Abstract
BACKGROUND AND PURPOSE:
Assessment of cerebral venous sinus thrombosis on MR imaging can be challenging. The aim of this study was to evaluate the diagnostic accuracy of high-resolution 3D T2 sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE) in patients with cerebral venous sinus thrombosis and to compare its performance with contrast-enhanced 3D T1-MPRAGE.
MATERIALS AND METHODS:
We performed a blinded retrospective analysis of T2-SPACE and contrast-enhanced MPRAGE sequences from patients with cerebral venous sinus thrombosis and a control group. The results were compared with a reference standard, which was based on all available sequences and clinical history. Subanalyses were performed according to the venous segment involved and the clinical stage of the thrombus.
RESULTS:
Sixty-three MR imaging examinations from 35 patients with cerebral venous sinus thrombosis and 51 examinations from 40 control subjects were included. The accuracy, sensitivity, and specificity calculated from the initial MR imaging examination for each patient were 100% each for T2-SPACE and 95%, 91%, and 98%, respectively, for contrast-enhanced MPRAGE. The interrater reliability was high for both sequences. In the subanalysis, the accuracy for each venous segment involved and if subdivided according to the clinical stage of thrombus was ≥95% and ≥85% for T2-SPACE and contrast-enhanced MPRAGE, respectively.
CONCLUSIONS:
Both T2-SPACE and contrast-enhanced MPRAGE offer high accuracy for the detection and exclusion of cerebral venous sinus thrombosis; however, T2-SPACE showed a better overall performance and thus could be a useful tool if included in a multiparametric MR imaging protocol for the diagnosis of cerebral venous sinus thrombosis, especially in scenarios where gadolinium administration is contraindicated.
Cerebral venous sinus thrombosis (CVST) is an infrequently occurring but potentially life-threatening condition.1 Around 0.5% of acute strokes are caused by CVST.2 However, excluding CVST is one of the most frequent reasons for referral to the neuroradiology department. CVST has also recently been reported in patients with coronavirus disease 2019 (COVID-19)3 and after COVID-19 vaccination in the setting of thrombosis and thrombocytopenia syndrome.4 Both CT and MR imaging can be used for the diagnosis, but in many centers and in certain circumstances, such as in younger patients and pregnant women, MR imaging has become the method of choice.5 A combination of MR imaging sequences is usually used to verify the diagnosis.6 This combination aims to visualize the thrombus, detect absent flow in the segments involved, and identify the concomitant changes in the brain parenchyma.5
Absent flow is assessed using phase-contrast or TOF MR venography, but these flow-sensitive sequences are subject to multiple pitfalls.7 Contrast-enhanced (CE) sequences, such as venous angiography8 or CE-MPRAGE have provided better results;9 therefore, a contrast agent is usually introduced to exclude venous thrombosis. However, administration of a contrast agent is not always appropriate, for example during pregnancy10 or in patients with allergic reactions11 and impaired renal function.11 Furthermore, patients may refuse the administration of contrast, following the widespread discussions regarding gadolinium deposition in the brain.12 In such cases, there is uncertainty in the diagnosis or exclusion of CVST. Therefore, non-contrast-dependent sequences with a higher sensitivity for the detection of CVST are required.
The conventional T2 spin-echo sequence depicts signal changes within the thrombotic material,5 with absence of the physiologic flow void. However, due to the hypointense signal in thrombi at certain stages, which mimic a flow void,8,13 as well as the complex blood flow in the venous sinuses, radiologists cannot solely rely on this sequence.5 Another drawback is the limitation of visualization of small venous structures in 2D sequences due to the partial volume effect. However, the data obtained on the utility of the conventional T2 sequences is based on older techniques.13-17
In recent years, MR imaging manufacturers have developed an optimized single-slab 3D FSE sequence that has a clinically acceptable acquisition time.18,19 This technique provides a high-resolution 3D volume with millimeter section thickness with adequate SNR and optimized tissue contrast,18 without a limitation in the specific absorption rate.18,20 One such sequence is the so-called 3D T2 sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE) sequence. We hypothesized that this sequence would have high accuracy in the detection of CVST. Thus, we aimed to evaluate the performance of T2-SPACE in comparison with one of the widely used CE gradient-echo sequences, namely 3D CE-MPRAGE.9,21
MATERIALS AND METHODS
Patients
This retrospective study was approved by the Bern University Hospital medical ethics committee. A search was performed in the PACS of our tertiary hospital as well as the local stroke registry for 2 groups of participants: 1) patients with a final diagnosis of CVST, and 2) a control group of subjects who underwent MR imaging but were negative for CVST. The inclusion criterion for both groups was the presence of both T2-SPACE and CE-MPRAGE in the same scanning protocol. Absence of one or both of these sequences was the exclusion criterion. To avoid bias during imaging interpretation due to the possibility of the presence of parenchymal lesions (eg, venous edema) in patients with CVST, the presence of parenchymal lesions in the control group was not an exclusion criterion. The first available MR imaging, the “baseline-MR imaging” containing both sequences was considered for the primary analysis. Further follow-up MRIs from the same patient, with both sequences acquired during each examination, were also considered for further subanalysis regarding the clinical stage of the thrombus.
MR Imaging Protocol
Patients were examined on either 1.5T or 3T scanners (Magnetom Avanto, Aera, Skyra, Prisma, or Vida; Siemens). The scanning parameters are shown in the Online Supplemental Data. The MR imaging protocol also included other sequences that varied according to the clinical indication.
Index Test: Imaging Interpretation
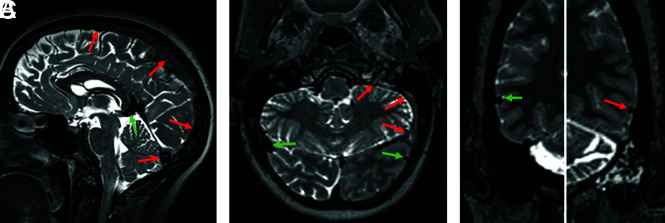
Images from patients with CVST and the control group were anonymized and randomized. Two readers, blinded to the clinical information and final diagnosis, reviewed the images independently. Reader 1 was a board-certified neuroradiologist with >14 years of experience, and reader 2 was a neuroradiology resident with 1 year of experience in vascular neuroradiology. The rating began with T2-SPACE for all patients, while raters were blinded to the other sequences. On T2-SPACE, the diagnosis of thrombosis was defined by the absence of the physiologic flow void with an inhomogeneous signal in the venous segment involved (Fig 1). The images were analyzed in the multiplanar reconstruction tool to evaluate each segment in multiple projections. Studies from the same patient were rated during different sessions. Rating of CE-MPRAGE began a month after finishing the rating of the T2-SPACE analyses. The readers assessed the following venous segments: superior sagittal sinus, torcular Herophili, transverse sinus, sigmoid sinus, jugular vein, straight sinus, vein of Galen, internal cerebral veins, vein of Labbé, and cortical veins including the vein of Trolard and reported their final impression regarding the presence or absence of CVST. Disagreement between the 2 readers was resolved by a third senior reader with 25 years of experience in neuroradiology.
FIG 1.
T2-SPACE obtained from different patients. The normal segments (green arrows) show a homogeneous physiologic flow void. In the thrombosed segments (red arrows, A), superior sagittal sinus and torcular; left sigmoid sinus and jugular vein (B); and the left vein of Labbé (C), the flow void is absent and the signal is mostly inhomogeneous. According to the stage of the thrombus, expansion of the sinus may be seen (superior sagittal sinus in A).
Reference Standard
Reader 1 and the senior reader established the reference standard, which was the final diagnosis and location of CVST based on the evaluation of all available MR imaging sequences (T2-SPACE, T1, FLAIR, DWI, SWI, TOF, or phase-contrast venography; 3D CE-MPRAGE; and CE venous angiography) from each examination. A valid reference standard included at least T2-SPACE, 3D CE-MPRAGE, and ≥1 venous angiography sequence (TOF, phase-contrast, and/or CE venous angiography). In only 3 examinations was venous angiography not performed on the examination; however, in these 3 cases, it was performed within 2 days. All MR imaging examinations and clinical data were taken into consideration during the evaluation of each venous segment. To avoid misinterpretation of the segment involved due to evolution of the thrombus, the reference standard was performed for each MR imaging examination included in the study.
Clinical Data
Clinical data from the group of patients with CVST were obtained from the Swiss Stroke Registry. Data included symptom onset, which was used to estimate the clinical stage of the thrombus according to the interval between symptom onset and imaging as follows: acute = 0–5 days; subacute = 6–15 days; chronic, >15 days, or late chronic, >1 year. 5
Statistical Analysis
The results obtained from each sequence (T2-SPACE and CE-MPRAGE) based on the baseline MR imaging were compared with the reference standard to obtain the sensitivity, specificity, accuracy, and the positive and negative predictive values. Interrater reliability was calculated using the Cohen κ coefficient. All available examinations (ie, the baseline MR imaging as well as follow-ups) were included to perform 2 different subanalyses according to the clinical stage and the location of the thrombus.
RESULTS
From May 2016 to September 2019, thirty-five patients with CVST fulfilled the inclusion criteria and 20 patients were initially examined on a 1.5T and 15 on a 3T scanner. In total, 16/35 patients (45.7%) showed parenchymal lesions (vasogenic edema, cytotoxic edema, and/or hemorrhage). A total of 63 examinations from the patients with CVST were included in the subanalysis. Table 1 and the Online Supplemental Data summarize the patient characteristics.
Table 1:
Characteristics of patients included in the analysis
| Control | CVST | |
|---|---|---|
| No. of patients | 41 | 35 |
| Male/female ratio | 14:27 | 14:21 |
| Age (median) (interquartile range) (yr) | 38.5 (27–56) | 43.5 (34–61) |
| No. of MR imaging examinations | 51 | 63 |
| 1.5T/3T | 34:17 | 37:26 |
The clinical stage of thrombus was identified as acute (n = 16), subacute (n = 13), chronic (n = 23), and late chronic (n = 10). In 1 examination, the stage was uncertain. The Online Supplemental Data summarize the stage and location of the thrombi. Very few examinations revealed thrombosis of the vein of Galen and internal cerebral veins. These were excluded from the subanalysis.
Fifty-one MRIs obtained from 41 individuals without CVST (control group) were included. Around half of these examinations were performed to exclude CVST. Thirty-two studies revealed no parenchymal lesions, while the other 19 examinations revealed other parenchymal lesions such as perifocal edema due to hemorrhage or other pathologies. In total, 114 examinations obtained from 76 patients were included.
T2-SPACE
Based on the baseline MR imaging, the final diagnosis of CVST was accurately identified in all study participants (ie, 35 patients with CVST and all 41 controls) on T2-SPACE (Fig 1, Table 2, and the Online Supplemental Data), with the Cohen κ = 0.92 (Online Supplemental Data).
Table 2:
Results of T2-SPACE and CE-MPRAGE based on the first available MR imaging examinationsa
| T2-SPACE | CE-MPRAGE | |
|---|---|---|
| Sensitivity | 1 (0.9–1) | 0.91 (0.8–1) |
| Specificity | 1 (0.9–1) | 0.98 (0.9–1) |
| Accuracy | 1 (0.9–1) | 0.95 (0.9–1) |
| Positive predictive value | 1 (0.9–1) | 0.97 (0.8–1) |
| Negative predictive value | 1 (0.9–1) | 0.93 (0.8–1) |
Data in parentheses are 95% confidence intervals.
In the subanalysis, the venous segment involved was correctly identified in >95% of cases for each segment (Online Supplemental Data). The negative predictive value was >90% for all segments.
In the subanalysis regarding the clinical stage (Fig 2), the final diagnosis of the acute and subacute stages was correct in 100% of cases (Online Supplemental Data). In the chronic and late chronic stages, the accuracy reached 99% and 97%, respectively. The interrater reliability was better in the acute (κ = 0.81) and chronic stages (κ = 0.82) than in the subacute (κ = 0.79) and late chronic (κ = 0.67) stages (Online Supplemental Data). The Online Supplemental Data provide examples showing false-positive and false-negative results of T2-SPACE.
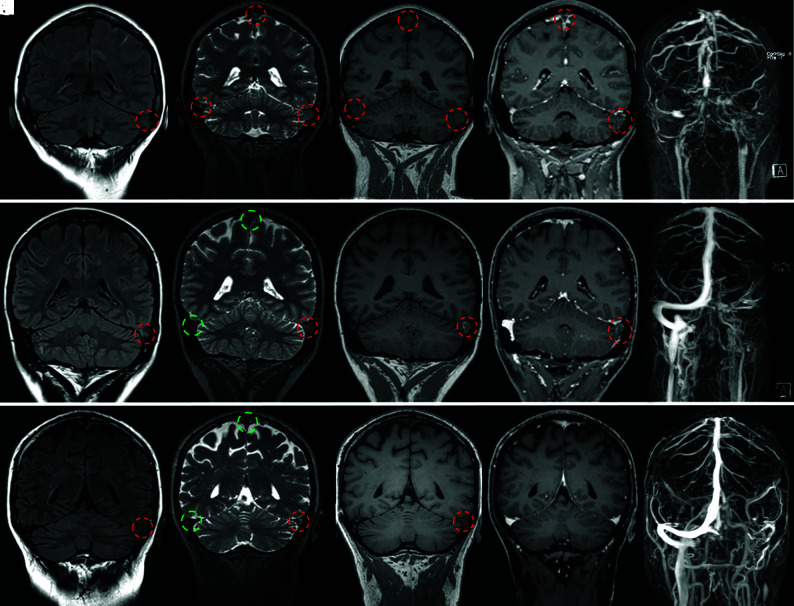
FIG 2.
Coronal MR imaging from 3 different patients showing 3 phases of CVST. From left to right, FLAIR (A, F, K), T2-SPACE (B, G, L), T1-SPACE (C, H, M), CE T1-MPRAGE (D, I, N), and MIP of CE MRA (E, J, O). Thrombosed and patent veins are marked with red and green circles, respectively. The latter exhibit a physiologic flow void, clearly visible on SPACE images. Upper row, A–E, Acute CVST in a 45-year-old woman with headache and vomiting. MR imaging, performed on the day of symptom onset shows extensive thrombosis (red circles) of the transverse and sigmoid sinuses, jugular vein, torcular Herophili, and superior sagittal sinus. The flow void is absent on the T1 and T2 sequences. There are filling defects on CE-MPRAGE. The extension of the thrombosis is seen on the CE-MRA. Middle row, F–J, Subacute CVST in a 30-year-old woman who presented initially with headache. A follow-up MR imaging 13 days after the initial presentation shows thrombosis with an absent flow void on SPACE of the left transverse/sigmoid sinus and jugular vein. Lower row, K–O, Chronic CVST in a 63-year-old male patient who initially presented with headache. A follow-up MR imaging after 200 days shows a thrombus in the left transverse sinus. The flow void is absent on T2 and T1, but CVST is not detectable on CE-MPRAGE.
CE-MPRAGE
In the final diagnosis based on the initial MR imaging, CE-MPRAGE correctly identified 32/35 patients with CVST and 40/41 controls. The accuracy, sensitivity, and specificity were 95%, 91%, and 98%, respectively, with the Cohen κ = 0.87 (Table 2 and Online Supplemental Data). The performance of 3T was better than that of the 1.5T scanner (accuracy, 100% versus 91%) (Online Supplemental Data).
Subanalysis showed the correct diagnosis in ≥85% for each segment (Online Supplemental Data). The lowest accuracy was 85% in the torcular. The specificity was at the lowest in the sigmoid sinus, reaching 95%; this was higher than the lowest sensitivity, which dropped to <50% for the cortical veins.
In the subanalysis regarding the clinical stage of the thrombus (Fig 2), the accuracy in the acute phase was 97% (Online Supplemental Data), while in the subacute and chronic phases, the accuracy was 92%. In the late chronic stage, the accuracy was 85%. The interrater reliability was better in the acute (κ = 0.93) and chronic (κ = 0.9) stages than in the subacute (κ = 0.8) and late chronic (κ = 0.74) stages (Online Supplemental Data).
DISCUSSION
The main findings of this study are that T2-SPACE has a slightly higher sensitivity and specificity than CE-MPRAGE in the diagnosis of CVST. The interrater agreement was slightly better for CE-MPRAGE than for T2-SPACE. For T2-SPACE, agreement ranged from moderate (in the subacute and late chronic phases) to strong (in the acute and chronic phases). The accuracy of T2-SPACE was >95% in a subanalysis of all venous vascular segments, and it exceeded 95% accuracy for each of the different clinical stages of thrombus. The results also highlight the disadvantages of relying solely on contrast-based sequences for excluding CVST and emphasize the potential value of adding T2-SPACE to CVST protocols.
Exclusion of CVST is one of the common reasons for referrals to the radiology department during pregnancy and the postpartum period.22 However, during pregnancy and lactation, gadolinium-based contrast agents should be used with caution and only if the diagnosis cannot be made without them.10,11,23 Administration of contrast is also not desired in patients with renal impairment11 and in patients who refuse gadolinium due to increasing evidence of its deposition in the brain.12 Therefore, finding an MR imaging protocol that reduces the need for gadolinium is of utmost importance. The addition of the T2-SPACE to the routine CVST protocol can increase the sensitivity and specificity of the MR imaging, especially in the above-mentioned situations.24,25
T2-SPACE is an isotropic 3D FSE technique characterized by very long echo-train lengths, ultrashort echo spacing, and reduced flip angles.26 It has a high sampling efficiency and a high turbo factor due to use of nonselective short refocusing pulse trains with variable flip angles. One of the advantages of this sequence is that it combines the properties of spin-echo with the advantage of speedy acquisition by reading multiple lines in phase-encoding following each excitation pulse.18 T2 spin-echo-based sequences are important because they provide optimal image contrast27and are resistant to magnetic field inhomogeneities.28,29 There is intrinsic blood suppression,18,28 allowing the reduction of flow artifacts. Therefore, T2-SPACE is not only valuable for evaluating brain parenchyma but also for the assessment of the vessel lumen. One of its important advantages is the delineation of the outer wall of the vein or sinus, which enables the differentiation between lumen narrowing due to a thrombus or a hypoplastic sinus (Online Supplemental Data), thus avoiding one of the pitfalls in angiographic imaging. Furthermore, expansion of the vein lumen by a clot in T2-SPACE is useful in interpretation (Fig 1A and Fig 2B). The 3D and isotropic imaging allow retrospective postprocessing, even of small veins, in different projections so that the acquisition of one 3D sequence replaces multiple conventional 2D T2 series.18 Another advantage over 2D T2 is the coverage of the jugular vein (which is usually not covered in conventional axial 2D T2). Filling defects in TOF or phase-contrast venography due to arachnoid granulations are correctly classified in T2-SPACE (Online Supplemental Data). Moreover, as in the case of dark-blood sequences,30 T2-SPACE offers advantages when evaluating vascular segments adjacent to tumors, allowing reliable assessment of sinus invasion (Online Supplemental Data).
Disadvantages of T2-SPACE include motion artifacts, as is the case with any 3D sequences. Collateralization and recanalization could be difficult to evaluate (Online Supplemental Data). Collateralization is usually assessed on an angiographic sequence with background suppression.31 Recanalization is also usually assessed using an angiographic sequence31,32 and could be difficult to evaluate using solely T2-SPACE due to the possibility of inhomogeneous signal of the thrombus. However, using T2-SPACE in a multiparametric approach (signal intensity, signal void, and filling defects on MR venography) could help in detecting recanalization and in assessing the thrombus load score.33 In our study, we did not perform longitudinal comparisons to calculate the thrombus load score due to the relative inhomogeneity of data regarding the clinical stage of thrombosis, but this could be of interest for future studies.
A thrombus demonstrates signal evolution with time. The detection of an acute or chronic thrombus with an iso- or hypointense signal on conventional T1 and T2 imaging could be troublesome; thus, conventional angiography is usually required.8 However, in our patient sample, the accuracy of the T2-SPACE in the final diagnosis was very high for thrombi at all clinical stages. We suggest that the signal changes in T2-SPACE are probably related not only to the signal of the thrombus but also to flow alterations. Furthermore, a thrombus at certain stages may be missed in contrast-based sequences when the thrombus signal is hyperintense on T1 or due to enhancement of the thrombus (Fig 3). In general, subacute thrombus with the presence of methemoglobin is well-depicted on precontrast T1 and also on SWI due to the presence of blooming artifacts.7,34 Diffusion restriction can be identified, especially in the subacute clots.5 Here, multiparametric imaging plays a very important role because it increases the sensitivity and specificity. A protocol to exclude sinus thrombosis without the need for contrast administration should be based on multiparametric MR imaging, using FLAIR, T1, DWI, SWI, phase-contrast venography, and adding T2-SPACE instead of conventional 2D T2. This choice will increase the accuracy and reduce the number of cases in which contrast is needed because the high negative predictive value of T2-SPACE makes it suitable for thrombus exclusion when combined with other sequences.
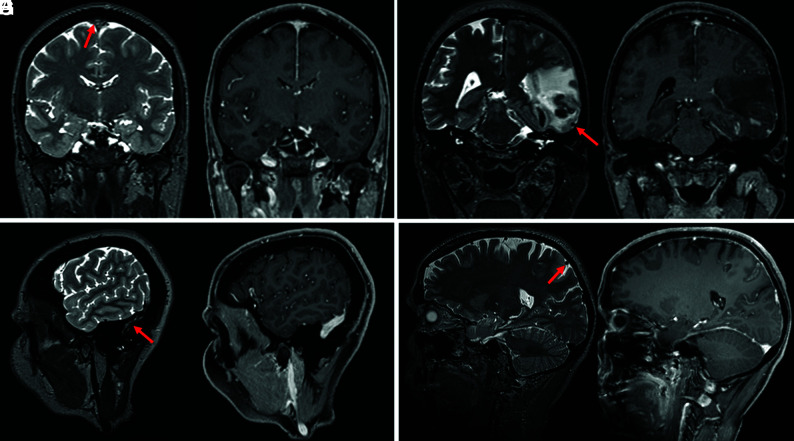
FIG 3.
Examples from 4 different patients showing a thrombus (red arrows) on the T2-SPACE in the superior sagittal sinus (A), the vein of Labbé (C), the sigmoid sinus (E), and the cortical vein (G). CVST was missed on the CE T1-MPRAGE (B, D, F, and H).
Strengths and Limitations
In this study, we included T2-SPACE and CE-MPRAGE from the same patient sample, enabling a fair comparison between the 2 isotropic sequences with the same rater experience. The inclusion of the control group with parenchymal lesions also allowed an unbiased comparison because the presence of edema or hemorrhage in the CVST group could influence the diagnosis.
The study has the limitations typical of a single-center retrospective analysis. The sample size was relatively small, given that CVST is an uncommon condition, and the size was further reduced by the inclusion criterion that the same MR imaging examination should include both T2-SPACE and CE-MPRAGE. Including multiple MRIs from the same patient is also a substantial limitation; however, the main aim here was to allow subanalysis according to the stage of thrombus. Clinical data (ie, time from initial symptoms) were used to define the clinical stage of the thrombus, which could be challenging in patients with nonspecific symptoms. Another limitation was the lack of patients with involvement of the cavernous sinus or cerebellar veins as well as the small number of cases with involvement of the vein of Galen and internal cerebral veins, meaning that the accuracy of these subgroups cannot be calculated. This study had aimed to assess the sensitivity and specificity of T2-SPACE as a potential sequence to be used in pregnancy. However, no pregnant patients were included in the study; in our institution, pregnant patients do not usually receive contrast agents and thus did not fulfill the inclusion criteria.
CONCLUSIONS
T2-SPACE has a high accuracy in the detection and exclusion of CVST at all clinical stages of thrombus and should be added to the routine CVST MR imaging protocol, especially if a contrast injection is contraindicated or undesirable.
ABBREVIATIONS:
- CE
contrast-enhanced
- CVST
cerebral venous sinus thrombosis
- SPACE
sampling perfection with application-optimized contrasts using different flip angle evolution
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1. Bonneville F. Imaging of cerebral venous thrombosis. Diagn Interv Imaging 2014;95:1145–50 10.1016/j.diii.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 2. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol 2007:6:162–70 10.1016/S1474-4422(07)70029-7 [DOI] [PubMed] [Google Scholar]
- 3. Al-Mufti F, Amuluru K, Sahni R, et al. Cerebral venous thrombosis in COVID-19: a New York Metropolitan Cohort Study. AJNR Am J Neuroradiol 2021;42:1196–1200 10.3174/ajnr.A7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sánchez van Kammen M, Aguiar de Sousa D, Poli S, et al. ; Cerebral Venous Sinus Thrombosis with Thrombocytopenia Syndrome Study Group. Characteristics and outcomes of patients with cerebral venous sinus thrombosis in SARS-CoV-2 vaccine–induced immune thrombotic thrombocytopenia. JAMA Neurol 2021;78:1314 10.1001/jamaneurol.2021.3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linn J, Brückmann H. Cerebral venous and dural sinus thrombosis: state-of-the-art imaging. Clin Neuroradiol 2010;20:25–37 10.1007/s00062-010-9035-7 [DOI] [PubMed] [Google Scholar]
- 6. Patel D, Machnowska M, Symons S, et al. Diagnostic performance of routine brain MRI sequences for dural venous sinus thrombosis. AJNR Am J Neuroradiol 2016;37:2026–32 10.3174/ajnr.A4843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dmytriw AA, Song JS, Yu E, et al. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology 2018;60:669–85 10.1007/s00234-018-2032-2 [DOI] [PubMed] [Google Scholar]
- 8. Leach JL, Fortuna RB, Jones BV, et al. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 2006;26(Suppl 1):S19–41 10.1148/rg.26si055174 [DOI] [PubMed] [Google Scholar]
- 9. Sari S, Verim S, Hamcan S, et al. MRI diagnosis of dural sinus-cortical venous thrombosis: immediate post-contrast 3D GRE T1-weighted imaging versus unenhanced MR venography and conventional MR sequences. Clin Neurol Neurosurg 2015;134:44–54 10.1016/j.clineuro.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 10. Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016;316:952–61 10.1001/jama.2016.12126 [DOI] [PubMed] [Google Scholar]
- 11. Thomsen H, Morcos SK; European Society of Urogenital Radiology. USUR guidelines on contrast agents. Abdom Imaging 2006;31:131–40 10.1007/s00261-005-0380-y [DOI] [PubMed] [Google Scholar]
- 12. Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–41 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 13. Hinman JM, Provenzale JM. Hypointense thrombus on T2-weighted MR imaging: a potential pitfall in the diagnosis of dural sinus thrombosis. Eur J Radiol 2002;41:147–52 10.1016/S0720-048X(01)00365-5 [DOI] [PubMed] [Google Scholar]
- 14. Bianchi D, Maeder P, Bogousslavsky J, et al. Diagnosis of cerebral venous thrombosis with routine magnetic resonance: an update. Eur Neurol 1998;40:179–90 10.1159/000007978 [DOI] [PubMed] [Google Scholar]
- 15. Vogl TJ, Bergman C, Villringer A, et al. Dural sinus thrombosis: value of venous MR angiography for diagnosis and follow-up. AJR Am J Roentgenol 1994;162:1191–98 10.2214/ajr.162.5.8166009 [DOI] [PubMed] [Google Scholar]
- 16. Isensee C, Reul J, Thron A. Magnetic resonance imaging of thrombosed dural sinuses. Stroke 1994;25:29–34 10.1161/01.str.25.1.29 [DOI] [PubMed] [Google Scholar]
- 17. Dormont D, Anxionnat R, Evrard S, et al. MRI in cerebral venous thrombosis [in French]. J Neuroradiol 1994:21;81–99 [PubMed] [Google Scholar]
- 18. Mugler JP 3rd. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging 2014;39:745–67 10.1002/jmri.24542 [DOI] [PubMed] [Google Scholar]
- 19. Mugler JP, Bao S, Mulkern RV, et al. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology 2000;216:891–99 10.1148/radiology.216.3.r00au46891 [DOI] [PubMed] [Google Scholar]
- 20. Mugler JP, Wald LL, Brookeman JR. T2-weighted 3D spin-echo train imaging of the brain at 3 Tesla: reduced power deposition using low flip-angle refocusing RF pulses. In: Proceedings of the 9th Annual Meeting of International Society for Magnetic Resonance in Medicine, Glasgow, Scotland. April 21–27, 2001 [Google Scholar]
- 21. Saindane AM, Mitchell BC, Kang J, et al. Performance of spin-echo and gradient-echo T1-weighted sequences for evaluation of dural venous sinus thrombosis and stenosis. AJR Am J Roentgenol 2013;201:162–69 10.2214/AJR.12.9095 [DOI] [PubMed] [Google Scholar]
- 22. Hacein-Bey L, Varelas PN, Ulmer JL, et al. Imaging of cerebrovascular disease in pregnancy and the puerperium. AJR Am J Roentgenol 2016;206:26–38 10.2214/AJR.15.15059 [DOI] [PubMed] [Google Scholar]
- 23. American College of Radiology. ACR Manual on Contrast Media. 2021. https://www.acr.org/Clinical-Resources/Contrast-Manual. Accessed July 29, 2021
- 24. Hand JW, Li Y, Thomas EL, et al. Prediction of specific absorption rate in mother and fetus associated with MRI examinations during pregnancy. Magn Reson Med 2006;55:883–93 10.1002/mrm.20824 [DOI] [PubMed] [Google Scholar]
- 25. Barrera CA, Francavilla ML, Serai SD, et al. Specific absorption rate and specific energy dose: comparison of 1.5-T versus 3.0-T fetal MRI. Radiology 2020;295:664–74 10.1148/radiol.2020191550 [DOI] [PubMed] [Google Scholar]
- 26. Lichy MP, Horger W, Mugler JP, et al. T2-weighted 3D MR imaging of the Torso–First Clinical Experiences with SPACE. CLINICAL SPACE. 2005;1. https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000000081895/b135e0f62913/T2-weighted_3D_MR_Imaging_of_the_Torso_Page_58-60_1800000000081895.pdf. Accessed April 28, 2020 [Google Scholar]
- 27. Bitar R, Leung G, Perng R, et al. MR pulse sequences: what every radiologist wants to know but is afraid to ask. Radiographics 2006;26:513–37 10.1148/rg.262055063 [DOI] [PubMed] [Google Scholar]
- 28. Mihai G, Winner MW, Raman SV, et al. Assessment of carotid stenosis using three-dimensional T2-weighted dark blood imaging: initial experience. J Magn Reson Imaging 2012;35:449–55 10.1002/jmri.22839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morelli JN, Runge VM, Ai F, et al. An image-based approach to understanding the physics of MR artifacts. Radiographics 2011;31:849–66 10.1148/rg.313105115 [DOI] [PubMed] [Google Scholar]
- 30. Wang D, Lu Y, Yin B, et al. 3D fast spin-echo T1 black-blood imaging for the preoperative detection of venous sinus invasion by meningioma. Clin Neuroradiol 2019;29:65–73 10.1007/s00062-017-0637-1 [DOI] [PubMed] [Google Scholar]
- 31. Qureshi AI, Qureshi Z, Center SR. A classification scheme for assessing recanalization and collateral formation following cerebral venous thrombosis. J Vasc Interv Neurol 2010;3:1–2 [PMC free article] [PubMed] [Google Scholar]
- 32. Ferro JM, Bendszus M, Jansen O, et al. ; RE-SPECT CVT Study Group. Recanalization after cerebral venous thrombosis: a randomized controlled trial of the safety and efficacy of dabigatran etexilate versus dose-adjusted warfarin in patients with cerebral venous and dural sinus thrombosis. Int J Stroke 2022;17:189–97 10.1177/17474930211006303 [DOI] [PubMed] [Google Scholar]
- 33. Aguiar de Sousa D, Lucas Neto L, Arauz A, et al. Early recanalization in patients with cerebral venous thrombosis treated with anticoagulation. Stroke 2020;51:1174–81 10.1161/STROKEAHA.119.028532 [DOI] [PubMed] [Google Scholar]
- 34. Ghoneim A, Straiton J, Pollard C, et al. Imaging of cerebral venous thrombosis. Clin Radiol 2020;75:254–64 10.1016/j.crad.2019.12.009 [DOI] [PubMed] [Google Scholar]