PURPOSE
The incidence of cancer in sub-Saharan Africa is increasing rapidly, yet cancer research in the region continues to lag. One contributing factor is limited exposure to clinical research among trainees. We describe implementation and results of a virtual clinical research training program for Zambian clinical oncology fellows developed jointly by the Cancer Diseases Hospital in Zambia and the MD Anderson Cancer Center to address this need.
METHODS
The clinical research training program consisted of 14 weekly virtual lectures, development of research questions by Zambian clinical oncology fellows, assignment of faculty and peer mentors, longitudinal mentorship of research protocols, and anonymous precourse and postcourse surveys. The paired t-test was used to analyze the change in academic self-efficacy scores.
RESULTS
Fourteen Zambian clinical oncology fellows participated. Senior fellows were paired with research mentors, leading to the development of eight research protocols. A total of 70 meetings and 126 hours of mentorship occurred with a median of seven meetings and 15 hours per pairing. The precourse and postcourse survey response rates were 86% and 79%, respectively. There were statistically significant increases in nine of 12 academic self-efficacy domains. The largest gains were in ability to independently perform research (P < .001) and research mentorship (P = .02) with an average increase of 1.5 points on a five-point scale in both domains.
CONCLUSION
The Cancer Diseases Hospital MD Anderson Cancer Center clinical research training program for Zambian clinical oncology fellows led to increases in multiple academic self-efficacy domains among participants, formation of longitudinal mentorship groups with both faculty and peer mentors, and development of Zambian-led research protocols, demonstrating the feasibility of implementing a virtual model. This may be especially relevant because of shifting international collaboration paradigms after the COVID-19 pandemic.
INTRODUCTION
The burden of cancer is quickly growing in Africa and presents a significant public health challenge to the continent today and in the coming decades if the status quo remains.1 Although cancer has historically received less attention and resources because of the competing risk of communicable diseases in Africa, the present-day risk of mortality from cancer among African women is already similar to that of North American women, demonstrating the immediacy of the problem.2,3 Furthermore, countries located in sub-Saharan Africa are projected to have the greatest relative increase in global cancer incidence between 2020 and 2040 by a significant margin, which will exacerbate existing limitations in cancer care.3 Clinical research is essential to develop effective, evidence-based interventions to the unique cancer care challenges that occur in Africa and should be prioritized. Rather than being regarded as a luxury because of a shortage of resources, it is counterintuitively more important to appropriately allocate scarce resources and develop cost-effective and culturally aligned solutions.4
CONTEXT
Key Objective
To determine whether a virtual format is a feasible and effective format for an international clinical research training program for clinical oncology fellows at a cancer teaching hospital in Zambia.
Knowledge Generated
A virtual clinical research training program for Zambian oncology fellows had key advantages over an in-person format, including the ability to leverage the full breadth of the institution to identify lecturers and mentors, allow for longitudinal collaboration, and reduce costs associated with implementation. The program led to marked increases in academic self-efficacy domains among participants, development of Zambian-led research protocols, and support through longitudinal mentorship by peer trainee and faculty mentors.
Relevance
A virtual clinical research training model is feasible and effective and has certain advantages over an in-person format, which may be especially relevant because of shifting international collaboration paradigms because of the COVID-19 pandemic.
African countries, however, are significantly under-represented in cancer research because of historical and persistent inequalities.5,6 Barriers to clinical research in Africa include lack of adequate funding to build research infrastructure, procure equipment, implement high-quality training programs, maintain research personnel and collaborations, and consistently collect health data.1 Among these, training physicians in clinical research has been identified as a sustainable method to strengthen clinical research capacity as local physicians are best equipped to define and investigate important research issues within their own country.7,8 There have been numerous successful partnerships between developed countries and countries in Africa for the training of doctoral fellows, epidemiology and operational researchers, and health care workers.9-16 However, there are few published experiences of clinical research training programs for African oncologists, and even fewer delivered through a virtual platform.
The MD Anderson Cancer Center (MDA) in Houston, TX, and the Cancer Diseases Hospital (CDH) in Lusaka, Zambia, have an established academic partnership that includes telehealth mentoring through Project ECHO17 and an annual high-yield course in radiation biology and physics.18 The CDH Specialized Training Program in Clinical and Radiation Oncology enrolled its first fellows in 2018 and will graduate the country's first cohort of locally trained oncologists in 2022. A significant area of need was jointly identified as a structured clinical research training program. Here, we describe the implementation and results from the initial yearlong virtual clinical research training program for Zambian clinical oncology fellows created as a collaboration between MDA and CDH.
METHODS
Training Program and Participants
The virtual clinical research training program consisted of five core components (Fig 1), including (1) the weekly virtual lecture series, (2) development of research questions by the Zambian oncology fellows, (3) identification and assignment of faculty and peer mentors, (4) joint longitudinal mentorship of research protocols, and (5) mixed qualitative and quantitative anonymous precourse and postcourse surveys. The Zambian oncology fellows were given protected time to attend the lecture series, and a requirement for graduation was the completion of a research thesis.
FIG 1.
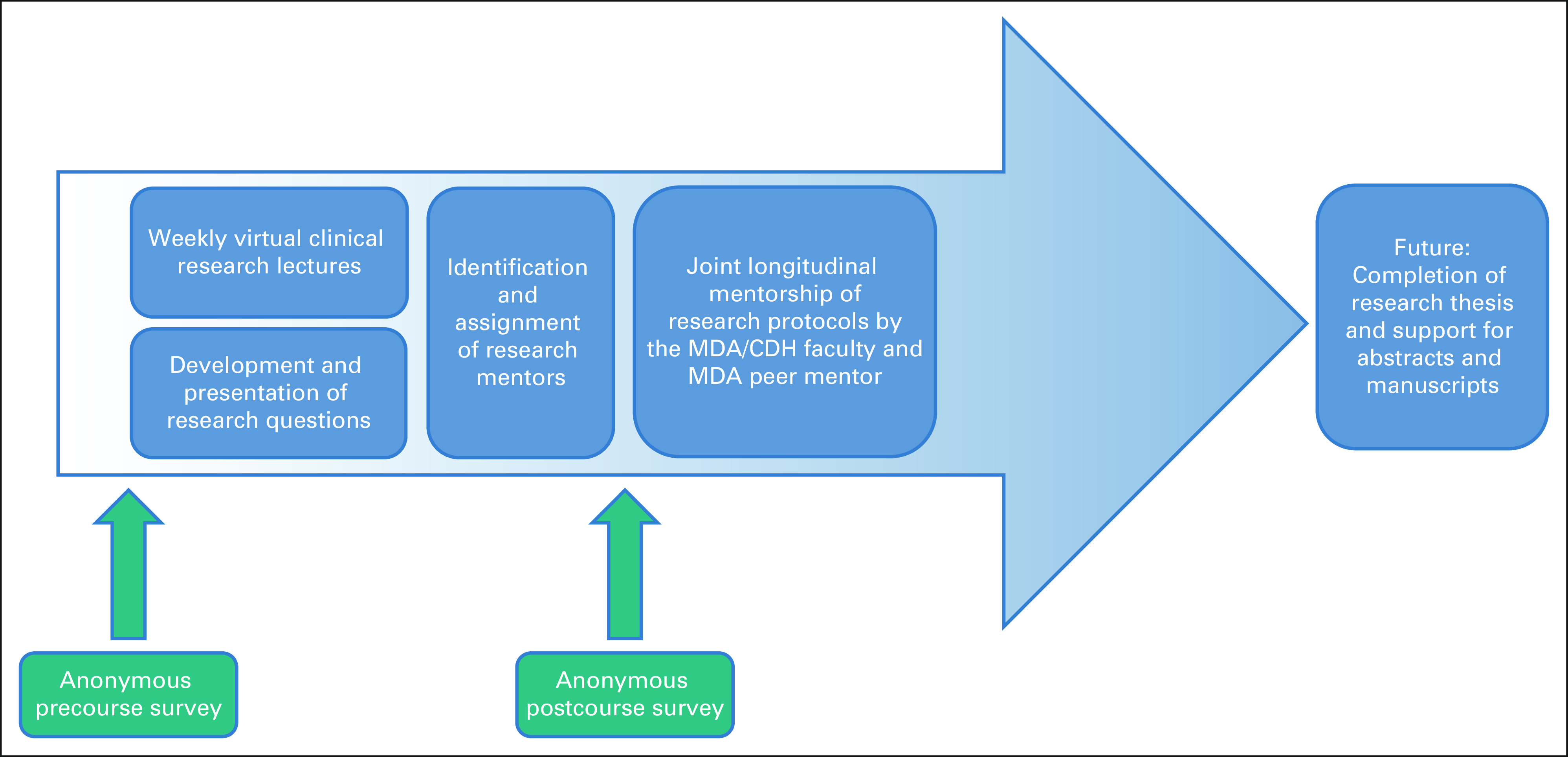
The CDH-MDA virtual clinical research training program schema. CDH, Cancer Diseases Hospital; MDA, MD Anderson Cancer Center.
Virtual Lectures
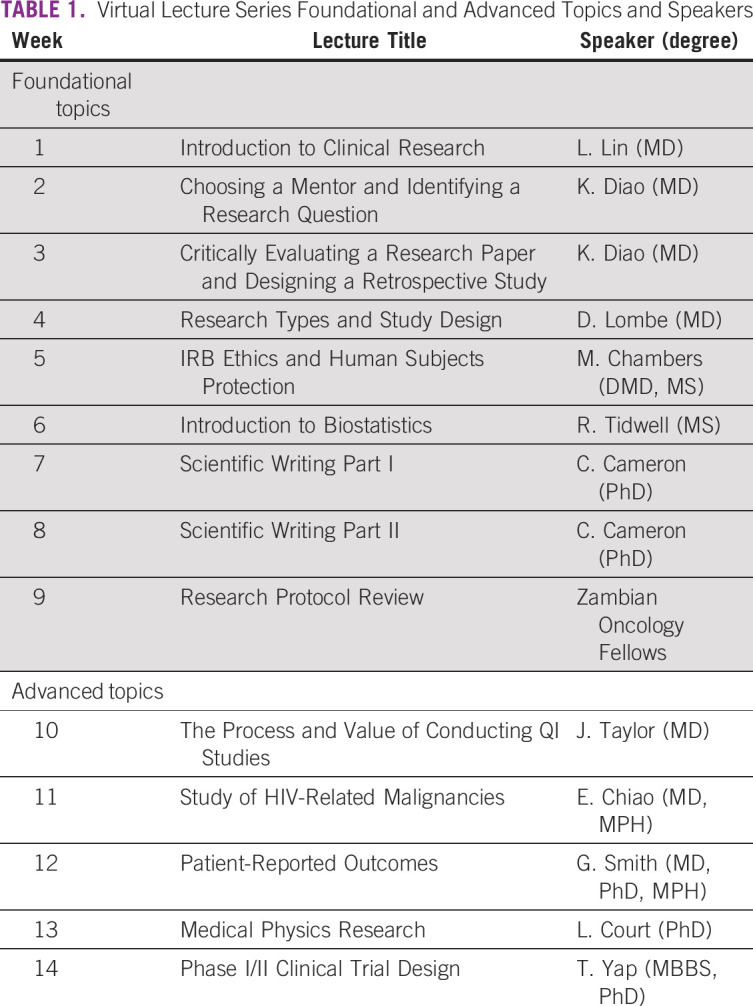
The lecture component consisted of 14 weekly lectures that were delivered over teleconference (Zoom Video Communications Inc, San Jose, CA). The lecture topics were chosen by a core group of senior faculty from MDA and CDH. The lectures were given by nine different MDA faculty members, one CDH faculty member, and one MDA resident physician. One session was led by CDH fellows presenting their research proposals. The lectures were broadly categorized into foundational and advanced topics (Table 1). The foundational topics focused on research skills that could be interdisciplinary, with incorporation of examples involving oncology, whereas the advanced topics developed more specific clinical or radiation oncology research skills. Each session was allotted up to 90 minutes of time, with the lecture component generally lasting between 30 and 60 minutes and additional questions and discussion another 30 minutes.
TABLE 1.
Virtual Lecture Series Foundational and Advanced Topics and Speakers
Mentorship Program
Although all Zambian oncology fellows were encouraged to participate in the training program, only senior oncology fellows were expected to develop a research question and be paired with research mentors. Fellows initially developed a research question during the weekly lecture series with assistance from CDH faculty supervisors. Afterward, they were assigned to both an MDA faculty and an MDA peer mentor (a resident or fellow in an oncologic discipline at MDA with a background in clinical research). Mentor groups were encouraged to meet every 2-3 weeks on a virtual platform to develop and execute their research protocol. Peer mentors were elicited for feedback at regular intervals and asked to estimate their total number of research meetings and hours spent mentoring.
Survey Design and Analysis
Under institutional review board approval and with written consent, anonymous precourse and postcourse surveys were administered electronically via Research Electronic Data Capture before and after the lecture series. Both surveys included 12 5-point Likert scale academic self-efficacy questions (Data Supplement). The precourse survey included background research experience questions, and the postcourse survey included six 5-point Likert scale questions on course feedback in addition to open-ended feedback questions. The paired t-test was used to test differences in precourse and postcourse academic self-efficacy inventory scores, with P < .05 considered statistically significant.
RESULTS
The initial yearlong training program took place between August 2020 and July 2021 with the weekly lecture series occurring between August 2020 and November 2020 and was attended by all 14 Zambian clinical oncology fellows at the time. This included eight senior (second or third year) and six junior (first year) fellows in the 4-year program.
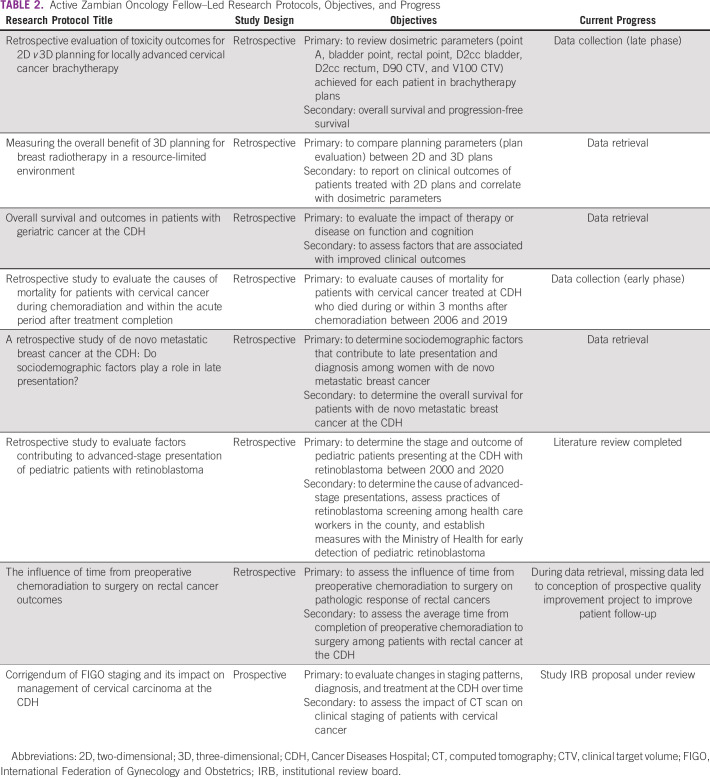
At the conclusion of the weekly lecture series, all eight senior fellows had developed a research question and were successfully paired with a local Zambia faculty mentor, a MD Anderson faculty mentor, and a MD Anderson peer trainee mentor on the basis of their area of research interest. Between November 2020 and July 2021, a total of 70 virtual meetings and 126 hours of mentorship occurred. The median number of meetings and hours of mentorship for each pairing were seven (range, 3-21) and 15 (range, 8-38) hours, respectively. Table 2 lists the current Zambian fellow-led active research proposals including their progress as of August 2021.
TABLE 2.
Active Zambian Oncology Fellow–Led Research Protocols, Objectives, and Progress
The precourse survey response rate was 12 of 14 (86%), and the postcourse survey response rate was 11 of 14 (79%). All survey nonrespondents were first-year fellows. None of the respondents reported previous participation in clinical research projects or lectures on clinical research or statistical methods.
Average scores precourse and postcourse for the 12 Likert scale academic self-efficacy questions are reported with statistically significant increases in nine domains including comfort in interpreting research; ability to generate a research hypothesis, develop a research question, collect and maintain data, summarize and report data, publish results of research, and independently perform research; the presence of research mentorship and guidance; and professional satisfaction (Fig 2).
FIG 2.
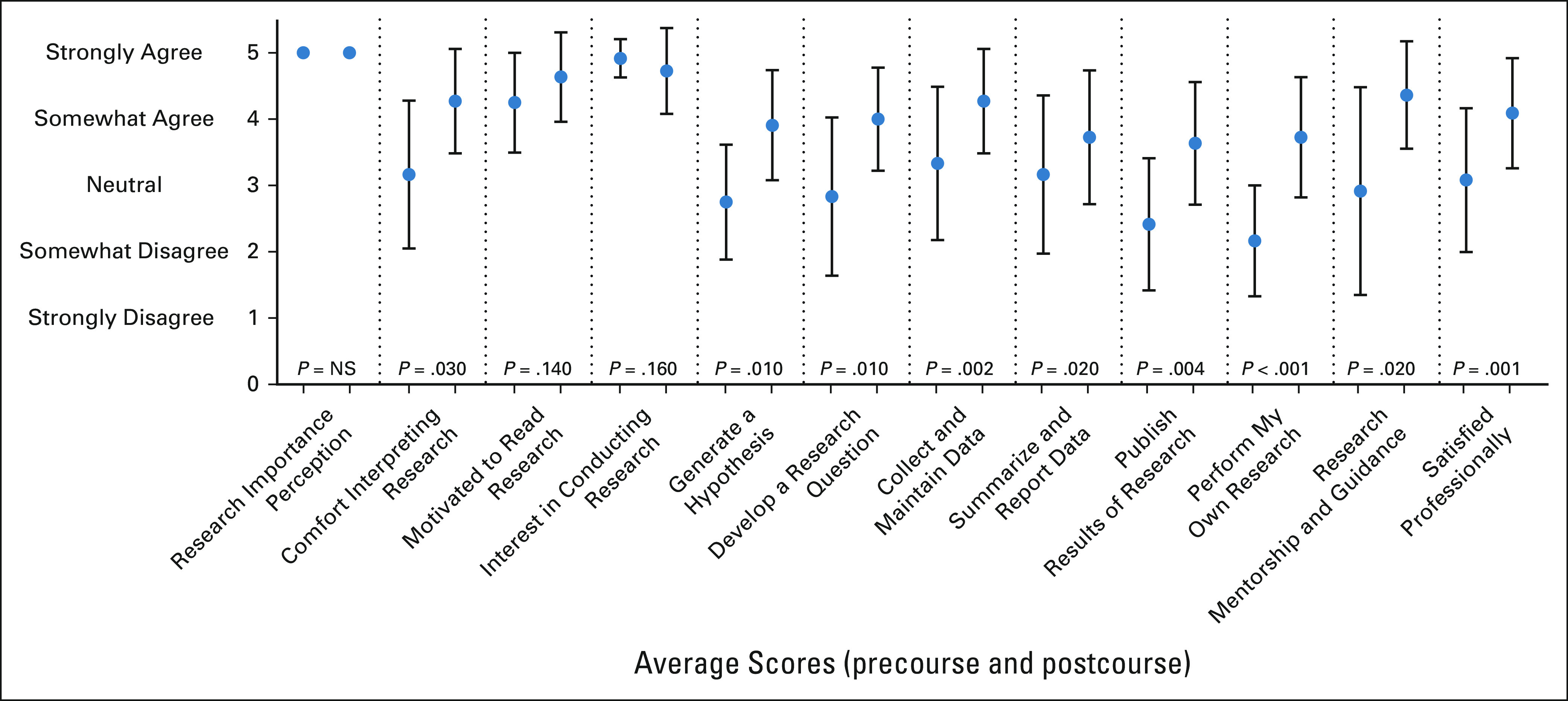
Average scores precourse (left hashmark within each category) and postcourse (right hashmark within each category) for 5-point Likert scale academic self-efficacy domains. NS, not significant.
The largest gains were noted in ability to independently perform research and the presence of research mentorship with an average increase of 1.5 points in both domains postcourse compared with precourse. In the three domains without a statistically significant difference, both high baseline and postcourse scores were observed in perception of research importance, motivation to read medical research, and interest in conducting research.
In the postcourse survey, the average Likert scale scores for “I have more knowledge about research after taking the course,” “The course stimulated a greater interest in me for research,” and “I would recommend this course to others” were 4.5 of 5.0, 4.8 of 5.0, and 5.0 of 5.0, respectively.
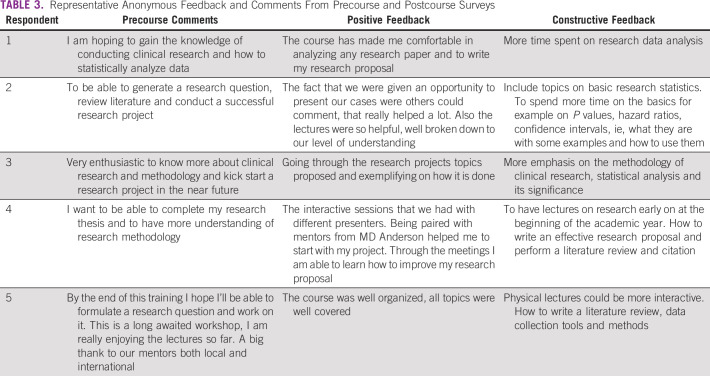
Representative anonymous comments of individual respondents from the precourse survey and positive and constructive feedback from the postcourse survey are summarized in Table 3. For example, respondent 1 stated “I am hoping to gain the knowledge of conducting clinical research and how to statistically analyze data” before the start of the lecture series and “The course has made me comfortable in analyzing any research paper and to write my research proposal” after the conclusion of the lecture series but desired “More time spent on research data analysis.”
TABLE 3.
Representative Anonymous Feedback and Comments From Precourse and Postcourse Surveys
Challenges identified by MDA peer mentors included the logistical difficulties associated with finding mutual times to meet because of busy clinical schedules and the time difference, perceived lack of protected time for faculty and registrars to meet and conduct research, limited experience of the mentees with practical topics such as literature review and data analysis, need for formal statistical support, and concerns regarding data quality and infrastructure.
DISCUSSION
The virtual CDH-MDA clinical research training program for Zambian oncology fellows led to marked increases in multiple academic self-efficacy domains among participants, formation of longitudinal mentorship groups with both peer trainees and faculty mentors, and development of numerous Zambian-led research protocols, demonstrating the feasibility of implementing a virtual clinical research training model, which may be especially relevant because of shifting international collaboration paradigms as a result of the COVID-19 pandemic.19 The burden of cancer in Africa is well-documented to be increasing rapidly, and among low human development index countries, most of which are located in sub-Saharan Africa, the incidence of cancer is projected to increase by 95% between 2020 and 2040.3 Despite this, Africa is responsible for only 2% of all research output globally.20 Developing local clinical research capacity has been identified as an important means to allow countries to define their own greatest health issues, propose cost-effective and evidence-based solutions, implement the interventions, and investigate their impact.21
The CDH-MDA academic partnership was first established in 2015 and has included in-country clinical education workshops and telementoring17 and an annual radiation biology and physics course.18 In addition, core faculty members from CDH and MDA meet weekly via teleconference to discuss joint initiatives and research. We found that the longstanding partnership between CDH and MDA and consistent communication were instrumental in the success of the present program by allowing for shared planning and program development, allocation of resources (ie, protected time for registrars), and rapid response to issues as they arose. The clinical research training program was initially planned to be an in-person workshop taking place over one week, but because of the ongoing COVID-19 global pandemic, the workshop was adapted to a virtual format. We found that a virtual format provided several key advantages, including the ability to engage true content experts to deliver the weekly lectures, leverage the full breadth of the institution to identify faculty mentors for all senior Zambian oncology fellows, engage peer trainee mentors in the program, allow for longitudinal collaboration over the course of the year, and reduce costs associated with implementation. For example, the biostatistics lecture was given by a senior biostatistician with a degree in education, the scientific writing lectures by a National Institutes of Health–funded investigator of scientific communication, the research ethics lecture by the Chairman of the MDA institutional review board, and the clinical trials lecture by a faculty participant in the ASCO/American Association for Cancer Research Methods in Clinical Cancer Research Workshop.22
There is growing interest in global health among US health professional trainees, and it is increasingly viewed as a viable academic career pathway.23 We found that the peer mentorship program generated significant, bilateral value by providing US peer mentors with a global health experience that can serve as a foundation for future academic opportunities and providing Zambian mentees with a highly accessible mentor at a comparable level of training. Peer mentorship has several distinct advantages, including being more approachable, increased time available for one-on-one teaching, closer proximity to age of mentee and level of understanding of subject matter, and high levels of energy and enthusiasm. Importantly, all peer mentors serving in the program had significant clinical research experience including previous peer-reviewed publications. The Zambian oncology fellows were able to receive a level of guidance on their research protocols that would have been difficult to provide with a faculty mentor alone. Mentors found that flexibility with the route of communication was helpful, as the Zambian fellows were typically more comfortable communicating through mobile apps than e-mail. Still, mentors noted challenges with coordinating meetings and responsiveness to communication, which might have in part been due to cultural factors that can significantly affect the mentorship relationship.24 As a result, we are implementing a global, culturally sensitive mentorship curriculum for peer mentors in the program.
We found high rates of baseline perception of research importance and interest in clinical research among all respondents. Although other similar training programs have used a competitive application process for selection of participants, our results demonstrate that even among an unselected group of postgraduate physician trainees in Africa, there may be high rates of perception of research importance and that furthermore, participating in a structured research training program may foster an interest in research that would not have otherwise occurred without the opportunity to participate in research.25 However, baseline scores in other clinical research domains were lower and demonstrated statistically significant gains postcourse compared with precourse. The largest numeric gains were made in ability to independently perform research and the presence of mentorship with an average increase of 1.5 points in both domains on a 5-point scale postcourse compared with precourse, indicating a highly relevant difference. These results suggest that our weekly lecture series and longitudinal mentorship program were successful in developing research skills.
Positive themes from open-ended postcourse feedback included clear lectures, meaningful relationships with mentors, and utility of presenting research proposals for feedback, whereas constructive themes included desire for more teaching on foundational topics, including statistical analysis, literature review, and data collection, and incorporation of self-assessment modules, which will be addressed in subsequent years of the program. Other research programs in development should consider spending additional time on fundamental research skills in this trainee population. Although the progress made in active research protocols thus far has been modest, with the most advanced protocol to date nearing the completion of data collection and no protocols having yet been completed, the Zambian clinical oncology fellows are also juggling a full-time clinical load and examinations. Our experience demonstrates an achievable yearlong timeline for research among clinical trainees and can be used to establish goals for other nascent training programs. The timeline also highlights the importance of initiating trainees in research early on in their program, longitudinal mentorship, and a long-term collaboration between partner institutions.
There have been numerous successful research training partnerships between developed countries and countries in Africa.9,10,14-16 Although these initiatives have shown the potential positive impact of north-south research partnerships, there remains a clear need for highly accessible clinical research training among African oncology fellows. However, to our knowledge, the only other published experience of a distant clinical research training program for oncology trainees has been the Accra-Toronto collaboration.25 This initiative by Vulpe et al involved a distant clinical research curriculum for five Ghanaian radiation oncology residents with 13 weekly seminars over videoconference followed by a 1-year long mentorship program for the top two residents. At the end of the program, two manuscripts were finalized and one was published. The Accra-Toronto collaboration and the present CDH-MDA partnership share core principles, and the success of both programs strengthens the case for the effectiveness of a distant-learning partnership model of clinical research training. Differences between the CDH-MDA partnership and Accra-Toronto collaboration include size (14 v five participants), allocation of mentorship (all eight senior fellows v the top two residents), and type of mentorship (combined faculty and peer v faculty only), respectively. There are merits for both the more inclusive CDH-MDA approach and the more selective Accra-Toronto approach, and either can be considered when developing a program on the basis of available resources and program goals.
Semistructured interviews with participants will be performed to understand the experience of African oncology trainees in a virtual, international clinical research training program, the results of which will be reported separately and will guide expansion of the program in subsequent years. A virtual education platform will host high-fidelity studio recordings of lectures; additional lectures on biostatistics, literature review, and data management; individual self-assessment modules; and a handbook on biostatistical methods. These resources will be made publicly available. Funding support will be available for resulting abstracts, conference presentations, and manuscripts. Future goals include the development of culturally sensitive training programs for peer mentors and establishment of structured mentorship expectations, goals, and timelines. The role of an in-person component to the program will be re-evaluated after the COVID-19 pandemic.
There was no competitive application process, meaning that participants were unselected in terms of level of interest in clinical research—this may represent a strength (inclusiveness and part of core fellowship curriculum, particularly important given the small number of oncologists in Africa and need to train leaders in clinical research) or limitation (more resource-intensive, relatively less likely to lead to a publication for the effort) depending on the context. The results described here were at the end of the initial year of the training program, and longer-term follow-up will be required to determine the impact of the course on participant careers, research productivity, and patient care in Zambia. There were shortcomings with the initial training program that we were made aware of through feedback from participants and mentors, which included topics that needed to be covered in greater detail, desired resources, and challenges with mentorship including baseline research knowledge, communication, and finding time to meet. We hope to address many of these issues in subsequent iterations of the training program.
In conclusion, clinical research rwill be essential to develop effective, evidence-based interventions to confront the rising burden of cancer and unique cancer challenges in Africa. The results from the initial virtual CDH-MDA clinical research training program for Zambian clinical oncology fellows, which led to marked increases in multiple academic self-efficacy domains, formation of longitudinal mentorship groups, and development of numerous Zambian-led research protocols, demonstrate the feasibility of implementing a virtual clinical research training model that features peer trainee mentors. A virtual approach has key advantages including ability to (1) engage content experts to deliver lectures, (2) leverage the full breadth of the institution to identify faculty and peer mentors, (3) allow for longitudinal collaboration, and (4) reduce costs associated with implementation. Our model can be a framework for other initiatives that seek to increase clinical research capacity in Africa during and after the era of COVID-19 with shifting international collaboration paradigms.
ACKNOWLEDGMENT
The authors would like to thank Chidinma Anakwenze, Lauren Andring, and Han Cun for their contributions as peer research mentors and Mark Chambers, Laurence Court, Grace Smith, Jolyn Taylor, Rebecca Tidwell, and Timothy Yap for their contributions as lecturers.
Dorothy C. Lombe
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Susan C. Msadabwe
Consulting or Advisory Role: Market Access Africa
Lilie L. Lin
Research Funding: AstraZeneca (Inst), Pfizer/Calithera (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune
No other potential conflicts of interest were reported.
SUPPORT
Supported by MD Anderson Cancer Center Support Grant No. P30CA016672. Funding for future development of the training program was provided by the Derek Harwood-Nash International Education Scholar Grant through the Radiological Society of North America (RSNA) and a MD Anderson Cancer Center Department of Radiation Oncology Strategic Initiatives (ROSI) grant.
AUTHOR CONTRIBUTIONS
Conception and design: Kevin Diao, Dorothy C. Lombe, Catherine K. Mwaba, Juliana Wu, Carrie A. Cameron, Elizabeth Y. Chiao, Susan C. Msadabwe, Lilie L. Lin
Administrative support: Elizabeth Y. Chiao, Susan C. Msadabwe, Kevin Diao, Dorothy C. Lombe, Lilie L. Lin
Provision of study materials or patients: Kevin Diao, Dorothy C. Lombe, Catherine K. Mwaba, Elizabeth Y. Chiao, Susan C. Msadabwe
Collection and assembly of data: Kevin Diao, Dorothy C. Lombe, Susan C. Msadabwe, Lilie L. Lin
Data analysis and interpretation: Kevin Diao, Dorothy C. Lombe, Darya A. Kizub, Elizabeth Y. Chiao, Susan C. Msadabwe, Lilie L. Lin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Dorothy C. Lombe
This author is a member of the JCO Global Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Susan C. Msadabwe
Consulting or Advisory Role: Market Access Africa
Lilie L. Lin
Research Funding: AstraZeneca (Inst), Pfizer/Calithera (Inst)
Travel, Accommodations, Expenses: AstraZeneca/MedImmune
No other potential conflicts of interest were reported.
REFERENCES
- 1. Adewole I, Martin DN, Williams MJ, et al. Building capacity for sustainable research programmes for cancer in Africa. Nat Rev Clin Oncol. 2014;11:251–259. doi: 10.1038/nrclinonc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamdi Y, Abdeljaoued-Tej I, Zatchi AA, et al. Cancer in Africa: The untold story. Front Oncol. 2021;11:650117. doi: 10.3389/fonc.2021.650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4. Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries—A systematic review. Int J Equity Health. 2018;17:37. doi: 10.1186/s12939-018-0748-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowsher G, Papamichail A, El Achi N, et al. A narrative review of health research capacity strengthening in low and middle-income countries: Lessons for conflict-affected areas. Glob Health. 2019;15:23. doi: 10.1186/s12992-019-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kayamba V, Mutale W, Cassell H, et al. Systematic review of cancer research output from Africa, with Zambia as an example. JCO Glob Oncol. 2021;7:802–810. doi: 10.1200/GO.21.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirigia JM, Wambebe C. Status of national health research systems in ten countries of the WHO African Region. BMC Health Serv Res. 2006;6:135. doi: 10.1186/1472-6963-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lingwood RJ, Boyle P, Milburn A, et al. The challenge of cancer control in Africa. Nat Rev Cancer. 2008;8:398–403. doi: 10.1038/nrc2372. [DOI] [PubMed] [Google Scholar]
- 9. Adedokun B, Nyasulu P, Maseko F, et al. Sharing perspectives and experiences of doctoral fellows in the first cohort of Consortium for Advanced Research Training in Africa: 2011-2014. Glob Health Action. 2014;7:25127. doi: 10.3402/gha.v7.25127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kayem ND, Rojek A, Denis E, et al. Clinical REsearch During Outbreaks (CREDO) training for low- and middle-income countries. Emerg Infect Dis. 2019;25:2084–2087. doi: 10.3201/eid2511.180628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fish M, Parkes J, Dharsee N, et al. POETIC (Program for Enhanced Training in Cancer): An initial experience of supporting capacity building for oncology training in sub-Saharan Africa. Oncologist. 2019;24:1557–1561. doi: 10.1634/theoncologist.2019-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Binagwaho A, Kyamanywa P, Farmer PE, et al. The human resources for health program in Rwanda—New partnership. N Engl J Med. 2013;369:2054–2059. doi: 10.1056/NEJMsr1302176. [DOI] [PubMed] [Google Scholar]
- 13. Manabe YC, Nambooze H, Okello ES, et al. Group mentorship model to enhance the efficiency and productivity of PhD research training in sub-Saharan Africa. Ann Glob Health. 2018;84:170–175. doi: 10.29024/aogh.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odhiambo J, Amoroso CL, Barebwanuwe P, et al. Adapting operational research training to the Rwandan context: The Intermediate Operational Research Training programme. Glob Health Action. 2017;10:1386930. doi: 10.1080/16549716.2017.1386930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramsay A, Harries AD, Zachariah R, et al. The structured operational research and training initiative for public health programmes. Public Health Action. 2014;4:79–84. doi: 10.5588/pha.14.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okello CD, Ddungu H, Omoding A, et al. Capacity building for hematologic malignancies in Uganda: A comprehensive research, training, and care program through the Uganda Cancer Institute-Fred Hutchinson Cancer Research Center collaboration. Blood Adv. 2018;2(suppl 1):8–10. doi: 10.1182/bloodadvances.2018GS111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lopez MS, Baker ES, Milbourne AM, et al. Project ECHO: A telementoring program for cervical cancer prevention and treatment in low-resource settings. J Glob Oncol. 2017;3:658–665. doi: 10.1200/JGO.2016.005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stecklein SR, Taniguchi CM, Melancon AD, et al. Radiation sciences education in Africa: An assessment of current training practices and evaluation of a high-yield course in radiation biology and radiation physics. JCO Glob Oncol. 2020;6:1631–1638. doi: 10.1200/GO.20.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bump JB, Friberg P, Harper DR. International collaboration and covid-19: What are we doing and where are we going? BMJ. 2021;372:n180. doi: 10.1136/bmj.n180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasprowicz VO, Chopera D, Waddilove KD, et al. African-led health research and capacity building—Is it working? BMC Public Health. 2020;20:1104. doi: 10.1186/s12889-020-08875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franzen SRP, Chandler C, Siribaddana S, et al. Strategies for developing sustainable health research capacity in low and middle-income countries: A prospective, qualitative study investigating the barriers and enablers to locally led clinical trial conduct in Ethiopia, Cameroon and Sri Lanka. BMJ Open. 2017;7:e017246. doi: 10.1136/bmjopen-2017-017246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Von Hoff DD, Clark GM, Coltman CA, et al. A grant-based experiment to train clinical investigators: The AACR/ASCO methods in clinical cancer research workshop. Clin Cancer Res. 2021;27:5472–5481. doi: 10.1158/1078-0432.CCR-21-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah SK, Nodell B, Montano SM, et al. Clinical research and global health: Mentoring the next generation of health care students. Glob Public Health. 2011;6:234–246. doi: 10.1080/17441692.2010.494248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sawatsky AP, Parekh N, Muula AS, et al. Cultural implications of mentoring in sub-Saharan Africa: A qualitative study. Med Educ. 2016;50:657–669. doi: 10.1111/medu.12999. [DOI] [PubMed] [Google Scholar]
- 25. Vulpe H, Vanderpuyne V, Yarney J, et al. Design and implementation of a distant-learning clinical research mentorship program: The Accra-Toronto Collaboration. JCO Glob Oncol. 2020;6:919–928. doi: 10.1200/JGO.19.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]