Abstract
Hippocampal plasticity and memory are modulated by the potent estrogen 17β-estradiol (E2). Research on the molecular mechanisms of hippocampal E2 signaling has uncovered multiple intracellular pathways that contribute to these effects, but few have questioned the role that extracellular signaling processes may play in E2 action. Modification of the extracellular matrix (ECM) by proteases like matrix metalloproteinase-9 (MMP-9) is critical for activity-dependent remodeling of synapses, and MMP-9 activity is required for hippocampal learning and memory. Yet little is known about the extent to which E2 regulates MMP-9 in the hippocampus, and the influence this interaction may have on hippocampal memory. Here, we examined the effects of hippocampal MMP-9 activity on E2-induced enhancement of spatial and object recognition memory consolidation. Post-training bilateral infusion of an MMP-9 inhibitor into the dorsal hippocampus of ovariectomized female mice blocked the enhancing effects of E2 on object placement and object recognition memory, supporting a role for MMP-9 in estrogenic regulation of memory consolidation. E2 also rapidly increased the activity of dorsal hippocampal MMP-9 without influencing its protein expression, providing further insight into hippocampal E2/MMP-9 interactions. Together, these results provide the first evidence that E2 regulates MMP-9 to modulate hippocampal memory and highlight the need to further study estrogenic regulation of extracellular modification.
Keywords: Estrogen, Hippocampus, Extracellular matrix, Object recognition, Spatial memory, Mouse
1. Introduction
The hippocampus is remarkably sensitive to the modulatory effects of the potent estrogen 17β-estradiol (E2). E2 induces changes in dendritic morphology and synaptic signaling that are associated with memory formation (Frankfurt and Luine, 2015; Frick, 2015), and work by our lab and others has shown consistently that E2 enhances hippocampus-dependent spatial and object recognition memory consolidation in ovariectomized (OVX) female rodents (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Walf et al., 2006). Our understanding of the molecular mechanisms driving these effects has grown significantly over the last two decades, with a number of synaptic mechanisms and intracellular signaling cascades being linked to E2 regulation of hippocampal structure and function (Finney et al., 2020; Frick, 2015; Frick et al., 2018). However, an area that remains largely unexplored is the potential contribution of extracellular signaling processes, such as regulation of the extracellular matrix (ECM), to E2-induced hippocampal plasticity and memory.
The ECM is a dense network of proteins and molecules that provide structure and biochemical support to cells, and which undergoes dynamic modification and restructuring by extracellular proteases, such as matrix metalloproteinases (MMP). MMPs have been increasingly recognized as important mediators of synaptic plasticity (Ferrer-Ferrer and Dityatev, 2018; Huntley, 2012), with MMP-dependent breakdown of ECM components and associated membrane proteins contributing to long-term potentiation (LTP) and dendritic spine remodeling (Conant et al., 2010; Meighan et al., 2006; Tian et al., 2007; Wang et al., 2008). MMP-9 is highly expressed in the hippocampus (Szklarczyk et al., 2002), where it localizes to excitatory synapses (Bozdagi et al., 2007) and has been directly linked to hippocampal plasticity and memory (Huntley, 2012). MMP-9 knockout mice have impaired hippocampal LTP and contextual fear conditioning (Nagy et al., 2006), and inhibition of hippocampal MMP-9 in male rats impairs maintenance of LTP and acquisition of a Morris water maze spatial memory task (Bozdagi et al., 2007; Meighan et al., 2006; Wright et al., 2007). Hippocampal MMP-9 also regulates dendritic spine morphology. Enhanced MMP-9 activity results in dendritic spine elongation in the CA1 region of the hippocampus in male rats (Michaluk et al., 2011), and MMP-9 activity during LTP supports spine enlargement in hippocampal slices (Wang et al., 2008). However, much remains to be learned about the role of MMP-9 in hippocampal synaptic plasticity and memory, including how findings in male rodents extend to the female hippocampus and how MMP-9 might contribute to specific memory processes, such as consolidation. In addition, considerable overlap of the literature on estrogenic enhancement of hippocampal memory and on the molecular effects of hippocampal MMP-9 indicates that MMP-9 may be an overlooked mediator of E2 action in the hippocampus, as both MMP-9 and E2 enhance LTP, dendritic spine remodeling, and spatial memory formation in this region (Frick et al., 2018).
Relatively few studies have directly examined the relationship between E2 and MMP-9 in the nervous system, and the existing literature largely focuses on the neuroprotective effects of E2. In neuroblastoma cells, E2 increases both MMP-9 expression and activity, which is necessary for E2-induced enhancement of amyloid-β degradation (Merlo and Sortino, 2012). Further, in models of stroke and immune activation, E2 appears to have a stabilizing effect on MMP-9 by reducing MMP-9 levels that are heightened by injury and inflammation (Liu et al., 2005; Vegeto et al., 2003). These data provide initial evidence that MMP-9 may be an important mediator of E2 effects in the brain, but how this interaction may influence normal hippocampal function is largely unexplored. A recent study shed light on this question by demonstrating that E2-induced synaptic facilitation in the male rat hippocampus is dependent on MMP-2/9 activity and subsequent signaling through β1-integrins (Wang et al., 2016). However, the extent of the interaction between E2 and MMP-9 in the hippocampus, and whether MMP-9 contributes to the memory enhancing effects of E2, is unknown. Here, we combine hippocampus-dependent object memory tasks with post-training infusions of E2 and an MMP-9 inhibitor to determine if MMP-9 activity in the dorsal hippocampus (DH) is required for E2 enhancement of hippocampal memory consolidation in OVX mice. The effects of E2 administration on dorsal hippocampal MMP-9 expression and activity were also examined. Our findings suggest that MMP-9 critically mediates E2-induced memory enhancement, perhaps via rapidly increased hippocampal MMP-9 activity.
2. Methods
2.1. Subjects
Female C57BL/6 mice (n = 84 across all experiments) were obtained from Taconic Biosciences at 9 weeks of age. Mice were maintained on a 12:12 light-dark cycle with food and water ad libitum. All procedures were performed during the light phase of the cycle. Mice were housed in groups of up to 5 prior to surgery, after which they were singly housed. All procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin-Milwaukee.
2.2. Surgery
At 10 weeks of age, mice were bilaterally ovariectomized and implanted with indwelling guide cannulae targeting the DH and/or dorsal third ventricle using previously described methods (Boulware et al., 2013; Gross et al., 2021; Kim et al., 2019). Mice were anesthetized with 5% isoflurane in pure oxygen and placed into a stereotaxic apparatus; anesthesia was maintained at 2–3% isoflurane throughout surgery. Following bilateral ovariectomy, guide cannulae (C232G; 22 gauge; Plastics One) were placed at coordinates targeting the DH alone (−1.7 mm AP, +/− 1.5 mm ML, −2.3 mm DV) or the DH and dorsal third ventricle (intracerebroventricular (ICV); − 0.9 mm AP, 0.0 mm ML, − 0.2.3 mm DV). After implantation, cannulae were secured with dental cement, which also served to close the wound. Dummy cannulae (C232DC, Plastics One) were inserted into the guides to maintain patency of the cannulae throughout experiments. Cannula placements were verified visually during tissue collection. Mice were allowed to recover for a minimum of one week before beginning behavioral testing.
2.3. Drugs and infusions
For intracranial infusions, mice were gently restrained, dummy cannulae were removed, and an infusion cannula (C313l; DH: 28 gauge, extending 0.8 mm beyond the 1.5 mm guide; ICV: 28 gauge, extending 1.0 mm beyond the 1.8 mm guide; Plastics One) was inserted. Infusion cannulae were attached to PE50 polyethylene tubing mounted on a 10 μl Hamilton syringe. Infusions were controlled by a microinfusion pump (KDS Legato 180, KD Scientific). As described previously (Boulware et al., 2013; Fortress et al., 2013), infusions of 0.5 μl were made into each hemisphere of the DH at a rate of 0.5 μl/min (infusion time: 1 min); ICV infusions were 1 μl in volume and administered at a rate of 0.5 μl/min (infusion time: 2 min). At the end of infusion, the cannula was left in place for 1 min to allow for drug diffusion. In triple infusion experiments using both an inhibitor and E2, the inhibitor was infused first bilaterally into the DH, followed immediately by ICV E2 infusion. This triple infusion protocol was used to prevent tissue damage from multiple infusions into the DH in rapid succession (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Zhao et al., 2010).
The MMP-9 inhibitor, MMP-9 Inhibitor II (MMPI, Calbiochem), was dissolved in 100% DMSO for a stock solution of 4 μg/μl and stored light protected at −20 °C. On the day of the experiment, dilutions at concentrations of 1 and 2 μg/μl in 80% DMSO were prepared. MMPI was infused into the DH at doses of 0.5 or 1 μg/hemisphere with 80% DMSO as vehicle control. Cyclodextrin-encapsulated E2 (Sigma-Aldrich) was dissolved at a concentration of 10 μg/μl in 0.9% sterile saline and infused at a dose of 5 μg/hemisphere (DH) or 10 μg (ICV). For vehicle control, 2-hydroxypropyl-beta-cyclodextrin (HBC; Sigma-Aldrich) was dissolved in 0.9% sterile saline at an equivalent concentration as the encapsulated E2 solution.
2.4. Object recognition (OR) and object placement (OP)
Object recognition (OR) and object placement (OP) tasks were used to assess object recognition and spatial memory. Tasks were conducted as described previously (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Gross et al., 2021; Kim et al., 2019). Experimenters were blind to treatment group, and all mice completed both tasks, with the order of tasks counterbalanced within each group. Mice were first briefly handled for three days to habituate to the experimenters. To habituate mice to objects, a Lego Duplo block was placed into the home cage on the second day of handling and then removed at the start of behavioral training. Following handling, mice were habituated to the empty testing arena for 5 min on each of 2 consecutive days. On the day of behavioral training, mice were briefly exposed to the empty arena for 1 min, removed, and then placed back into the arena with two identical objects placed near the upper right and left corners. To ensure that mice received equal amounts of exposure to the objects, mice remained in the arena until 30 s of object exploration was accumulated or the trial was ended after 20 min. Exploration was defined as when the mouse was immediately adjacent to an object with its front paws and/or nose directed at or touching the object, and was manually scored with ANY-maze tracking software (Stoelting). Mice with 30 s of object exploration were immediately infused to target the memory consolidation period and then returned to their home cage.
During testing, one of the objects was either moved to a lower corner of the testing arena (OP) or replaced with a novel object (OR). Behavioral testing was conducted at timepoints previously established by our lab to observe either impairment or facilitation of memory consolidation (Boulware et al., 2013; Fortress et al., 2013; Gross et al., 2021). For experiments on the memory impairing effects of MMP-9 inhibition, mice were tested after either a 4 hr (OP) or 24 hr (OR) delay. At these delays, control mice demonstrate intact memory of the training objects, spending more time than chance (15 s) with the moved or novel objects due to an innate preference for novelty. For experiments examining the faciliatory effects of E2 on memory, mice were tested at either 24 hr (OP) or 48 hr (OR), times at which control mice do not demonstrate memory of training (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Gross et al., 2021). Mice explored the objects until 30 s of exploration time was reached and were then returned to their home cages. Behavioral tests were separated by two weeks to allow for dissipation of acute effects of drug infusion.
2.5. Western blot
In mice that previously underwent behavioral training and testing, tissue was collected at a minimum of 2 weeks following the last behavioral testing. Mice received intracranial infusions as described above and 1 hr later were cervically dislocated, decapitated, and brains were removed for DH dissection. The DH was dissected rapidly on ice and frozen at − 80 °C until further processing. Cannula placements were visually verified during dissection and no missed placements were noted during the study. Western blotting was performed as described previously (Boulware et al., 2013; Fortress et al., 2013; Gross et al., 2021; Kim et al., 2019). Tissue was homogenized in a hypotonic lysis buffer containing PMSF and a protease inhibitor cocktail (ThermoFisher Scientific) via sonication. A Bradford assay was used to determine total protein concentrations and then homogenates were electrophoresed on 4–15% Tris-HCl precast gels. Protein was transferred to a PVDF membrane and blocked with 5% milk before incubation with an MMP-9 (Cell Signaling Technology, #13667, 1:1000) or GAPDH (Cell Signaling Technology, #5174, 1:10000) primary antibody overnight at 4 °C. The next day, blots were incubated with an appropriate HRP-conjugated secondary antibody (anti-rabbit IgG, Cell Signaling Technology, 1: 5000) and developed with Clarity Max enhanced chemiluminescence substrate (Bio-Rad). Membranes were imaged on a ChemiDoc MP gel imager and Image Lab software (Bio-Rad, Image Lab version 5.2) was used to perform densitometry analysis of MMP-9 normalized to GAPDH. For statistical analysis, normalized proteins were expressed as a percentage relative to vehicle control.
2.6. MMP-activity assay
MMP-9 activity was measured by a fluorometric immunocapture assay using methods described by Hawkins et al. (2013). Dissected DH tissue was homogenized in a Tris-CaC12-NaCl-Brij buffer (TCNB; 50 mM Tris, 10 mM CaC12, 150 mM NaCl, 0.05% Brij L23) with 0.1% Triton X-100 and HALT protease/phosphatase inhibitor (Thermo Scientific). Samples were prepared with 100 μg of total protein in 100 μl of TCNB buffer. Additional aliquots of 20 μg protein were reserved for western blot analysis of MMP-9 protein expression as described above. As the assay substrate is not specific to MMP-9, we first immunoprecipitated MMP-9 from samples using a primary antibody to isolate specific MMP-9 activity. Black 96-well IgG-coupled plates were rinsed with TCNB and then incubated with a primary antibody solution of 2 μg MMP-9 primary antibody (Abcam, #228402) in 100 μl TCNB/well on a shaker at room temperature for 2 hrs. Plates were washed with TCNB and then blocked with 5% BSA in TBS at room temperature for 1 hr. Plates were then incubated with protein samples (100 μg) overnight at 4 °C on a plate shaker. The next morning, samples were removed, and the plate was washed with TCNB. A volume of 100 μl of assay buffer (50 mM Tris–HCl pH 7.6, 200 mM NaCl, 5 mM CaC12, 20 μM ZnCl, and 0.05% Brij L23) was added to each well and the plate was incubated at 37 °C for 30 min. Then, the peptide substrate (5-FAM/QXL™520 MMP FRET peptide III; Cat. No. 60570; AnaSpec) was added at a concentration of 1 μM/well and incubated for 18–24 hrs at 37 °C. Endpoint fluorescence activity was measured on a Synergy H1 plate reader (Biotek) at 485 nm excitation/528 nm emission. Protein samples were run in duplicate with recombinant MMP-9 protein used as a positive control. A substrate control well was used to subtract baseline fluorescence.
2.7. Statistical analysis
All statistical analyses were conducted in GraphPad Prism 8. Outliers, defined by ± 2 standard deviations from the mean, were removed from all data sets prior to further analysis (n = 3 across all experiments). Behavioral data was first analyzed for within group learning effects with one sample t-tests comparing individual group means to chance (15 s). This analysis was used because time spent with the objects is not independent; time spent with one object necessarily reduces time spent with the other (Frick and Gresack, 2003). Behavioral data were further analyzed for between-group differences with one- or two-way ANOVAs. For MMPI dose-response data, one-way ANOVAs were followed by post-hoc Dunnett’s test comparing treatment doses to vehicle control. For E2/MMPI behavior experiments, two-way ANOVAs (hormone x drug) were followed by planned post-hoc comparisons using Fisher’s LSD test. Western blot and MMP-9 activity assay data were analyzed using paired samples t-tests. Significance was determined at p < 0.05.
3. Results
3.1. MMP-9 inhibition impairs object memory consolidation in OVX mice
As MMP-9 is known to contribute to hippocampal memory formation (Meighan et al., 2006; Nagy et al., 2006; Wright et al., 2007), experiments examining interactions of MMP-9 with estrogenic enhancement of memory could be confounded by a general inhibitory effect of MMP inhibition on memory consolidation. Therefore, we first determined a dose of the specific MMP-9 inhibitor, MMPI, that does not impair object placement or object recognition memory on its own for use in later experiments. OVX female mice were trained with two identical objects and then immediately received bilateral DH infusion of vehicle or MMPI (0.5 or 1 μg/hemisphere) after removal from the training arena. Mice were tested either 4 hrs later for OP or 24 hrs later for OR, as control mice at these timepoints demonstrate intact memory for the training conditions, which allows for measurement of memory impairing interventions (Boulware et al., 2013; Gross et al., 2021; Kim et al., 2019). Mice infused with vehicle or the low dose (0.5 μg) of MMPI spent significantly more time than chance with the moved (vehicle: t(7) = 5.28, p = 0.001; 0.5 μg MMPI: t(8) = 2.37, p = 0.045; Fig. 1A) or novel objects (vehicle: t(10) = 2.51, p = 0.031; 0.5 μg MMPI: t(9) = 3.13, p = 0.012; Fig. 1B) during testing, indicating successful memory consolidation in both groups. However, mice treated with the higher dose of MMPI (1 μg) did not differ from chance in time spent with the moved (t(7) = 0.06, p = 0.947) or novel (t(10) = 1.54, p = 0.156) objects, demonstrating that this dose impairs memory consolidation. Further analysis with one-way ANOVA revealed a significant main effect of treatment for both OP (F(2,22) = 3.80, p = 0.038) and OR (F(2,29) = 6.17, p = 0.006), and post-hoc analyses found that mice treated with 1 μg MMPI spent significantly less time with the moved (p < 0.05) or novel (p < 0.05) objects when compared to vehicle-treated mice. These results establish a specific role for MMP-9 activity in hippocampal memory consolidation and provided a sub-threshold dose of MMPI for use in subsequent studies with E2.
Fig. 1.
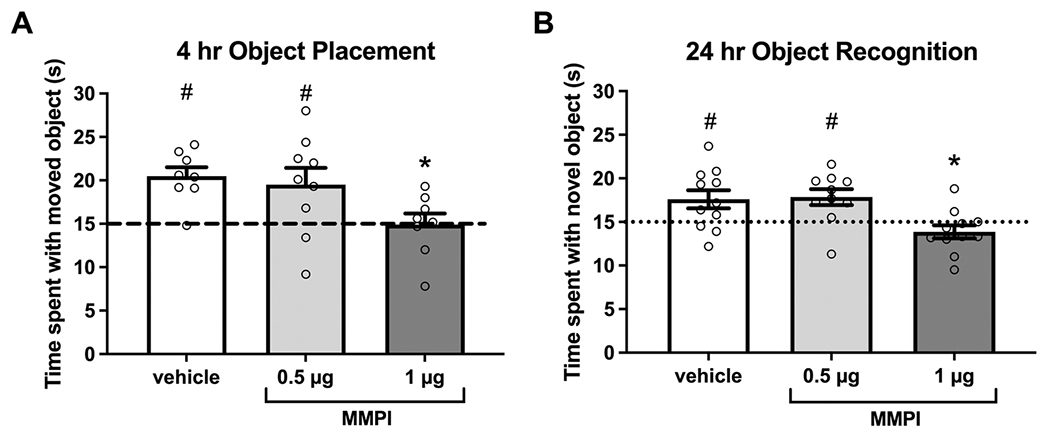
MMP-9 is essential for spatial and object recognition memory consolidation. MMP-9 inhibition with 1 μg, but not 0.5 μg, of the specific MMP-9 inhibitor MMPI blocked consolidation of object placement and object recognition memory. OVX female mice (n = 8–12/group) received bilateral DH infusion of vehicle or MMPI (0.5 or 1 μg/hemisphere) immediately following object training. In an object placement test 4 hr later (A) or an object recognition test 24 h later (B), mice receiving vehicle (OP: n = 8; OR: n = 11) or 0.5 μg MMPI (OP: n = 9; OR: n = 10) spent significantly more time with the moved or novel object compared to chance (dotted line at 15 s, #p < 0.05 relative to chance), whereas mice receiving 1 μg of MMPI (OP: n = 8; OR: n = 11) did not, suggesting that the high dose impaired memory consolidation. Mice treated with 1 μg MMPI also spent significantly less time with the moved or novel object compared to vehicle-treated mice (*p < 0.05). Error bars indicate mean ± SEM.
3.2. E2 enhancement of memory consolidation depends on MMP-9 activity and is not associated with changes in MMP-9 expression
To determine if MMP-9 activity is required for E2 enhancement of hippocampal memory consolidation, OVX female mice received ICV infusion of vehicle or E2 and a bilateral DH infusion of vehicle or 0.5 μg/hemisphere MMPI immediately following object training (Fig. 2A). Mice were tested either 24 hrs later for OP or 48 hrs later for OR. These extended time points allow for observation of the memory-enhancing effects of E2, as only OVX mice treated with E2, but not vehicle, will exhibit successful memory at these times (Boulware et al., 2013; Gross et al., 2021). For both tasks, mice treated with vehicle+vehicle or vehicle+MMPI were indistinguishable from chance in time spent with the moved (vehicle+vehicle: t(8) = 0.732, p = 0.485; vehicle+MMPI: t(7) = 0.341, p = 0.743, Fig. 2B) or novel objects (vehicle+vehicle: t(8) = 0.416, p = 0.688; vehicle+MMPI: t(9) = 1.28, p = 0.382, Fig. 2C). In contrast, mice treated with E2+vehicle spent significantly more time with the moved (t(10) = 3.02, p = 0.013) and novel (t(7) = 3.82, p = 0.007) objects, suggesting enhanced memory consolidation. However, in E2-treated mice also infused with MMPI, this effect was abolished, with mice spending significantly less time than chance with the moved object (t(8) = 2.49, p = 0.038) and not differing from chance in time spent with the novel object (t(7) = 0.93, p = 0.38). Further analysis by two-way ANOVA confirmed a significant main effect of MMP-9 inhibition (F(1,33) = 6.95, p = 0.013) and hormone x drug interaction for OP (F(1, 33) = 6.75, p = 0.014) and significant main effects of both E2 (F(1,31) = 11.72, p = 0.002) and MMP-9 inhibition (F(1,31) = 5.44, p = 0.026) for OR. Planned post-hoc comparisons found that the E2 +vehicle group spent more time with the moved and novel objects compared to the vehicle+vehicle (OP: p = 0.0063, OR: p = 0.0026) and E2+MMPI groups (OP: p = 0.0005, OR: p = 0.020).
Fig. 2.
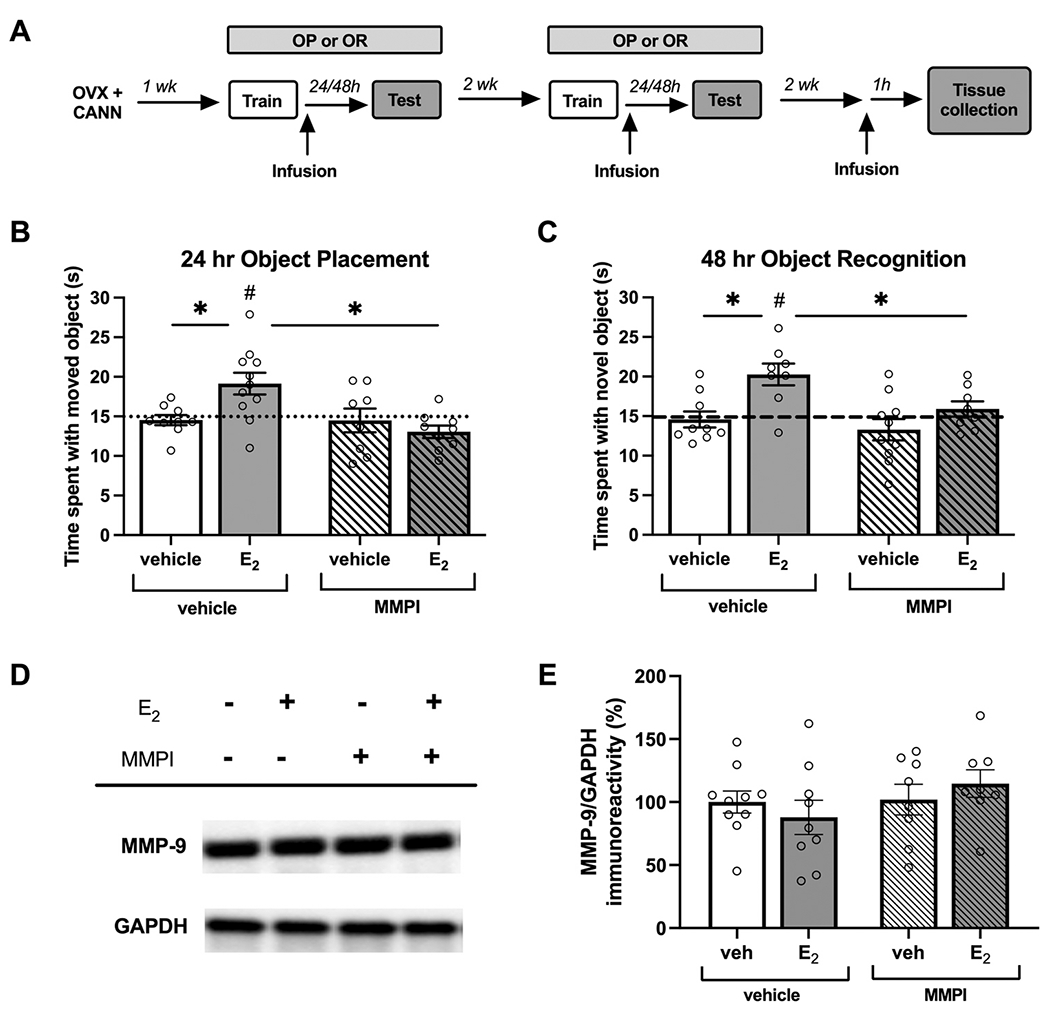
Estradiol depends on MMP-9 activity to enhance hippocampal memory consolidation but does not influence MMP-9 expression. OVX female mice (n = 8–11/group) received ICV infusion of vehicle or E2 (10 μg) and DH infusion of vehicle or MMPI (0.5 μg/hemisphere) immediately following object training (A). In an object placement test 24 hr later (B) or an object recognition test 48 h later (C), mice infused with E2 +vehicle (OP: n = 11; OR: n = 8), but not E2 +MMPI (OP: n = 8; OR: n = 8), spent significantly more time than chance with the moved or novel objects (dotted line at 15 s, #p < 0.05). Mice treated with E2 +vehiele also spent significantly more time with the moved or novel objects compared to vehiele+vehiele (OP: n = 9; OR: n = 9) or E2 +MMPI groups (OP: n = 8; OR: n = 10; * p < 0.05). DH tissue collected 1 hr after infusion showed no change to MMP-9 protein expression following E2 and MMPI treatment (D, E). Error bars indicate mean ± SEM.
To examine whether the interaction of E2 and MMP-9 was correlated with changes to MMP-9 protein expression, mice again received an ICV infusion of vehicle or E2 and a DH infusion of vehicle or MMP-9, and DH tissue was collected 1 hr later. At this time point, no significant differences were observed in MMP-9 protein expression as assessed via western blot (main effect E2: F(1,31) = 0.00, p = 0.98; main effect MMPI: F(1,31) = 1.59, p = 0.22; interaction: F(1,31) = 1.9, p = 0.28. Fig. 2D, E). Together, these results indicate that MMP-9 activity in the DH is required for E2-induced enhancement of object recognition and spatial memory consolidation in OVX mice, but that this effect is not associated with a change in MMP-9 expression.
3.3. E2 increases MMP-9 activity, not expression
To further examine how E2 interacts with MMP-9, we directly measured MMP-9 enzyme activity levels in the DH following E2 treatment. Because MMP-9 is largely expressed as an inactive pro-enzyme, we performed MMP-9 immunocapture followed by a highly sensitive fluorescent substrate assay that allows for observation of the small pool of endogenously active MMP-9 (Hawkins et al., 2013). A set of behaviorally naïve OVX mice received bilateral DH infusion of vehicle or E2, and DH tissue was collected 30 min later (Fig. 3A). Again, E2 treatment did not affect MMP-9 protein levels in the DH at this time point (t(12) = 0.71, p = 0.94, Fig. 3B, C). However, E2 treatment did significantly increase MMP-9 activity (t(12) = 3.74, p = 0.0028, Fig. 3D). Overall, these data suggest that E2 rapidly increases hippocampal MMP-9 activity without influencing its protein expression.
Fig. 3.

Estradiol increases hippocampal MMP-9 enzyme activity. OVX female mice (n = 7/group) received bilateral DH infusion of vehicle (V) or E2 (5 μg/hemisphere) and DH tissue was collected 30 min later (A). E2 treatment did not affect levels of MMP-9 protein expression (B,C), but did increase levels of MMP-9 enzyme activity (D). (**p < 0.01) Error bars indicate mean ± SEM.
4. Discussion
Previous literature has elucidated numerous intracellular mechanisms essential for E2 to influence hippocampal plasticity and memory, but whether regulation of the extracellular space also plays a role has yet to be examined. Here, we began to study this putative relationship by examining the ability of E2 to facilitate hippocampal-dependent memory consolidation in OVX mice in the presence of an inhibitor for the extracellular matrix modifier, MMP-9. We found that MMP-9 inhibition in the DH could impair memory consolidation on its own and blocked the memory-enhancing of effects of E2. Experiments testing the regulation of MMP-9 by E2 found that a single infusion of E2 rapidly increased dorsal hippocampal MMP-9 activity without an associated increase in MMP-9 protein expression. Together, these results demonstrate that MMP-9 is a critical mediator of both memory consolidation and E2-induced memory consolidation, and begin to define how E2 regulates hippocampal MMP-9.
Prior work in rodent models has demonstrated that MMP-9 plays an important role in hippocampal learning and memory. Training in inhibitory avoidance, contextual fear conditioning, and Morris water maze tasks increases levels of active MMP-9 in the male rodent hippocampus (Ganguly et al., 2013; Nagy et al., 2007; Zhang et al., 2013), and in intact female rats, MMP-9 activity is increased in the hippocampus following Morris water maze training or object exploration (Wright et al., 2004). ICV or DH infusion of a non-specific MMP inhibitor impairs acquisition in the Morris water maze in male rodents (Meighan et al., 2006; Wright et al., 2007), and male mice with full MMP-9 knockout exhibit reduced freezing following hippocampus-dependent contextual fear conditioning but not cued fear conditioning (Nagy et al., 2006), suggesting a specific role for MMP-9 in hippocampal memory. Whether hippocampal MMP-9 activity is particularly required for memory consolidation is less clear, as previous studies administered MMP inhibitors prior to training and did not specifically manipulate the consolidation phase. Here, we used the one-trial learning tasks object placement and object recognition, which are ideal for examining the molecular mechanisms of memory consolidation because they offer a temporally discrete window of time post-training to manipulate consolidation processes. We found that immediate post-training infusion of 1 μg of a specific MMP-9 inhibitor into the dorsal hippocampus impaired object placement and object recognition memory in OVX mice, demonstrating a necessity for MMP-9 activity during the memory consolidation period. Although a lower dose of the MMP-9 inhibitor (0.5 μg) left consolidation memory intact when infused on its own, this dose blocked the memory-enhancing effects of E2, again supporting the importance of MMP-9 for memory consolidation. These data build on previous work by extending tests of MMP-9 inhibition to female rodents and by defining a specific role for MMP-9 in hippocampal memory consolidation.
We next examined whether MMP-9 contributes to E2 enhancement of hippocampal memory consolidation in these tasks. Immediate post-training infusion of E2 promoted memory consolidation as observed previously (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Gross et al., 2021). However, in mice treated with a low dose of MMP-9 inhibitor, this effect was abolished, suggesting that estrogenic enhancement of memory depends on activity of dorsal hippocampal MMP-9. This result is consistent with previous findings that E2-mediated degradation of β-amyloid is dependent on MMP-9 (Merlo and Sortino, 2012) and that MMP-2/9 inhibition blocks E2 facilitation of hippocampal CA1 excitatory postsynaptic potentials (Wang et al., 2016). In this latter study, E2-induced CA1 excitatory synaptic facilitation was also dependent on β1-integrin signaling, which can be activated by MMP-9-meditated production of integrin ligands in the ECM (Dityatev et al., 2010; Wang et al., 2016). Whether MMP-9 activation of integrin signaling is also involved in E2-induced memory enhancement is a topic for future work, as is the examination of other substrates of MMP-9 activity that may contribute to memory consolidation. For instance, MMP-9 has been shown to influence hippocampal plasticity and memory by targeting cellular adhesion molecules, like ICAM-5 and nectin-3. MMP-9-mediated ICAM-5 breakdown is associated with hippocampal LTP induction and dendritic spine elongation (Conant et al., 2010; Tian et al., 2007), whereas MMP-9 dependent nectin-3 cleavage in the hippocampus is associated with stress-induced memory impairment (van der Kooij et al., 2014). Interestingly, MMP-9 can also extracellularly process proforms of growth factors into mature, active forms, including the conversion of pro-BDNF to mature BDNF, which is associated with increased activation of its receptor TrkB (Hwang et al., 2005). In the hippocampus, MMP-9 upregulation or overexpression is associated with increased production of mature BDNF (mBDNF) following environmental enrichment, seizure activity, and in a mouse model of Alzheimer’s disease (Cao et al., 2014; Fragkouli et al., 2014; Mizoguchi et al., 2011). As BDNF and TrkB signaling are known to be important mediators of E2-induced hippocampal plasticity and memory consolidation (Gross et al., 2021; Kramár et al., 2013; Murphy et al., 1998; Sato et al., 2007), determining a role for MMP-9-induced generation of mBDNF in the memory-enhancing effects of E2 may be a particularly interesting avenue for future studies.
To better define the interactions between E2 and MMP-9 in the dorsal hippocampus, we also examined MMP-9 expression and activity following E2 treatment. We found that E2 increased MMP-9 activity, but not protein expression, 30 min after a single infusion into the DH. Classical E2 signaling is characterized by slow, modulatory effects on gene expression via intracellular receptors, however, current work shows that E2 modulation of hippocampal memory largely depends on rapid recruitment of membrane-receptor-initiated intracellular signaling cascades (Taxier et al., 2020). E2 initiates processes that support memory consolidation such as ERK phosphorylation, mTOR activation, histone acetylation, and dendritic spine formation within 5–30 min of infusion into the dorsal hippocampus (Fernandez et al., 2008; Fortress et al., 2013; Tuscher et al., 2016; Zhao et al., 2010). Our observed effect of E2 on MMP-9 activation is consistent with this time course, as well as the time course of rapid E2 synaptic facilitation, which is dependent on MMP-2/9 signaling (Wang et al., 2016). Thus, rapid signaling at membrane-localized estrogen receptors might underlie the rapid increase observed in MMP-9 activity following E2 infusion, however, direct testing of this hypothesis and a further understanding of the time course of E2-induced MMP-9 regulation is needed. As our study focused on relatively early timepoints following E2 administration, it remains unknown whether E2 infusion might alter hippocampal MMP-9 gene or protein expression at later time points.
The mechanisms through which E2 might increase hippocampal MMP-9 activity remain unclear. Regulation of MMP-9 activity can occur not only through altered gene expression, but also via changes in MMP-9 cellular compartmentalization, cleavage of inactive zymogens (pro-MMPs), and regulation of MMP-9 degradation. Although previous studies have found that increased MMP-9 activity correlates with increased MMP-9 expression (Ganguly et al., 2013; Nagy et al., 2007), we do not find evidence that E2 increased MMP-9 protein expression at either 30 min or 1 h post-treatment. Because previous observations of learning-induced increases in hippocampal MMP-9 expression peaked at 6–24 h, it is possible that our relatively rapid increase in MMP-9 activity is fueled by alternative mechanisms. For instance, E2 could rapidly activate MMP-9 by reducing its endogenous inhibition by tissue inhibitors of matrix metalloproteinases (TIMPs). E2 reduces TIMP activity in both uterine and breast cancer cells (Nilsson et al., 2007; Zhang et al., 2007), and in the mouse uterus, this effect is accompanied by an increase in MMP-9 activity (Zhang et al., 2007). Dynamic control of MMP-9 activity by TIMP inhibition also appears to be important for promoting hippocampal plasticity. Work in hippocampal slice and culture finds that a short period of high MMP-9 activity and low TIMP inhibition, followed by an increase in TIMP-1 inhibition to prevent excessive proteolysis, is necessary for successful LTP and maturation of dendritic spines (Magnowska et al., 2016). Thus, E2 might transiently control MMP-9 inhibition in a similar way to regulate hippocampal MMP-9 activity.
E2 could also regulate active MMP-9 by increasing cleavage of pro-MMP-9 to yield more active enzyme. Numerous proteases can cleave pro-MMP-9, including other MMPs and serine proteases like tissue plasminogen activator (tPA). In particular, tPA is the primary driver of MMP-9 activation following cerebral ischemia (Tsuji et al., 2005), and interestingly, E2 administration following ischemia modulates this relationship by reducing excessive upregulation of MMP-9 activity (Liu et al., 2011; Liu et al., 2010). In postmenopausal women, estrogen therapy and acute E2 administration increase endothelial tPA release (Hoetzer et al., 2003), further suggesting that E2 can dynamically regulate tPA activity. Characterizing E2 regulation of pro-MMP-9, tPA, and other MMP subtypes in the hippocampus will be required to determine whether pro-enzyme cleavage contributes to E2-induced MMP-9 activity.
In summary, the results of the present study provide important new insights into the mechanisms of E2 signaling in the hippocampus, as well as the signaling processes essential for E2 to regulate object recognition and spatial memory consolidation. We found that MMP-9 signaling was necessary both for memory consolidation on its own, as well as for E2-induced enhancement of memory consolidation in OVX mice. Moreover, we found that E2 rapidly induces hippocampal MMP-9 activity without affecting MMP-9 protein levels, suggesting that rapid actions of E2 on extracellular signaling may influence its effects on intracellular signaling. These results provide the first direct evidence that E2 regulates hippocampal MMP-9 and further our understanding of the mechanisms of estrogenic memory enhancement. However, many questions remain to be addressed in future work, for example, defining the mechanisms through which E2 regulates MMP-9 and extending these findings to studies of male rodents and local estrogen synthesis. Nevertheless, the addition of MMP-9, a modulator of the extracellular space, fundamentally alters the existing model of E2 signaling in the dorsal hippocampus and highlights the importance of considering extracellular modifications as a potentially critical substrates for estrogenic regulation of memory.
Acknowledgements
This work was supported by the National Institutes of Health (R01MH107886, F32MH118782, 2R15GM118304–02), the Alzheimer’s Association (SAGA-17-419092), the University of Wisconsin-Milwaukee Office of Undergraduate Research, and the University of Wisconsin-Milwaukee College of Letters and Science. The authors would like to thank Dr. Fred Helmstetter for use of his Synergy H1 plate reader.
Declarations of interest
KMF is a co-founder of, and shareholder in, Estrigenix Therapeutics, Inc., a company which aims to improve women’s health by developing safe, clinically proven treatments for the mental and physical effects of menopause. She also serves as the company’s Chief Scientific Officer. The other authors have no interests to declare.
Footnotes
CRediT authorship contribution statement
KMF: Funding acquisition, Conceptualization, Project administration, Supervision, Writing – review & editing. KSG: Conceptualization, Data collection, Formal analysis, Supervision, Writing – original draft, Writing – review & editing, Investigation; CML, MMA, and GEG: Data collection, Writing – review & editing, Investigation.
References
- Boulware MI, Heisler JD, Frick KM, 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci 33, 15184–15194. 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Nagy V, Kwei KT, Huntley GW, 2007. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol 98, 334–344. 10.1152/jn.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Duan J, Wang X, Zhong X, Hu Z, Huang F, Wang H, Zhang Juan, Li F, Zhang Jianyi, Luo X, Li C-Q, 2014. Early enriched environment induces an increased conversion of proBDNF to BDNF in the adult rat’s hippocampus. Behav. Brain Res 265, 76–83. 10.1016/j.bbr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP, Lim ST, 2010. MMP-dependent shedding of ICAM-5 occurs with LTP. Neuroscience 166, 508–521. 10.1016/j.neuroscience.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P, 2010. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat. Rev. Neurosci 11, 735–746. 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci 28, 8660–8667. 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Ferrer M, Dityatev A, 2018. Shaping synapses by the neural extracellular matrix. Front. Neuroanat 12, 40. 10.3389/fnana.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney CA, Shvetcov A, Westbrook RF, Jones NM, Morris MJ, 2020. The role of hippocampal estradiol in synaptic plasticity and memory: a systematic review. Front. Neuroendocrinol 56, 100818 10.1016/j.yfrne.2019.100818. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM, 2013. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn. Mem 20, 147–155. 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkouli A, Tsilibary EC, Tzinia AK, 2014. Neuroprotective role of MMP-9 overexpression in the brain of Alzheimer’s 5xFAD mice. Neurobiol. Dis 70, 179–189. 10.1016/j.nbd.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, Luine V, 2015. The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm. Behav 74, 28–36. 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, 2015. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm. Behav 74, 4–18. 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Gresack JE, 2003. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav. Neurosci 117, 1283–1291. 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Koss WA, 2018. Estradiol and hippocampal memory in female and male rodents. Curr. Opin. Behav. Sci 23, 65–74. 10.1016/j.cobeha.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Rejmak E, Mikosz M, Nikolaev E, Knapska E, Kaczmarek L, 2013. Matrix metalloproteinase (MMP) 9 transcription in mouse brain induced by fear learning. J. Biol. Chem 288, 20978–20991. 10.1074/jbc.M113.457903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gross KS, Alf RL, Polzin TR, Frick KM, 2021. 17β-estradiol activation of dorsal hippocampal TrkB is independent of increased mature BDNF expression and is required for enhanced memory consolidation in female mice.Psychoneuroendocrinology 125, 105110. 10.1016/j.psyneuen.2020.105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins KE, DeMars KM, Yang C, Rosenberg GA, Candelario-Jalil E, 2013. Fluorometric immunocapture assay for the specific measurement of matrix metalloproteinase-9 activity in biological samples: application to brain and plasma from rats with ischemic stroke. Mol. Brain 6, 14. 10.1186/1756-6606-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoetzer GL, Stauffer BL, Irmiger HM, Ng M, Smith DT, DeSouza CA, 2003. Acute and chronic effects of oestrogen on endothelial tissue-type plasminogen activator release in postmenopausal women. J. Physiol 551, 721–728. 10.1113/jphysiol.2003.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW, 2012. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci 13, 743–757. 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JJ, Park M-H, Choi S-Y, Koh J-Y, 2005. Activation of the Trk signaling pathway by extracellular zinc. Role of metalloproteinases. J. Biol. Chem 280, 11995–12001. 10.1074/jbc.M403172200. [DOI] [PubMed] [Google Scholar]
- Kim J, Schalk JC, Koss WA, Gremminger RL, Taxier LR, Gross KS, Frick KM, 2019. Dorsal hippocampal actin polymerization is necessary for activation of G-protein-coupled estrogen receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J. Neurosci 39, 9598–9610. 10.1523/JNEUROSCI.2687-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramár EA, Babayan AH, Gall CM, Lynch G, 2013. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 239, 3–16. 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang Z, Sun W, Koehler RC, Huang J, 2011. 17β-estradiol attenuates breakdown of blood–brain barrier and hemorrhagic transformation induced by tissue plasminogen activator in cerebral ischemia. Neurobiol. Dis 44, 277–283. 10.1016/j.nbd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu Q, He S, Simpkins JW, Yang S-H, 2010. Combination therapy of 17β-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J. Pharmacol. Exp. Ther 332, 1006–1012. 10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang S-H, 2005. 17β-Estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia–reperfusion injury in female rats. Brain Res. 1060, 55–61. 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Magnowska M, Gorkiewicz T, Suska A, Wawrzyniak M, Rutkowska-Wlodarczyk I, Kaczmarek L, Wlodarczyk J, 2016. Transient ECM protease activity promotes synaptic plasticity. Sci. Rep 6, 27757. 10.1038/srep27757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan SE, Meighan PC, Choudhury P, Davis CJ, Olson ML, Zornes PA, Wright JW, Harding JW, 2006. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem 96, 1227–1241. 10.1111/j.1471-4159.2005.03565.x. [DOI] [PubMed] [Google Scholar]
- Merlo S, Sortino MA, 2012. Estrogen activates matrix metalloproteinases-2 and −9 to increase beta amyloid degradation. Mol. Cell. Neurosci 49, 423–429. 10.1016/j.mcn.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P, Mercik K Medvedev N, Wilczek E, Roo MD, Zuschratter W, Muller D, Wilczynski GM, Mozrzymas JW, Stewart MG, Kaczmarek L, Wlodarczyk J, 2011. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell. Sci 124, 3369–3380. 10.1242/jcs.090852. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, Sawada M, Sato J, Yamada K, 2011. Matrix Metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J. Neurosci 31, 12963–12971. 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Segal M, 1998. Brain-derived neurotrophic factor mediates estradiol-induced dendritic spine formation in hippocampal neurons. Proc. Natl. Acad. Sci. USA 95, 11412–11417. 10.1073/pnas.95.19.11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Huntley GW, 2007. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn. Mem 14, 655–664. 10.1101/lm.678307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW, 2006. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory.J. Neurosci 26, 1923–1934. 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson UW, Garvin S, Dabrosin C, 2007. MMP-2 and MMP-9 activity is regulated by estradiol and tamoxifen in cultured human breast cancer cells. Breast Cancer Res. Treat 102, 253–261. 10.1007/s10549-006-9335-4. [DOI] [PubMed] [Google Scholar]
- Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K, 2007. β-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 1150, 108–120. 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RDG, Kaczmarek L, 2002. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci 22, 920–930. 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxier LR, Gross KS, Frick KM, 2020. Oestradiol as a neuromodulator of learning and memory. Nat. Rev. Neurosci 21, 535–550. 10.1038/s41583-020-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H, Libert C Itohara S, Mishina M, Rauvala H, Gahmberg CG, 2007. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J. Cell Biol 178, 687–700. 10.1083/jcb.200612097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Aoki T, Tejima E, Arai K, Lee S-R, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH, 2005. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke 36, 1954–1959. 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM, 2016. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus.J. Neurosci 36, 1483–1489. 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij MA, Fantin M, Rejmak E, Grosse J, Zanoletti O, Fournier C, Ganguly K, Kalita K, Kaczmarek L, Sandi C, 2014. Role for MMP-9 in stress-induced downregulation of nectin-3 in hippocampal CA1 and associated behavioural alterations. Nat. Commun 5, 4995. 10.1038/ncomms5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A, 2003. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 100, 9614–9619. 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA, 2006. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem 86, 35–46. 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kantorovich S, Babayan AH, Hou B, Gall CM, Lynch G, 2016. Estrogen’s effects on excitatory synaptic transmission entail integrin and TrkB transactivation and depend upon β1-integrin function. Neuropsychopharmacology 41, 2723–2732. https://doi.org/10.1038/npp.2016.83, 10.1038/npp.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW, 2008. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. USA 105, 19520–19525. 10.1073/pnas.0807248105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Brown TE, Harding JW, 2007. Inhibition of hippocampal matrix metalloproteinase-3 and −9 disrupts spatial memory. Neural Plast. 2007, 73813. 10.1155/2007/73813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Murphy ES, Elijah IE, Holtfreter KL, Davis CJ, Olson ML Muhunthan K, Harding JW, 2004. Influence of hippocampectomy on habituation, exploratory behavior, and spatial memory in rats. Brain Res. 1023, 1–14. 10.1016/j.brainres.2004.06.083. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang X, Jiang W, 2013. Propofol impairs spatial memory consolidation and prevents learning-induced increase in hippocampal matrix metalloproteinase-9 levels in rat. NeuroReport 24, 831–836. 10.1097/WNR.0b013e328364fe69. [DOI] [PubMed] [Google Scholar]
- Zhang X, Christenson LK, Nothnick WB, 2007. Regulation of MMP-9 expression and activity in the mouse uterus by estrogen. Mol. Reprod. Dev 74, 321–331. 10.1002/mrd.20582. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM, 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc. Natl. Acad. Sci. USA 107, 5605–5610. 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]


