Abstract
We studied phosphopeptidomannans (PPMs) of two Saccharomyces cerevisiae NCYC 625 strains (S. diastaticus): a wild type strain grown aerobically, anaerobically, and in the presence of antimycin and a [rho0] mutant grown aerobically and anaerobically. The aerobic wild-type cultures were highly flocculent, but all others were weakly flocculent. Ligands implicated in flocculation of mutants or antimycin-treated cells were not aggregated as much by concanavalin A as were those of the wild type. The [rho0] mutants and antimycin-treated cells differ from the wild type in PPM composition and invertase, acid phosphatase, and glucoamylase activities. PPMs extracted from different cells differ in the protein but not in the glycosidic moiety. The PPMs were less stable in mitochondrion-deficient cells than in wild-type cells grown aerobically, and this difference may be attributable to defective mitochondrial function during cell wall synthesis. The reduced flocculation of cells grown in the presence of antimycin, under anaerobiosis, or carrying a [rho0] mutation may be the consequence of alterations of PPM structures which are the ligands of lectins, both involved in this cell-cell recognition phenomenon. These respiratory chain alterations also affect peripheral, biologically active glycoproteins such as extracellular enzymes and peripheral PPMs.
Yeast cell wall composition and organization depend on the strain, the culture medium, and growth conditions. Saccharomyces cerevisiae is a fermentative yeast that generates ATP by glycolysis in aerobiosis. The cell wall of this yeast consists of approximately equal amounts of glucans and mannans and a small amount of chitin. The mannans are highly branched polymers that often are phosphorylated (1) and linked to proteins by N- or O-glycosidic linkages. They are commonly termed phosphopeptidomannans (PPMs).
Yeast flocculation is a natural active process of reversible cell-cell aggregation resulting from a lectin-like interaction (23). Lectins are carbohydrate-binding proteins other than enzymes or antibodies. A mannose-specific agglutinin has recently been extracted from Saccharomyces cell walls (29). The ligands of this lectin are the cell wall PPMs, which must have a specific structure to be recognized by the lectin (34).
Yeast cell wall synthesis, flocculation, and mitochondrial function are interrelated. Flocculation requires mitochondrial function and the synthesis of cytoplasmic proteins (2). Cells treated with drugs that inhibit mitochondrial function (7) or cells that carry deletions in the mitochondrial genes oli1 and oxi2 (14) have reduced or no flocculation abilities. Aerobically and anaerobically grown strains differ in cell wall structure (37), probably at the PPM level. Glycoproteins are synthesized and glycosylated intracellularly and transported to the cell surface via a secretory route. If mitochondria are involved in cell wall synthesis, then they will also indirectly affect cell wall composition, structure, and flocculation. However, differences in PPMs attributable to differences in mitochondrial function have not been described.
Our objectives in this study were (i) to determine if mitochondrial mutations or inhibition of the respiratory chain results in structural modifications of PPMs, (ii) to determine if PPMs have the same type and level of lectin-binding activity, and (iii) to determine if the enzymatic activity of the cell wall-associated glycoproteins is altered by changes in mitochondrial function.
MATERIALS AND METHODS
Strains.
Wild-type S. cerevisiae NCYC 625 (formerly S. diastaticus [9]) and a [rho0] mutant induced with ethidium bromide (31) were provided by J. P. Guiraud, GBS-A-Microbiologie et Biochimie Industrielle, Université Montpellier II, Montpellier, France.
Growth conditions.
Yeasts were maintained at 4°C on agar slants containing glucose at 2% (wt/vol) and Bacto Peptone (Difco, Detroit, Mich.) at 1% (wt/vol). Yeasts were grown at 30°C in a 2-liter fermentor containing 1.5 liters of medium containing (wt/vol) 14% glucose, 1% yeast extract, 0.5% ammonium sulfate, 0.25% MgSO4 · 7H2O, and 0.2% KH2PO4. Antimycin (15 μM; Sigma, St. Louis, Mo.), a respiratory chain inhibitor (30), was added when specified. Under aerobic conditions, cultures were constantly aerated with sterilized air at 10 liters/h. Under anaerobic conditions, a flux of nitrogen was injected into the medium and agitation at 50 rpm was applied. When the cultures reached stationary phase, cells were harvested at 4°C by centrifugation (2,500 × g for 10 min), washed twice with distilled water, and then lyophilized.
Measurement of flocculation.
Flocculation was measured either directly in the culture medium or in Helm’s acetate buffer (15). The flocculation degree (FD)—0 (nonflocculent yeast) to 5 (strongly flocculent yeast)—was determined as previously described (11).
Flocculation tests.
Tests of coflocculation or of flocculation with concanavalin A were performed as described by Stratford (33) using a 1:1 ratio of flocculent and less-flocculent strains in 2 ml of 100 mM succinate buffer, pH 4.0. Aggregation tests with concanavalin A were done by adding 150 mg of concanavalin A per liter. The proportion of less-flocculent yeasts which coflocculated with flocculent strains was determined by counting cells after dilution in 25 mM EDTA.
Enzyme activities.
Extracytoplasmic enzyme activities were determined as excreted activity and as cell wall-bound activity (13).
(i) Invertase.
We measured invertase activity as previously described (13) by using saccharose (3%, wt/vol) as the substrate in 20 mM phosphate buffer, pH 5. The released glucose was estimated with a colorimetric method (32). The same medium, containing a high level of glucose, despite catabolite repression of this enzyme, was used for determination of invertase activity.
(ii) Acid phosphatase activity.
To 0.2 ml of medium or 0.2 ml of cell suspension, we added 1.2 ml of 0.2 M acetate buffer, pH 4.5, and 0.5 ml of 8 mM p-nitrophenol phosphate. After a 10- or 20-min incubation at room temperature, 2 ml of 1 M Tris-HCl, pH 9, containing Na2CO3 and 0.4 M K2HPO4 was added. The A405 was measured, and enzymatic activity was determined by comparison to a calibration curve obtained with standard p-nitrophenol.
(iii) Glucoamylase activity.
Glucoamylase activity was determined by using starch (1.2%, wt/vol) as the substrate in 20 mM phosphate buffer, pH 5. After 30 min at 30°C, the released glucose was estimated colorimetrically (32).
Extraction of PPMs.
PPMs were extracted by autoclaving whole cells (10 g [dry weight]) for 2 h at 120°C in 100 ml of 20 mM citrate buffer, pH 7 (28). PPMs were precipitated with 3 volumes of ethanol (4°C) for 12 h and washed twice with 60% ethanol, and the precipitate was collected by centrifugation (1,500 × g for 15 min), solubilized in distilled water, precipitated with ethanol, and then lyophilized.
Fractional precipitation of PPM extracts with cetyltrimethylammonium bromide (CTAB).
PPM extracts were precipitated with CTAB as described previously (20). Three fractions, termed FA, FB, and FC, were obtained.
Ultrafiltration and fractionation.
Samples of PPM extract (100 mg) were dissolved in 100 ml of distilled water and filtered on a membrane with a nominal cutoff of 100 kDa by using a tangential Minitan Ultrafiltration system (Millipore, Bedford, Mass.). The retained and concentrated products (molecular mass, >100 kDa) were lyophilized. One milligram of ultrafiltered PPM was dissolved in 1 ml of 0.2 M β-mercaptoethanol. After incubation at room temperature for 15 min, the solutions were applied to a Biogel A 0.5 M column (17 by 2 cm; Bio-Rad, Richmond, Calif.). The column was equilibrated with 0.02% natrium azoture (NaN3). Samples were eluted at room temperature with the same solution at a flow rate of 0.2 ml/min, and 2-ml fractions were collected. The column was calibrated with the following molecular mass markers: blue dextran (2,000 kDa) and dextrans with molecular masses of 465, 162, and 10 kDa.
Analytical procedures.
Total carbohydrate content was determined by a phenol-sulfuric acid method (6) after hydrolysis of the samples with 2 M HCl at 100°C under vacuum for 2 h. Free amino groups were identified with a 2,4-dinitrofluorobenzene reagent (10) after hydrolysis with 6 M HCl at 100°C under vacuum for 8 h. Protein levels of the samples were estimated from the A280. Amino acid analyses were done by high-performance liquid chromatography on phenylisothiocyanate derivatives after hydrolysis of the samples with 6 M HCl in the presence of 1% (wt/vol) phenol at 105°C under vacuum for 15 h. For cysteine analyses, the samples were analyzed by high-performance liquid chromatography following hydrolysis with 6 M HCl in the presence of 2% (vol/vol) dimethyl sulfoxide at 105°C under vacuum for 15 h. Phosphorus was determined after mineralization of the samples at 210°C with perchloric acid (21), using an ammonium molybdate-ascorbic acid reagent. The phosphate level on the cell surface also was estimated by alcian blue staining (1).
RESULTS
Growth and flocculation of yeasts.
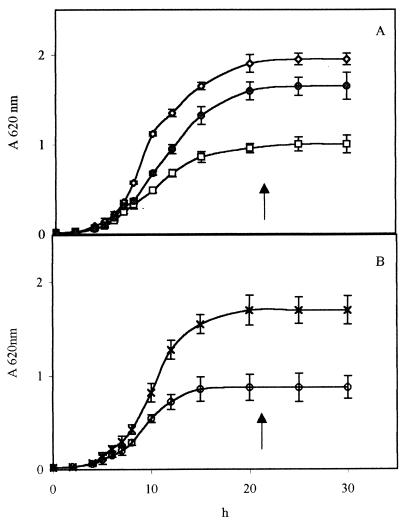
The aerobic growth rates of the wild type and the [rho0] mutant and the growth rate of the wild type in the presence of antimycin were similar (Fig. 1). The anaerobic growth rate was 20% lower; furthermore, at stationary phase, i.e., after growth for 22 h, the harvested biomass was about half of that obtained with aerobic growth (Table 1).
FIG. 1.
Growth curves of wild-type and [rho0] mutant S. cerevisiae NCYC 625 cells in aerobiosis, anaerobiosis, and anaerobiosis in the presence of antimycin. (A) Symbols: ◊, □, and ●, wild-type cells grown in aerobiosis, anaerobiosis, and aerobiosis in the presence of antimycin, respectively. (B) Symbols: ×, mA; ○, mN; [rho0] mutant cells grown in aerobiosis and anaerobiosis, respectively; ↑, cell harvesting time for PPM extraction.
TABLE 1.
Growth, FD, and alcian blue staining of cells with respect to strain and culture conditions
| Strain and culture conditions | Mean biomass dry wt (g) ± SDa | Mean growth rate (h−1) ± SD | Alcian blue stainingb | FDc |
|---|---|---|---|---|
| Wild type | ||||
| Aerobiosis | 14 ± 2d | 0.44 ± 0.05 | +++ | 4 |
| Anaerobiosis | 6.5 ± 1 | 0.37 ± 0.03 | + | 2 |
| Anaerobiosis + antimycin | 11 ± 1 | 0.44 ± 0.04 | + | 1 |
| [rho0] mutant | ||||
| Aerobiosis | 11 ± 1.5 | 0.49 ± 0.05 | + | 2 |
| Anaerobiosis | 4.5 ± 1 | 0.37 ± 0.03 | + | 1 |
Cells were harvested in the stationary growth phase. Results are expressed in lyophilized biomass harvested from a 1.5-liter fermentor.
Staining was done as described in reference 1.
FD was estimated as described in reference 11.
Assays were performed in the culture medium and in Helm’s acetate buffer. The values shown are means of at least three determinations.
We stained cells with alcian blue and measured flocculation properties (Table 1). The aerobically grown wild-type strain was the most pigmented following alcian blue staining. The FDs were similar when measured in the culture medium and in Helm’s acetate buffer (pH 4.5). Flocculation intensity depended on the strain and the growth conditions, with the most flocculent cells being those of the aerobically grown wild type. Wild-type cells cultured aerobically in the presence of antimycin had phosphate levels and flocculation properties similar to those of the aerobically grown [rho0] mutant (Table 1).
Coflocculation and aggregation by concanavalin A.
Aerobic mixtures of wild-type cells with [rho0] mutant or antimycin-treated cells combined into flocs (Table 2). The mutant and antimycin-treated cells alone were weakly flocculent; [rho0] mutant and antimycin-treated cells were less aggregated with concanavalin A than the wild-type cells.
TABLE 2.
Coflocculation assays and aggregation by concanavalin A of untreated or antimycin-treated wild-type and [rho0] mutant S. cerevisiae NCYC 625
| Cells | Mean % of cells in suspension ± SD (FD)
|
|
|---|---|---|
| Coflocculationa | Aggregation by concanavalin Ab | |
| Controls | ||
| Wild type | 29 ± 4.3c (4) | 57 ± 4.4 (3) |
| [rho0] mutant | 65 ± 9.5 (2) | 82 ± 3.9 (1) |
| Antimycin-treated wild type | 90 ± 8.5 (1) | 93 ± 6.1 (0–1) |
| Mixtures | ||
| Untreated + antimycin-treated wild type | 63 ± 8.2 (2) | 74 ± 8.4 (2) |
| Wild type + mutant | 48 ± 5.3 (3) | 62 ± 6.6 (2) |
Cells were mixed at a 1:1 ratio in 100 mM succinate buffer (pH 4.0) for 4 to 6 h at 25°C.
Aggregation was started by addition of concanavalin A at 150 mg/liter.
The values shown are means of three determinations.
Enzyme activities.
Invertase, acid phosphatase, and glucoamylase activities were determined in the media (extracellular activities) or in whole cells (cell wall bound activities) after 18 h of culture (Table 3). Invertase and acid phosphatase may be cell wall-bound and/or extracellular glycoproteins, but glucoamylase is only extracellular. Invertase and acid phosphatase total activities were always affected in respiratory chain-deficient cells; for glucoamylase, which was only extracellular, a decrease was also observed in respiratorily deficient cells. In general, cell wall-bound activity appeared to decrease whenever extracellular activity increased (Table 3). For both invertase and acid phosphatase, the rate of excretion in the growth medium was higher in the [rho0] and antimycin-treated cells than in the wild type. For extracellular invertase, increases of 10 to 20% and for extracellular acid phosphatase, increases of 40 and 16% were observed in mutant and antimycin-treated cells, respectively.
TABLE 3.
Extracellular and cell wall-bound invertase, acid phosphatase, and glucoamylase activities in wild-type untreated and antimycin-treated and [rho0] mutant S. cerevisiae NCYC 625 cellsa
| Enzyme | Extracellular activity
|
Cell wall-bound activity
|
Total extracytoplasmic activity
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| wtA | mA | wtAant | wtA | mA | wtAant | wtA | mA | wtAant | |
| Invertaseb | 24 ± 5 | 16 ± 3 | 32 ± 5 | 89 ± 7 | 37 ± 5 | 48 ± 6 | 113 | 53 | 80 |
| Acid phosphatasec | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | 3.2 ± 0.1 | 0.4 ± 0.0 | 1.6 ± 0.1 | 4.7 | 1.6 | 3.1 |
| Glucoamylased | 57 ± 5 | 4 ± 0 | 47 ± 3 | 0 | 0 | 0 | 57 | 4 | 47 |
Values are the means of three determinations. Abbreviations: wtA, wild-type cells grown under aerobiosis; mA, mutant cells grown under aerobiosis; wtAant, wild-type cells grown under aerobiosis and treated with antimycin.
Results are expressed as nanomoles of glucose released per minute per milligram (dry weight) of cells.
Results are expressed as micromoles of p-nitrophenol released per minute per milligram (dry weight) of cells.
Results are expressed as micromoles of glucose released per minute per milligram (dry weight) of cells.
Extraction and chemical composition of PPMs.
Respiratorily deficient cells synthesized slightly more PPM (about 10%) than did wild-type cells grown under aerobic conditions. PPM chemical composition also depended upon the strain and the culture conditions (Table 4).
TABLE 4.
Yield and global chemical composition of PPM extractsa
| Parameter | Wild type
|
[rho0] mutant
|
|||
|---|---|---|---|---|---|
| wtA | wt N | wtAant | m A | m N | |
| Yieldb | 9.0 ± 0.12 | 11 ± 0.34 | 9.7 ± 0.24 | 10 ± 0.11 | 11 ± 0.15 |
| Carbohydratec | 42 ± 1.8 | 39 ± 2.5 | 45 ± 2.2 | 60 ± 4.1 | 40 ± 2.9 |
| Proteind | 16 ± 1.9 | 21 ± 1.7 | 19 ± 0.8 | 12 ± 1.3 | 22 ± 0.2 |
| Phosphatee | 4.3 ± 0.6 | 3.3 ± 0.4 | 2.3 ± 0.3 | 2.4 ± 0.2 | 2.8 ± 0.2 |
The results shown are means ± standard deviations. Carbohydrate, protein, and phosphate are expressed as percentages of lyophilized crude PPM extracts. Phosphate results are the means of three determinations. Yield; carbohydrate, and protein results are means of four determinations. The abbreviations are the same as in Table 3, except that N indicates anaerobiosis.
Values are percentages of lyophilized yeast cells.
Determined by the phenol-sulfuric acid method (6).
Determined by the 2,4-dinitrofluorobenzene method (10).
Determined by the ammonium molybdate-ascorbic acid reagent method (21).
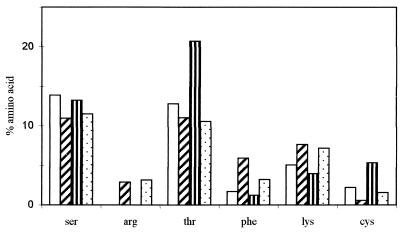
PPMs of the wild type and [rho0] mutant produced during aerobic growth had a higher carbohydrate content but a lower protein content than after culture under anaerobic conditions. In the PPMs of wild-type cells, the carbohydrate/protein ratio was 2.6 following aerobic growth and 1.9 following anaerobic growth. The amino acid compositions of the wild-type and mutant PPMs (Fig. 2) were similar, with Ser, Thr, and Cys less frequent in PPM from anaerobic cells than in PPM from aerobic cells, and Phe, Lys, and Arg were more frequent. The main changes in global protein content and in amino acid profiles resulted from aerobic-anaerobic growth conditions and not from the [rho0] mutation. Cysteine, the level of which was 3.5 times higher in the mutant PPMs than in wild-type PPMs, was the sole exception. PPMs from aerobically grown wild-type cells had the highest levels of phosphate.
FIG. 2.
Amino acid composition profiles of PPMs of wild-type and [rho0] mutant S. cerevisiae NCYC 625. Symbols: □ and  , wild-type cells grown in aerobiosis and anaerobiosis, respectively;
, wild-type cells grown in aerobiosis and anaerobiosis, respectively;  and
and  , [rho0] mutant cells grown in aerobiosis and anaerobiosis, respectively. Results are expressed as percentages of the total amino acid content.
, [rho0] mutant cells grown in aerobiosis and anaerobiosis, respectively. Results are expressed as percentages of the total amino acid content.
Fractionation of PPMs. (i) CTAB treatment.
We obtained three fractions: FA was precipitated with CTAB, FB was precipitated with CTAB at pH 8.8 in the presence of boric acid, and FC was soluble in CTAB and not precipitated at pH 8.8 (Table 5). FA fractions showed the most stable percentage, i.e., 20% ± 1%, and contained the highest phosphate levels, 5.1 to 6.1%. The amino acid profiles and the hexosamine percentages were similar in all FA fractions.
TABLE 5.
Yields and global chemical compositions of fractions obtained by CTAB fractionation of PPM extractsa
| Fraction | Yieldb (%) | Carbohydratec (%) | Proteind (%) | Phosphatee (%) |
|---|---|---|---|---|
| FA-wt A | 19 | 41 ± 1 | 12 ± 0.1 | 5.4 ± 0.1 |
| FA-wt N | 21 | 21 ± 1 | 12 ± 0.1 | 5.1 ± 0.3 |
| FA-wt Aant | 21 | 31 ± 0 | 12 ± 0.3 | 5.5 ± 0.1 |
| FA-m A | 21 | 34 ± 5 | 11 ± 0.7 | 6.1 ± 0.4 |
| FA-m N | 20 | 20 ± 0 | 17 ± 0.5 | 5.5 ± 0.2 |
| FB-wt A | 27 | 84 ± 2 | 16 ± 0.8 | 0.43 ± 0.05 |
| FB-wt N | 25 | 78 ± 2 | 22 ± 0.1 | 0.11 ± 0.02 |
| FB-wt Aant | 32 | 82 ± 3 | 18 ± 0.5 | 0.21 ± 0.03 |
| FB-m A | 37 | 86 ± 2 | 14 ± 0.5 | 0.22 ± 0.05 |
| FB-m N | 19 | 80 ± 1 | 20 ± 0.1 | 0.21 ± 0.01 |
| FC-wt A | 16 | 34 ± 3 | 65 ± 2.1 | 0.21 ± 0.08 |
| FC-wt N | 15 | 42 ± 5 | 58 ± 0.2 | 0.13 ± 0.02 |
| FC-wt Aant | 16 | 66 ± 3 | 34 ± 0.5 | 0.62 ± 0.04 |
| FC-m A | 11 | 82 ± 2 | 19 ± 0.7 | 0.26 ± 0.05 |
| FC-m N | 25 | 48 ± 6 | 51 ± 0.7 | 0.27 ± 0.02 |
Results are the mean values of three determinations ± the standard deviations. The abbreviations are the same as those in Table 4.
Values are percentages of lyophilized PPM extracts.
Determined by the phenol-sulfuric acid method (6).
Determined by the 2,4-dinitrofluorobenzene method (10).
Determined by the ammonium molybdate-ascorbic acid reagent method (21).
FB fractions had the highest level of carbohydrates and were similar to the global PPM extracts in phosphate and protein levels, as well as amino acid profiles.
FC fractions contained the highest protein levels, and the amino acid profile of the FC fraction showed an increase of Ser and Thr in the mutant compared to the wild type.
(ii) Ultrafiltration and fractionation of PPMs.
Following tangential ultrafiltration (nominal cutoff of 100 kDa) and gel filtration (Biogel A 0.5 M column), protein and carbohydrate eluted in a single peak with a molecular mass of 940 kDa for all of the PPMs, except for the PPMs from the anaerobically grown mutant, which had a second small peak at 50 kDa.
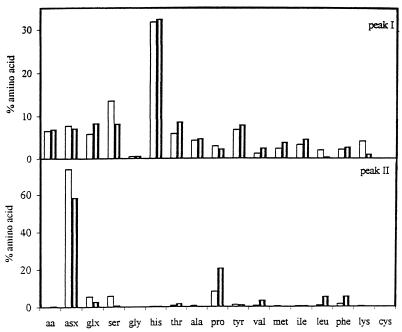
After mercaptoethanol treatment (Fig. 3), the PPMs resolved into two peaks, one (peak I) with a molecular mass of 940 kDa that contained carbohydrates and proteins and a second (peak II) that contained primarily proteins with molecular masses of 20 to 50 kDa. Using the area of the protein peaks, the peak II/peak I ratios of the mutant PPMs were higher (3.7 for aerobic growth and 1.4 for anaerobic growth) than the peak II/peak I ratios of the wild-type strain PPMs (approximately 0.5). Almost all of the carbohydrate and most of the phosphate were recovered in peak I (results not shown). Further filtration of the compounds of peaks I did not separate the proteins from the carbohydrates and the phosphates. The amino acid profiles of peaks I and II were quite different (Fig. 3). In both the mutant and wild-type strains, a peptide or protein enriched in Glx (glutamic acid or glutamine) and tyrosine was released from the PPMs; however, in the [rho0] mutant PPMs, the level of the released peptidic subunits was much higher than in the wild-type PPM.
FIG. 3.
Amino acid composition profiles of peaks I and II obtained after mercaptoethanol treatment of PPMs extracted from wild-type or [rho0] mutant S. cerevisiae NCYC 625 grown in aerobiosis. Symbols: □ and  , wild-type and [rho0] mutant cells grown in aerobiosis, respectively. Results are expressed as percentages of the total amino acid content.
, wild-type and [rho0] mutant cells grown in aerobiosis, respectively. Results are expressed as percentages of the total amino acid content.
DISCUSSION
In the present study, we observed decreased flocculation (lower self-FD; lower coflocculation and aggregation with concanavalin A) of S. cerevisiae NCYC 625 after [rho0] mutation and after antimycin treatment; this drop resulted from mitochondrial alterations that affected the synthesis of the cell wall rather than lectin. These data can be related to those of Evans et al. (8), who showed that defective mitochondria can affect cell surface characteristics of yeast, e.g., concanavalin A agglutinability, cell movement in a biphasic polymer system, and cell adhesion. We also observed that three extracytoplasmic enzymes indicated an alteration of excretion and activities of these glycoproteins in [rho0] mutant or antimycin-treated cells. Defects in excretion seemed to be due to changes in cell wall components or structure, as suggested by Tammi et al. (35). The requirement of mitochondrial function for sugar use, flocculation, and enzyme secretion was described previously (8), particularly for the expression of STA, a gene encoding an excreted glucoamylase (1,4-α-d-glucohydrolase) (27). Our flocculation and enzyme activity results are consistent with the hypothesis that defects in the respiratory chain lead to changes in cell wall structure.
To identify structural changes in the cell wall, we analyzed the PPMs, which are the ligands of lectins in the flocculation phenomenon. PPMs, as well as the whole cell surface of respiratory chain-deficient cells, decreased in phosphate content. According to Ballou (1), S. cerevisiae mnn (mannan-defective) mutants cannot bind alcian blue dye because the peripheral peptidomannans are not properly phosphorylated. Concerning amino acid content, similar profiles were obtained under aerobic conditions but under anaerobic conditions, the level of the protein moiety was increased and the amino acid profiles were altered. These results are similar to those for other cell wall proteins of S. cerevisiae, which also increase under anaerobiosis conditions, such as TIP1 (4, 24) or TIR1 (24).
When PPMs were treated with CTAB, three fractions resulted. The FA fraction appeared to be constant (except for the carbohydrate moiety of the PPM), while the FB and FC fractions varied. Previous studies (25) of PPMs found that the FB fractions were the major mannan protein constituent of the CTAB fractions. Changes in the FB and FC fractions may be responsible for the properties and roles of the PPMs at the periphery of yeast cells.
Following treatment with mercaptoethanol (or the physiological redox agent glutathione [results not shown]), PPMs isolated from mitochondrion-deficient cells were more sensitive to dissociation than those isolated from the wild-type strain. We hypothesize that in the native PPMs, disulfur linkages associate small peptidic subunits to a much larger peptidomannan backbone. Our present results indicate that treatment with monothiols dissociates the PPMs through reduction of oxidized sulfhydryl groups and that larger quantities of peptidic subunits are released as a consequence of an alteration of the structure of these cell wall polymers in respiratorily (or mitochondrion) deficient cells. By comparison, agglutinins involved in sexual aggregation in S. cerevisiae have similar structures in which small peptidic subunits are bound by disulfur bridges to a larger mannoprotein structure (3).
Cell wall synthesis requires transport of material from the cytosol to the cell periphery, and mitochondria could be indirectly involved in this translocation, which follows a secretory pathway (18) in which motor proteins are used. Alteration of the motor protein Myo1p or a deficit in gene Myo2 induced misdeposit of the material in the yeast cell wall (16). Furthermore, Drubin et al. (5) suggested that actin-myosin interactions might underlie mitochondrial organization in S. cerevisiae. Mutants with double deletions in the MYO3 and MYO5 genes, coding for myosin in yeast, had phenotypes associated with actin disorganization, including accumulation of intracellular membranes and vesicles, defects in chitin and cell wall deposition, and invertase secretion (12). Preliminary fluorescence microscopy observations on wild-type and [rho0] mutant S. cerevisiae NCYC 625 cells showed an abnormal distribution of actin in cells containing mitochondria (results not shown). We cannot exclude the hypothesis that mitochondrial DNA products also affect the expression of nuclear genes that encode proteins involved in cell wall synthesis. Indeed, regulation of the expression of CIT1 and CIT2, which encode citrate synthase, is altered in [rho0] mutant cells (19) while transcription of the human lysozyme gene on an expression plasmid under the control of the GAL10 promoter increased in [rho0] cells (17). Promoter plasmids are more abundant in [rho0] than in [rho+] cells (22), and Parikh et al. (26) hypothesized the existence of a retrograde path of communication from the mitochondria to the nucleus in yeast. Thus, the mitochondrial genome influences the expression of nuclear genes, including those encoding cell wall components (36).
In S. cerevisiae NCYC 625, [rho0] mutation and respiratory chain alteration affect biologically active glycoproteins, e.g., cell wall or extracellular enzymes, and structural glycoproteins, e.g., peripheral PPMs, which are ligands of lectins involved in flocculation. Our results demonstrate not only that the carbohydrate moiety of these heteropolymers plays a role in this phenomenon but also that the protein or peptide moiety has a significant role in this cell-cell recognition process.
REFERENCES
- 1.Ballou C E. Isolation, characterization and properties of Saccharomyces cerevisiae mnn mutants with non-conditional protein glycosylation defects. Methods Enzymol. 1990;185:440–470. doi: 10.1016/0076-6879(90)85038-p. [DOI] [PubMed] [Google Scholar]
- 2.Calleja G B. Role of mitochondria in the sex-directed flocculation of a yeast. Arch Biochem Biophys. 1973;154:382–386. doi: 10.1016/0003-9861(73)90070-2. [DOI] [PubMed] [Google Scholar]
- 3.Capellaro C, Hauser K, Mrsa V, Watzele M, Watzele G, Gruber C, Tanner W. Saccharomyces cerevisiae a-agglutinin and alpha-agglutinin. Characterization of their molecular interaction. EMBO J. 1991;10:4081–4088. doi: 10.1002/j.1460-2075.1991.tb04984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donzeau M, Bourdineaud J P, Lauquin G J M. Regulation by low temperatures and anaerobiosis of a yeast gene specifying a putative GPI-anchored plasma membrane protein. Mol Microbiol. 1996;20:449–459. doi: 10.1111/j.1365-2958.1996.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 5.Drubin D G, Jones H D, Wertman K F. Actin structure and functions: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol Biol Cell. 1993;4:1277–1294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 7.Egilsson V, Evans I H, Wilkie D. Toxic and mutagenic effects of carcinogens on the mitochondria of Saccharomyces cerevisiae. Mol Gen Genet. 1979;174:39–46. doi: 10.1007/BF00433303. [DOI] [PubMed] [Google Scholar]
- 8.Evans I H, Diala E S, Earl A, Wilkie D. Mitochondrial control of cell surface characteristics in Saccharomyces cerevisiae. Biochim Biophys Acta. 1980;602:201–206. doi: 10.1016/0005-2736(80)90302-8. [DOI] [PubMed] [Google Scholar]
- 9.Fontana A, Bidenne C, Ghommidh C, Guiraud J, Vezhinhet F. Study of the flocculation of Saccharomyces diastaticus. J Inst Brew. 1992;98:401–407. [Google Scholar]
- 10.Ghuysen J, Tipper D, Birge C, Strominger J. Structure of the cell wall of Staphylococcus aureus strain Copenhagen. IV. The soluble glycopeptide and its sequential degradation by peptidases. Biochemistry. 1965;4:2245–2254. doi: 10.1021/bi00879a016. [DOI] [PubMed] [Google Scholar]
- 11.Gilliland R B. Proceedings of European Brewing Conventions Congress of Brighton. 1951. The flocculation characteristics of brewing yeast during formation; pp. 35–55. [Google Scholar]
- 12.Goodson H V, Anderson B L, Warrick H M, Pon L A, Spudich J A. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin Iproteins are required for polarization of the actin cytoskeleton. J Cell Biol. 1996;133:1277–1291. doi: 10.1083/jcb.133.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiraud J P, Fontana A. Isolement et caractérisation d’un mutant floculant de Saccharomyces diastaticus. Res Microbiol. 1992;143:81–91. doi: 10.1016/0923-2508(92)90037-o. [DOI] [PubMed] [Google Scholar]
- 14.Hinrichs J, Stahl U, Esser K. Flocculation in Saccharomyces cerevisiae and mitochondrial DNA structure. Appl Microbiol Biotechnol. 1988;29:48–54. [Google Scholar]
- 15.Hussain T, Salhi O, Lematre J, Charpentier C, Bonaly R. Comparative studies of flocculation and deflocculation of Saccharomyces uvarum and Kluyveromyces bulgaricus. Appl Microbiol Biotechnol. 1986;23:269–273. [Google Scholar]
- 16.Johnston B F, Prender Gast J A, Singer R A. The Saccharomyces cerevisiae MY02 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaisho Y, Yoshimura K, Nakahama K. Increase in gene expression by respiratory-deficient mutation. Yeast. 1989;5:91–98. doi: 10.1002/yea.320050204. [DOI] [PubMed] [Google Scholar]
- 18.Klis F M. Cell wall assembly in yeast. Yeast. 1994;10:851–869. doi: 10.1002/yea.320100702. [DOI] [PubMed] [Google Scholar]
- 19.Liao X, Small W C, Srere P A, Butow R A. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:38–46. doi: 10.1128/mcb.11.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lloyd Kenneth O. Isolation, characterisation and partial structure of peptidogalactomannans from the yeast form of Cladosporium werneckii. Biochemistry. 1970;9:3446–3453. doi: 10.1021/bi00819a025. [DOI] [PubMed] [Google Scholar]
- 21.Mac Clare C. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971;39:527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- 22.Marczynski G T P, Schultz P W, Jaehning J A. Use of yeast nuclear DNA sequences to define the mitochondrial RNA polymerase promoter in vitro. Mol Cell Biol. 1989;9:3193–3202. doi: 10.1128/mcb.9.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miki B L A, Poon N H, James A P, Seligy V L. Possible mechanism for flocculation interactions governed by gene FLOI in Saccharomyces cerevisiae. J Bacteriol. 1982;150:878–889. doi: 10.1128/jb.150.2.878-889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz-Dorado J, Kondo K, Inouye M, Sone H. Identification of cis- and trans-acting elements involved in the expression of cold shock-inducible TIP1 gene of yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1994;22:449–459. doi: 10.1093/nar/22.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okubo Y, Shibata N, Ichikawa T, Chaki S, Suzuki S. Immunochemical study on baker’s yeast mannan prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch Biochem Biophys. 1981;212:204–215. doi: 10.1016/0003-9861(81)90360-x. [DOI] [PubMed] [Google Scholar]
- 26.Parikh V S, Morgan M M, Scott R, Clements L S, Butow R A. The mitochondrial genotype can influence nuclear gene expression in yeast. Science. 1987;235:576–580. doi: 10.1126/science.3027892. [DOI] [PubMed] [Google Scholar]
- 27.Patel D, Evans I H, Bevan E A. A genetic analysis of glucoamylase activity in the diastatic yeast Saccharomyces cerevisiae NCYC625. Curr Genet. 1990;17:281–288. doi: 10.1007/BF00314873. [DOI] [PubMed] [Google Scholar]
- 28.Peat S, Whelan, Edward S T. Polysaccharides of baker’s yeast. Part IV. Mannan. J Chem Soc. 1961;1:29–34. [Google Scholar]
- 29.Shankar C S, Umesh-Kumar S. A surface lectin associated with flocculation in brewing strains of Saccharomyces cerevisiae. Microbiology. 1994;140:1097–1101. doi: 10.1099/13500872-140-5-1097. [DOI] [PubMed] [Google Scholar]
- 30.Slater E C. Application of inhibitors and uncouplers for a study of oxidative phosphorylation. Methods Enzymol. 1967;10:48–57. [Google Scholar]
- 31.Slonimisky P, Perroding G, Croft J N. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient nonchromosomal ‘Petites’. Biochem Biophys Res Commun. 1968;30:232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- 32.Somogyi M. Notes on sugar determination. J Biol Chem. 1952;195:19–23. [PubMed] [Google Scholar]
- 33.Stratford M. Yeast flocculation: receptor definition by mnn mutants and concanavalin A. Yeast. 1992;8:635–645. doi: 10.1002/yea.320080807. [DOI] [PubMed] [Google Scholar]
- 34.Stratford M, Assinder S. Yeast flocculation: FLO 1 and new FLO phenotypes and receptor structure. Yeast. 1991;7:559–574. doi: 10.1002/yea.320070604. [DOI] [PubMed] [Google Scholar]
- 35.Tammi M, Ballou L, Taylor A, Ballou C E. Effect of glycosylation on yeast invertase oligomer stability. J Biol Chem. 1987;262:4395–4401. [PubMed] [Google Scholar]
- 36.Wilkie D, Evans I. Mitochondria and the yeast cell surfaces: implications for carcinogenesis. Trends Biochem Sci. 1982;7:147–151. [Google Scholar]
- 37.Zaamoun S, Tran Thi X, Reisinger O, Guiraud J P, Fontana A, Bonaly R. Influence of aeration and [rho0] mutation on the structure of the cell wall of Saccharomyces cerevisiae and Saccharomyces diastaticus. Mycol Res. 1995;99:492–500. [Google Scholar]