Abstract
Background:
Mortality rates for patients presenting with acute myocardial infarction (AMI) and cardiogenic shock (CS) remain high despite advances in revascularization strategies and mechanical circulatory support (MCS) devices.
Objectives:
To elucidate the association between comorbid peripheral artery disease (PAD) and outcomes in CS and AMI.
Methods:
PAD status was defined in Medicare beneficiaries hospitalized with CS and AMI from 10/1/2015–6/30/2018. Primary outcomes ascertained through 12/31/2018 included in- and out-of-hospital mortality. Secondary outcomes included bleeding, amputation, stroke, and peripheral revascularization. Multivariable regression models with adjustment for confounders were used to estimate risk. Subgroup analyses included patients with MCS and those who underwent coronary revascularization.
Results:
Among 71,690 patients, 5.9% (N=4,259) had PAD. Mean age was 77.8±7.9 years, 58.7% were male and 84.3% were white. Cumulative in-hospital mortality was 47.2%, with greater risk among those with PAD (56.3% vs 46.6% without PAD; adjusted odds ratio [aOR] 1.50, 95% confidence interval [CI]: 1.40–1.59). PAD patients also had greater risk of in-hospital amputation (1.6% vs 0.2%; aOR 7.0, 95% CI: 5.26–9.37) and out-of-hospital mortality (67.9% vs 40.7%; adjusted hazard ratio: 1.78, 95% CI: 1.67–1.90). MCS was less frequently utilized in PAD patients (21.5% vs. 38.6% without PAD, p<0.001) and was associated with higher mortality and amputation risk. Findings were consistent in patients who underwent coronary revascularization.
Conclusions:
Among patients presenting with AMI and CS, PAD was associated with worse limb outcomes and survival. In addition to lower MCS utilization rates, those with PAD who received MCS had increased mortality, peripheral revascularization, and amputation rates.
Keywords: Peripheral artery disease, Myocardial infarction, Cardiogenic shock, Mechanical circulatory support, Percutaneous coronary intervention, Coronary artery bypass grafting
Condensed Abstract:
In patients with acute myocardial infarction complicated by cardiogenic shock, coronary revascularization remains critical. Comorbid lower extremity peripheral artery disease (PAD) is a significant risk factor for adverse events among these patients, and is associated with worse limb outcomes as well as poorer short- and long-term survival compared with those without PAD. Mechanical circulatory support, an important tool in the management of cardiogenic shock, is less often used in this patient population, and when employed, is associated with worse outcomes. These findings highlight the importance of identifying and considering PAD as a risk factor in prognostication and multidisciplinary decision making.
Introduction:
Among patients with coronary artery disease (CAD) presenting with acute myocardial infarction (AMI), cardiogenic shock (CS) remains the leading cause of death, with in-hospital mortality estimated as high as 50% (1–3). More than 40% of patients with CAD have comorbid lower extremity peripheral artery disease (PAD) and suffer from an even greater burden of cardiovascular events and overall mortality (4). To date, however, the relationship between PAD and outcomes following AMI complicated by CS has been poorly characterized.
Coronary revascularization remains critical to the management of AMI. The co-existence of PAD among patients with CAD undergoing revascularization significantly influences revascularization strategies and outcomes. This may be related to access site issues, atherosclerotic burden in coronary arteries and other vital organs (e.g. carotid, renal arteries), and high-risk comorbid illnesses related to the development PAD. Not surprisingly, patients with PAD who undergo percutaneous coronary intervention have a greater incidence of major adverse cardiovascular events and a doubling of in- and out-of-hospital mortality when compared to patients without PAD (5–7).
Mechanical circulatory support (MCS) utilization is increasing in the management of CS, now used in upwards of one-third of cardiogenic shock patients (1,8). Large bore access, a requirement for MCS, may lead to an increased incidence of limb ischemia, amputation, bleeding complications and mortality among patients with PAD (9–11). The utilization of MCS devices among CS patients with PAD and the rates of associated outcomes is unknown.
As such, we sought to understand the morbidity and mortality associated with comorbid PAD among patients presenting with AMI and CS. Separately, we aimed to determine how MCS is being used in this population compared with similar patients without PAD. We hypothesized that comorbid PAD is associated with higher mortality and portended greater risk of limb-related complications, with consistent findings among those treated with MCS.
Methods:
Study Population:
All Medicare fee-for-service beneficiaries aged ≥65 years who were hospitalized at short-term acute care hospitals across the United States from October 1, 2015 to June 30, 2018 with a principal diagnosis of AMI and a secondary diagnosis of CS based on the International Classification of Disease (ICD), Tenth Revision codes were identified in the Medicare Provider and Analysis Review (MedPAR) files (Appendix Table 1) (12). For patients with sequential hospital visits (i.e. transfers), patients were identified by the principle diagnosis of the index hospital, and subsequent interventions and outcomes were tracked across hospitals. All patients with a presentation for CS associated with AMI in the year preceding the index hospitalization were excluded. The study was exempt by the institutional review board of Beth Israel Deaconess Medical Center, with a waiver of informed consent for retrospective data analysis.
Explanatory Variables:
Patients with established PAD were identified based on the presence of either a corresponding ICD-9-CM (for patient presentation prior to October 1, 2015) or ICD-10-CM claims code in the year preceding the date of the index CS/AMI admission (Appendix Table 1). Baseline chronic comorbid conditions were identified by linkage of beneficiary data to the Chronic Conditions Data Warehouse (CCW). Race/ethnicity was reported as collected and classified by the CMS MedPAR files. Smoking status, prior surgical or endovascular peripheral revascularization, and prior amputation were assessed during a 1-year lookback period using the corresponding and previously validated diagnosis or procedure codes (Appendix Table 2) (13). Procedural variables ascertained during the index admission include coronary revascularization (percutaneous or surgical) and MCS utilization (12). MCS utilization was identified based on ICD-10-PCS codes for extracorporeal membrane oxygenation (ECMO), percutaneous ventricular assist device (pVAD), and intra-aortic balloon pump (IABP) (Appendix Table 3). In the case of multiple devices, the following hierarchy was used to define the primary device that was then tied to outcomes: 1. ECMO, 2. pVAD, and 3. IABP. A separate analysis was performed of in- and out-of-hospital outcomes stratified by PAD status in patients who received PCI and separately, coronary artery bypass grafting (CABG), during their index hospitalization.
Outcomes:
The primary outcomes were all-cause in-hospital mortality and out-of-hospital mortality. Secondary in-hospital outcomes included peripheral surgical or endovascular intervention, amputation, major bleeding, cerebrovascular accident (CVA)/transient ischemic attack (TIA), and cardiac arrest. Lower extremity revascularization consisted of endovascular interventions, surgical interventions, or a combination approach during the same hospitalization. Secondary out-of-hospital outcomes included hospitalization for heart failure (HF) or AMI, and need for hospital readmission. Outcomes were determined based on the corresponding and previously validated or clinically relevant ICD-10 claims codes listed in Appendix Table 4 (13–16). All outcomes were assessed through December 31, 2018. Patients were censored at the time of death or at the end of the study period, whichever came first. As Medicare has near complete information on patient’s vital status, patients were considered alive and contributing data until the end of the study period unless a death occurred. As all included participants were required to be Medicare fee-for-service beneficiaries, changes in insurance were unlikely during the study period.
Statistical Methods:
Categorical variables were reported as counts and percentages, and continuous variables as means with standard deviations. Univariate comparisons of characteristics between patients with and without PAD were performed using Student’s t-test for continuous variables and chi-squared tests for categorical variables.
For in-hospital outcomes, logistic regression models were used to estimate the unadjusted and adjusted odds ratios with confidence intervals for each in-hospital endpoint. The regression models first adjusted only for age and sex, followed by the addition of comorbidities selected as potential confounders based on literature review and clinical expertise. These included race, chronic kidney disease, diabetes mellitus, ischemic heart disease, congestive heart failure, tobacco use history, prior cerebrovascular accident/transient ischemic attack, atrial fibrillation, and hyperlipidemia. The number of comorbidities selected for inclusion in the regression models were restricted due to limited event rates and to avoid over-adjustment.
For out-of-hospital outcomes, survival methods were applied, taking into account the competing risk of death. For long-term survival, traditional Kaplan Meier estimates of the cumulative incidence of death at each time point were reported. For non-death outcomes, cumulative incidences at each time point were reported and cumulative incidence functions were estimated by Fine-Gray methods. Cox regression models were then used to estimate the associated hazard ratios of PAD versus no PAD for all outcomes. For non-death outcomes, sub-distribution hazard models were constructed based on Fine-Gray methods (17). Adjustment was then performed first for age and sex alone, and then with the addition of the potential confounders listed above.
Subgroup analyses were performed among those who underwent treatment with MCS and those who underwent coronary revascularization during the index admission. Similar methods as reported above were used to estimate the adjusted risks of PAD and the study endpoints among patients in each subgroup.
A p value <0.05 was considered significant for all analyses. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all analyses.
Results:
Study Population:
During the study period, 71,690 patients met inclusion criteria, of which 4,259 patients (5.9%) had a prior diagnosis of PAD (Appendix Figure 1). The mean age of patients in both cohorts was 77.8±7.9 years and 59.7% of patients were male. Patients with PAD were more commonly non-white or Hispanic, and had a greater burden of cardiovascular conditions and risk factors, including prior AMI, chronic kidney disease, chronic obstructive pulmonary disease, congestive heart failure, diabetes, ischemic heart disease, and hyperlipidemia (Table 1). Furthermore, patients with PAD more frequently used tobacco, had prior amputations and had undergone previous surgical or endovascular peripheral interventions. During the index hospitalization, 53.0% of all patients underwent coronary revascularization with either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Patients without PAD were more likely to undergo coronary revascularization (CABG: 13.2% without PAD vs 6.6% with PAD; PCI: 42.7% without PAD vs 27.3% with PAD; p<0.001 for both) (Table 1).
Table 1:
Baseline characteristics overall and stratified by patients with and without lower extremity peripheral artery disease (PAD).
| Subject Characteristic | Total (N = 71,690) | Without PAD (n=67,431) | PAD (n=4,259) | p-value |
|---|---|---|---|---|
| Age | 77.8±7.9 | 77.8±8.0 | 77.7±7.6 | 0.388 |
| Female | 29595 (41.3%) | 41.4% | 40.1% | 0.101 |
| Race | <.001 | |||
| White | 60426 (84.3%) | 84.3% | 83.4% | |
| Black | 5607 (7.8%) | 7.6% | 10.8% | |
| Hispanic | 1477 (2.1%) | 2.0% | 2.3% | |
| Other + Unknown | 4180 (5.8%) | 6.0% | 3.6% | |
| AMI | 951 (1.3%) | 1.2% | 3.4% | <.001 |
| Alzheimer’s | 2074 (2.9%) | 2.8% | 3.8% | <.001 |
| Dementia | 6242 (8.7%) | 8.4% | 13.0% | <.001 |
| Atrial Fibrillation | 5690 (7.9%) | 7.5% | 15.5% | <.001 |
| Chronic Kidney Disease | 15388 (21.5%) | 20.2% | 40.9% | <.001 |
| COPD | 8078 (11.3%) | 10.4% | 24.3% | <.001 |
| CHF | 12444 (17.4%) | 16.2% | 36.1% | <.001 |
| Diabetes | 20011 (27.9%) | 26.8% | 45.9% | <.001 |
| Ischemic Heart Disease | 22525 (31.4%) | 30.0% | 54.4% | <.001 |
| Stroke/TIA | 2602 (3.6%) | 3.3% | 8.3% | <.001 |
| Anemia | 13090 (18.3%) | 17.3% | 33.5% | <.001 |
| Asthma | 2190 (3.1%) | 2.9% | 5.4% | <.001 |
| Hyperlipidemia | 24453 (34.1%) | 33.1% | 50.2% | <.001 |
| Tobacco Use | 30267 (42.2%) | 40.7% | 65.8% | <.001 |
| Prior Amputation | 1198 (1.7%) | 0.9% | 13.9% | <.001 |
| Prior Peripheral Surgical Revascularization | 403 (0.6%) | 0.3% | 5.4% | <.001 |
| Prior Peripheral Endovascular Revascularization | 1276 (1.8%) | 1.2% | 11.1% | <.001 |
| CABG | 8625 (12.0%) | 13.2% | 6.6% | <.001 |
| PCI | 29356 (40.9%) | 42.7% | 27.3% | <.001 |
(AMI: Acute myocardial infarction; COPD: Chronic obstructive pulmonary disease, CHF: Congestive heart failure; CABG: Coronary artery bypass grafting; PCI: Percutaneous coronary intervention
In-Hospital Outcomes:
Overall, in-hospital mortality was 47.2%, and was significantly higher among patients with PAD (56.3%) compared to patients without PAD (46.6%, p<0.001). PAD patients also experienced higher rates of major bleeding (2.2% vs. 1.4%, p<0.001), amputation (1.6% vs. 0.4%, p<0.001), lower extremity revascularization (4.06% vs. 1.85%, p<0.001). Rates of cerebrovascular events (TIA/CVA) and cardiac arrest were comparable between the two groups (Appendix Table 5A).
After adjustment for age and sex, patients with PAD compared to patients without PAD had a 1.5-fold increased odds of in-hospital death (95% CI: 1.40–1.59, p<0.001) and a 7.0-fold higher odds of in-hospital amputation (95% CI: 5.26–9.37, p<0.001). Patients with PAD were also more likely to experience a bleeding complication (OR: 1.56, 95% CI: 1.26–1.93, p<0.001) and require lower extremity revascularization (OR: 2.25, 95% CI: 1.91–2.65, p<0.001). There was no difference between groups in age and sex adjusted risk of TIA/CVA (OR: 1.28, 95% CI: 0.59–2.76, p=0.532) or in-hospital cardiac arrest (OR: 1.39, 95% CI: 0.95–2.05, p=0.09)
After multivariable adjustment, the findings were comparable. Patients with PAD had a 1.4-fold increased risk of in-hospital death (95% CI: 1.29–1.47, p<0.001), 7.0-fold higher risk of amputation (95%: 5.16–9.62, p<0.001), 1.4-fold increased risk of bleeding (95% CI: 1.11–1.74, p<0.01), and 2.2-fold higher risk of lower extremity revascularization (95% CI: 1.82–2.54, p<0.001). The risk of TIA/CVA (OR: 1.29, 95% 0.58–2.85, p=0.53) and cardiac arrest (OR: 1.26, 95% CI: 0.84–1.88, p=0.26) was comparable (Figure 1, Central Illustration).
Figure 1: Risk of In-hospital Outcomes in Patients with PAD.
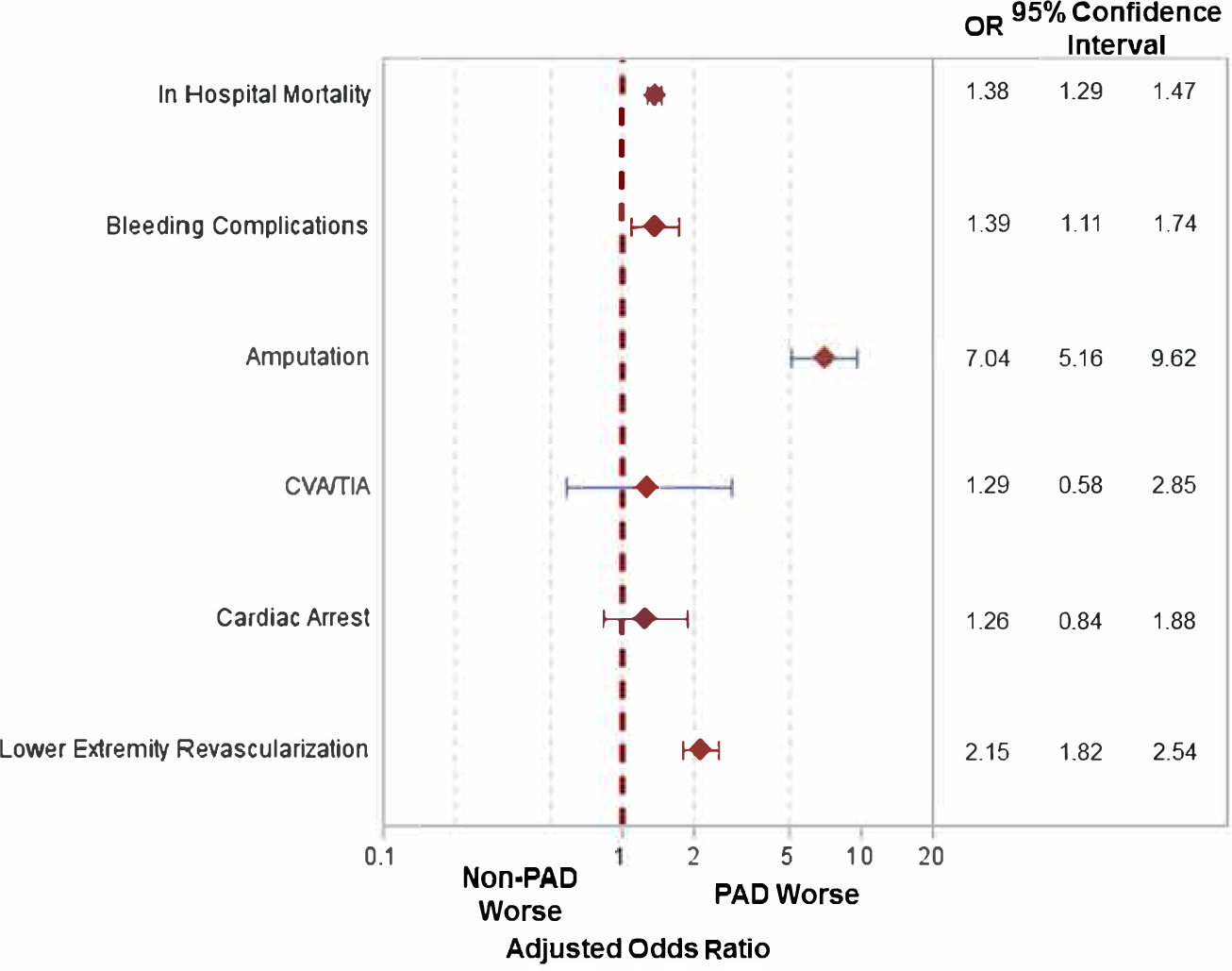
Adjusted odds ratios for in hospital events for patients with versus without lower extremity peripheral arterial disease (PAD). [CVA/TIA: Cerebrovascular accident/transient ischemic attack]
Central Illustration: PAD Outcomes in Cardiogenic Shock & Myocardial Infarction.
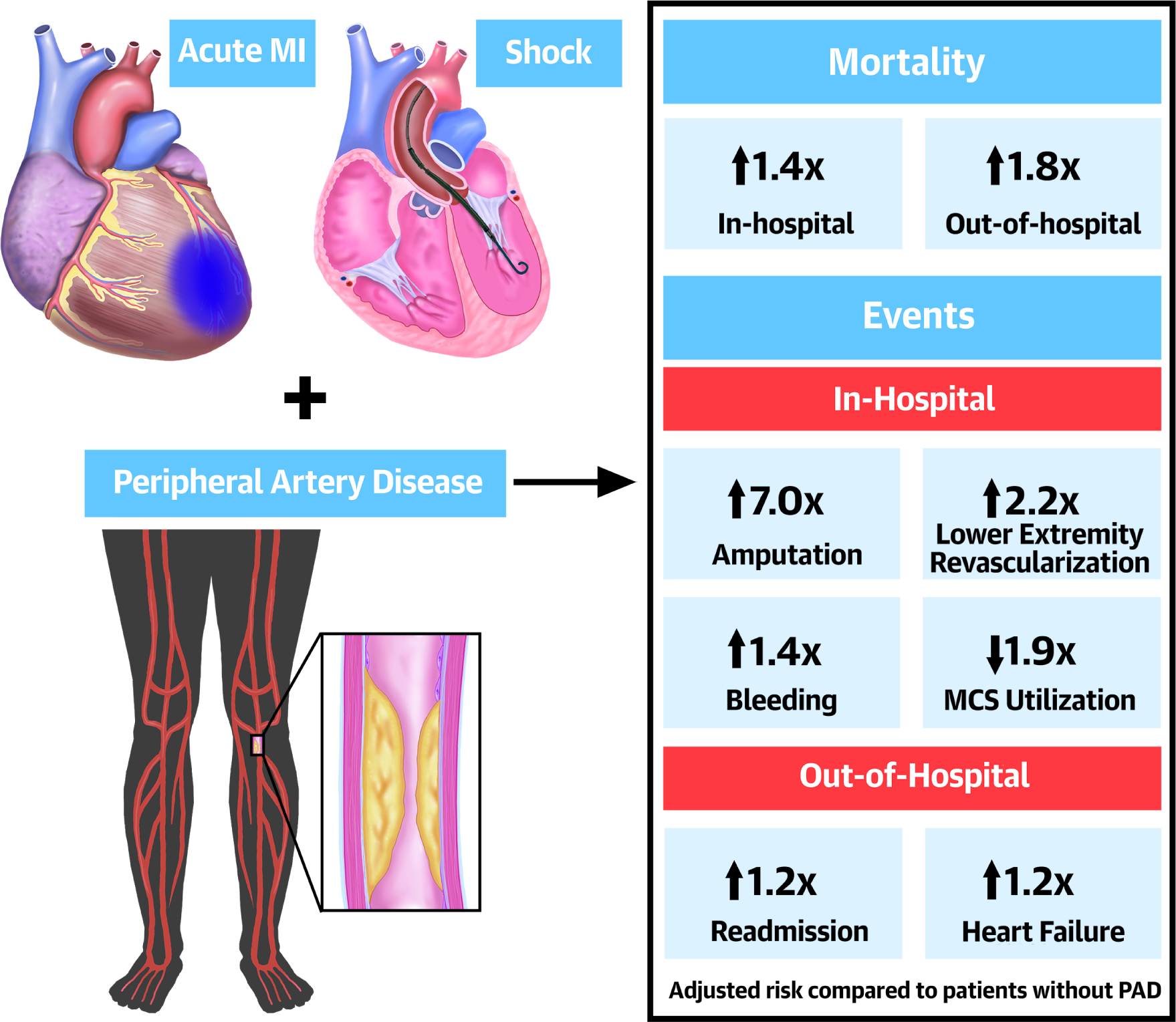
In-Hospital and out-of-hospital outcomes in patients with lower extremity peripheral artery disease (PAD) presenting with acute myocardial infarction (MI) and cardiogenic shock.
Out-of-Hospital Outcomes:
In addition to a greater risk of in-hospital death, patients with PAD had a higher unadjusted cumulative incidence of out-of-hospital mortality compared to patients without PAD (67.9% vs. 40.7%, unadjusted HR: 2.13, 95% CI: 2.01–2.26, p<0.0001; Figure 2A). Patients with PAD also had higher cumulative incidences of subsequent AMI (8.9% vs. 8.0%, unadjusted HR: 1.22, 95% CI: 1.03–1.44, p=0.02; Figure 2B), HF (26.1% vs. 18.9%, unadjusted HR: 1.50, 95% CI: 1.36–1.65, p<0.0001; Figure 2C) and readmissions (62.0% vs. 56.7%, unadjusted HR: 1.33, 95% CI: 1.25–1.41, p<0.0001; Figure 2D) when compared to those without PAD (Appendix Table 5B).
Figure 2: Risk of Out-of-Hospital Outcomes in Patients with PAD.
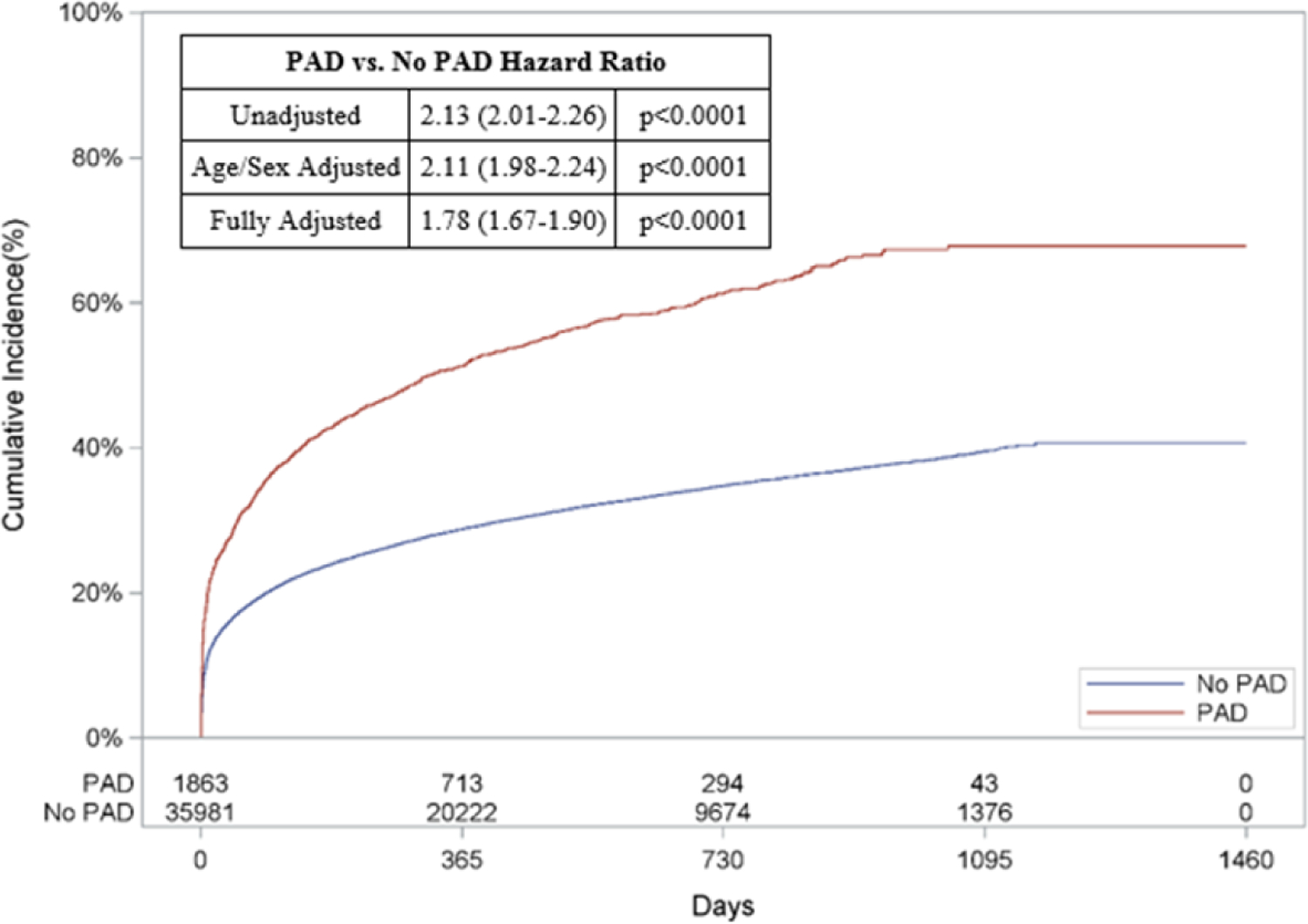
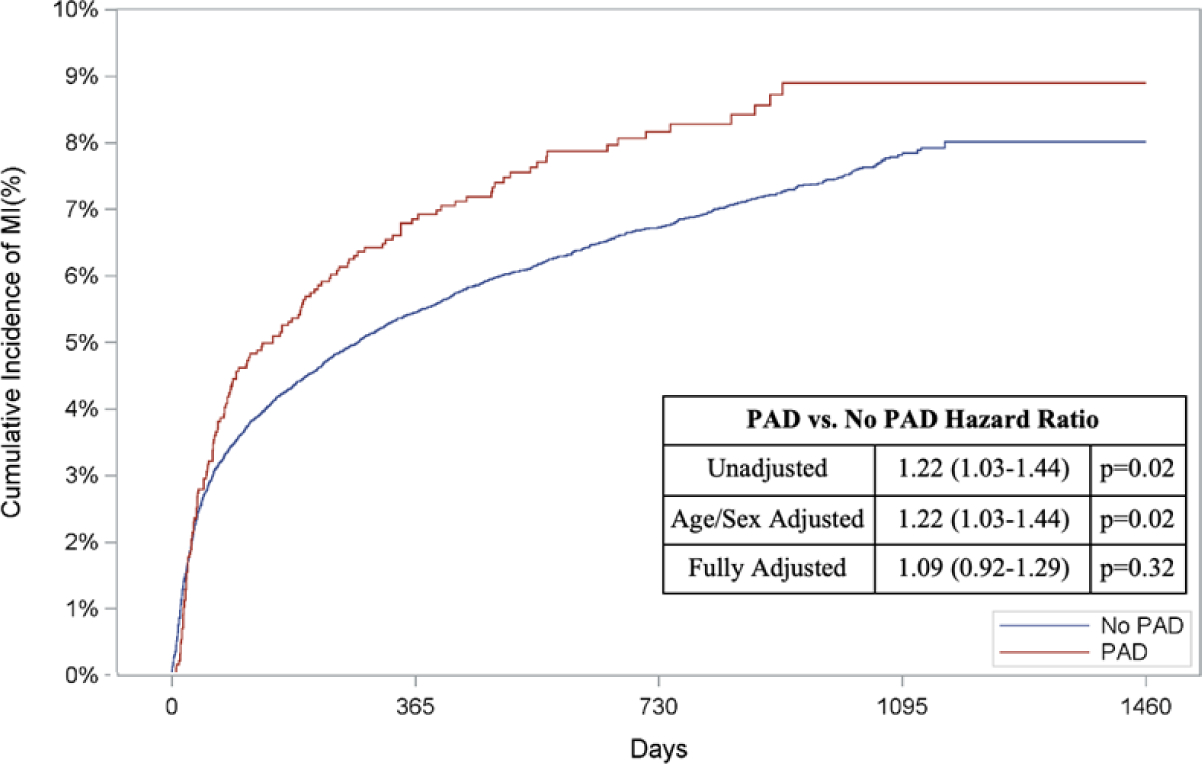
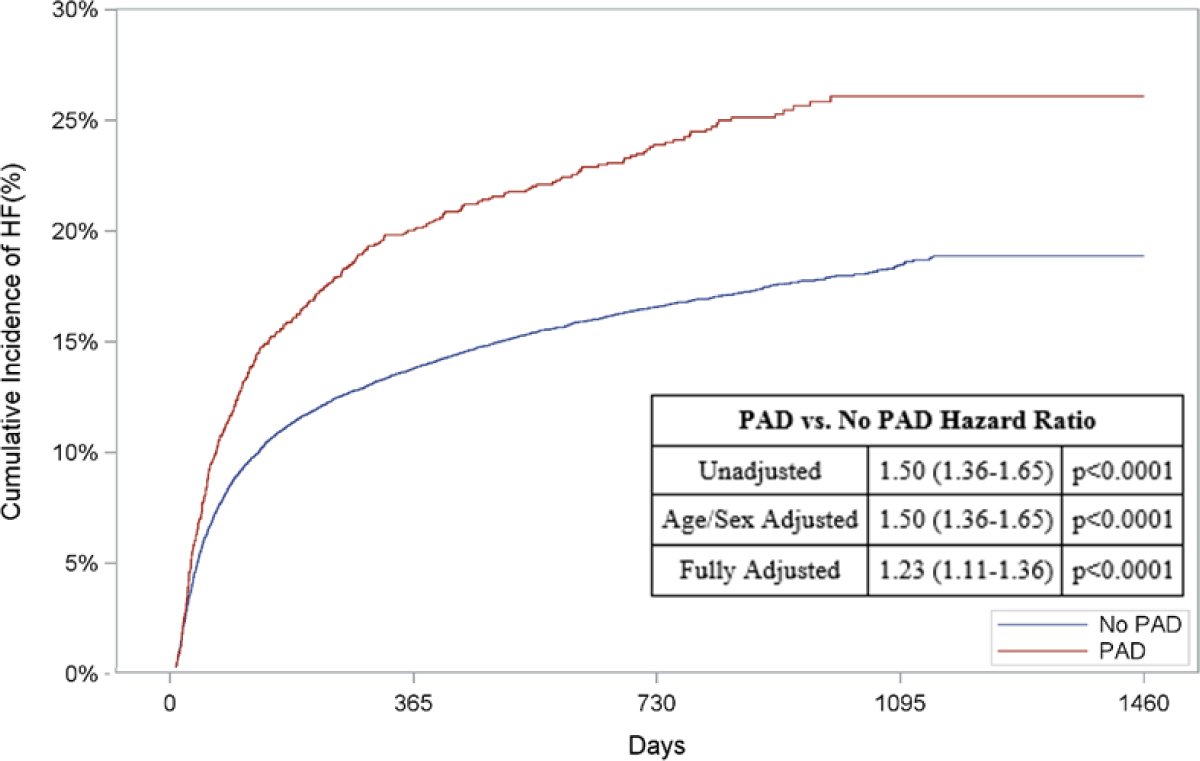
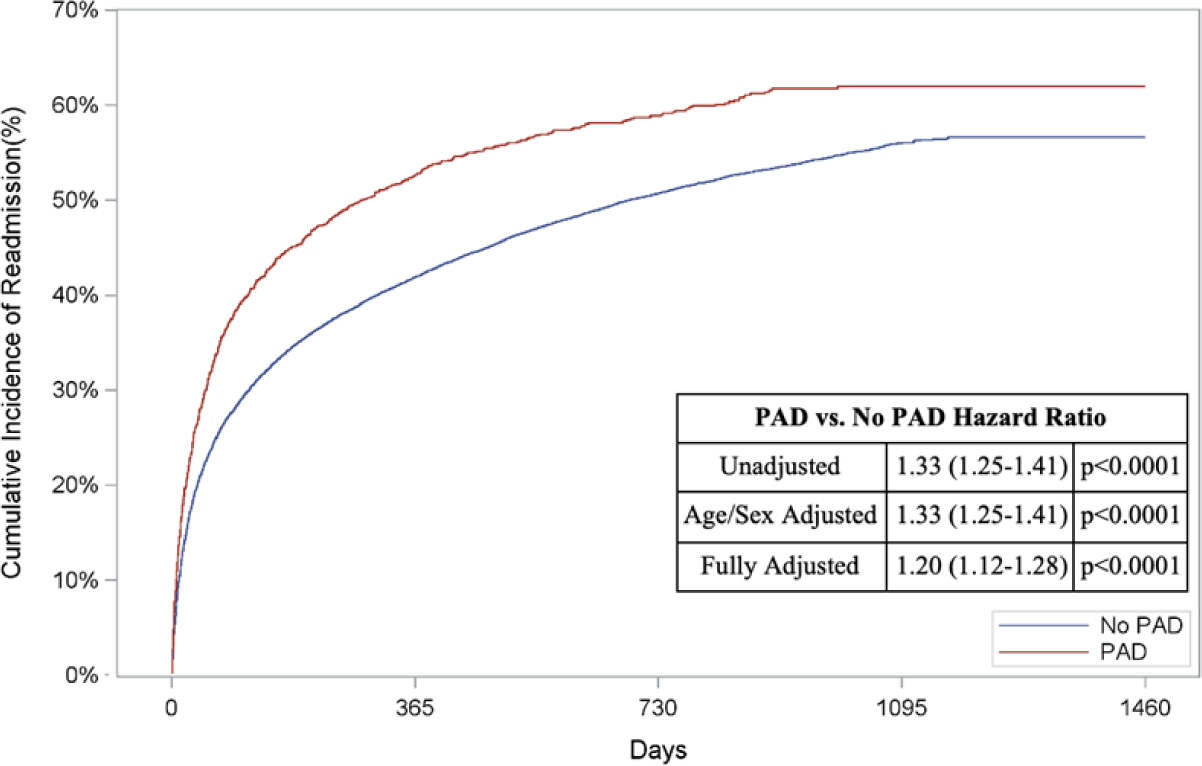
Cumulative incidence curves and hazard ratios for out-of-hospital outcomes stratified by the presence of lower extremity peripheral artery disease (PAD): a.) Out-of-hospital mortality, b.) Hospitalization for myocardial infarction (MI), c.) Hospitalization for heart failure (HF), and d.) Readmission. For out-of-hospital mortality, cumulative incidence curves estimated using Kaplan Meier methods are displayed. For non-death-related outcomes, cumulative incidence function curves are displayed, which account for the competing risk of death. In addition, all hazard ratios for non-death outcomes are based on sub-distribution hazards models using Fine-Gray methods to account for the competing risk of death.
When adjusted for age and sex, patients with PAD had a higher risk of long-term death (HR: 2.11, 95% CI: 1.98–2.24, p<0.0001; Figure 2A), AMI (HR: 1.22, 95% CI: 1.03–1.44, p=0.02; Figure 2B), HF (HR: 1.50, 95% CI: 1.36–1.65, p<0.0001; Figure 2C), and readmission (HR: 1.33, 95% CI: 1.25–1.41, p<0.0001; Figure 2D)
After multivariable adjustment, PAD remained associated with an increased risk of long-term death (HR: 1.78, 95% CI: 1.67–1.90, p<0.0001, Figure 2A), HF (HR: 1.23, 95% CI: 1.11–1.36, p<0.0001, Figure 2C), and readmission (HR: 1.20, 95% CI: 1.12–1.28, p<0.0001, Figure 2D). The risk of AMI was comparable between the two groups after full adjustment (HR: 1.09, 95% CI: 0.92–1.29, p=0.32, Figure 2B).
<.0001
MCS Subgroup:
Of the study population, 37.6% (n=26,957) of patients were treated with MCS. IABP was the most commonly utilized MCS therapy (18.6%; n=13,365), followed by ECMO (12.1%; n=8,646) and pVAD (6.9%; n=4,946) (Appendix Table 6). Of all patients who received ECMO, 9.5% (N=805) also received a pVAD. Patients with PAD were less likely to be treated with MCS (21.5% of all PAD patients) compared to those without PAD (38.6% of all non-PAD patients; OR: 0.53, 95% CI: 0.49–0.58, p<0.001). Baseline characteristics of patients who received MCS overall and subdivided by device type can be found in Appendix Table 7.
Among patients who received MCS, in-hospital mortality was 41.4%, and was significantly higher among patients with PAD compared to patients without PAD (51.9% vs 41.0%, respectively; p<0.001; Appendix Table 8). After multivariable adjustment, patients with PAD who received MCS had a 1.5-fold higher risk of death (95% CI: 1.27–1.67, p<0.001), 4.4-fold higher risk of amputation (95% CI: 1.63–11.82, p<0.01), and 2.3-fold higher risk of in-hospital lower extremity revascularization (95% CI: 1.73–2.97, p<0.001) when compared to patients without PAD. The risk of bleeding, TIA/CVA, and cardiac arrest was comparable (Figure 3). Findings were consistent when adjusted for age and sex only (Appendix Table 10A).
Figure 3: Risk of In-hospital Outcomes in Patients with PAD & MCS.
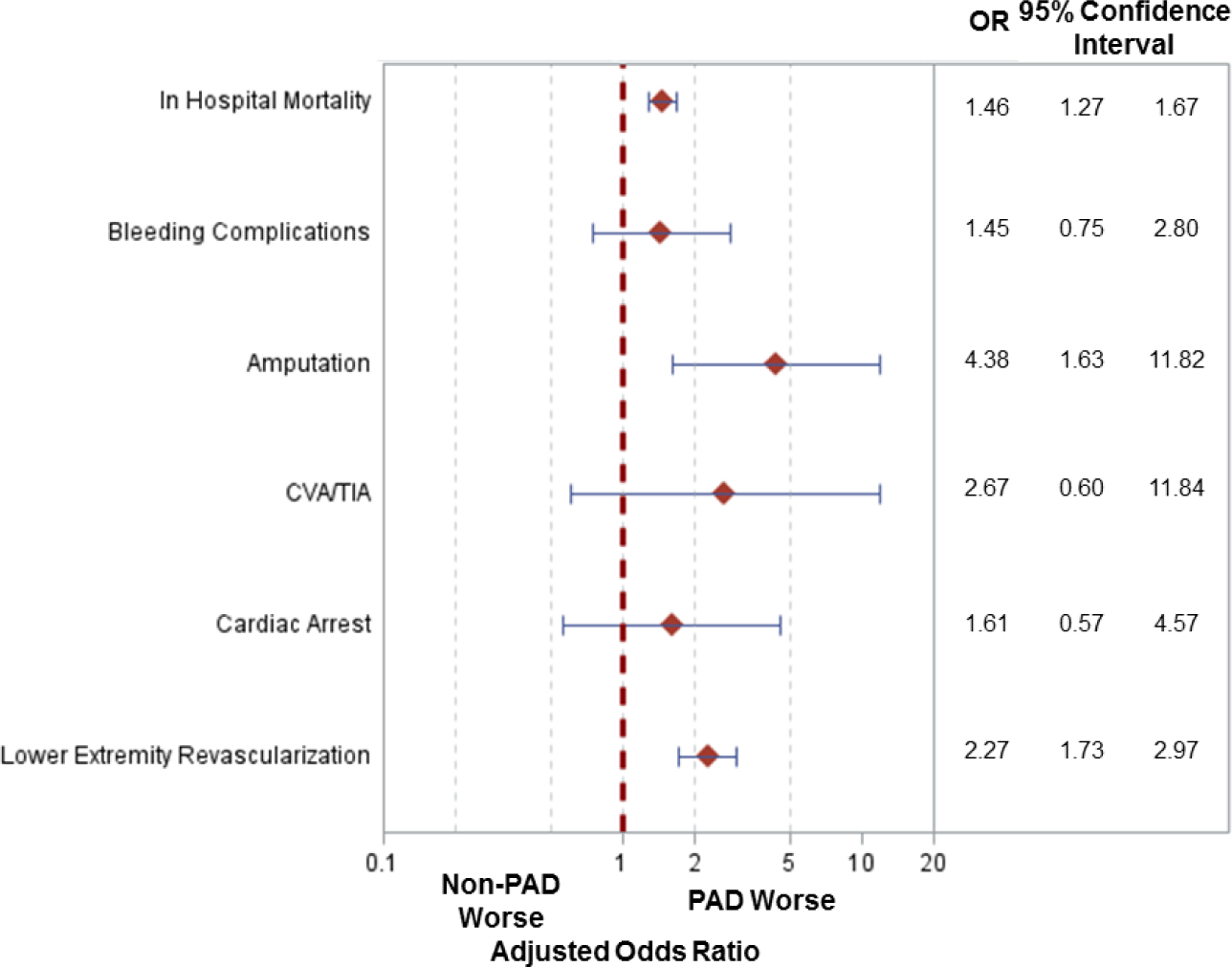
Adjusted odds ratios for in hospital events amongst patients who received mechanical circulatory support (MCS) with versus without lower extremity peripheral arterial disease (PAD). [CVA/TIA: Cerebrovascular accident/transient ischemic attack]
With regards to out-of-hospital outcomes, patients with versus without PAD who received MCS had an increased risk of out-of-hospital death (HR: 1.91, 95% CI: 1.64–2.24, p<0.0001; Figure 4A), HF (HR: 1.80, 95% CI 1.51–2.15, p<0.0001; Figure 4C), and readmission (HR: 1.42, 95% CI: 1.26–1.61, p,<0.0001; Figure 4D) when adjusted for age and sex. Following multivariable adjustment, patients with versus without PAD who received MCS had a 1.6-fold higher adjusted risk of out-of-hospital death (95% CI: 1.40–1.93, p<0.001; Figure 4A). Similarly, the adjusted risk of HF (HR: 1.50, 95% CI: 1.25–1.80, p<0.001, Figure 4C) and readmission (HR: 1.26, 95% CI: 1.12–1.43, p<0.001, Figure 4D) was higher amongst patients with PAD who received MCS compared to patients without PAD treated with MCS. The adjusted risk of AMI was comparable between the two groups after adjustment (HR: 1.11, 95% CI: 0.8–1.54, p=0.52, Figure 4B). Unadjusted and adjusted in-of-hospital and out-of-hospital outcomes subdivided by device type can be found in Appendix Table 9 & 10 and Appendix Figures 2.
Figure 4: Risk of Out-of-Hospital Outcomes in Patients with PAD & MCS.
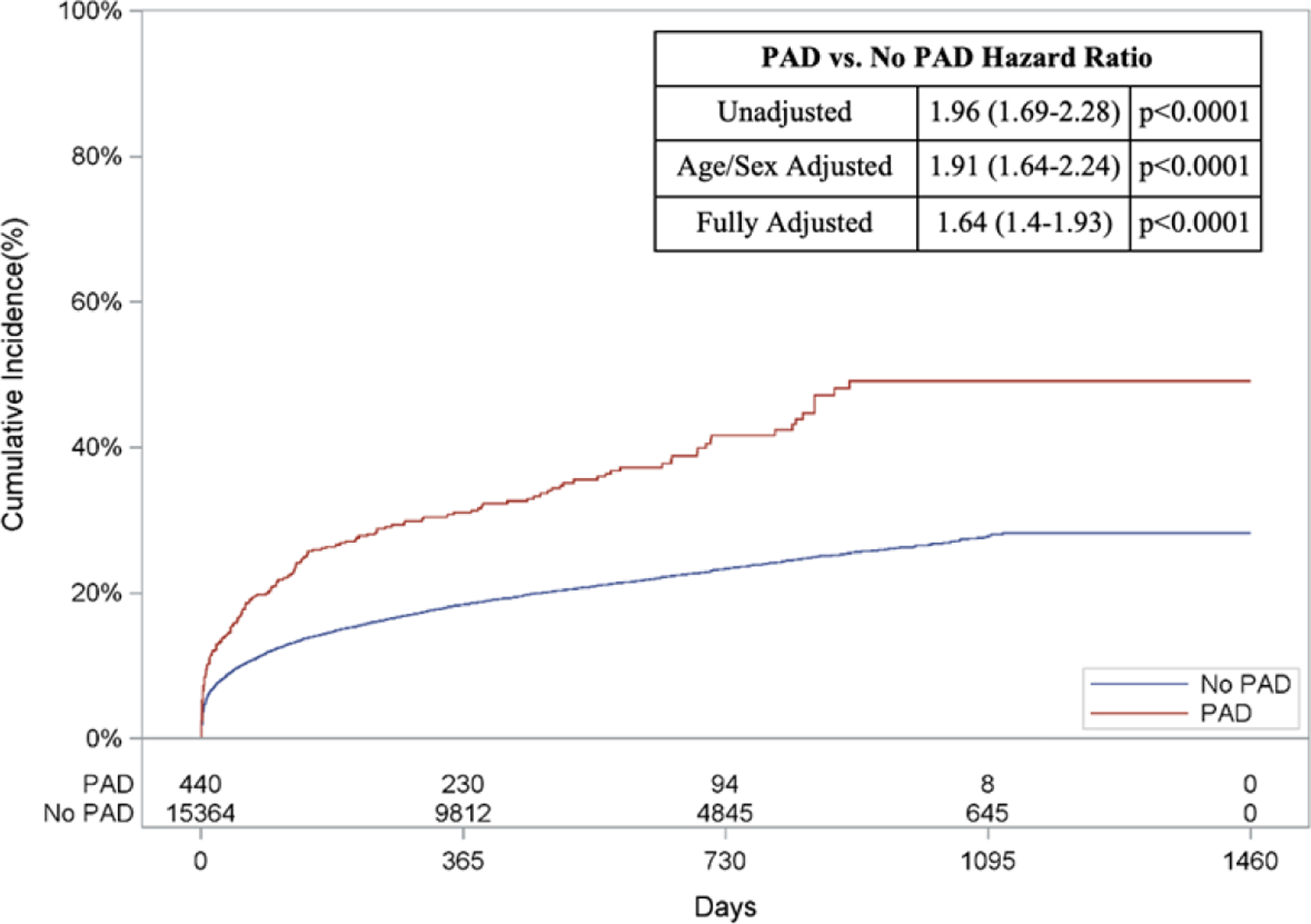
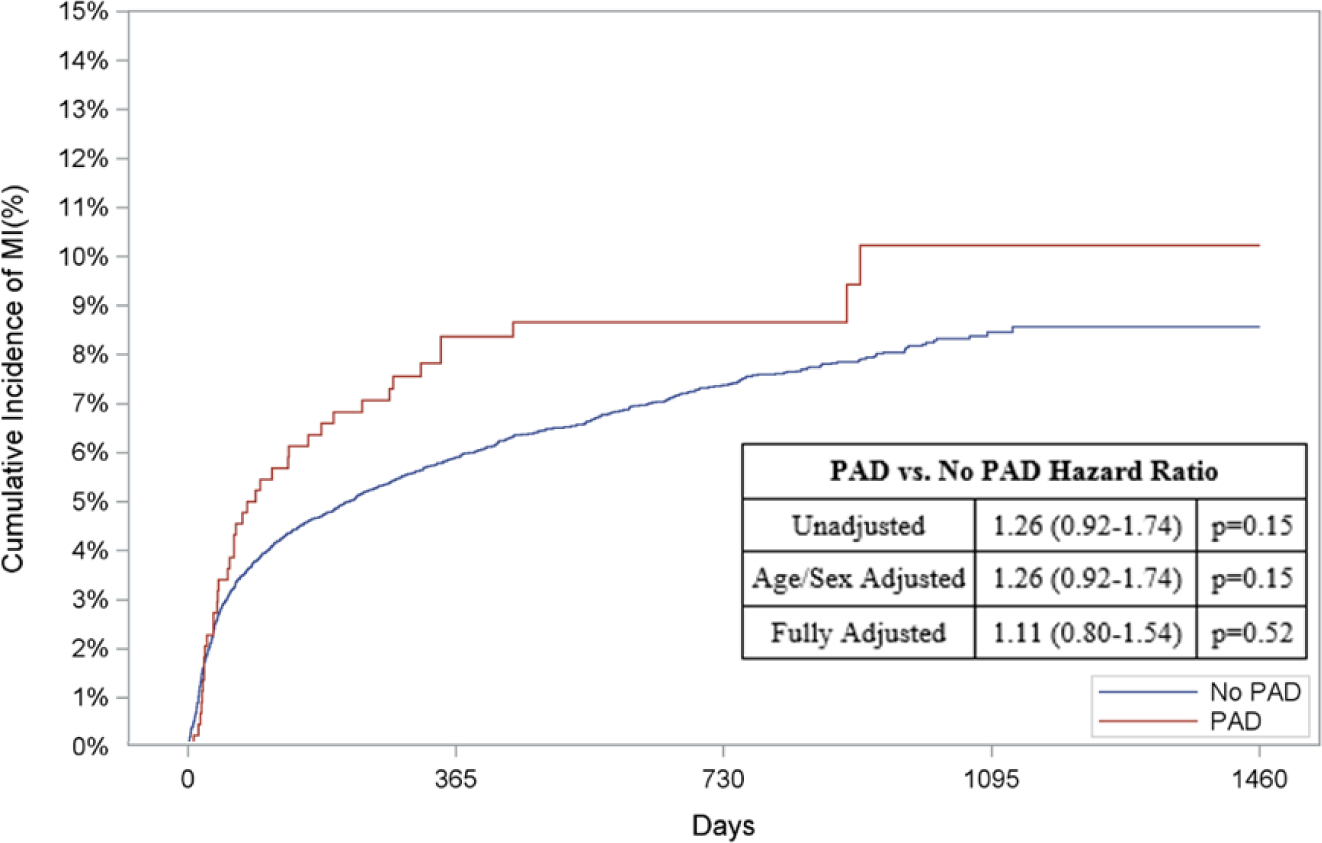
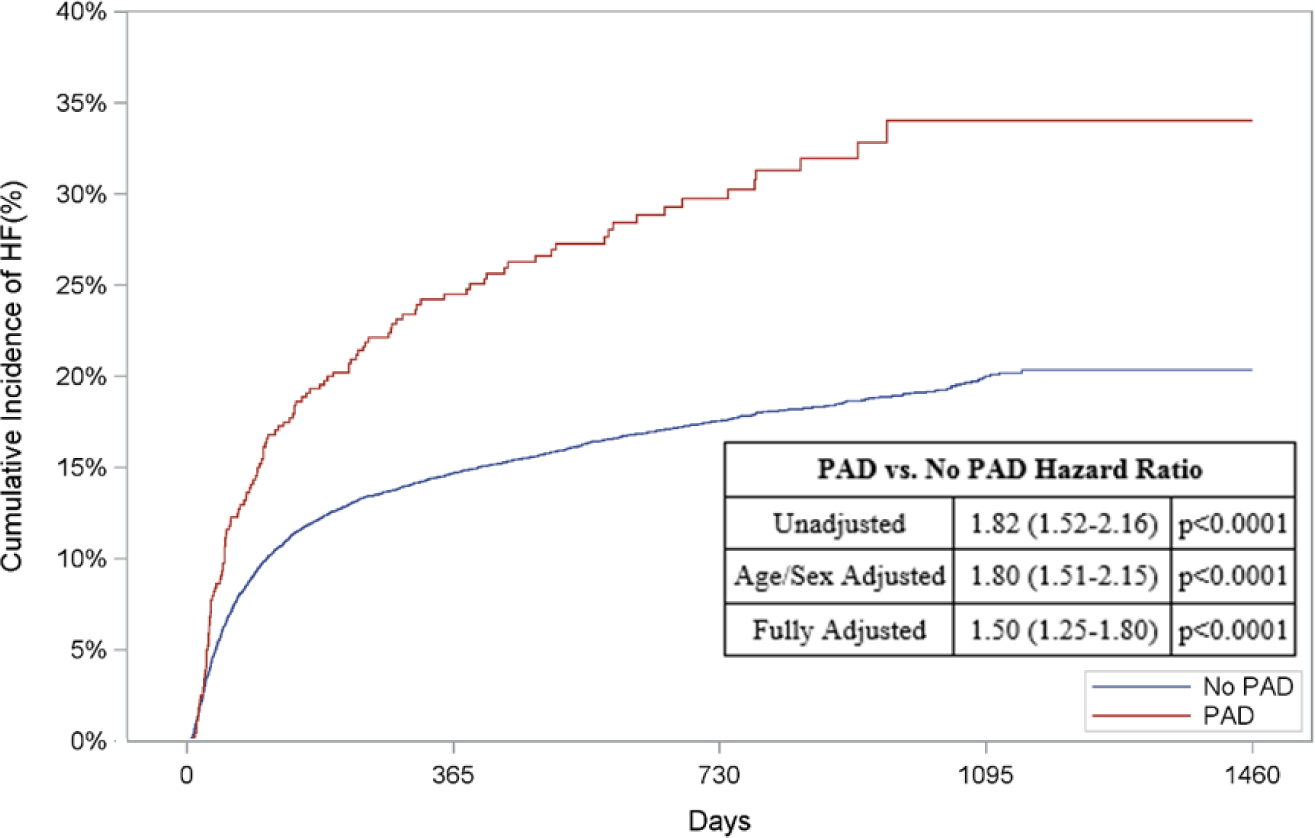
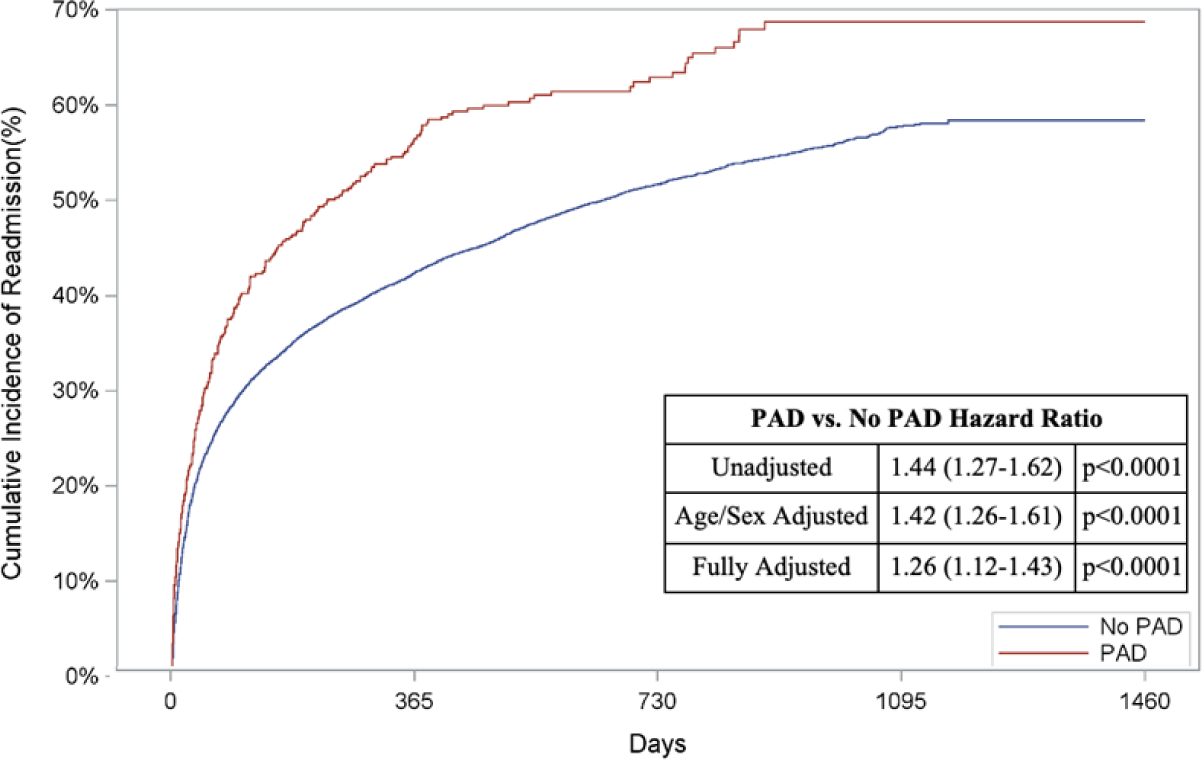
Cumulative incidence curves and hazard ratios for out-of-hospital outcomes in patients receiving mechanical circulatory support stratified by the presence of lower extremity peripheral artery disease (PAD): a.) Out-of-hospital mortality, b.) Hospitalization for myocardial infarction (MI), c.) Hospitalization for heart failure (HF), and d.) Readmission. For out-of-hospital mortality, cumulative incidence curves estimated using Kaplan Meier methods are displayed. For non-death-related outcomes, cumulative incidence function curves are displayed, which account for the competing risk of death. In addition, all hazard ratios for non-death outcomes are based on sub-distribution hazards models using Fine-Gray methods to account for the competing risk of death.
Coronary Revascularization Subgroup
Among all study patients, 40.9% (n=29,356) underwent PCI and 12.0% (n=8,625) underwent CABG. Of all those who underwent PCI, 4.0% (n=1,161) had PAD. After multivariable adjustment, patients with PAD who underwent PCI had higher risk of in-hospital mortality (OR: 1.43, 95% CI: 1.27–1.61, p<0.001), amputation (OR: 8.09, 95% CI: 3.86–16.95, p<0.001), and need for lower extremity revascularization (OR: 2.72, 95%: 2.1–3.5, p<0.001). Similarly, patients with PAD who underwent CABG had higher risk of in-hospital mortality (OR: 1.33, 95% CI: 1.01–1.74, p=0.04) compared to patients without PAD. MCS was utilized in 53% of patients who underwent PCI and 93% of patients who underwent CABG. Adjusted in- and out-of-hospital mortality, subsequent HF, and readmissions remained increased in patients with PAD who received MCS independent of revascularization strategy. Amputation and lower extremity revascularization risk was higher in patients with PAD who underwent PCI and received MCS (Appendix Table 11).
Discussion:
In this analysis of Medicare patients presenting with CS and AMI, 5.9% were found to have previously diagnosed PAD. These patients experienced a greater risk of both in-hospital and out-of-hospital death compared with patients without PAD. Moreover, patients with PAD had a 7.0 fold increased risk of in-hospital amputation in addition to higher risk of bleeding and need for lower extremity revascularization. Risks of adverse events persisted after discharge, with patients with PAD having higher longitudinal risks of subsequent heart failure hospitalization and readmission.
The management of patients presenting with CS and AMI requires an upfront simultaneous risk assessment of therapeutic benefit versus harm of coronary revascularization and mechanical circulatory support. Paramount to the treatment of patients presenting with AMI remains early implementation of a revascularization strategy (18). Outcomes in this analysis may be influenced by the lower rates of both surgical and percutaneous coronary revascularization in patients with PAD. This selection bias likely has multifactorial underpinnings that relate to decreased revascularization rates in patients that are older, female, or non-white and clinical equipoise in optimal timing and strategy of revascularization particularly in complex disease (1,19). It is known that the presence of PAD predicts more severe coronary disease, including left main CAD or complex CAD as quantified by SYNTAX score and it is further known that multivessel CAD is tied to worse outcomes in AMI and cardiogenic shock (19,20). Thus, lower rates of both percutaneous and surgical coronary revascularization amongst PAD patients may reflect an upfront bias in management strategies, a potential avoidance of MCS utilization, and the overall high mortality associated with the presenting syndrome. Our findings, however, reinforce the association of PCI in patients with PAD with reduced procedural success and increased risk of adverse outcomes including bleeding, limb ischemia, and in-hospital and out-of-hospital mortality (21).
In parallel with revascularization, outcomes in this population may also be influenced by MCS utilization among those with PAD. Despite temporal trends reflecting increasing utilization of MCS overall, MCS was used in patients with PAD at nearly half the rate of patients without PAD (22). When used, it was associated with increased risk of amputation. These findings may be influenced in part by the common femoral artery serving as the primary vascular access site of choice for MCS. Atherosclerotic and calcified common femoral and iliac arteries are prominent in patients with severe PAD and increase the risk for vascular complications, a risk that may be exacerbated by large bore vascular access required for MCS (9,23). This may dissuade the utilization of MCS in this patient population. These findings echo the demonstrated association between periprocedural outcomes, including increases in mortality, length of stay, and cost in patients who undergo large-bore transcatheter interventions (9).
These results, the largest study to date of PAD in AMI and CS, highlight the complexities inherent in the clinical prognostication and management of patients with PAD and the associated comorbidities. Patients with PAD often have polyvascular disease along with high rates of comorbid cardiovascular disorders, observations reflected in the baseline characteristics of this analysis (24). Notwithstanding, our findings have several important implications. First, the association between pre-existing PAD and worse short and long-term outcomes may help cardiologists inform patients and their healthcare proxies about expected prognosis and the risks and benefits of invasive treatments such as MCS. Second, these findings reinforce the importance of meticulous techniques in vascular access, the consideration of alternative access sites if large-bore access is needed, and the need to be aware of the risk of adverse limb outcomes in patients with PAD hospitalized with cardiogenic shock. In patients undergoing revascularization or for whom MCS is deemed necessary, early consideration of alternative vascular access, including transradial, transaxillary or transcaval access, may be beneficial and has suggested promise in cardiogenic shock and high-risk PCI in patients with severe PAD (25,26). Ultimately, the optimal management strategy for patients with PAD presenting with cardiogenic shock and AMI may be multidisciplinary, with early involvement from vascular specialists, until prospective data can inform optimal revascularization and mechanical circulatory support decision-making.
Study Limitations:
Our study has several important limitations. First, in this retrospective analysis, unmeasured confounders may be present in our population, including factors that determine treatment selection, such as patient frailty, and procedural risk (i.e. access site). Second, the generalizability of these findings may be limited to an older patient population with greater risk of death. Nonetheless, there is an established association between increased age and peripheral vascular disease, and this analysis thus likely encompasses a large proportion of the PAD population (27). Thirdly, patients were defined by claims codes for PAD in the year prior to their admission. This strategy may have identified a particularly higher-risk subset of patients with symptomatic PAD due to the need for treatment for their condition in the year preceding presentation. In addition, while our rates of PAD are comparable to previously published data, under-diagnosis of PAD is possible (11). Further studies are needed to define the complete epidemiology of symptomatic and asymptomatic PAD in AMI and cardiogenic shock.
Conclusions:
Comorbid established PAD is a significant risk factor for adverse events among patients who present with CS and AMI, and is associated with worse limb outcomes as well as poorer short- and long-term survival compared with those without PAD. MCS, an important tool in the management of cardiogenic shock, was less often used in this patient population, and when employed, was associated with worse outcomes. These findings highlight the importance of identifying and considering PAD as a risk factor and underscores the need for a multidisciplinary approach to managing these patients.
Supplementary Material
Clinical Perspectives.
Competency in Patient Care:
Patients with lower extremity peripheral artery disease hospitalized with acute myocardial infarction and cardiogenic shock face a high risk of mortality and limb complications, particularly when managed with mechanical circulatory support.
Translational Outlook:
Further studies are needed to evaluate strategies to reduce the risk of limb amputation and improve clinical outcomes in patients with peripheral artery disease hospitalized with acute myocardial infarction and cardiogenic shock.
Sources of Funding/Conflict of Interest:
The following authors has no conflicts of interest to declare: RM, SC, YS. NM has received funding from the National Institutes of Health (grant T32HL007208). AJK reports Institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical. In addition to research grants, institutional funding includes fees paid to Columbia University and/or Cardiovascular Research Foundation for speaking engagements and/or consulting. Personal: Travel Expenses/Meals from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, CathWorks, Siemens, Philips, ReCor Medical, Chiesi, OpSens, Zoll, and Regeneron. SAP reports institutional research support from Abbott Vascular, TriReme Medical, SurModics, and Shockwave Medical; is an advisory board member for Abbott Vascular, Boston Scientific, Cardinal Health, Medtronic, Janssen, CSI, and Philips; and receives honoraria from Abiomed and Terumo. KR has served as a consultant to or on the scientific advisory board for Access Vascular, Althea Medical, Angiodynamics; BMS-Pfizer; Boston Scientific; Contego; Janssen; InspireMD; Magneto; Mayo Clinic; Neptune Medical; Philips; Summa Therapeutics; Surmodics; Thrombolex; Terumo; Truvic, he has received institutional grants from the NIH, Philips, Intact Vascular, and Boston Scientify and has equity in Equity: Accolade; Access Vascular; Althea Medical; Contego; Cruzar Systems; Embolitech; Endospan; Eximo; InspireMG; JanaCare; Magneto; Micell; Neptune Medical; Orchestra; PQ Bypass; Prosomnus; Shockwave; Summa Therapeutics; Thrombolex; Truvic; Valcare, and serves as a board member for the National PERT Consortium. RWY has served on scientific advisory boards, consulted for, and received research support from Abbott Vascular, AstraZeneca, Boston Scientific, and Medtronic. He also receives funding from the National Heart, Lung, and Blood Institute (grant R01HL136708) and the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. EAS has receiving consulting and/or speaking fees from Bard, Cook Medical, CSI, Medtronic and Phillips. He receives research support from AstraZeneca, Bard, Boston Scientific, Cook Medical, CSI, Medtronic and Philips.
Abbreviations:
- AMI
Acute myocardial infarction
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CVA
Cerebrovascular accident
- CS
Cardiogenic shock
- ECMO
Extracorporeal membrane oxygenation
- IABP
Intra-aortic balloon pump
- PAD
Peripheral artery disease
- MCS
Mechanical circulatory support
- PCI
Percutaneous coronary intervention
- pVAD
Percutaneous ventricular assist device
- TIA
Transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kolte D, Khera S, Aronow WS et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc 2014;3:e000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg RJ, Makam RCP, Yarzebski J, McManus DD, Lessard D, Gore JM. Decade-Long Trends (2001–2011) in the Incidence and Hospital Death Rates Associated with the In-Hospital Development of Cardiogenic Shock after Acute Myocardial Infarction. Circulation: Cardiovascular Quality and Outcomes 2016;9:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aissaoui N, Puymirat E, Tabone X et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J 2012;33:2535–2543. [DOI] [PubMed] [Google Scholar]
- 4.Poredos P, Jug B. The prevalence of peripheral arterial disease in high risk subjects and coronary or cerebrovascular patients. Angiology 2007;58:309–15. [DOI] [PubMed] [Google Scholar]
- 5.Jeremias A, Gruberg L, Patel J, Connors G, Brown DL. Effect of peripheral arterial disease on in-hospital outcomes after primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol 2010;105:1268–1271. [DOI] [PubMed] [Google Scholar]
- 6.Saw J, Bhatt DL, Moliterno DJ et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol 2006;48:1567–1572. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero M, Harjai K, Stone GW et al. Usefulness of the presence of peripheral vascular disease in predicting mortality in acute myocardial infarction patients treated with primary angioplasty (from the Primary Angioplasty in Myocardial Infarction Database). Am J Cardiol 2005;96:649–654. [DOI] [PubMed] [Google Scholar]
- 8.Strom JB, Zhao Y, Shen C et al. Hospital Variation in the Utilization of Short-Term Nondurable Mechanical Circulatory Support in Myocardial Infarction Complicated by Cardiogenic Shock. Circ Cardiovasc Interv 2019;12:e007270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redfors B, Watson BM, McAndrew T et al. Mortality, Length of Stay, and Cost Implications of Procedural Bleeding After Percutaneous Interventions Using Large-Bore Catheters. JAMA Cardiology 2017;2:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2005;26:1276–1283. [DOI] [PubMed] [Google Scholar]
- 11.Abaunza M, Kabbani LS, Nypaver T et al. Incidence and prognosis of vascular complications after percutaneous placement of left ventricular assist device. J Vasc Surg 2015;62:417–423. [DOI] [PubMed] [Google Scholar]
- 12.Hlatky MA, Ray RM, Burwen DR et al. Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes 2014;7:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secemsky EA, Raja A, Shen C et al. Rationale and Design of the SAFE-PAD Study. Circ Cardiovasc Qual Outcomes 2021;14:e007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strom JB, Zhao Y, Faridi KF et al. Comparison of Clinical Trials and Administrative Claims to Identify Stroke Among Patients Undergoing Aortic Valve Replacement: Findings From the EXTEND Study. Circ Cardiovasc Interv 2019;12:e008231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faridi KF, Tamez H, Butala NM et al. Comparability of Event Adjudication Versus Administrative Billing Claims for Outcome Ascertainment in the DAPT Study: Findings From the EXTEND-DAPT Study. Circulation Cardiovascular quality and outcomes 2021;14:e006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess CN, Cannon CP, Beckman JA et al. Effectiveness of Blood Lipid Management in Patients With Peripheral Artery Disease. Journal of the American College of Cardiology 2021;77:3016–3027. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 18.Zeymer U, Hochadel M, Karcher AK et al. Procedural Success Rates and Mortality in Elderly Patients With Percutaneous Coronary Intervention for Cardiogenic Shock. JACC Cardiovasc Interv 2019;12:1853–1859. [DOI] [PubMed] [Google Scholar]
- 19.Khera R, Secemsky EA, Wang Y et al. Revascularization Practices and Outcomes in Patients With Multivessel Coronary Artery Disease Who Presented With Acute Myocardial Infarction and Cardiogenic Shock in the US, 2009–2018. JAMA Intern Med 2020;180:1317–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim EK, Song PS, Yang JH et al. Peripheral Artery Disease in Korean Patients Undergoing Percutaneous Coronary Intervention: Prevalence and Association with Coronary Artery Disease Severity. Journal of Korean Medical Science 2013;28:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nikolsky E, Mehran R, Mintz GS et al. Impact of symptomatic peripheral arterial disease on 1-year mortality in patients undergoing percutaneous coronary interventions. J Endovasc Ther 2004;11:60–70. [DOI] [PubMed] [Google Scholar]
- 22.Strom JB, Zhao Y, Shen C et al. National trends, predictors of use, and in-hospital outcomes in mechanical circulatory support for cardiogenic shock. EuroIntervention 2018;13:e2152–e2159. [DOI] [PubMed] [Google Scholar]
- 23.Genereux P, Webb JG, Svensson LG et al. Vascular complications after transcatheter aortic valve replacement: insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. Journal of the American College of Cardiology 2012;60:1043–52. [DOI] [PubMed] [Google Scholar]
- 24.Suárez C, Zeymer U, Limbourg T et al. Influence of polyvascular disease on cardiovascular event rates. Insights from the REACH Registry. Vasc Med 2010;15:259–265. [DOI] [PubMed] [Google Scholar]
- 25.Cheney AE, McCabe JM. Alternative Percutaneous Access for Large Bore Devices. Circ Cardiovasc Interv 2019;12:e007707. [DOI] [PubMed] [Google Scholar]
- 26.Kajy M, Laktineh A, Blank N et al. Deploying Mechanical Circulatory Support Via the Axillary Artery in Cardiogenic Shock and High-Risk Percutaneous Coronary Intervention. Am J Cardiol 2020;128:127–133. [DOI] [PubMed] [Google Scholar]
- 27.Savji N, Rockman CB, Skolnick AH et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 2013;61:1736–1743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


