Abstract
The effects of pulsed electric field (PEF) treatment and processing factors on the inactivation kinetics of Listeria innocua NCTC 11289 were investigated by using a pilot plant PEF unit with a flow rate of 200 liters/h. The electric field strength, pulse length, number of pulses, and inlet temperature were the most significant process factors influencing the inactivation kinetics. Product factors (pH and conductivity) also influenced the inactivation kinetics. In phosphate buffer at pH 4.0 and 0.5 S/m at 40°C, a 3.0-V/μm PEF treatment at an inlet temperature of 40°C resulted in ≥6.3 log inactivation of strain NCTC 11289 at 49.5°C. A synergistic effect between temperature and PEF inactivation was also observed. The inactivation obtained with PEF was compared to the inactivation obtained with heat. We found that heat inactivation was less effective than PEF inactivation under similar time and temperature conditions. L. innocua cells which were incubated for a prolonged time in the stationary phase were more resistant to the PEF treatment, indicating that the physiological state of the microorganism plays a role in inactivation by PEF. Sublethal injury of cells was observed after PEF treatment, and the injury was more severe when the level of treatment was increased. Overall, our results indicate that it may be possible to use PEF in future applications in order to produce safe products.
Nonthermal food-processing techniques are receiving a lot of attention in the food industry since inactivation of microorganisms takes place under reduced-temperature conditions. Consequently, not only do food products retain a fresher appearance, but there is also less loss of flavor; both of these factors are currently in high demand by consumers. The results of preliminary studies have suggested that the pulsed-electric-field (PEF) technique is a promising nonthermal decontamination method and that it can replace or partially replace thermal processes (19). The PEF procedure involves applying pulses with very high field strengths (2.0 to 5.0 V/μm) for a very short time (2 to 4 μs) to foods placed between two electrodes.
Most previous studies of PEF treatment were carried out by using small amounts of samples in parallel plate chambers (3, 5, 6, 12, 16). During the last few years, there has been considerable progress in the development of equipment in which PEF treatment takes place in a continuous-treatment chamber (1, 15, 20). PEF treatment in continuous systems is more effective in terms of inactivation than PEF treatment in static systems is because the electric field is more uniform in continuous systems than in static systems (11, 14); therefore, PEF treatment in continuous systems offers more possibilities for scaling up the technology. However, very little systematic microbial kinetic data obtained with a continuous-treatment chamber has been reported previously (15). Furthermore, the data that are available are sometimes contradictory, and most of the data have been obtained with laboratory-scale PEF units. Moreover, there is insufficient information concerning the resistance of microorganisms to PEF treatment and the factors that influence this resistance, which is necessary for predicting the death rates of organisms under PEF conditions.
The underlying mechanism of inactivation of microorganisms by PEF has not been fully elucidated. The most commonly accepted theory is that local instabilities in the membranes of the microorganisms are formed by electromechanical compression and electrical field-induced tension, which form pores in the membrane (21). These instabilities are created only when the applied electrical field induces a certain critical membrane potential. This critical membrane potential depends on the cell size, the surface charge of the membrane, and the electrical conductivities of the membrane, cytoplasm, and suspending liquid medium (2, 10).
Clearly, any industrial application of PEF would require a process that is approved by governmental authorities, as current thermal treatments are. Although PEF offers many advantages compared to thermal treatments, further research is essential in order to develop safe processes.
In this study a number of product and process parameters that influence the inactivation kinetics of Listeria innocua NCTC 11289 were thoroughly investigated by using a pilot plant PEF unit with a continuous-treatment chamber. L. innocua is a nonpathogenic species which was used instead of the pathogenic organism Listeria monocytogenes. In addition, we compared the inactivation of L. innocua obtained with PEF and the inactivation obtained with heating at a similar temperature for a similar length of time. Experiments were also performed with several other microorganisms, including Escherichia coli, Lactobacillus plantarum, and Saccharomyces cerevisiae, in order to gain some insight into whether PEF had different effects on gram-negative, gram-positive, and eukaryotic microorganisms.
MATERIALS AND METHODS
Bacteria, growth conditions, and media.
The following strains were obtained from the Unilever culture collection: L. innocua VBLLi 02-53; S. cerevisiae SU 51, and L. plantarum LA10-11. The latter strain was isolated from spoiled onion ketchup and was identified by the American Type Culture Collection. E. coli NCTC 9001 and L. innocua NCTC 11289 were obtained from the National Collection of Type Cultures, London, England. Inocula were prepared 3 days before each experiment by starting with organisms which were stored in vials with 10% (vol/vol) glycerol at −20°C. L. innocua and E. coli were inoculated into 10 ml of Trypticase soy broth (TSB) (Becton Dickinson, Cockeysville, Md.) and incubated for 18 h at 30°C. The L. plantarum inoculum was prepared in a similar manner, except that the medium used was De Man-Rogosa-Sharpe (MRS) broth (Difco Laboratories, Detroit, Mich.). The S. cerevisiae inoculum was prepared in yeast extract-peptone-dextrose medium (yeast extract, peptone, and dextrose were all purchased from Difco Laboratories) supplemented with 2% (vol/vol) glucose (YPD-G), and the incubation temperature was 25°C. The cultures were inoculated (0.4%) into 500 ml of TSB (for L. innocua and E. coli), MRS broth (for L. plantarum), or YPD-G (for S. cerevisiae) and incubated for 48 h at 30°C (or 25°C for S. cerevisiae) in order to obtain cells in the stationary phase. The cells were harvested by centrifugation at 13,000 × g for 10 min at 5°C and resuspended in 50 ml of TSB (or MRS broth for L. plantarum or YPD-G for S. cerevisiae) to obtain approximately 109 CFU/ml. Each inoculum was kept on ice until inoculation (maximum, 2 h).
PEF treatment.
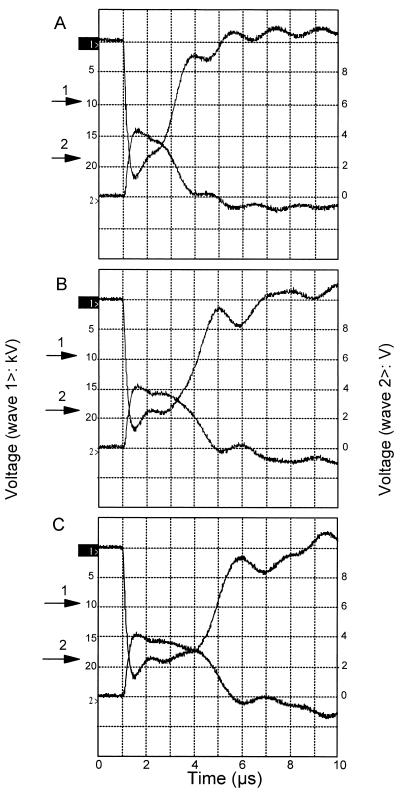
Cells were treated in a CoolPure apparatus (PurePulse Technologies Inc., San Diego, Calif.), which is a pilot plant scale continuous-treatment PEF apparatus with a capacity of 30 to 200 liters per h. The system runs with a coaxial treatment chamber with a gap of 5 mm. The power supplies are rated at 10 kJ/s, and the maximum output voltage is 40 kV. The electric field strength, pulse length, and pulse wave shape were determined with an oscilloscope (model TDS 220; Tektronix). The system was able to generate a pulse which approached a square wave, as shown in Fig. 1. The pulse length used were 2, 3, and 3.9 μs, which were obtained by changing the capacitance from 0.48, 0.72, and 0.96 μF, respectively. The PEF treatment was performed in a 10 mM Na2HPO4 · 2H2O-NaH2PO4 · 2H2O buffer solution. The conductivity of the solution was adjusted to 0.27, 0.51, or 0.79 S/m at 40°C by using 3 M KCl, and the pH was adjusted to 4.0, 4.4, 5.0, or 6.0 by using either 4 N HCl or 4 N NaOH. Approximately 175 liters of buffer was necessary to perform one PEF experiment; 50 liters of sterile buffer (which was not inoculated) was used to start the process and adjust the equipment settings, and 125 liters of buffer was inoculated with the microorganism. The final pH and conductivity of the suspension were measured prior to each experiment. The phosphate buffer was sterilized in the product tank and cooled to room temperature. Part of the buffer (50 liters) was pumped into a sterile tank and used in the start-up procedure prior to inoculation. During the start-up procedure, the desired system settings (i.e., pressure, flow rate, charge voltage, inlet medium temperature, and pulse frequency) were set. When the system was running steadily, the valves were adjusted so that buffer containing the inoculum was added. Experiments were performed with either one or two treatment chambers in series. The inlet temperature of the buffer was adjusted to 20, 30, or 40°C by using a heat exchanger prior to the PEF treatment. Between the two treatment chambers the buffer was cooled to the required inlet temperature by using a second heat exchanger. During each experiment the following parameters were measured continuously: flow rate, pressure, inlet and outlet medium temperatures for both treatment chambers, and conductivity at a certain temperature. The data and the pulse wave shape of each PEF treatment were entered into the computer. The energy input was calculated from the difference between the inlet and outlet medium temperatures and was multiplied by the heat capacitance of water (i.e., 4.18 J/ml). To determine the inoculum level in the buffer, samples were taken before and after the PEF treatment from the tank containing the inoculum. The concentrations of viable cells, expressed as the number of CFU per milliliter, were determined before and after the PEF treatment by using pour plates containing plate count agar (PCA) (Difco Laboratories) for L. innocua and E. coli, MRS agar (Merck, Darmstadt, Germany) for L. plantarum, and yeast extract-peptone-dextrose agar for S. cerevisiae. The plates were incubated aerobically at 25°C for S. cerevisiae and at 30°C for the other microorganisms for 5 days.
FIG. 1.
Typical wave shapes with different pulse lengths, including 2 μs (A), 3 μs (B), and 3.9 μs (C), generated with the PEF system during this study. The voltage pulse shape is indicated by wave 1>, and the current pulse shape is indicated by wave 2>. The pulse length (arrow 1) and the voltage used for the field strength calculations (arrow 2) are also indicated. The voltage-to-current ratio of wave 2 is 0.7 V/kA.
Heat treatment.
Heat treatments were performed by using the method described by Kooiman and Geers (8). The inactivation kinetics of L. innocua NCTC 11289 were investigated for various time intervals ranging from 0 to 600 s at 55, 57.5, 60, and 62.5°C by using 10 mM sodium phosphate buffer (Na2HPO4 · 2H2O-NaH2PO4 · 2H2O) (pH 4.0; 0.2 S/m) at 25°C. After each heat treatment, tubes were immediately placed in ice water. The concentrations of viable cells were determined before and after heat treatment by using pour plates containing PCA; the plates were incubated aerobically for 5 days at 30°C. A linear regression analysis was performed with survival plots (log surviving cell count versus heating time at each temperature), and decimal reduction times (D values) were calculated by determining the negative reciprocals of the slopes. The z value, which expressed the temperature dependence of D values, was calculated in a similar manner from the regression line obtained from the thermal inactivation curves (plots of log D values versus corresponding heating temperatures).
Sublethal injury after PEF.
After a PEF treatment in sodium phosphate buffer (pH 5.0) with a conductivity of 0.79 S/m at 40°C and an electric field strength of 2.0 to 2.5 V/μm, the samples were plated onto PCA at different pH values (adjusted with HCl). The pH values used were 5.0, 5.2, and 5.4, as well as the optimum pH, pH 6.8. The plates were incubated for 5 days at 30°C.
Statistical analyses.
The significance of differences in the PEF treatment results was tested by using the Student t test at P levels of 0.1 and 0.01.
RESULTS
Effect of electric field strength on inactivation of L. innocua NCTC 11289.
The effect of electric field strength on inactivation of L. innocua NCTC 11289 in pH 5 treatment medium with a conductivity of 0.51 S/m at 40°C was determined (Fig. 2). Inactivation of the microorganism clearly increased as the field strength increased. However, there appeared to be a threshold level (3.0 V/μm) above which no differences in inactivation could be detected despite increasing field strength (P < 0.1).
FIG. 2.
Inactivation kinetics of L. innocua NCTC 11289 at different electric field strengths. Symbols: ■, 2.0 to 2.1 V/μm; ⧫, 2.3 to 2.5 V/μm; ●, 2.7 to 3.0 V/μm; ✶, 3.2 to 3.5 V/μm; ■, 3.6 to 3.9 V/μm. The pH of the treatment medium was 5, the conductivity was 0.51 S/m at an inlet temperature of 40°C, and the pulse length was 3.9 μs. The inoculum contained 1.4 × 106 CFU/ml. The results are the means of data from two experiments, and standard deviations are indicated by error bars.
Effect of the conductivity of the treatment medium on inactivation of L. innocua NCTC 11289.
The effect of the conductivity of the treatment medium on inactivation of L. innocua was investigated by using a constant electric field strength, 2.3 to 2.6 V/μm. This relatively wide range of constant electric field strength could be explained by the different temperatures during the experiment. When high numbers of pulses were applied, the temperature increased more; consequently, the conductivity was higher, and the electric field strength was lower. The electric field strength was kept constant in three experiments in which the conductivities were 0.79, 0.51, and 0.27 S/m at 40°C. The inactivation data obtained with the different conductivities are shown in Fig. 3. At an outlet temperature of 50°C, a 2-log reduction was observed in a medium with a conductivity of 0.79 S/m at 40°C, whereas a reduction of almost 4.5 logs was observed in a medium with a conductivity of 0.27 S/m at 40°C. Thus, higher conductivity of the treatment medium resulted in a decrease in inactivation of microorganisms.
FIG. 3.
Effect of conductivity on the inactivation kinetics of L. innocua NCTC 11289. Symbols: ■, 0.79 S/m; ⧫, 0.51 S/m; ●, 0.27 S/m. The electric field strength was 2.3 to 2.6 V/μm, the inlet temperature of the treatment medium was 40°C, the pH was 5.0, and the pulse length was 3.9 μs. The inoculum contained 1.6 × 106 CFU/ml. The results are the means of data from two experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; N0, number of cells before treatment.
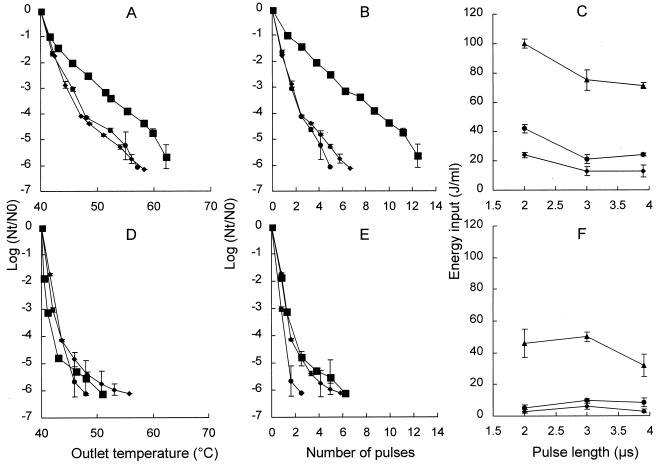
Effect of the pulse length on inactivation of L. innocua NCTC 11289.
The effect of the pulse length on inactivation of L. innocua NCTC 11289 was investigated at two electric field strengths, 2.8 and 3.6 V/μm, by using a pH 5.0 treatment medium (Fig. 4). The pulse lengths studied were 2, 3, and 3.9 μs. At a field strength of 2.8 V/μm, a shorter pulse resulted in less inactivation of L. innocua NCTC 9001 compared to the 3- and 3.9-μs pulse lengths (Fig. 4A). At an outlet temperature of 55°C, a 3.9-log reduction was observed with a pulse length of 2-μs, whereas a 5.2-log reduction was observed with pulse lengths of 3 and 3.9 μs. More pulses and a higher energy input were necessary in order to obtain similar inactivation if short pulses (2 μs) were used (Fig. 4B and C).
FIG. 4.
Inactivation kinetics of L. innocua NCTC 11289 with different pulse lengths. Data were obtained after PEF treatment with electric field strengths of 2.8 V/μm (A through C) and 3.6 V/μm (D through F). (A, B, D, and E) Log reduction plotted versus the temperature after treatment (outlet temperature) (A and D) and versus the number of pulses (B and E). Symbols: ■, 2-μs pulse; ⧫, 3-μs pulse; ●, 3.9-μs pulse. (C and F) Relationship between the energy input and the pulse length at various levels of inactivation levels. Symbols: ⧫, 2-log reduction; ●, 3-log reduction; ▴, 6-log reduction. The pH of the treatment medium was 5, and the inlet medium temperature was 40°C. The inoculum contained 1.4 × 106 CFU/ml. The results are means based on data from two experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; N0, number of cells before treatment.
Pulse length had no effect on inactivation of L. innocua NCTC 11289 at a field strength of 3.6 V/μm (Fig. 4D), and an approximately 5-log reduction was observed at an outlet temperature of 45°C with all three pulse lengths. However, when the pulse length was 3.9 μs, fewer pulses were needed (P < 0.01) to obtain a similar level of inactivation (Fig. 4E). By plotting the energy input versus the pulse length, we found that the energy inputs required to obtain a certain level of inactivation were almost the same for all three pulse lengths (Fig. 4F). We concluded that the pulse length influenced the inactivation kinetics depending on the electric field strength, and we noticed that the relationship between energy input and pulse length during inactivation depended on the electric field strength. The same effect was observed for a lower electric field strength, 2.3 V/μm (data not shown).
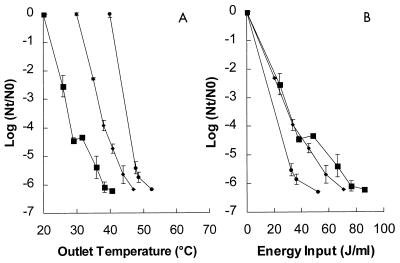
Effect of the inlet temperature of the treatment medium on inactivation of L. innocua NCTC 11289.
The effects of inlet temperatures of the treatment medium of 20, 30, and 40°C on inactivation of L. innocua NCTC 11289 were also studied. It was not possible to study lower temperatures with the equipment used, and higher inlet temperatures may have resulted in reductions in the number of CFU or in injury to the microorganisms. The effect of the inlet temperature was studied at an electric field strength of 4.7 V/μm with the conductivity adjusted to 0.27 S/m at the inlet temperature and with the pH of the buffer adjusted to 5.0 (Fig. 5A). At an inlet temperature of 20°C, a ≥6-log reduction was observed with an outlet temperature of 40°C; at an inlet temperature of 30°C, a similar reduction was observed when the outlet temperature was approximately 47°C; and at an inlet temperature of 40°C a similar reduction was observed with an outlet temperature of approximately 52°C. When the reduction was plotted versus the energy input (Fig. 5B), we found that with an energy input of 40 J/ml inactivation was greater at an inlet temperature of 40°C than at lower inlet temperatures (P < 0.01). Thus, the inlet temperature of the treatment medium had a substantial effect on the inactivation kinetics and energy efficiency. There was a strong synergism between PEF treatment and heat.
FIG. 5.
Effect of the inlet temperature of the treatment medium on the inactivation kinetics of L. innocua NCTC 11289 at various outlet medium temperatures (A) and at various energy input levels (B). The electric field strength of the PEF treatment was 4.7 V/μm, the pH of the treatment medium was 5, and the pulse length was 3.9 μs. Symbols: ■, 20°C; ⧫, 30°C; ●, 40°C. The inoculum contained 1.6 × 106 CFU/ml. The results are means based on data from two experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; N0, number of cells before treatment.
Calculation of the mean residence time after PEF treatment.
In order to compare heat inactivation kinetics with PEF inactivation kinetics, the residence time after the PEF treatment must be known. This was calculated with the following formula, in which the flow rate (Qv) is 5.556 × 10−5 m3/s and Vt is the volume: mean residence time = Qv/Vt. The volume of the processing line was calculated from the diameters and lengths of the pipes. Between treatment chamber 1 and the sampling point, the calculated residence time was 3.2 s. The sampling time was estimated to be 10 s, and the total residence time (at a certain temperature) before placement on ice was 13.2 s. The temperature decreased during this time; however, in the calculations we assumed that the temperature remained equal to the treatment temperature, which led to slight overestimates.
Comparison of the heat inactivation kinetics with the PEF inactivation kinetics.
The heat treatments were performed at pH 4.0 and 0.2 S/m, since the greatest PEF inactivation was observed under these conditions (see below). The calculated D values and the z value after various heat treatments of L. innocua NCTC 11289 are shown in Table 1. The D values and the z value were used to calculate the predicted inactivation by heat after treatment for 13.2 s, the total residence time of the PEF process described above. The results of the comparison of heat inactivation and PEF inactivation are shown in Table 2. After PEF treatment with an outlet temperature of 55°C in pH 4 buffer with an electric field strength of 2.2 to 2.4 V/μm, ≥6-log inactivation was observed, while inactivation was less than 0.05 log after heat treatment alone at 55°C for 13.2 s. At 57.5 and 60°C only slight inactivation was observed after the heat treatment. Thus, there was a significant difference between heat inactivation kinetics and PEF inactivation kinetics.
TABLE 1.
D values and z value for L. innocua NCTC 11289a
| Temp (°C) | D value (s) | z value (°C) |
|---|---|---|
| 57.5 | 62.3 | |
| 60 | 32.6 | 7.70 |
| 62.5 | 14.0 |
Heat treatments were performed in a 10 mM Na2HPO4 · 2H2O-NaH2PO4 · 2H2O solution (pH 4.0; 0.2 S/m at 25°C). Duplicate experiments were performed, and the values are means.
TABLE 2.
Comparison of observed PEF inactivation and predicted heat inactivation of L. innocua NCTC 11289 at different temperatures after treatment for 13.2 sa
| Temp (°C) | PEF inactivation (log) | Heat inactivation (log) |
|---|---|---|
| 55 | ≥6 | <0.05 |
| 57.5 | ≥6 | 0.2 |
| 60 | ≥6 | 0.4 |
| 62.5 | ≥6 | 0.9 |
The predicted values were calculated by using the D values and z value in Table 1. Both the PEF treatment and the heat treatment were performed in a 10 mM Na2HPO4 · 2H2O-NaH2PO4 · 2H2O solution (pH 4.0; 0.2 S/m at 25°C).
Effect of the pH of the treatment medium on inactivation of L. innocua NCTC 11289 and other microorganisms.
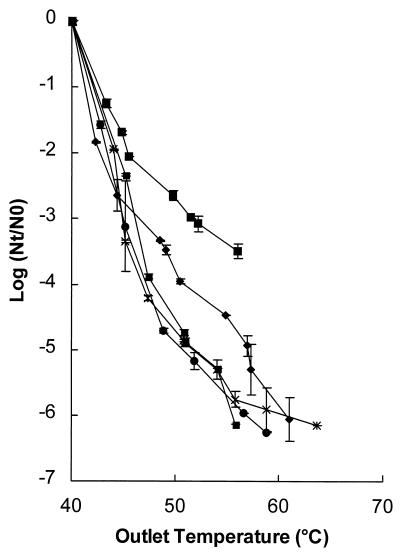
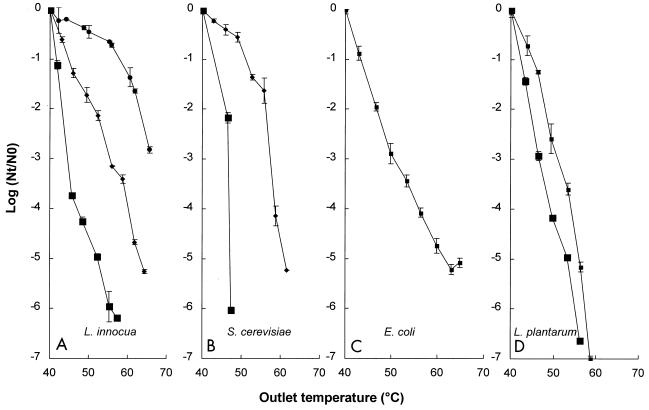
The relationship between the inactivation kinetics for L. innocua NCTC 11289, a representative gram-positive microorganism, and the pH of the buffer is shown in Fig. 6. Inactivation was studied in pH 4.0, 4.4, 5.0, and 6.0 phosphate buffers with a conductivity of 0.78 S/m at 40°C. For L. innocua NCTC 11289 at pH 4.0, a ≥6-log reduction in inactivation was observed at an outlet temperature of 55°C, whereas at pH 6.0 only a 0.6-log reduction was observed at 55°C. Higher pH values resulted in increased survival, which demonstrated that pH plays an important role in determining the inactivation kinetics.
FIG. 6.
Inactivation kinetics of L. innocua NCTC 11289 (A), S. cerevisiae SU 51 (B), E. coli NCTC 9001 (C), and L. plantarum LA 10-11 (D) in phosphate buffers at different pH values. Symbols: ■, pH 4.0; ■, pH 4.4; ⧫, pH 5.0; ●, pH 6.0. The electric field strength was 2.2 to 2.7 V/μm, the conductivity of the phosphate buffer was 0.78 S/m at an inlet medium temperature of 40°C, and the pulse length was 3.9 μs. The L. innocua NCTC 11289 inoculum contained 1.6 × 106 CFU/ml, the S. cerevisiae inoculum contained 106 CFU/ml, the E. coli inoculum contained 1.2 × 105 CFU/ml, and the L. plantarum inoculum contained 4.2 × 106 CFU/ml (pH 4.0) or 1.0 × 107 CFU/ml (pH 4.4). The results are means based on data from two experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; N0, number of cells before treatment.
The effects of PEF on S. cerevisiae SU 51, a representative eukaryotic microorganism, E. coli NCTC 9001, a representative gram-negative microorganism, and L. plantarum LA 10-11, another gram-positive microorganism, were also investigated (Fig. 6). The yeast S. cerevisiae SU 51 seemed to be the microorganism that was most sensitive to PEF treatment at pH 4.0 (Fig. 6B). The gram-positive bacteria L. plantarum LA 10-11 and L. innocua NCTC 11289 exhibited similar levels of resistance at pH 4.0 (Fig. 6A and D). However, it is noteworthy that another L. innocua strain, VBLLi 02-53, was much more resistant and its survival rate was 2.5 logs greater when it was tested under the same conditions (data not shown). The gram-negative organism E. coli NCTC 9001 and the gram-positive organism L. plantarum LA 10-11 exhibited similar levels of resistance at pH 4.4 (Fig. 6C and D). At pH 5.0, the yeast strain exhibited approximately the same level of resistance as the gram-positive organism L. innocua NCTC 11289 (Fig. 6A and B). In order to confirm that the pH effect was not due to the presence of Cl− ions, we performed some experiments with a 70 mM Na2HPO4 · 2H2O-NaH2PO4 · 2H2O buffer solution in which the final pH was adjusted to 4.0 with 4 N H2SO4. Similar inactivation of L. innocua NCTC 11289 was observed under these conditions (data not shown). Thus, the efficiency of PEF inactivation depended on the type of organism, the strain, and, in general, lower pH values.
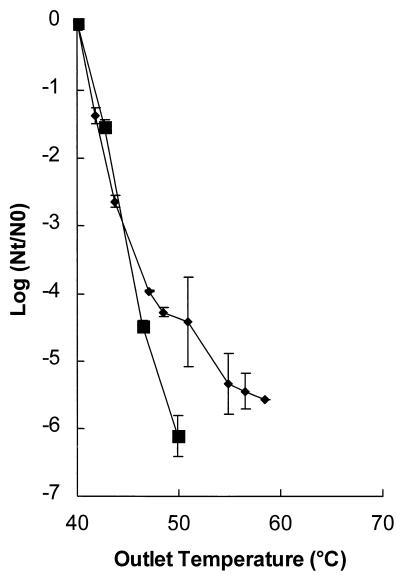
Effect of length of incubation of the inoculum on inactivation kinetics.
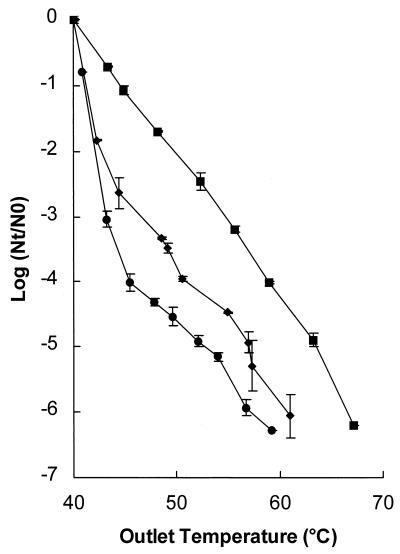
L. innocua NCTC 11289 inocula were incubated for two different lengths of time. In addition to incubation for 48 h, which was used in all of the other experiments, this organism was also incubated for only 12 h; during this time the cells were in the early stationary phase. A shorter incubation time resulted in a higher level of inactivation (Fig. 7). At an outlet temperature of 50°C a ≥6-log reduction was observed with 12 h of incubation, whereas only a 4.4-log reduction was observed with incubation for 48 h (P < 0.01). Thus, the physiological state of the microorganisms influenced the inactivation kinetics.
FIG. 7.
Inactivation of L. innocua NCTC 11289 after different growth times. Symbols: ■, 12 h; ⧫, 48 h. The electric field strength was 2.8 to 3.1 V/μm, the pH of the treatment medium was 5.0, the conductivity was 0.53 S/m at an inlet medium temperature of 40°C, and the pulse length was 3.9 μs. The contained 1.3 × 106 CFU/ml. The results are means based on data from two experiments, and standard deviations are indicated by error bars. Nt, number of survivors after treatment; N0, number of cells before treatment.
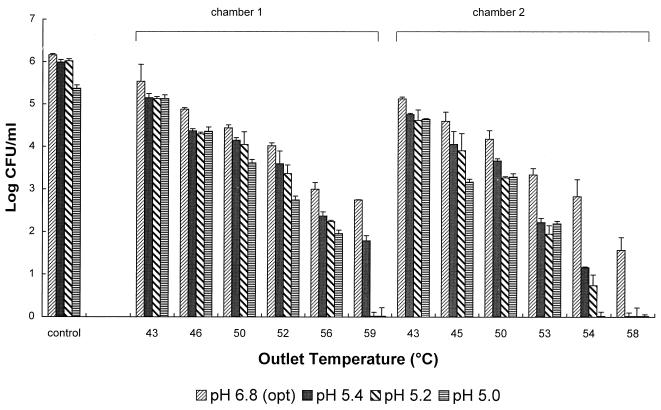
Sublethal injury after PEF treatment.
Sublethal injury of L. innocua NCTC 11289 was studied by plating a treated cell suspension onto media with different pH values (Fig. 8). Samples were collected after the first and second treatment chambers. The treatment protocol in both chambers was similar, and between the two chambers the treatment medium was cooled to 40°C. After PEF treatment with an outlet temperature of 59°C, no growth was observed on pH 5.2 outgrowth medium, whereas on the optimum growth medium (pH 6.8), 2.8 log survivors were observed. The effect of a lower pH of the outgrowth medium was less pronounced at lower outlet temperatures. The number of survivors was further reduced after the second treatment. Higher numbers of injured cells were found after the second treatment than after the first treatment, which was demonstrated by the lower rate of survival on the pH 5.0 outgrowth medium at an outlet temperature of 54°C. This indicates that the synergistic effects of PEF and temperature contributed to sublethal damage in L. innocua cells.
FIG. 8.
Survival of L. innocua NCTC 11289 after PEF treatment at 2.0 to 2.5 V/μm and different outgrowth medium pH values. The pH of the treatment medium was 5.0, the conductivity was 0.79 S/m at an inlet medium temperature of 40°C, and the pulse length was 3.9 μs. The results are means based on data from two experiments, and standard deviations are indicated by error bars. opt, optimum.
DISCUSSION
In this paper we describe in detail the effects of a range of product and process parameters in a continuous PEF system on the inactivation kinetics of microorganisms. Clearly, the electric field strength was the most important electrical parameter investigated that influenced the inactivation kinetics. The pulse length also influenced the inactivation kinetics, depending on the strength of the electric field. However, at higher electric field strengths, the effect of the pulse length became less pronounced, indicating that the relationship between energy input and pulse length depended on the electric field strength. Schoenbach et al. (17) reported that the electric energy in their batch PEF system required for lysing E. coli decreased with shorter pulse times, which were as short as 60 ns. However, these authors estimated that the electric field strengths required for a 1-order of magnitude reduction in the E. coli population would be 16 V/μm for 60-ns pulses, 10.7 V/μm for 300-ns pulses, and 6.6 V/μm for 2 μs pulses. These field strengths were very high and probably necessary to compensate for the reduced energy input with the shorter pulse lengths. Conversely, in our experiments, in which the field strength was kept constant and three different pulse lengths were used, the energy input necessary to obtain a certain level of reduction was higher with shorter pulse lengths.
In contrast to previous research (16), in several recent reports the authors demonstrated that the temperature of the medium at the start of a batch PEF treatment had a significant effect on the inactivation kinetics (4, 6, 13). Jayaram et al. (6) and Pothakamury et al. (13) found that PEF treatment performed at increased start temperatures increased the inactivation effect in a batch treatment system in which the temperature was kept constant. In our experiments with the continuous-treatment system, we examined the effects of different inlet medium temperatures, and when pulses were applied, the temperature automatically increased. Less energy was necessary to obtain a similar level of inactivation when the experiment was started with a higher medium temperature. Thus, our procedure allowed us to use the synergistic effects of the PEF treatment and the heat from the inlet medium and the effects generated by the pulses. On the other hand, if some factors in the food product were heat labile, for the sake of product quality it would be beneficial to start with lower inlet medium temperatures and use more pulses to obtain the necessary inactivation. It is important to realize that the lethal effect of PEF was not simply due to heat and that there was a real synergism between the two treatments. This was confirmed when the inactivation after a PEF treatment was compared to the inactivation after a similar time-temperature heat treatment, which demonstrated that 6-log-greater inactivation was obtained with PEF than with heat.
The electric resistivity of the treatment medium decreases as the ionic strength increases. Consequently, it is more difficult to build electric field strength when the conductivity of a medium or product is too high. Our equipment allowed us to treat solutions with conductivities up to approximately 1.0 S/m at 40°C, but higher conductivities resulted in arcing of the solution. Jayaram et al. (7) investigated inactivation of Lactobacillus brevis by using a batch system and solutions with various ionic strengths and observed that maximum inactivation was obtained in solutions with the lowest conductivity; they speculated that this was primarily due to conductivity influencing the membrane permeability. In our experiments, the electric field strength was kept constant by changing the conductivity of the buffer. However, we also observed that the inactivation rate was lower when the conductivity of the solution was higher. Thus, our results support the hypothesis that the conductivity of the medium or product is important in determining the inactivation rate for the continuous-treatment system.
In previous studies in which batch PEF systems were used the authors found that pH had no effect on inactivation of microorganisms (4, 16). However, Vega-Mercado et al. (18) used a continuous PEF system and observed that inactivation of E. coli was greater at pH 5.69 than at pH 6.82. Our results obtained with L. innocua NCTC 11289 and several other vegetative microorganisms confirmed this finding and demonstrated that the pH of the medium has a substantial positive effect on inactivation for a continuous PEF system. Recently, Liu et al. (9) demonstrated that high electric field pulses and the presence of benzoic or sorbic acid at pH 3.4 had a synergistic effect on killing E. coli O157:H7. Using the synergistic effects of low pH and organic acids or combinations of factors offers excellent possibilities for enhancing inactivation of spoilage microorganisms with the PEF technology. In the present study, complete inhibition of growth of L. innocua NCTC 11289 cells was observed when the pH of the outgrowth medium was 5.4 after PEF treatment with temperatures lower than the temperatures used for pasteurization.
Hülsheger and Niemann (3) demonstrated that when solutions containing chloride (Cl−) compounds were treated in a batch PEF system, hypochloric acid (HClO) was produced in a secondary step from a reaction of chlorine (Cl2) with water. The concentration of HClO, which is more bactericidal than Cl− ions, depended on the amount of chlorine in the solution and the pH. These authors suggested that this hypochloric acid contributed to inactivation by PEF, and they also noticed an increase in the pH of the solution following the electric pulses; they speculated that this increase was due to the production of undissociated HClO and the remaining Cl2, together with completely dissociated NaOH at the cathode. However, in our experiments, in which we used a continuous PEF system, an increase in the pH was never observed. Furthermore, the inactivation kinetics observed in our experiments performed with buffers containing chloride ions (HCl was deliberately added) were essentially the same as the inactivation kinetics observed when the pH was adjusted with acids which do not generate chloride ions. Thus, in our continuous PEF system the observed inactivation of the microorganisms and the synergistic effects at lower pH values were not due to creation of toxic compounds from chlorine ions present in the treatment solution.
In this study, we decided to work with cells which were in the stationary phase of growth, since these cells are generally more resistant to physical inactivation treatments. Indeed, this effect was demonstrated for PEF treatments, as younger L. innocua cultures were more sensitive to PEF inactivation. This is just one example of how the physiological state of a treated cell suspension can influence the inactivation kinetics. More research is necessary to establish the effects of different kinds of stresses on inactivation kinetics so that a safe preservation process can be designed.
In this research we demonstrated that if the correct parameters are used for PEF treatment, it is possible to completely and reproducibly inactivate an L. innocua strain. If only vegetative microorganisms are the major targets to be inactivated, PEF treatment is already a feasible alternative decontamination technique for low-pH products. Our experiments carried out with a simple buffer system allowed us to accurately assess the major parameters that influence inactivation kinetics. Based on the information obtained, the effects of PEF in real food systems are now being investigated.
ACKNOWLEDGMENTS
This research was supported in part by a grant from EU FAIR project 97-3044 (high electric field pulses: food safety, quality, and critical process parameters).
We thank Leon Gorris for critically reading the manuscript. We gratefully acknowledge Ad Bos and Alex Volanschi for helpful discussions throughout the study. We also thank Cees den Hollander, Sebo Poel, and Jan Siebesma for technical assistance with the PEF apparatus.
REFERENCES
- 1.Dunn J E, Pearlman J S. Methods and apparatus for extending the shelf life of fluid food products. U.S. patent 4,695,472. September 1987. [Google Scholar]
- 2.Gross D. Electromobile surface charge alters membrane and potential changes induced by applied electric fields. Biophys J. 1988;54:879–884. doi: 10.1016/S0006-3495(88)83024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hülsheger H, Niemann E G. Lethal effects of high-voltage pulses on E. coli K12. Radiat Environ Biophys. 1980;18:281–288. doi: 10.1007/BF01324271. [DOI] [PubMed] [Google Scholar]
- 4.Hülsheger H, Potel J, Niemann E-G. Killing of bacteria with electric pulses of high field strength. Radiat Environ Biophys. 1981;20:53–65. doi: 10.1007/BF01323926. [DOI] [PubMed] [Google Scholar]
- 5.Jacob H-E, Förster W, Berg H. Microbiological implications of electric field effects. Z Allg Mikrobiol. 1981;21:225–233. doi: 10.1002/jobm.3630210308. [DOI] [PubMed] [Google Scholar]
- 6.Jayaram S, Castle G S P, Margaritis A. Kinetics of sterilization of Lactobacillus brevis cells by application of high voltage pulses. Biotechnol Bioeng. 1992;40:1412–1420. doi: 10.1002/bit.260401116. [DOI] [PubMed] [Google Scholar]
- 7.Jayaram S, Castle G S P, Margaritis A. The effects of high field DC pulse and medium conductivity on survivability of Lactobacillus brevis. Appl Microbiol Biotechnol. 1993;40:117–122. [Google Scholar]
- 8.Kooiman W J, Geers J M. Simple and accurate technique for determination of heat resistance of bacterial spores. J Appl Bacteriol. 1975;38:185–189. doi: 10.1111/j.1365-2672.1975.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Yousef A E, Chism G W. Inactivation of Escherichia coli O157:H7 by the combination of organic acids and pulsed electric fields. J Food Safety. 1997;16:287–299. [Google Scholar]
- 10.Lojewska Z, Farkas D L, Ehrenberg B, Loew L M. Analysis of the effect of medium and membrane conductance on the amplitude and kinetics of membrane potentials induced by externally applied electric fields. Biophys J. 1989;56:121–128. doi: 10.1016/S0006-3495(89)82657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin O, Qin B L, Chang F J, Barbosa-Cánovas G V, Swanson B G. Inactivation of Escherichia coli in skim milk by high intensity pulsed electric fields. J Food Process Eng. 1997;20:317–336. [Google Scholar]
- 12.Mizuno A, Hori Y. Destruction of living cells by pulsed high-voltage application. IEEE (Inst Electr Electron Eng) Trans Ind Applic. 1988;24:387–394. [Google Scholar]
- 13.Pothakamury U R, Vega H, Zhang Q, Barbosa-Cánovas G V, Swanson B G. Effect of growth stage and processing temperature on the inactivation of E. coli by pulsed electric fields. J Food Protect. 1996;59:1167–1171. doi: 10.4315/0362-028X-59.11.1167. [DOI] [PubMed] [Google Scholar]
- 14.Qin B-L, Zhang Q, Barbosa-Cánovas G V, Swanson B G, Pedrow P D. Pulsed electric field treatment chamber design for liquid food pasteurization using a finite element method. Trans ASAE (Am Soc Agric Eng) 1995;38:557–565. [Google Scholar]
- 15.Qin B-L, Barbosa-Cánovas G V, Swanson B G, Pedrow P D, Olsen R G. Inactivating microorganisms using a pulsed electric field continuous treatment system. IEEE (Inst Electr Electron Eng) Trans Ind Applic. 1998;34:43–50. [Google Scholar]
- 16.Sale A J H, Hamilton W A. Effect of high electric fields on microorganisms. I. Killing of bacteria and yeast. Biochim Biophys Acta. 1967;148:781–788. [Google Scholar]
- 17.Schoenbach K H, Peterkin F E, Alden III R W, Beebe S J. The effect of pulsed electric fields on biological cells: experiments and applications. IEEE (Inst Electr Electron Eng) Trans Plasma Sci. 1997;25:284–293. [Google Scholar]
- 18.Vega-Mercado H, Pothakamury U R, Chang F-J, Barbosa-Cánovas G V, Swanson B G. Inactivation of Escherichia coli by combining pH, ionic strength and pulsed electric fields hurdles. Food Res Int. 1996;29:117–121. [Google Scholar]
- 19.Wouters P C, Smelt J P P M. Inactivation of microorganisms with pulsed electric fields: potential for food preservation. Food Biotechnol. 1997;11:193–229. [Google Scholar]
- 20.Yin Y, Zhang Q H, Sastry S K. High voltage pulsed electric field treatment chambers for the preservation of liquid food products. U.S. Patent 5,690,978. November 1997. [Google Scholar]
- 21.Zimmerman U. Electric breakdown, electropermeabilization and electrofusion. Rev Physiol Biochem Pharmacol. 1986;105:175–256. [PubMed] [Google Scholar]