Introduction
Fibromyalgia (FM) is a very common pain disorder that has been undergoing extensive research within the literature over the last several years. FM is characterized by widespread and chronic musculoskeletal pain, but these symptoms are also observed in a wide-array of pathologies (Borchers & Gershwin, 2015), resulting in heterogenous diagnostic standards. FM is most likely to be diagnosed in young (Arout, Sofuoglu, Bastian, & Rosenheck, 2018) women than in any other subgroup. Symptoms that accompany the disease include cognitive disturbances, psychiatric disorders, and multiple somatic symptoms, including pain and fatigue (Häuser et al., 2015). Despite increased awareness and investigations into FM, more than 75% of people suffering from this disorder remain undiagnosed (Clauw, Arnold, & McCarberg, 2011). In addition, FM is widespread across the world, with its prevalence (Häuser & Fitzcharles, 2018) being around 2–4% of the general populations. The main symptom (El-Rabbat, Mahmoud, & Gheita, 2018) of FM comes in the form of chronic pain, which is found in 15–30% of FM patients. Other symptoms include depression, anxiety, sleep disorders, and irritable bowel syndrome (Häuser et al., 2015). Along with the added strain of the chronic symptoms of FM, the disease also presents challenges in-terms of its cost of treatment and reduced economic productivity of FM patients. Total healthcare costs per year for FM patients is five times higher than non-FM patients, resulting in FM families paying almost $2000 dollars a year in additional costs. FM patients who had a paid job also lost an average of 5.6 days of productivity every three months due to pain, which is almost 24 days a year (Berger, Dukes, Martin, Edelsberg, & Oster, 2007).
The initial clinical criteria for the diagnosis of FM issued by the American College of Rheumatology in 1990 was intended for research classification and not as “checklist” for clinical practice with a well-defined line between those patients that do and those that do not fulfill them. These initial criteria relied on the presence of tender-points and have been revised over time to become more comprehensive and include a survey, including other symptoms that compose the syndrome, such as fatigue, sleep disturbances, cognitive difficulties and mood alterations (Galvez-Sánchez & Reyes Del Paso, 2020). Clinicians, therefore, often suspect of FM when pain is present in different locations of the body, without a clear relationship with trauma or systemic inflammation (Clauw, 2014). One of the main characteristics of FM patients is not having a biological alteration in routine exams. Laboratory testing is used thus in the clinical setting to rule-out differential diagnosis, not to confirm FM.
Once the diagnosis of FM is established, a multidisciplinary approach to treatment is recommended and symptom severity is monitored to adjust the therapy over time. Observational studies have shown that FM symptoms vary considerably over time and from one patient to another (Walitt et al., 2011). 15% of FM patients at one point during their treatments improve their symptoms to the point of failing to meet the criteria for FM; but 60% out of those get worse again and return to criteria fulfillment after that, demonstrating the cyclical nature of the symptoms. This transitory state of symptom improvement and worsening presented by some patients can lead to difficulties in their longitudinal assessment, either in the research or clinical setting. Considering this dynamic nature of clinical manifestations and the high individual variability, the need of an objective and reliable biomarker is a priority pending task in FM research. In this editorial we provide a summary of potential biomarkers associated with Fibromyalgia, with an emphasis in neurophysiological studies.
The current search for biomarkers in Fibromyalgia
Clinical and mechanistic research in FM have made continuing efforts to find sensitive and reliable biomarkers that can help to improve diagnostic accuracy and adequate treatment selection. This is a relevant area of research in the field as such markers have the potential to reshape care in FM. For instance, diagnostic markers can help the implementation of early intervention measures leading to substantial savings on health care expenses (Annemans et al., 2008). As the ideal biomarkers are yet to be found, the existing ones have advantages but also disadvantages, which prevent them from being commonly used.
Inflammatory markers
The main laboratory inflammatory markers that have been related to FM are Interleukins 6, 8, 10, and TNF-α (O’Mahony, Srivastava, Mehta, & Ciurtin, 2021). However, these markers are also associated with other systemic inflammatory diseases, limiting their role as diagnostic tools. More recently, there have been metabolomics studies (Malatji et al., 2017) attempting to discover through analysis of blood plasma or urine samples specific metabolic indicators to help with an understanding of FM etiology, as well as a more objective diagnosis. So far, these studies have shown good accuracy (75–90%) of certain groups of metabolites in predicting FM in experimental settings. However, it remains to be seen if such results are reproducible in a clinical setting and if they can also predict response to treatment in longitudinal analyses.
Neuroimaging markers
Neuroimaging studies with magnetic resonance imaging (MRI) in FM patients with a long-term history of chronic pain have shown structural changes with decreased cortical thickness in certain brain areas, such as the anterior cingulate cortex (ACC), and increased cortical thickness in other brain areas, such as the parts of the frontal and temporal lobes, possibly due to compensatory mechanisms (Jensen et al., 2013). Other studies have shown anatomical changes with lower gray matter volumes in the cingulate and insular cortices, associated with affective and sensory processing, in FM patients in comparison to healthy controls (Robinson, Craggs, Price, Perlstein, & Staud, 2011). The mechanisms leading to these volumetric changes are not completely known, thus functional neuroimaging studies assessing abnormal nociceptive processing have also been extensively studied in this condition (Jorge & Amaro, 2012).
Imaging techniques measuring brain activity include single photon computed tomography (SPECT), positron emission tomography (PET), and functional magnetic resonance imaging (fMRI). These techniques indirectly evaluate brain activity by measuring blood flow or changes in glucose or oxygen concentrations in specific brain areas (Staud, 2011). These measures of brain activity can be pain-related, for example, in response to an experimentally induced nociceptive stimulation. Other measures can be done in resting-state (i.e., spontaneous brain activity is measured without any task), in which the connectivity between brain regions involved in pain processing can be assessed. Studies of functional connectivity have shown, for example, altered connectivity between the periaqueductal gray matter (PAG) and the insula, ACC, and anterior prefrontal cortex, suggesting an abnormal descending pain inhibitory system (Truini et al., 2016). Interestingly, a similar analysis has shown normalization of this abnormal connectivity state after a program involving physical therapy (Flodin et al., 2015).
Neurophysiological markers
Electroencephalogram (EEG) has been described as a potential tool to elucidate the mechanism and a biomarker for the disease and progression. It provides information about brain functioning through the graphical representation of differences in voltage between electrodes placed on the scalp, and automated processing of signals can be done, for example, in power spectral analysis, to assess functional changes associated with mental states. EEG can be done either during rest, sensory stimulation, motor function, or cognitive tasks. Martin-Brufau et al. 2021 (Martín-Brufau, Gómez, Sanchez-Sanchez-Rojas, & Nombela, 2021) studied EEG activity in resting state comparing 23 FM patients with 23 healthy controls, they found a generalized lower frequency power of theta, beta and alpha bands. Uygur-Kucukseymen et al. 2020 (Uygur-Kucukseymen et al., 2020) showed in FM patients a decrease in alpha power in the frontal, central and parietal regions; a decrease in beta power in the central region, despite no significant results in the theta band. These bands have a relationship with cortical neuronal interaction and its networks and neuroimaging studies have found that FM patients have a decrease in white and gray matter connectivity, as well as neuronal loss (Murga, Guillen, & Lafuente, 2017). Since FM patients have a less inhibitory function, this could be evaluated by EEG event-related potentials (ERPs). Some studies have evaluated auditory, somatosensory, visual and motor ERPs, however, given the variability and the lack of an established methodology, they are difficult to compare and to reproduce. Lenoir et al. 2020 (Lenoir et al., 2020) collected 18 studies for a systematic review on nociceptive stimulation in chronic pain patients using ERPs, including 4 of them in FM patients, one study found a reduced amplitude of N1-P1 compared to healthy controls (Üçeyler et al., 2013) and a previous study found a larger amplitude of N2-P3 (Gibson, Littlejohn, Gorman, Helme, & Granges, 1994), however, the systematic review concluded that more studies are needed in FM patients. De Tommaso et al. 2017 (de Tommaso et al., 2017) found a higher pain-related ERP amplitude in FM patients (peak to peak N2P2 amplitude) over the vertex compared with healthy subjects; also, they report a short latency period. These findings suggested an impaired cortical pain processing of pain stimulus and cortical excitability in FM patients indexed by pain-related ERP alterations (Fig A). Also, quantitative EEG (qEEG) can be used in motor-related tasks to evaluate this inhibitory function. One study used motor tasks (i.e., motor observation, motor imagery, and motor execution) and measured event-related desynchronization. Higher event-related desynchronization (ERD) is known to be needed during an optimal cortical inhibitory activity. This study found a significant negative correlation, meaning higher pain intensity with lower theta and delta ERD in central regions during the fixation period on the motor imagery and motor observation tasks (Uygur-Kucukseymen et al., 2020). However, variability in the results might be explained by the inclusion and exclusion criteria of the sample, the different characteristics of the disease as patients might present with more than one symptom, and the methodology and variability of the auditory, somatosensory, visual and motor ERPs.
Figure 1.
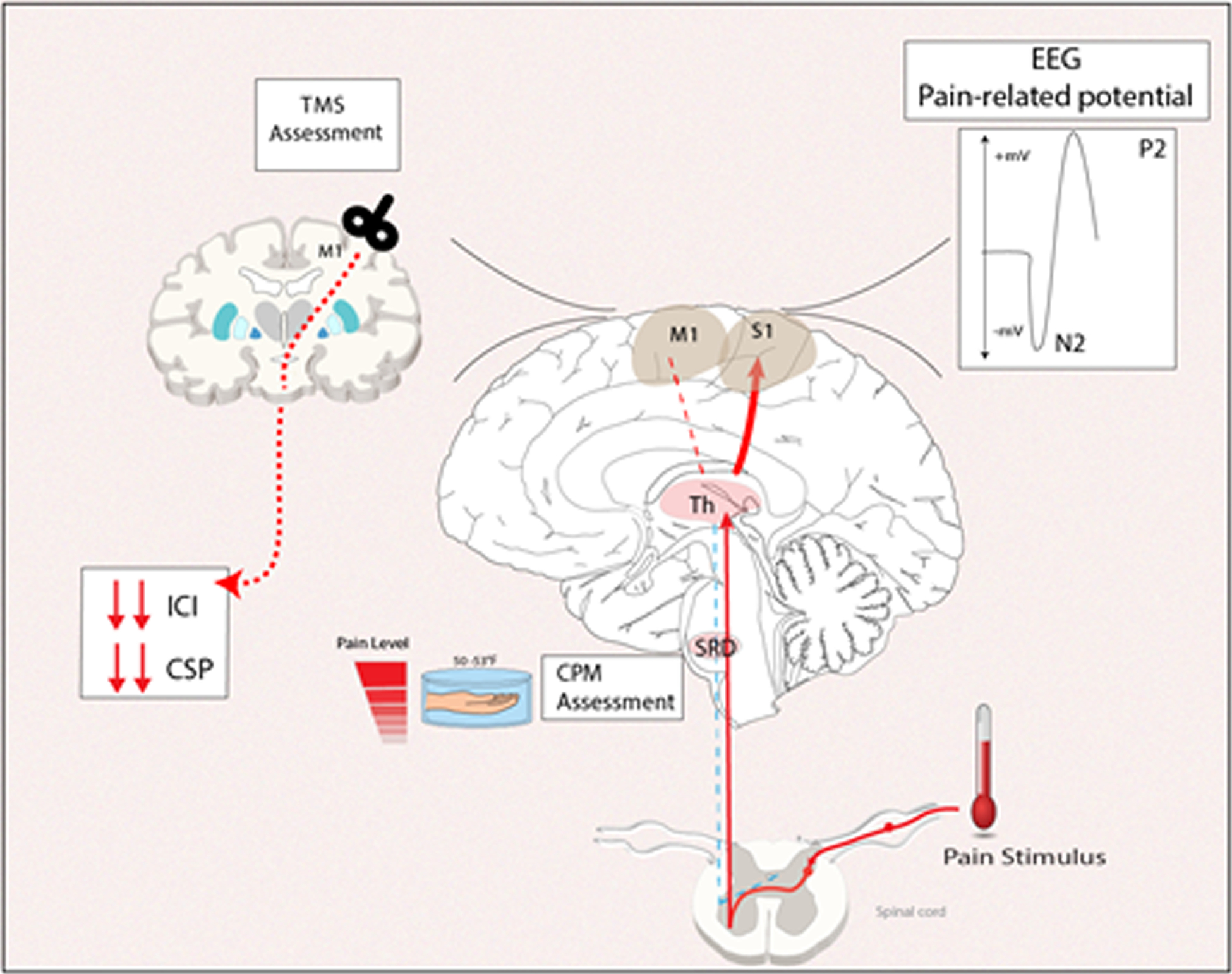
A. Neurophysiological markers found in fibromyalgia patients. Source: Spaulding Neuromodulation Lab. ACR, KPB
Another potential neurophysiological marker is the use of transcranial magnetic stimulation (TMS). This tool measures motor thresholds, motor evoked potentials as well as excitatory and inhibitory function as measured by paired-pulse the short-interval intracortical inhibition (SICI), and Intracortical facilitation (ICF) in FM patients. For example, Cardinal et al. 2019 (Cardinal et al., 2019) compared the motor cortex inhibition indexed by SICI and ICF in FM patients, patients with major depression, and healthy controls. Less intracortical inhibition (high ICI ratio) was observed in FM patients than in healthy controls. Another study compared the cortical excitability parameters differences assessed by TMS in FM, myofascial pain syndrome, osteoarthritis, and healthy controls, showing significantly decreased SICI, higher SICF, and shorter CSP in FM patients compared to healthy controls (Caumo et al., 2016). These results suggest that motor cortex inhibition parameters indexed by TMS are likely to be helpful as a biomarker for inhibitory control in FM patients (See Figure 1).
Quantitative sensory testing markers
In FM, chronic pain is thought to be caused by impairment of the endogenous descending inhibitory pain pathway (O’Brien, Deitos, Triñanes Pego, Fregni, & Carrillo-de-la-Peña, 2018). Therefore, the use of experimental pain protocols, involving quantitative sensory testing (QST) have been thought of as useful surrogate markers to assess the effectiveness of Central Nervous System (CNS) pain pathways (Hackshaw, 2021). Consistently, FM patients have been shown to have an increased response to repetitive experimental pain stimuli and lowered, or inefficient, response to Conditioned pain modulation (CPM), supporting the role of CNS pain inhibitory mechanisms in the pathophysiology of the disease as well as its implicated deficits (O’Brien et al., 2018). The enhanced response and after sensations caused by repetitive painful stimuli in FM patients can occur from a bottom-up response which can be attributed to defective muscle nociceptors, triggering central sensitization patterns that in turn exaggerate their response to pain (Bosma et al., 2016; Price et al., 2002). Regarding mechanical pain thresholds, in line with other QST responses, FM patients appear to have lower pain thresholds on their tender points, once again highlighting the important role of central sensitization in the disease (Terzi, Terzi, & Kale, 2015).
Moreover, these experimental pain assessments can also be used to identify different subgroups within FM, given its significant heterogeneity in presentation (Hurtig, Raak, Kendall, Gerdle, & Wahren, 2001). Although all individuals with FM have a deficit in their endogenous pain inhibition pathways, the extent of this deficit varies, and QST protocols have been used to classify these individuals (O’Brien et al., 2018). Thus, experimental pain protocols are currently important contenders for future FM biomarkers. However, although widely investigated, few studies have used these processes as actual biomarkers, as there are still various steps ahead needed to be done for their validation (Ou, Michiels, Shyr, Adjei, & Oberg, 2021), leaving room for further investigation of these protocols as potential FM biomarkers through future studies.
Emerging biomarkers
There is evidence of genetic components underlying the pathophysiology of FM syndrome. Replicated data shows that FM tends to cluster in families, with co-occurrence of comorbid conditions like psychological symptoms, physical functioning, fatigue, and irritable bowel syndrome, in first-degree relatives of patients with FM (Kato, Sullivan, Evengård, & Pedersen, 2006). In fact, there’s data pointing to 8.5 higher odds of familial aggregation in FM than rheumatoid arthritis (Arnold et al., 2004) and an estimated sibling recurrence-risk ratio of 13.6 (Arnold et al., 2013). Several pain-related and non-related genes have been associated with FM (Tanwar, Mattoo, Kumar, Dada, & Bhatia, 2021), although none have shown to consistently correlate to disease activity.
Regarding invasive procedures that require tissue samples, researchers investigating the intraepidermal region of skin biopsy have shown a decrease in the small nerve fiber concentrations in FM patients compared to controls (Oaklander, Herzog, Downs, & Klein, 2013). Small Nerve fiber polyneuropathy is a distinct entity, and these findings still cannot explain the histopathological processes occurring in FM patients. Another study demonstrated that increased IL-5 predicts increased general pain scores and decreased physical function in previously diagnosed FM patients (Merriwether et al., 2021). Also, gut microbiome alterations associated with a decrease in the bacteria population linked to short-chain fatty acid metabolism are shown in FM patients compared to normal individuals (Clos-Garcia et al., 2019). Although these biological alterations correlate with the condition, they do not explain the physiopathology of the illness, and further studies are necessary to create solid evidence for pathognomonic biomarkers.
Conclusion
There are several promising FM biomarkers under study, but with inherent challenges and limitations. While some existing biomarkers may be reliable and sensitive, its clinical applicability may be limited. Functional magnetic resonance imaging, for example, has been shown to be useful in detecting the amplification of pain processing activity that is seen in FM. Its disadvantages include high cost and clinically unfeasible. On the other hand, evoked pain measures that are commonly used clinically, such as the tender point counts, have had their validity questioned (Wolfe, 1997). Other markers like pain pressure thresholds are largely used in research but have not yet achieved a consensus on a uniform and reproducible technique to be used as a clinical parameter. For other markers such as heart rate variability, pro-inflammatory chemokines and genetic associations, despite the supporting evidence to its correlation with signs and symptoms of FM, they are also known to be influenced by many different factors, making them uncertain measures. Current literature suggests the FM is likely a heterogeneous dynamic phenotype that can be approached through diverse pathogenetic mechanisms; thus, the combination of multiple biomarkers may be needed depending on clinical presentation and comorbidities to characterize this condition. Future explorations of combined biomarker performance and endotyping methods are necessary to finally validate and implement the most appropriate diagnostic and prognostic biomarkers in FM clinical care. Among the presented potential markers, we believe that electrophysiological methods such as qEEG and quantitative sensory tests such as CPM, due to their low cost, have the potential to become widely available in research and clinical practice. These methods can therefore play in an important role in treatment response prediction and tailoring of individualized dynamic therapy regimens.
Funding
This work is supported by NIH grant R01 AT009491-01A1.
REFERENCES
- Annemans L, Wessely S, Spaepen E, Caekelbergh K, Caubère JP, Le Lay K, & Taïeb C (2008). Health economic consequences related to the diagnosis of fibromyalgia syndrome. Arthritis Rheum, 58(3), 895–902. doi: 10.1002/art.23265 [DOI] [PubMed] [Google Scholar]
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, … Iyengar SK (2013). The fibromyalgia family study: a genome-wide linkage scan study. Arthritis Rheum, 65(4), 1122–1128. doi: 10.1002/art.37842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LM, Hudson JI, Hess EV, Ware AE, Fritz DA, Auchenbach MB, … Keck PE Jr. (2004). Family study of fibromyalgia. Arthritis Rheum, 50(3), 944–952. doi: 10.1002/art.20042 [DOI] [PubMed] [Google Scholar]
- Arout CA, Sofuoglu M, Bastian LA, & Rosenheck RA (2018). Gender Differences in the Prevalence of Fibromyalgia and in Concomitant Medical and Psychiatric Disorders: A National Veterans Health Administration Study. Journal of women’s health (2002), 27(8), 1035–1044. doi: 10.1089/jwh.2017.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Dukes E, Martin S, Edelsberg J, & Oster G (2007). Characteristics and healthcare costs of patients with fibromyalgia syndrome. International journal of clinical practice, 61(9), 1498–1508. doi: 10.1111/j.1742-1241.2007.01480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers AT, & Gershwin ME (2015). Fibromyalgia: A Critical and Comprehensive Review. Clinical Reviews in Allergy & Immunology, 49(2), 100–151. doi: 10.1007/s12016-015-8509-4 [DOI] [PubMed] [Google Scholar]
- Bosma RL, Mojarad EA, Leung L, Pukall C, Staud R, & Stroman PW (2016). FMRI of spinal and supra-spinal correlates of temporal pain summation in fibromyalgia patients. Hum Brain Mapp, 37(4), 1349–1360. doi: 10.1002/hbm.23106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal TM, Antunes LC, Brietzke AP, Parizotti CS, Carvalho F, De Souza A, … Caumo W (2019). Differential Neuroplastic Changes in Fibromyalgia and Depression Indexed by Up-Regulation of Motor Cortex Inhibition and Disinhibition of the Descending Pain System: An Exploratory Study. Frontiers in Human Neuroscience, 13. doi: 10.3389/fnhum.2019.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussán-Sarria JA, … Fregni F (2016). Motor Cortex Excitability and BDNF Levels in Chronic Musculoskeletal Pain According to Structural Pathology. Front Hum Neurosci, 10, 357. doi: 10.3389/fnhum.2016.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ (2014). Fibromyalgia: a clinical review. Jama, 311(15), 1547–1555. doi: 10.1001/jama.2014.3266 [DOI] [PubMed] [Google Scholar]
- Clauw DJ, Arnold LM, & McCarberg BH (2011). The science of fibromyalgia. Mayo Clin Proc, 86(9), 907–911. doi: 10.4065/mcp.2011.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, Abecia L, Lavín JL, van Liempd S, … Falcón-Pérez JM (2019). Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine, 46, 499–511. doi: 10.1016/j.ebiom.2019.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tommaso M, Ricci K, Libro G, Vecchio E, Delussi M, Montemurno A, … Iannone F (2017). Pain Processing and Vegetative Dysfunction in Fibromyalgia: A Study by Sympathetic Skin Response and Laser Evoked Potentials. Pain research and treatment, 2017, 9747148–9747148. doi: 10.1155/2017/9747148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rabbat MS, Mahmoud NK, & Gheita TA (2018). Clinical significance of fibromyalgia syndrome in different rheumatic diseases: Relation to disease activity and quality of life. Reumatol Clin (Engl Ed), 14(5), 285–289. doi: 10.1016/j.reuma.2017.02.008 [DOI] [PubMed] [Google Scholar]
- Flodin P, Martinsen S, Mannerkorpi K, Löfgren M, Bileviciute-Ljungar I, Kosek E, & Fransson P (2015). Normalization of aberrant resting state functional connectivity in fibromyalgia patients following a three month physical exercise therapy. Neuroimage Clin, 9, 134–139. doi: 10.1016/j.nicl.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez-Sánchez CM, & Reyes Del Paso GA (2020). Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J Clin Med, 9(4). doi: 10.3390/jcm9041219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S, Littlejohn G, Gorman M, Helme R, & Granges G (1994). Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain, 58(2), 185–193. [DOI] [PubMed] [Google Scholar]
- Hackshaw KV (2021). The Search for Biomarkers in Fibromyalgia. Diagnostics (Basel, Switzerland), 11(2), 156. doi: 10.3390/diagnostics11020156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuser W, Ablin J, Fitzcharles M-A, Littlejohn G, Luciano JV, Usui C, & Walitt B (2015). Fibromyalgia. Nature reviews Disease primers, 1(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Häuser W, & Fitzcharles M-A (2018). Facts and myths pertaining to fibromyalgia. Dialogues in clinical neuroscience, 20(1), 53–62. doi: 10.31887/DCNS.2018.20.1/whauser [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtig IM, Raak RI, Kendall SA, Gerdle B, & Wahren LK (2001). Quantitative sensory testing in fibromyalgia patients and in healthy subjects: identification of subgroups. Clin J Pain, 17(4), 316–322. doi: 10.1097/00002508-200112000-00005 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Srinivasan P, Spaeth R, Tan Y, Kosek E, Petzke F, … Kong J (2013). Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheum, 65(12), 3293–3303. doi: 10.1002/art.38170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge LL, & Amaro E Jr. (2012). Brain imaging in fibromyalgia. Curr Pain Headache Rep, 16(5), 388–398. doi: 10.1007/s11916-012-0284-9 [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengård B, & Pedersen NL (2006). Chronic widespread pain and its comorbidities: a population-based study. Arch Intern Med, 166(15), 1649–1654. doi: 10.1001/archinte.166.15.1649 [DOI] [PubMed] [Google Scholar]
- Lenoir D, Willaert W, Coppieters I, Malfliet A, Ickmans K, Nijs J, … Cagnie B (2020). Electroencephalography during nociceptive stimulation in chronic pain patients: a systematic review. Pain Medicine, 21(12), 3413–3427. [DOI] [PubMed] [Google Scholar]
- Malatji BG, Meyer H, Mason S, Engelke UFH, Wevers RA, van Reenen M, & Reinecke CJ (2017). A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol, 17(1), 88. doi: 10.1186/s12883-017-0863-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Brufau R, Gómez MN, Sanchez-Sanchez-Rojas L, & Nombela C (2021). Fibromyalgia Detection Based on EEG Connectivity Patterns. J Clin Med, 10(15). doi: 10.3390/jcm10153277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriwether EN, Agalave NM, Dailey DL, Rakel BA, Kolker SJ, Lenert ME, … Sluka KA (2021). IL-5 mediates monocyte phenotype and pain outcomes in fibromyalgia. Pain, 162(5), 1468–1482. doi: 10.1097/j.pain.0000000000002089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murga I, Guillen V, & Lafuente J-V (2017). Cambios en la resonancia magnética cerebral asociados al síndrome de fibromialgia. Medicina Clínica, 148(11), 511–516. [DOI] [PubMed] [Google Scholar]
- O’Brien AT, Deitos A, Triñanes Pego Y, Fregni F, & Carrillo-de-la-Peña MT (2018). Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J Pain, 19(8), 819–836. doi: 10.1016/j.jpain.2018.01.010 [DOI] [PubMed] [Google Scholar]
- O’Mahony LF, Srivastava A, Mehta P, & Ciurtin C (2021). Is fibromyalgia associated with a unique cytokine profile? A systematic review and meta-analysis. Rheumatology, 60(6), 2602–2614. doi: 10.1093/rheumatology/keab146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaklander AL, Herzog ZD, Downs HM, & Klein MM (2013). Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain, 154(11), 2310–2316. doi: 10.1016/j.pain.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou FS, Michiels S, Shyr Y, Adjei AA, & Oberg AL (2021). Biomarker Discovery and Validation: Statistical Considerations. J Thorac Oncol, 16(4), 537–545. doi: 10.1016/j.jtho.2021.01.1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, & Vierck CJ (2002). Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain, 99(1–2), 49–59. doi: 10.1016/s0304-3959(02)00053-2 [DOI] [PubMed] [Google Scholar]
- Robinson ME, Craggs JG, Price DD, Perlstein WM, & Staud R (2011). Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain, 12(4), 436–443. doi: 10.1016/j.jpain.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staud R (2011). Brain imaging in fibromyalgia syndrome. Clin Exp Rheumatol, 29(6 Suppl 69), S109–117. [PubMed] [Google Scholar]
- Tanwar S, Mattoo B, Kumar U, Dada R, & Bhatia R (2021). Does human serotonin-1A receptor polymorphism (rs6295) code for pain and associated symptoms in fibromyalgia syndrome? Reumatismo, 73(1), 24–31. doi: 10.4081/reumatismo.2021.1312 [DOI] [PubMed] [Google Scholar]
- Terzi H, Terzi R, & Kale A (2015). The relationship between fibromyalgia and pressure pain threshold in patients with dyspareunia. Pain Res Manag, 20(3), 137–140. doi: 10.1155/2015/302404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, La Cesa S, … Di Franco M (2016). Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol, 34(2 Suppl 96), S129–133. [PubMed] [Google Scholar]
- Üçeyler N, Zeller D, Kahn A-K, Kewenig S, Kittel-Schneider S, Schmid A, … Sommer C (2013). Small fibre pathology in patients with fibromyalgia syndrome. Brain, 136(6), 1857–1867. [DOI] [PubMed] [Google Scholar]
- Uygur-Kucukseymen E, Castelo-Branco L, Pacheco-Barrios K, Luna-Cuadros MA, Cardenas-Rojas A, Giannoni-Luza S, … Fregni F (2020). Decreased neural inhibitory state in fibromyalgia pain: A cross-sectional study. Neurophysiologie clinique = Clinical neurophysiology, 50(4), 279–288. doi: 10.1016/j.neucli.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walitt B, Fitzcharles MA, Hassett AL, Katz RS, Häuser W, & Wolfe F (2011). The longitudinal outcome of fibromyalgia: a study of 1555 patients. J Rheumatol, 38(10), 2238–2246. doi: 10.3899/jrheum.110026 [DOI] [PubMed] [Google Scholar]
- Wolfe F (1997). The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis, 56(4), 268–271. doi: 10.1136/ard.56.4.268 [DOI] [PMC free article] [PubMed] [Google Scholar]


