SUMMARY:
Despite their small size, the mammillary bodies play an important role in supporting recollective memory. However, they have typically been overlooked when assessing neurologic conditions that present with memory impairment. While there is increasing evidence of mammillary body involvement in a wide range of neurologic disorders in adults, very little attention has been given to infants and children. Literature searches of PubMed and EMBASE were performed to identify articles that describe mammillary body pathology on brain MR imaging in children. Mammillary body pathology is present in the pediatric population in several conditions, indicated by signal change and/or atrophy on MR imaging. The main causes of mammillary body pathology are thiamine deficiency, hypoxia-ischemia, direct damage due to masses or hydrocephalus, or deafferentation resulting from pathology within the wider Papez circuit. Optimizing scanning protocols and assessing mammillary body status as a standard procedure are critical, given their role in memory processes.
The mammillary bodies (MBs) were arguably the first brain region to be implicated in memory, on the basis of the pathology observed in Korsakoff syndrome around the end of the 19th century. However, since then, they have been consistently overlooked during neuropathologic assessments, with memory impairment often being attributed to hippocampal involvement. More recent studies have re-confirmed the importance of the MBs for memory in adults.1,2 Furthermore, it has been demonstrated that MB pathology is by no means restricted to Korsakoff syndrome/thiamine deficiency but can occur in numerous neurologic conditions, including strokes,3 craniopharyngiomas,4 colloid cysts,5,6 schizophrenia,7 Alzheimer disease,8 multiple sclerosis,9 and following acute or repetitive/prolonged hypoxia.10,11
Despite this wide-ranging involvement of the MBs in neurologic disorders in adults, far less attention has been given to the MBs in infants and children. However, several recent studies have underlined the importance of assessing the MBs in younger patients. For example, the MBs appear particularly sensitive to neonatal hypoxia-ischemia.12,13 Volume loss in the MBs, the hippocampus, and fornices, is also associated with cognitive impairment, including episodic memory deficit in school-age children with a history of neonatal hypoxic-ischemic encephalopathy.14 MB atrophy has also been reported following the Fontan procedure, with MB volume related to the degree of memory impairment.15
These recent studies have identified the importance of assessing the status of the MBs in infants and children, and as with adult cases of MB pathology, there is likely a wide range of conditions that can impact the MBs in younger patients. This review will identify those conditions in which MB pathology has been observed in infants and children. The focus is on human literature and includes studies that reported MB pathology on brain MR imaging in children (see the Online Supplemental Data for an overview of search terms and inclusion criteria and the list of articles identified). The conditions associated with MB pathology will be summarized below as well as implications for radiologic assessment.
Mammillary Body Embryology, Anatomy, and Function
The MBs are a paired round structure located at the undersurface of the diencephalon. They are separated along the midline by the intermammillary sulcus. The MBs contain 2 gray matter structures, the medial and lateral nuclei; these are encapsulated by white matter and form the mammillothalamic and mammillotegmental tracts.
In humans, both the lateral and medial mammillary bodies are present at 10 weeks' gestation, though at this stage, the cells are undifferentiated.16 By 11–14 weeks, the MBs comprise a group of homogeneous neurons; by 16 weeks' gestation, they are clearly differentiated. By 24–33 weeks, the MBs take on an appearance similar to that in adult MBs, ie, they are well-developed at the time of full-term birth.
There is some natural variation in the anatomy of the MBs in the general population.17,18 In the axial plane, they can appear more circular or elliptic, and there is also variation in the depth of the intermammillary sulcus.17 The MBs can also appear asymmetric without an underlying pathologic cause; for example, Tagliamonte et al17 reported MB asymmetry in 13.9% of their young, healthy cohort. In most cases, this asymmetry arose from the abnormal course of the posterior cerebral artery, which resulted in dorsal displacement of the MBs. In 4 of the 78 participants, the asymmetry appeared to reflect a loss of volume of one of the MBs.
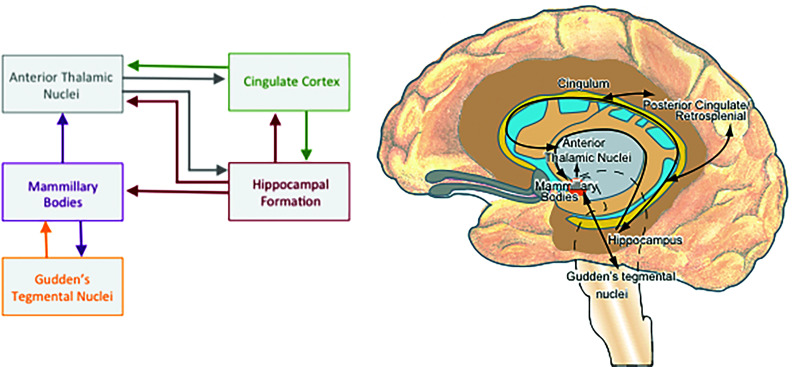
In addition to primary damage, the MBs can be affected by damage to their principal input and output (Fig 1). For example, anterograde degeneration can occur following injury to the hippocampus or fornix,19 though the resultant MB atrophy is thought to reflect a loss of white matter rather than neuronal loss.20 In contrast, neuronal loss in the MBs can occur as a result of retrograde degeneration following damage to the mammillothalamic tract.21
FIG 1.
The principal connections of the mammillary bodies.
Consistent with the anatomic connectivity of the MBs with the hippocampus, fornix, and mammillothalamic tract, the MBs also form a functional network with these structures, ie, the Papez circuit (Fig 1). The MBs have an important role in integrating input from the hippocampus and the Gudden tegmental nuclei and for coordinating oscillatory activity in the hippocampus and cortex.2,22 The MBs appear to be particularly important for episodic (ie, event) memory,2,23 including temporal and contextual memory.24
MB Pathology
Thiamine Deficiency.
Korsakoff syndrome, arising from thiamine (vitamin B1) deficiency, is the most common condition associated with MB pathology. Indeed, almost half of the articles identified in this literature search referred to MB abnormalities in children with thiamine deficiency. Although Korsakoff syndrome is typically associated with alcoholism in adults, many other conditions have been identified that can result in similar thiamine deficiency and resultant neuropathology in children.25,26
Thiamine deficiency may present as infantile encephalopathic beriberi (dry beriberi) or as Wernicke encephalopathy. Wernicke encephalopathy is difficult to diagnose and often goes untreated in clinical practice. This lack of detection and treatment is evidenced by the finding that Wernicke encephalopathy is first diagnosed postmortem in >80% of cases in adults27,28 and >40% in children.29 Because patients usually respond well to acute thiamine replacement therapy, it highlights the importance of making an early diagnosis.
Wernicke encephalopathy in children typically arises from nutritional deficiency, which can occur due to vomiting (eg, due to anorexia nervosa, hyperemesis, gastrointestinal obstruction),30-32 chronic gastrointestinal disease,33 and following bariatric surgery.34 Wernicke encephalopathy has also been reported following acute liver failure35 and pancreatitis.36 The MR imaging characteristics of thiamine deficiency include symmetric T2 hyperintensities in the dorsal medial thalamus, MBs, periaqueductal gray matter, and tectal plate,26 with the MBs being involved in 17%–58% of cases.37-39 High-signal-intensity changes on T2-weighted images are the most frequent pathologic findings seen on MR imaging compared with low-signal-intensity changes on T1-weighted images.37
Contrast enhancement of the MBs, which is characteristic in alcohol-related Wernicke encephalopathy, occurs less frequently in Wernicke encephalopathy in nonalcoholic populations.39,40 However, contrast enhancement has also been reported in children with Wernicke encephalopathy, resulting from malnutrition, for example.31,33 In addition to signal changes, MB volume loss has also been reported in patients with anorexia nervosa as well as recovery of MB volume in individuals who regained weight.30 Recovery of MB volume has also been reported in patients with acute liver failure following treatment with thiamine.35 Furthermore, MB volume was found to correlate with blood thiamine levels.32 Consistent with this observed recovery of MB volume after thiamine treatment, signal changes in Wernicke encephalopathy have also been shown to improve41 or even resolve after treatment.26,38 This finding suggests that the signal changes may reflect reversible vasogenic edema as opposed to acute ischemia.32
MB Pathology in Infants with Perinatal Asphyxia.
Severe perinatal asphyxia can result in hypoxic-ischemic encephalopathy (HIE). The prevalence of HIE is approximately 1.5 per 1000 live-birth-term neonates.42 The brain areas that are typically involved in HIE include the deep gray nuclei or white matter and cortex, depending on the severity and duration of the insult. Two main patterns of injury have been distinguished on MR imaging:43 The first is the basal ganglia–thalamus pattern, which is mainly associated with acute, severe perinatal asphyxia such as uterine rupture; the second is the watershed-predominant pattern of injury, which is more common after prolonged partial asphyxia. This second pattern involves the vascular watershed zones of the anterior MCA and posterior MCA. In the most severe cases, both patterns are visible—this is called a “near total” pattern of injury because on diffusion-weighted MR imaging, the cerebellum is mostly spared.
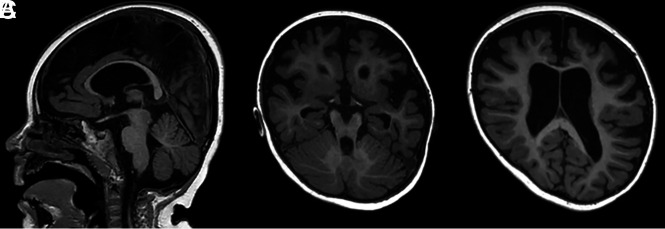
The limbic system can also be affected following HIE, with MRIs showing diffusion restriction on DWI in the hippocampal region.44,45 Recent studies have also shown signal change in the MBs after perinatal asphyxia (Fig 2).12,13 Molavi et al12 reported a hyperintense signal change in the MBs on the T2-weighted sequences in 13.2% of neonates with HIE. In those cases with abnormal MB signal, the most common MR imaging pattern (in 41.9% of cases) was found to be abnormal signal on either T2-weighted imaging or DWI restricted to the MBs, with no other abnormalities observed. Lequin et al13 performed a multicenter study looking into signal changes on both T1- and T2-weighted images as well as restricted diffusion on DWI in the MBs after HIE and therapeutic hypothermia. They observed abnormal MB signal in approximately 40% of the cooled neonates. Involvement of the MBs was not related to the severity of encephalopathy or the severity of hypoxic-ischemic brain injury, and there was no relation to the pattern of brain injury. In both studies, the MB abnormalities were only identified retrospectively, when the MBs were specifically assessed. Thus, it is likely that many infants with MB abnormalities are currently being overlooked, with their MRI findings reported as looking normal. These results emphasize the need for optimized protocols with sufficiently thin section series (<2 mm) and for the MBs to be routinely assessed.
FIG 2.
Images from a term neonate initially scanned at 6 days of age. A, A T2-weighted image with a hyperintense aspect of the MBs. B, A b=1000 image shows a hyperintense aspect on the ADC map (not shown), consistent with ischemia. C, Ischemia is also seen symmetrically in the thalami and basal ganglia. Follow-up at 3 months shows atrophy of the MBs (D) with flattening on the sagittal view (E). Tissue loss and gliosis in the lentiform nucleus can also be seen (F).
Annink et al14 performed a retrospective observational study of neonates with HIE with follow-up at 10 years of age. Of the infants with abnormal neonatal MBs, 76% had MB atrophy at 10 years of age. MB atrophy was seen in 38% of all 10-year-old patients (50% of those who had undergone therapeutic hypothermia and 17% of those who not had hypothermia treatment). The study also showed that children with a history of HIE have long-term neurodevelopmental problems despite therapeutic hypothermia. Hippocampal volume and MB atrophy were strongly associated with neurocognitive outcome and episodic memory at 10 years of age. These findings suggest that abnormal T2 signal of the MBs in the acute phase is associated with cognitive and memory deficits later in life.
Dzieciol et al46 assessed children and adolescents with developmental amnesia, which is marked by extensive bilateral damage to the hippocampus as a result of early life exposure to hypoxic-ischemic events. Developmental amnesia is characterized by impaired episodic memory with relative sparing of semantic memory.47 Dzieciol et al found the MBs absent in 12 of the 18 patients with developmental amnesia. The remaining 6 patients had visible MBs, but as a group, they were significantly smaller than those in controls. Of note, all 12 patients in whom the MBs were absent experienced a hypoxic-ischemic event perinatally, whereas for the 3 patients who experienced the hypoxic-ischemic event later in life (4–15 years of age), the MBs were visible. Geva et al48 also noted that the MBs were small in 10 of their 20 patients with hippocampal atrophy resulting from perinatal hypoxia-ischemia.
Congenital Central Hypoventilation Syndrome.
Congenital central hypoventilation syndrome (CCHS) is defined as the failure of automatic control of breathing, with ventilation most severely affected during quiet sleep, when automatic neural control is predominant.49 CCHS can disrupt blood pressure, glucose and temperature control, reduce the sensitivity to carbon dioxide and oxygen, as well affect intestinal motility causing malabsorption.50 Patients with CCHS often show multiple cognitive impairments including learning and memory problems.51,52 Initial studies on this patient group reported structural and functional changes in the hippocampus and anterior thalamus.53-55 In a subsequent study, Kumar et al56 found the MBs and fornix volume to be significantly reduced in patients with CCHS, suggesting that pathology within the Papez circuit, including the MBs, contributes to the memory impairment observed in this patient group.
Although only 1 study has examined the MBs in patients with CCHS, the pathology appears similar to that of other sleep-disordered breathing conditions such as obstructive sleep apnea as well as heart failure and beriberi. In addition to the chronic hypoxia/hypoxemia present in these conditions, patients can also present with intestinal absorption abnormalities as well as low levels of thiamine and magnesium,57,58 which are likely to contribute to the pathology in these patient groups.
Congenital Heart Disease.
Single ventricular heart disease (SVHD) is considered one of the most challenging types of congenital heart disease, with an incidence of approximately 1 per 2000 live births.59 Patients typically require at least 3 staged palliative operations; the Fontan procedure has the greatest impact on life expectancy. Patients with SVHD are at greater risk of brain injury and neurocognitive deficits as a direct result of the condition and comorbidities60 as well as from the multiple surgical procedures.61 Adolescents with SVHD exhibit brain lesions on MR imaging in approximately 60% of cases.62 This finding could be due to the delayed brain maturation observed in complex heart disease, which can make the brain more vulnerable to injury.63
Cabrera-Mino et al15 found reduced MB volumes in adolescents with SVHD compared with controls. Cognitive test scores were also significantly lower in the SVHD group, and performance on verbal and delayed recall memory tests correlated with MB volume, ie, poorer memory with smaller MBs. In a related study, Singh et al64 investigated DTI-based diffusivity measures. Multiple brain regions including the limbic system and MBs showed changes consistent with chronic tissue injury. Given that the MRIs in this study were acquired more than a decade after the patient's last surgical procedure, the assumption was that the pathology reflected chronic changes associated with this condition, predominantly to myelin.
Metabolic Disease.
MB abnormalities have only been reportedin a few metabolic diseases. One case showed diffusion restriction in the MBs in a patient with Leigh disease arising from a mutation in the sulfide:quinone oxidoreductase enzyme.65 A further case showed enhancement in the MBs in a patient with Alexander disease,66 while Inui et al67 found high signal intensities on T2-weighted images in the MBs of a young patient with a case of chronic infantile fucosidosis. Finally, 2 patients with biotinidase deficiency were reported showing bilateral abnormal signal intensity in the MBs and area postrema of the dorsal medulla as well as bilateral symmetric optic neuritis.68
Epilepsy.
There is a well-documented relationship between MB abnormalities and epilepsy in adults. As will be discussed in the next section, patients with refractory mesial temporal lobe epilepsy often undergo temporal lobe surgery or laser interstitial thermal therapy (LiTT) as part of their treatment. The resultant hippocampal loss can cause anterograde degeneration in the MBs. There is some evidence that the MBs may also be implicated in epilepsy before treatment, possibly due to anterograde degeneration from the pre-existing hippocampal pathology or as a more direct effect of the epilepsy.69 Indeed, a case from our own hospital demonstrates this finding of compromised MBs in a patient with epilepsy who had not undergone temporal lobe surgery or LiTT (Fig 3).
FIG 3.
T1-weighted images show generalized atrophy of the brain with atrophy of the MBs, which are completely flattened (A and B), and ex-vacuo dilation of the ventricles (C) in a 2.5-year-old patient with severe epilepsy.
Mesial Temporal Sclerosis.
Mesial temporal sclerosis (MTS) involves neuronal loss and gliosis of the hippocampus and is the most common disease associated with mesial temporal lobe epilepsy. MB and fornix asymmetry have been repeatedly reported in MTS.18,70 Ozturk et al18 found asymmetry of the MBs (37.1% of cases) and fornix (34.3% of cases) when examining patients with MTS presurgery. This reported asymmetry was significantly higher than that found in a control group (6.5% for MBs and 7.9% for fornix). Kim et al71 performed a similar study but observed asymmetric MBs in only 3% of patients with MTS presurgery. This discrepancy may be due to Ozturk et al using 1.5-mm slices to evaluate the MB asymmetry compared with 3-mm slices in the Kim et al study, increasing the likelihood of detecting asymmetries.
Refractory mesial temporal lobe epilepsy is the most common form of surgically treated epilepsy and is caused by MTS in ∼65% of the patients undergoing surgery.72 In a blinded retrospective analysis of 20 patients who underwent amygdalohippocampal LiTT, the seizure-free group showed an average 35% reduction in ipsilateral MB volume compared with 8% reduction in patients with continued seizures. This finding suggests that MB volume change could be a marker for successful ablation surgery.73 In contrast, Urbach et al74 did not find MB volume after amygdalo-hippocampectomy predictive of postsurgical seizure outcome in patients with unilateral MTS. Because the latter study involved twice the number of patients, there might be a selection bias in the former study; alternatively, the discrepancy could reflect differences between LiTT and amygdalo-hippocampectomy.
Epilepsy Related to MB Pathology.
Mamourian and Brown75 reported a case study of a 4.5-year-old boy who presented with staring spells and abnormal electrocardiogram activity in the right parietal region. MR imaging showed a total absence of the right MB, while the temporal lobes appeared normal. The authors raised the possibility that the seizure activity was related to the shrunken right MB. There have been other studies that have directly linked the MBs to epileptic activity rather than being indirectly affected via the hippocampus. For example, epileptiform discharges have been recorded directly from the MBs,76 and a fluorodeoxyglucose PET/CT study of a 50-year-old woman found the MBs to be the focus of epileptic activity.77
Further associations between the MBs and seizures come from an adolescent with a 2-year history of complex partial seizures whose MR imaging showed encephalomalacia in the right anteromedial thalamus, gliosis of the mammillothalamic tract, and a right-sided atrophic MB.78 The mammillothalamic tract and MB pathology were thought to be the result of retrograde degeneration following a thalamic infarct. Because there was no evidence of hippocampal involvement, the partial seizures were assumed to be due to the mammillothalamic pathology.
Hypothalamic hamartomas can also be associated with epilepsy; Freeman et al79 found that the common factor in a large group of patients who presented with hypothalamic hamartoma and epilepsy was involvement of the MBs, suggesting that they may be the focus of the epileptic activity. Linear defects in the anterior thalamus and associated MB and fornix atrophy have also been identified in patients with chronic seizures.80
One further study reported congenital aplasia of the MBs in a young child with early infantile epileptic encephalopathy (Ohtahara syndrome), who also presented with dentato-olivary dysplasia.81 The absence of the MBs, both macroscopically and microscopically, was noted at postmortem examination, leading the authors to recommend high-resolution MR imaging and detailed postmortem assessment in this patient group.81
Masses Involving the Hypothalamic Region, Postoperative MB Abnormalities, and Other Iatrogenic Causes of MB Pathology.
The MBs can be displaced and/or compressed in numerous conditions that result in masses forming in adjacent structures, for example, hypothalamic hamartomas and suprasellar arachnoid cysts.79,82-86 The MBs are an important landmark for neurosurgeons when operating on these suprasellar masses.84,87 Although compression occurs, the structure of the MBs can remain intact,88 and after decompression, the MBs can appear normal.84
Craniopharyngiomas are rare embryogenic malformations of the sellar/parasellar area with low-grade histologic malignancy.89 Despite high survival rates, the quality of life can be affected by optic chiasm and hypothalamic pathology, resulting in visual impairment and hypothalamic obesity, respectively.90,91 In a mixed population of pediatric and adult patients with craniopharyngiomas, the authors classified the MBs as “dislocated” in 34% of cases and “unrecognizable” in 19% of cases.92 Involvement of the MBs was one of the main factors associated with postoperative obesity. This was in accordance with Müller et al,93 who found that the extent of hypothalamic damage increased the risk of postsurgical obesity in a group of 120 children. In addition to the development of hypothalamic obesity, children with high-grade hypothalamic involvement also showed poorer performance on tests of executive function and reduced functional capabilities for daily life actions, and these cognitive impairments appear to be associated with MB involvement.94
The MBs can be directly affected by not only these masses but also the treatment protocols, including radiation therapy and chemotherapy, likely causing additional MB damage. For example, intrathecal chemotherapy for childhood acute lymphoblastic leukemia has been shown to affect the MBs.95 Ciesielski et al95 found the MBs and prefrontal cortex significantly reduced in volume in the patient group with acute lymphoblastic leukemia compared with matched controls. The patient group also performed significantly worse on both visual and verbal memory tasks.
Personal Experience
In our institution (Wilhelmina Children's Hospital), as a standard practice, we use imaging protocols that enable us to visualize the MBs in detail. Furthermore, the MBs are routinely assessed in all pediatric MRIs of the brain. This practice has resulted in MB pathology being observed in conditions that have not previously been reported in the literature. For example, when assessing the scans of neonates with an MCA infarct, we have identified several cases with damage to the MBs. One example involved a girl with hereditary spherocytosis who was severely anemic at 6 weeks and presented with right-sided hemiparesis at 2 months of age. A scan at 1 year of age showed sequelae of a large left-sided MCA infarct with little remaining basal ganglia and left-sided atrophy of the body of corpus callosum, thalamus, hippocampus, and MB (Fig 4).
FIG 4.
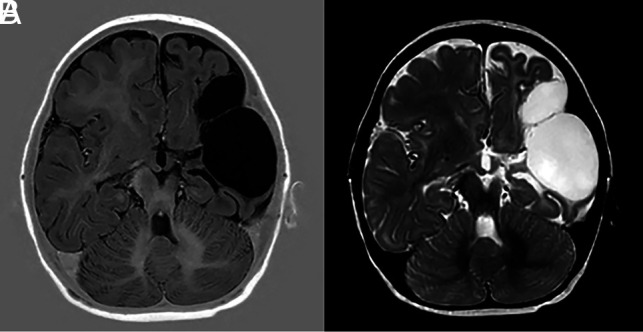
T1-weighted (A) and T2-weighted (B) images showing extensive loss of tissue due to a left-sided MCA with atrophy of the left MB. There was narrowing of the proximal MCA on MRA (not shown).
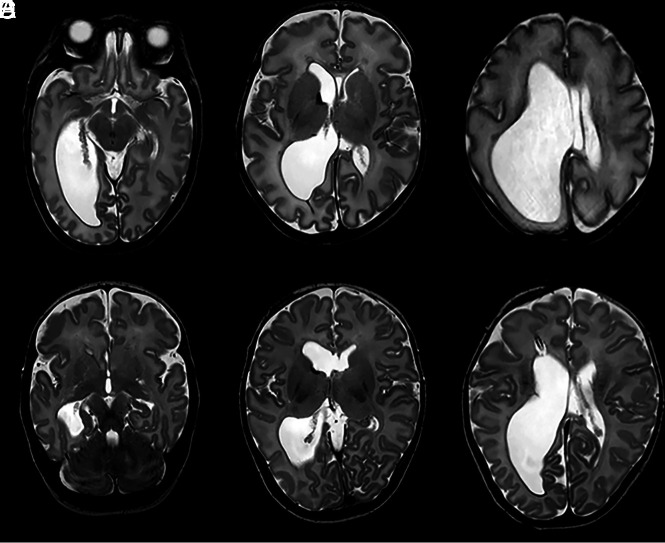
Chronic bilirubin encephalopathy (kernicterus) can also cause bilateral MB abnormalities. A term neonate with an uneventful pregnancy and an uncomplicated delivery was admitted for an exchange transfusion for severe hyperbilirubinemia. The patient showed opisthotonus and convulsions/seizures. A brain MR imaging at 8 days of age showed a bilateral hypointense aspect of the MBs on T1-weighted imaging and a hyperintense T2 aspect in addition to a T1 hyperintense aspect of the globus pallidus bilaterally, consistent with kernicterus (Fig 5). At a 7-month follow-up, the MBs appeared atrophic. To our knowledge, there are no other reports of MB involvement in kernicterus.
FIG 5.
A T2-weighted image at the level of the MBs, acquired when the patient was 8 days of age, showing a hyperintense aspect of the MBs (A). A T1-weighted image (B) shows a hypointense MB signal. There is a bilateral hyperintense signal of the globus pallidus on a T1-weighted image (C), consistent with kernicterus. On follow-up after 7 months, there is atrophy with complete flattening of the MBs as seen on the sagittal T1-weighted image (D). An axial T2-weighted image shows persistent high signal of the globus pallidus bilaterally, with accompanying volume loss (E).
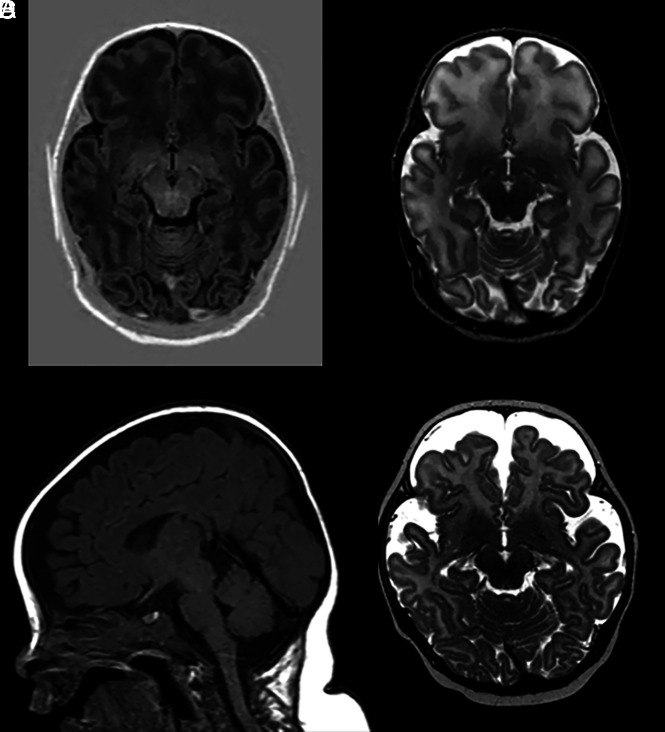
Hydrocephalus can often affect the MBs, causing compression and making them difficult to visualize. However, it is only possible to determine whether there is long-lasting structural damage to MBs after the onset of treatment and reduction of intracranial pressure. The MBs are at risk from direct pressure from hydrocephalus, which can reduce blood flow to the MBs and cause local ischemia, but also from the interventional treatment procedures. A 32-week preterm neonate was born with unilateral ventricular dilation at our institution. No MB abnormalities were observed on 2-day postnatal MR imaging. Because of the suspected obstruction at the foramen of Monro, a ventricular access device was inserted. MR imaging after 2.5 months showed atrophy with an abnormal signal intensity of the left MB (Fig 6).
FIG 6.
A, T2-weighted image of unilateral dilation of the right lateral ventricle with normal MBs. B, There is a suggestion of an obstruction at the foramen of Monro and increased signal intensity of the periventricular white matter due to the increased pressure. Small remains of germinal matrix hemorrhage are seen on the right. C, Intact septum. At follow-up, after insertion of a ventricular access device and a neuroendoscopic septostomy procedure, an asymmetric aspect of the MBs is found with T2 hyperintense signal in the left MB, which is also smaller than the contralateral right MB (D). Decreased ventricular dilation is also seen on E after septostomy and placement of the ventricular access device (F).
We have also found the MBs to be affected by group B streptococcus, the leading cause of neonatal meningitis, and herpes simplex encephalitis. One such patient was scanned at 11 weeks after group B streptococcus sepsis and possible meningitis (Fig 7). On MR imaging, the basal ganglia appeared normal, but there was minor supratentorial generalized loss of white and gray matter with subtle dilation of the lateral ventricles, and both MBs were atrophic.
FIG 7.
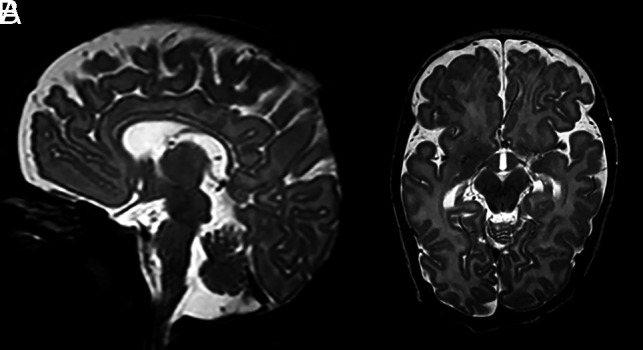
Preterm infant (gestational age of 34 + 4 weeks) scanned at 11 weeks after group B streptococcus sepsis and possible meningitis. T2-weighted images at the level of the MBs show severe atrophy with flattening of the MBs (A and B).
Finally, as previously described, metabolic disorders might also impact the MBs. From MR imaging studies, MB abnormalities have been described in Leigh disease, Alexander disease, and fucosidosis. A postmortem study, however, found necrosis and spongiosis of the MBs in a patient with methylmalonic acidemia.96 Similarly, we had a patient with propionic acidemia who showed bilateral volume loss of the MBs on a 3-month follow-up scan. No MB abnormalities were seen on an initial scan at 2 weeks, but this may have been outside the window of the acute stage (Fig 8).
FIG 8.
A patient with propionic acidemia. There is a normal aspect of the MBs on T1-weighted (A) and T2-weighted (B) images at 2 weeks of age. On follow-up 3 months later, volume loss is seen on the sagittal T1-weighted image (C) and axial T2-weighted image (D).
DISCUSSION
While the MBs have been implicated in a wide range of neurologic conditions in adults, their involvement in neurologic conditions in infants and children is less well-documented. Thus, we have performed a review of conditions in younger patients in whom MB pathology has been reported. Perhaps unsurprisingly, given that the MBs and their connections are well-developed at birth, many of the conditions that can cause MB pathology in adults also can affect the MBs in younger individuals. While numerous diverse conditions have been associated with MB pathology across age groups, this pathology appears to typically arise from a limited number of factors: thiamine deficiency, hypoxia-ischemia direct damage due to masses or hydrocephalus, or deafferentation resulting from pathology within the wider Papez circuit.
MB pathology is most frequently associated with thiamine deficiency; indeed, MB pathology was first noted in Korsakoff syndrome at the end of the 19th century, and this provided the first link between the MBs and memory.97 However, the exact mechanism by which thiamine deficiency impacts the MBs remains uncertain. It is likely that the effects of thiamine deficiency are multifactorial, given that it can cause cell loss via various mechanisms, including mitochondrial dysfunction, glycolysis, acidosis, increased oxidative stress, excitotoxity, and inflammation.98,99 Suboptimal thiamine reserves or borderline thiamine deficiency can present in many conditions and can be exacerbated by glucose infusion,100 which is often given to children on hospitalization.
There is increasing evidence that the MBs are affected by hypoxia-ischemia, particularly in neonates.12,13 What is striking is that the MBs can often be the only structure to show abnormal signal, with the rest of the brain appearing normal, highlighting the sensitivity of the MBs.12,13 While hypothermia treatment appears to be effective in protecting wider brain areas, the MBs do not appear to benefit from this treatment to the same extent,12 as such, there is a need to identify additional treatment approaches.
Both thiamine deficiency and hypoxia can cause neural cell loss via the activation of several similar signaling pathways that result in necrosis and apoptosis. As with thiamine deficiency, hypoxia also increases mitochondrial dysfunction, excitotoxity, oxidative stress, and acidosis.101 Furthermore, both conditions disrupt the blood-brain barrier and alter levels of hypoxia-inducible factor 1α.102,103 Given the sensitivity of the MBs to thiamine deficiency and hypoxia, the most parsimonious conclusion is that the MBs are affected by similar underlying mechanisms in both conditions; however, as the cell loss likely occurs via multiple complex mechanisms, there may be selective pathways that are preferentially involved in the different conditions and even across different patients with the same condition.
A further cause of MB pathology is via direct damage, eg, due to a mass, hydrocephalus, or radiation therapy, or indirectly via anterograde or retrograde degeneration. In adults, damage to the hippocampus or fornix typically results in a loss of white matter in the MBs, with a volume reduction of up to 50%.20 However, it is possible that in neonates, the MBs are more sensitive to deafferentation, and greater atrophy might be observed following damage to the hippocampus or fornix. Direct damage to the MBs should be minimized when possible, for example, when targeting masses in the vicinity of the MBs. However, there are some inconsistencies as to whether compression of the MBs can cause long-term pathology once the pressure on the MBs is removed. Therefore, further work is needed to determine those situations in which the MBs are most vulnerable to both direct and indirect damage and the time windows during which long-term pathology can be avoided.
While damage to the MBs appears to arise from similar etiologies in adults, children, and infants, it is not yet clear whether age differentially affects the sensitivity of the MBs. There is some suggestion that neonatal brains are more resilient to some forms of damage, for example, those arising from hypoxia, because there are differing metabolic demands in cells and a different propensity to excitotoxity.104 However, the blood-brain barrier is also immature during the neonatal period,105 which could exacerbate the impact of hypoxia and thiamine deficiency. Given the high rate of MB involvement in neonatal hypoxia-ischemia, it is possible that the MBs are more sensitive in this age group; however, it is difficult to directly compare with adults, given the difference in prevalence. Animal models may be required to determine whether MBs in neonates are more sensitive to hypoxia-ischemia as well as deafferentation.
The purpose of this review was to identify conditions in which MB pathology occurred rather than specifically address the functions of the MBs; indeed, most of the included studies described pathology but did not perform detailed cognitive tests. From the evidence that is available, however, it seems that little if any functional compensation occurs when MB pathology occurs earlier in life. In both children and adults, the profile of memory impairment seems remarkably similar, with episodic and recollective memory being particularly affected. However, there are very few cognitive assessments of individuals with early-acquired MB pathology in which the damage is selective. In most cases there is co-occurring pathology in the hippocampus, making it difficult to determine the specific contribution of the MBs. This issue highlights the need to assess the extent of memory impairment in cases in which pathology is restricted to the MBs.
A limitation of this review is that many of the studies comprise case reports. Furthermore, some of the studies included mixed age groups that included not only children but also adults. In some cases, the authors did not distinguish between the pathology observed in children and adults, making it difficult to determine the relative involvement across age groups. A further complicating factor when interpreting unilateral MB pathology is that asymmetric MBs can occur in the general population, though this appears to generally reflect MB displacement rather than actual atrophy. Good-quality MRIs should help distinguish between these scenarios.
Unfortunately, the size and location of the MBs can often result in poor visualization on MRI, which is exacerbated when scan slices are not sufficiently thin. Furthermore, partial volume effects can also affect the interpretation of MRI if imaging protocols are not optimized. Together, these are likely to have resulted in MB pathology going undetected. Given the importance of the MBs for memory, accurate identification of MB pathology is necessary to provide appropriate follow-up assessment and support for children and their families. The MBs need to be protected from damage when possible, and thiamine supplementation is an easily implemented measure for critically ill children. A better understanding of the specific pathways that are most likely to result in MB pathology is also needed so more targeted treatments can be developed.
ABBREVIATIONS:
- CCHS
congenital central hypoventilation syndrome
- HIE
hypoxic-ischemic encephalopathy
- LiTT
laser interstitial thermal therapy
- MB
mammillary body
- MTS
mesial temporal sclerosis
- SVHD
single ventricular heart disease
Footnotes
S.D. Vann is supported by a Wellcome Trust Senior Research Fellowship (212273/Z/18/).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org
References
- 1. Tsivilis D, Vann SD, Denby C, et al. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci 2008;11:834–42 10.1038/nn.2149 [DOI] [PubMed] [Google Scholar]
- 2. Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia 2010;48:2316–27 10.1016/j.neuropsychologia.2009.10.019 [DOI] [PubMed] [Google Scholar]
- 3. Male S, Zand R. Isolated mammillary body infarct causing global amnesia: a case report. J Stroke Cerebrovasc Dis 2017;26:e50–52 10.1016/j.jstrokecerebrovasdis.2016.11.115 [DOI] [PubMed] [Google Scholar]
- 4. Tanaka Y, Miyazawa Y, Akaoka F, et al. Amnesia following damage to the mammillary bodies. Neurology 1997;48:160–65 10.1212/wnl.48.1.160 [DOI] [PubMed] [Google Scholar]
- 5. Vann SD, Tsivilis D, Denby CE, et al. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci U S A 2009;106:5442–47 10.1073/pnas.0812097106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denby CE, Vann SD, Tsivilis D, et al. The frequency and extent of mammillary body atrophy associated with surgical removal of a colloid cyst. AJNR Am J Neuroradiol 2009;30:736–43 10.3174/ajnr.A1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernstein HG, Krause S, Krell D, et al. Strongly reduced number of parvalbumin-immunoreactive projection neurons in the mammillary bodies in schizophrenia: further evidence for limbic neuropathology. Ann N Y Acad Sci 2007;1096:120–27 10.1196/annals.1397.077 [DOI] [PubMed] [Google Scholar]
- 8. Baloyannis SJ, Mavroudis I, Baloyannis IS, et al. Mammillary bodies in Alzheimer's disease: a Golgi and electron microscope study. Am J Alzheimers Dis Other Demen 2016;31:247–56 10.1177/1533317515602548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dineen RA, Bradshaw CM, Constantinescu CS, et al. Extra-hippocampal subcortical limbic involvement predicts episodic recall performance in multiple sclerosis. PLoS One 2012;7:e44942 10.1371/journal.pone.0044942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johkura K, Naito M. Wernicke's encephalopathy-like lesions in global cerebral hypoxia. J Clin Neurosci 2008;15:318–19 10.1016/j.jocn.2006.10.022 [DOI] [PubMed] [Google Scholar]
- 11. Schmidtke K. Wernicke-Korsakoff syndrome following attempted hanging. Rev Neurol (Paris) 1993;149:213–16 [PubMed] [Google Scholar]
- 12. Molavi M, Vann SD, de Vries LS, et al. Signal change in the mammillary bodies after perinatal asphyxia. AJNR Am J Neuroradiol 2019;40:1829–34 10.3174/ajnr.A6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lequin MH, Steggerda SJ, Severino M, et al. Mammillary body injury in neonatal encephalopathy: a multicentre, retrospective study. Pediatr Res 2021. Mar 2. [Epub ahead of print] 10.1038/s41390-021-01436-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Annink KV, de Vries LS, Groenendaal F, et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci Rep 2021;11:5017 10.1038/s41598-021-83982-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabrera-Mino C, Roy B, Woo MA, et al. Reduced brain mammillary body volumes and memory deficits in adolescents who have undergone the Fontan procedure. Pediatr Res 2020;87:169–75 10.1038/s41390-019-0569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koutcherov Y, Mai JK, Paxinos G. Hypothalamus of the human fetus. J Chem Neuroanat 2003;26:253–70 10.1016/j.jchemneu.2003.07.002 [DOI] [PubMed] [Google Scholar]
- 17. Tagliamonte M, Sestieri C, Romani GL, et al. MRI anatomical variants of mammillary bodies. Brain Struct Funct 2015;220:85–90 10.1007/s00429-013-0639-y [DOI] [PubMed] [Google Scholar]
- 18. Ozturk A, Yousem DM, Mahmood A, et al. Prevalence of asymmetry of mamillary body and fornix size on MR imaging. AJNR Am J Neuroradiol 2008;29:384–87 10.3174/ajnr.A0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bachevalier J, Meunier M. Cerebral ischemia: are the memory deficits associated with hippocampal cell loss? Hippocampus 1996;6:553–60 [DOI] [PubMed] [Google Scholar]
- 20. Loftus M, Knight RT, Amaral DG. An analysis of atrophy in the medial mammillary nucleus following hippocampal and fornix lesions in humans and nonhuman primates. Exp Neurol 2000;163:180–90 10.1006/exnr.2000.7361 [DOI] [PubMed] [Google Scholar]
- 21. Vann SD. Dismantling the Papez circuit for memory in rats. Elife 2013;2:e00736 10.7554/eLife.00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dillingham CM, Milczarek MM, Perry JC, et al. Mammillothalamic disconnection alters hippocampocortical oscillatory activity and microstructure: implications for diencephalic amnesia. J Neurosci 2019;39:6696–6713 10.1523/JNEUROSCI.0827-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci 2004;5:35–44 10.1038/nrn1299 [DOI] [PubMed] [Google Scholar]
- 24. Dillingham CM, Milczarek MM, Perry JC, et al. Time to put the mammillothalamic pathway into context. Neurosci Biobehav Rev 2021;121:60–74 10.1016/j.neubiorev.2020.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zuccoli G, Siddiqui N, Bailey A, et al. Neuroimaging findings in pediatric Wernicke encephalopathy: a review. Neuroradiology 2010;52:523–29 10.1007/s00234-009-0604-x [DOI] [PubMed] [Google Scholar]
- 26. Lallas M, Desai J. Wernicke encephalopathy in children and adolescents. World J Pediatr 2014;10:293–98 10.1007/s12519-014-0506-9 [DOI] [PubMed] [Google Scholar]
- 27. Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl 2000;35:2–7 10.1093/alcalc/35.supplement_1.2 [DOI] [PubMed] [Google Scholar]
- 28. Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry 1986;49:341–45 10.1136/jnnp.49.4.341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vasconcelos MM, Silva KP, Vidal G, et al. Early diagnosis of pediatric Wernicke's encephalopathy. Pediatr Neurol 1999;20:289–94 10.1016/S0887-8994(98)00153-2 [DOI] [PubMed] [Google Scholar]
- 30. Khalsa SS, Kumar R, Patel V, et al. Mammillary body volume abnormalities in anorexia nervosa. Int J Eat Disord 2016;49:920–29 10.1002/eat.22573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamdhade S, Almulla A, Alroughani R. Recurrent Wernicke's encephalopathy in a 16-year-old girl with atypical clinical and radiological features. J Neurol Sci 2013;333:e627 10.1155/2014/582482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oka M, Terae S, Kobayashi R, et al. Diffusion-weighted MR findings in a reversible case of acute Wernicke encephalopathy. Acta Neurol Scand 2001;104:178–81 10.1034/j.1600-0404.2001.00098.x [DOI] [PubMed] [Google Scholar]
- 33. Sparacia G, Banco A, Lagalla R. Reversible MRI abnormalities in an unusual paediatric presentation of Wernicke's encephalopathy. Pediatr Radiol 1999;29:581–84 10.1007/s002470050652 [DOI] [PubMed] [Google Scholar]
- 34. Samanta D. Dry beriberi preceded Wernicke encephalopathy: thiamine deficiency after laparoscopic sleeve gastrectomy. J Pediatr Neurosci 2015;10:297–99 10.4103/1817-1745.165732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Srivastava A, Yadav SK, Borkar VV, et al. Serial evaluation of children with ALF with advanced MRI, serum proinflammatory cytokines, thiamine, and cognition assessment. J Pediatr Gastroenterol Nutr 2012;55:580–86 10.1097/MPG.0b013e31825f4c3e [DOI] [PubMed] [Google Scholar]
- 36. Arana-Guajardo AC, Cámara-Lemarroy CR, Rendón-Ramírez EJ, et al. Wernicke encephalopathy presenting in a patient with severe acute pancreatitis. JOP 2012;13:104–107 [PubMed] [Google Scholar]
- 37. Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke's encephalopathy: review of the literature. AJR Am J Roentgenol 2009;192:501–08 10.2214/AJR.07.3959 [DOI] [PubMed] [Google Scholar]
- 38. Fei GQ, Zhong C, Jin L, et al. Clinical characteristics and MR imaging features of nonalcoholic Wernicke encephalopathy. AJNR Am J Neuroradiol 2008;29:164–69 10.3174/ajnr.A0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zuccoli G, Gallucci M, Capellades J, et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol 2007;28:1328–31 10.3174/ajnr.A0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zuccoli G, Santa Cruz D, Bertolini M, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol 2009;30:171–76 10.3174/ajnr.A1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gliebus G, Faerber EN, Valencia I, et al. Ataxia, ophthalmoplegia, and impairment of consciousness in a 19-month-old American boy. Semin Pediatr Neurol 2014;21:139–43 10.1016/j.spen.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 42. Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:329–38 10.1016/j.earlhumdev.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 43. de Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology 2010;52:555–66 10.1007/s00234-010-0674-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alderliesten T, Nikkels PG, Benders MJ, et al. Antemortem cranial MRI compared with postmortem histopathologic examination of the brain in term infants with neonatal encephalopathy following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed 2013;98:F304–09 10.1136/archdischild-2012-301768 [DOI] [PubMed] [Google Scholar]
- 45. Kasdorf E, Engel M, Heier L, et al. Therapeutic hypothermia in neonates and selective hippocampal injury on diffusion-weighted magnetic resonance imaging. Pediatr Neurol 2014;51:104–08 10.1016/j.pediatrneurol.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 46. Dzieciol AM, Bachevalier J, Saleem KS, et al. Hippocampal and diencephalic pathology in developmental amnesia. Cortex 2017;86:33–44 10.1016/j.cortex.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cooper JM, Gadian DG, Jentschke S, et al. Neonatal hypoxia, hippocampal atrophy, and memory impairment: evidence of a causal sequence. Cereb Cortex 2015;25:1469–76 10.1093/cercor/bht332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geva S, Jentschke S, Argyropoulos GP, et al. Volume reduction of caudate nucleus is associated with movement coordination deficits in patients with hippocampal atrophy due to perinatal hypoxia-ischaemia. Neuroimage Clin 2020;28:102429 10.1016/j.nicl.2020.102429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fleming PJ, Cade D, Bryan MH, et al. Congenital central hypoventilation and sleep state. Pediatrics 1980;66:425–28 10.1542/peds.66.3.425 [DOI] [PubMed] [Google Scholar]
- 50. O'Brien LM, Holbrook CR, Vanderlaan M, et al. Autonomic function in children with congenital central hypoventilation syndrome and their families. Chest 2005;128:2478–84 10.1378/chest.128.4.2478 [DOI] [PubMed] [Google Scholar]
- 51. Ruof H, Hammer J, Tillmann B, et al. Neuropsychological, behavioral, and adaptive functioning of Swiss children with congenital central hypoventilation syndrome. J Child Neurol 2008;23:1254–59 10.1177/0883073808318048 [DOI] [PubMed] [Google Scholar]
- 52. Vanderlaan M, Holbrook CR, Wang M, et al. Epidemiologic survey of 196 patients with congenital central hypoventilation syndrome. Pediatr Pulmonol 2004;37:217–29 10.1002/ppul.10438 [DOI] [PubMed] [Google Scholar]
- 53. Macey PM, Woo MA, Macey KE, et al. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol (1985) 2005;98:958–69 10.1152/japplphysiol.00969.2004 [DOI] [PubMed] [Google Scholar]
- 54. Woo MA, Macey PM, Macey KE, et al. FMRI responses to hyperoxia in congenital central hypoventilation syndrome. Pediatr Res 2005;57:510–18 10.1203/01.PDR.0000155763.93819.46 [DOI] [PubMed] [Google Scholar]
- 55. Kumar R, Macey PM, Woo MA, et al. Elevated mean diffusivity in widespread brain regions in congenital central hypoventilation syndrome. J Magn Reson Imaging 2006;24:1252–58 10.1002/jmri.20759 [DOI] [PubMed] [Google Scholar]
- 56. Kumar R, Lee K, MacEy PM, et al. Mammillary body and fornix injury in congenital central hypoventilation syndrome. Pediatr Res 2009;66:429–34 10.1203/PDR.0b013e3181b3b363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fatouleh RH, Hammam E, Lundblad LC, et al. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin 2014;6:275–83 10.1016/j.nicl.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kumar R, Woo MA, Birrer BV, et al. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis 2009;33:236–42 10.1016/j.nbd.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85–92 10.1161/CIRCULATIONAHA.107.738559 [DOI] [PubMed] [Google Scholar]
- 60. Marelli A, Miller SP, Marino BS, et al. Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation 2016;133:1951–62 10.1161/CIRCULATIONAHA.115.019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gaynor JW, Stopp C, Wypij D, et al. ; International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 2015;135:816–25 10.1542/peds.2014-3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bellinger DC, Watson CG, Rivkin MJ, et al. Neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc 2015;4:e002302 10.1161/JAHA.115.002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sethi V, Tabbutt S, Dimitropoulos A, et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res 2013;73:661–67 10.1038/pr.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Singh S, Roy B, Pike N, et al. Altered brain diffusion tensor imaging indices in adolescents with the Fontan palliation. Neuroradiology 2019;61:811–24 10.1007/s00234-019-02208-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Friederich MW, Elias AF, Kuster A, et al. Pathogenic variants in SQOR encoding sulfide: quinone oxidoreductase are a potentially treatable cause of Leigh disease. J Inherit Metab Dis 2020;43:1024–36 10.1002/jimd.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Poloni CB, Ferey S, Haenggeli CA, et al. Alexander disease: early presence of cerebral MRI criteria. Eur J Paediatr Neurol 2009;13:556–58 10.1016/j.ejpn.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 67. Inui K, Akagi M, Nishigaki T, et al. A case of chronic infantile type of fucosidosis: clinical and magnetic resonance image findings. Brain Dev 2000;22:47–49 10.1016/S0387-7604(99)00082-0 [DOI] [PubMed] [Google Scholar]
- 68. Shah S, Khan N, Lakshmanan R, et al. Biotinidase deficiency presenting as neuromyelitis optica spectrum disorder. Brain Dev 2020;42:762–66 10.1016/j.braindev.2020.07.007 [DOI] [PubMed] [Google Scholar]
- 69. Kodama F, Ogawa T, Sugihara S, et al. Transneuronal degeneration in patients with temporal lobe epilepsy: devaluation by MR imaging. Eur Radiol 2003;13:2180–85 10.1007/s00330-003-1875-y [DOI] [PubMed] [Google Scholar]
- 70. Oikawa H, Sasaki M, Tamakawa Y, et al. The circuit of Papez in mesial temporal sclerosis: MRI. Neuroradiology 2001;43:205–10 10.1007/s002340000463 [DOI] [PubMed] [Google Scholar]
- 71. Kim JH, Tien RD, Felsberg GJ, et al. Clinical significance of asymmetry of the fornix and mamillary body on MR in hippocampal sclerosis. AJNR Am J Neuroradiol 1995;16:509–15 [PMC free article] [PubMed] [Google Scholar]
- 72. Blumcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 2002;12:199–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Grewal SS, Gupta V, Vibhute P, et al. Mammillary body changes and seizure outcome after laser interstitial thermal therapy of the mesial temporal lobe. Epilepsy Res 2018;141:19–22 10.1016/j.eplepsyres.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 74. Urbach H, Siebenhaar G, Koenig R, et al. Limbic system abnormalities associated with Ammon's horn sclerosis do not alter seizure outcome after amygdalohippocampectomy. Epilepsia 2005;46:549–55 10.1111/j.0013-9580.2005.29104.x [DOI] [PubMed] [Google Scholar]
- 75. Mamourian AC, Brown DB. Asymmetric mamillary bodies: MR identification. AJNR Am J Neuroradiol 1993;14:1332–35; discussion 1336–42 [PMC free article] [PubMed] [Google Scholar]
- 76. van Rijckevorsel K, Abu Serieh B, de Tourtchaninoff M, et al. Deep EEG recordings of the mammillary body in epilepsy patients. Epilepsia 2005;46:781–85 10.1111/j.1528-1167.2005.45704.x [DOI] [PubMed] [Google Scholar]
- 77. Jha P, Agarwal KK, Sahoo MK, et al. Mammillary body: chronic refractory epilepsy seizure focus detected by 18F-FDG PET-CT. Clin Nucl Med 2016;41:419–20 10.1097/RLU.0000000000001142 [DOI] [PubMed] [Google Scholar]
- 78. Assis ZA, Sevick R. Association of transsynaptic degeneration of the Papez circuit with anterior thalamic encephalomalacia. JAMA Neurol 2018;75:1437–38 10.1001/jamaneurol.2018.2262 [DOI] [PubMed] [Google Scholar]
- 79. Freeman JL, Coleman LT, Wellard RM, et al. MR imaging and spectroscopic study of epileptogenic hypothalamic hamartomas: analysis of 72 cases. AJNR Am J Neuroradiol 2004;25:450–62 [PMC free article] [PubMed] [Google Scholar]
- 80. Tschampa HJ, Greschus S, Sassen R, et al. Thalamus lesions in chronic and acute seizure disorders. Neuroradiology 2011;53:245–54 10.1007/s00234-010-0734-1 [DOI] [PubMed] [Google Scholar]
- 81. Trinka E, Rauscher C, Nagler M, et al. A case of Ohtahara syndrome with olivary-dentate dysplasia and agenesis of mamillary bodies. Epilepsia 2001;42:950–53 10.1046/j.1528-1157.2001.042007950.x [DOI] [PubMed] [Google Scholar]
- 82. Wang JC, Heier L, Souweidane MM. Advances in the endoscopic management of suprasellar arachnoid cysts in children. J Neurosurg 2004;100:418–26 10.3171/ped.2004.100.5.0418 [DOI] [PubMed] [Google Scholar]
- 83. Maixner W. Hypothalamic hamartomas–clinical, neuropathological and surgical aspects. Childs Nerv Syst 2006;22:867–73 10.1007/s00381-006-0129-0 [DOI] [PubMed] [Google Scholar]
- 84. Ozek MM, Urgun K. Neuroendoscopic management of suprasellar arachnoid cysts. World Neurosurg 2013;79(2 Suppl): S19e1318 10.1016/j.wneu.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 85. Perez FA, Elfers C, Yanovski JA, et al. MRI measures of hypothalamic injury are associated with glucagon-like peptide-1 receptor agonist treatment response in people with hypothalamic obesity. Diabetes Obes Metab 2021;23:1532–41 10.1111/dom.14366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Roth CL, Eslamy H, Pihoker C, et al. Semi-quantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring). 2015;23:1226–33 10.1002/oby.21067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Roth J, Bercu MM, Constantini S. Combined open microsurgical and endoscopic resection of hypothalamic hamartomas. J Neurosurg Pediatr 2013;11:491–94 10.3171/2013.2.PEDS12275 [DOI] [PubMed] [Google Scholar]
- 88. Leal AJ, Moreira A, Robalo C, et al. Different electroclinical manifestations of the epilepsy associated with hamartomas connecting to the middle or posterior hypothalamus. Epilepsia 2003;44:1191–95 10.1046/j.1528-1157.2003.66902.x [DOI] [PubMed] [Google Scholar]
- 89. Garre ML, Cama A. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr Opin Pediatr 2007;19:471–79 10.1097/MOP.0b013e3282495a22 [DOI] [PubMed] [Google Scholar]
- 90. Roth C, Wilken B, Hanefeld F, et al. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. Eur J Endocrinol 1998;138:89–91 10.1530/eje.0.1380089 [DOI] [PubMed] [Google Scholar]
- 91. Muller HL, Faldum A, Etavard-Gorris N, et al. Functional capacity, obesity and hypothalamic involvement: cross-sectional study on 212 patients with childhood craniopharyngioma. Klin Padiatr 2003;215:310–14 10.1055/s-2003-45499 [DOI] [PubMed] [Google Scholar]
- 92. Mortini P, Gagliardi F, Bailo M, et al. Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine 2016;51:148–62 10.1007/s12020-015-0683-x [DOI] [PubMed] [Google Scholar]
- 93. Müller HL, Gebhardt U, Teske C, et al. ; Study Committee of KRANIOPHARYNGEOM 2000. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol 2011;165:17–24 10.1530/EJE-11-0158 [DOI] [PubMed] [Google Scholar]
- 94. Ozyurt J, Thiel CM, Lorenzen A, et al. Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J Pediatr 2014;164:876–81 10.1016/j.jpeds.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 95. Ciesielski KT, Lesnik PG, Benzel EC, et al. MRI morphometry of mamillary bodies, caudate nuclei, and prefrontal cortices after chemotherapy for childhood leukemia: multivariate models of early and late developing memory subsystems. Behav Neurosci 1999;113:439–50 10.1037/0735-7044.113.3.439 [DOI] [PubMed] [Google Scholar]
- 96. Larnaout A, Mongalgi MA, Kaabachi N, et al. Methylmalonic acidaemia with bilateral globus pallidus involvement: a neuropathological study. J Inherit Metab Dis 1998;21:639–44 10.1023/A:1005428432730 [DOI] [PubMed] [Google Scholar]
- 97. Vann SD, Nelson AJ. The mammillary bodies and memory: more than a hippocampal relay. Prog Brain Res 2015;219:163–85 10.1016/bs.pbr.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health 2003;27:134–42 [PMC free article] [PubMed] [Google Scholar]
- 99. Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med 2001;1:197–207 10.2174/1566524013363870 [DOI] [PubMed] [Google Scholar]
- 100. Schabelman E, Kuo D. Glucose before thiamine for Wernicke encephalopathy: a literature review. J Emerg Med 2012;42:488–94 10.1016/j.jemermed.2011.05.076 [DOI] [PubMed] [Google Scholar]
- 101. Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol 2011;69:743–58 10.1002/ana.22419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang X, Ma J, Fu Q, et al. Role of hypoxia-inducible factor-1alpha in autophagic cell death in microglial cells induced by hypoxia. Mol Med Rep 2017;15:2097–2105 10.3892/mmr.2017.6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zera K, Zastre J. Stabilization of the hypoxia-inducible transcription factor-1 alpha (HIF-1alpha) in thiamine deficiency is mediated by pyruvate accumulation. Toxicol Appl Pharmacol 2018;355:180–88 10.1016/j.taap.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 104. Haddad GG, Jiang C. O2 deprivation in the central nervous system: on mechanisms of neuronal response, differential sensitivity and injury. Prog Neurobiol 1993;40:277–318 10.1016/0301-0082(93)90014-j [DOI] [PubMed] [Google Scholar]
- 105. Ben-Zvi A, Liebner S. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J Intern Med 2021. Mar 4 [Epub ahead of print] 10.1111/joim.13263 [DOI] [PubMed] [Google Scholar]