Background:
Whether the combination of different blood pressure and arterial stiffness (AS) status is independently associated with diabetes has not been fully investigated so far. This study aimed at investigating the status of hypertension and AS in determining diabetes.
Methods:
This prospective cohort study included 11 156 participants from the Kailuan study. AS was measured by brachial-ankle pulse wave velocity. We compared the risk of diabetes between individuals with ideal vascular function (defined as normotension with normal AS), normotension with elevated AS, hypertension with normal AS, and hypertension with elevated AS.
Results:
After a median follow-up of 6.16 years, diabetes occurred in 768 participants. Compared with ideal vascular function group, the highest risk of diabetes was observed in hypertension with elevated AS group (hazard ratio, 2.42 [95% CI, 1.93–3.03]), followed by normotension with elevated AS group (hazard ratio, 2.11 [95% CI, 1.68–2.66]), hypertension with normal AS group exhibited the lowest risk of diabetes (hazard ratio, 1.48 [95% CI, 1.08–2.02]). Multiple sensitivity and subgroup analyses yielded similar results. Furthermore, the additional of AS to a conventional model including traditional risk factors had a higher incremental effect on the predictive value for diabetes than the addition of hypertension (the C statistics was 0.707 versus 0.695; the integrated discrimination improvement was 0.65% versus 0.28%; net reclassification improvement was 40.48% versus 34.59%).
Conclusions:
Diabetes is associated with not only hypertension but also AS. Additionally, AS shows a better predictive ability than hypertension in predicting diabetes.
Keywords: arterial stiffness, blood pressure, diabetes, hypertension, prospective study
Novelty and Relevance.
What Is New?
This prospective study found that diabetes is associated with not only hypertension but also arterial stiffness, additionally, arterial stiffness shows a better predictive ability than hypertension in predicting diabetes.
Individuals with the isolated systolic, isolated diastolic, high systolic and diastolic, controlled and uncontrolled hypertensives, the combination of hypertension and elevated arterial stiffness showed a higher risk of diabetes than other groups.
What Is Relevant?
Our present findings emphasized the importance of hypertension status and elevated arterial stiffness in the development of diabetes. Combination control of hypertension and AS may help reduce the risk of diabetes.
Clinical/pathophysiological implications?
In a community-based population, we found that the risk of diabetes was significantly associated with elevated arterial stiffness measured by brachial-ankle pulse wave velocity as well as hypertension. In addition, AS performed better than hypertension in predicting diabetes, which provided novel insight into future preventive strategies against diabetes.
Diabetes is an important global public health problem with high morbidity and disability. The World Health Organization estimated that the prevalence of diabetes in adults was 9.3% in 2019. Due to the aging population and unhealthy lifestyles, the prevalence of diabetes tends to continue to rise worldwide, which is estimated to reach 10.9% by 2045.1 Diabetes can mediate multiple organ damage, leading to cardiovascular events, kidney disease, and cerebrovascular complications.2–4 Therefore, early identification of individuals at high-risk of developing diabetes is of clinical importance, for early risk assessment and intervention can help prevent the onset and slow the progress of diabetes.
Hypertension and diabetes frequently coexist in the same individuals.5 The 2 diseases have etiological aspects in common, such as obesity, inflammation, oxidative stress, insulin resistance, and factors associated with increased microvascular and macrovascular impairment.6 Studies have demonstrated that hypertensive subjects were at higher risk of developing diabetes than the normotensive subjects.7,8 Therefore, hypertension may be considered as the greatest provoking factors for incident diabetes. Arterial stiffness (AS), a common finding in patients with hypertension, is also reported to be associated with the risk of insulin resistance and diabetes.9–12 Path analysis showed that brachia-ankle pulse wave velocity (baPWV), a widely used method for assessing AS,13 appeared to precede the increase in fasting blood glucose (FBG).12 These results indicate that both hypertension and AS may predict the risk of diabetes.
Furthermore, a study extending the findings showed the presence of hypertensive target organ damage (such as carotid atherosclerosis) increased the risk of diabetes, indicating that preclinical diabetes may be attributable to hypertension and the vascular damage.14 AS has been reported as a better index than blood pressure (BP) in predicting adverse clinical events.15–18 However, whether AS was better than BP in predicting diabetes has not been investigated up to now. Therefore, we performed the current study to investigate the status of hypertension and AS (measured by baPWV) in determining future risk of diabetes based on a community-based population in China.
Research Design and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Population
We used data from the Kailuan study, which is an ongoing prospective study conducted in Tangshan, China. The details of the study have been described elsewhere.19,20 Participants aged ≥18 years were included in the first survey from the Kailuan community and were followed up biennially. Since the third survey in 2010, participants who consented to join nested studies on vascular health received a measurement of baPWV to assess the health status of artery wall. In the present study, a total of 22 310 participants with at least 1 survey from 2010 to 2014 were included, after excluding 9306 participants without synchronized data, 1848 participants with previous prediabetes or diabetes, a total of 11 156 participants were enrolled in the final analysis (Figure S1). The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006-05) and Beijing Tiantan Hospital (approval number: 2010-014-01). All the participants agreed to take part in the study and provided written informed consent.
BP Measurement
BP was measured in the left upper arm using a calibrated mercury sphygmomanometer in a sitting position. At least 2 BP measurements were taken after 5 minutes of rest. BP was then measured again if the difference between the two measurements was ≥5 mm Hg, then the average value was taken. Mean arterial pressure was calculated as 1/3 systolic BP + 2/3 diastolic BP. Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg, any use of the antihypertensive drug, or a self-reported history of hypertension.
BaPWV Measurement
BaPWV was measured by a BP-203 RPE III networked AS detection device (Omron Health Medical [China], Co, LTD), as detailed elsewhere.12,21,22 Participants of the baPWV subcohort received repeated measurements following same standards. The procedure was performed by specially trained nurses from 7 to 9 am on the examination day, following the manufacture’s recommendations. After being seated for at least 5 minutes in a room with the temperature controlled between 22 °C and 25 °C, participants in thin clothes were asked to lie down on the examination couch in a supine position and remain quiet during the measurement. Cuffs were wrapped on both arms and ankles. The lower edges of the arm cuffs were positioned 2 to 3 cm above the transverse striation of the cubital fossa, while the lower edges of the ankle cuffs were positioned 1 to 2 cm above the superior aspect of the medial malleolus. One heart sound detector was placed at the left edge of the sternum. Measurements were repeated twice for each at a 5-minute interval, with the second data considered the final value. The maximum of left and right sides of baPWV was used for analysis. The normal range of baPWV was defined as <1400 cm/s, and elevated AS was defined as baPWV ≥1400 cm/s.12,23,24
Definition of Diabetes
Fasting blood samples (8–10 hours) of 5 mL were taken from the antecubital vein on an empty stomach from 7 to 9 am. The supernatant was centrifuged at room temperature and measured within 4 hours. FBG was measured using the hexokinase/glucose-6-phosphate dehydrogenase method (Mind Bioengineering Co. Ltd, Shanghai, China) with an upper limit of detection of 30.07 mmol/L.25 Diabetes was defined as either FBG ≥7.0 mmol/L, self-report of a physician diagnosis, or self-report use of antidiabetic medication. The primary outcomes of the current study was incident diabetes during the follow-up period among those without history of diabetes.
Covariate Measurement
Information on birth date, sex, smoking status, alcohol consumption, and past medical history (hypertension, diabetes, dyslipidemia, antihypertensive, hypoglycemic, and lipid-lowering medications, etc) were collected via a structural questionnaire. Smoking and drinking status were stratified into 2 levels: current and never or former. Heart rate, height, weight, and waist circumference were measured by trained nurses and body mass index was calculated as weight in kilograms divided by height in meters squared. Concentrations of total cholesterol, triglyceride, LDL-C (low-density lipoprotein cholesterol), HDL-C (high-density lipoprotein cholesterol), serum uric acid, and hs-CRP (high-sensitivity C-reactive protein) were measured by auto analyzer (Hitachi 747, Hitachi, Tokyo, Japan). Estimated glomerular filtration rate was calculated by the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.26
Statistical Analysis
The participants were divided into 4 groups, according to their hypertension (BP ≥140 mm Hg systolic or ≥90 mm Hg diastolic or usage of antihypertensive medication) and AS status: (1) ideal vascular function (IVF) group: normotension and baPWV <1400 cm/s; (2) normotension with elevated AS group: normotension and baPWV ≥1400 cm/s; (3) hypertension and normal AS group: hypertension and baPWV<1400 cm/s; (4) hypertension and elevated AS (HTAS) group: hypertension and baPWV ≥1400 cm/s. Continuous variables with normal and skewed distributions were described as the mean±SD and the medians with interquartile ranges, respectively, whereas categorical variables were described as frequencies and percentages. One-way ANOVA, Kruskal-Wallis test, and χ2 test were used to compare the differences in characteristics across baseline groups. Person-years was calculated from baseline to the first occurrence of diabetes, mortality, or the end of the study (December 31, 2017), whichever came first. The incidence rate of diabetes was calculated by dividing the number of incident cases by the total follow-up duration (person-years).
Time-to-event data were analyzed by the Kaplan-Meier estimator, with the difference between groups compared by the log-rank test. Cox proportional hazard regression models were used to compare the risk of incident diabetes across groups. Hazard ratios (HRs) with 95% CIs were calculated, with IVF group as the reference group. The proportional hazard assumptions were evaluated by visualization of Schoenfeld residuals, and no potential violation was observed. Three models were constructed step-by-step. Model 1 was unadjusted; model 2 was adjusted for age, sex, body mass index, heart rate, smoking status, and alcohol consumption; model 3 was further adjusted for dyslipidemia, total cholesterol, LDL-C, HDL-C, serum uric acid, hs-CRP, and estimated glomerular filtration rate. Additionally, considering baPWV is dependent on distending pressure of the artery, we added another 2 analyses by adjusted for mean arterial pressure (model 4) and DBP (model 5), separately.
Several sensitivity analyses were performed to test the robustness of the findings. First, hypertension was redefined as BP ≥130/80 mm Hg or by the usage of antihypertensive medications. Second, AS was defined used an age- and sex- specific cut point of baPWV according to a previous report about the normal and reference values of baPWV based on the healthy individuals of the Kailuan cohort (Table S1).27 Third, to explore the potential impact of reverse causality, we repeated the primary analysis using a 1-year lag period by excluding participants who developed diabetes within the first 1 year of follow-up. Fourth, the competing risk model with nondiabetes death being regarding as a competing risk event was applied. Fifth, the primary analysis was repeated by refining diabetes as the presence of new diabetes in 2 consecutive examinations during the follow-up period. Finally, AS was classified into three categories according to baPWV values: baPWV <1400 cm/s indicated normal AS, 1400 to 1800 indicated moderate AS, and ≥1800 cm/s indicated severe AS. Then participants were divided into 6 groups according to hypertension and AS status.
To explore the contribution of AS to hypertension, using systolic BP <140 mm Hg and DBP <90 mm Hg as a cutoff point, subgroup analyses were performed in the hypertensive controlled group and the uncontrolled group, and the effect of isolated systolic, isolated diastolic, high systolic and diastolic hypertension were performed as well. In each subgroup, individuals in the IVF group were taken as reference. Additionally, we used C statistics, integrated discrimination improvement, and net reclassification index to compare the incremental predictive value of hypertension, AS and their combined effect beyond other conventional risk factors.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). All the statistical tests were 2-sided, and P<0.05 was considered statistical significance.
Results
Baseline Characteristics
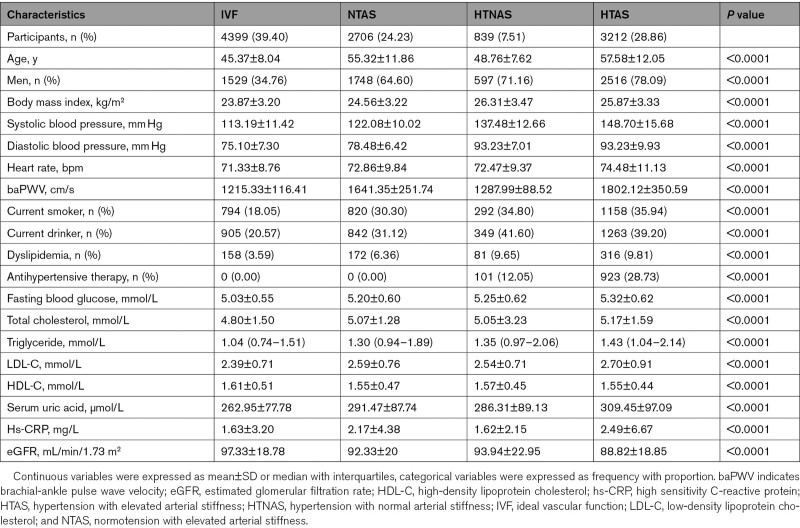
A total of 11 166 participants were enrolled in this study. A comparison of the differences between included and excluded participants due to missing information was presented in Table S2. Most baseline characteristics were balanced between the 2 groups, except for age, sex, the proportion of current smokers and drinkers. The baseline characteristics across the 4 groups are presented in Table 1. Participants in the HTAS group tended to be older, men, more obese with higher body mass index, have a higher heart rate, more current smokers and drinkers. Likewise, significant differences in biological parameters were observed among the groups, participants in the HTAS group had a high level of FBG, total cholesterol, LDL-C, serum uric acid, and hsCRP, and a lower level of HDL-C and estimated glomerular filtration rate than other groups.
Table 1.
Baseline Characteristics of the Study Population
Risk of Diabetes in Groups by baPWV and Hypertension Status
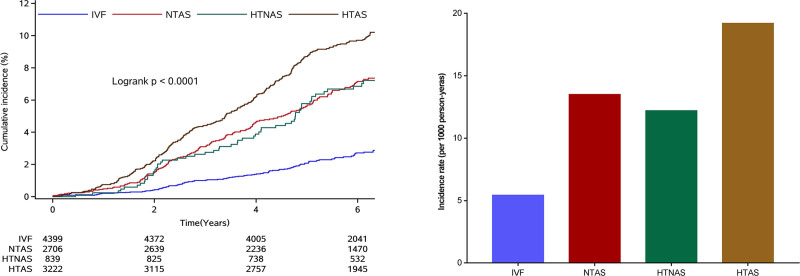
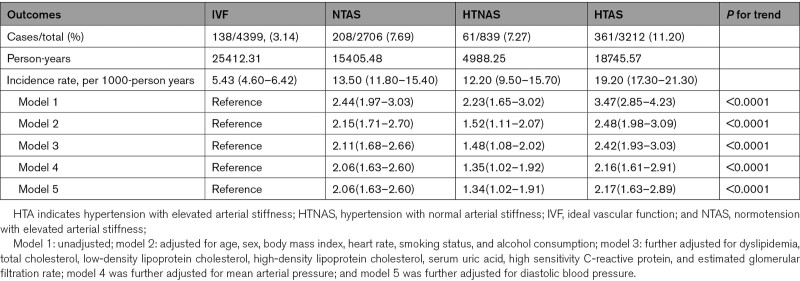
During a median follow-up of 6.16 years (interquartile range: 5.02–7.00), 768 (6.88%) diabetes were identified. Participants in the HTAS group experienced a higher risk of diabetes than participants of other groups during the follow-up period (P<0.0001 for log rank, Figure 1). After adjusted for potential variables, when comparing with IVF group, participants in the HTAS had the highest risk of diabetes (HR, 2.42 [95% CI, 1.93–3.03]), followed by participants in the normotension with elevated AS group (HR, 2.11 [95% CI, 1.64–2.61]), the lowest risk of diabetes was observed in the hypertension and normal AS group (HR, 1.48 [95% CI, 1.08–2.02]). The results did not change materially after adjusted for mean arterial pressure or DBP (Table 2).
Figure 1.
Cumulative incidence of diabetes by hypertension and arterial stiffness status. HTAS indicates hypertension with elevated arterial stiffness; HTNAS, hypertension with normal arterial stiffness; IVF, ideal vascular function; and NTAS, normotension with elevated arterial stiffness.
Table 2.
Association of Different Hypertension and Arterial Stiffness Status With Risk of Diabetes
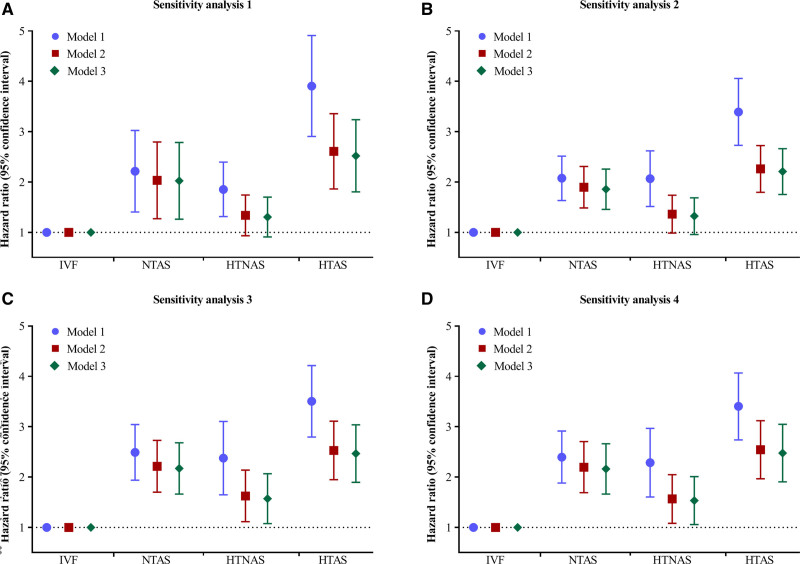
Sensitivity analysis using 130/80 mm Hg or antihypertensive medication as another definition of hypertension, and using age- and sex-specific cutoff points of AS yielded similar results in the crude model, after adjusting for potential confounders, only the individuals in the HTAS group exerted a higher risk of diabetes (Figure 2, Tables S3 and S4). Sensitivity analyses by excluding incident diabetes within the first follow-up (n=79), with competing risk model yielded similar results, and redefining diabetes as the presence of new diabetes in 2 consecutive examinations (Figure 2, Tables S5 through S7). In sensitivity analysis classifying participants into 6 groups, the highest risk of diabetes was observed for participants with hypertension and severe AS had (adjusted HR, 3.21 [95% CI, 2.46–4.19]), followed by those with normotension and severe AS (adjusted HR, 2.78 [95% CI, 1.96–3.95]), the lowest risk of incident diabetes was observed for those with hypertension and normal AS (adjusted HR, 1.48 [95% CI, 1.08–2.02]; Table S8).
Figure 2.
Sensitivity analyses for the association of hypertension and arterial stiffness status with diabetes. Sensitivity analysis 1 was using 130/80 mm Hg as cutoff point to redefine hypertension. Sensitivity analysis 2 was using an age- and sex- specific cutoff point to redefine arterial stiffness status. Sensitivity analysis 3 was performed by excluding participants who developed diabetes cases within the first 1 y of follow-up. Sensitivity analysis 4 was performed using competing risk model by taking nondiabetes related death as competing risk event rather than censoring. Model 1: unadjusted; model 2: adjusted for age, sex, body mass index, heart rate, smoking status, and alcohol consumption; model 3: further adjusted for dyslipidemia, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum uric acid, high sensitivity C-reactive protein, and estimated glomerular filtration rate. HTAS indicates hypertension with elevated arterial stiffness; HTNAS, hypertension with normal arterial stiffness; IVF, ideal vascular function; NTAS, normotension with elevated arterial stiffness.
Subgroup Analysis
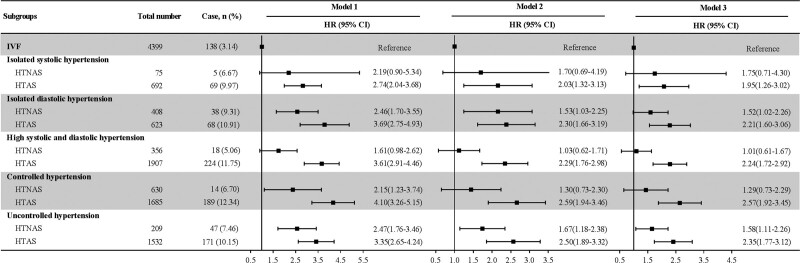
As presented in Figure 3, the analysis of isolated systolic hypertension, isolated diastolic hypertension, individuals with high systolic and diastolic hypertension, controlled, and uncontrolled hypertension showed that the risk of diabetes (HR [95% CI] was 1.95 [1.26–3.02], 2.21 [1.60–3.06], 2.24 [1.72–2.92], 2.57 [1.92–3.45], and 2.35 [95% CI, 1.77–3.12], respectively) in the HTAS group was elevated significantly. For the individuals of isolated systolic hypertension, high systolic and diastolic hypertension, and controlled, the difference of IVF and HTNAS did not reach statistical significance.
Figure 3.
Association of arterial stiffness status combined with isolated systolic, isolated diastolic, high systolic and diastolic, controlled and uncontrolled hypertension with diabetes. In each subgroup analysis, participants with IVF were taken as reference. Model 1: unadjusted; model 2: adjusted for age, sex, body mass index, heart rate, smoking status, and alcohol consumption; model 3: further adjusted for dyslipidemia, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum uric acid, high sensitivity C-reactive protein, and estimated glomerular filtration rate. HTAS indicates hypertension with elevated arterial stiffness; HTNAS, hypertension with normal arterial stiffness; IVF, ideal vascular function; and NTAS, normotension with elevated arterial stiffness.
Incremental Predictive Value of AS and Hypertension
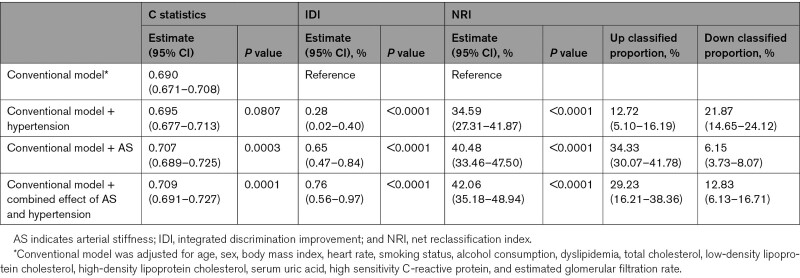
We compared the predictive value of hypertension and AS (Table 3). The C statistics by the conventional model significantly improve with the addition of AS (from 0.690 to 0.707, P=0.0003) but not significantly improve with addition of hypertension (P=0.0807). The discriminatory power and risk reclassification of AS appeared to be substantial better than hypertension, the integrated discrimination improvement for hypertension was 0.28% (95% CI, 0.02%–0.40%; P<0.0001) and 0.65% (95% CI, 0.47%–0.84%; P<0.0001), the corresponding net reclassification index was 34.59% (95% CI, 27.31%–41.87%; P<0.0001) and 40.48% (95% CI, 33.46%–47.50%; P<0.0001). Moreover, the addition of the combined effect of hypertension and AS can significantly further improve the C statistics, the discriminatory power and risk reclassification. Additionally, we also compared the hypertension versus AS, the results showed that shows the C statistics by the conventional model with hypertension significantly improved by the conventional model with AS (from 0.695 to 0.707, P=0.0010), the discriminatory power and risk reclassification was also substantially better, the integrated discrimination improvement was 0.48% (P<0.0001) and the net reclassification index was 40.03% (P<0.0001). This results provided strong evidence on the conclusion that AS had a higher incremental effect on the predictive value of diabetes versus hypertension.
Table 3.
Reclassification and Discrimination Statistics for Arterial Stiffness and Hypertension Status
Discussion
Our prospective study found that compared with individuals with IVF, those with increased AS were at a higher risk in reaching diabetes. Hypertension status amplified these association after a comprehensive adjustment of cardiovascular risk factors. Further analysis showed the conventional risk factors with the addition of AS had a higher predictive value than with the addition of hypertension, indicating AS may be superior to BP in determining future risk of diabetes. The results were robust in multiple sensitivity and subgroup analyses.
There is no consensus on the associations of AS and hypertension with the risk of diabetes. Some studies showed a positive association between AS and diabetes,9–12 demonstrating that PWV of prediabetic and diabetic patients is higher than that of population with normal FBG, and even nondiabetic individuals with normal glucose tolerance test and insulin resistance still had stiff aorta.28–30 Nevertheless, some studies failed to demonstrated a significant association. A Brazilian study found that differences in PWV progression after a 5-year follow-up in a subset of diabetics compared with nondiabetics were not significant.31 The inconclusive results were also observed for hypertension, most studies, but not all showed that participants with elevated BP were at higher risk of developing diabetes after adjusting for some covariates.32,33 Our findings added evidence on the literatures, supporting the hypothesis that AS and hypertension were associated with an increasing risk of diabetes. This was supported by the China Stroke Primary Prevention Trial, which presented a significant positive association between baPWV and the risk of new-onset diabetes in hypertensive patients.34
Several previous studies have reported that AS is superior to BP in predicting future cognitive decline, end organ damage, and cardiovascular events.15–17 AS was measured with different methods in these studies. Some used an invasive method, which is difficult to be applied to clinical practice.15 Some studies used a more complex noninvasive measurement method, namely carotid femoral pulse wave velocity (cfPWV). Although cfPWV is served as a gold standard technique to measure AS, it requires experienced operators and cannot explore the repeatability of measurement results, intraobserver and interobserver differences.17,18 In contrast, baPWV uses brachial cuff-based waveform analysis, can simplify the procedure and provide better reproducibility, making it more popular in some regions. Moreover, evidence has shown that the predictive value of baPWV is similar to that of cfPWV for predicting clinical outcomes.13 Using baPWV as the measuring method, our study found that elevated AS may be superior to high BP in determining diabetes, suggesting that AS is an important determinant in health life span.
The associations between AS and BP has been largely documented. A post hoc analysis of a cross-sectional multicenter study showed that systolic BP was significantly associated with AS in patients with diabetes.35 Another Japanese study showed longitudinal increase of AS appears to be associated with longitudinal elevation of BP to the hypertension range.36 Here, we reported that compared with individuals with IVF, those with increased AS have a higher risk of diabetes, and the individuals with a combination of hypertension and increased AS were at the highest risk in all analyses. Furthermore, a cross-lagged analysis investigated the causal relationship between AS and hypertension and showed that AS played a decisive role in the pathogenesis of hypertension.21 Elevated BP may be the consequence of elevated AS, these may explained our finding that AS was a better predictor than BP in predicting diabetes.
Biological evidence suggests that aging exerts functional and structural influences on large elastic arteries, aging is also associated with oxidative stress and systematic inflammatory.21 These mechanisms may contribute to the development of AS, hypertension, as well as diabetes. In our study, we used age- and sex-specific cutoff point of AS measured by baPWV to investigate the association of AS and hypertension with risk of diabetes, the results were consistent with the primary analysis, indicating that the pathway from AS or hypertension to diabetes did not significantly modified by aging.
Studies have proposed several preventive strategies for AS. Studies have suggested that, compared with β-receptor blockers and diuretics, antihypertensive medications can lower AS, early counter pulsation or central arterial pressure, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and calcium channel blockers, could lower risk of diabetes. Use of these types of drugs in hypertensive population may bring additional beneficial effect for antidiabetes at the same time of preventing AS and lowering BP.37 Apart from that, lifestyle modification, such as smoking cessation,38 weight loss,39 and physical exercise,40 has been shown to exert a modulatory effect on AS. Early detection and possible slowing of the vascular stiffening process with pharmacological agents and lifestyle interventions may reduce the associated risks for diabetes. However, there is still not a paradigm for the best protective prescriptions for vascular function. Although there are studies implying that chronic inflammation and increased oxidative stress are associated with increased AS,41 there is little evidence on treatment specifically aimed to alleviate AS, which may be a result of too much emphasis on risk rather than on protection. Future researches are needed to explore protective prescriptions for vascular function.
The exact mechanisms underlying the relationship of baPWV with risk of diabetes remain to be elucidated. Some previously proposed potential mechanisms are outlined here. First, AS could lead to increased arterial pulse pressure and pulsatile shear, resulting in endothelial dysfunction and metabolic dysregulation.42 Second, AS could cause damage to capillary diastolic dysfunction, shrinkage, or sparse distribution, which then lead to the damage of low-resistance organs, such as pancreas.12 Then the endothelial dysfunction and impaired endothelium-dependent vasodilation may exacerbate insulin resistance by limiting the delivery of glucose to key target tissues, which precede the occurrence of diabetes.43 In addition, AS and diabetes may share the same genetic background. A Mendelian randomization study showed that genetically determined decrease in insulin secretion was association with AS.44 More studies are needed to confirm our findings, and to further investigating the underlying mechanisms involved in this association.
This study has several limitations. First, because we did not assess oral glucose tolerance test and hemoglobin A1c level, there might be un-diagnosed diabetes patients included at baseline in our study group. However, we excluded participants diagnosed with diabetes <1 years to avoid misclassification at baseline and increase specificity of diabetes diagnosis, and performed another sensitivity analysis by redefining diabetes as the presence of new diabetes in 2 consecutive examinations, the results did not change materially. Second, we used baPWV as measurement of AS instead of cfPWV. Although cfPWV was the golden standard, baPWV is reported to be well associated with cfPWV, and the American Heart Association had included baPWV in the recommended criteria for evaluating AS. Third, we did not analyze the effect of antihypertensive agents on AS, considering there was a meta-analysis reporting that the antihypertensive agents have no effect on reducing AS45; such analysis may provide further insight into clinical practice. Finally, hypertension and AS were determined based on a single measurement of baPWV and BP, thus prone to some misclassification bias respect to hypertension and AS status.
In conclusion, our community-based population study showed that the risk of diabetes was associated with not only hypertension, but also AS. Individuals with the combination of HTAS exerted the highest risk, which hold true in isolated systolic, isolated diastolic, high systolic and diastolic, controlled and uncontrolled hypertensives. In addition, AS performed better than hypertension in predicting diabetes, which provided novel insight into future preventive strategies against diabetes.
Perspectives
Whether the combination of different BP and AS status is independently associated with diabetes has not been fully investigated so far. Using data from the Kailuan study, we found that the risk of diabetes was associated with not only hypertension, but also AS. Participants with increased AS and hypertension exerted the highest risk of diabetes, which holds true in hold true in isolated systolic, isolated diastolic, high systolic and diastolic, controlled and uncontrolled hypertensives. Further analysis showed that AS may be superior to BP in determining future risk of diabetes. These findings indicated that preventive strategies on hypertension and AS may also contribute to the prevention of diabetes.
Article Information
Acknowledgments
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group; and the project development and management teams at the Beijing Tiantan Hospital and the Kailuan Group.
Source of Funding
This work was supported by National Key Research and Development Program of China (2018YFC1312400, 2018YFC1312402, and 2017YFC1310902) and Beijing Municipal Administration of Hospitals Incubating Program (PX2020021).
Disclosures
None.
Supplemental Materials
Tables S1–S8
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AS
- arterial stiffness
- baPWV
- brachial-ankle pulse wave velocity
- BP
- blood pressure
- cfPWV
- carotid femoral pulse wave velocity
- FBG
- fasting blood glucose
- HDL-C
- high-density lipoprotein cholesterol
- HR
- hazard ratio
- hs-CRP
- high-sensitivity C-reactive protein
- HTAS
- hypertension and elevated arterial stiffness
- IVF
- ideal vascular function
- LDL-C
- low-density lipoprotein cholesterol
X. Tian and Y. Zuo contributed equally.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.122.19256.
For Sources of Funding and Disclosures, see page 1495.
Reference
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. ; IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Fox CS. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the Framingham Heart Study. Trends Cardiovasc Med. 2010;20:90–95. doi: 10.1016/j.tcm.2010.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukkar L, Kang A, Hockham C, Young T, Jun M, Foote C, Pecoits-Filho R, Neuen B, Rogers K, Pollock C, et al. ; EXTEND45 Study Steering Committee. Incidence and associations of chronic kidney disease in community participants with diabetes: a 5-year prospective analysis of the EXTEND45 Study. Diabetes Care. 2020;43:982–990. doi: 10.2337/dc19-1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardigan T, Ward R, Ergul A. Cerebrovascular complications of diabetes: focus on cognitive dysfunction. Clin Sci (Lond). 2016;130:1807–1822. doi: 10.1042/CS20160397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601–610. doi: 10.1016/S0140-6736(12)60987-8 [DOI] [PubMed] [Google Scholar]
- 6.Moreno B, de Faria AP, Ritter AMV, Yugar LBT, Ferreira-Melo SE, Amorim R, Modolo R, Fattori A, Yugar-Toledo JC, Coca A, et al. Glycated hemoglobin correlates with arterial stiffness and endothelial dysfunction in patients with resistant hypertension and uncontrolled diabetes mellitus. J Clin Hypertens (Greenwich). 2018;20:910–917. doi: 10.1111/jch.13293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women’s Health Study. Eur Heart J. 2007;28:2937–2943. doi: 10.1093/eurheartj/ehm400 [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Chen J, Pan A, Wu JHY, Zhao F, Xie Y, Wang Y, Ye Y, Pan XF, Yang CX. Association between higher blood pressure and risk of diabetes mellitus in middle-aged and elderly Chinese Adults. Diabetes Metab J. 2020;44:436–445. doi: 10.4093/dmj.2019.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236 [DOI] [PubMed] [Google Scholar]
- 10.Muhammad IF, Borné Y, Östling G, Kennbäck C, Gottsäter M, Persson M, Nilsson PM, Engström G. Arterial stiffness and incidence of diabetes: a population-based cohort study. Diabetes Care. 2017;40:1739–1745. doi: 10.2337/dc17-1071 [DOI] [PubMed] [Google Scholar]
- 11.Yasuno S, Ueshima K, Oba K, Fujimoto A, Hirata M, Ogihara T, Saruta T, Nakao K. Is pulse pressure a predictor of new-onset diabetes in high-risk hypertensive patients?: a subanalysis of the Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) trial. Diabetes Care. 2010;33:1122–1127. doi: 10.2337/dc09-1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng M, Zhang X, Chen S, Song Y, Zhao Q, Gao X, Wu S. Arterial stiffness preceding diabetes: a longitudinal study. Circ Res. 2020;127:1491–1498. doi: 10.1161/CIRCRESAHA.120.317950 [DOI] [PubMed] [Google Scholar]
- 13.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359–364. doi: 10.1291/hypres.25.359 [DOI] [PubMed] [Google Scholar]
- 14.Izzo R, de Simone G, Trimarco V, Gerdts E, Giudice R, Vaccaro O, De Luca N, Trimarco B. Hypertensive target organ damage predicts incident diabetes mellitus. Eur Heart J. 2013;34:3419–3426. doi: 10.1093/eurheartj/eht281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflections, assessed with a novel method for pulse wave separation, are associated with end-organ damage and clinical outcomes. Hypertension. 2012;60:534–541. doi: 10.1161/HYPERTENSIONAHA.112.194571 [DOI] [PubMed] [Google Scholar]
- 16.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension. 2016;67:171–175. doi: 10.1161/HYPERTENSIONAHA.115.06277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342 [DOI] [PubMed] [Google Scholar]
- 18.Niiranen TJ, Kalesan B, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Relative Contributions of arterial stiffness and hypertension to cardiovascular disease: the framingham heart study. J Am Heart Assoc. 2016;5:e004271. doi: 10.1161/JAHA.116.004271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris-Etherton PM, Wu Y, Jin C, Huang S, Hu FB, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open. 2019;2:e194758. doi: 10.1001/jamanetworkopen.2019.4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, Li Y, Yao S, Chen S, Wu S, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75:2921–2930. doi: 10.1016/j.jacc.2020.04.038 [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Jin C, Li S, Zheng X, Zhang X, Cui L, Gao X. Aging, arterial stiffness, and blood pressure association in Chinese Adults. Hypertension. 2019;73:893–899. doi: 10.1161/HYPERTENSIONAHA.118.12396 [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20:146. doi: 10.1186/s12933-021-01342-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong YD, Bian AL, Hu HY, Ma Y, Zhou XZ. A cross-sectional study of the relationships between different components of sarcopenia and brachial ankle pulse wave velocity in community-dwelling elderly. BMC Geriatr. 2020;20:115. doi: 10.1186/s12877-020-01525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Chen Z, Yuan J, Zhang C, Chen H, Wu W, Chen Z, Liu Y, Zheng M, Chen S, et al. Association Between Body Mass Index (BMI) and Brachial-Ankle Pulse Wave Velocity (baPWV) in males with hypertension: a community-based cross-section study in North China. Med Sci Monit. 2019;25:5241–5257. doi: 10.12659/MSM.914881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris-Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40:1565–1572. doi: 10.2337/dc17-0610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Xing AJ, Zhang JN, Xia WH, Su C, Xu SY, Zhang XY, Chen SH, Huang Z, Qian XX, et al. Hypertension, arterial stiffness, and clinical outcomes: a cohort study of chinese community-based population. Hypertension. 2021;78:333–341. doi: 10.1161/HYPERTENSIONAHA.121.17131 [DOI] [PubMed] [Google Scholar]
- 28.Ferreira MT, Leite NC, Cardoso CR, Salles GF. Correlates of aortic stiffness progression in patients with type 2 diabetes: importance of glycemic control: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care. 2015;38:897–904. doi: 10.2337/dc14-2791 [DOI] [PubMed] [Google Scholar]
- 29.Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C. The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care. 2003;26:2133–2138. doi: 10.2337/diacare.26.7.2133 [DOI] [PubMed] [Google Scholar]
- 30.Stakos DA, Boudoulas KD, Gaillard TR, Schuster DP, Osei K, Boudoulas H. Regional and overall aortic function in nondiabetic individuals with insulin resistance and normal glucose tolerance. J Clin Endocrinol Metab. 2013;98:4457–4463. doi: 10.1210/jc.2013-2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Oliveira Alvim R, Santos PCJL, Musso MM, de Sá Cunha R, Krieger JE, Mill JG, Pereira AC. Impact of diabetes mellitus on arterial stiffness in a representative sample of an urban Brazilian population. Diabetol Metab Syndr. 2013;5:45. doi: 10.1186/1758-5996-5-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullican DR, Lorenzo C, Haffner SM. Is prehypertension a risk factor for the development of type 2 diabetes? Diabetes Care. 2009;32:1870–1872. doi: 10.2337/dc09-0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee WY, Kwon CH, Rhee EJ, Park JB, Kim YK, Woo SY, Kim S, Sung KC. The effect of body mass index and fasting glucose on the relationship between blood pressure and incident diabetes mellitus: a 5-year follow-up study. Hypertens Res. 2011;34:1093–1097. doi: 10.1038/hr.2011.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, He P, Li Y, Zhang Y, Li J, Liang M, Wang G, Tang G, Song Y, Wang B, et al. Positive association between baseline brachial-ankle pulse wave velocity and the risk of new-onset diabetes in hypertensive patients. Cardiovasc Diabetol. 2019;18:111. doi: 10.1186/s12933-019-0915-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ushigome E, Fukui M, Hamaguchi M, Tanaka T, Atsuta H, Mogami S, Tsunoda S, Yamazaki M, Hasegawa G, Nakamura N. Maximum home systolic blood pressure is a useful indicator of arterial stiffness in patients with type 2 diabetes mellitus: post hoc analysis of a cross-sectional multicenter study. Diabetes Res Clin Pract. 2014;105:344–351. doi: 10.1016/j.diabres.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 36.Tomiyama H, Shiina K, Matsumoto-Nakano C, Ninomiya T, Komatsu S, Kimura K, Chikamori T, Yamashina A. The contribution of inflammation to the development of hypertension mediated by increased arterial stiffness. J Am Heart Assoc. 2017;6:e005729. doi: 10.1161/JAHA.117.005729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, et al. ; ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1 [DOI] [PubMed] [Google Scholar]
- 38.Tomiyama H, Hashimoto H, Tanaka H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A. Continuous smoking and progression of arterial stiffening: a prospective study. J Am Coll Cardiol. 2010;55:1979–1987. doi: 10.1016/j.jacc.2009.12.042 [DOI] [PubMed] [Google Scholar]
- 39.Yamada J, Tomiyama H, Matsumoto C, Yoshida M, Koji Y, Shiina K, Nagata M, Yamashina A. Overweight body mass index classification modifies arterial stiffening associated with weight gain in healthy middle-aged Japanese men. Hypertens Res. 2008;31:1087–1092. doi: 10.1291/hypres.31.1087 [DOI] [PubMed] [Google Scholar]
- 40.Cavero-Redondo I, Tudor-Locke C, Álvarez-Bueno C, Cunha PG, Aguiar EJ, Martínez-Vizcaíno V. Steps per day and arterial stiffness. Hypertension. 2019;73:350–363. doi: 10.1161/HYPERTENSIONAHA.118.11987 [DOI] [PubMed] [Google Scholar]
- 41.Zanoli L, Rastelli S, Granata A, Inserra G, Empana JP, Boutouyrie P, Laurent S, Castellino P. Arterial stiffness in inflammatory bowel disease: a systematic review and meta-analysis. J Hypertens. 2016;34:822–829. doi: 10.1097/HJH.0000000000000867 [DOI] [PubMed] [Google Scholar]
- 42.Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–584. doi: 10.1016/j.cjca.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Häring HU. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101:1780–1784. doi: 10.1161/01.cir.101.15.1780 [DOI] [PubMed] [Google Scholar]
- 44.Xu M, Huang Y, Xie L, Peng K, Ding L, Lin L, Wang P, Hao M, Chen Y, Sun Y, et al. Diabetes and risk of arterial stiffness: a mendelian randomization analysis. Diabetes. 2016;65:1731–1740. doi: 10.2337/db15-1533 [DOI] [PubMed] [Google Scholar]
- 45.Ye L, Yang X, Hu J, Chen Q, Wang J, Li X. Impact of antihypertensive agents on arterial stiffness in hypertensive patients. Int J Cardiol. 2018;273:207–212. doi: 10.1016/j.ijcard.2018.06.092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.