Background:
Previous studies suggested blood pressure variability (BPV) might help reveal interactions between blood pressure fluctuation and white matter lesions, and the impact of elevated BPV on white matter hyperintensity (WMH) or cerebral arterial dilation is unclear.
Methods:
This retrospective observational study involved 2634 stroke-free individuals (68.6±11.1 years, 50.3% female), who underwent magnetic resonance imaging and magnetic resonance angiography scans, from a single center in Shanghai, China. Measurements for variability of blood pressure were made based on 7 days blood pressure recordings. WMHs were quantified from T2-FLAIR images and further classified as periventricular WMH or deep WMH. M1 segment of middle cerebral artery dilation was assessed from magnetic resonance angiography images. General linear model was used to examine the associations.
Results:
Both increased systolic and diastolic BPV were associated with increased WMH volume (systolic: β=0.02 [95% CI, 0.004–0.03], P=0.01; diastolic: β=0.05 [95% CI, 0.03–0.08], P<0.001). Only periventricular WMH was associated with BPV (systolic: β=0.02 [95% CI, 0.005–0.04], P=0.01; diastolic: β=0.06 [95% CI, 0.04–0.09], P<0.001). MCA dilation was found in 125 individuals (4.75%). Systolic BPV was associated with MCA dilation only in the hypertensive individuals (β=0.11 [95% CI, 0.06–0.17], P<0.001). Increased WMH volume was found associated with dilated MCA (β=0.17 [95% CI, 0.11–0.23], P<0.001).
Conclusions:
Increased BPV might be one of the pathophysiological phenomena involving in the small vessel disease independent of hypertension. Increased BPV might independently contribute to intracranial arterial dilation. Management of BPV might be a target to preserve cerebrovascular wellness.
Keywords: angiography, blood pressure, cerebral small vessel disease, dementia, hypertension
Novelty and Relevance.
What Is New?
High blood pressure variability is linked to white matter hyperintensity and intracranial arterial dilation independent of hypertension.
What Is Relevant?
Even in nonhypertensive individuals, diastolic blood pressure variability was associated with increased white matter lesion volume. Moreover, excessive variability in systolic blood pressure was associated with intracranial arterial dilation only in the hypertensive individuals.
Clinical/Pathophysiological Implications?
Besides hypertension, management of blood pressure variability might be a target to preserve cerebrovascular wellness.
Hypertension affects around one-third of adults worldwide.1 Being a leading cerebrovascular risk, hypertension has been reported to have deleterious effects on the cerebral vessels, resulting in cerebrovascular morbidities including stroke and cerebral small vessel disease (CSVD).2,3 Episodic hypertension, based on the average of blood pressure (BP) measurements upon hospital admission, is commonly used to define hypertension.4 Yet, such definition may, very often, not reliably reflect the real BP of the patients.5 Not to mention it also disregards fluctuations in the BP levels.6
However, as an organ with high blood flow, the brain is vulnerable, not only to elevated BP, but also to rapid changes in BP.7 Excessive fluctuations in BP would affect vessels supplying the deep white matter or periventricular regions due to their anatomic features.8 Previous studies have suggested that extreme BP variability (BPV) over time could lead to higher risks of dementia and stroke.9,10 Moreover, extreme BPV was reported to impact large vessels, increasing arterial stiffness which predisposes risks for future stroke.11 Intracranial arterial dolichoectasia (IADE), distinct from intracranial atherosclerotic stenosis and characterized as an excessive increase in length or diameter of at least one intracranial artery, is a dilative arteriopathy.12,13 IADE has a different pathophysiological changes of arterial walls compared with intracranial atherosclerotic stenosis and, therefore, may be influenced by different risk factors commonly reported to contribute to intracranial atherosclerotic stenosis.13 However, only aging was consistently found to be correlated with increased cerebral arterial diameters in previous studies.14,15 The association between BPV and IADE has less been reported.
Increased BPV, meanwhile, could also affect vessels of smaller sizes. CSVD, characterized by several neuroimaging features, including recent small subcortical infarcts, cerebral microbleeds, lacunes, and white matter hyperintensities (WMHs), results in significantly increased risk of stroke and is found involved in approximately half of the dementia cases.16,17 As the most commonly observed clinical feature of CSVD, WMHs have been under extensive investigation, and their relationship with hypertension has frequently been reported.18,19 The relationship between WMHs and BPV, however, has been largely debated.20
To investigate the complex nature of lesions of both large and small cerebral vessels and their relations with extreme BP fluctuations, we monitored the BP of subjects for 7 consecutive days and analyzed the day-to-day BPV in a large group of patients free of stroke.21 We hypothesize that increased BPV would result in lesions of the both large and small cerebral vessels, manifested as larger WMH lesion volume and more severe IADE. WMHs were classified as the periventricular WMH (PWMH) or deep WMH (DWMH) to further study the differential effects of BPV on anatomically distinctive vessels, and the impact of BPV on WMH spatial distribution was reported.
Materials and Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
This retrospective, observational study involves data from the Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai, China. Patients hospitalized in the department of neurology and underwent brain magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) between January 2017 and June 2021 were included as the candidates. Subjects with acute stroke, dementia, carotid, or cerebral artery stenosis were excluded. Cerebral artery stenosis was defined as any degree stenosis in at least one of the following arteries: intracranial carotid artery, middle cerebral artery (MCA), anterior artery, intracranial segment of vertebral artery, basilar artery, and posterior cerebral artery.22 The subjects involved in this study were mostly elderly individuals with traditional cerebrovascular risk factors. The main reasons for their visits were due to headache, dizziness, sleep disorders, palpitation, gait disturbances, memory loss, or concentration problems. Patients with CT-confirmed signs of CSVD were admitted for hospitalization, and conventional BP and heart rate monitoring and brain MRI were performed for further evaluation of CSVD. Neuropsychological test, sleep monitoring, and gait analysis were performed only for part of the subjects. The study uses only previously collected data, with no additional tests or interventions undertaken on any participants and thus would inflict no impact on patient care or the outcome. The Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine approved the current study (Number: 2020-060).
Data Collection
Demographic characteristics, personal and family medical history, and lifestyle risk factors were collected in the clinic. Cigarette use, alcohol consumption, hypertension, diabetes, hyperlipidemia, history of cardiovascular disease (coronary artery disease, atrial fibrillation, heart failure, or heart valve disease) were assessed. Hypertension was defined as self-reported hypertension, treatment with antihypertensive agents, systolic BP ≥140 mm Hg, or diastolic BP ≥90 mm Hg. Venous blood samples were taken by certified nurses between 7 am and 8 am after overnight fasting and used for the determination of lipid profile (including total cholesterol, HDL [high-density lipoprotein] cholesterol, triglycerides), creatinine, glucose, and glycated hemoglobin.
BP was measured after the subject rested at least 5 minutes in a seated position. An appropriately sized cuff was placed on the dominant forearm arranged at the horizontal level of the fourth intercostal space at the sternum and BP was measured using a validated digital electronic tensiometer OMRON (OMRON Corp., Kyoto, Japan). In the following days, BP was taken from the same arm, at a similar time. BPV was calculated based on first 7 consecutive measurements after admission. Three commonly used BPV parameters were assessed including within-individual SD (mm Hg), coefficient of variation (CoV; SD/mean×100, %) and variation independent of mean (VIM, mm Hg). VIM was calculated as the SD divided by the within-individual mean to the power p and multiplied the average value of SBP in the cohort to the power p, which is obtained by fitting a curve of SD against the within-individual mean SBP.11
Brain MRI and Analysis
Individuals underwent brain MRI and MRA scan on one 3.0T MR scanner (Philips Ingenia, Philips Healthcare, Best, the Netherlands). The MRI protocol included T1-weighted structural imaging, T2 FLAIR, and time-of-flight MRA with more protocol details in the Supplemental Material.
WMH lesions were automatically segmented from the T2 FLAIR images. The total WMH, PWMH, and DWMH lesion volume were obtained after registration of T2 FLAIR images to the Montreal Neurological Institute brain template.23 The presence of WMH in the basal ganglia region was defined as at least 0.01 mL of WMH in this region. Voxel-wise regressions were performed to investigate the impact of BPV on WMH spatial distributions,24 with more details in the Supplemental Material.
The M1 segment of the MCA was manually quantified from the vascular segmentation on MRA images (Figure S1). The mean diameter of each vascular segment was assessed. The sum of mean diameter of left and right M1 segment was used. Presence of MCA dilation was defined as the within-individual diameter ≥ mean diameter in the cohort + 2 SD in the cohort.13 The internal carotid artery and basilar artery were not included in the analysis as their origin could not be clearly defined in the MRA images. For more detailed information for the MRI analysis, please see Methods in the Supplemental Material.
Statistical Analysis
Multivariable linear regression analysis was used to assess the association of BPV with cerebral artery dilation and WMH. Due to the skewed distribution of WMH lesion volume, logarithmic transformation was applied. General linear model with logistic link function and binomial distribution was used for the binary response variable. The results of normally distributed continuous variables were presented as mean and SD or as median and interquartile range. For categorical variables, frequencies and proportions were presented. Two-sample t test or χ2 test was applied to evaluate group differences according to requirement. All tests were 2-sided, and a P value of <0.05 was considered significant. All statistical analysis was undertaken using Matlab (version 2021a, MathWorks, Natick, MA).
Results
Individual Characteristics
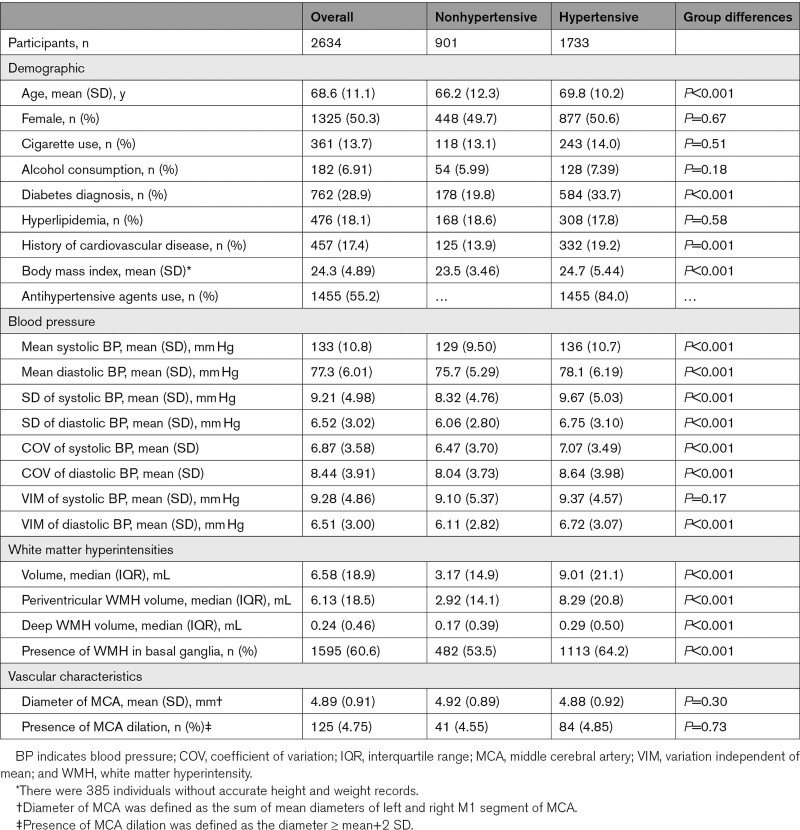
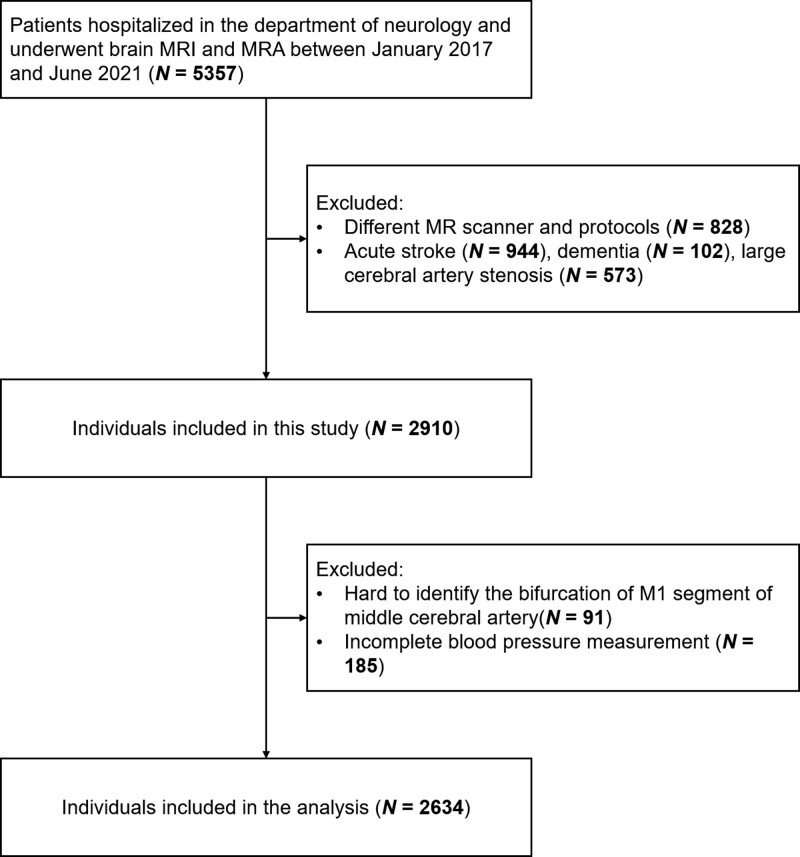
The individual characteristics are presented in Table 1. Figure 1 summarizes the enrollment process of the current study. A total of 2910 individuals were initially included; of these, 91 individuals for whom the bifurcations of MCA could not be identified and 185 individuals with incomplete BP measurement were excluded. Therefore, 2634 individuals were included in the subsequent analysis. The mean (SD) age was 68.6 (11.1) years and 1325 individuals were female (50.3%).
Table 1.
Participants Characteristics
Figure 1.
Flow chart of enrollment in the current study. MRA indicates magnetic resonance angiography; and MRI, magnetic resonance imaging.
Age, sex, and hypertension were found to be associated with BPV in bivariable models (Table S1). In multivariable models, age remained associated with both systolic and diastolic BPV, and sex was associated with systolic BPV.
Association Between BPV and WMH
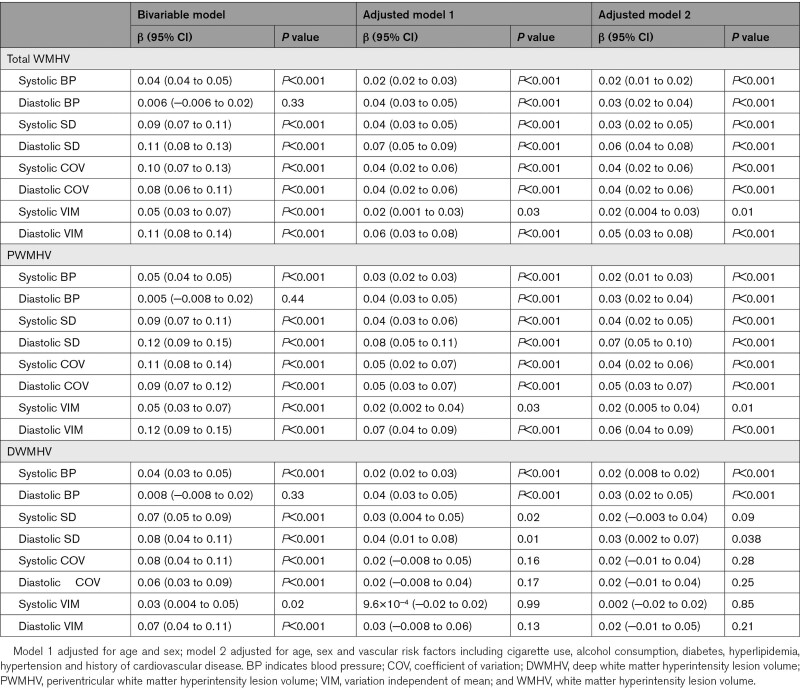
In general, higher SD, CoV, and VIM were found to be associated with increased total WMH volume, after adjusting for age, sex, and risk factors for both systolic (systolic SD: β=0.03 [95% CI, 0.02–0.05], P<0.001; systolic CoV: β=0.04 [95% CI, 0.02–0.06], P<0.001; systolic VIM: β=0.02 [95% CI, 0.004–0.03], P=0.01) and diastolic BPV (diastolic SD: β=0.06 [95% CI, 0.04–0.08], P<0.001; diastolic CoV: β=0.04 [95% CI, 0.02–0.06], P<0.001; diastolic VIM: β=0.05 [95% CI, 0.03–0.08], P<0.001), as shown in the Table 2. When considering PWMHs and DWMHs, BPV was significantly associated with only PWMH volume. Only SD was found to be associated with DWMH volume after adjusting for age and sex (systolic SD: β=0.03 [95% CI, 0.004–0.05], P=0.02; diastolic SD: β=0.04 [95% CI, 0.01–0.08], P=0.01), and the association remained for diastolic SD after adjusted for risk factors (β=0.03 [95% CI, 0.002–0.07], P=0.038).
Table 2.
Association Between Blood Pressure Variability and White Matter Hyperintensity
These associations were further examined in hypertensive and nonhypertensive individuals (Tables S2 and S3). In the hypertensive individuals, these associations remained, whereas in the nonhypertensive individuals, only diastolic BPV was associated with WMH volume. Nevertheless, no significant interaction between BPV and hypertension status on WMH volume was observed (Table S4).
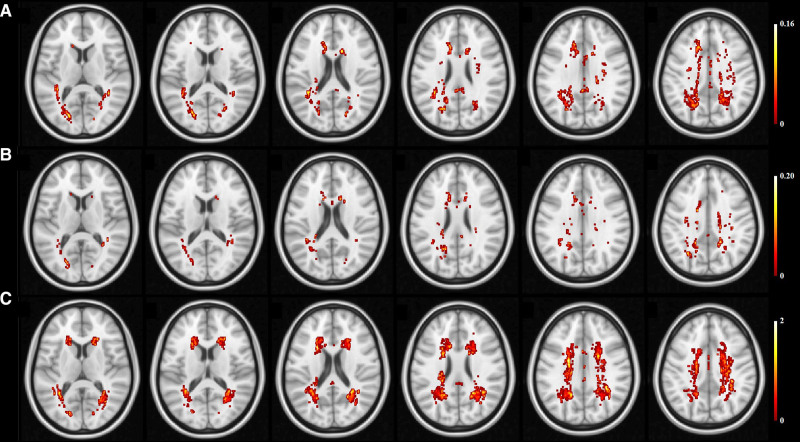
Figure 2 shows BPV and hypertension associated distribution of WMH. WMHs associated with SBP VIM and DBP VIM mainly were found in the anterior and posterior horns of the lateral ventricles and the centrum semiovale, and most were on the posterior white matter. Hypertension or high BP associated WMHs were noted in the area around the anterior horns of the lateral ventricles, the posterior horns of the ventricles and the centrum semiovale. Other BPV features associated distribution of WMH was presented in the Figure S2.
Figure 2.
Blood pressure variability and hypertension associated distribution of white matter hyperintensity. A, Variation independent of mean (VIM) of systolic blood pressure. B, VIM of diastolic blood pressure. C, hypertension status.
Association Between BPV and Cerebral Arterial Dilation
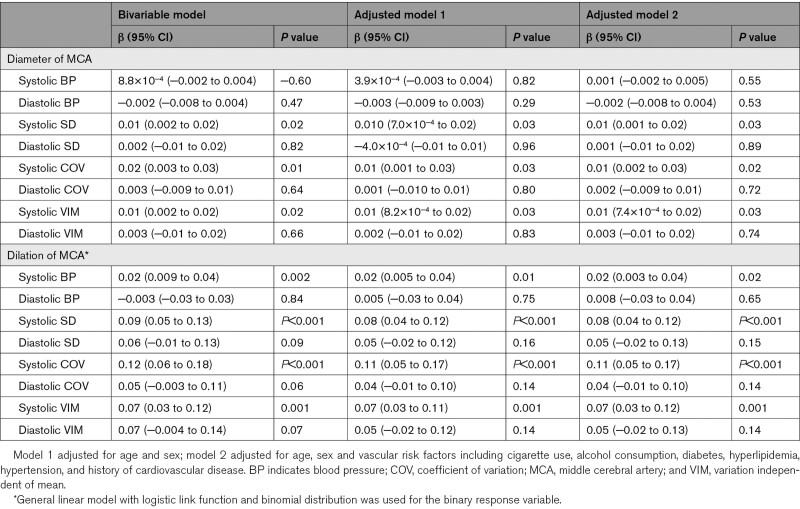
Association between BPV and cerebral arterial dilation was presented in Table 3. Only systolic BPV measures were associated with the diameter of MCA (systolic SD: β=0.01 [95% CI, 0.002–0.02], P=0.02; systolic CoV: β=0.02 [95% CI, 0.003–0.03], P=0.01; systolic VIM: β=0.01 [95% CI, 0.002–0.02], P=0.02). After adjusted for age, sex and risk factors, higher systolic SD (systolic SD: β=0.01 [95% CI, 0.001–0.02], P=0.03; systolic CoV: β=0.01 [95% CI, 0.002–0.03], P=0.02; systolic VIM: β=0.01 [95% CI, 7.4×10−4 to 0.02], P=0.03) was still associated with increased MCA diameter. Meanwhile, presence of MCA dilation was associated with only three systolic BPV measures (systolic SD: β=0.09 [95% CI, 0.05–0.13], P<0.001; systolic CoV: β=0.12 [95% CI, 0.06–0.18], P<0.001; systolic VIM: β=0.07 [95% CI, 0.03–0.12], P=0.001), and such associations remained after adjusted for age, sex and risk factors (systolic SD: β=0.08 [95% CI, 0.04–0.12], P<0.001; systolic CoV: β=0.11 [95% CI, 0.05–0.17], P<0.001; systolic VIM: β=0.07 [95% CI, 0.03–0.12], P=0.001). In the subgroup with BMI information, after adjusted for BMI and all risk factors, presence of MCA dilation still associated with elevated BPV, while no significant association between MCA diameter and BPV was found as shown in the Table S5.
Table 3.
Association Between Blood Pressure Variability and Cerebral Arterial Dilation
As shown in the Table S6, in hypertensive individuals, increased systolic VIM was associated with increased diameter of MCA (β=0.02 [95% CI, 0.004–0.03], P=0.01) after adjusted for age, sex, and risk factors. No significant association between BPV measures and cerebral arterial dilation was found in nonhypertensive individuals (Table S7), and no significant interactions were observed (Table S8).
Cerebral Arterial Dilation and WMH
The relationships between cerebral arterial dilation and WMH were further examined as shown in Table 4. Total WMH was associated with MCA diameter before (β=0.22 [95% CI, 0.14–0.29], P<0.001) and after adjusted for age, sex, and risk factors (β=0.17 [95% CI, 0.11–0.23], P<0.001). Higher total WMH volume was also found in individuals with dilated MCA (β=0.96 [95% CI, 0.62–1.3], P<0.001). Such patterns were similarly observed for PWMH, but not DWMH.. Only MCA diameter was associated with DWMH volume after adjusted for age, sex and risk factors (β=0.11 [95% CI, 0.02–0.20], P=0.01). In a more focal view, the presence of WMH in the basal ganglia region was significantly associated with increased MCA diameter (β=0.28 [95% CI, 0.18–0.37], P<0.001) and MCA dilation (β=1.0 [95% CI, 0.55–1.44], P<0.001).
Table 4.
Association Between Cerebral Arterial Dilation and White Matter Hyperintensity
Discussion
In this study, BPV indices including CoV, SD, and VIM were calculated based on consecutive measurements of BP over 7 days. CoV allows us to see how great the dispersion is around the mean, as larger CoV values indicate greater variability in BP. VIM, however, was used to help assess the associations of BPV with WMH and arterial dilation irrespect of the mean BP levels. Consequently, significant associations of VIM with several indices of vascular lesions identified in this study suggest large fluctuations in BP alone would increase the liability of the cerebrovasculature to damages. The current study provides preliminary data for future prospective studies investigating the driving forces for spatially distinct clusters of WM lesions and also suggests more active investigations on stabilizing BP for hypertensive individuals as a possible means to preserve the soundness of the cerebrovasculature should be carried out.
BPV and WMH
High BP during mid-life is widely recognized as a modifiable risk for late-life cerebrovascular disease.25,26 To date, the association between high BP and CSVD in elderly individuals has been well established in cross-sectional and longitudinal studies.27,28 In addition to higher BP, large variations in BP from consecutive measures may also contribute to CSVD.29–31 Nonetheless, such relationship between BPV and WMH is still largely debated. Zhou et al20 suggested that long-term or short-term SD of BP are not associated with WMH features measured, and similarly, others reported no association between 24-hour SD of BP and the presence of WMH.32 In this study, increases in all three short-term measurements of BPV in systolic or diastolic BP were found to be associated with increased WMH lesion volume independent of hypertension status, and the results are the most pronounced in hypertensive patients. Such results may suggest that increased mean BP may increase the vulnerability of the vascular system to large fluctuations in BP.
Various BPV measurements have been used in previous studies which might be the reason for the inconsistencies found in the reported association between BPV and cerebral lesions. For example, Zhou et al20 used SD based on seven days home BP measurements, while Filomena et al30 used several BPV features including SD, CoV, and weighted-SD based on 24 hours BP monitoring. Another possible reason for these inconsistent results was the differences in the measurement of WMH. In this study, after dividing the WMHs into PWMHs and DWMHs, elevated BPV, as indicated by all 3 BPV indices used, was only found associated with higher PWMH volume. Moreover, the spatial distribution pattern of WMH associated with elevated BPV is distinct from that associated with hypertension, suggesting the spatial distinct clusters of WMHs might be implicated in different pathological processes. PWMH was associated with mean arterial pressure, since the periventricular region were supplied by long straight arteries with very few branches, resulting in greater pressure transmitted directly to the small resistance vessels.8,33 On the contrary, the small arterioles of the cortex usually branch off long arteries with numerous branches, which resulted in a drop in BP.8 Variations in BP would, thus, expected to have diminished effects on the white matter. The heterogeneous nature of WMHs could probably underpin the discrepancies found in previous studies. Future studies should be cautious in evaluating WMH, while more attention is needed to explore other mechanisms associated with DWMH.
BPV and Cerebral Artery Dilation
The cerebral artery dilation discussed in the current study is the one of the common used imaging diagnostic criteria of IADE.12,13 The prevalence of MCA dilation in the current study was nearly 5%, similar to the prevalence reported in the stroke-free population (5.4%) and lower than that in patients with stroke (12%).34,35
In this study, systolic BPV was associated with intracranial arterial dilation. In previous studies, risk factors for IADE have been mainly investigated in patient with stroke.13 Aging, hypertension, and a history of myocardial infarction have been associated with IADE.34 In a stroke-free cohort study, only aging was found to be associated with IADE.35 Nevertheless, BPV has been associated with the functions of vessel wall, including endothelial function and smooth muscle function,36 which may be one of the reasons for the link between BPV and IADE. Furthermore, BPV was found to be associated with the prevalence of MCA dilation only in hypertensive individuals. This result suggested that elevated BPV might be more likely to induce intracranial vasodilation in hypertensive individuals. Since the arterial stiffness is associated with hypertension,37 fragile vessels walls might be more vulnerable to elevated BPV, leading to the development of excessive arterial dilation. However, the mechanism of MCA dilation in normotensive individuals remains unclear.
Cerebral Artery Dilation and WMH
Previous studies have reported the association between carotid dilation and CSVD.38,39 In this study, except for carotid dilation, intracranial arterial diameters were assessed. Increasement of MCA diameter was associated with increased WMH lesion volume, adding the additional knowledge that intracranial arterial dilation was associated not only with the presence of WMH but also with the severity of WMH. There were at least 2 possible explanations for this finding.
First, proximal vessels might undergo compensatory dilation when distal small vessel disease happened. In the experimental study, cerebral hypoperfusion due to unilateral common carotid artery occlusion is gradually restored over several months by an increase in diameter of the contralateral internal carotid artery.40 The association of increased diameter of the carotid artery with CSVD is also thought to be a compensatory mechanism to counteract the increase in stiffness and thickness of the arterial wall to maintain normal arterial compliance.38
Second, proximal vascular dilatation might lead to increased distal BP, which results in the fragile distal vessels being more vulnerable to the impact of BP. The cerebral circulation is thought to be susceptible to pressure damage, since the torrential circulation receives less vascular resistance.41 According to the Poiseuille’s law, the pressure drop is inversely proportional to the fourth power of the lumen radius. As a result, the dilated proximal vessels inevitably have a reduced partial pressure capacity, and thus elevating the BP in the distal vessels would lead to the disruption of small vessels. Moreover, it has been suggested that elevated BP may affect CSVD by reshape the large intracranial arteries.42
Nevertheless, it is more likely that the above 2 processes interact with each other, forming a vicious circle, which promotes the growth of WMH. A similar vicious cycle related to morphological modifications between large and small arteries caused by hypertension has been reported previously.43 Future longitudinal studies could help to distinguish the sequence of these two processes, and thus allowing for different interventions to reduce damage to the cerebrovascular system.
Limitation
There are several limitations to be addressed. First, day-to-day BPV measures can only be obtained from hospitalized subjects in this current study, since home BP measuring requires training of the subjects and the quality/credibility of data cannot be gauranteed. Further investigations are needed to determine the relationships of BPV over longer periods or 24-hour BPV with WMH and IADE. Second, the assessments of cerebral arterial dilation were based on internal diameter of the vessels. It is not yet available to direct assess modifications in the vessel wall from our current MRA scans. The use of additional imaging modalities and techniques will facilitate direct analysis of the intracranial vascular wall. Third, the configuration of the circle of Willis was not included in the analysis. The effect of the circle of Willis on the cerebral arterial dilation still needs further studies. Fourth, causal relationships between BPV and the vasculopathies could not be established in this study due to the retrospective design and observational nature. Since increased BPV might be a result of disturbed cerebral autoregulation caused by damaged vascular services. Further longitudinal studies will help distinguish the sequence of these 2 pathological alterations, and thus allowing for different interventions to reduce damage to the cerebrovascular system. Although a large sample was drawn (2634), most of them were elder Chinese residing in Shanghai, with two-thirds of them being hypertensive. As a result, our findings were not pronounced in the normotensive subpopulation and should also be interpreted with caution. Future studies involving more normotensive individuals are required to establish the association between BPV and cerebral vasculopathy.
Perspectives
This study contributes to elucidating the impact of BPV on the cerebral vasculature. Currently, higher BP have been associated with increased CSVD. This study indicated that variation in BP, independent with BP level, was associated with both large cerebral arterial modification and small vessel disease, suggesting that elevated BPV might be one of the common pathophysiological phenomena involving in these cerebral vasculopathy. Cautions, though, were warranted since our study, limited by the retrospective design, was incable of establishing a causal relationship between excessive BPV and cerebral vasculopathy. Future longitudinal studies investigating the temporal sequence of cerebral vascular lesions and BPV changes would be required for better clinical relevance.
Article Information
Acknowledgments
We are greatly thankful to all the members in our research group at Fudan University and Yueyang Hospital who helped to accomplish the study.
Sources of Funding
This work was supported by the National Key R&D Program of China (No. 2018YFC1312900), National Natural Science Foundation of China (No. 81971583), Shanghai Natural Science Foundation (No. 20ZR1406400), Shanghai Municipal Science and Technology Major Project (No.2017SHZDZX01, No.2018SHZDZX01) and ZJLab.
Disclosures
None.
Supplemental Materials
Detailed Methods
Tables S1–S8
Figures S1 and S2
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- BPV
- blood pressure variability
- CoV
- coefficient of variation
- CSVD
- cerebral small vessel disease
- DWMH
- deep white matter hyperintensity
- HDL
- high-density lipoprotein
- IADE
- intracranial arterial dolichoectasia
- MCA
- middle cerebral artery
- MRA
- magnetic resonance angiography
- MRI
- magnetic resonance imaging
- PWMH
- periventricular white matter hyperintensity
- VIM
- variation independent of mean
- WMH
- white matter hyperintensity
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.122.19269.
For Sources of Funding and Disclosures, see page 1463.
References
- 1.Williams B, Mancia G. Ten commandments of the 2018 ESC/ESH HTN guidelines on hypertension in adults. Eur Heart J. 2018;39:3007–3008. doi: 10.1093/eurheartj/ehy439 [DOI] [PubMed] [Google Scholar]
- 2.Meissner A. Hypertension and the brain: a risk factor for more than heart disease. Cerebrovasc Dis. 2016;42:255–262. doi: 10.1159/000446082 [DOI] [PubMed] [Google Scholar]
- 3.Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88:669–674. doi: 10.1136/jnnp-2016-315324 [DOI] [PubMed] [Google Scholar]
- 4.Kario K, Wang JG. Could 130/80 mm hg be adopted as the diagnostic threshold and management goal of hypertension in consideration of the characteristics of asian populations? Hypertension. 2018;71:979–984. doi: 10.1161/HYPERTENSIONAHA.118.11203 [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X [DOI] [PubMed] [Google Scholar]
- 6.Beckett LA, Rosner B, Roche AF, Guo S. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol. 1992;135:1166–1177. doi: 10.1093/oxfordjournals.aje.a116217 [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, Papatheodorou S. Blood pressure variability and cerebral small vessel disease: a systematic review and meta-analysis of population-based cohorts. Stroke. 2020;51:82–89. doi: 10.1161/STROKEAHA.119.026739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco PJ, Müller LO, Spence JD. Blood pressure gradients in cerebral arteries: a clue to pathogenesis of cerebral small vessel disease. Stroke Vasc Neurol. 2017;2:108–117. doi: 10.1136/svn-2017-000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, Shibata M, Ohtsubo T, Kitazono T, Kiyohara Y, Ninomiya T. Day-to-day blood pressure variability and risk of dementia in a general japanese elderly population: the hisayama study. Circulation. 2017;136:516–525. doi: 10.1161/CIRCULATIONAHA.116.025667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes D, Judge C, Murphy R, Loughlin E, Costello M, Whiteley W, Bosch J, O’Donnell MJ, Canavan M. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323:1934–1944. doi: 10.1001/jama.2020.4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedla YG, Yano Y, Carnethon M, Greenland P. Association between long-term blood pressure variability and 10-year progression in arterial stiffness: the multiethnic study of atherosclerosis. Hypertension. 2017;69:118–127. doi: 10.1161/HYPERTENSIONAHA.116.08427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez J, Sacco RL, Wright CB. Dolichoectasia-an evolving arterial disease. Nat Rev Neurol. 2011;7:41–50. doi: 10.1038/nrneurol.2010.181 [DOI] [PubMed] [Google Scholar]
- 13.Pico F, Labreuche J, Amarenco P. Pathophysiology, presentation, prognosis, and management of intracranial arterial dolichoectasia. Lancet Neurol. 2015;14:833–845. doi: 10.1016/S1474-4422(15)00089-7 [DOI] [PubMed] [Google Scholar]
- 14.Bullitt E, Zeng D, Mortamet B, Ghosh A, Aylward SR, Lin W, Marks BL, Smith K. The effects of healthy aging on intracerebral blood vessels visualized by magnetic resonance angiography. Neurobiol Aging. 2010;31:290–300. doi: 10.1016/j.neurobiolaging.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Sun J, Hippe DS, Balu N, Yuan Q, Yuan I, Zhao X, Li R, He L, Hatsukami TS, et al. Quantitative assessment of the intracranial vasculature in an older adult population using iCafe. Neurobiol Aging. 2019;79:59–65. doi: 10.1016/j.neurobiolaging.2019.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. ; STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos D, Wolters FJ, Darweesh SKL, Vernooij MW, de Wolf F, Ikram MA, Hofman A. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimers Dement. 2018;14:1482–1492. doi: 10.1016/j.jalz.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 18.Wartolowska KA, Webb AJS. Midlife blood pressure is associated with the severity of white matter hyperintensities: analysis of the UK Biobank cohort study. Eur Heart J. 2021;42:750–757. doi: 10.1093/eurheartj/ehaa756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, Malone IB, Lu K, James SN, Keshavan A, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 british birth cohort (insight 46): an epidemiological study. Lancet Neurol. 2019;18:942–952. doi: 10.1016/S1474-4422(19)30228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou TL, Rensma SP, van der Heide FCT, Henry RMA, Kroon AA, Houben AJHM, Jansen JFA, Backes WH, Berendschot TTJM, Schouten JSAG, et al. Blood pressure variability and microvascular dysfunction: the Maastricht Study. J Hypertens. 2020;38:1541–1550. doi: 10.1097/HJH.0000000000002444 [DOI] [PubMed] [Google Scholar]
- 21.Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–155. doi: 10.1038/nrcardio.2013.1 [DOI] [PubMed] [Google Scholar]
- 22.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643. [PMC free article] [PubMed] [Google Scholar]
- 23.Griffanti L, Jenkinson M, Suri S, Zsoldos E, Mahmood A, Filippini N, Sexton CE, Topiwala A, Allan C, Kivimäki M, et al. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: A study in older adults. Neuroimage. 2018;170:174–181. doi: 10.1016/j.neuroimage.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 24.Rostrup E, Gouw AA, Vrenken H, van Straaten EC, Ropele S, Pantoni L, Inzitari D, Barkhof F, Waldemar G; LADIS study group. The spatial distribution of age-related white matter changes as a function of vascular risk factors–results from the LADIS study. Neuroimage. 2012;60:1597–1607. doi: 10.1016/j.neuroimage.2012.01.106 [DOI] [PubMed] [Google Scholar]
- 25.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2 [DOI] [PubMed] [Google Scholar]
- 27.Wright SN, Kochunov P, Mut F, Bergamino M, Brown KM, Mazziotta JC, Toga AW, Cebral JR, Ascoli GA. Digital reconstruction and morphometric analysis of human brain arterial vasculature from magnetic resonance angiography. Neuroimage. 2013;82:170–181. doi: 10.1016/j.neuroimage.2013.05.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legdeur N, Visser PJ, Woodworth DC, Muller M, Fletcher E, Maillard P, Scheltens P, DeCarli C, Kawas CH, Corrada MM. White matter hyperintensities and hippocampal atrophy in relation to cognition: the 90+ study. J Am Geriatr Soc. 2019;67:1827–1834. doi: 10.1111/jgs.15990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein IB, Bartzokis G, Hance DB, Shapiro D. Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke. 1998;29:765–772. doi: 10.1161/01.str.29.4.765 [DOI] [PubMed] [Google Scholar]
- 30.Filomena J, Riba-Llena I, Vinyoles E, Tovar JL, Mundet X, Castañé X, Vilar A, López-Rueda A, Jiménez-Baladó J, Cartanyà A, et al. ; ISSYS Investigators. Short-term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66:634–40; discussion 445. doi: 10.1161/HYPERTENSIONAHA.115.05440 [DOI] [PubMed] [Google Scholar]
- 31.Chokesuwattanaskul A, Cheungpasitporn W, Thongprayoon C, Vallabhajosyula S, Bathini T, Mao MA, Cato LD, Chokesuwattanaskul R. Impact of circadian blood pressure pattern on silent cerebral small vessel disease: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e016299. doi: 10.1161/JAHA.119.016299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sierra C, de La Sierra A, Mercader J, Gómez-Angelats E, Urbano-Márquez A, Coca A. Silent cerebral white matter lesions in middle-aged essential hypertensive patients. J Hypertens. 2002;20:519–524. doi: 10.1097/00004872-200203000-00028 [DOI] [PubMed] [Google Scholar]
- 33.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539 [DOI] [PubMed] [Google Scholar]
- 34.Pico F, Labreuche J, Touboul PJ, Amarenco P; GENIC Investigators. Intracranial arterial dolichoectasia and its relation with atherosclerosis and stroke subtype. Neurology. 2003;61:1736–1742. doi: 10.1212/01.wnl.0000103168.14885.a8 [DOI] [PubMed] [Google Scholar]
- 35.Zhai FF, Yan S, Li ML, Han F, Wang Q, Zhou LX, Ni J, Yao M, Zhang SY, Cui LY, et al. Intracranial arterial dolichoectasia and stenosis: risk factors and relation to cerebral small vessel disease. Stroke. 2018;49:1135–1140. doi: 10.1161/STROKEAHA.117.020130 [DOI] [PubMed] [Google Scholar]
- 36.Diaz KM, Veerabhadrappa P, Kashem MA, Thakkar SR, Feairheller DL, Sturgeon KM, Ling C, Williamson ST, Kretzschmar J, Lee H, et al. Visit-to-visit and 24-h blood pressure variability: association with endothelial and smooth muscle function in african americans. J Hum Hypertens. 2013;27:671–677. doi: 10.1038/jhh.2013.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brisset M, Boutouyrie P, Pico F, Zhu Y, Zureik M, Schilling S, Dufouil C, Mazoyer B, Laurent S, Tzourio C, Debette S. Large-vessel correlates of cerebral small-vessel disease. Neurology. 2013;80:662–669. doi: 10.1212/WNL.0b013e318281ccc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai FF, Yang M, Wei Y, Wang M, Gui Y, Han F, Zhou LX, Ni J, Yao M, Zhang SY, et al. Carotid atherosclerosis, dilation, and stiffness relate to cerebral small vessel disease. Neurology. 2020;94:e1811–e1819. doi: 10.1212/WNL.0000000000009319 [DOI] [PubMed] [Google Scholar]
- 40.Struys T, Govaerts K, Oosterlinck W, Casteels C, Bronckaers A, Koole M, Van Laere K, Herijgers P, Lambrichts I, Himmelreich U, et al. In vivo evidence for long-term vascular remodeling resulting from chronic cerebral hypoperfusion in mice. Journal of Cerebral Blood Flow and Metabolism. 2017;37:726–739. doi: 10.1177/0271678x16638349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65 [DOI] [PubMed] [Google Scholar]
- 42.Zhang B, Wang Y, Wang B, Chu Y-H, Jiang Y, Cui M, Wang H, Chen X. MRI-based investigation of association between cerebrovascular structural alteration and white matter hyperintensity induced by high blood pressure. Journal of Magnetic Resonance Imaging. 2021;54:1516–1526. doi: 10.1002/jmri.27815 [DOI] [PubMed] [Google Scholar]
- 43.Maass A, Düzel S, Goerke M, Becke A, Sobieray U, Neumann K, Lövden M, Lindenberger U, Bäckman L, Braun-Dullaeus R, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20:585–593. doi: 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.