Abstract
Random clones of 16S ribosomal DNA gene sequences were isolated after PCR amplification with eubacterial primers from total genomic DNA recovered from samples of the colonic lumen, colonic wall, and cecal lumen from a pig. Sequences were also obtained for cultures isolated anaerobically from the same colonic-wall sample. Phylogenetic analysis showed that many sequences were related to those of Lactobacillus or Streptococcus spp. or fell into clusters IX, XIVa, and XI of gram-positive bacteria. In addition, 59% of randomly cloned sequences showed less than 95% similarity to database entries or sequences from cultivated organisms. Cultivation bias is also suggested by the fact that the majority of isolates (54%) recovered from the colon wall by culturing were related to Lactobacillus and Streptococcus, whereas this group accounted for only one-third of the sequence variation for the same sample from random cloning. The remaining cultured isolates were mainly Selenomonas related. A higher proportion of Lactobacillus reuteri-related sequences than of Lactobacillus acidophilus- and Lactobacillus amylovorus-related sequences were present in the colonic-wall sample. Since the majority of bacterial ribosomal sequences recovered from the colon wall are less than 95% related to known organisms, the roles of many of the predominant wall-associated bacteria remain to be defined.
Our understanding of the complex natural communities of bacteria which colonize the intestines of monogastric mammals such as pigs and humans is still far from complete. While the better-studied gastrointestinal pathogens are of obvious importance, the roles of commensal anaerobic bacteria in host nutrition, colonic health, and gut development is becoming increasingly recognized (reviewed in references 8, 9, and 12). Culture-based methods have been used to identify and enumerate commensal components of the fecal flora and have revealed considerable species diversity (29, 30, 38). A small number of studies have concentrated on the bacteria present in the cecum or colon, including investigation of wall-associated populations (7, 33, 34, 36). Culture-based methods are extremely time-consuming, and since they only provide information on bacteria that are readily cultivated, they may give a biased view of microbial diversity. Increasingly, molecular methodologies that examine the diversity of the gut microflora independent of any cultural bias are being exploited (45). While molecular approaches based on PCR can introduce different types of bias (13, 39, 41), they provide powerful tools for revealing the true phylogenetic diversity of microorganisms within environmental samples (22, 25, 26, 42, 46).
The present work is the first attempt to identify the main types of bacteria present by direct retrieval and analysis of small-subunit ribosomal DNA (rDNA) sequences, free of cultural bias, and to compare these with cultured isolates from the pig colon. We report an analysis of the adherent population of the colonic wall and of the colonic and cecal luminal contents. We show here that a significant fraction of the microbial diversity, particularly in the colonic-wall sample, is only distantly related to cultured bacterial types whose sequences are available through the databases. Phylogenetic analysis is used to determine the positions of the unidentified bacterial strains relative to known rDNA sequences.
MATERIALS AND METHODS
Bacterial strains.
The origins of the stock Prevotella and Bacteroides spp. have been described previously (40, 47).
Collection and processing of samples.
Colonic samples were obtained initially from three adult feeder pigs (4 to 5 months old; 46 to 55 kg) obtained from the Rowett Research Institute. Samples from pig 1 only were used for microbiological isolations and molecular analysis by random 16S rDNA cloning. These pigs were fed a standard diet of wheat (40%), barley (28.25%), soybean meal (20%), white fishmeal (5%), soybean oil (2.0%), molasses (2.0%), vitamin-mineral supplement (2.0%), and salt (0.75%). The pigs were not fed after 10 p.m. the evening before they were sacrificed.
A 10- to 20-cm-long section of pig colon (1 m from the cecum) and the entire cecum were ligated and removed aseptically. The luminal contents or sections of tissue (about 5 cm2) were transferred into preweighed Universal bottles containing 9 ml of anaerobic diluting fluid consisting of the anaerobic rumen fluid-containing medium 2 of Hobson (20) prepared under O2-free CO2 (5) and modified by the omission of sugars and lactate. The samples were vortex mixed to break down bacterial clumps and to remove loosely attached bacteria from the tissue sections. The tissue was then quickly transferred, in an anaerobic chamber (Coy Laboratory Products Inc., Grass Lake, Mich.) in an atmosphere of 55% CO2, 40% N2, and 5% H2, to a second, similar bottle containing anaerobic diluent and disintegrated by homogenization (top-bladed homogenizer; Silverson, Chesham, United Kingdom) to release firmly adherent bacteria.
Bacterial analyses.
Anaerobic roll tubes (5) were prepared in 16- by 125-mm Hungate tubes sealed with butyl septum stoppers (Bellco Glass Inc., Vineland, N.J.) with M2GSC medium (28) and inoculated with 0.5-ml aliquots of appropriate serial dilutions of the samples. Undiluted aliquots (1 ml) were dispensed into 1.5-ml Eppendorf tubes and centrifuged (13,000 × g; 10 min) to pellet the bacteria, which were stored at −70°C prior to DNA extraction. The roll tubes were incubated at 38°C for 48 h, after which 20 colonies were picked from each colonic-sample site and 50 colonies were picked from the cecal-lumen samples. Many cecal-lumen samples proved extremely difficult to purify to homogeneity, and effort was therefore concentrated on obtaining pure cultures from the colonic-wall isolates. Cultures picked and grown in broths of M2GSC were used for examination of gram-stained smears, for inoculation of API test strips (under 100% CO2), for DNA extraction (API 20A; BioMerieux Ltd., Basingstoke, United Kingdom), and for determination of fermentation products by capillary gas chromatography (32).
DNA extraction.
DNA was extracted from the frozen pellets by a modification of the method of Stahl et al. (40). Pellets of collected material were thawed on ice and resuspended in 1 ml of sterile distilled water. The bacterial suspension was transferred to a capped tube containing sterile zirconium beads (0.1-mm diameter) so that the tube was completely filled. The samples were beaten for 30 s followed by chilling on ice for at least 1 min on a minibead beater (Biospec Corporation, Statech Scientific, Luton, United Kingdom); this procedure was repeated until the supernatant appeared clear. The supernatant was extracted twice with phenol-chloroform-isoamyl alcohol (25:24:1) and ethanol precipitated. The DNA pellets were resuspended in sterile distilled water, and humic material present within the samples was removed with the wizard DNA purification kit (Promega) subsequent to PCR.
Control experiments were performed in which cultures of gram-positive (Enterococcus faecalis JH2-SS, kindly provided by Abigail Salyers, University of Illinois) and gram-negative (Bacteroides vulgatus 1447) bacteria were mixed in different proportions before DNA extraction by the method described above. PCR amplification confirmed that there was no significant difference from the bead-beating method in either the yield of DNA obtained or amplification of the genomic DNA from both gram-positive and gram-negative organisms (results not shown). This indicated that the method showed no bias in DNA extraction between gram-positive and gram-negative organisms. Comparison of the bead-beating method with a Triton X-100 lysis procedure (43) confirmed that bead beating yielded significantly higher amounts of DNA from both gram-positive and gram-negative bacteria.
Random cloning of isolated DNA.
Total genomic DNA (50 ng) was used as a target for amplification of approximately 720 bp of 16S rDNA with the eubacterial primers P3-Mod (5′ CGCGCCGCATTAGATACCCTDGTAGTCC 3′ [Escherichia coli positions 787 to 814]) and PC5 (5′ GCGGCCGCTACCTTGTTACGACTT 3′ [E. coli positions 1515 to 1492]) (45). Amplification was carried out as described previously (16). 20 cycles of PCR were used to minimize the risk of certain 16S rDNA types being preferentially amplified.
Amplified PCR products were obtained from different regions of the pig gut, purified with the Wizard PCR product purification kit (Promega), and then cloned into a pGEM-T vector plasmid (Promega). Ligation was done at 16°C overnight followed by transformation into competent E. coli JM109 cells. The clones were screened for α-complementation of β-galactosidase by using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactopyranoside).
Positive clones were selected from each region of the pig colon and cecum and grown in Luria broth with ampicillin selection. Plasmid DNA was isolated from 20 selected positive clones of each gut region by a plasmid DNA miniprep procedure (10). Insert DNA was sequenced with the automated ABI 373A sequencer. The sequence reactions employed the P3-Mod and PC5 primers and the PRISM Ready Reaction DyeDeoxy Terminator cycle-sequencing kit (Perkin-Elmer). Sequences were determined in the same manner for DNA isolated from 20 pure cultures obtained by conventional microbiology. Only isolates and clones yielding unambiguous sequence data were included in Table 1.
TABLE 1.
Microbial diversity of the pig colon from clones of PCR-amplified rDNA
| Clone(s) sequenced | No. (%) froma:
|
|||
|---|---|---|---|---|
| Isolated cultures (Co wall) | Random cloning of 16S rDNA
|
|||
| Co wall | Co lumen | Ca lumen | ||
| >95% homologous to EMBL database | 15 (88) | 10 (42) | 9 (53) | 6 (30) |
| Unidentified | 1 (6) | 0 | 0 | 1 (5) |
| Lactobacillus sp. | 6 (35) | 6 (25) | 2 (12) | 5 (25) |
| Streptococcus sp. | 3 (17) | 2 (8) | 5 (29) | 0 |
| Ruminococcus sp. | 0 | 0 | 1 (6) | 0 |
| Eubacterium sp. | 0 | 0 | 1 (6) | 0 |
| Clostridium sp. | 0 | 2 (8) | 0 | 0 |
| Selenomonas sp. | 5 (30) | 0 | 0 | 0 |
| >90% and <95% homologous to database sequences | 1 (6) | 11 (46) | 7 (41) | 11 (55) |
| <90% homologous to database sequences | 1 (6) | 3 (12) | 1 (6) | 3 (15) |
| Total | 17 | 24 | 17 | 20 |
Co, colonic samples; Ca, cecal sample.
Specific PCR amplification of Bacteroides and Prevotella spp.
The Bacteroides- and Prevotella-specific primer BacPre(2) reverse complement, rBacPre (5′ TCACCGTTGCCGGCGTACTC 3′; positions 887 to 907), was used in combination with the universal eubacterial primer fD1 (5′ AGAGTTTGATCCTGGCTCAG 3′; positions 7 to 26) (44) to amplify a portion of the 16S rDNA gene. The amplification protocol was described previously (47).
PCR-restriction fragment length polymorphism (RFLP) analysis.
PCR products were digested to completion with the enzyme HhaI and analyzed by electrophoresis in a 1.5% agarose gel.
Phylogenetic analysis.
The partial rDNA sequences corresponding to E. coli 16S rRNA bases 1197 to 1492 (4) were compared directly with the EMBL and GenBank nonredundant nucleotide databases by using BLAST (18) and the ribosomal database project (27). All sequences from this study contained identified bases; however, some database sequences contained internal “N” bases. The most similar sequences were then directly aligned with the cloned sequences over equalized lengths to determine the percentage of identity.
Sequences derived from previously cultured and described organisms of known phylogeny which corresponded to major subdivisions of the domain Bacteria were included in the phylogenetic analysis of the pig data. The sequence data approximating to E. coli positions 1197 to 1492 were aligned with CLUSTAL V (19), and a phylogenetic tree was generated with software from the PHYLIP package (15). The DNADIST program analyzed distance with the Kimura-Nei correction (24); trees were generated from distance matrices by the neighbor-joining method (37). Sequence data for the distance matrix and analysis were subjected to bootstrap resampling (data were resampled 100 times) with the SEQBOOT program, and consensus trees were generated by the CONSENSE program (14).
Nucleotide sequence accession numbers.
The 16S rDNA gene sequences of clones and isolates used in phylogenetic analysis have been deposited in the EMBL data library under accession no. AJ241718 to AJ241786. The reference strains used in phylogenetic analysis were also from the EMBL database.
RESULTS
16S rDNA sequence diversity of the pig hindgut microflora.
Samples of porcine colonic wall (cow), colonic lumen (col), and cecal lumen (cal) were obtained as described in Materials and Methods. Total bacterial counts (CFU per gram [wet weight]) estimated on anaerobic rumen fluid medium M2GMC were 8.8 × 108, 2.3 × 1010, and 5.3 × 1010 for cow, col, and cal samples, respectively. These numbers are similar to those reported by previous investigators (1, 34, 35).
Part of each sample was used for extraction of nucleic acids, by bead beating followed by phenol-chloroform extraction and Wizard microcolumn purification as described in Materials and Methods. Extracted nucleic acid from samples was then used as the template for amplification by PCR of a region of approximately 720 bp of the 16S rRNA gene with primers designed to recognize all eubacterial 16S sequences (45). Amplified material was cloned into a plasmid vector, and approximately 20 clones from each sample were subjected to partial sequencing. Sequence analysis revealed that all sequences showed conserved regions typical of eubacterial 16S rDNA, and no chimeric clones were found with the program CHECK (27).
The presumptive relationships of these sequences were obtained from database comparisons. In Table 1, sequences that show more than 95% identity with existing EMBL-GenBank database entries have been assigned to the closest genus, and on this basis the largest identifiable group of sequences belonged to Lactobacillus and Streptococcus spp. On the other hand, a very significant proportion of the sequences from all three samples (58% for cow, 47% for col, and 70% for cal) showed less than 95% relatedness to database sequences from cultured bacteria.
Analysis of cultured isolates from the colonic wall.
Seventeen isolates were obtained in pure culture from the colon wall samples. Fifteen of these 17 isolates showed >95% homology in their 16S rDNA sequences to sequences in the EMBL database. These isolates included one unidentified gram-negative rod and three streptococci; the remaining strains were identified as either lactobacilli or selenomonads based on morphological and biochemical data and on their 16S rDNA sequences (see Materials and Methods). The five Selenomonas-like isolates (*cow 6, *cow 8, *cow 11, *cow 15, and *cow 17) were indole-, gelatin-, and urease-negative gram-negative curved rods which produced acetate, propionate, and lactate as fermentation products. These characteristics are typical of Selenomonas strains.
Five of the Lactobacillus isolates (*cow 2, *cow 4, *cow 10, *cow 18, and *cow 19) were gram-positive rods which produced predominantly lactic acid and showed 99 to 100% homology with Lactobacillus reuteri. Sequence analysis suggested that the sixth Lactobacillus isolate, cow 3, was related to Lactobacillus amylovorus; the culture characteristics were not determined.
Comparison with the data obtained from the parallel analysis of randomly cloned sequences from the colonic wall (Table 1) showed that the random cloning revealed a greater diversity of sequences. The sequences that have no close relatives in database searches are present almost exclusively in the randomly cloned material. This would be predicted if a proportion of isolates from this sample were difficult to culture under the conditions used.
Phylogenetic analysis.
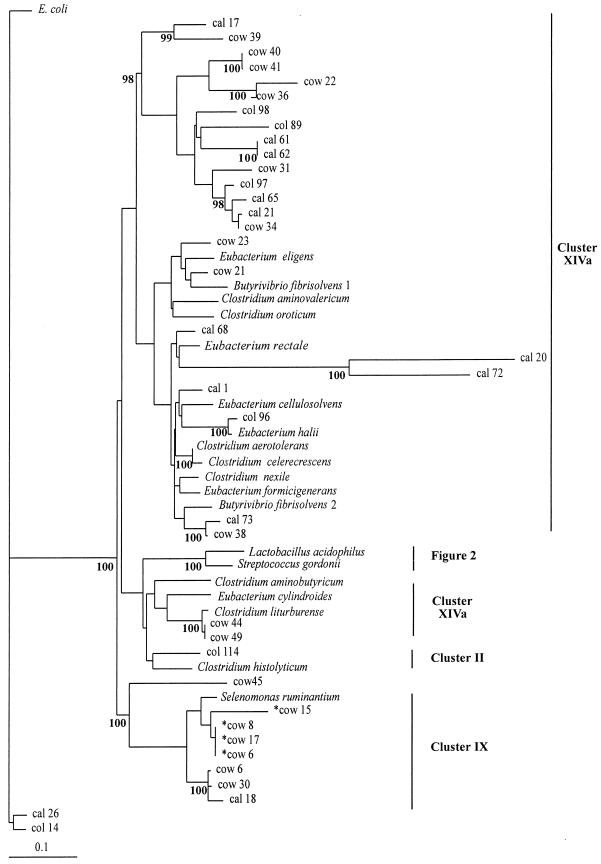
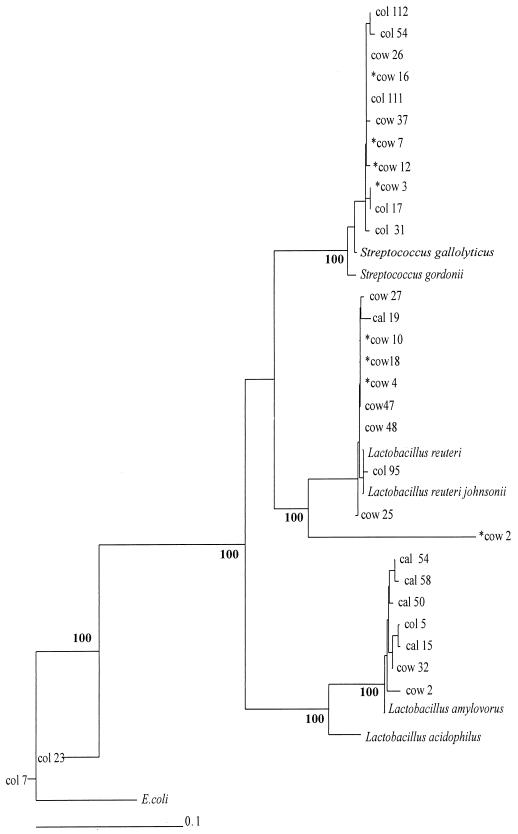
Sixty-seven sequences from this study, including 12 from cultured isolates obtained from the colonic wall, were used to construct phylogenetic trees by the neighbor-joining method, as shown in Fig. 1 and 2. Reference sequences were also included, in particular, those identified as being the closest relatives of the cloned sequences in database searches. For the Lactobacillus and Streptococcus group (Fig. 2), the randomly cloned sequences, sequences for cultured isolates, and database sequence entries are generally interspersed, indicating that the porcine isolates are quite close relatives of well-recognized species. Interestingly, while seven of the nine L. reuteri-like sequences were from the colonic-wall sample, only two of seven L. amylovorus and Lactobacillus acidophilus sequences were from the colonic wall.
FIG. 1.
Phylogenetic tree showing 16S rDNA sequences from porcine hindgut samples for low-G+C-content bacteria. The tree was constructed by neighbor-joining analysis of a distance matrix obtained from a multiple-sequence alignment. Bootstrap values (expressed as percentages of 100 replications) are shown at branch points: values of 90% or more were considered significant. Sequences derived from the database are shown in italics. The cultured isolates from the colonic wall are prefixed by asterisks. E. coli is used as the outgroup sequence. The scale bar represents substitutions per 100 nucleotides.
FIG. 2.
Phylogenetic tree showing 16S rDNA sequences from porcine hindgut samples, for Streptococcus- and Lactobacillus-related sequences. The tree was constructed by neighbor-joining analysis of a distance matrix obtained from a multiple-sequence alignment. Bootstrap values (expressed as percentages of 100 replications) are shown at branch points: values of 90% or more were considered significant. Sequences derived from the database are shown in italics. The cultured isolates from the colonic wall are prefixed by asterisks. E. coli is used as the outgroup sequence. The scale bar represents substitutions per 100 nucleotides.
The remaining sequences show a general relationship with other groups of low-G+C-content gram-positive bacteria, in particular Clostridium and Eubacterium spp. (Fig. 1). Twenty-six sequences fall into cluster XIVa, which includes many Clostridium spp. together with Eubacterium spp., Butyrivibrio spp., Peptostreptococcus spp., and Ruminococcus spp. (6). At least seven sequences fall into a heterogeneous group of organisms including Selenomonas sp. and Megasphaera sp. (cluster IX) (6). Cluster IX contains mainly representatives from the colonic wall. A large number of the cloned sequences, however, occupy branches in the phylogenetic tree that contain few if any cultured strains. In particular, a major cluster comprising 15 sequences (including 7 from the colonic wall) is apparently not related to sequences from cultured organisms. This implies that these sequences belong to bacterial groupings that are seriously underrepresented within the sequence databases, either because they belong to cultured organisms that have received little attention from molecular taxonomists or because they belong to organisms that are difficult to culture or that remain so far uncultured. Cultivation bias is also suggested by the fact that Streptococcus and Lactobacillus species were the most abundant among the sequences from cultured isolates while they accounted for only 35% of the randomly cloned sequence diversity (Table 1). Furthermore, the only other group of isolates recovered by cultivation from the colonic wall were Selenomonas spp., whereas the sequence analysis (Fig. 1) indicates a far greater degree of bacterial diversity.
Although sequences from a particular site were found in many branches of the phylogenetic trees, there was some evidence of clustering, especially among the colonic-wall isolates. This would be consistent with the expectation that bacteria of particular phylogenetic groups occupy particular niches or microhabitats within the gut.
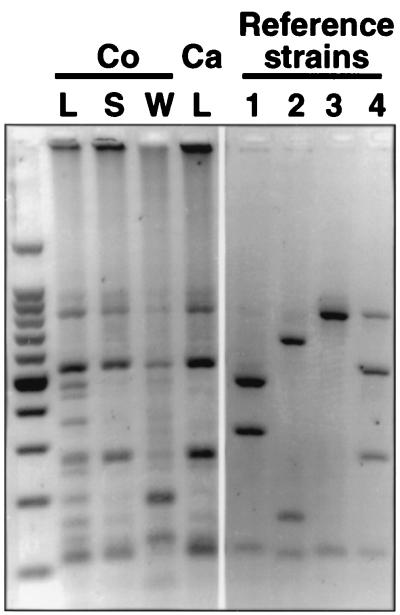
In previous studies of the pig colonic flora (34, 36, 38, 47) Bacteroides spp. accounted for 2.4 to 12.5% of the colonic flora. Our limited analysis of the cloned isolates did not indicate the presence of Bacteroides species. However, PCR amplification of DNA extracted from the colonic lumen, wall, and surface and cecal lumen with Bacteroides- and Prevotella-specific primers (47) and subsequent RFLP analysis revealed that this bacterial group could be detected and profiled in our study (Fig. 3). It seems that Bacteroides sp. representatives were present within our random cloned isolates but not identified in our limited study. Figure 3 further indicates differences between the predominant Bacteroides and Prevotella ribotypes in the colonic wall and lumen. Although profiles for only one pig are shown in Fig. 3, comparable profiles were obtained with samples from another two pigs (data not shown).
FIG. 3.
PCR-RFLP profiles of Bacteroides and Prevotella strains from different sites in the pig hindgut digested with restriction endonuclease HhaI. Regions of extracted DNA are denoted as colonic (Co) lumen (L), wall (W), and wall surface (S) and cecal (Ca) lumen. The reference strains are as follows: lane 1, Prevotella ruminicola 23; lane 2, Prevotella albensis M384; lane 3, Bacteroides uniformis 1100; lane 4, Bacteroides thetaiotaomicron 5482. Some residual uncut material is present in lane 4.
DISCUSSION
To our knowledge, this is the first application of 16S rDNA sequence analysis to the pig gut microflora. Previous studies have defined the culturable diversity of the normal flora of pig feces, colonic wall, and cecum (10, 31, 33, 34). The aim of the present study, which examines samples from a single animal, was not to survey the diversity of the porcine flora but to compare the information gained from cultural and molecular analyses. Studies of a larger number of pigs would be required to test the generality of the conclusions discussed here.
The isolates obtained here from the colonic-wall sample are entirely consistent with expectations from earlier work. Previous culture-based studies have suggested that gram-positive bacteria dominate the pig colonic flora under normal conditions, with lactobacilli and streptococci accounting for 40% of fecal isolates (30) and 60% of isolates adherent to the colonic wall (33, 34). Lactobacillus fermentum was reported to be the predominant lactobacillus adherent to the colon wall of the pigs (34), but distinction between L. fermentum and L. reuteri is difficult by physiological tests alone (23). Streptococcus intestinalis (35), to which three strains from the present study show significant homology, was first isolated (as Streptococcus sp. group U-2) from the colonic epithelia of normal pigs (34), where it accounted for over 50% of the cultivable flora. Selenomonas spp. were detected in all of these studies as a significant proportion of the microflora, particularly in the cecum (33, 34). Bacteroides and Prevotella species are the most abundant group of gram-negative bacteria recovered previously, being particularly common in the cecum (34, 36, 38). This group was not detected among the predominant cultured isolates from the animal studied here, although its presence was demonstrated by PCR amplification with primers selective for the Bacteroides and Prevotella grouping (47) (Fig. 3) and subsequent RFLP analysis. Possibly, the Bacteroides and Prevotella species represent a small proportion of the microflora in the hindguts of the pigs studied here and could only be detected with specific primers. It has been shown that variations occur between the cultivable bacterial gut flora of different pigs, even when dietary variations are avoided (30, 34, 36, 38). On the other hand, although precautions were taken to minimize PCR bias, it is also possible that some bacterial groups were preferentially amplified with the universal primer combination. To resolve this question would require using alternative molecular methods, such as dot blot or whole-cell in situ hybridization to quantify the abundance of the sequences due to different bacteria in the pig gut.
The main conclusion of the present study is that the culture approach, despite employing strictly anaerobic conditions, failed to reflect much of the diversity present in the colonic-wall sample and is likely to overestimate the contributions of certain groups, including lactobacilli and streptococci and possibly Selenomonas, to the flora. Furthermore, at least one important cluster, and several minor clusters, that include many sequences from the colonic wall remain unidentified or perhaps even uncultured. These findings have profound implications for the use of probiotics in pigs (21), in particular, whether existing laboratory isolates are the most appropriate to use in attempts to adjust the intestinal microflora. The use of a wider range of culture media (1) might yet reveal the presence of further cultivatable species, and there is also a case for 16S rDNA sequencing of existing culture collection isolates from pigs.
When applied to human fecal microflora, similar approaches have also suggested that a somewhat greater diversity of organisms is present than is recovered by culture approaches, but the discrepancy was not as great as that reported here (45). The human colonic microflora, as reflected by culturable fecal bacteria, has probably been better studied than that of the pig. On the other hand the contrast found here between the microflora of the colonic wall and that of the colonic and cecal lumen provides a strong indication that the fecal microflora, even if it reflects the microflora of the colonic lumen accurately, may not reflect that of the colonic wall. The bacterial communities associated with the intestinal wall are potentially of great significance because this is the main site of interaction with the host (3). The majority of bacterial pathogens, both invasive and noninvasive, attach to the mucosa or to mucus, while the nonpathogenic bacterial populations are assumed to compete for attachment sites and to be important in defense against pathogens. The intestinal wall is also the site of interactions with the immune system (17) and of nutritional and metabolic interactions between bacteria and the host, as well as between different bacteria represented in what may constitute an attached biofilm. The finding that the majority of bacterial ribosomal sequences recovered from this site in the pig are not closely related to known organisms could have profound implications for the nutrition and health of the host.
ACKNOWLEDGMENTS
We thank Sylvia Duncan for the culture counts and for help and guidance with culture characterization, Jenny Martin for the processing of the samples, and Gail Skene for access to unpublished data on some of the isolates.
This work was supported by a Scottish Office Agriculture, Environment and Fisheries Department (SOAEFD) flexible fund grant.
REFERENCES
- 1.Allison M J, Robinson I M, Bucklin J A, Booth G D. Comparison of bacterial populations of the pig cecum and colon based upon enumeration with specific energy sources. Appl Environ Microbiol. 1979;37:1142–1151. doi: 10.1128/aem.37.6.1142-1151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avgustin G, Wright F, Flint H J. Genetic diversity and phylogenetic relationships among strains of Prevotella (Bacteroides) ruminicola from the rumen. Int J Syst Bacteriol. 1994;47:284–288. doi: 10.1099/00207713-44-2-246. [DOI] [PubMed] [Google Scholar]
- 3.Bengmark S. Econutrition and health maintenance—a new concept to prevent GI inflammation, ulceration and sepsis. Clin Nutr. 1996;15:1–10. doi: 10.1016/s0261-5614(96)80253-6. [DOI] [PubMed] [Google Scholar]
- 4.Brosius J, Palmer M L, Kennedy P J, Noller H F. Complete nucleotide sequence of a 16S ribosomal gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant M P. Commentary on the Hungate technique for cultivation of anaerobic bacteria. Am J Clin Nutr. 1972;25:1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- 6.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 7.Croucher S C, Houston A P, Bayliss C E, Turner R J. Bacterial populations associated with different regions of the human colon wall. Appl Environ Microbiol. 1983;45:1025–1033. doi: 10.1128/aem.45.3.1025-1033.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings J H, MacFarlane G T. Colonic microflora: nutrition and health. Nutrition. 1997;13:476–478. doi: 10.1016/s0899-9007(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J H, MacFarlane G T. Role of intestinal bacteria in nutrient metabolism. Clin Nutr. 1997;16:3–11. [Google Scholar]
- 10.Doyle K, editor. Promega protocols and applications guide. 3rd ed. Madison, Wis: Promega; 1996. [Google Scholar]
- 11.Durmic Z, Pethick J R, Hampson D J. Changes in bacterial populations of pigs fed different sources of dietary fibre, and the development of swine dysentery after experimental infection. J Appl Microbiol. 1998;85:574–582. doi: 10.1046/j.1365-2672.1998.853539.x. [DOI] [PubMed] [Google Scholar]
- 12.Edwards C A, Gibson G, Champ M, Jensen B B, Mathers J C, Nagengast F, Rumney C, Quehl A. In vitro method for quantitation of the fermentation of starch by human fecal bacteria. J Sci Food Agric. 1996;71:209–217. [Google Scholar]
- 13.Farrelly V, Rainey F A, Stackebrandt E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl Environ Microbiol. 1995;61:2798–2801. doi: 10.1128/aem.61.7.2798-2801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein J. PHYLIP—Phylogeny Inference Package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 16.Frothingham R, Allen R L, Wilson K H. Rapid 16S ribosomal DNA sequencing from a single colony without DNA extraction or purification. BioTechniques. 1991;11:40–44. [PubMed] [Google Scholar]
- 17.Gaskins H R. Immunological aspects of host/microbiota interactions at the intestinal epithelium. In: Mackie R I, White B A, Isaacson R E, editors. Gastrointestinal microbiology. Vol. 2. New York, N.Y: Chapman and Hall; 1996. pp. 537–587. [Google Scholar]
- 18.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 19.Higgins D G, Bleasby A J, Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 20.Hobson P N. Rumen bacteria. Methods Microbiol. 1969;3B:133–149. [Google Scholar]
- 21.Jonsson E, Conway P L. Probiotics for pigs. In: Fuller R, editor. Probiotics. London, United Kingdom: Chapman and Hall; 1992. pp. 260–316. [Google Scholar]
- 22.Jurgens G, Linstrom K, Saano A. Novel group within the kingdom Crenarchaeota from boreal forest soil. Appl Environ Microbiol. 1997;63:803–805. doi: 10.1128/aem.63.2.803-805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandler O, Weiss N. Genus Lactobacillus Beijerinck 1901, 212AL. In: Sneath P H A, Mair N S, Sharpe M E, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams and Wilkins; 1986. pp. 1209–1234. [Google Scholar]
- 24.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones G, Lau P C K. A molecular view of microbial diversity in a dynamic landfill in Québec. FEMS Microbiol Lett. 1998;172:219–226. doi: 10.1111/j.1574-6968.1998.tb13002.x. [DOI] [PubMed] [Google Scholar]
- 26.MacGregor B J, Moser D P, Alm E W, Nealson K H, Stahl D A. Crenarchaeota in Lake Michigan sediments. Appl Environ Microbiol. 1997;63:1178–1181. doi: 10.1128/aem.63.3.1178-1181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maidak B L, Larsen N, McCaughey M J, Overbeek R, Olsen G J, Forgel K, Blandy J, Woese C R. The ribosomal database project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki K, Martin J C, Marinsek-Logar R, Flint H J. Degradation and utilization of xylans by the rumen anaerobe Prevotella bryantii (formerly P. ruminicola subsp. brevis) B14. Anaerobe. 1997;3:373–381. doi: 10.1006/anae.1997.0125. [DOI] [PubMed] [Google Scholar]
- 29.Moore W E C, Holdeman L V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Environ Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore W E C, Moore L V H, Cato E P, Wilkins T D, Kornegay E T. Effect of high-fiber and high-oil diets on the fecal flora of swine. Appl Environ Microbiol. 1987;53:1638–1644. doi: 10.1128/aem.53.7.1638-1644.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore W E C, Moore L H. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61:3202–3207. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson A J, Calder A G, Stewart C S, Smith A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett Appl Microbiol. 1989;9:5–8. [Google Scholar]
- 33.Robinson I M, Allison M J, Bucklin J A. Characterization of the cecal bacteria of normal pigs. Appl Environ Microbiol. 1981;41:950–955. doi: 10.1128/aem.41.4.950-955.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson I M, Whipp S C, Bucklin J A, Allison M J. Characterization of predominant bacteria from the colons of normal and dysenteric pigs. Appl Environ Microbiol. 1984;48:964–969. doi: 10.1128/aem.48.5.964-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson I M, Stromley J M, Varel V H, Cato E P. Streptococcus intestinalis, a new species from the colons and feces of pigs. Int J Syst Bacteriol. 1988;38:245–248. [Google Scholar]
- 36.Russell E G. Types and distribution of anaerobic bacteria in the large intestine of pigs. Appl Environ Microbiol. 1979;37:187–193. doi: 10.1128/aem.37.2.187-193.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitou N, Nei M. The neighbor joining method: a new method for constructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 38.Salanitro J P, Blake I G, Muirhead P A. Isolation and identification of fecal bacteria from adult swine. Appl Environ Microbiol. 1977;33:79–84. doi: 10.1128/aem.33.1.79-84.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stackebrandt E, Grobel B M. A place for DNA-DNA reassociation and 16S ribosomal-RNA sequence analysis in the present species identification in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 40.Stahl D A, Flesher B, Mansfield H R, Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki M T, Giovanni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voordouw G, Armstrong S M, Reimer M F, Fouts B, Telang A J, Shen Y, Gevertz D. Characterization of 16S rRNA genes from the oil field microbial communities indicates the presence of a variety of sulfate-reducing, fermentative, and sulfide-oxidizing bacteria. Appl Environ Microbiol. 1996;62:1623–1629. doi: 10.1128/aem.62.5.1623-1629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang R F, Cao W W, Cernaglia C E. PCR detection and quantitation of predominant and anaerobic bacteria in human and animal fecal samples. Appl Environ Microbiol. 1996;62:1242–1247. doi: 10.1128/aem.62.4.1242-1247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson K H, Blitchington R B. Human colonic biota studied by ribosomal DNA sequence analysis. Appl Environ Microbiol. 1996;62:2273–2278. doi: 10.1128/aem.62.7.2273-2278.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wise M G, McArthur J V, Shimkets L J. Bacterial diversity of a Carolina bay as determined by 16S rRNA gene analysis: confirmation of novel taxa. Appl Environ Microbiol. 1997;63:1505–1514. doi: 10.1128/aem.63.4.1505-1514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood J, Scott K P, Avgustin G, Newbold C J, Flint H J. Estimation of the relative abundance of different Prevotella ribotypes in gut samples by restriction enzyme profiling of PCR-amplified 16S rRNA gene sequences. Appl Environ Microbiol. 1998;64:3683–3689. doi: 10.1128/aem.64.10.3683-3689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]