Abstract
Purpose
An increase in the incidence of pediatric complicated appendicitis (CA) during the COVID-19 pandemic has been reported in many countries. We investigated how the pandemic has affected Japan.
Methods
We retrospectively reviewed children of ≤ 15 years old treated for acute appendicitis across 5 medical centers during the pandemic period (January to October in 2020), with the pre-pandemic period (January to October in 2017 to 2019) evaluated as a historical control. The incidence of CA and disease characteristics were then compared between the periods.
Results
The total number of patients was 55 in 2020 and 192 in 2017–2019. In all centers, the incidence of acute pediatric CA in the pandemic period significantly increased compared to the pre-pandemic period (18.2% vs. 32.7%, p = 0.02). On limiting our evaluation to the 3 institutions with reductions in patient numbers, the incidence of CA increased (16.3% vs. 37.9%, p = 0.01), and the duration of pre-operative symptoms was prolonged (1.3% vs. 1.7 days, p = 0.03). There were no significant differences in the age, sex, white blood cell count, C-reactive protein, or body temperature. No cases were diagnosed with SARS-CoV-2.
Conclusions
The incidence of acute pediatric CA increased during the pandemic period. This may be related to an extended duration of symptoms due to individuals fearing contracting COVID-19 while visiting a hospital.
Keywords: Acute appendicitis, Complicated appendicitis, COVID-19, Duration of symptoms, Pediatrics
Introduction
In December 2019, a novel coronavirus infection (COVID-19) was first confirmed in Wuhan, China [1]. The infection subsequently spread worldwide. The first case of COVID-19 in Japan was confirmed on January 15, 2020 [2]. With the subsequent spread of infection in Japan, avoidance of three Cs (closed spaces, crowded places, and close-contact settings) and staying home were recommended as measures against infection. The Japan Surgical Society and American College of Surgeons also recommended that elective surgery be considered from various angles, and at facilities dealing with pediatric surgical diseases, measures were taken to postpone non-emergency surgery, such as inguinal hernia [3, 4].
Acute appendicitis (AA) in children is a disease that may require emergency surgery, and the incidence of complicated appendicitis (CA) in children is reported to be high (12.5%–30%) [5, 6]. According to reports from other countries, where the number of people infected with COVID-19 is much higher than that in Japan, there are some studies showing an increase in the incidence of pediatric CA during the COVID-19 pandemic period [7–9].
We therefore examined the impact of COVID-19 on pediatric AA in our facilities during the pandemic period.
Methods
The study population included children of ≤ 15 years old who were hospitalized and treated under a diagnosis of AA at 5 facilities in the Tokyo metropolitan region, including a main hospital (A), 2 branch university hospitals (B, C), and 2 affiliated community hospitals (D, E). To evaluate the chance that any potential difference might be due to COVID-19 and not to temporal or seasonal changes, we retrospectively compared the cases treated during the COVID-19 pandemic period from January to October 2020 (pandemic period) with those treated during the same period from 2017 to 2019, before the pandemic (pre-pandemic period).
The diagnosis of AA was made based on the findings of a physical examination (right lower abdominal pain, tenderness, and muscular defense), blood tests and imaging studies (swelling of appendix, fecalith, and abscess formation) by ultrasonography and/or computed tomography. The definition of CA was based on the imaging results (abscess formation and/or existence of free air by perforation) reviewed by a radiologist and/or intraoperative and histopathological findings of perforation, if surgery was performed.
Basically, the decision to perform conservative treatment with intravenous antibiotics or surgical intervention was decided by each surgeon, and the surgical procedure varied from facility to facility. In AA with abscess formation, conservative treatment with intravenous antibiotics was usually introduced, and delayed laparoscopic appendectomy was planned.
The evaluation items were the period from the appearance of symptoms to hospital visit (duration of symptoms), the incidence of CA, age, sex, white blood cell (WBC) count, C-reactive protein (CRP) level, body temperature at admission, pathological diagnosis in surgical cases, and SARS-CoV-2 infection. Data were recorded prospectively and analyzed retrospectively.
For the statistical analysis, continuous data (the duration of symptoms, age, body temperature, WBC, CRP, and length of stay in hospital) were analyzed using Student’s t test, and categorical data (the perforation rate and sex) were analyzed using the Chi-squared test. p values of < 0.05 were considered to indicate statistical significance.
This study was approved by the institutional review board (Approval No. 31-182(9681)).
Results
The total number of hospitalizations for AA was 192 during the pre-pandemic period from 2017 to 2019 (annual average 64 patients) and 55 during the pandemic period.
In the region where our facilities are located, the pandemic first appeared from April to May 2020, and continuous infection expansion occurred from July 2020 (Fig. 1) [10]. To determine the presence of an increase in the incidence of CA coinciding with the period of explosive infection in 2020, we evaluated the incidence of CA every 2 months. We noted an increasing trend from spring to summer during the pre-pandemic period. In 2020, after decreasing to the level observed in April 2020, when the pandemic first began, fewer patients than usual were encountered. Before the pandemic, the incidence of CA tended to be lower in summer than in other seasons, but it was constant in 2020, and in the second half of the year during the period of infection expansion, a marked increase was observed (Fig. 2). The comparison of each facility revealed that the number of hospitalizations for AA in the pandemic period decreased in three institutes (C, D, and E) and increased in two (A, and B) (Fig. 3).
Fig. 1.

Changes in the number of COVID-19-positive cases from January 1 to October 31, 2020, in the prefectures where the facilities are located
Fig. 2.
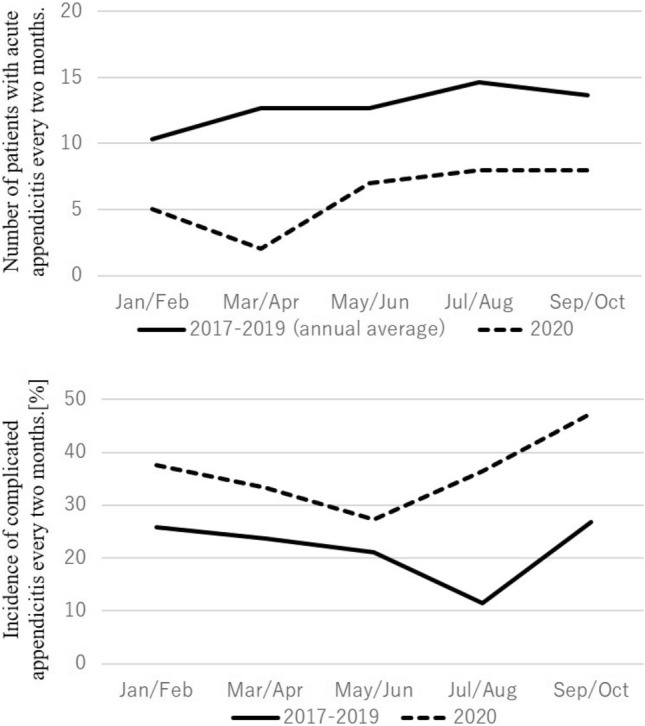
Changes in the number of hospitalized patients with acute appendicitis and incidence of complicated appendicitis every 2 months
Fig. 3.
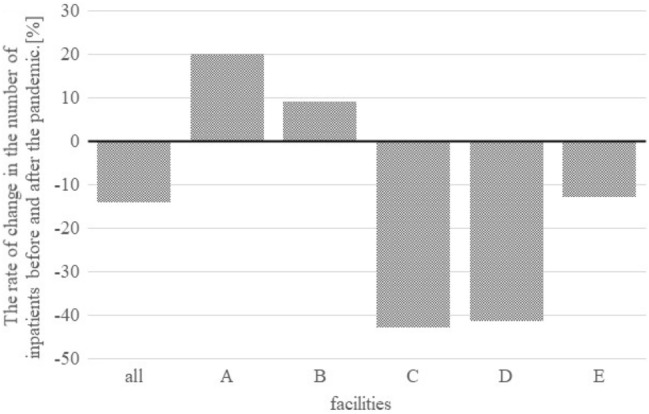
Rate of change in the number of inpatients at each institution before and after the pandemic period [what is ‘after the pandemic’? The pandemic is still ongoing. Weren’t you comparing before and during the pandemic?]. Facilities A and B are in urban areas, whereas facilities C, D, and E are in the suburbs
Next, we compared the clinical findings of the pre-pandemic and pandemic periods in all facilities and in the three institutes where the number of AA cases decreased separately (Table 1). There were no significant differences in the median age (10 vs. 11 years old, p = 0.31) or in the rate of male sex (57% vs. 56%, p = 0.90). Regarding the clinical findings, there were no significant differences in the WBC, CRP, body temperature, or length of stay in the hospital; however, the incidence of CA was significantly higher during the pandemic period than in the pre-pandemic period (18.2% vs. 32.7%, p = 0.02).
Table 1.
Clinical findings in all facilities and the three facilities where the number of hospitalizations decreased during both the pandemic and non-pandemic periods
| All facilities | Three facilities with decreased hospitalizations | |||||
|---|---|---|---|---|---|---|
| 2017–2019 (n = 192) | 2020 (n = 55) | p value | 2017–2019 (n = 122) | 2020 (n = 29) | p value | |
| Age (years) | 10 (9–13) | 11 (7–13) | 0.31 | 10.5 (9–13) | 11 (7–12) | 0.23 |
| Male gender | 110 (57%) | 31 (56%) | 0.90 | 71 (58%) | 15 (52%) | 0.53 |
| Incidence of CA | 35 (18.2%) | 18 (32.7%) | 0.02 | 20 (16.4%) | 11 (37.9%) | 0.01 |
| Duration of PSa (days) | 1.4 ± 1.2 | 1.5 ± 1.1 | 0.28 | 1.3 ± 1.1 | 1.7 ± 1.0 | 0.03 |
| WBC | 14,500 (12,300–17,500) | 15,700 (12,950–17,900) | 0.11 | 14,700 (12,850–17,775) | 15,600 (12,700–18,100) | 0.24 |
| CRP | 2.06 (0.28–6.66) | 2.53 (0.42–8.64) | 0.48 | 1.62 (0.24–5.65) | 3.08 (0.66–7.42) | 0.22 |
| BT | 37.5 (37.0–38.0) | 37.5 (37.0–38.3) | 0.61 | 37.4 (37.0–37.9) | 37.4 (37.0–37.7) | 0.50 |
| Length of stay (days) | 6.0 (5.0–7.0) | 6.0 (5.0–8.0) | 0.56 | 5.0 (4.0–7.0) | 6.5 (5.0–8.8) | 0.11 |
Data are expressed as the median (interquartile range)
PS pre-operative symptoms, BT body temperature
aData are expressed as the mean ± standard deviation
Interestingly, when our evaluation was limited to the 3 facilities (C, D, and E) where the number of hospitalizations for AA decreased, in addition to an increase in the incidence of CA (16.3% vs. 37.9%, p = 0.01), a prolonged duration of pre-operative symptoms (1.3 vs. 1.7 days, p = 0.03) and longer duration of hospitalization (5.0 vs. 6.5 days, p = 0.11) were observed. The CRP values at admission during the spread of infection tended to be higher at these facilities than before the pandemic at all facilities (1.62 vs. 3.08, p = 0.22), which is consistent with the fact that the illness period was prolonged, because CRP levels increase with prolonged illness.
There was no significant difference in the incidence of CA between 2017–2019 and 2020 at the facility where the number of patients increased (21.4% vs. 26.9%, p = 0.57). In addition, the incidence of CA in 2020 compared with the increased hospitalization facility and the reduced hospitalization facility was 26.9% and 37.9% (p = 0.39), respectively, suggesting that the incidence of CA tended to rise.
The histology in appendectomy was either phlegmonous or gangrenous, with no cases of catarrhal. In the 3 hospitals where the number of hospitalizations for AA decreased, the ratio of gangrenous appendicitis during the pandemic period was greater than before the pandemic, although without significance (43.7% vs. 66.7%, p = 0.11).
No SARS-CoV-2-positive cases were found in these patients.
Discussion
This study demonstrated that the incidence of pediatric CA during the pandemic period increased significantly in comparison to that in the pre-pandemic periods, especially in the three facilities where the number of hospitalizations for AA decreased. In general, the longer the period until treatment, the higher the possibility of perforation. We therefore focused on the duration of pre-operative symptoms as the cause of this increase in the incidence of CA.
We observed that the duration of symptoms during the pandemic period was significantly extended at the three facilities with a decreased number of hospitalizations. Similar results have been reported from other countries. In a report from Fisher et al., a 3-center study in New York showed a significant increase in perforation rate (45% vs. 27%) and the duration of pre-operative symptoms (71 ± 39 vs. 47 ± 27 h) in children during the pandemic compared with before the pandemic [7]. They suggested a relationship between barriers to a prompt assessment (from combined external restrictions and reluctance to seek care) and an increase in symptom duration and resultant perforation rates during the COVID-19 period. Saleem et al. reported a significant increase in the perforation rate (58.3%) in pediatric patients at a facility in Pakistan [8]. They concluded that the reasons for this included lockdown policies restricting access to the hospital, fear of an epidemiological SARS-CoV-2 infection while visiting the hospital, a reduction in operation theater slots, patients leaving the hospital against medical advice when counseled regarding the policy regarding mandatory SARS-CoV-2 testing prior to surgery, and a decrease in patients seen in tele-clinics. Snapiri et al. showed that three Israeli institutions reported double the incidence of complications, such as perforation or abscess formation, compared with before the pandemic [9]. They recognized that the reasons for a delayed diagnosis included parental concern about the possibility of contracting COVID-19 in public places, such as in the clinic or emergency room, inadequate clinical evaluations, and also due to problems associated with the conditions of consultation, such and the use of telemedicine.
There have been no reports suggesting a direct relationship between COVID-19 and AA; thus, the number of AA cases would not be expected to have changed markedly due to the pandemic. However, the number of hospitalizations for an AA diagnosis in 2020 decreased in three institutes (C, D, and E) and increased in two (A and B). Facilities A and B are general hospitals that provide emergency medical care and can hospitalize patients 24 h a day in a city center and suburban area, where the largest number of COVID-19 patients was found in Japan. Since the surrounding hospitals were busy dealing with patients with COVID-19, it is possible that patients with pediatric AA were referred to these hospitals. A similar situation was seen in a report from France by Montalva et al. [11]. There are multiple general hospitals of the same size in the vicinity. Therefore, the same number of hospitalizations was thought to have been maintained, even during the period of infectious spread, by referring children who were considered difficult to accept at other hospitals.
Facilities C, D, and E are also general hospitals that provide 24 h emergency medical care and are located in suburban and urban areas, where referrals from local clinics account for a large proportion of the cases. The number of perforations increased in 2020 at these three facilities. The increase in the perforation rate may be because there were some mild cases of appendicitis that improved simply by resting at home and did not require a trip to the hospital. Some authors have proposed that milder forms of appendicitis can be treated conservatively at home or using antibiotics [12]. In addition, Neufeld et al. reported that the pandemic was associated with a decreased incidence of uncomplicated appendicitis and suggested that some cases of uncomplicated appendicitis may resolve without progression to complicated disease, even in the absence of treatment [13]. Although the reasons underlying the decrease in hospitalization for AA during the pandemic remain unclear, we hypothesized that the decrease was due to the consciousness of patients, who may have refrained from visiting a doctor out of fear of contracting COVID-19 while doing so. According to a questionnaire survey released by the Japan Medical Association, the number of pediatric clinic visits during from April to August 2020 continued to decrease by more than 30% every month in comparison to the same month of the previous year [14], which supports our hypothesis. Furthermore, it was considered that the psychological distance of the patients and their family led to the extension of the duration of pre-operative symptoms and contributed to the increase in the incidence of CA.
The present study was associated with some limitations, including its retrospective design. First, it has been reported that AA progresses rapidly and causes perforation in 36–48 h in children [15, 16]. We evaluated the duration of pre-operative symptoms in days; however, evaluating this value in hours might have provided more insight. Unfortunately, it is difficult to determine the exact timing of symptoms onset retrospectively. In addition, the process leading up to the visit to our facility (for example, the history of treatment at the clinic and the presence or absence of oral antibiotics) was not examined; thus, it cannot be mentioned. It is believed that multi-center and multidisciplinary studies would be able to investigate the effects of COVID-19 more accurately and would not be associated with the biases seen in some hospitals and regions. However, because there is no common protocol for the treatment of pediatric AA, decisions regarding the indication of hospitalization may differ among institutions. Thus, the number of cases diagnosed with AA may not be accurate, as inpatient treatment may not have been indicated in some cases at an outpatient visit. Strictly speaking, not all patients with AA are hospitalized, and outpatient treatment may be selected instead. The decrease in hospitalization may not be equivalent to the decrease in the number of consultations, as hospitalization may have been refused during the pandemic period. Furthermore, the accurate diagnosis of AA and perforation is generally made during surgery, which is followed by a pathological examination. This study included some patients who were treated conservatively without surgery, which may have influenced the results of the comparison between the pandemic and pre-pandemic groups. Finally, although this study involved five institutions, the study population was relatively small, and the roles of each facility in the region and the medical care system during the pandemic period varied. Further research is therefore needed to validate our conclusions in a larger cohort from multiple facilities.
In conclusion, AA in children was shown to be exacerbated during the COVID-19 pandemic period compared with before the pandemic. This may be related to an extended duration of pre-operative symptoms due to individuals refraining from seeing a doctor based on fear of contracting COVID-19 while visiting a hospital.
Acknowledgements
None.
Declarations
Conflicts of Interest
The authors declare no conflicts of interest in association with the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furuse Y, Ko YK, Saito M, Shobugawa Y, Jindai K, Saito T, et al. Epidemiology of COVID-19 outbreak in Japan, from January–March 2020. Jpn J Infect Dis. 2020;73:391–393. doi: 10.7883/yoken.JJID.2020.271. [DOI] [PubMed] [Google Scholar]
- 3.Surgery recommendations for coronavirus positive and suspected patients. Japan Surgical Society web site (in Japanese). http://www.jssoc.or.jp/aboutus/coronavirus/info20200402.html. Accessed 10 Apr 2020.
- 4.COVID-19: elective case triage guidelines for surgical care. American College of Surgeons web site. https://www.facs.org/covid-19/clinical-guidance/elective-case. Accessed Mar 24 2020.
- 5.Körner H, Söndenaa K, Söreide JA, Andersen E, Nysted A, Lende TH, et al. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg. 1997;21:313–317. doi: 10.1007/s002689900235. [DOI] [PubMed] [Google Scholar]
- 6.Kulik DM, Uleryk EM, Maguire JL. Does this child have appendicitis? A systematic review of clinical prediction rules for children with acute abdominal pain. J Clin Epidemiol. 2013;66:95–104. doi: 10.1016/j.jclinepi.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Jason CF, Sandra ST, Howard BG, Alex G, David W, Keith AK. Increase in pediatric perforated appendicitis in the New York city metropolitan region at the epicenter of the COVID-19 outbreak. Ann Surg. 2020;273:410–415. doi: 10.1097/SLA.0000000000004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleem A, Sajid MI, Arshad M. Acute appendicitis during SARS-CoV-2: a brief communication of patients and changes in clinical practice from a single institute in Pakistan. J Pediatr Surg. 2020;55:2844–2845. doi: 10.1016/j.jpedsurg.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snapiri O, Danziger CR, Krause I, Kravarusic D, Yulevich A, Balla U, et al. Delayed diagnosis of paediatric appendicitis during the COVID-19 pandemic. Acta Paediatr. 2020;109:1672–1676. doi: 10.1111/apa.15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Changes in the number of infected people by prefecture. NHK (Japan Broadcasting Corporation) web site (in Japanese). https://www3.nhk.or.jp/news/special/coronavirus/data. Accessed 20 Aug 2021.
- 11.Montalva L, Haffreingue A, Ali L, Clariot S, Marsollier FJ, Ghoneimi AE, et al. The role of a pediatric tertiary care center in avoiding collateral damage for children with acute appendicitis during the COVID-19 outbreak. Pediatr Surg Int. 2020;36:1397–1405. doi: 10.1007/s00383-020-04759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tankel J, Keinan A, Blich O, Koussa M, Helou B, Shay S, et al. The decreasing incidence of acute appendicitis during COVID-19: a retrospective multi-centre study. World J Surg. 2020;44:2458–2463. doi: 10.1007/s00268-020-05599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neufeld MY, Bauerle W, Eriksson E, Azar FK, Evans HL, Johnson M, et al. Where did the patients go? Changes in acute appendicitis presentation and severity of illness during the coronavirus disease pandemic: a retrospective cohort study. Surgery. 2019 doi: 10.1016/j.surg.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Impact of new coronavirus infection on clinic management-July-August 2020-(in Japanese). Japan Medical Association Research Institute web site. https://www.jmari.med.or.jp/research/research/wr_717.html. Accessed 19 Nov 2020.
- 15.Rothrock SG, Skeoch G, Rush JJ, Johnson NE. Clinical features of misdiagnosed appendicitis in children. Ann Emerg Med. 1991;20:45–50. doi: 10.1016/S0196-0644(05)81117-5. [DOI] [PubMed] [Google Scholar]
- 16.Pepper VK, Stanfill AB, Pearl RH. Diagnosis and management of pediatric appendicitis, intussusception, and Meckel diverticulum. Surg Clin North Am. 2012;92:505–526. doi: 10.1016/j.suc.2012.03.011. [DOI] [PubMed] [Google Scholar]


