflicts of interest are declared. This research was supported by the National Heart, Lung, and Blood Institute (U01 HL097973 and R01 HL116511), the Robert Wood Johnson Health and Society Scholars Program, the Lisa and John Pritzker Family Fund, the National Center for Advancing Translational Sciences–National Institutes of Health (UCSF-CTSI UL1 TR000004), the Tauber Family Foundation, and the Lisa Stone Pritzker Family Foundation.
Presentation Information: Results from this study were presented within a paper symposium to the Society for Research on Child Development’s biennial meeting held in Spring 2021.
Contributor Information
Michael Coccia, Email: michael.coccia@ucsf.edu.
Elissa Epel, Email: Elissa.Epel@ucsf.edu.
Cassandra Vieten, Email: cassandra.vieten@johnwbrickfoundation.org.
Nancy E. Adler, Email: Nancy.Adler@ucsf.edu.
Barbara Laraia, Email: blaraia@berkeley.edu.
Karen Jones-Mason, Email: kjmason@sbcglobal.net.
Abbey Alkon, Email: abbey.alkon@ucsf.edu.
Nicole R. Bush, Email: nicole.bush@ucsf.edu.
REFERENCES
- 1.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective—2015 Curt Richter Award Paper. Psychoneuroendocrinology 2015;62:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eberle C, Fasig T, Brüseke F, Stichling S. Impact of maternal prenatal stress by glucocorticoids on metabolic and cardiovascular outcomes in their offspring: a systematic scoping review. PLoS One 2021;16:e0245386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, Schwab M. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev 2020;117:26–64. [DOI] [PubMed] [Google Scholar]
- 4.Almond D, Currie J. Killing me softly: the fetal origins hypothesis. J Econ Perspect 2011;25:153–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EP, Narayan AJ. Pregnancy as a period of risk, adaptation, and resilience for mothers and infants. Dev Psychopathol 2020;32:1625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huizink AC, de Rooij SR. Prenatal stress and models explaining risk for psychopathology revisited: generic vulnerability and divergent pathways. Dev Psychopathol 2018;30:1041–62. [DOI] [PubMed] [Google Scholar]
- 7.van Bodegom M, Homberg JR, Henckens MJAG. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 2017;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hambleton MT, Reynolds EW, Sithisarn T, Traxel SJ, Patwardhan AR, Crawford TN, Mendiondo MS, Bada HS. Autonomic nervous system function following prenatal opiate exposure. Front Pediatr 2013;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stéphan-Blanchard E, Chardon K, Djeddi D-D, Léké A, Delanaud S, Bach V, Telliez F. The dynamics of cardiac autonomic control in sleeping preterm neonates exposed in utero to smoking. Clin Neurophysiol 2016;127:2871–7. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol 2006;572:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, Coleman-Phox K, Wadhwa PD, Laraia BA, Adler NE, Epel ES. Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Dev Psychopathol 2017;29:1553–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suurland J, van der Heijden KB, Smaling HJA, Huijbregts SCJ, van Goozen SHM, Swaab H. Infant autonomic nervous system response and recovery: associations with maternal risk status and infant emotion regulation. Dev Psychopathol 2017;29:759–73. [DOI] [PubMed] [Google Scholar]
- 13.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 2012;59:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Propper CB, Holochwost SJ. The influence of proximal risk on the early development of the autonomic nervous system. Dev Rev 2013;33:151–67. [Google Scholar]
- 15.Alkon A, Boyce WT, Tran L, Harley KG, Neuhaus J, Eskenazi B. Prenatal adversities and Latino children’s autonomic nervous system reactivity trajectories from 6 months to 5 years of age. PLoS One 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry 2011;52:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Inf Dent 2004;6:319–31. [Google Scholar]
- 18.Bergman K, Sarkar P, O’connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry 2007;46:1454–63. [DOI] [PubMed] [Google Scholar]
- 19.Brenner SL, Beauchaine TP. Pre-ejection period reactivity and psychiatric comorbidity prospectively predict substance use initiation among middle-schoolers: a pilot study. Psychophysiology 2011;48:1588–96. [DOI] [PubMed] [Google Scholar]
- 20.Sijtsema JJ, Van Roon AM, Groot PF, Riese H. Early life adversities and adolescent antisocial behavior: the role of cardiac autonomic nervous system reactivity in the TRAILS study. Biol Psychol 2015;110:24–33. [DOI] [PubMed] [Google Scholar]
- 21.Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J, Verhulst FC. Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology 2015;51:135–50. [DOI] [PubMed] [Google Scholar]
- 22.Nikolić M, de Vente W, Colonnesi C, Bögels SM. Autonomic arousal in children of parents with and without social anxiety disorder: a high-risk study. J Child Psychol Psychiatry 2016;57:1047–55. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz J, Tuschen-Caffier B, Wilhelm FH, Blechert J. Taking a closer look: autonomic dysregulation in socially anxious children. Eur Child Adolesc Psychiatry 2013;22:631–40. [DOI] [PubMed] [Google Scholar]
- 24.de Vente W, Majdandžić M, Bögels SM. Intergenerational transmission of anxiety: linking parental anxiety to infant autonomic hyperarousal and fearful temperament. J Child Psychol Psychiatry 2020;61:1203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry NB, Dollar JM, Calkins SD, Bell MA. Developmental cascade and transactional associations among biological and behavioral indicators of temperament and maternal behavior. Child Dev 2018;89:1735–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev 2010;81:270–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bush NR, Boyce WT. Differential sensitivity to context: implications for developmental psychopathology. In: Cicchetti D, editor. Developmental Psychopathology [Internet]. Hoboken, NJ: John Wiley & Sons, Inc.; 2016:1–31. [Google Scholar]
- 28.Zhou Y, Xie G, Wang J, Yang S. Cardiovascular risk factors significantly correlate with autonomic nervous system activity in children. Can J Cardiol 2012;28:477–82. [DOI] [PubMed] [Google Scholar]
- 29.Nagai N, Matsumoto T, Kita H, Moritani T. Autonomic nervous system activity and the state and development of obesity in Japanese school children. Obes Res 2003;11:25–32. [DOI] [PubMed] [Google Scholar]
- 30.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer 2006;6:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischoff SC, Boirie Y, Cederholm T, Chourdakis M, Cuerda C, Delzenne NM, Deutz NE, Fouque D, Genton L, Gil C, Koletzko B, Leon-Sanz M, Shamir R, Singer J, Singer P, Stroebele-Benschop N, Thorell A, Weimann A, Barazzoni R. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin Nutr 2017;36:917–38. [DOI] [PubMed] [Google Scholar]
- 32.Epel E, Laraia B, Coleman-Phox K, Leung C, Vieten C, Mellin L, Kristeller JL, Thomas M, Stotland N, Bush N, Lustig RH, Dallman M, Hecht FM, Adler N. Effects of a mindfulness-based intervention on distress, weight gain, and glucose control for pregnant low-income women: a quasi-experimental trial using the ORBIT model. Int J Behav Med 2019;26:461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dijk AE, van Eijsden M, Stronks K, Gemke RJ, Vrijkotte TG. Prenatal stress and balance of the child’s cardiac autonomic nervous system at age 5–6 years. PLoS One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Womens Ment Health 2008;11:67–74. [DOI] [PubMed] [Google Scholar]
- 35.Matvienko-Sikar K, Lee L, Murphy G, Murphy L. The effects of mindfulness interventions on prenatal well-being: a systematic review. Psychol Health 2016;31:1415–34. [DOI] [PubMed] [Google Scholar]
- 36.Beddoe AE, Paul Yang C-P, Kennedy HP, Weiss SJ, Lee KA. The effects of mindfulness-based yoga during pregnancy on maternal psychological and physical distress. J Obstet Gynecol Neonatal Nurs 2009;38:310–9. [DOI] [PubMed] [Google Scholar]
- 37.Guardino CM, Dunkel Schetter C, Bower JE, Lu MC, Smalley SL. Randomised controlled pilot trial of mindfulness training for stress reduction during pregnancy. Psychol Health 2014;29:334–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youngwanichsetha S, Phumdoung S, Ingkathawornwong T. The effects of mindfulness eating and yoga exercise on blood sugar levels of pregnant women with gestational diabetes mellitus. Appl Nurs Res 2014;27:227–30. [DOI] [PubMed] [Google Scholar]
- 39.DiPietro JA, Costigan KA, Nelson P, Gurewitsch ED, Laudenslager ML. Fetal responses to induced maternal relaxation during pregnancy. Biol Psychol 2008;77:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Heuvel MI, Johannes MA, Henrichs J, Van den Bergh BR. Maternal mindfulness during pregnancy and infant socio-emotional development and temperament: the mediating role of maternal anxiety. Early Hum Dev 2015;91:103–8. [DOI] [PubMed] [Google Scholar]
- 41.US Department of Health and Human Services . Poverty Guidelines, Research, and Measurement: The 2011 HHS Poverty Guidelines. Washington, DC: US Department of Health and Human Services; 2011. [Google Scholar]
- 42.Daubenmier J, Moran PJ, Kristeller J, Acree M, Bacchetti P, Kemeny ME, Dallman M, Lustig RH, Grunfeld C, Nixon DF, Milush JM, Goldman V, Laraia B, Laugero KD, Woodhouse L, Epel ES, Hecht FM. Effects of a mindfulness-based weight loss intervention in adults with obesity: a randomized clinical trial. Obesity (Silver Spring) 2016;24:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord 2011;19:49–61. [DOI] [PubMed] [Google Scholar]
- 44.Vieten C, Laraia BA, Kristeller J, Adler N, Coleman-Phox K, Bush NR, Wahbeh H, Duncan LG, Epel E. The mindful moms training: development of a mindfulness-based intervention to reduce stress and overeating during pregnancy. BMC Pregnancy Childbirth 2018;18:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laraia BA, Adler NE, Coleman-Phox K, Vieten C, Mellin L, Kristeller JL, Thomas M, Stotland NE, Lustig RH, Dallman MF, Hecht FM, Bush NR, de Groat CL, Epel E. Novel interventions to reduce stress and overeating in overweight pregnant women: a feasibility study. Matern Child Health J 2018;22:670–8. [DOI] [PubMed] [Google Scholar]
- 46.Felder JN, Roubinov D, Bush NR, Coleman-Phox K, Vieten C, Laraia B, Adler NE, Epel E. Effect of prenatal mindfulness training on depressive symptom severity through 18-months postpartum: a latent profile analysis. J Clin Psychol 2018;74:1117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry 1978;17:1–13. [DOI] [PubMed] [Google Scholar]
- 48.Rudd KL, Alkon A, Abrams B, Bush NR. Infant weight-for-length gain associated with autonomic nervous system reactivity. Pediatr Res 2021;90:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bar-Haim Y, Marshall PJ, Fox NA. Developmental changes in heart period and high-frequency heart period variability from 4 months to 4 years of age. Dev Psychobiol 2000;37:44–56. [DOI] [PubMed] [Google Scholar]
- 50.Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: the psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology 1994;31:412–9. [DOI] [PubMed] [Google Scholar]
- 51.Weinberg MK, Tronick EZ. Infant affective reactions to the resumption of maternal interaction after the still-face. Child Dev 1996;67:905–14. [PubMed] [Google Scholar]
- 52.Pinheiro J Bates D DebRoy S Sarkar D, Core R Team . nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 53.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 2017;9:378–400. [Google Scholar]
- 54.Rudd KL, Yates TM. The implications of sympathetic and parasympathetic regulatory coordination for understanding child adjustment. Dev Psychobiol 2018;60:1023–36. [DOI] [PubMed] [Google Scholar]
- 55.Kahle S, Miller JG, Lopez M, Hastings PD. Sympathetic recovery from anger is associated with emotion regulation. J Exp Child Psychol 2016;142:359–71. [DOI] [PubMed] [Google Scholar]
- 56.Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: the coming of age of a discipline. J Child Psychol Psychiatry 2009;50(1–2):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry 2007;46:737–46. [DOI] [PubMed] [Google Scholar]
- 58.Chan KP. Prenatal meditation influences infant behaviors. Infant Behav Dev 2014;37:556–61. [DOI] [PubMed] [Google Scholar]
- 59.Bush NR, Caron ZK, Blackburn KS, Alkon A. Measuring cardiac autonomic nervous system (ANS) activity in toddlers—resting and developmental challenges. J Vis Exp 2016;53652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones-Mason K, Alkon A, Coccia M, Bush NR. Autonomic nervous system functioning assessed during the still-face paradigm: a meta-analysis and systematic review of methods, approach and findings. Dev Rev 2018;50:113–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bigelow AE, Power M. Effects of maternal responsiveness on infant responsiveness and behavior in the still-face task. Inf Dent 2014;19:558–84. [Google Scholar]
- 62.Porges SW. The polyvagal perspective. Biol Psychol 2007;74:116–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glover V, O’Donnell KJ, O’Connor TG, Fisher J. Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—a global perspective. Dev Psychopathol 2018;30:843–54. [DOI] [PubMed] [Google Scholar]
- 64.Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev 2005;29:237–58. [DOI] [PubMed] [Google Scholar]
- 65.Hornberger LK. Maternal diabetes and the fetal heart. Heart 2006;92:1019–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gouveia MJ, Carona C, Canavarro MC, Moreira H. Self-compassion and dispositional mindfulness are associated with parenting styles and parenting stress: the mediating role of mindful parenting. Mind 2016;7:700–12. [Google Scholar]
- 67.DeVoe JE, Baez A, Angier H, Krois L, Edlund C, Carney PA. Insurance + access not equal to health care: typology of barriers to health care access for low-income families. Ann Fam Med 2007;5:511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodgkinson S, Godoy L, Beers LS, Lewin A. Improving mental health access for low-income children and families in the primary care setting. Pediatrics 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Center for Health Statistics . Health, United States, 2017: With Special Feature on Mortality. Hyattsville, MD: National Center for Health Statistics; 2018. [PubMed] [Google Scholar]
- 70.Ham J, Tronick E. Infant resilience to the stress of the still-face: infant and maternal psychophysiology are related. Ann N Y Acad Sci 2006;1094:297–302. [DOI] [PubMed] [Google Scholar]
- 71.Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, Wright RJ. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev 2014;90:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev 2011;14:1–27. [DOI] [PubMed] [Google Scholar]


