Abstract
Magnetic resonance imaging (MRI) has a key role to play at multiple steps of the radiotherapy (RT) treatment planning and delivery process. Development of high-precision RT techniques such as intensity-modulated RT, stereotactic ablative RT, and particle beam therapy has enabled oncologists to escalate RT dose to the target while restricting doses to organs at risk (OAR). MRI plays a critical role in target volume delineation in various disease sites, thus ensuring that these high-precision techniques can be safely implemented. Accurate identification of gross disease has also enabled selective dose escalation as a means to widen the therapeutic index. Morphological and functional MRI sequences have also facilitated an understanding of temporal changes in target volumes and OAR during a course of RT, allowing for midtreatment volumetric and biological adaptation. The latest advancement in linear accelerator technology has led to the incorporation of an MRI scanner in the treatment unit. MRI-guided RT provides the opportunity for MRI-only workflow along with online adaptation for either target or OAR or both. MRI plays a key role in post-treatment response evaluation and is an important tool for guiding decision making. In this review, we briefly discuss the RT-related applications of MRI in the management of brain, prostate, and GI malignancies.
INTRODUCTION
Magnetic resonance imaging (MRI) is an imaging modality on the basis of the principle of nuclear magnetic resonance. As hydrogen atoms constitute the major share of the human body, it enables the use of nuclear magnetic resonance for clinical imaging.1 Since the first human MRI images were acquired in 1977, MRI has evolved rapidly with the development of relatively faster image acquisition, increase in magnetic field strength, and improved image processing techniques.2,3 The ability of noninvasive characterization of internal anatomy attributed to better soft-tissue clarity and the development of functional sequences to capture the internal physiology have facilitated numerous clinical applications of MRI. In clinical practice, MRI plays a pivotal role in diagnosis, disease staging, treatment planning, response monitoring, and surveillance after treatment completion.
CONTEXT
Key Objective
Magnetic resonance imaging (MRI) constitutes an integral role in contemporary oncology practice. This review was aimed at discussing radiotherapy (RT)–related applications of MRI in the context of brain, prostate, and GI malignancies.
Knowledge Generated
MRI plays a crucial role in decision making, RT planning and delivery, and response evaluation. MRI protocols including standardized sequences and slice thickness are required for target volume and organs at risk delineation for individual disease sites. The advent of MRI-linear accelerator (MR-Linac) provides opportunities for precise treatment delivery and real-time treatment adaptation, potentially improving therapeutic ratio. The merits of using MR-Linac are being investigated in ongoing clinical trials, with recent evidence suggesting reduction in acute toxicities with prostate stereotactic body radiotherapy delivered on MR-Linac.
Relevance
This review summarizes the current and expanding role of MRI in brain, prostate, and GI malignancies to allow optimum integration with RT practice.
Radiation oncology practice is deeply intertwined with imaging, primarily aiding in target volume and organs at risk (OAR) delineation and computing radiotherapy (RT) doses in the planning process (Fig 1). With the advancement and development of conformal and high-precision techniques such as intensity-modulated radiotherapy, stereotactic radiosurgery, stereotactic body radiotherapy (SBRT), and particle beam therapy, the need for imaging modalities with better anatomical information has become essential.4,5 Insights from molecular imaging such as positron emission tomography (PET) and functional MRI have paved the way toward dose painting.6 Similarly, midtreatment volumetric and biological adaptation using morphological and functional MRI sequences accounting for changes to the target volumes and OAR can help improve the therapeutic ratio in the form of adaptive RT.7 Image-guided radiotherapy involving online imaging before treatment delivery has improved the precision and accuracy of treatment delivery.8 The traditional platforms for image-guided radiotherapy involve computed tomography (CT) built in the treatment unit. The integration of a compatible MRI scanner with a linear accelerator (linac) device has successfully led to an MR-linear accelerator (MR-Linac), which had been introduced in clinical practice, popularly known as MRI-guided radiotherapy (MRgRT).9,10 In this review, we briefly discuss the clinical applications of MRI in the management of brain, prostate, and GI malignancies from the perspective of radiation oncologists.
FIG 1.
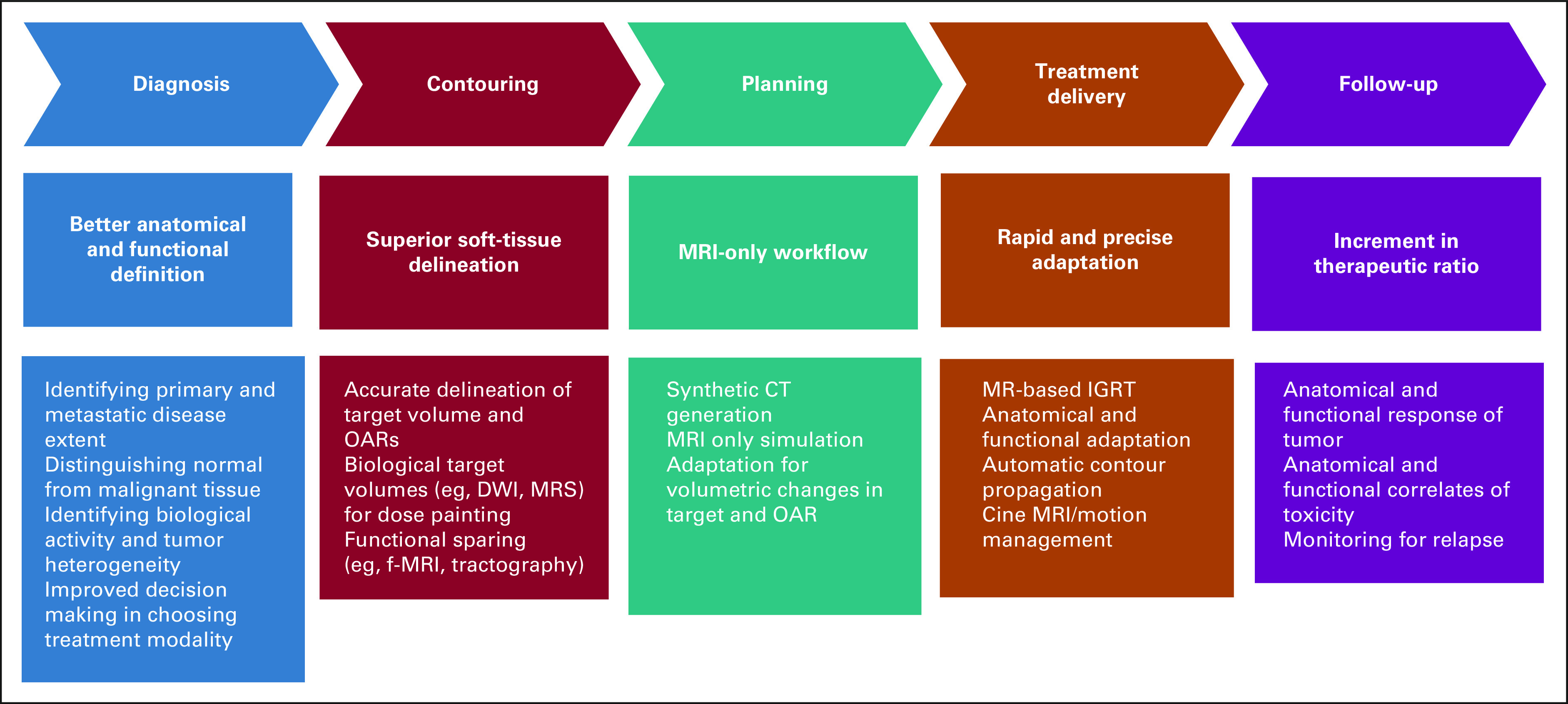
ROLE of MRI in radiation oncology workflow. CT, computed tomography; DWI, diffusion-weighted imaging; f-MRI, functional MRI; IGRT, image-guided radiotherapy; MRI, magnetic resonance imaging; MRS, spectroscopy; OAR, organs at risk.
ROLE OF MRI IN CONTEMPORARY RADIATION ONCOLOGY PRACTICE
CNS Malignancies
Rationale and parameters.
MRI forms an indispensable part of the contemporary management of CNS tumors. The superior soft-tissue resolution, true multiplanar imaging capability, and the capacity for innate multiparametric functional imaging such as diffusion-weighted imaging (DWI), intravoxel incoherent motion, perfusion imaging, chemical exchange saturation transfer, and blood oxygenation level-dependent predicate a decisive advantage for MRI over all other forms of cross-sectional imaging in this regard. Typically, field strengths of 1.5-3T are used for routine imaging of CNS tumors, with no clear advantage of the higher field strength in clinical practice.11 Imaging for diagnosis and response assessment usually requires axial T1 (pregadolinium and postgadolinium), axial T2, and fluid-attenuated inversion recovery (FLAIR) sequences at minimum, with DWI, perfusion-weighted imaging, and MR spectroscopy assisting initial diagnosis and later distinction between tumor progression and pseudoprogression.12,13 Thin slice (1-2 mm width) volumetric sequences such as 3-dimensional fast spoilt gradient (3D FSPGR) and 3D FLAIR offer the advantages of rapid acquisition, good image reconstruction, and increased lesion detection rates, facilitating accurate delineation and ultra high-precision RT planning.14-16 Additional sequences such as steady-state free precession sequences (CISS/FIESTA sequence as used by vendors) and fat suppression sequences are often used in the delineation of skull base tumors or targets in close relation to the brain stem and cranial nerves (eg, chordoma, chondrosarcoma, meningioma, and schwannoma) because of anatomical clarity. Acquisition of thin-slice MRI sequences aids in accurate estimation of lesion size as higher slice width can lead to overestimation of the delineated structures because of interslice interpolation.17 Initial imaging should ideally be performed no later than 72 hours after surgery, else delayed by 2 weeks to avoid obfuscation of imaging findings by blood products and postoperative changes.12 Imaging follow-up is usually performed at 4-6 weeks after RT conclusion, at completion of planned adjuvant therapy, and on clinical suspicion of progression or symptomatic worsening.18 Diffusion tensor imaging has proven beneficial in detecting radiation-induced demyelination and axonal degeneration,19,20 resulting in neurocognitive deterioration providing a window of changes before clinical manifestation and early interventions.
Clinical applications.
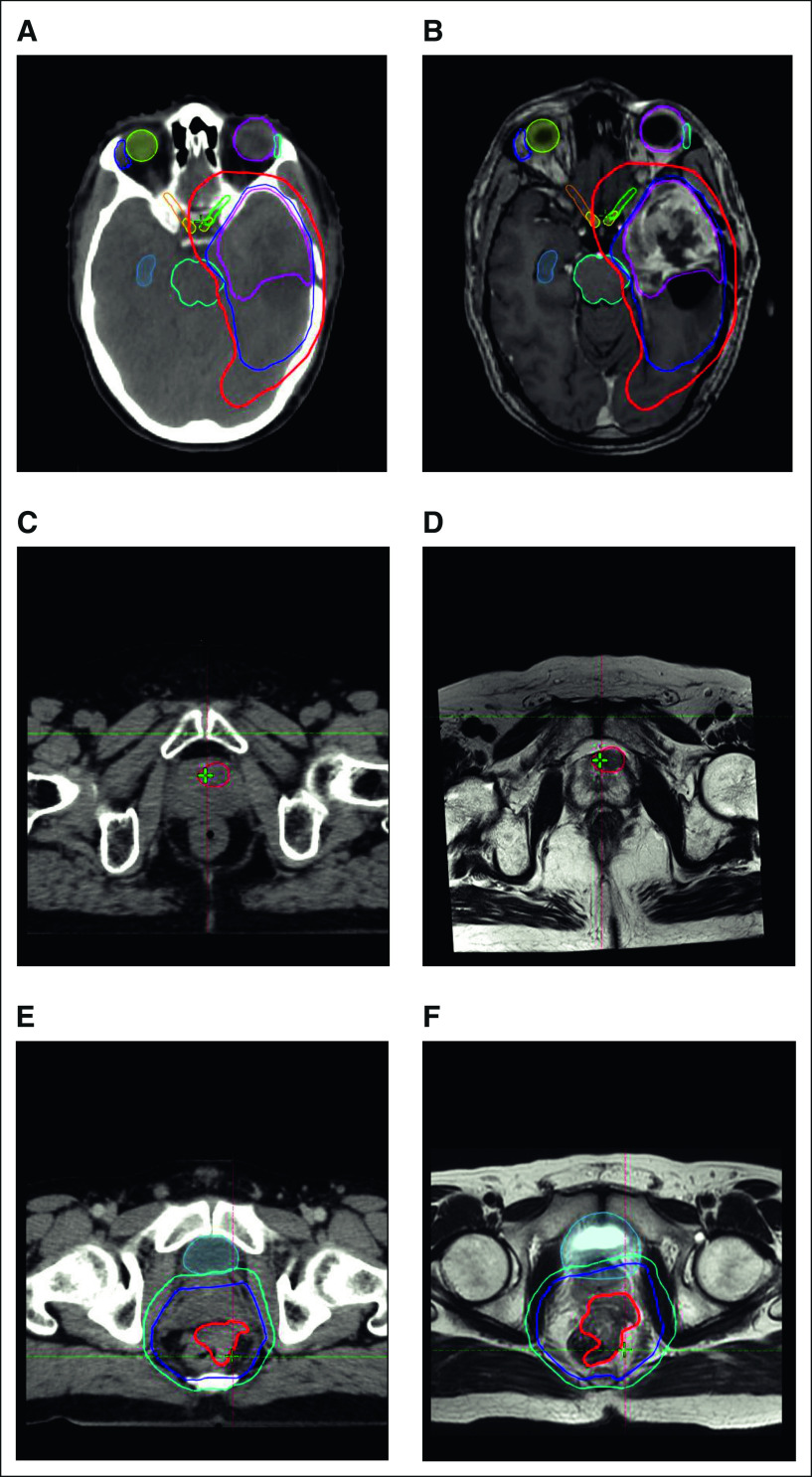
MRI forms a crucial part of target delineation in primary and metastatic tumors in the CNS. Complementary sequences (T1 postcontrast MRI to delineate the enhancing residual and areas of leptomeningeal dissemination and T2/FLAIR to distinguish areas of infiltrative and nonenhancing tumors) are typically used for comprehensive delineation.21,22 MRI image fusion is typically achieved with a high degree of accuracy in the brain and is facilitated by the presence of rigid bony anatomical markers and limited movement of the brain within the calvarium. Rigid registration algorithms are usually sufficient for image fusion.23,24 The accuracy of image fusion is confirmed by matching standard anatomical references (clinoid processes, bony sella, tentorium cerebelli, and vertebral artery). The standard imaging protocols share the common caveat of inability to distinguish between infiltrative disease and vasogenic edema with reliability while delineating the clinical target volume. Additionally, contemporary recommendations on MRI anatomy facilitate delimitation of the clinical target volume with respect to anatomical barriers and provide useful adjuncts as practice shifts to MRI-based planning and delivery in the current decade25 (Figs 2A and 2B).
FIG 2.
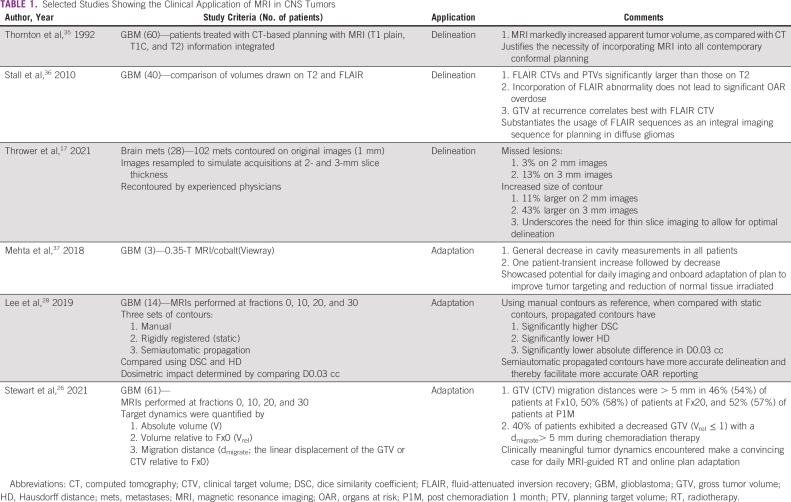
Composite diagram of CT and MRI used for radiation planning for brain, prostate, and GI malignancies. Representative CT and MRI scans acquired during radiation planning process. (A) Axial CT and (B) corresponding axial MRI T1-weighted contrast-enhanced sequence for a patient with glioblastoma. The target volumes are seen over the left temporal lobe: GTV (magenta), CTV (blue), and PTV (red). The extension of PTV in the basifrontal region was a result of expansion from extension of CTV in the superior slices (not seen in this image). (C) Axial CT and (D) corresponding axial T2W MRI for a patient with prostate cancer. Dominant intraprostatic lesion clearly visualized (in red) on the MRI, whereas it could not be discerned on the planning CT images, thus facilitating dose escalation to the DIL. (E) Axial CT and (F) corresponding axial T2W MRI for a patient with locally advanced rectal adenocarcinoma. Target volumes include GTV (red), CTV (purple), and PTV (light blue). The extension of the gross disease into the prostate could be accurately seen only on the MRI. CT, computed tomography; CTV, clinical target volume; DIL, dominant intraprostatic lesion; MRI, magnetic resonance imaging; PTV, planning target volume.
The target volumes (postoperative cavity, surrounding infiltrative disease/vasogenic edema) in gliomas can undergo significant changes during the course of RT.26 The dynamic changes offer the opportunity to modify elective target volumes as areas of putative tumor tissue reduce in responders to therapy. Similarly, special consideration is to be given to cystic tumors such as craniopharyngioma, wherein a significant proportion of patients have been reported to have changes in cyst dimensions requiring treatment modifications during a course of fractionated RT spanning over several weeks.27 Automatic contour propagation facilitates real-time adaptation with accurate delineation in this regard.28 The significant challenges in this regard remain the accurate determination of the shifting tumor-normal tissue interface on anatomic and functional imaging. Limited evidence does suggest that it may be possible to distinguish the former from the latter through higher-order radiomic analysis, allowing one to potentially de-escalate RT in areas of response (reducing radiation necrosis and corticosteroid and bevacizumab usage rates) while intensifying therapy in voxels suggestive of radioresistance.29 Although anatomical variations in the target and OARs are demonstrated during the course of fractionated RT, the clinical merits either in terms of improving disease control or reducing toxicities need to be proven from prospective clinical trials.
Functional MRI constitutes a profusion of sequences that allow for comprehensive biological assessment of a tumor and is emerging as a useful adjunct for optimizing treatment. It has long been known that metabolic abnormalities (increased choline, reduced n-acetyl aspartate, and increased lipid lactate) exist beyond the tumor.30 In addition, areas with increased choline: n-acetyl aspartate ratios have also been found to correlate with adverse outcomes and are currently being targeted for dose escalation in clinical trials.31 Additional emerging areas for assessing tumor response include chemical exchange saturation transfer MRI, which can potentially detect both early response and tumor progression without the necessity for exogenous contrast.32 Noncontrast-based studies providing a combination of both diffusion and perfusion matrices such as intravoxel incoherent motion33 provide another powerful tool for assessing response to treatment.34 It is quite likely that such protocols in isolation or combination will provide opportunities for real-time biological adaptation in the setting of proliferation of MRI-based RT delivery systems with rapid onboard functional imaging capability. Table 1 shows selected studies for the role of MRI related to RT for brain tumors.
TABLE 1.
Selected Studies Showing the Clinical Application of MRI in CNS Tumors
Prostate Cancer
Rationale and parameters.
MRI is central to external beam radiotherapy planning for prostate cancer. Multiparametric MRI (mp-MRI) is the recommended technique in prostate cancer combining anatomical with functional imaging. This includes a high-resolution T2-weighted imaging (T2WI) with at least two functional MRI techniques. DWI, dynamic contrast-enhanced (DCE) perfusion imaging, and occasionally MR spectroscopy are commonly used. 3-T scanners are preferred to 1.5-T as they provide a higher signal to noise ratio allowing for better structural and functional details.38 The study is performed on an external-phased array coil; using an endorectal coil does not offer significant benefit and may be avoided. Thin-slice (3 mm without interslice gap) T2W images with a small field of view (FOV) are used to depict prostate anatomy.39 The high spatial resolution enables accurate assessment of extracapsular extension and seminal vesicle invasion. Addition of DWI aids in differentiating malignant from benign lesions with the former having restricted diffusion.40
Clinical applications.
MRI plays a pivotal role in target volume delineation in prostate RT. The apex and base of the prostate are often poorly visualized on CT. MRI helps to differentiate the prostatic apex from the genitourinary diaphragm and the penile bulb and the base of the prostate from the bladder wall.
One of the biggest challenges for the use of MRI for volume delineation is the accuracy of CT and MRI coregistration as pelvic organs have nonrigid anatomy. The CT and T2W MRI images are fused using rigid automatic registration algorithm (on the basis of bony landmarks) and thereafter can be adjusted manually. For patients who have gold fiducial seeds implanted before the simulation scans, the images are aligned on the basis of the midpoint of the gold seeds. The prostate is then evaluated in all three planes to ensure precise anatomical superimposition. The presence of gold fiducial markers offers advantages for image coregistration as it overcomes the issue of accurately defining bony landmarks on MRI. Wegener et al41 showed that with the use of gold markers, the CT and MRI matching precision was within 2 mm.
Some of the issues precluding accurate CT-MRI coregistration are variations in rectal and bladder filling and patient position for the two scans with CT scan usually performed on a flat couch with knee support and the MRI being performed on a rounded table top unless using a dedicated MRI simulator. Chen et al42 showed that significant fusion uncertainties of > 4 mm were seen in 8.6% (anteroposterior direction) and 11.4% (superoinferior direction) of the patients with higher difference when scans were performed on different days. Patients with gold seed markers in situ had less prostate fusion uncertainties.
Prostate volume, as delineated on MRI, is nearly 30%-40% smaller than that delineated on CT with less interobserver variability.43-47 The maximum discrepancy in the two volumes is in the region of the prostatic apex and at the base of the seminal vesicles. Reduction in the target volumes potentially translates into better OAR sparing. Steenbakkers et al48 showed that the rectal wall for CT-delineated prostate plans received 5.1 Gy higher equivalent uniform dose, and the penile bulb received 11.6 Gy higher mean dose than the MRI-delineated prostate plans. This also meant that allowing for the same rectal wall dose, the planning target volume (PTV) dose could be escalated from 78 Gy to 85 Gy using plans on the basis of MRI delineation of the prostate. Ali et al49 compared intensity-modulated radiotherapy plans generated using CT-MRI delineation versus CT alone and found a statistically significant reduction in dose to the bladder and rectum with an approximately 22% reduction in Gr2 GU toxicity for CT-MRI patients, as compared with CT alone.
Patients with prostate cancer who develop local recurrence tend to do so at the site of the dominant intraprostatic lesion (DIL).50 mp-MRI provides excellent visualization of the DIL and has allowed the escalation of dose to the DIL to > 90 Gy51,52 (Figs 2C and 2D). The FLAME trial randomly assigned patients with localized prostate cancer to either standard RT (77 Gy to the entire prostate) or an additional integrated focal boost to the DIL to a dose of upto 95 Gy. The dose-escalation arm showed a superior biochemical disease-free survival (92% v 85%, P < .001) at 5 years with no difference in overall survival or toxicity.53 Most studies for DIL boost have used a combination of T2WI plus DCE plus DWI for delineation of the DIL. However, recent studies have suggested that the actual DIL may correlate better with the volume delineated on Ga 68 prostate membrane–specific antigen PET-CT. Zamboglou et al54 reported the combined use of prostate membrane–specific antigen PET CT and mp-MRI for the delineation of DIL and correlated it with the tumor control probability (TCP) on the basis of histology. On average, 86% ± 10%, 74% ± 17%, and 93% ± 5% of GTV as seen on the histology specimen overlapped with PTV generated on PET, MRI, and combined PET/MRI, respectively. The plan generated using combined information from PET and MRI had significantly higher TCP values than either PET or MRI alone.
MRI as the sole imaging modality for RT treatment planning is gaining ground saving additional CT scan required for RT planning and eliminating the uncertainties from coregistration. As part of the MRI-only workflow, a pseudo-CT or synthetic CT is generated for dose computation. The methods for generating synthetic CT can be classified into voxel-based, atlas-based, and hybrid methods,55 with expected dose differences performed on synthetic images compared with standard CT being within 1%.56,57 MRI-only workflow also enables automatic delineation of prostate and OARs which can be manually adjusted. Patient setup for treatment is achieved by matching synthetic DRRs with a success rate of > 90%58,59 with constraints in remaining 10% because of inability to accurately identify gold fiducial markers appearing as signal void. Other issues include artifacts generated by metallic implants,59 large separation resulting in body contour reaching outside the FOV, and image distortion related to motion artifacts.
Interfraction and intrafraction variation of the prostate during RT has significant dosimetric and clinical implications. The use of MR-Linac can improve accuracy of delivery and combine it with real-time adaptive planning. Online matching of prostate using MRI is more accurate and thus can also be a factor in reducing PTV margins.60 The use of cine-MRI during beam delivery affords the option to intervene in the event of extreme anatomical changes. Using motion monitoring and gating, it has been reported that 2D shifts during treatment are required in > 20% of all delivered fractions.61 Table 2 shows selected studies highlighting the role of MRI in various aspects of RT for prostate cancer.
TABLE 2.
Selected Studies Showing the Clinical Application of MRI in Prostate Cancer
Multiple prospective studies exploring the utility of MR-Linac for prostate RT are currently underway. Recently, the interim analysis from the phase III randomized MIRAGE study was presented65 comparing SBRT for localized prostate cancer (40 Gy/5 fr) using CT versus MRI guidance. The primary end point was acute grade ≥ 2 GU toxicity within 90 days. One hundred patients were evaluated (51 CT and 49 MRI arm). MRI-guided SBRT had significantly lower acute grade ≥ 2 GU and GI toxicity (22.4% v 47.1%, P = .01 and 0 vs 13.7%, P = .01, respectively). Patient-reported outcome in the form of EPIC-26 bowel domain scores was also in favor of the MRI-guided SBRT arm. Of note, the PTV margins used for MRI guidance were smaller than those for CT guidance (2 mm and 4 mm, respectively) and could have contributed to the large difference in the effect between the two arms.
GI Malignancies
Rationale and parameters.
MRI has had a far-reaching impact in the management of GI malignancies right from staging, RT treatment planning, and execution to follow-up. MRI for rectal cancer should be performed using a 1.5-T or 3-T scanner with phased array coil positioned from the sacral promontory to 10 cm below the pubic symphysis.66 The use of an endorectal coil is not beneficial.67 The standard rectal MRI protocol includes 2D FSE T2-weighted non-fat–suppressed sequences. The sagittal series from one pelvic sidewall to another locates the tumor. Axial images are taken with a large FOV covering the entire pelvis and a smaller FOV with < 3 mm slice thickness axial and coronal to the long axis of the tumor. For low-lying rectal tumors, high-resolution coronal images are used to demonstrate levator muscles, sphincter complex, and intersphincteric plane. These sequences have high accuracy for identifying invasion of adjacent organs and mesorectal fascia and for extramural vascular invasion.67
Traditionally, for liver lesions, multiphase CT or MRI is used for imaging. MRI has been shown to have higher sensitivity compared with CT scan for the diagnosis of hepatocellular carcinoma, especially for lesions < 1 cm.68 The minimum specifications for liver MRI include the use of a minimum 1.5-T scanner with phased array torso coil. The minimum sequences to be acquired are T2-weighted (with and without fat saturation), T1-weighted in- and out-of-phase images, dynamic postcontrast gadolinium T1-weighted gradient echo sequence (3D preferable), and preferably DWI. The dynamic sequences would include late arterial phase (30-35 seconds postcontrast), portal venous phase (60-70 seconds postcontrast), and delayed phase (3-5 minutes) with < 5 mm slice thickness.69 Patients need to hold breath similarly for each sequence. The addition of DWI increases the detection rates, especially for smaller tumors.70 There is also emerging use of hepatobiliary-specific contrast agents such as gadoxetate disodium, which is progressively transported into hepatocytes and excreted through the bile ducts.
Clinical applications.
MRI is becoming a cornerstone in the RT planning process for upper abdominal tumors. Voroney et al compared MRI and CT-derived target volumes for liver tumors (primary and metastases) and found significant differences in the median percentage surface area difference. The median values for the percentage of surface area differing by 3 mm and 5 mm in spatial position between CT-GTV and MRI-GTV were 55% and 26%, respectively, with certain tumor foci visible only on MRI.71 Pech et al72 showed that the volume of liver metastases contoured using MRI was significantly larger than that on CT, with the difference between the target volumes being 181% for T1w images, 178% for contrast-enhanced T1w, and 246% for T2w sequences.
MRI-based target volume delineation in rectal cancer has been studied in a limited number of patients, and it has been shown that the MRI-derived volume is smaller than CT with significant differences when anal canal and sigmoid are involved73 (Figs 2E and 2F). Issues with CT and MRI coregistration owing to bladder filling and rectal distention at the time of the two scans preclude the routine use of MRI for external beam radiotherapy planning in rectal cancers. The best use of MRI for RT planning in rectal cancers may be for dose escalation wherein the T2 intermediate bright tumor can be accurately delineated and selectively boosted to a higher dose.74
Managing motion of upper abdominal organs is a significant issue during RT planning and execution. Breathing-related motion artifacts during planning CT acquisition lead to incorrect target delineation, altered dosimetry, and eventually excessive PTV margins. Cine-MRI can be used to directly visualize the 3-dimensional tumor motion. Studies for liver motion using cine-MRI have demonstrated that four-dimensional CT scan underestimated motion while fluoroscopy overestimated motion relative to cine-MRI.75 Pancreatic motion as assessed using cine MRI ranges from 6 to 34 mm, suggesting individualized PTV margins.76
Table 3 summarizes selected studies highlighting the role of MRI in various aspects of RT for GI malignancies.
TABLE 3.
Selected Studies Showing the Clinical Application of MRI in GI Tumors
MR-guided RT is most suitable for sites where isodensity on CT does not allow discrimination of targets, especially if mobile. This makes the upper abdomen an ideal candidate for the application of this modality, considering the growing role of SBRT for primary or metastatic liver tumors and pancreatic cancers to improve therapeutic ratio. A panel of radiation oncologists and radiologists with experience in MRgRT have published an atlas for OAR contouring of upper abdomen.79 Peristalsis-related motion artifacts create difficulties for delineation in MR online workflow. However, drinking a glass of water shortly before the treatment fraction may help in visualizing structures, and antiperistaltic agents may reduce motion artifacts. Continuous real-time 2D cine-MRI is used to monitor target motion, thus obviating the need for implanting fiducial markers.80 Changes in stomach filling and bowel distention in close proximity to targets call for online adaptation, especially for peripheral liver tumors and pancreatic lesions. There are two kinds of adaptive workflows: adapt to shape and adapt to position. Adapt to shape entails an adaptation of structures as seen on the day of treatment, whereas adapt to position refers to an isocenter shift because of the inability to shift the couch on the 1.5-T MR-Linac. Henke et al demonstrated the use of MRgART in their study of SBRT (50 Gy/5 fr) for metastases or unresectable abdominal tumors, wherein all constraints were met on initial radiation planning. However, for 81 of the 97 fractions, a daily adapted plan was deemed superior. Three quarters of the plans were adapted because of violation of an OAR constraint while the rest were performed to improve target coverage. No ≥ grade 3 toxicities were observed in the 15-month follow-up period.77
MRI in conjunction with sigmoidoscopy for response evaluation after neoadjuvant chemoradiation in rectal cancers has heralded the wait and watch policy for patients with complete clinical response.81,82
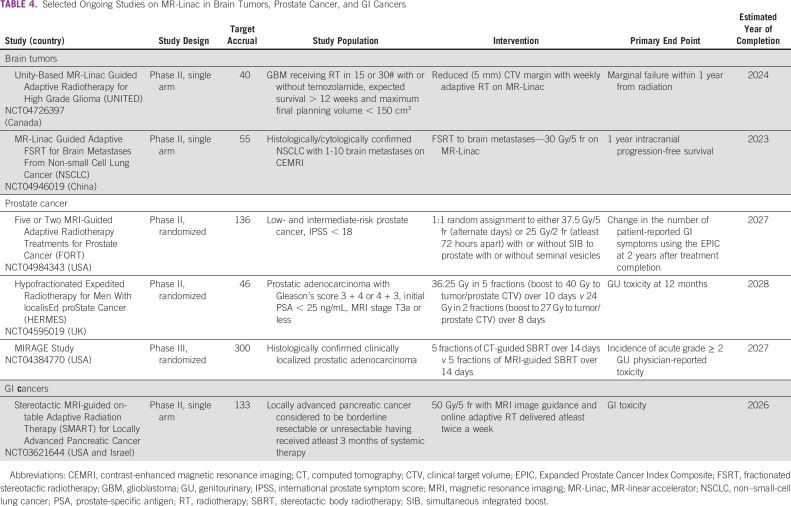
Table 4 summarizes selected ongoing studies on MR-Linac for brain, prostate, and GI malignancies.
TABLE 4.
Selected Ongoing Studies on MR-Linac in Brain Tumors, Prostate Cancer, and GI Cancers
FUTURE DIRECTIONS
The field of medical imaging and MRI is undergoing continuous refinements with contributions from physics, computer science, and related disciplines. Ultra high-field MRI systems using 7T have been introduced in clinical practice, and 10.5T MRI has been tested in humans recently to generate better structural and functional information from enhanced signal-to-noise and contrast-to-noise ratios.83,84 The contribution of artificial intelligence in quantitative analysis of medical imaging is an active area of research and is more popularly known as radiomics.85 With MRI used in multiple steps of radiation oncology practice, radiomic analysis is expected to have a significant impact in the future to lead the way toward personalized radiation therapy.85,86 One of the major advances in the recent era in the therapeutic delivery of RT has been the introduction of MR-Linac in clinical practice. As described in previous sections, the clinical application of MRgRT is still in its infancy, with two MR-Linacs introduced for patient treatment in the past 5 years. Generation of TCP and normal tissue complication probability models from daily MRgRT along with rapid contour propagation and plan optimization algorithms provide the window for real-time treatment adaptation as well as dose modification (escalation/de-escalation) for target and OARs87,88 (Fig 3). With ongoing conceptual refinements and applications, improving the precision of treatment delivery and better provision of adaptive RT, the actual clinical merits in toxicity reduction and/or better control rates need to be solicited in the future, compared with the available linac-based RT. Inspired by the IDEAL (Idea, Development, Exploration, Assessment, and Long-term evaluation) recommendations as described for the surgical development process,89 the concept of R-IDEAL framework has been introduced for radiation oncology innovations.90 Given the higher cost of the commercially available MR-Linacs compared with standard linear accelerators, it will be necessary to critically analyze the forthcoming evidence in the context of cost-benefit analysis, which is included in stage 3 of the R-IDEAL framework. The MR-Linac platform entails higher time on couch for the patient and demands increased human resources with the involvement of therapists, physicists, and oncologists. With artificial intelligence–based algorithms, fast and robust real-time optimization procedures, modifications of contour, and planning can potentially make the adaptive workflow more efficient and less time-consuming. Further technological advances in the accelerator device of the MR-Linac with the ability to deliver higher-energy beams, thin-width microleaf collimators are desired in the future to improve radiation conformality. Finally, MRI provides opportunities for delivery of anticancer therapies such as MR-guided focused ultrasound, which can be used to induce hyperthermia, temporary opening of the blood-brain barrier, triggering drug delivery, and microbubble (ultrasound contrast) stimulation as radiosensitizers.91-94
FIG 3.
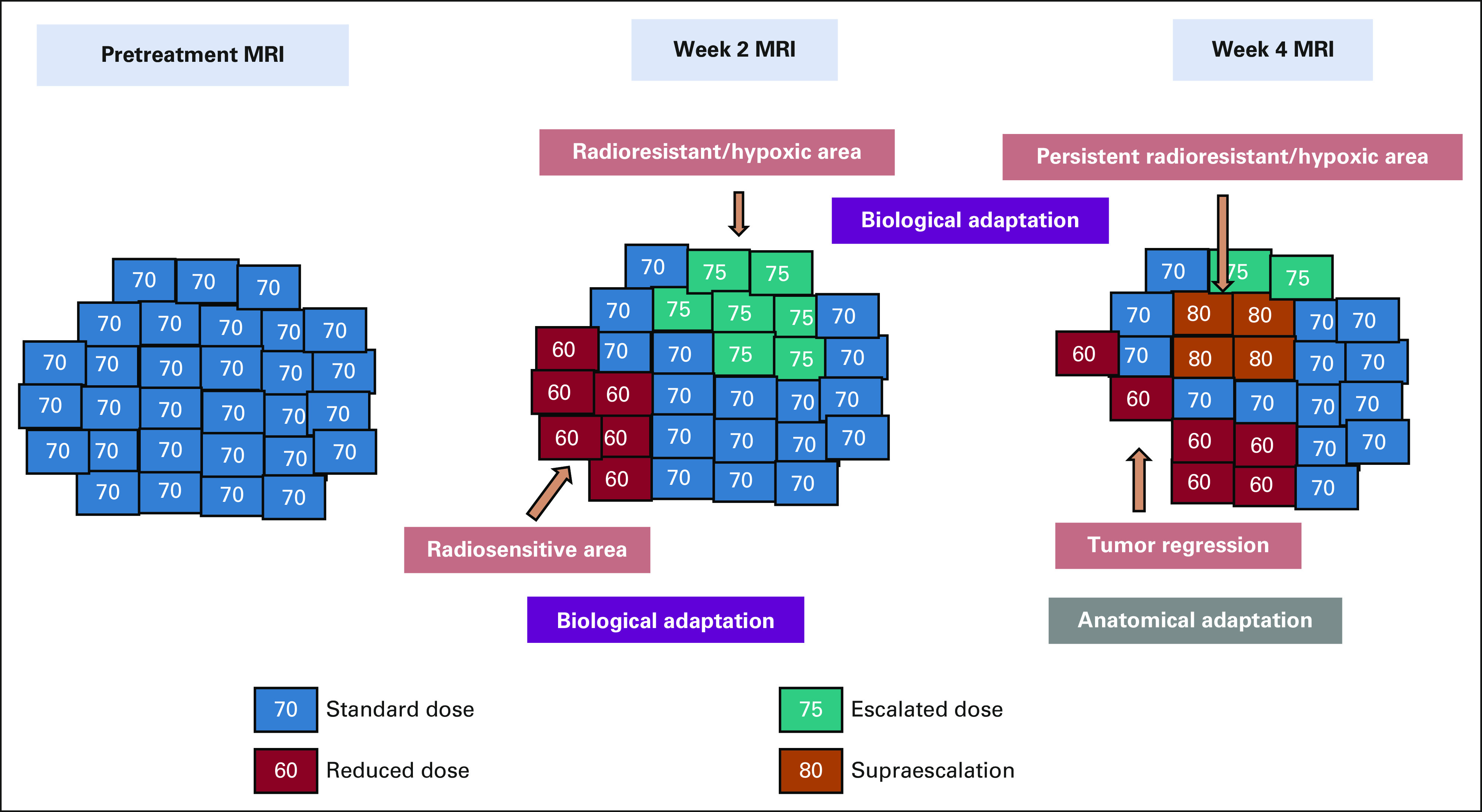
Schematic workflow for potential MRI-guided adaptive radiotherapy undertaken in a course of fractionated RT MRI is performed at week 2 and week 4 during radiation. Anatomical adaptation is undertaken for morphological changes as detected on midtreatment MRI for target volumes and organs at risk. Biological adaptation with different dose levels is performed on the basis of findings from functional MRI, with areas of refractory disease escalated to a higher dose and areas with response de-escalated to a relatively lower dose level. MRI, magnetic resonance imaging; RT, radiotherapy.
In conclusion, the contemporary practice of radiation oncology involves MRI in multiple instances including diagnosis, treatment planning, treatment delivery, midtreatment adaptation, response assessment, and surveillance. MR-Linac has been introduced in clinical practice recently is promising for real-time adaptation, improving the therapeutic ratio, although future clinical studies are warranted to establish the clinical advantages. Further developments in functional imaging sequences and quantitative imaging analysis incorporating artificial intelligence strategies are expected to have significant contributions in the future to pave the way toward precision oncology.
Shashank Srinivasan
Employment: GlaxoSmithKline
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Shashank Srinivasan, Archya Dasgupta, Akshay Baheti, Tejpal Gupta, Vedang Murthy
Provision of study materials or patients: Shashank Srinivasan, Archya Dasgupta, Vedang Murthy
Collection and assembly of data: Shashank Srinivasan, Archya Dasgupta, Abhishek Chatterjee, Akshay Baheti, Vedang Murthy
Data analysis and interpretation: Shashank Srinivasan, Akshay Baheti, Reena Engineer, Vedang Murthy
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Shashank Srinivasan
Employment: GlaxoSmithKline
No other potential conflicts of interest were reported.
REFERENCES
- 1. Pooley RA. AAPM/RSNA physics tutorial for residents: Fundamental physics of MR imaging. Radiographics. 2005;25:1087–1099. doi: 10.1148/rg.254055027. [DOI] [PubMed] [Google Scholar]
- 2. Edelman RR. The history of MR imaging as seen through the pages of radiology. Radiology. 2014;273(2 suppl):S181–S200. doi: 10.1148/radiol.14140706. [DOI] [PubMed] [Google Scholar]
- 3. Plewes DB, Kucharczyk W. Physics of MRI: A primer. J Magn Reson Imaging. 2012;35:1038–1054. doi: 10.1002/jmri.23642. [DOI] [PubMed] [Google Scholar]
- 4. Garibaldi C, Jereczek-Fossa BA, Marvaso G, et al. Recent advances in radiation oncology. Ecancermedicalscience. 2017;11:785. doi: 10.3332/ecancer.2017.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. FitzGerald TJ, Rosen MA, Bishop-Jodoin M. The influence of imaging in the modern practice of radiation oncology. Int J Radiat Oncol Biol Phys. 2018;102:680–682. doi: 10.1016/j.ijrobp.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 6. Bentzen SM, Gregoire V. Molecular imaging-based dose painting: A novel paradigm for radiation therapy prescription. Semin Radiat Oncol. 2011;21:101–110. doi: 10.1016/j.semradonc.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keall P, Poulsen P, Booth JT. See, think, and act: Real-time adaptive radiotherapy. Semin Radiat Oncol. 2019;29:228–235. doi: 10.1016/j.semradonc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 8. Jaffray DA. Image-guided radiotherapy: From current concept to future perspectives. Nat Rev Clin Oncol. 2012;9:688–699. doi: 10.1038/nrclinonc.2012.194. [DOI] [PubMed] [Google Scholar]
- 9. Corradini S, Alongi F, Andratschke N, et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat Oncol. 2019;14:92. doi: 10.1186/s13014-019-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall WA, Paulson ES, van der Heide UA, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: A review. Eur J Cancer. 2019;122:42–52. doi: 10.1016/j.ejca.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tselikas L, Souillard-Scemama R, Naggara O, et al. Imaging of gliomas at 1.5 and 3 Tesla—A comparative study. Neuro-Oncol. 2015;17:895–900. doi: 10.1093/neuonc/nou332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 13. Lundy P, Domino J, Ryken T, et al. The role of imaging for the management of newly diagnosed glioblastoma in adults: A systematic review and evidence-based clinical practice guideline update. J Neurooncol. 2020;150:95–120. doi: 10.1007/s11060-020-03597-3. [DOI] [PubMed] [Google Scholar]
- 14. Metcalfe P, Liney GP, Holloway L, et al. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12:429–446. doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tome WA, Mehta MP, Meeks SL, et al. Fractionated stereotactic radiotherapy: A short review. Technol Cancer Res Treat. 2002;1:153–172. doi: 10.1177/153303460200100301. [DOI] [PubMed] [Google Scholar]
- 16. Chagla GH, Busse RF, Sydnor R, et al. Three-dimensional fluid attenuated inversion recovery imaging with isotropic resolution and nonselective adiabatic inversion provides improved three-dimensional visualization and cerebrospinal fluid suppression compared to two-dimensional flair at 3 Tesla. Invest Radiol. 2008;43:547–551. doi: 10.1097/RLI.0b013e3181814d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thrower SL, Al Feghali KA, Luo D, et al. The effect of slice thickness on contours of brain metastases for stereotactic radiosurgery. Adv Radiat Oncol. 2021;6:100708. doi: 10.1016/j.adro.2021.100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–29. doi: 10.1016/S1470-2045(17)30194-8. [DOI] [PubMed] [Google Scholar]
- 19. Mabbott DJ, Noseworthy MD, Bouffet E, et al. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: Correlation with IQ. Neuro Oncol. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chapman CH, Zhu T, Nazem-Zadeh M, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol. 2016;120:234–240. doi: 10.1016/j.radonc.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kruser TJ, Bosch WR, Badiyan SN, et al. NRG brain tumor specialists consensus guidelines for glioblastoma contouring. J Neurooncol. 2019;143:157–166. doi: 10.1007/s11060-019-03152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niyazi M, Brada M, Chalmers AJ, et al. ESTRO-ACROP guideline “target delineation of glioblastomas.”. Radiother Oncol. 2016;118:35–42. doi: 10.1016/j.radonc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 23. Iqbal K, Saima A, Aqeel M, et al. Brain gliomas CT-MRI image fusion for accurate delineation of gross tumor volume in three dimensional conformal radiation therapy. OMICS J Radiol. 2015;4 [Google Scholar]
- 24. Guo L, Shen S, Harris E, et al. A tri-modality image fusion method for target delineation of brain tumors in radiotherapy. PLoS One. 2014;9:e112187. doi: 10.1371/journal.pone.0112187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tseng C-L, Stewart J, Whitfield G, et al. Glioma consensus contouring recommendations from a MR-Linac International Consortium Research Group and evaluation of a CT-MRI and MRI-only workflow. J Neurooncol. 2020;149:305–314. doi: 10.1007/s11060-020-03605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stewart J, Sahgal A, Lee Y, et al. Quantitating interfraction target dynamics during concurrent chemoradiation for glioblastoma: A prospective serial imaging study. Int J Radiat Oncol Biol Phys. 2021;109:736–746. doi: 10.1016/j.ijrobp.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 27. Winkfield KM, Linsenmeier C, Yock TI, et al. Surveillance of craniopharyngioma cyst growth in children treated with proton radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:716–721. doi: 10.1016/j.ijrobp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 28. Lee S, Stewart J, Lee Y, et al. Improved dosimetric accuracy with semi-automatic contour propagation of organs-at-risk in glioblastoma patients undergoing chemoradiation. J Appl Clin Med Phys. 2019;20:45–53. doi: 10.1002/acm2.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dasgupta A, Geraghty B, Maralani PJ, et al. Quantitative mapping of individual voxels in the peritumoral region of IDH-wildtype glioblastoma to distinguish between tumor infiltration and edema. J Neurooncol. 2021;153:251–261. doi: 10.1007/s11060-021-03762-2. [DOI] [PubMed] [Google Scholar]
- 30. Chaumeil MM, Lupo JM, Ronen SM. Magnetic resonance (MR) metabolic imaging in glioma. Brain Pathol. 2015;25:769–780. doi: 10.1111/bpa.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laprie A, Ken S, Filleron T, et al. Dose-painting multicenter phase III trial in newly diagnosed glioblastoma: The SPECTRO-GLIO trial comparing arm A standard radiochemotherapy to arm B radiochemotherapy with simultaneous integrated boost guided by MR spectroscopic imaging. BMC Cancer. 2019;19:167. doi: 10.1186/s12885-019-5317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan RW, Chen H, Myrehaug S, et al. Quantitative CEST and MT at 1.5T for monitoring treatment response in glioblastoma: Early and late tumor progression during chemoradiation. J Neurooncol. 2021;151:267–278. doi: 10.1007/s11060-020-03661-y. [DOI] [PubMed] [Google Scholar]
- 33. Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 34. Liu Z-C, Yan L-F, Hu Y-C, et al. Combination of IVIM-DWI and 3D-ASL for differentiating true progression from pseudoprogression of glioblastoma multiforme after concurrent chemoradiotherapy: Study protocol of a prospective diagnostic trial. BMC Med Imaging. 2017;17:10. doi: 10.1186/s12880-017-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thornton AF, Sandler HM, Ten Haken RK, et al. The clinical utility of magnetic resonance imaging in 3-dimensional treatment planning of brain neoplasms. Int J Radiat Oncol Biol Phys. 1992;24:767–775. doi: 10.1016/0360-3016(92)90727-y. [DOI] [PubMed] [Google Scholar]
- 36. Stall B, Zach L, Ning H, et al. Comparison of T2 and FLAIR imaging for target delineation in high grade gliomas. Radiat Oncol. 2010;5:5. doi: 10.1186/1748-717X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mehta S, Gajjar SR, Padgett KR, et al. Daily tracking of glioblastoma resection cavity, cerebral edema, and tumor volume with MRI-guided radiation therapy. Cureus. 2018;10:e2346. doi: 10.7759/cureus.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rouvière O, Hartman RP, Lyonnet D. Prostate MR imaging at high-field strength: Evolution or revolution? Eur Radiol. 2006;16:276–284. doi: 10.1007/s00330-005-2893-8. [DOI] [PubMed] [Google Scholar]
- 39. Hoeks CMA, Barentsz JO, Hambrock T, et al. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 40. Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013;37:1035–1054. doi: 10.1002/jmri.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wegener D, Zips D, Thorwarth D, et al. Precision of T2 TSE MRI-CT-image fusions based on gold fiducials and repetitive T2 TSE MRI-MRI-fusions for adaptive IGRT of prostate cancer by using phantom and patient data. Acta Oncol. 2019;58:88–94. doi: 10.1080/0284186X.2018.1518594. [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Xue J, Chen L, et al. CT-MRI fusion uncertainty in prostate treatment planning for different image guidance techniques. Int J Radiat Oncol. 2013;87:S718. [Google Scholar]
- 43. Rasch C, Barillot I, Remeijer P, et al. Definition of the prostate in CT and MRI: A multi-observer study. Int J Radiat Oncol Biol Phys. 1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 44. Roach M, Faillace-Akazawa P, Malfatti C, et al. Prostate volumes defined by magnetic resonance imaging and computerized tomographic scans for three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 1996;35:1011–1018. doi: 10.1016/0360-3016(96)00232-5. [DOI] [PubMed] [Google Scholar]
- 45. Sannazzari GL, Ragona R, Ruo Redda MG, et al. CT-MRI image fusion for delineation of volumes in three-dimensional conformal radiation therapy in the treatment of localized prostate cancer. Br J Radiol. 2002;75:603–607. doi: 10.1259/bjr.75.895.750603. [DOI] [PubMed] [Google Scholar]
- 46. Hentschel B, Oehler W, Strauss D, et al. Definition of the CTV prostate in CT and MRI by using CT-MRI image fusion in IMRT planning for prostate cancer. Strahlenther Onkol. 2011;187:183–190. doi: 10.1007/s00066-010-2179-1. [DOI] [PubMed] [Google Scholar]
- 47. Pathmanathan AU, McNair HA, Schmidt MA, et al. Comparison of prostate delineation on multimodality imaging for MR-guided radiotherapy. Br J Radiol. 2019;92:20180948. doi: 10.1259/bjr.20180948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steenbakkers RJHM, Deurloo KEI, Nowak PJCM, et al. Reduction of dose delivered to the rectum and bulb of the penis using MRI delineation for radiotherapy of the prostate. Int J Radiat Oncol Biol Phys. 2003;57:1269–1279. doi: 10.1016/s0360-3016(03)01446-9. [DOI] [PubMed] [Google Scholar]
- 49. Ali AN, Rossi PJ, Godette KD, et al. Impact of magnetic resonance imaging on computed tomography-based treatment planning and acute toxicity for prostate cancer patients treated with intensity modulated radiation therapy. Pract Radiat Oncol. 2013;3:e1–e9. doi: 10.1016/j.prro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 50. Cellini N, Morganti AG, Mattiucci GC, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int J Radiat Oncol Biol Phys. 2002;53:595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 51. Feutren T, Herrera FG. Prostate irradiation with focal dose escalation to the intraprostatic dominant nodule: A systematic review. Prostate Int. 2018;6:75–87. doi: 10.1016/j.prnil.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pickett B, Vigneault E, Kurhanewicz J, et al. Static field intensity modulation to treat a dominant intra-prostatic lesion to 90 Gy compared to seven field 3-dimensional radiotherapy. Int J Radiat Oncol Biol Phys. 1999;44:921–929. doi: 10.1016/s0360-3016(98)00502-1. [DOI] [PubMed] [Google Scholar]
- 53. Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the FLAME randomized phase III trial. J Clin Oncol. 2021;39:787–796. doi: 10.1200/JCO.20.02873. [DOI] [PubMed] [Google Scholar]
- 54. Zamboglou C, Thomann B, Koubar K, et al. Focal dose escalation for prostate cancer using 68Ga-HBED-CC PSMA PET/CT and MRI: A planning study based on histology reference. Radiat Oncol. 2018;13:81. doi: 10.1186/s13014-018-1036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Owrangi AM, Greer PB, Glide-Hurst CK. MRI-only treatment planning: Benefits and challenges. Phys Med Biol. 2018;63:05TR01. doi: 10.1088/1361-6560/aaaca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dowling JA, Lambert J, Parker J, et al. An atlas-based electron density mapping method for magnetic resonance imaging (MRI)-alone treatment planning and adaptive MRI-based prostate radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83:e5–e11. doi: 10.1016/j.ijrobp.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 57. Korhonen J, Kapanen M, Keyriläinen J, et al. A dual model HU conversion from MRI intensity values within and outside of bone segment for MRI-based radiotherapy treatment planning of prostate cancer: HU conversion from MRI data. Med Phys. 2013;41:011704. doi: 10.1118/1.4842575. [DOI] [PubMed] [Google Scholar]
- 58. Tyagi N, Zelefsky MJ, Wibmer A, et al. Clinical experience and workflow challenges with magnetic resonance-only radiation therapy simulation and planning for prostate cancer. Phys Imaging Radiat Oncol. 2020;16:43–49. doi: 10.1016/j.phro.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tenhunen M, Korhonen J, Kapanen M, et al. MRI-only based radiation therapy of prostate cancer: Workflow and early clinical experience. Acta Oncol. 2018;57:902–907. doi: 10.1080/0284186X.2018.1445284. [DOI] [PubMed] [Google Scholar]
- 60. Murray J, Tree AC. Prostate cancer—Advantages and disadvantages of MR-guided RT. Clin Transl Radiat Oncol. 2019;18:68–73. doi: 10.1016/j.ctro.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tetar SU, Bruynzeel AME, Lagerwaard FJ, et al. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imaging Radiat Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rischke HC, Nestle U, Fechter T, et al. 3 Tesla multiparametric MRI for GTV-definition of dominant intraprostatic lesions in patients with prostate cancer—An interobserver variability study. Radiat Oncol. 2013;8:183. doi: 10.1186/1748-717X-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gunnlaugsson A, Persson E, Gustafsson C, et al. Target definition in radiotherapy of prostate cancer using magnetic resonance imaging only workflow. Phys Imaging Radiat Oncol. 2019;9:89–91. doi: 10.1016/j.phro.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bruynzeel AME, Tetar SU, Oei SS, et al. A prospective single-arm phase 2 study of stereotactic magnetic resonance guided adaptive radiation therapy for prostate cancer: Early toxicity results. Int J Radiat Oncol Biol Phys. 2019;105:1086–1094. doi: 10.1016/j.ijrobp.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 65. Kishan AU, Lamb J, Casado M, et al. Magnetic resonance imaging-guided versus computed tomography-guided stereotactic body radiotherapy for prostate cancer (MIRAGE): Interim analysis of a phase III randomized trial. J Clin Oncol. 2022;40 suppl 6; abstr 255. [Google Scholar]
- 66. Arya S, Das D, Engineer R, et al. Imaging in rectal cancer with emphasis on local staging with MRI. Indian J Radiol Imaging. 2015;25:148–161. doi: 10.4103/0971-3026.155865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Taylor FGM, Swift RI, Blomqvist L, et al. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 68. Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 69. Wald C, Russo MW, Heimbach JK, et al. New OPTN/UNOS policy for liver transplant allocation: Standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 70. Park M-S, Kim S, Patel J, et al. Hepatocellular carcinoma: Detection with diffusion-weighted versus contrast-enhanced magnetic resonance imaging in pretransplant patients. Hepatology. 2012;56:140–148. doi: 10.1002/hep.25681. [DOI] [PubMed] [Google Scholar]
- 71. Voroney J-P, Brock KK, Eccles C, et al. Prospective comparison of computed tomography and magnetic resonance imaging for liver cancer delineation using deformable image registration. Int J Radiat Oncol. 2006;66:780–791. doi: 10.1016/j.ijrobp.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 72. Pech M, Mohnike K, Wieners G, et al. Radiotherapy of liver metastases: Comparison of target volumes and dose-volume histograms employing CT- or MRI-based treatment planning. Strahlenther Onkol. 2008;184:256–261. doi: 10.1007/s00066-008-1849-8. [DOI] [PubMed] [Google Scholar]
- 73. Tan J, Lim Joon D, Fitt G, et al. The utility of multimodality imaging with CT and MRI in defining rectal tumour volumes for radiotherapy treatment planning: A pilot study. J Med Imaging Radiat Oncol. 2010;54:562–568. doi: 10.1111/j.1754-9485.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- 74. Couwenberg AM, Burbach JPM, Berbee M, et al. Efficacy of dose-escalated chemoradiation on complete tumor response in patients with locally advanced rectal cancer (RECTAL-BOOST): A phase 2 randomized controlled trial. Int J Radiat Oncol. 2020;108:1008–1018. doi: 10.1016/j.ijrobp.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 75.Coolens C, Hawkins M, Ockwell C.Volume and motion definition in helical CT, 4DCT and MR imaging in upper gastrointestinal radiotherapy planning. Presented at 9th Biennial ESTRO Meeting, Barcelona, Spain, September 8–13, 2007.
- 76. Heerkens HD, van Vulpen M, van den Berg CAT, et al. MRI-based tumor motion characterization and gating schemes for radiation therapy of pancreatic cancer. Radiother Oncol. 2014;111:252–257. doi: 10.1016/j.radonc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 77. Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126:519–526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 78. de Jong EA, ten Berge JCEM, Dwarkasing RS, et al. The accuracy of MRI, endorectal ultrasonography, and computed tomography in predicting the response of locally advanced rectal cancer after preoperative therapy: A metaanalysis. Surgery. 2016;159:688–699. doi: 10.1016/j.surg.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 79. Lukovic J, Henke L, Gani C, et al. MRI-based upper abdominal organs-at-risk atlas for radiation oncology. Int J Radiat Oncol. 2020;106:743–753. doi: 10.1016/j.ijrobp.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 80. Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): An international multicentre registry study. The Lancet. 2018;391:2537–2545. doi: 10.1016/S0140-6736(18)31078-X. [DOI] [PubMed] [Google Scholar]
- 82. Fernandez LM, São Julião GP, Figueiredo NL, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: A retrospective, international, multicentre registry study. Lancet Oncol. 2021;22:43–50. doi: 10.1016/S1470-2045(20)30557-X. [DOI] [PubMed] [Google Scholar]
- 83. Ladd ME, Bachert P, Meyerspeer M, et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1–50. doi: 10.1016/j.pnmrs.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 84. Grant A, Metzger GJ, Van de Moortele P-F, et al. 10.5 T MRI static field effects on human cognitive, vestibular, and physiological function. Magn Reson Imaging. 2020;73:163–176. doi: 10.1016/j.mri.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]
- 86. Baumann M, Krause M, Overgaard J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 87. Winkel D, Bol GH, Kroon PS, et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brock KK. Adaptive radiotherapy: Moving into the future. Semin Radiat Oncol. 2019;29:181–184. doi: 10.1016/j.semradonc.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: The IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 90. Verkooijen HM, Kerkmeijer LGW, Fuller CD, et al. R-IDEAL: A framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol. 2017;7:59. doi: 10.3389/fonc.2017.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Elsherbini AAM, Saber M, Aggag M, et al. Magnetic nanoparticle-induced hyperthermia treatment under magnetic resonance imaging. Magn Reson Imaging. 2011;29:272–280. doi: 10.1016/j.mri.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 92. Meng Y, Pople CB, Lea-Banks H, et al. Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. J Control Release. 2019;309:25–36. doi: 10.1016/j.jconrel.2019.07.023. [DOI] [PubMed] [Google Scholar]
- 93. Thanou M, Gedroyc W. MRI-guided focused ultrasound as a new method of drug delivery. J Drug Deliv. 2013;2013:616197. doi: 10.1155/2013/616197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. McNabb E, Al-Mahrouki A, Law N, et al. Ultrasound-stimulated microbubble radiation enhancement of tumors: Single-dose and fractionated treatment evaluation. PLoS One. 2020;15:e0239456. doi: 10.1371/journal.pone.0239456. [DOI] [PMC free article] [PubMed] [Google Scholar]