PURPOSE
An expert panel on breast cancer and COVID-19 disease was convened to address the impact of the COVID-19 pandemic for early breast cancer (eBC) management.
METHODS
To ensure that the most clinically relevant information was addressed, essential information was drawn from several of the latest national and international guidelines and another technical document. The expert panel met in five virtual closed sessions from November 2020 to May 2021 to consult on the relevant data from evidence-based results. The data gathered were discussed on an online platform.
RESULTS
This article reports the expert panel's highlights of these meetings' discussions. In addition, it provides practical recommendations covering topics regarding diagnosis, treatment, and management of patients with eBC in clinical settings routinely encountered by health care professionals amid the COVID-19 pandemic.
CONCLUSION
This article provided guidance on several topics regarding eBC management amid the COVID-19 pandemics to inform safer care practices.
INTRODUCTION
In 2020, the predicted number of new breast cancer (BC) cases was 2.3 million worldwide, with an estimated age-standardized rate incidence of 47.8 per 100,000 person-years and an age-standardized rate mortality of 13.6 per 100,000 person-year with 684,996 deaths predicted.1 The COVID-19 pandemic has challenged the medical community on many fronts, significantly affecting access to cancer diagnosis and treatment.2 The fear of becoming infected while using health care facilities, fueled by the rising number of infected individuals seeking medical care, is one of the main factors delaying cancer diagnosis and treatment.3-5 A significant decrease in cancer diagnoses has been observed during the COVID-19 pandemic, with the most marked decline seen in BC care (51.8%).6
CONTEXT
Key Objective
To discuss relevant evidence-based data on the management of early breast cancer (eBC) during the COVID-19 pandemic.
Knowledge Generated
We provided expert panel recommendations regarding the best practices on eBC management during the COVID-19 pandemic, concerning both patient and health care professionals' health and safety.
Relevance
Our results contribute with evidence-based information that supports the development of protocols and algorithms to adapt the management of eBC during the COVID-19 pandemic or during times of higher restrictions.
Surgery remains the primary curative treatment for BC.7 However, because of the COVID-19 pandemic, BC teams have been forced to review triage for surgical procedures to optimize clinical resource usage. This move has entailed assessing risks and deciding which surgery cases should be postponed,8 such as elective surgeries9 and taking preventive measures for potentially infected nondeferrable surgery candidates.10,11 Brazil has registered more than 600,000 deaths, with more than 4,000 daily obits during the worst moments of the pandemic.12 The purpose of this review is to provide an evidence-based update on the management of early BC (eBC) during the COVID-19 outbreak, with a particular emphasis on avoiding risks to both patients and health care professionals (HCPs).
METHODS
With the aim of pooling information on the host of clinical scenarios in which patients with eBC may present during the COVID-19 pandemic, a group of specialists in Brazil was invited to join an expert panel. To ensure that the most clinically relevant information was addressed, essential information was drawn from several of the latest national and international guidelines and other technical documents.4,9,10,13-31 The data gathered were discussed on an online platform (Within3), covering topics regarding diagnosis, treatment, and management of patients with BC in clinical settings routinely encountered by HCPs amid the COVID-19 pandemic.
Thirteen recognized experts joined an online expert panel and worked collaboratively in five virtual closed sessions from November 18 to May 25, 2021, in five virtual closed sessions. A three-step process was conducted: (1) prework, in which all relevant material was shared and notes on crucial aspects were acknowledged; (2) steering committee meeting, where participants discussed and shared clinical expertise, drafting recommendations; and (3) meeting convening all experts, in which a comprehensive review of all evidence provided was performed online and resultant recommendations were discussed and refined.
RESULTS
Clinical Presentation of BC
BC is a heterogeneous disease with different subtypes. Most patients with BC are asymptomatic (findings from screening mammography), whereas others may present with a palpable lump at diagnosis. eBC (stages I and II) represents more than 75% of cases in most parts of the world.32 The management of eBC is well-defined according to international protocols.13,14,33 Human epidermal growth factor receptor 2 (HER2)–positive and triple-negative (TN) BC are biologically more aggressive tumors, whereas luminal cancers (which express hormone receptors) are more indolent.34 On the basis of the Ki-67 proliferation index, the St Gallen Consensus defines two luminal subtypes: luminal A (better prognosis) and luminal B (more aggressive disease).34 Surgery is the mainstay treatment for eBC, and the procedure may be performed upfront or after neoadjuvant therapy (chemotherapy or endocrine therapy). As a rule, HER2-positive, luminal B, and TN patients are priority categories for urgent BC therapy.33
Pathophysiology
Patients with cancer have dysregulated immunity with depleted immune cells, such as CD8+ T cells, CD4+ T cells, natural killer cells, and others.35 COVID-19 disease in patients with cancer significantly increases inflammatory factors and cytokines (high-sensitivity C-reactive protein, procalcitonin, interleukin [IL]-2, IL-6, and IL-8), possibly explaining the poorer prognosis in individuals with cancer relative to those without cancer.36 SARS-CoV-2 infection can enter the cell by mediating spike proteins using the angiotensin-converting enzyme 2 receptor via plasma membrane fusion or endosomes.37 SARS-CoV-2 infection stimulates the innate immune system and antigen-specific responses of B and T cells through a mechanism similar to that seen for the influenza virus.38 The development of virus-neutralizing antibodies is essential for protection against viral infections, and clinical studies of SARS-CoV-2 vaccines have been pursuing this therapeutic target.39
Management
Assessment and diagnosis.
In the context of the COVID-19 pandemic, the management of patients with eBC has become more complex, as SARS-CoV-2 infection can be symptomatic or asymptomatic.40 A summary of the recommendations discussed in the sections below is presented in Table 1.
TABLE 1.
Summary of Specialist Panel Recommendations
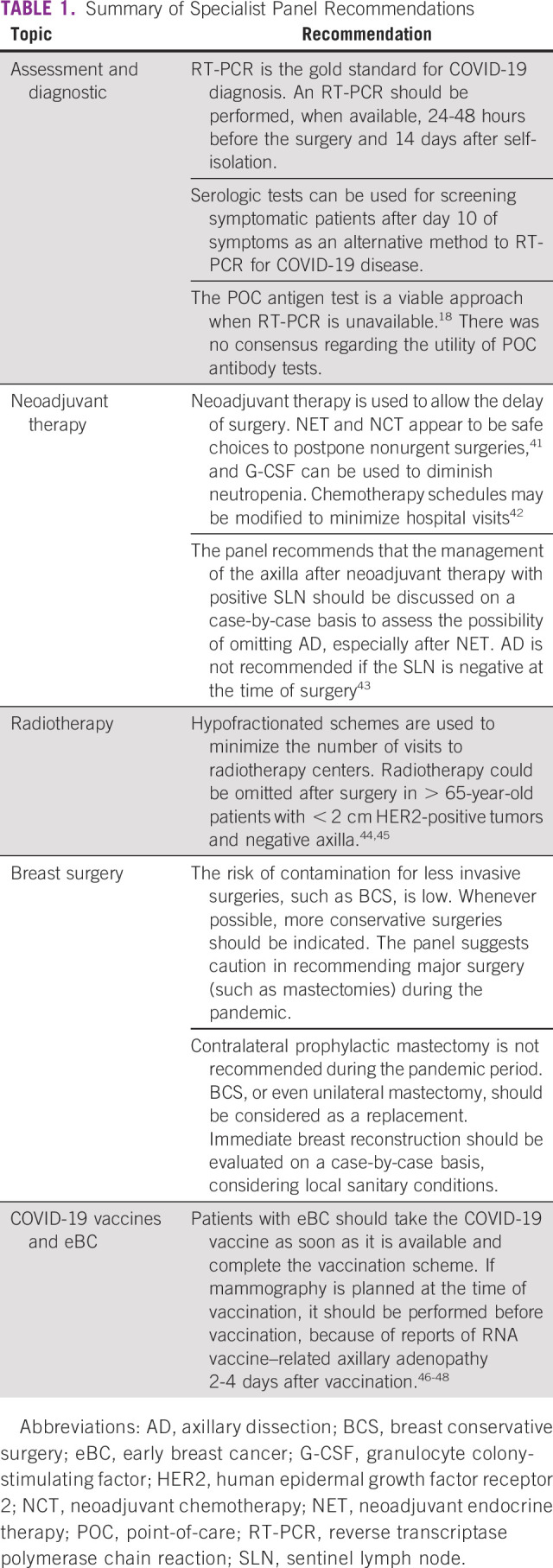
The diagnosis of SARS-CoV-2 infection can be established on the basis of the reverse transcription-polymerase chain reaction (RT-PCR) test for symptomatic or asymptomatic patients exposed within 5-10 days to SARS-CoV-2–infected individuals.49,50 An RT-PCR should be performed, when available, 24-48 hours before the surgery and 14 days after self-isolation.15 Considering that RT-PCR has a false-negative rate of 20%-30%,51 < 10% of COVID-19–infected patients will inadvertently undergo surgery during the incubation period with this approach.16
Serologic tests can be used for screening symptomatic patients after day 10 of symptoms as an alternative method to RT-PCR for COVID-19 diagnosis (gold standard).50 However, serologic tests alone are not recommended because they are less sensitive before 10 days of symptom onset and given the possibility of false positives.22
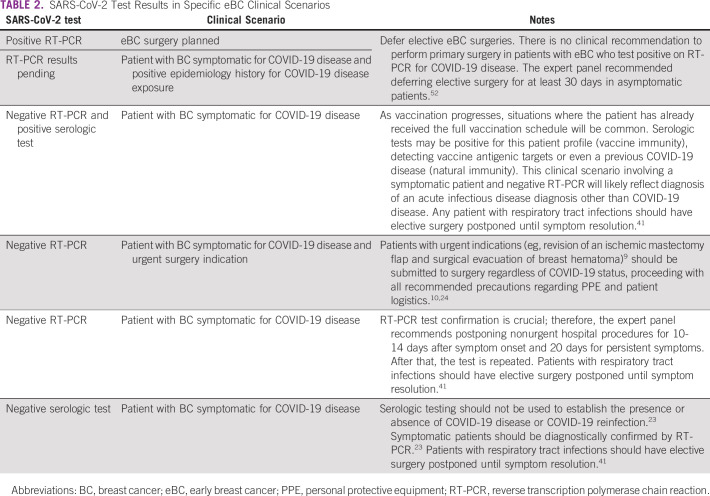
Another practical approach is to assess eBC management in those cases with SARS-CoV-2 test results available (positive or negative) and a more controversial clinical scenario (Table 2). The risk of overall postoperative mortality is increased up to 6 weeks after SARS-CoV-2 infection.52 However, longer delays could negatively affect disease progression and patient outcome.53 This delay should be considered when deciding whether to postpone elective and nonurgent eBC surgeries in patients with preoperative positive SARS-CoV-2 diagnosis.
TABLE 2.
SARS-CoV-2 Test Results in Specific eBC Clinical Scenarios
In addition, the decision to defer a surgical operation because of COVID-19 disease should be based on positive RT-PCR results (or antigen point-of-care [POC] tests when RT-PCR is unavailable) and clinical symptoms. Serologic testing results should not guide decision making, considering increased seroconversion of the population as vaccination progresses and other issues related to antibody tests discussed below.
Considerations on POC antigen and antibody testing as a replacement for RT-PCR.
Antigen detection for the diagnosis of SARS-CoV-2 infection using POC tests provides a workable solution that could enable patients to self-isolate earlier and reduce the spread of infection,17 representing an option accessible to most outbreak areas compared with standard nucleic acid amplification tests, such as RT-PCR assays.18 However, the trade-off is a loss of sensitivity compared with nucleic acid amplification tests, particularly among asymptomatic patients.54 Trained professionals should carry out these tests.
The POC antigen test is a viable approach when RT-PCR is unavailable in the following scenarios18:
Patients presenting with 5- to 7-day onset of symptoms;
Positive results need confirmation by RT-PCR assays (ideally);
Outbreak areas and remote settings, where POC testing constitutes an alternative to RT-PCR.
On the other hand, serology tests have limited application diagnosis-wise, particularly in the acute phase,55 as most patients will develop an antibody response within 1-3 weeks after infection.19 Crucial windows of opportunity for clinical intervention and isolation measures might have already been missed.19
There is also a possibility of cross-reaction with other pathogens, such as other human coronaviruses, increasing the odds for false positives.55 There was no consensus among the experts regarding the clinical utility of POC antibody tests. Some authors agreed that this technology could be considered in some situations, despite its limitations in19
determining the extent of infection in patients not diagnosed using RT-PCR,
determining infection fatality rate, and
supporting the development of vaccines.
Treatment
Neoadjuvant therapy to allow the delay of surgery.
The clinical management guidelines for BC were recently updated in the COVID-19 era. Clinical cases eligible for neoadjuvant treatment are9,24 as follows:
TNBC, HER2-positive, and luminal B tumors ≥ 2 cm and/or with positive axilla (≥ N1).
Luminal A tumors stage T1-T2 and N0-N1 (neoadjuvant endocrine therapy [NET] may be recommended, especially in postmenopausal patients).
Inflammatory and locally advanced BC (NET or neoadjuvant chemotherapy [NCT]).
Any type—to complete NCT that has already been initiated.
Specifically, for estrogen receptor–positive and HER2-negative patients, both the European Society for Medical Oncology and the American Cancer Society have stated that NET is an option to enable deferral of surgery by 6-12 months in clinical stage I or II BCs according to menopausal status.23,24 In addition, the Johns Hopkins Women's Malignancies Program has developed a guideline for BC management during the COVID-19 pandemic on the basis of tumor biology and stage.56
Although constraints are often present in terms of resources, workforce, and hospital bed availability in the COVID-19 pandemic, causing a delay in procedures, both NET and NCT appear to be safe choices to postpone surgery in nonurgent indications of estrogen receptor–positive early-stage BC, also potentially contributing to a reduction in outpatient visits.41
When NCT is proposed, there is a suggestion for using granulocyte colony-stimulating factor as support to diminish neutropenia.42 Regarding choices of chemotherapy regimens for early-stage BC, especially for TN, luminal B, and HER2-positive BCs, the recommendation is to follow the usual guidelines for these biologic subtypes. Chemotherapy schedules may be modified from weekly to every 3-week schedule, for example, to minimize hospital visits.42
Managing axilla after neoadjuvant systemic therapy.
As sentinel lymph node biopsy (SLNB) techniques become more widely practiced, invasive surgical methods for nodal staging such as axillary dissection (AD) are progressively de-escalated and restricted to specific scenarios.57 Surgeries have been a concern because of the risk of patient infection and human and resource restrictions during the COVID-19 pandemic. A multicenter retrospective study demonstrated that perioperative COVID-19–positive patients who underwent hip fracture surgeries had significantly higher postoperative morbidity and mortality.58
According to the panel, AD is not recommended if SLNB is negative at surgery, even in the previously positive axilla. However, if the sentinel lymph node (SLN) is positive, the course of action should be discussed on a case-by-case basis, especially after NET.43
Studies of adjuvant therapy in residual disease cases after NCT59-61 have demonstrated the importance of minimizing SLNB false-negative rates (FNRs).61 Failure in identifying residual disease in the axilla may alter clinical outcomes, as these patients would not be selected for additional treatment with trastuzumab emtansine, capecitabine, or olaparib. On the other hand, using chemotherapy regimens with lower odds of immunosuppression during the pandemic could decrease the complete pathologic response rate (pathologic complete response [pCR]) in these patients. An option to minimize the negative impact of modified chemotherapy regimens over pCR in axilla-positive patients during the pandemic is to clip the lymph node before NCT. This approach reduces the FNR from 2% to 8%.62,63 Another alternative would be to perform SLNB with dual tracer. A meta-analysis of 1,921 patients showed an 11% FNR with dual tracer and 4% when three or more lymph nodes were harvested for biopsy.64 It is worth highlighting that assessing the breast sample is crucial to identify residual disease, as it is uncommon to simultaneously observe breast pCR and residual disease in the axilla.65
With the increasing interest in omitting AD after NCT in the past few years, even in patients with residual disease on SLNB, a recent American study demonstrated that the use of isolated positive SLN after NCT has an upward trend after publication results of ACOSOG Z0011.66,67 The Z0011 study demonstrated excellent local and locoregional control with isolated sentinel lymph node biopsy but excluded patients who underwent neoadjuvant systemic treatment (NCT or NET).67 In women undergoing NCT, the residual axillary disease can be associated with resistance, and there are no data on cancer safety when omitting AD at this time.
A retrospective review evaluated residual disease burden in positive SLN after NCT. It demonstrated an additional high disease burden, whether micrometastasis (59%) or macrometastasis (63%), possibly an indication for AD.68 Another analysis showed that the likelihood of non–SLN-centered metastasis at axillary lymph node dissection was high across all tumor subtypes.69 The core point is whether AD would play a role in residual lymph node disease cases or whether axillary radiation therapy could replace surgery in such cases. For instance, a retrospective study using data from the National Cancer Database (NCDB), with 1,617 women with N1 disease after NCT, compared patients who received AD associated with nodal radiotherapy with those who received only SLNB and radiotherapy, similar to the design of an ongoing randomized study of the ALLIANCE group (A11202)70 showing increased survival in women undergoing AD.71 However, in an exploratory analysis, the authors found that SLN was comparable with AD in luminal tumors with single metastases. The panel recommends caution in omitting AD in such cases.
On the other hand, after NET, pCR is generally not expected after systemic treatment.72 The question is whether these patients match the ACOSOG Z0011 study profile or otherwise. The data in this scenario are limited. A study using the NCDB and Dana-Farber/Brigham and Women's Cancer Center database evaluated tumor burden after NET and the type of axillary surgery performed (SLNB or AD): more than 90% of patients who had cN0 axilla at initial presentation, in both cohorts, they had < 3 positive lymph nodes in the final pathology, with no difference in overall survival regardless of the type of axillary surgery.43 In another study, using the NCDB for stages 2 and 3, SLNB use after NET was similar to that for upfront surgery and, among those with pathological node-positive disease, the NET patients were less likely to undergo AD.73 In this scenario, the panel recommended a case-by-case assessment, with the possibility of omitting AD, especially in the initially clinically negative axilla. As NET and NCT become more common approaches during the COVID-19 pandemic, understanding nodal staging in these scenarios is even more relevant.
Radiotherapy.
COVID-19 is a highly transmissible disease. Potential outbreaks within health care facilities such as radiotherapy services have been a concern since the pandemic, as inpatients and outpatients outside of COVID-19–restricted areas can get ill or further bring the virus to their communities. Thus, the panel recommended using hypofractionated schemes to minimize the number of visits to radiotherapy centers. Five-fraction schemes once a week for 5 weeks (FAST trial)74 or daily fractions for one week (FAST forward trial)75 would be viable options for breast conservative surgery (BCS) in patients with negative axilla. A controversial topic is hypofractionation in chest wall after breast reconstruction. The panel believes that hypofractionation would be acceptable (eg, 15 fractions for three weeks)44 in this pandemic context. Elderly patients (> 65 years old) with < 2 cm HER2-negative tumors and negative axilla could have radiotherapy omitted after conservative surgery.45
Management of breast cancer surgeries in hospital restriction scenarios.
The COVID-19 pandemic has demanded hospitals reallocate health care resources, with a sudden reorganization of all clinical activities, including oncologic units.76 The restrictions differ depending on the regional level of acuity of the pandemic and resources availability.
BCS and risk of infection by COVID-19 disease.
BCS is associated with lower rates of hospital stay and visits after surgery and hospitalization than mastectomy77: a study with patients undergoing nipple-sparing mastectomy had total complication rates of 47% and reoperations around 9%.78 Regarding the use of oncoplastic surgery, complication rates also tend to be higher than in BCS. In a study using the American College of Surgeons National Surgical Quality Improvement Program database, complications within 30 days were more significant in patients undergoing oncoplastic surgery than BCS (3.8% v 2.6%; P < .001).79 Another prospective cohort (TeaM Study) identified a reoperation rate of 2.8%.80 In a survey conducted during the pandemic among mastologists from the Brazilian Society of Mastology, 75% of surgeons would recommend partial reconstruction after BCS; however, 54% of those would contraindicate mammoplasty techniques during the pandemic.81 The panel recommends caution in recommending major surgery during the pandemic.
Although there are still limited data on this subject, it is possible to infer that the risk of contamination for less invasive surgeries, such as BCS, is low because of risks of procedure complications and lower surgery time. In addition, all precautions mentioned previously should also be taken for this surgical procedure.
Elective surgeries that cannot be delayed.
Elective surgeries, by definition, can be postponed for up to 8 weeks. A few elective situations are considered essential and require planned or immediate medical assistance surgery-wise. Emergency or urgent surgeries might compromise patient survivorship if not performed. Examples of this type of surgery are a revision of an ischemic mastectomy flap, surgical evacuation of breast hematoma, drainage of breast abscess, and revascularization of an autologous tissue flap.9
Bilateral mastectomy.
Regarding patients with contralateral prophylactic mastectomy in unilateral BC indication, although there are still limited data on this subject, historically, these cases have a more extended hospital stay than to breast-conserving surgery or unilateral mastectomy and have more postsurgery visits and higher rates of hospitalization.77 This potential increase in patient exposure could lead to a greater risk of infection by COVID-19 disease.25 The expert panel suggested that a contralateral prophylactic mastectomy is not recommended during this period, and conservative breast surgery or even unilateral mastectomy should be carried out instead. The panel recommended that immediate breast reconstruction is evaluated on a case-by-case basis, according to the local conditions or resource availability because of the pandemics.
COVID-19 vaccines and breast cancer.
According to the panel, patients with BC should receive the COVID-19 vaccine as soon as it becomes available since benefits are likely to outweigh the risks of adverse effects from SARS-CoV-2 vaccination.82 The National Comprehensive Cancer Network and the European Society for Medical Oncology recently reinforced this position.26,27 It is essential to point out that limited clinical data support COVID-19 vaccination in patients with cancer.83 A multicenter, observational, prospective study has shown that SARS-CoV-2–specific immunoglobulin G antibody response after natural infection does not differ in patients with cancer and healthy control patients.84 Two prospective observational studies have demonstrated that oncologic patients develop poor SARS-CoV-2 spike protein seroconversion after one dose of the BNT162b2 (Pfizer–BioNTech, Mainz, Germany) vaccine, but remarkably increased after the second dose, highlighting the importance of completing the vaccination scheme.85,86 However, it is uncertain whether long-term protection can be achieved in the oncologic population, as these studies rely on immunogenicity data alone, and real-world data on the long-term protection of vaccinated cancer patients against COVID-19 disease are limited.83 In the same vein, data from influenza vaccinations indicate the development of a protective immune response in patients with cancer, and, although potentially not the same level as the general population, it is generally safe.28,87-89 Again, there are long-term uncertainties, and the protection may vary depending on antineoplastic therapies, administration timing, disease stage, and comorbidities.90
It is important to note that patients who received monoclonal antibodies or convalescent plasma as part of COVID-19 treatment should defer vaccination for at least 90 days as stated by the Centers for Disease Control and prevention recommendations.29 After the final dose is received, an individual is considered fully vaccinated after a minimum of 2 weeks.30 If the patient is asymptomatic and has not been in close contact with someone with SARS-CoV-2 infection in the past 14 days, the panel deemed it safe to conduct a surgical procedure. Patients with cancer and surgical patients, especially those undergoing chemotherapy or with chemotherapy planned within 8 weeks, are confirmed to be particularly at risk of infection and might have a negative outcome.91 A prospective cohort demonstrated that 30-day adjusted mortality was higher in patients with preoperative SARS-CoV-2 infection who had surgery 0-2 weeks, 3-4 weeks, and 5-6 weeks after the diagnosis of the infection (odds ratio [95% CI], 4.1 [3.3 to 4.8]; 3.9 [2.6 to 5.1], and 3.6 [2.0 to 5.2], respectively) compared with the mortality rate in patients without preoperative SARS-CoV-2 infection of 1.5% (95% CI, 1.4 to 1.5).52
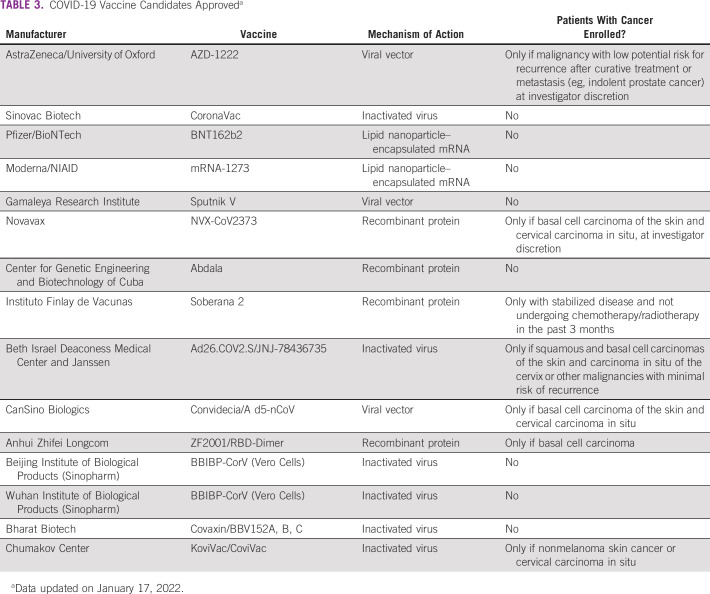
Vaccination reduces the odds of SARS-CoV-2 infection and negative outcomes of COVID-19 disease. The expert panel recommends that patients with eBC take the COVID-19 vaccine as soon as it is available to them and complete the vaccination scheme. Indeed, they are considered a priority group in national vaccination strategies.92 Although vaccinated individuals have a lower risk, the panel states that patients with eBC should keep social distancing, masks, and other protective measures. Table 3 summarizes the main vaccines approved worldwide on January 17, 2022.
TABLE 3.
COVID-19 Vaccine Candidates Approveda
Recently, an unexpectedly high incidence of axillary adenopathy findings after Moderna and Pfizer-BioNTech COVID-19 vaccines occurred.46 A solicited adverse event for patients receiving the Moderna vaccine was reported in 11.6% versus 5.0% for placebo after dose 1 and 16.0% versus 4.3% for placebo after dose 2.47 Adenopathy occurred in the arm and neck 2-4 days after vaccination with a median duration of 1-2 days.46 For those receiving the Pfizer-BioNTech vaccine, resultant lymphadenopathy lasted for a mean of 10 days. However, in the Pfizer-BioNTech study, adenopathy was only reported as an unsolicited adverse event.46 A single-institution report found similar findings, and the authors are considering magnetic resonance imaging–detected isolated unilateral lymphadenopathy ipsilateral to the vaccination arm to be most likely COVID-19 vaccine–related if within 4 weeks of either dose.48
Five cases of COVID-19 vaccine–related axillary lymphadenopathy that mimicked metastasis in a vulnerable oncologic patient group have been described.93 Because of widescale vaccination, axillary lymphadenopathy because of COVID-19 vaccination is likely to be encountered in screening or diagnostic mammography. A recent retrospective study reported a vaccine axillary adenopathy incidence rate of 3% among women who underwent mammography after at least one vaccine dose. This study included data from 750 women, and most women with lymph nodes had received two vaccine doses (18 out 23 patients).94 Despite these findings, experts do not recommend postponing either vaccination or mammography but ideally performing mammography before vaccination.95
Few recommendations have been made to obtain supplementary information specific to the COVID-19 vaccine on the patient anamnesis, such as vaccination status date(s) of vaccination(s), type of vaccine, injection site (left or right arm), and any history of palpable axillary adenopathy. Radiologists and oncologists should be aware of this secondary effect of vaccination to avoid false-positive results and unnecessary changes in management, patient emotional stress, or biopsy.96,97
What Is the Role of Postvaccine Antibody Quantification Tests in Patients With eBC?
The current evidence supports that seroconversion rates among patients with cancer are similar to those without the disease, particularly in solid tumors like BC.98 Vaccine-wise, serologic tests can often be misinterpreted as they might not distinguish between past infection and postvaccination immunologic response.23 Furthermore, serologic testing does not evaluate cellular immune response. When performed against nucleocapsid protein, these tests will not detect immune responses resulting from vaccination and are unsuitable for vaccine decision making.29 Most experts do not see a clinical application for these tests.
DISCUSSION
In conclusion, we have provided guidance on several topics regarding eBC management amid the COVID-19 pandemic to inform safer care practices for both patients and HCPs.
ACKNOWLEDGMENT
All the authors contributed to writing the article and were approved to submit it for publication. In addition, the authors thank Dr Alexandre Ferreira Oliveira, Dr Gustavo Aguiar Campana, Dr Reitan Ribeiro, and Dr Ruffo Freitas Jr for their review and inputs. CoreBox Medical Communications provided medical writing assistance.
Francisco Pimentel Cavalcante
Consulting or Advisory Role: Pfizer, Roche, MSD Oncology
Speakers' Bureau: Roche, Pfizer, Gencell Pharma, Libbs
Travel, Accommodations, Expenses: Roche, Gencell Pharma
Carlos Eduardo dos Santos Ferreira
Speakers' Bureau: Roche Diagnostica Brasil, Beckman Coulter, Abbott Diagnostics
Gilberto Amorim
Stock and Other Ownership Interests: Pfizer, AstraZeneca
Honoraria: Roche, Novartis, Lilly, Sanofi/Aventis, Pfizer, MSD Oncology
Consulting or Advisory Role: Novartis, Roche, MSD Oncology
Travel, Accommodations, Expenses: Roche, Novartis
Luciana Landeiro
Consulting or Advisory Role: GlaxoSmithKline
Álvaro Pulchinelli Jr
Consulting or Advisory Role: Roche, Thermo Fisher Scientific, bioMerieux, BD Biosciences
Daniela Dornelles Rosa
Consulting or Advisory Role: Roche, Novartis, AstraZeneca, Lilly, GlaxoSmithKline, Sanofi, Libbs, Pfizer, Amgen, Zodiac Pharma
Speakers' Bureau: Novartis, Lilly, Pfizer
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by Diagnostics (Grant No.: 12233444555).
PREPRINT VERSION
https://www.authorea.com/users/420763/articles/527023-impact-of-covid-19-in-early-breast-cancer-management-a-summary-of-the-current-evidence. doi: 10.22541/au.162430528.81312830/v1.
AUTHOR CONTRIBUTIONS
Conception and design: Francisco Pimentel Cavalcante, Edson Abdala, Carlos Eduardo dos Santos Ferreira, Gilberto Amorim, Vilmar Marques de Oliveira, Gisah Guilgen, Luciana Landeiro, Álvaro Pulchinelli Jr, Rafael Souza, Daniela Dornelles Rosa
Provision of study materials or patients: Gilberto Amorim, Gisah Guilgen, Álvaro Pulchinelli Jr, Daniela Dornelles Rosa
Collection and assembly of data: Edson Abdala, Leonardo Weissmann, Carlos Eduardo dos Santos Ferreira, Gilberto Amorim, Gisah Guilgen, Luciana Landeiro, João Renato Rebello Pinho, Rafael Souza
Data analysis and interpretation: Francisco Pimentel Cavalcante, Edson Abdala, Carlos Eduardo dos Santos Ferreira, Gilberto Amorim, Gisah Guilgen, Luciana Landeiro, João Renato Rebello Pinho, Álvaro Pulchinelli Jr, Heber Ribeiro, Rafael Souza
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Francisco Pimentel Cavalcante
Consulting or Advisory Role: Pfizer, Roche, MSD Oncology
Speakers' Bureau: Roche, Pfizer, Gencell Pharma, Libbs
Travel, Accommodations, Expenses: Roche, Gencell Pharma
Carlos Eduardo dos Santos Ferreira
Speakers' Bureau: Roche Diagnostica Brasil, Beckman Coulter, Abbott Diagnostics
Gilberto Amorim
Stock and Other Ownership Interests: Pfizer, AstraZeneca
Honoraria: Roche, Novartis, Lilly, Sanofi/Aventis, Pfizer, MSD Oncology
Consulting or Advisory Role: Novartis, Roche, MSD Oncology
Travel, Accommodations, Expenses: Roche, Novartis
Luciana Landeiro
Consulting or Advisory Role: GlaxoSmithKline
Álvaro Pulchinelli Jr
Consulting or Advisory Role: Roche, Thermo Fisher Scientific, bioMerieux, BD Biosciences
Daniela Dornelles Rosa
Consulting or Advisory Role: Roche, Novartis, AstraZeneca, Lilly, GlaxoSmithKline, Sanofi, Libbs, Pfizer, Amgen, Zodiac Pharma
Speakers' Bureau: Novartis, Lilly, Pfizer
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3. Aguiar S, Baiocchi G, Duprat JP, et al. Value of preoperative testing for SARS-CoV-2 for elective surgeries in a cancer center during the peak of pandemic in Brazil. J Surg Oncol. 2020;122:1293–1295. doi: 10.1002/jso.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavalcante FP, Novita GG, Millen EC, et al. Management of early breast cancer during the COVID-19 pandemic in Brazil. Breast Cancer Res Treat. 2020;184:637–647. doi: 10.1007/s10549-020-05877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020;184:249–254. doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaufman HW, Chen Z, Niles J, et al. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czajka ML, Pfeifer C. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. Breast cancer surgery. [PubMed] [Google Scholar]
- 8. Bartlett DL, Howe JR, Chang G, et al. Management of cancer surgery cases during the COVID-19 pandemic: Considerations. Ann Surg Oncol. 2020;27:1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Surgeons . COVID-19 Guidelines for Triage of Breast Cancer Patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/breast-cancer [Google Scholar]
- 10.Pryor A. SAGES and EAES Recommendations Regarding Surgical Response to COVID-19 Crisis. 2020. https://www.sages.org/recommendations-surgical-response-covid-19 [Google Scholar]
- 11. Hwang ES, Balch CM, Balch GC, et al. Surgical oncologists and the COVID-19 pandemic: Guiding cancer patients effectively through turbulence and change. Ann Surg Oncol. 2020;27:2600–2613. doi: 10.1245/s10434-020-08673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ministério da Saúde, Brasil . Coronavírus Brasil. 2022. https://covid.saude.gov.br/ [Google Scholar]
- 13.National Comprehensive Cancer Network . NCCN Guidelines: Breast Cancer, Version 1.2021—January 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Google Scholar]
- 14. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence . COVID-19 Rapid Guideline: Arranging Planned Care in Hospitals and Diagnostic Services: NICE Guideline [NG179] https://www.nice.org.uk/guidance/ng179 [PubMed] [Google Scholar]
- 16. Ribeiro R, Wainstein AJA, de Castro Ribeiro HS, et al. Perioperative cancer care in the context of limited resources during the COVID-19 pandemic: Brazilian Society of Surgical Oncology recommendations. Ann Surg Oncol. 2020;28:1289–1297. doi: 10.1245/s10434-020-09098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database Syst Rev. 2020;3:CD013705. doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays: Interim Guidance. 2020. https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays [Google Scholar]
- 19.Centers for Disease Control and Prevention . Using Antibody Tests for COVID-19. 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests.html [Google Scholar]
- 20.WHO . Advice on the Use of Point-of-Care Immunodiagnostic Tests for COVID-19: Scientific Brief. 2020. https://www.who.int/publications/i/item/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19-scientific-brief [Google Scholar]
- 21.Centers for Disease Control and Prevention . Sequence for Putting on Personal Protective Equipment (PPE) https://www.cdc.gov/hai/pdfs/ppe/ppe-sequence.pdf [Google Scholar]
- 22.Centers for Disease Control and Prevention . Clinical Questions about COVID-19: Questions and Answers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html [Google Scholar]
- 23.Centers for Disease Control and Prevention . Interim Guidelines for COVID-19 Antibody Testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html [Google Scholar]
- 24.European Society for Medical Oncology . ESMO Management and Treatment Adapted Recommendations in the COVID-19 Era: Breast Cancer. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/breast-cancer-in-the-covid-19-era [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention . Interim U.S. Guidance for Risk Assessment and Work Restrictions for Healthcare Personnel with Potential Exposure to SARS-CoV-2. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html [Google Scholar]
- 26.National Comprehensive Cancer Network . Recommendations of the NCCN COVID-19 Vaccination Advisory Committee. https://www.nccn.org/covid-19 [Google Scholar]
- 27.European Society for Medical Oncology . ESMO Statements for Vaccination Against COVID-19 in Patients With Cancer. https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination [Google Scholar]
- 28. Bitterman R, Eliakim-Raz N, Vinograd I, et al. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst Rev. 2018;2:CD008983. doi: 10.1002/14651858.CD008983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention . Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html [Google Scholar]
- 30.Centers for Disease Control and Prevention . Updated Healthcare Infection Prevention and Control Recommendations in Response to COVID-19 Vaccination. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-after-vaccination.html [Google Scholar]
- 31.Centers for Disease Control and Prevention . Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html [Google Scholar]
- 32. Simon SD, Bines J, Werutsky G, et al. Characteristics and prognosis of stage I-III breast cancer subtypes in Brazil: The AMAZONA retrospective cohort study. Breast. 2019;44:113–119. doi: 10.1016/j.breast.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 33. Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181:487–497. doi: 10.1007/s10549-020-05644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcus A, Gowen BG, Thompson TW, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cai G, Gao Y, Zeng S, et al. Immunological alternation in COVID-19 patients with cancer and its implications on mortality. Oncoimmunology. 2021;10:1854424. doi: 10.1080/2162402X.2020.1854424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han HJ, Nwagwu C, Anyim O, et al. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int Immunopharmacol. 2021;90:107247. doi: 10.1016/j.intimp.2020.107247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferreira CE, Bonvehi PE, de la Torre JCG, et al. Algorithms for testing COVID-19 focused on use of RT-PCR and high-affinity serological testing: A consensus statement from a panel of Latin American experts. Int J Infect Dis. 2021;103:260–267. doi: 10.1016/j.ijid.2020.11.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mills GH. Respiratory complications of anaesthesia. Anaesthesia. 2018;73(suppl 1):25–33. doi: 10.1111/anae.14137. [DOI] [PubMed] [Google Scholar]
- 42. Curigliano G, Cardoso MJ, Poortmans P, et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kantor O, Wakeman M, Weiss A, et al. Axillary management after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer. Ann Surg Oncol. 2021;28:1358–1367. doi: 10.1245/s10434-020-09073-6. [DOI] [PubMed] [Google Scholar]
- 44. Haviland JS, Owen JR, Dewar JA, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-Year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 45. Kunkler IH, Williams LJ, Jack WJL, et al. PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 46.Grimm L, Destounis S, Dogan B, et al. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination, 2022. https://www.sbi-online.org/Portals/0/Position%20Statements/2022/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination_updatedFeb2022.pdf
- 47.Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine. CDC; 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html [Google Scholar]
- 48. Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021;217:831–834. doi: 10.2214/AJR.21.25604. [DOI] [PubMed] [Google Scholar]
- 49. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323:2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 50. Mattioli IA, Hassan A, Oliveira ON, et al. On the challenges for the diagnosis of SARS-CoV-2 based on a review of current methodologies. ACS Sens. 2020;5:3655–3677. doi: 10.1021/acssensors.0c01382. [DOI] [PubMed] [Google Scholar]
- 51. Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92:903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. COVIDSurg Collaborative, GlobalSurg Collaborative Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anaesthesia. 2021;76:748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Finley C, Prashad A, Camuso N, et al. Guidance for management of cancer surgery during the COVID-19 pandemic. Can J Surg. 2020;63(2 suppl 1):S2–S4. doi: 10.1503/cjs.005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pray IW. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses—Wisconsin, September–October 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1642–1647. doi: 10.15585/mmwr.mm695152a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cheng MP, Yansouni CP, Basta NE, et al. Serodiagnostics for severe acute respiratory syndrome–related coronavirus-2. Ann Intern Med. 2020;173:450–460. doi: 10.7326/M20-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sheng JY, Santa-Maria CA, Mangini N, et al. Management of breast cancer during the COVID-19 pandemic: A stage- and subtype-specific approach. JCO Oncol Pract. 2020;16:665–674. doi: 10.1200/OP.20.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jatoi A, Ritter H, Dueck A, et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9) Lung Cancer. 2010;68:234–239. doi: 10.1016/j.lungcan.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kayani B, Onochie E, Patil V, et al. The effects of COVID-19 on perioperative morbidity and mortality in patients with hip fractures. Bone Joint J. 2020;102-B:1136–1145. doi: 10.1302/0301-620X.102B9.BJJ-2020-1127.R1. [DOI] [PubMed] [Google Scholar]
- 59. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 60. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 61. Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–1461. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–1078. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tee SR, Devane LA, Evoy D, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105:1541–1552. doi: 10.1002/bjs.10986. [DOI] [PubMed] [Google Scholar]
- 65. Barron AU, Hoskin TL, Day CN, et al. Association of low nodal positivity rate among patients with ERBB2-positive or triple-negative breast cancer and breast pathologic complete response to neoadjuvant chemotherapy. JAMA Surg. 2018;153:1120–1126. doi: 10.1001/jamasurg.2018.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kantor O, Pesce C, Liederbach E, et al. Are the ACOSOG Z0011 trial findings being applied to breast cancer patients undergoing neoadjuvant chemotherapy? Breast J. 2017;23:554–562. doi: 10.1111/tbj.12793. [DOI] [PubMed] [Google Scholar]
- 67. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Moo TA, Edelweiss M, Hajiyeva S, et al. Is low-volume disease in the sentinel node after neoadjuvant chemotherapy an indication for axillary dissection? Ann Surg Oncol. 2018;25:1488–1494. doi: 10.1245/s10434-018-6429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Moo TA, Pawloski KR, Flynn J, et al. Is residual nodal disease at axillary dissection associated with tumor subtype in patients with low volume sentinel node metastasis after neoadjuvant chemotherapy? Ann Surg Oncol. 2021;28:6044–6050. doi: 10.1245/s10434-021-09910-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Comparison of Axillary Lymph Node Dissection With Axillary Radiation for Patients With Node-Positive Breast Cancer Treated With Chemotherapy. 2021. https://clinicaltrials.gov/ct2/show/NCT01901094 [Google Scholar]
- 71. Almahariq MF, Levitin R, Quinn TJ, et al. Omission of axillary lymph node dissection is associated with inferior survival in breast cancer patients with residual N1 nodal disease following neoadjuvant chemotherapy. Ann Surg Oncol. 2021;28:930–940. doi: 10.1245/s10434-020-08928-2. [DOI] [PubMed] [Google Scholar]
- 72. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bi Z, Liu J, Chen P, et al. Neoadjuvant chemotherapy and timing of sentinel lymph node biopsy in different molecular subtypes of breast cancer with clinically negative axilla. Breast Cancer. 2019;26:373–377. doi: 10.1007/s12282-018-00934-3. [DOI] [PubMed] [Google Scholar]
- 74. Brunt AM, Haviland J, Sydenham M, et al. FAST phase III RCT of radiotherapy hypofractionation for treatment of early breast cancer: 10-Year results (CRUKE/04/015) Int J Radiat Oncol Biol Phys. 2018;102:1603–1604. [Google Scholar]
- 75. Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol. 2016;120:114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fregatti P, Gipponi M, Giacchino M, et al. Breast cancer surgery during the COVID-19 pandemic: An observational clinical study of the breast surgery clinic at Ospedale Policlinico San Martino—Genoa, Italy. In Vivo. 2020;34(3 suppl):1667–1673. doi: 10.21873/invivo.11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tuttle TM, Burke EE. Bilateral mastectomy: Doubling down on complications? Ann Surg Oncol. 2015;22:3407–3408. doi: 10.1245/s10434-015-4629-6. [DOI] [PubMed] [Google Scholar]
- 78. Valero MG, Muhsen S, Moo TA, et al. Increase in utilization of nipple-sparing mastectomy for breast cancer: Indications, complications, and oncologic outcomes. Ann Surg Oncol. 2020;27:344–351. doi: 10.1245/s10434-019-07948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Angarita FA, Acuna SA, Cordeiro E, et al. Thirty-day postoperative morbidity and mortality in elderly women with breast cancer: An analysis of the NSQIP database. Breast Cancer Res Treat. 2018;170:373–379. doi: 10.1007/s10549-018-4747-5. [DOI] [PubMed] [Google Scholar]
- 80. O'Connell RL, Baker E, Trickey A, et al. Current practice and short-term outcomes of therapeutic mammaplasty in the international TeaM multicentre prospective cohort study. Br J Surg. 2018;105:1778–1792. doi: 10.1002/bjs.10959. [DOI] [PubMed] [Google Scholar]
- 81. Cavalcante FP, Novita GG, Millen EC, et al. Breast reconstruction and coronavirus pandemic. J Plast Reconstr Aesthet Surg. 2021;74:644–710. doi: 10.1016/j.bjps.2020.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Desai A, Gainor JF, Hegde A, et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18:313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Corti C, Crimini E, Tarantino P, et al. Current perspectives: SARS-CoV-2 vaccines for cancer patients: A call to action. Eur J Cancer. 2021;148:316–327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Marra A, Generali DG, Zagami P, et al. LBA77 Anti-SARS-CoV-2 antibody response in patients with cancer and oncology healthcare workers: A multicenter, prospective study. Ann Oncol. 2020;31:S1206. [Google Scholar]
- 85. Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goshen-Lago T, Waldhorn I, Holland R, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1507–1513. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Miraglia JL, Abdala E, Hoff PM, et al. Immunogenicity and reactogenicity of 2009 influenza A (H1N1) inactivated monovalent non-adjuvanted vaccine in elderly and immunocompromised patients. PLoS One. 2011;6:e27214. doi: 10.1371/journal.pone.0027214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Brydak LB, Guzy J, Starzyk J, et al. Humoral immune response after vaccination against influenza in patients with breast cancer. Support Care Cancer. 2001;9:65–68. doi: 10.1007/s005200000186. [DOI] [PubMed] [Google Scholar]
- 89. Ward EM, Flowers CR, Gansler T, et al. The importance of immunization in cancer prevention, treatment, and survivorship. CA Cancer J Clin. 2017;67:398–410. doi: 10.3322/caac.21407. [DOI] [PubMed] [Google Scholar]
- 90. Loulergue P, Alexandre J, Iurisci I, et al. Low immunogenicity of seasonal trivalent influenza vaccine among patients receiving docetaxel for a solid tumour: Results of a prospective pilot study. Br J Cancer. 2011;104:1670–1674. doi: 10.1038/bjc.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Spolverato G, Capelli G, Restivo A, et al. The management of surgical patients during the coronavirus disease 2019 (COVID-19) pandemic. Surgery. 2020;168:4–10. doi: 10.1016/j.surg.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ministério da Saúde (Brasil) Plano Nacional de Operacionalização da Vacina contra a Covid-19—4 a Edição. https://www.gov.br/saude/pt-br/Coronavirus/vacinas/plano-nacional-de-operacionalizacao-da-vacina-contra-a-covid-19 [Google Scholar]
- 93. Özütemiz C, Krystosek LA, Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: Diagnostic dilemma in oncologic patients. Radiology. 2021;300:E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Robinson KA, Maimone S, Gococo-Benore DA, et al. Incidence of axillary adenopathy in breast imaging after COVID-19 vaccination. JAMA Oncol. 2021;7:1395–1397. doi: 10.1001/jamaoncol.2021.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nota técnica—Informações atualizadas sobre vacinação contra COVID-19 e Mamografia. SBM; https://www.sbmastologia.com.br/noticias/nota-tecnica-informacoes-atualizadas-sobre-vacinacao-contra-covid-19-e-mamografia/ [Google Scholar]
- 96. Seely JM, Barry MH. The Canadian Society of Breast Imaging recommendations for the management of axillary adenopathy in patients with recent COVID-19 vaccination—Update. Can Assoc Radiol J. 2021;72:601–602. doi: 10.1177/0846537121998949. [DOI] [PubMed] [Google Scholar]
- 97. Ko G, Hota S, Cil TD. COVID-19 vaccination and breast cancer surgery timing. Breast Cancer Res Treat. 2021;188:825–826. doi: 10.1007/s10549-021-06293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2:392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]