Abstract
Disruption of intracellular Ca2+ homeostasis plays an important role as an upstream pathology in Alzheimer’s disease (AD), and correction of Ca2+ dysregulation has been increasingly proposed as a target of future effective disease-modified drugs for treating AD. Calcium dysregulation is also an upstream pathology for the COVID-19 virus SARS-CoV-2 infection and replication, leading to host cell damage. Clinically available drugs that can inhibit the disturbed intracellular Ca2+ homeostasis have been repurposed to treat COVID-19 patients. This narrative review aims at exploring the underlying mechanism by which lithium, a first line drug for the treatment of bipolar disorder, inhibits Ca2+ dysregulation and associated downstream pathology in both AD and COVID-19. It is suggested that lithium can be repurposed to treat AD patients, especially those afflicted with COVID-19.
Keywords: Lithium, Mitochondria, Endosome, Calcium, Amyloid, Tau, SARS-CoV-2, Infection, Replication
Introduction
Alzheimer’s disease (AD) is the 6th leading cause of death in the United States and the 5th leading cause of death among those age 65 and older, without disease-modifying treatment1. In 2020, the costs of treating dementia in the United States were projected to be about $256.7 billion1. Most (>95%) cases of AD are sporadic (SAD); while <5% cases are familial AD (FAD)2,3. FAD arises from genetic mutations in the amyloid β precursor protein (APP), and presenilin 1 and 2 (PSEN1 and PSEN2), resulting in increased amyloid β peptide (Aβ42) fragments which aggregate into soluble intracellular amyloid oligomers and/or insoluble extracellular plaques4–8. Pathological tau phosphorylation results in the formation of neurofibrillary tangles9–12. Although pathological markers are common in both FAD and SAD, the etiology of SAD and associated AD pathology, including synaptic and cognitive dysfunctions, is largely unknown, which impedes the development of new effective drugs for AD treatment13,14. The apolipoprotein E4 (ApoE4) allele is considered a predominant risk factor for SAD among all other recognized risk factors15–17. This tends to shift current research focus from amyloid pathologies to tau pathologies or to a combination of both amyloid and tau alongside other related downstream AD pathologies14. In turn, the following strategies have been proposed to develop new effective drugs for the treatment of AD patients13,18: 1) Targeting an upstream AD pathology that results in other multiple pathology pathways; 2) Utilizing a combination of drugs targeting different AD aberrant pathways, given the multifaceted etiology of AD; 3) The prevention or treatment of AD patients in the early stages of their disease, thanks to the improved techniques for the early diagnosis of AD19.
Besides AD, the ongoing COVID-19 pandemic has resulted in over 364 million infection cases and over 5.63 million deaths worldwide (https://covid19.who.int/), impacting every aspect of our societies. The elderly, notably the demented population, is one of the groups most at risk of having severe COVID-19 symptoms. AD patients have greater than seven-times the risk of being infected with the COVID-19 virus, and more than a two-fold rate of mortality20. Disruption of intracellular Ca2+ homeostasis is considered an upstream pathological pathway in not only AD, but also SARS-CoV-2 virus infection and replication in COVID-1921–23. Specifically, aberrant elevation of Ca2+ concentrations in the cytosol and endosome as well as associated amyloid pathology in AD promote SARS-CoV-2 virus binding to host cells, subsequent infection, and RNA replication in host cells22,24,25 – a potential underlying mechanism which could increase COVID-19 severity in AD patients. This narrative review addresses mechanisms underlying neuroprotection via lithium (the current first-line treatment for patients with bipolar disorder) against AD and, to a lesser extent, against COVID-19. We propose that lithium provides protection in both AD and COVID-19, at least in part, by restoring the disrupted intracellular Ca2+ homeostasis. Lithium is expected to inhibit both AD and COVID-19 pathologies and therefore may be utilized as a potential repurposed drug for the treatment of AD patients, especially those also infected with SARS-CoV-2.
Lithium as a Potential Therapeutic Drug for AD by Correcting Upstream Ca2+ Dysregulation and Aberrant Signaling Pathways
Changes of cytosolic Ca2+ concentrations ([Ca2+]c) regulate a variety of physiological functions, such as cell survival, cell death, cell division, neurogenesis, synaptogenesis and autophagy, among others26–29. As shown in Figure 1, pathological and prolonged elevation of [Ca2+]c and mitochondrial Ca2+ concentrations ([Ca2+]m) due to Ca2+ influx via over-activation of NMDAR30–34, AMPAR35–38 and metabotropic GluRs (mGluRs)39–42 glutamate receptors in AD results in multiple AD-like pathologies including neurodegeneration43–46, impaired neurogenesis34,47,48, disrupted autophagy34,49–51, and excessive inflammation, etc.33,52–55. Additionally, the SAD high risk protein, ApoE4, pathologically aggravates over-activation of NMDAR and subsequent activation of L type voltage-dependent Ca2+ channel (L-VDCC)30,56,57. Further, L-VDCC is increased in the hippocampus of AD transgenic mice58, which can be modulated by amyloid β peptide59. The Ca2+ release from the endoplasmic reticulum (ER) via InsP3 receptors (InsP3Rs) and/or ryanodine receptors (RyRs) is also pathologically increased in AD, due to the PSEN1 or PSEN2 mutation51,60–66. This Ca2+ dysregulation described above has been considered an upstream trigger for multiple AD pathologies, including activation of cyclin-dependent kinases 5 (CDK-5)67–70 and glycogen synthase kinase-3β (GSK-3β)71–74, tau hyperphosphorylation, and the spreading of tau pathology75,76, mitochondrial damage77–79, elevation of reactive oxygen species (ROS)46,80,81, and energy failure82,83 (Figure 1). These pathologies, especially when combined, result in the previously mentioned downstream AD pathologies, and eventually lead to synaptic/cognitive dysfunction61,84–87 (Figure 1). A drug that can inhibit upstream Ca2+ dysregulation13,76 and associated tau pathology14,88 is expected to be a good candidate for all above mentioned AD pathologies and to be an effective treatment of AD patients.
Figure 1.
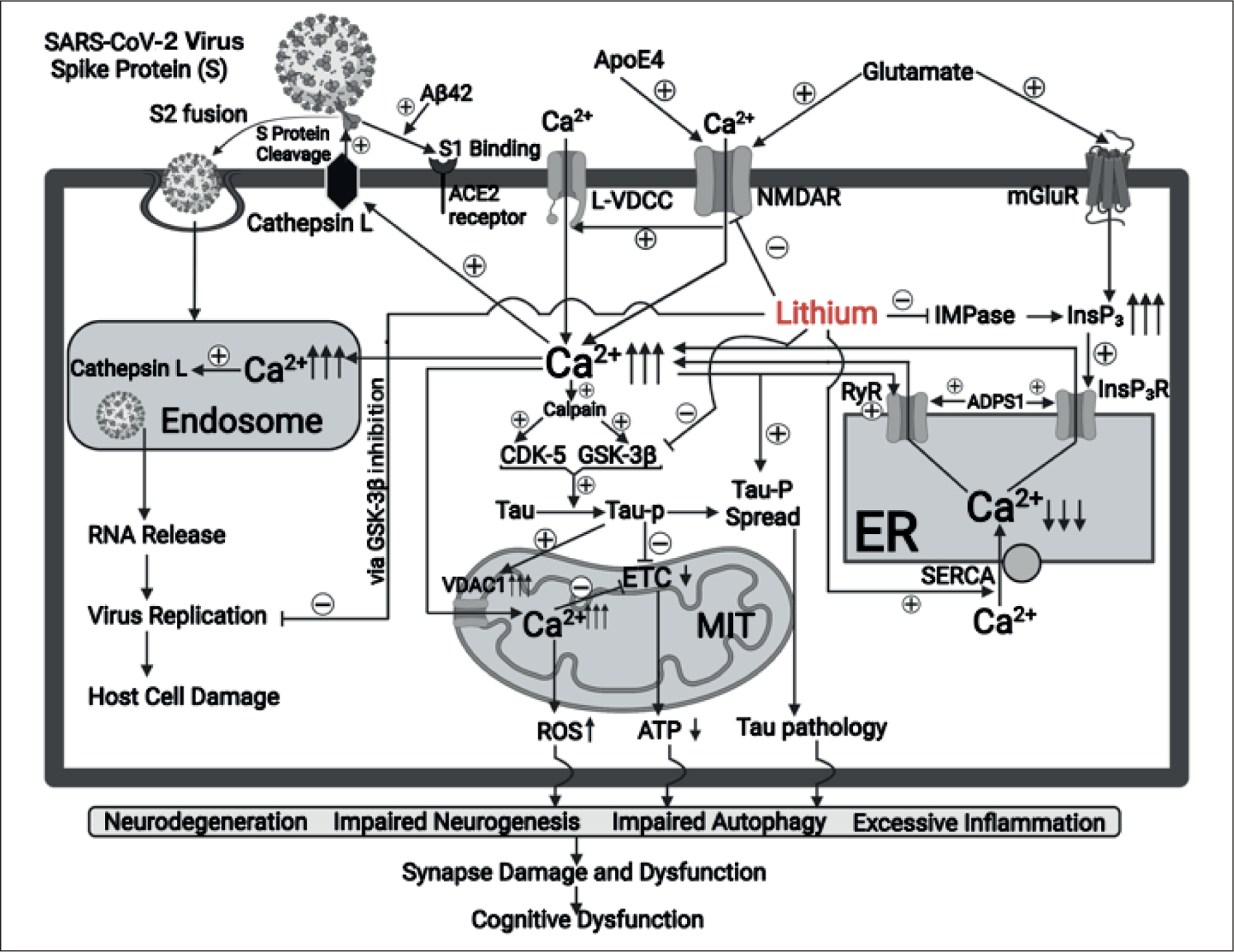
Proposed mechanisms underlying lithium inhibition of calcium dysregulation and associated pathological features in Alzheimer’s Disease (AD) and COVID-19.
Lithium has long been a primary drug for treating bipolar disorder and has been shown to exhibit neuroprotective properties in various neurodegenerative diseases, including AD89–94, stroke95–98, Parkinson’s disease72,99–102, Huntington’s disease103–105, and brain trauma106,107. In preclinical models of AD, lithium treatment has been reported to inhibit multiple pathological features of AD, including amyloid108–110 and tau71,111,112 pathology, oxidative stress113, autophagy impairment95, as well as synapse and learning/memory deficits110,114. In some clinical investigations, lithium at moderate doses improves cognitive function and memory performance in AD patients115,116. Although inhibition of GSK-3β and CDK-5 is thought to be one of the primary mechanisms for inducing lithium’s neuroprotective efficacies117,118, the role of Ca2+ modulation in mediating lithium-induced neuroprotection has been under-explored. Increasing evidence suggests that lithium also inhibits the upstream pathologically elevated [Ca2+]c and associated tau hyperphosphorylation, as well as other downstream AD pathological pathways93,95,119,120. As shown in Figure 1, lithium inhibits toxic glutamate-induced over-activation of NMDARs, both alone and when this NMDAR over-activation is aggravated by the AD high risk protein ApoE430,93. Lithium may inhibit NMDAR by inhibiting NMDA NR2B subunit tyrosine phosphorylation due to suppression of Src/Fyn tyrosine kinase119,121. Lithium also suppresses excessive Ca2+ release caused by over-activation of InsP3R in AD conditions by downregulating an aberrant level of the InsP3R agonist, insP3122. Additionally, the aforementioned effects of lithium indirectly reduce Ca2+ release from the ER via RyRs through inhibiting Ca2+-induced Ca2+ release (CICR)33,123. Moreover, lithium has also been demonstrated to increase the number and activity of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) pump and to facilitate the Ca2+ uptake from the cytosol to ER lumen, thus ameliorating cell damage due to significant ER Ca2+ depletion and associated ER stress124.
In neurophysiological conditions, transfer of Ca2+ from the ER into mitochondria through InsP3Rs/RyRs plays an important role in the generation of mitochondrial ATP as an energy source125,126. However, excessive transfer of Ca2+ from the ER into mitochondria, together with impaired electronic transfer chain (ETC) function caused by hyperphosphorylated tau in AD will impair mitochondrial function in energy production127–129. Furthermore, overloading mitochondria with Ca2+ due to elevated Ca2+ concentration in cytosolic space ([Ca2+]c), especially those transferred from the ER via InsP3Rs/RyRs, pathologically increases the generation of reactive oxygen species (ROS)130–133. Lithium can inhibit upstream abnormal Ca2+ influx from extracellular space by ameliorating dysfunctional changes of NMDARs93,119,134, AMPAR135, Kainite (KA) receptors136, mGluRs135,137, as well as excessive Ca2+ transfer from the ER into mitochondria via InsP3Rs/RyRs122,138,139. Lithium also promotes Ca2+ uptake into the ER lumen by increasing the SERCA Ca2+ pump activity124 and ameliorating ER stress and associated cell damage in AD120. Considering the ability of lithium to ameliorate the above-mentioned AD pathologies, it may be prudent to repurpose lithium as an effective disease-modifying drug for AD treatment72,91,93.
Intracellular Ca2+ homeostasis plays critical roles in determining cell survival and death140–144. Both an aberrant elevation of [Ca2+]c145,146 and Ca2+ concentration in mitochondria ([Ca2+]m)127,147, and the depletion of ER Ca2+143,148,149 contribute to neuronal death. Overloading mitochondria with Ca2+ collapses the mitochondria membrane potential and releases cytochrome c into the cytosol142,150, leading to caspase activation and apoptotic cell death127,150–152. Neurodegeneration and brain atrophy are commonly seen in AD patients153,154, and are key mechanisms underlying synapse/cognitive dysfunction85,155. Maintenance of cytosolic, especially mitochondrial Ca2+ homeostasis also plays prominent roles in neurogenesis and synaptogenesis48,156–159. Mounting evidence suggests that adult neurogenesis and synaptogenesis in AD are significantly impaired due to Ca2+ dysregulation34,47,48,77,84,160,161. Thus, drugs that restore intracellular Ca2+ homeostasis have been demonstrated to protect and/or promote neurogenesis/synaptogenesis in various AD models34,162. These drugs eventually improve synapse and cognitive dysfunction by restoring and/or promoting neurogenesis/synaptogenesis34,114,163–166. Through the correction of disrupted intracellular Ca2+ homeostasis, lithium is expected to inhibit neurodegeneration72,91,119,134,136 and impaired neurogenesis/synaptogenesis166–170, or even to further promote neurogenesis/synaptogenesis166,171.
Physiological autophagy plays a key role in maintaining protein homeostasis172–174, especially via the removal of harmful proteins, such as β-amyloid and tau proteins or their aggregates175–181. It is known that intracellular Ca2+ homeostasis, especially in the lysosome and mitochondria, helps to maintain normal autophagy49,51,182–187. Ca2+ dysregulation in the cytosolic space, mitochondria and/or lysosome in AD contributes to impaired autophagy49,51,182,188, leading to the accumulation of AD pathological proteins and a vicious cycle of Ca2+ dysregulation. This in turn ultimately results in cell and synapse damage as well as associated memory impairments32,34,164,177,179. Lithium has been proposed to suppress impaired autophagy in AD by ameliorating the upstream Ca2+ dysregulation and therefore restoring neuronal, synaptic, and cognitive functions90,95,189,190.
The over-expression of inflammation cytokines is likely involved in cell damage and synapse dysfunction in AD53,54,191–194. Intracellular Ca2+ homeostasis plays an important role in regulating levels of cytokine production and inflammation130,195–198. On the other hand, some pathologically elevated cytokines further disrupt intracellular Ca2+ homeostasis, forming a vicious cycle196,199–201. The upstream Ca2+ dysregulation contributes to the excessive production of toxic cytokines (TNF-α, Il-1, Il-6, etc.) and associated neuroinflammation55,195,197,202,203, leading to neuronal and glial cell damages192,194,204. As shown in Figure 1, lithium can suppress excessive inflammation in AD brains via normalizing upstream Ca2+ dysregulation, eventually resulting in improvement of synaptic function and cognitive performance168,190,205–207.
Potential Utility of Lithium in Treating COVID-19 Patients by Ameliorating the Upstream Pathology of Ca2+Dysregulation
COVID-19 is a systemic disease, involving multiple organ failures. Massive inflammation (cytokine storm) and cell damage or death in various organs likely contribute to COVID-19-related mortality21,208–212. Although multiple mechanisms and pathways are likely involved in the infection, replication and host cell damage caused by the COVID-19 virus SARS-CoV-223,213–216, Ca2+ dysregulation has been proposed to be an integral upstream pathological event21,23,198,217–219. Infection of host cells by SARS-CoV-2 requires initial binding of spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor on the plasma membrane and subsequent cleavage of S protein into S1 and S2 by the transmembrane proteases, serine 2 (TMPRSS2) and/or cathepsin L220,221. S1 binds to ACE-2 which can be promoted by the amyloid protein25, while S2 fuses with the plasma membrane and facilitates the endocytosis and invasion of the virus into the host cells220,221 (Figure 1). Activation of cathepsin L is dependent upon the elevation of [Ca2+]c caused by Ca2+ influx from various glutamate receptor subtypes or voltage-dependent Ca2+ channels (VDCC)22,24,217–219,222, and pathologically increased Ca2+ release from the ER via InsP3R/RyRs21,24,223. Activation of the L type Ca2+ channel facilitates the SARS-CoV-2 viral entry and spread in host cells218. Endocytosis of the SARS-CoV-2 virus inside the endosome and cytosol also depends on high levels of Ca2+ in the endosome lumen, which originates from elevated [Ca2+]c21,224. This Ca2+−dependent pathological process eventually promotes virus entry and spread, leading to host cell damage or death21,22,217,218. COVID-19 viral replication appears to require GSK-3β-mediated phosphorylation of the viral N protein of SARS-CoV-2 and accordingly GSK-3β inhibitors including lithium suppress the viral replication by blocking this GSK-3β-dependent event225,226. Additionally, lithium dose-dependently inhibited replication of foot-and-mouth disease virus (FMDV), a single strand RNA virus227, and replication of herpes simplex virus (a DNA virus) by suppression of DNA polymerase228. As shown in Figure 1, lithium can suppress both the fusion of SARS-CoV-2 with the host cell plasma membrane and subsequent virus replication inside host cells and thus reduces cell damage by normalizing the described upstream Ca2+ dysregulation. Therefore, lithium is expected to protect against host cell damage and associated multiple organ failures in COVID-19 patients225,226,229–231. A recent preliminary clinical study reported that lithium treatment of a small group of COVID patients showed significant benefits including improvement of inflammatory activity and the immune response231.
Conclusions
Aged people, especially those in nursing homes, are disproportionately affected by the COVID-19 pandemic232,233. Currently, 45 million people in the world suffer from AD, and this number is expected to triple by 20501,234,235. Unfortunately, no disease-modifying drugs have been developed for effective treatment of AD. A drug that can inhibit the pathologies of both AD and COVID-19 is expected to benefit those AD patients infected, or at high risk of being infected with SARS-CoV-2 virus. As shown in Figure 1 and discussed above, lithium inhibits the upstream pathology Ca2+ dysregulation in both AD and COVID-19 via its ability to restore intracellular Ca2+ homeostasis and could have the potential to be repurposed to treat AD patients suffering with COVID-19. Further timely preclinical and clinical investigations of this possibility are warranted.
Funding
This work was supported by grants to HW from the National Institution Aging (R01AG061447, 3R01AG061447-03S1). The support from the Intramural Research Program of NIMH, NIH to D-M Chuang is appreciated.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The views expressed in this review do not necessarily represent the views of the NIH, HHS, or the United States Government.
References
- 1).[No authors listed]. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 2021; 17: 327–406. [DOI] [PubMed] [Google Scholar]
- 2).Nguyen KV. Special Issue: Alzheimer’s disease. AIMS Neurosci 2018; 5: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Wang K, Zhang W. Mitochondria-associated endoplasmic reticulum membranes: at the cross-road between familiar and sporadic Alzheimer’s disease. Synapse 2021; 75: e22196. [DOI] [PubMed] [Google Scholar]
- 4).Bloom GS. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 2014; 71: 505–508. [DOI] [PubMed] [Google Scholar]
- 5).Sepulveda-Falla D, Barrera-Ocampo A, Hagel C, Korwitz A, Vinueza-Veloz M, Zhou K, Schonewille M, Zhou H, Velazquez-Perez L, Rodriguez-Labrada R, Villegas A, Ferrer I, Lopera F, Langer T, Zeeuw C, Glatzel M. Familial Alzheimer’s disease-associated presenilin-1 alters cerebellar activity and calcium homeostasis. J Clin Invest 2014; 124: 1552–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Zatti G, Ghidoni R, Barbiero L, Giuliano B, Tulli P, Fasolato C, Pizzo P. The presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+ release from intracellular stores. Neurobiol Dis 2004; 15: 269–278. [DOI] [PubMed] [Google Scholar]
- 7).Nelson O, Supnet C, Liu H, Bezprozvanny I. Familial Alzheimer’s disease mutations in presenilins: effects on endoplasmic reticulum calcium homeostasis and correlation with clinical phenotypes. J Alzheimers Dis 2010; 21: 781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Zampese E, Fasolato C, Pozzan T, Pizzo P. Presenilin-2 modulation of ER-mitochondria interactions: FAD mutations, mechanisms and pathological consequences. Commun Integr Biol 2011; 4: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Hernandez F, Lucas JJ, Avila J. GSK3 and tau: two convergence points in Alzheimer’s disease. J Alzheimers Dis 2013; 33: S141–144. [DOI] [PubMed] [Google Scholar]
- 10).Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribé E, Dalfó E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res 2005; 2: 3–18. [DOI] [PubMed] [Google Scholar]
- 11).Chung SH. Aberrant phosphorylation in the pathogenesis of Alzheimer’s disease. BMB Rep 2009; 42: 467–474. [DOI] [PubMed] [Google Scholar]
- 12).Guan PP, Cao LL, Wang P. Elevating the Levels of Calcium Ions Exacerbate Alzheimer’s Disease via Inducing the Production and Aggregation of beta-Amyloid Protein and Phosphorylated Tau. Int J Mol Sci 2021; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Wei H New Approaches to Develop Drug Treatment for Alzheimer’s Disease: Targeting Calcium Dysregulation. Curr Alzheimer Res 2020; 17: 311–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Allgaier M, Allgaier C. An update on drug treatment options of Alzheimer’s disease. Front Biosci 2014; 19: 1345–1354. [DOI] [PubMed] [Google Scholar]
- 15).Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 2011; 10: 698–712. [DOI] [PubMed] [Google Scholar]
- 16).Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, Rueda R, Kritskiy O, Abdurrob F, Peng Z, Milo B, Yu CJ, Elmsaouri S, Dey D, Ko T, Yankner BA, Tsai LH. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018; 98: 1141–54 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 2000; 97: 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Matan BA, Liang S, Wei H. Approaches to Optimizing Dantrolene Neuroprotection for Treatment of Alzheimer’s Disease. Curr Alzheimer Res 2020; 17: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Yu M, Sporns O, Saykin AJ. The human connectome in Alzheimer disease - relationship to biomarkers and genetics. Nat Rev Neurol 2021; 17: 545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hardan L, Filtchev D, Kassem R, Bourgi R, Lukomska-Szymanska M, Tarhini H, Salloum-Yared F, Mancino D, Kharouf N, Haikel Y. COVID-19 and Alzheimer’s Disease: A Literature Review. Medicina (Kaunas) 2021; 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Jiang B, Liang S, Liang G, Wei H. Could dantrolene be explored as a repurposed drug to treat COVID-19 patients by restoring intracellular calcium homeostasis? Eur Rev Med Pharmacol Sci 2020; 24: 10228–10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Danta CC. Calcium Channel Blockers: A Possible Potential Therapeutic Strategy for the Treatment of Alzheimer’s Dementia Patients with SARS-CoV-2 Infection. ACS Chem Neurosci 2020; 11: 2145–2148. [DOI] [PubMed] [Google Scholar]
- 23).Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020; 11: 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Reiken S, Dridi H, Sittenfeld L, Liu X, Marks AR. Alzheimer’s-like remodeling of neuronal ryanodine receptor in COVID-19. bioRxiv 2021. [Google Scholar]
- 25).Hsu JT, Tien CF, Yu GY, Shen S, Lee YH, Hsu PC, Wang Y, Chao PK, Tsay HJ, Shie FS. The Effects of Abeta1–42 Binding to the SARS-CoV-2 Spike Protein S1 Subunit and Angiotensin-Converting Enzyme 2. Int J Mol Sci 2021; 22; 8226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 2003; 4: 552–565. [DOI] [PubMed] [Google Scholar]
- 27).Bagur R, Hajnóczky G. Intracellular Ca 2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Molecular Cell 2017; 66: 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Toth AB, Shum AK, Prakriya M. Regulation of neurogenesis by calcium signaling. Cell Calcium 2016; 59: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Yao Z, Klionsky DJ. The symphony of autophagy and calcium signaling. Autophagy 2015; 11: 973–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Qiu Z, Crutcher KA, Hyman BT, Rebeck GW. ApoE isoforms affect neuronal N-methyl-D-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience 2003; 122: 291–303. [DOI] [PubMed] [Google Scholar]
- 31).Mishizen-Eberz AJ, Rissman RA, Carter TL, Ikonomovic MD, Wolfe BB, Armstrong DM. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal sub-regions throughout progression of Alzheimer’s disease pathology. Neurobiol Dis 2004; 15: 80–92. [DOI] [PubMed] [Google Scholar]
- 32).Green KN. Calcium in the initiation, progression and as an effector of Alzheimer’s disease pathology. J Cell Mol Med 2009; 13: 2787–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Goussakov I, Miller MB, Stutzmann GE. NMDA-mediated Ca(2+) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci 2010; 30: 12128–12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Wang Y, Liang G, Liang S, Mund R, Shi Y, Wei H. Dantrolene ameliorates impaired neurogenesis and synaptogenesis in induced pluripotent stem cell lines derived from patients with Alzheimer’s Disease. Anesthesiology 2020; 132: 1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Qu W, Yuan B, Liu J, Liu Q, Zhang X, Cui R, Yang W, Li B. Emerging role of AMPA receptor subunit GluA1 in synaptic plasticity: implications for Alzheimer’s disease. Cell Prolif 2021; 54: e12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Yu J, Cho E, Kwon H, Jeon J, Seong Sin J, Kwon Park J, Kim JS, Woong Choi J, Jin Park S, Jun M, Choon Lee Y, Hoon Ryu J, Lee J, Moon M, Lee S, Hyun Cho J, Hyun Kim D. Akt and calcium-permeable AMPA receptor are involved in the effect of pinoresinol on amyloid beta-induced synaptic plasticity and memory deficits. Biochem Pharmacol 2021; 184: 114366. [DOI] [PubMed] [Google Scholar]
- 37).Tanaka H, Sakaguchi D, Hirano T. Amyloid-beta oligomers suppress subunit-specific glutamate receptor increase during LTP. Alzheimers Dement 2019; 5: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Schurmann B, Bermingham DP, Kopeikina KJ, Myczek K, Yoon S, Horan KE, Kelly CJ, Martin-de-Saavedra MD, Forrest MP, Fawcett-Patel JM, Smith KR, Gao R, Bach A, Burette AC, Rappoport JZ, Weinberg RJ, Martina M, Penzes P. A novel role for the late-onset Alzheimer’s disease (LOAD)-associated protein Bin1 in regulating postsynaptic trafficking and glutamatergic signaling. Mol Psychiatry 2020; 25: 2000–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Olajide OJ, Gbadamosi IT, Yawson EO, Arogun-dade T, Lewu FS, Ogunrinola KY, Adigun OO, Bamisi O, Lambe E, Arietarhire LO, Oluyomi OO, Idowu OK, Kareem R, Asogwa NT, Adeniyi PA. Hippocampal degeneration and behavioral impairment during Alzheimer-like pathogenesis involves glutamate excitotoxicity. J Mol Neurosci 2021; 71: 1205–1220. [DOI] [PubMed] [Google Scholar]
- 40).Temido-Ferreira M, Ferreira DG, Batalha VL, Marques-Morgado I, Coelho JE, Pereira P, Gomes R, Pinto A, Carvalho S, Canas PM, Cuvelier L, Buée-Scherrer V, Faivre E, Baqi Y, Müller CE, Pimentel J, Schiffmann SN, Buée L, Bader M, Outeiro TF, Blum D, Cunha RA, Marie H, Pousinha PA, Lopes LV. Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol Psychiatry 2020; 25: 1876–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Lee M, Lee HJ, Jeong YJ, Oh SJ, Kang KJ, Han SJ, Nam KR, Lee YJ, Lee KC, Ryu YH, Hyun IY, Choi JY. Age dependency of mGluR5 availability in 5xFAD mice measured by PET. Neurobiol Aging 2019; 84: 208–216. [DOI] [PubMed] [Google Scholar]
- 42).Bie B, Wu J, Foss JF, Naguib M. Activation of mGluR1 mediates C1q-dependent microglial phagocytosis of glutamatergic synapses in Alzheimer’s rodent models. Mol Neurobiol 2019; 56: 5568–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).MacManus A, Ramsden M, Murray M, Henderson Z, Pearson HA, Campbell VA. Enhancement of (45)Ca(2+) influx and voltage-dependent Ca(2+) channel activity by beta-amyloid-(1–40) in rat cortical synaptosomes and cultured cortical neurons. Modulation by the proinflammatory cytokine interleukin-1beta. J Biol Chem 2000; 275: 4713–4718. [DOI] [PubMed] [Google Scholar]
- 44).Lopez JR, Lyckman A, Oddo S, Laferla FM, Querfurth HW, Shtifman A. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J Neurochem 2008; 105: 262–271. [DOI] [PubMed] [Google Scholar]
- 45).Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E, Bezprozvanny I. Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2015; 45: 561–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci 1998; 18: 4439–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Wang JM, Sun C. Calcium and neurogenesis in Alzheimer’s disease. Front Neurosci 2010; 4: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Glaser T, Arnaud Sampaio VF, Lameu C, Ulrich H. Calcium signalling: a common target in neurological disorders and neurogenesis. Semin Cell Dev Biol 2019; 95: 25–33. [DOI] [PubMed] [Google Scholar]
- 49).Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA. Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Reports 2015; 12: 1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Xue Z, Guo Y, Fang Y. Moderate activation of autophagy regulates the intracellular calcium ion concentration and mitochondrial membrane potential in beta-amyloid-treated PC12 cells. Neurosci Lett 2016; 618: 50–57. [DOI] [PubMed] [Google Scholar]
- 51).Yang M, Wang Y, Liang G, Xu Z, Chu CT, Wei H. Alzheimer’s Disease presenilin-1 mutation sensitizes neurons to impaired autophagy flux and propofol neurotoxicity: role of calcium dysregulation. J Alzheimers Dis 2019; 67: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Bordji K, Becerril-Ortega J, Buisson A. Synapses, NMDA receptor activity and neuronal Abeta production in Alzheimer’s disease. Rev Neurosci 2011; 22: 285–294. [DOI] [PubMed] [Google Scholar]
- 53).Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer’s disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiol Aging 2000; 21: 427–432. [DOI] [PubMed] [Google Scholar]
- 54).Belinson H, Michaelson DM. ApoE4-dependent Abeta-mediated neurodegeneration is associated with inflammatory activation in the hippocampus but not the septum. J Neural Transm (Vienna) 2009; 116: 1427–1434. [DOI] [PubMed] [Google Scholar]
- 55).Simma N, Bose T, Kahlfuss S, Mankiewicz J, Lowinus T, Lühder F, Schüler T, Schraven B, Heine M, Bommhardt U. NMDA-receptor antagonists block B-cell function but foster IL-10 production in BCR/CD40-activated B cells. Cell Commun Signal 2014; 12: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. J Neurosci Res 2002; 67: 379–387. [DOI] [PubMed] [Google Scholar]
- 57).Xu D, Peng Y. Apolipoprotein E 4 triggers multiple pathway-mediated Ca2+ overload, causes CaMK II phosphorylation abnormity and aggravates oxidative stress caused cerebral cortical neuron damage. Eur Rev Med Pharmacol Sci 2017; 21: 5717–5728. [DOI] [PubMed] [Google Scholar]
- 58).Wang Y, Mattson MP. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol Aging 2014; 35: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Ishii M, Hiller AJ, Pham L, McGuire MJ, Iadecola C, Wang G. Amyloid-beta modulates low-threshold activated voltage-gated l-type calcium channels of arcuate neuropeptide Y neurons leading to calcium dysregulation and hypothalamic dysfunction. J Neurosci 2019; 39: 8816–8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Cheung KH, Shineman D, Muller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron 2008; 58: 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Shilling D, Muller M, Takano H, Mak DO, Abel T, Coulter DA, Foskett JK. Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J Neurosci 2014; 34: 6910–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 2000; 275: 18195–18200. [DOI] [PubMed] [Google Scholar]
- 63).D’Adamio L, Castillo PE. Presenilin-ryanodine receptor connection. Proc Natl Acad Sci U S A 2013; 110: 14825–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Del Prete D, Checler F, Chami M. Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 2014; 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium 2008; 44: 507–518. [DOI] [PubMed] [Google Scholar]
- 66).Muller M, Cheung KH, Foskett JK. Enhanced ROS generation mediated by Alzheimer’s disease presenilin regulation of InsP3R Ca2+ signaling. Antioxid Redox Signal 2011; 14: 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Bréchot P, Trebak M, Checler F, Benfenati F, Chami M. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 2012; 32: 11820–11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Medeiros R, Kitazawa M, Chabrier MA, Cheng D, Baglietto-Vargas D, Kling A, Moeller A, Green KN, LaFerla FM. Calpain inhibitor A-705253 mitigates Alzheimer’s disease-like pathology and cognitive decline in aged 3xTgAD mice. Am J Pathol 2012; 181: 616–625. [DOI] [PubMed] [Google Scholar]
- 69).Darios F, Muriel MP, Khondiker ME, Brice A, Ruberg M. Neurotoxic calcium transfer from endoplasmic reticulum to mitochondria is regulated by cyclin-dependent kinase 5-dependent phosphorylation of tau. J Neurosci 2005; 25: 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70).Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 2010; 30: 11938–11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Hartigan JA, Johnson GV. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J Biol Chem 1999; 274: 21395–21401. [DOI] [PubMed] [Google Scholar]
- 72).Camins A, Crespo-Biel N, Junyent F, Verdaguer E, Canudas AM, Pallas M. Calpains as a target for therapy of neurodegenerative diseases: putative role of lithium. Curr Drug Metab 2009; 10: 433–447. [DOI] [PubMed] [Google Scholar]
- 73).Feng Y, Xia Y, Yu G, Shu X, Ge H, Zeng K, Wang J, Wang X. Cleavage of GSK-3beta by calpain counteracts the inhibitory effect of Ser9 phosphorylation on GSK-3beta activity induced by H(2)O(2). J Neurochem 2013; 126: 234–242. [DOI] [PubMed] [Google Scholar]
- 74).Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. J Biol Chem 2007; 282: 22406–22413. [DOI] [PubMed] [Google Scholar]
- 75).Arnsten AFT, Datta D, Tredici KD, Braak H. Hypothesis: tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement 2021; 17: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Tong BC, Wu AJ, Li M, Cheung KH. Calcium signaling in Alzheimer’s disease & therapies. Biochim Biophys Acta Mol Cell Res 2018; 1865: 1745–1760. [DOI] [PubMed] [Google Scholar]
- 77).Begley JG, Duan W, Chan S, Duff K, Mattson MP. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem 1999; 72: 1030–1039. [DOI] [PubMed] [Google Scholar]
- 78).Calvo-Rodriguez M, Kharitonova EK, Bacskai BJ. Therapeutic Strategies to Target Calcium Dysregulation in Alzheimer’s Disease. Cells 2020; 9: 2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Wu AJ, Tong BC, Huang AS, Li M, Cheung KH. Mitochondrial calcium signaling as a therapeutic target for Alzheimer’s disease. Curr Alzheimer Res 2020; 17: 329–343. [DOI] [PubMed] [Google Scholar]
- 80).Birnbaum JH, Wanner D, Gietl AF, Saake A, Kündig TM, Hock C, Nitsch RM, Tackenberg C. Oxidative stress and altered mitochondrial protein expression in the absence of amyloid-beta and tau pathology in iPSC-derived neurons from sporadic Alzheimer’s disease patients. Stem Cell Res 2018; 27: 121–130. [DOI] [PubMed] [Google Scholar]
- 81).Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 2004; 58: 39–46. [DOI] [PubMed] [Google Scholar]
- 82).Ferreira IL, Resende R, Ferreiro E, Rego AC, Pereira CF. Multiple defects in energy metabolism in Alzheimer’s disease. Curr Drug Targets 2010; 11: 1193–1206. [DOI] [PubMed] [Google Scholar]
- 83).Picone P, Nuzzo D, Caruana L, Scafidi V, Di Carlo M. Mitochondrial dysfunction: different routes to Alzheimer’s disease therapy. Oxid Med Cell Longev 2014; 2014: 780179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Chakroborty S, Stutzmann GE. Early calcium dysregulation in Alzheimer’s disease: setting the stage for synaptic dysfunction. Sci China Life Sci 2011; 54: 752–762. [DOI] [PubMed] [Google Scholar]
- 85).Lane-Donovan C, Herz J. ApoE, ApoE receptors, and the synapse in Alzheimer’s Disease. Trends Endocrinol Metab 2017; 28: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 2012; 516: 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Shi Y, Zhang L, Gao X, Zhang J, Ben Abou M, Liang G, Meng Q, Hepner A, Eckenhoff MF, Wei H. Intranasal Dantrolene as a Disease-Modifying Drug in Alzheimer 5XFAD Mice. J Alzheimers Dis 2020; 76: 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Sala Frigerio C, De Strooper B. Alzheimer’s disease mechanisms and emerging roads to novel therapeutics. Annu Rev Neurosci 2016; 39: 57–79. [DOI] [PubMed] [Google Scholar]
- 89).Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar Disord 2002; 4: 153–165. [DOI] [PubMed] [Google Scholar]
- 90).Damri O, Shemesh N, Agam G. Is there justification to treat neurodegenerative disorders by repurposing drugs? The Case of Alzheimer’s Disease, Lithium, and Autophagy. Int J Mol Sci 2020; 22: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med 2004; 6: 1–18. [DOI] [PubMed] [Google Scholar]
- 92).Forlenza OV, Aprahamian I, de Paula VJ, Hajek T. Lithium, a therapy for AD: current evidence from clinical trials of neurodegenerative disorders. Curr Alzheimer Res 2016; 13: 879–886. [DOI] [PubMed] [Google Scholar]
- 93).Wallace J Calcium dysregulation, and lithium treatment to forestall Alzheimer’s disease - a merging of hypotheses. Cell Calcium 2014; 55: 175–181. [DOI] [PubMed] [Google Scholar]
- 94).Wittenberg SM, Toxopeus KA, Schulte PFJ. [Lithium and its protective effect in Alzheimer’s disease]. Tijdschr Psychiatr 2017; 59: 559–563. [PubMed] [Google Scholar]
- 95).Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther 2010; 128: 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Ji YB, Gao Q, Tan XX, Huang XW, Ma YZ, Fang C, Wang SN, Qiu LH, Cheng YX, Guo FY, Chang J. Lithium alleviates blood-brain barrier breakdown after cerebral ischemia and reperfusion by upregulating endothelial Wnt/beta-catenin signaling in mice. Neuropharmacology 2021; 186: 108474. [DOI] [PubMed] [Google Scholar]
- 97).Plotnikov EV, Litvak MM. [Lithium ascorbate as a cerebroprotective agent in a model of ischemic stroke]. Zh Nevrol Psikhiatr Im S S Korsakova 2020; 120: 29–32. [DOI] [PubMed] [Google Scholar]
- 98).Li M, Xia M, Chen W, Wang J, Yin Y, Guo C, Li C, Tang X, Zhao H, Tan Q, Chen Y, Jia Z, Liu X, Feng H. Lithium treatment mitigates white matter injury after intracerebral hemorrhage through brain-derived neurotrophic factor signaling in mice. Transl Res 2020; 217: 61–74. [DOI] [PubMed] [Google Scholar]
- 99).Guttuso T Jr., Andrzejewski KL, Lichter DG, Andersen JK. Targeting kinases in Parkinson’s disease: a mechanism shared by LRRK2, neurotrophins, exenatide, urate, nilotinib and lithium. J Neurol Sci 2019; 402: 121–130. [DOI] [PubMed] [Google Scholar]
- 100).Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, van de Berg WD. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener 2017; 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101).Vallee A, Vallee JN, Lecarpentier Y. Parkinson’s Disease: potential actions of lithium by targeting the WNT/beta-catenin pathway, oxidative stress, inflammation and glutamatergic pathway. Cells 2021; 10: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Soleimani M, Ghasemi N. Lithium chloride can induce differentiation of human immortalized Ren-Vm cells into dopaminergic neurons. Avicenna J Med Biotechnol 2017; 9: 176–180. [PMC free article] [PubMed] [Google Scholar]
- 103).Lauterbach EC. Neuroprotective effects of psychotropic drugs in Huntington’s disease. Int J Mol Sci 2013; 14: 22558–22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington’s disease. Mol Psychiatry 2004; 9: 371–385. [DOI] [PubMed] [Google Scholar]
- 105).Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, Chuang DM. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience 2001; 106: 603–612. [DOI] [PubMed] [Google Scholar]
- 106).Leeds PR, Yu F, Wang Z, Chiu CT, Zhang Y, Leng Y, Linares GR, Chuang DM. A new avenue for lithium: intervention in traumatic brain injury. ACS Chem Neurosci 2014; 5: 422–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Shim SS, Stutzmann GE. Inhibition of glycogen synthase kinase-3: an emerging target in the treatment of traumatic brain injury. J Neurotrauma 2016; 33: 2065–2076. [DOI] [PubMed] [Google Scholar]
- 108).Sun X, Sato S, Murayama O, Murayama M, Park JM, Yamaguchi H, Takashima A. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett 2002; 321: 61–64. [DOI] [PubMed] [Google Scholar]
- 109).Sofola-Adesakin O, Castillo-Quan JI, Rallis C, Tain LS, Bjedov I, Rogers I, Li L, Martinez P, Khericha M, Cabecinha M, Bähler J, Partridge L. Lithium suppresses Abeta pathology by inhibiting translation in an adult Drosophila model of Alzheimer’s disease. Front Aging Neurosci 2014; 6: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Pan Y, Short JL, Newman SA, Choy KHC, Tiwari D, Yap C, Senyschyn D, Banks WA, Nicolazzo JA. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of beta-amyloid. Brain Behav Immun 2018; 70: 36–47. [DOI] [PubMed] [Google Scholar]
- 111).Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem 1997; 272: 25326–25332. [DOI] [PubMed] [Google Scholar]
- 112).Lovestone S, Davis DR, Webster MT, Kaech S, Brion JP, Matus A, Anderton BH. Lithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrations. Biol Psych 1999; 45: 995–1003. [DOI] [PubMed] [Google Scholar]
- 113).Xiang J, Cao K, Dong YT, Xu Y, Li Y, Song H, Zeng XX, Ran LY, Hong W, Guan ZZ. Lithium chloride reduced the level of oxidative stress in brains and serums of APP/PS1 double transgenic mice via the regulation of GSK3beta/Nrf2/HO-1 pathway. Int J Neurosci 2020; 130: 564–573. [DOI] [PubMed] [Google Scholar]
- 114).Fessel J The potential for one drug, administered at the earliest preclinical stage, to prevent the subsequent decline of cognition that eventuates in dementia. Alzheimers Dement 2020; 6: e12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Forlenza OV, Radanovic M, Talib LL, Gattaz WF. Clinical and biological effects of long-term lithium treatment in older adults with amnestic mild cognitive impairment: randomised clinical trial. Br J Psychiatry 2019: 215: 668–674. [DOI] [PubMed] [Google Scholar]
- 116).Forlenza OV, Diniz BS, Radanovic M, Santos FS, Talib LL, Gattaz WF. Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J Psychiatry 2011; 198: 351–356. [DOI] [PubMed] [Google Scholar]
- 117).Sofola O, Kerr F, Rogers I, Killick R, Augustin H, Gandy C, Allen MJ, Hardy J, Lovestone S, Partridge L. Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer’s disease. PLoS Genet 2010; 6: e1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Matsunaga S, Fujishiro H, Takechi H. Efficacy and safety of glycogen synthase kinase 3 inhibitors for alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2019; 69: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 119).Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem 2002; 80: 589–597. [DOI] [PubMed] [Google Scholar]
- 120).Hiroi T, Wei H, Hough C, Leeds P, Chuang DM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J 2005; 5: 102–111. [DOI] [PubMed] [Google Scholar]
- 121).Hashimoto R, Fujimaki K, Jeong MR, Christ L, Chuang DM. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicity. FEBS Lett 2003; 538: 145–148. [DOI] [PubMed] [Google Scholar]
- 122).Sade Y, Toker L, Kara NZ, Einat H, Rapoport S, Moechars D, Berry GT, Bersudsky Y, Agam G. IP3 accumulation and/or inositol depletion: two downstream lithium’s effects that may mediate its behavioral and cellular changes. Transl Psychiatry 2016; 6: e968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123).Verkhratsky A, Shmigol A. Calcium-induced calcium release in neurones. Cell Calcium 1996; 19: 1–14. [DOI] [PubMed] [Google Scholar]
- 124).Hamstra SI, Kurgan N, Baranowski RW, Qiu L, Watson CJF, Messner HN, MacPherson REK, MacNeil AJ, Roy BD, Fajardo VA. Low-dose lithium feeding increases the SERCA2a-to-phospholamban ratio, improving SERCA function in murine left ventricles. Exp Physiol 2020; 105: 666–675. [DOI] [PubMed] [Google Scholar]
- 125).Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, Satrústegui J. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci 2013; 33: 13957–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126).Cardenas C, Miller RA, Smith I, Bui T, Molgó J, Müller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 2010; 142: 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127).Krieger C, Duchen MR. Mitochondria, Ca2+ and neurodegenerative disease. Eur J Pharmacol 2002; 447: 177–188. [DOI] [PubMed] [Google Scholar]
- 128).Khodorov B Glutamate-induced deregulation of calcium homeostasis and mitochondrial dysfunction in mammalian central neurones. Prog Biophys Mol Biol 2004; 86: 279–351. [DOI] [PubMed] [Google Scholar]
- 129).Bauer TM, Murphy E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ Res 2020; 126: 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130).Dada LA, Sznajder JI. Mitochondrial Ca(2)+ and ROS take center stage to orchestrate TNF-alpha-mediated inflammatory responses. J Clin Invest 2011; 121: 1683–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131).Rummel NG, Butterfield DA. Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid Redox Signal. 2021. Dec 21. doi: 10.1089/ars.2021.0177. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132).Ryan KC, Ashkavand Z, Norman KR. The Role of Mitochondrial Calcium Homeostasis in Alzheimer’s and Related Diseases. Int J Mol Sci 2020; 21: 9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133).Esteras N, Abramov AY. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells 2020; 9: 2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134).Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci U S A 1998; 95: 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135).Sourial-Bassillious N, Rydelius PA, Aperia A, Aizman O. Glutamate-mediated calcium signaling: a potential target for lithium action. Neuroscience 2009; 161: 1126–1134. [DOI] [PubMed] [Google Scholar]
- 136).Crespo-Biel N, Camins A, Canudas AM, Pallas M. Kainate-induced toxicity in the hippocampus: potential role of lithium. Bipolar Disord 2010; 12: 425–436. [DOI] [PubMed] [Google Scholar]
- 137).Lohr C, Deitmer JW. Intracellular Ca2+ release mediated by metabotropic glutamate receptor activation in the leech giant glial cell. J Exp Biol 1997; 200: 2565–2573. [DOI] [PubMed] [Google Scholar]
- 138).Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, Sharma Y, Szigeti-Buck K, Ehrlich BE. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest 2006; 116: 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139).Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 2005; 170: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140).Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci 2000; 23: 222–229. [DOI] [PubMed] [Google Scholar]
- 141).Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol Neurodegener 2009; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142).Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem 2002; 80: 207–218. [DOI] [PubMed] [Google Scholar]
- 143).Pan Z, Damron D, Nieminen AL, Bhat MB, Ma J. Depletion of intracellular Ca2+ by caffeine and ryanodine induces apoptosis of chinese hamster ovary cells transfected with ryanodine receptor. J Biol Chem 2000; 275: 19978–19984. [DOI] [PubMed] [Google Scholar]
- 144).Smaili SS, Pereira GJ, Costa MM, Rocha KK, Rodrigues L, do Carmo LG, Hirata H, Hsu YT. The role of calcium stores in apoptosis and autophagy. Curr Mol Med 2013; 13: 252–265. [DOI] [PubMed] [Google Scholar]
- 145).Wei HF, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem 1996; 67: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 146).Humeau J, Bravo-San Pedro JM, Vitale I, Nuñez L, Villalobos C, Kroemer G, Senovilla L. Calcium signaling and cell cycle: progression or death. Cell Calcium 2018; 70: 3–15. [DOI] [PubMed] [Google Scholar]
- 147).Gao X, Wang X, Zhang L, Liang G, Mund R, Wei H. Sevoflurane but not propofol provided dual effects of cell survival in human neuroblastoma SH-SY5Y cells. Curr Alzheimer Res 2020; 17: 1311–1319. [DOI] [PubMed] [Google Scholar]
- 148).SanMartin CD, Veloso P, Adasme T, Lobos P, Bruna B, Galaz J, García A, Hartel S, Hidalgo C, Paula-Lima AC. RyR2-Mediated Ca2+ release and mitochondrial ROS generation partake in the synaptic dysfunction caused by amyloid beta peptide oligomers. Front Mol Neurosci 2017; 10: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149).Ferreiro E, Oliveira CR, Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J Neurosci Res 2004; 76: 872–880. [DOI] [PubMed] [Google Scholar]
- 150).Lu YC, Lin ML, Su HL, Chen SS. ER-Dependent Ca++-mediated Cytosolic ROS as an effector for induction of mitochondrial apoptotic and ATM-JNK signal pathways in gallic acid-treated human oral cancer cells. Anticancer Res 2016; 36: 697–705. [PubMed] [Google Scholar]
- 151).Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun 2003; 304: 445–454. [DOI] [PubMed] [Google Scholar]
- 152).Shoshan-Barmatz V, Nahon-Crystal E, Shteinfer-Kuzmine A, Gupta R. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacol Res 2018; 131: 87–101. [DOI] [PubMed] [Google Scholar]
- 153).Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR Jr. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 2008; 70: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154).O’Neill C, Cowburn RF, Bonkale WL, Ohm TG, Fastbom J, Carmody M, Kelliher M. Dysfunctional intracellular calcium homoeostasis: a central cause of neurodegeneration in Alzheimer’s disease. Biochem Soc Symp 2001; 67: 177–194. [DOI] [PubMed] [Google Scholar]
- 155).Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet 2011; 20: 4515–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156).Leclerc C, Neant I, Moreau M. The calcium: an early signal that initiates the formation of the nervous system during embryogenesis. Front Mol Neurosci 2012; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157).Michaelsen K, Lohmann C. Calcium dynamics at developing synapses: mechanisms and functions. Eur J Neurosci 2010; 32: 218–223. [DOI] [PubMed] [Google Scholar]
- 158).Toth AB, Shum AK, Prakriya M. Regulation of neurogenesis by calcium signaling. Cell Calcium 2016; 59: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159).Toescu EC, Verkhratsky A. Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J Cell Mol Med 2004; 8: 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160).Zhang H, Sun S, Wu L, Pchitskaya E, Zakharova O, Fon Tacer K, Bezprozvanny I. Store-operated calcium channel complex in postsynaptic spines: a new therapeutic target for Alzheimer’s Disease treatment. J Neurosci 2016; 36: 11837–11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161).Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer’s disease. Neuromolecular Med 2002; 1: 125–135. [DOI] [PubMed] [Google Scholar]
- 162).Petrus DS, Fabel K, Kronenberg G, Winter C, Steiner B, Kempermann G. NMDA and benzodiazepine receptors have synergistic and antagonistic effects on precursor cells in adult hippocampal neurogenesis. Eur J Neurosci 2009; 29: 244–252. [DOI] [PubMed] [Google Scholar]
- 163).Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer’s disease mice. J Neurosci 2015; 35: 6893–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164).Wang Y, Shi Y, Wei H. Calcium dysregulation in Alzheimer’s Disease: a target for new drug development. J Alzheimers Dis Parkinsonism 2017; 7: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165).Schaeffer EL, Novaes BA, da Silva ER, Skaf HD, Mendes-Neto AG. Strategies to promote differentiation of newborn neurons into mature functional cells in Alzheimer brain. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 1087–1102. [DOI] [PubMed] [Google Scholar]
- 166).Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One 2010; 5: e14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167).Bauer M, Alda M, Priller J, Young LT; International Group For The Study Of Lithium Treated P. Implications of the neuroprotective effects of lithium for the treatment of bipolar and neurodegenerative disorders. Pharmacopsychiatry 2003; 36: S250–254. [DOI] [PubMed] [Google Scholar]
- 168).Kerr F, Bjedov I, Sofola-Adesakin O. Molecular mechanisms of lithium action: switching the light on multiple targets for dementia using animal models. Front Mol Neurosci 2018; 11: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169).Contestabile A, Greco B, Ghezzi D, Tucci V, Benfenati F, Gasparini L. Lithium rescues synaptic plasticity and memory in Down syndrome mice. J Clin Invest 2013; 123: 348–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170).Zhou K, Xie C, Wickstrom M, Dolga AM, Zhang Y, Li T, Xu Y, Culmsee C, Kogner P, Zhu C, Blomgren K. Lithium protects hippocampal progenitors, cognitive performance and hypothalamus-pituitary function after irradiation to the juvenile rat brain. Oncotarget 2017; 8: 34111–34127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171).Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem 2004; 89: 324–336. [DOI] [PubMed] [Google Scholar]
- 172).Zhang HL, Zhu YM, Zhou XY. Coordination of autophagy and other cellular Activities. Adv Exp Med Biol 2019; 1206: 697–727. [DOI] [PubMed] [Google Scholar]
- 173).Stavoe AKH, Holzbaur ELF. Axonal autophagy: mini-review for autophagy in the CNS. Neurosci Lett 2019; 697: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174).Cherra SJ III, Dagda RK, Chu CT. Review: autophagy and neurodegeneration: survival at a cost? Neuropathol Appl Neurobiol 2010; 36: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175).Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Näslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy - a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol 2005; 171: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176).Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 2010; 5: e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177).Zhang Z, Yang X, Song YQ, Tu J. Autophagy in Alzheimer’s disease pathogenesis: therapeutic potential and future perspectives. Ageing Res Rev 2021; 72: 101464. [DOI] [PubMed] [Google Scholar]
- 178).Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci 2008; 27: 1119–1130. [DOI] [PubMed] [Google Scholar]
- 179).Majumder S, Richardson A, Strong R, Oddo S. Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLoS One 2011; 6: e25416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180).Zhang X, Heng X, Li T, Li L, Yang D, Zhang X, Du Y, Doody RS, Le W. Long-term treatment with lithium alleviates memory deficits and reduces amyloid-beta production in an aged Alzheimer’s disease transgenic mouse model. J Alzheimers Dis 2011; 24: 739–749. [DOI] [PubMed] [Google Scholar]
- 181).Zhang J, Cai T, Zhao F, Yao T, Chen Y, Liu X, Luo W, Chen J. The role of alpha-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci 2012; 8: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182).McBrayer M, Nixon RA. Lysosome and calcium dysregulation in Alzheimer’s disease: partners in crime. Biochem Soc Trans 2013; 41: 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183).Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosa-to A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 2015; 17: 288–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184).Mustaly-Kalimi S, Littlefield AM, Stutzmann GE. Calcium signaling deficits in glia and autophagic pathways contributing to neurodegenerative disease. Antioxid Redox Signal 2018; 29: 1158–1175. [DOI] [PubMed] [Google Scholar]
- 185).Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1998; 1366: 177–196. [DOI] [PubMed] [Google Scholar]
- 186).Cardenas C, Foskett JK. Mitochondrial Ca(2+) signals in autophagy. Cell Calcium 2012; 52: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187).Zhang X, Yu L, Xu H. Lysosome calcium in ROS regulation of autophagy. Autophagy 2016; 12: 1954–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188).Reddy PH, Oliver DM. Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer’s Disease. Cells 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189).Chiu CT, Chuang DM. Neuroprotective action of lithium in disorders of the central nervous system. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011; 36: 461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190).Morris G, Berk M. The Putative Use of Lithium in Alzheimer’s Disease. Curr Alzheimer Res 2016; 13: 853–861. [DOI] [PubMed] [Google Scholar]
- 191).Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal 2012; 2012: 756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192).Verri M, Pastoris O, Dossena M, Aquilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno AI. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int J Immunopathol Pharmacol 2012; 25: 345–353. [DOI] [PubMed] [Google Scholar]
- 193).Arnsten AFT, Datta D, Preuss TM. Studies of aging nonhuman primates illuminate the etiology of early-stage Alzheimer’s-like neuropathology: An evolutionary perspective. Am J Primatol 2021; 83: e23254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194).Hashioka S, Wu Z, Klegeris A. Glia-Driven Neuroinflammation and Systemic Inflammation in Alzheimer’s Disease. Curr Neuropharmacol 2021; 19: 908–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195).Hotchkiss RS, Osborne DF, Lappas GD, Karl IE. Calcium antagonists decrease plasma and tissue concentrations of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 alpha in a mouse model of endotoxin. Shock 1995; 3: 337–342. [PubMed] [Google Scholar]
- 196).Pollock J, McFarlane SM, Connell MC, Zehavi U, Vandenabeele P, MacEwan DJ, Scott RH. TNF-alpha receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology 2002; 42: 93–106. [DOI] [PubMed] [Google Scholar]
- 197).Jiang Y, Li Z, Ma H, Cao X, Liu F, Tian A, Sun X, Li X, Wang J. Upregulation of TREM2 Ameliorates Neuroinflammatory Responses and Improves Cognitive Deficits Triggered by Surgical Trauma in Appswe/PS1dE9 Mice. Cell Physiol Biochem 2018; 46: 1398–1411. [DOI] [PubMed] [Google Scholar]
- 198).Chiba N, Matsuzaki M, Mawatari T, Mizuochi M, Sakurai A, Kinoshita K. Beneficial effects of dantrolene in the treatment of rhabdomyolysis as a potential late complication associated with COVID-19: a case report. Eur J Med Res 2021; 26: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199).Kim BC, Kim HT, Mamura M, Ambudkar IS, Choi KS, Kim SJ. Tumor necrosis factor induces apoptosis in hepatoma cells by increasing Ca(2+) release from the endoplasmic reticulum and suppressing Bcl-2 expression. J Biol Chem 2002; 277: 31381–31389. [DOI] [PubMed] [Google Scholar]
- 200).Wang Q, Downey GP, Choi C, Kapus A, McCulloch CA. IL-1 induced release of Ca2+ from internal stores is dependent on cell-matrix interactions and regulates ERK activation. FASEB J 2003; 17: 1898–1900. [DOI] [PubMed] [Google Scholar]
- 201).Gerard F, Hansson E. Inflammatory activation enhances NMDA-triggered Ca2+ signalling and IL-1beta secretion in primary cultures of rat astrocytes. Brain Res 2012; 1473: 1–8. [DOI] [PubMed] [Google Scholar]
- 202).Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, Verkhratsky A. Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol 2003; 170: 3029–3036. [DOI] [PubMed] [Google Scholar]
- 203).Saad El-Din S, Rashed L, Medhat E, Emad Aboulhoda B, Desoky Badawy A, Mohammed ShamsEldeen A, Abdelgwad M. Active form of vitamin D analogue mitigates neurodegenerative changes in Alzheimer’s disease in rats by targeting Keap1/Nrf2 and MAPK-38p/ERK signaling pathways. Steroids 2020; 156: 108586. [DOI] [PubMed] [Google Scholar]
- 204).Zhu YG, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia 2012; 60: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205).Forlenza OV, De-Paula VJ, Diniz BS. Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci 2014; 5: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206).Wilson EN, Do Carmo S, Iulita MF, Hall H, Austin GL, Jia DT, Malcolm J, Foret M, Marks A, Butterfield DA, Cuello AC. Microdose lithium NP03 diminishes pre-plaque oxidative damage and neuroinflammation in a rat model of Alzheimer’s-like amyloidosis. Curr Alzheimer Res 2018; 15: 1220–1230. [DOI] [PubMed] [Google Scholar]
- 207).Wilson EN, Do Carmo S, Welikovitch LA, Hall H, Aguilar LF, Foret MK, Iulita MF, Jia DT, Marks AR, Allard S, Emmerson JT, Ducatenzeiler A, Cuello AC. NP03, a microdose lithium formulation, blunts early amyloid post-plaque neuropathology in McGill-R-Thy1-APP Alzheimer-Like Transgenic Rats. J Alzheimers Dis 2020; 73: 723–739. [DOI] [PubMed] [Google Scholar]
- 208).Iwasaki M, Saito J, Zhao H, Sakamoto A, Hirota K, Ma D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 2021; 44:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209).Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020. 46:846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210).Ling L, So C, Shum HP, Chan PKS, Lai CKC, Kandamby DH, Ho E, So D, Yan WW, Lui G, Leung WS, Chan MC, Gomersall CD. Critically ill patients with COVID-19 in Hong Kong: a multicentre retrospective observational cohort study. Crit Care Resusc 2020. 22: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211).Zhu Y, Du Z, Zhu Y, Li W, Miao H, Li Z. Evaluation of organ function in patients with severe COVID-19 infections. Med Clin (Barc) 2020; 155: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212).Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol 2020; 51: 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213).Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol 2020; 92: 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214).Trbojevic-Akmacic I, Petrovic T, Lauc G. SARS-CoV-2 S glycoprotein binding to multiple host receptors enables cell entry and infection. Glycoconj J 2021; 38: 611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215).Theken KN, Tang SY, Sengupta S, FitzGerald GA. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J Lipid Res 2021: 62: 100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216).Mohan J, Wollert T. Membrane remodeling by SARS-CoV-2 - double-enveloped viral replication. Fac Rev 2021; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217).Dakal TC. SARS-CoV-2 attachment to host cells is possibly mediated via RGD-integrin interaction in a calcium-dependent manner and suggests pulmonary EDTA chelation therapy as a novel treatment for COVID 19. Immunobiology 2021; 226: 152021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218).Straus MR, Bidon MK, Tang T, Jaimes JA, Whittaker GR, Daniel S. Inhibitors of L-type calcium channels show therapeutic potential for treating SARS-CoV-2 infections by preventing virus entry and spread. ACS Infect Dis 2021; 7: 2807–2815. [DOI] [PubMed] [Google Scholar]
- 219).Danta CC. SARS-CoV-2, Hypoxia, and calcium signaling: the consequences and therapeutic options. ACS Pharmacol Transl Sci 2021; 4: 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220).Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 2022; 23: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221).Zhao MM, Yang WL, Yang FY, Zhang L, Huang WJ, Hou W, Fan CF, Jin RH, Feng YM, Wang YC, Yang JK. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct Target Ther 2021; 6: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222).Nifedipine Solaimanzadeh I. and amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID-19. Cureus 2020; 12: e8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223).Brimson JM, Prasanth MI, Malar DS, Brimson S, Thitilertdecha P, Tencomnao T. Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: the possible role of the sigma-1 receptor and autophagy. Expert Opin Ther Targets 2021; 25: 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224).Liu T, Luo S, Libby P, Shi GP. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol Ther 2020; 213: 107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225).Rudd CE. GSK-3 Inhibition as a Therapeutic Approach Against SARs CoV2: dual benefit of inhibiting viral replication while potentiating the immune response. Front Immunol 2020; 11: 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226).Liu X, Verma A, Garcia G Jr., Ramage H, Lucas A, Myers RL, Michaelson JJ, Coryell W, Kumar A, Charney AW, Kazanietz MG, Rader DJ, Ritchie MD, Berrettini WH, Schultz DC, Cherry S, Damoiseaux R, Arumugaswami V, Klein PS. Targeting the coronavirus nucleocapsid protein through GSK-3 inhibition. Proc Natl Acad Sci U S A 2021; 118: e2113401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227).Zhao FR, Xie YL, Liu ZZ, Shao JJ, Li SF, Zhang YG, Chang HY. Lithium chloride inhibits early stages of foot-and-mouth disease virus (FMDV) replication in vitro. J Med Virol 2017; 89: 2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228).Ziaie Z, Brinker JM, Kefalides NA. Lithium chloride suppresses the synthesis of messenger RNA for infected cell protein-4 and viral deoxyribonucleic acid polymerase in herpes simplex virus-1 infected endothelial cells. Lab Invest 1994; 70: 29–38. [PubMed] [Google Scholar]
- 229).Murru A, Manchia M, Hajek T, Nielsen RE, Rybakowski JK, Sani G, Schulze TG, Tondo L, Bauer M. Lithium’s antiviral effects: a potential drug for CoViD-19 disease? Int J Bipolar Disord 2020; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230).Rajkumar RP. Lithium as a candidate treatment for COVID-19: promises and pitfalls. Drug Dev Res 2020; 81: 782–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231).Spuch C, Lopez-Garcia M, Rivera-Baltanas T, Rodrigues-Amorim D, Olivares JM. Does lithium deserve a place in the treatment against COVID-19? A preliminary observational study in six patients, case report. Front Pharmacol 2020; 11: 557629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232).Piet E, Maillard A, Mallaval FO, Dusseau JY, Galas-Haddad M, Ducki S, Creton H, Lallemant M, Forestier E, Gavazzi G, Delory T. Outbreaks of COVID-19 in nursing homes: a cross-sectional survey of 74 nursing homes in a French area. J Clin Med 2021; 10: 4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233).Soto-Anari M, Camargo L, Ramos-Henderson M, Rivera-Fernández C, Denegri-Solís L, Calle U, Mori N, Ocampo-Barbá N, López F, Porto M, Caldichoury-Obando N, Saldías C, Gargiulo P, Castellanos C, Shelach-Bellido S, López N. Prevalence of Dementia and Associated Factors among Older Adults in Latin America during the COVID-19 Pandemic. Dement Geriatr Cogn Dis Extra 2021; 11: 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234).Emmady PD, Tadi P. Dementia. 2021 Nov 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Jan–. [Google Scholar]
- 235).Fajardo VA, Fajardo VA, LeBlanc PJ, MacPherson REK. Examining the Relationship between Trace Lithium in Drinking Water and the Rising Rates of Age-Adjusted Alzheimer’s Disease Mortality in Texas. J Alzheimers Dis 2018; 61: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]


