Abstract
Background
STAMPEDE has previously reported that radiotherapy (RT) to the prostate improved overall survival (OS) for patients with newly diagnosed prostate cancer with low metastatic burden, but not those with high-burden disease. In this final analysis, we report long-term findings on the primary outcome measure of OS and on the secondary outcome measures of symptomatic local events, RT toxicity events, and quality of life (QoL).
Methods and findings
Patients were randomised at secondary care sites in the United Kingdom and Switzerland between January 2013 and September 2016, with 1:1 stratified allocation: 1,029 to standard of care (SOC) and 1,032 to SOC+RT. No masking of the treatment allocation was employed. A total of 1,939 had metastatic burden classifiable, with 42% low burden and 58% high burden, balanced by treatment allocation. Intention-to-treat (ITT) analyses used Cox regression and flexible parametric models (FPMs), adjusted for stratification factors age, nodal involvement, the World Health Organization (WHO) performance status, regular aspirin or nonsteroidal anti-inflammatory drug (NSAID) use, and planned docetaxel use. QoL in the first 2 years on trial was assessed using prospectively collected patient responses to QLQ-30 questionnaire.
Patients were followed for a median of 61.3 months. Prostate RT improved OS in patients with low, but not high, metastatic burden (respectively: 202 deaths in SOC versus 156 in SOC+RT, hazard ratio (HR) = 0·64, 95% CI 0.52, 0.79, p < 0.001; 375 SOC versus 386 SOC+RT, HR = 1.11, 95% CI 0.96, 1.28, p = 0·164; interaction p < 0.001). No evidence of difference in time to symptomatic local events was found. There was no evidence of difference in Global QoL or QLQ-30 Summary Score. Long-term urinary toxicity of grade 3 or worse was reported for 10 SOC and 10 SOC+RT; long-term bowel toxicity of grade 3 or worse was reported for 15 and 11, respectively.
Conclusions
Prostate RT improves OS, without detriment in QoL, in men with low-burden, newly diagnosed, metastatic prostate cancer, indicating that it should be recommended as a SOC.
Trial registration
ClinicalTrials.gov NCT00268476, ISRCTN.com ISRCTN78818544.
Chris C Parker and colleagues report long-term findings on overall survival and local complications in men with metastatic prostate cancer treated with radiotherapy.
Author summary
Why was this study done?
Prostate cancer is the most common cancer in males.
Radiotherapy (RT) to the prostate is widely used as a radical treatment for nonmetastatic prostate cancer.
A comparison was added to the STAMPEDE protocol to assess whether RT to the prostate would also be helpful for males with metastatic prostate cancer. A benefit in survival was targeted.
The trial previously reported a clinically relevant, statistically significant overall survival (OS) benefit for patients with a low metastatic burden but not for men with a high metastatic burden.
This long-term analysis assesses survival with substantially longer follow-up and more events and looked also at complications of local disease.
What did the researchers do and find?
A randomised controlled trial of adding RT to the prostate to standard of care (SOC) was incorporated into the STAMPEDE protocol.
More than 2,000 patients joined the comparison between 2013 and 2016.
The data set was frozen in 2021 and analysed using standard methods.
There was a clear improvement in survival with prostate RT in the low metastatic burden group.
There was no improvement in survival with prostate RT in the high metastatic burden group.
Symptomatic local progression and the need for later local intervention were improved with RT in the low metastatic burden group.
In the low metastatic burden group, the improvement with RT was similar whether the RT was given with a daily schedule (over 4.5 weeks) or a weekly schedule (over 6 weeks).
The adverse effects of RT were manageable without any impact on long-term quality of life (QoL).
What do these findings mean?
Prostate RT is a relatively cheap, widely accessible, and well-tolerated treatment.
Prostate RT is indicated in patients with newly diagnosed prostate cancer with a low metastatic burden.
RT to the prostate is not routinely indicated for patients with a high metastatic burden.
Introduction
Prostate radiotherapy (RT) is recommended for men with newly diagnosed, low-burden, metastatic prostate cancer, but not for men with high-burden disease [1]. This recommendation is based largely on the initial results of the STAMPEDE trial, reported in 2018 [2]. In this randomised controlled trial of 2,061 men with newly diagnosed metastatic prostate cancer, prostate RT improved overall survival (OS) for men with low metastatic burden (hazard ratio [HR] 0.68, 95% CI 0.52 to 0.90; p = 0.007), with no evidence of a meaningful effect on survival in men with high metastatic burden (HR 1.07, 95% CI 0.90 to 1.28; p = 0.420). That initial analysis, triggered by a preplanned number of events, was done after a median follow-up of 37 months and was based on 761 events. Here, we report the final analysis of OS, with an additional 2 years follow-up.
We hypothesised that prostate RT would reduce the complications of local disease progression, such as urinary or bowel obstruction. If so, this could benefit men with metastatic disease, regardless of disease burden. Here, we report data on freedom from local interventions (e.g., urinary catheter, ureteric stents, nephrostomies, and colostomy).
Any benefits of prostate RT need to be weighed against the risk of treatment-related adverse events (AEs). We report, for the first time, data from the trial on quality of life (QoL).
The trial was stratified according to the choice of 1 of 2 RT dose-fractionation schedules, nominated prior to randomisation; 36 Gy in 6 fractions over 6 weeks, or 55 Gy in 20 fractions over 4 weeks. The 2 schedules were chosen in the expectation that they would be similarly effective. With the benefit of additional follow-up, and more events in the final analysis, we have tested for any differential impact on OS by choice of RT schedule.
Methods
Study participants
Eligible patients had prostate cancer that was newly diagnosed, with no previous radical treatment, had metastatic disease confirmed on a bone scintigraphic scan and soft tissue imaging, and were within 12 weeks after starting androgen deprivation therapy (ADT). Patients were required to have no contraindications to RT and no clinically significant cardiovascular history. Participants were recruited at secondary care sites in the UK and Switzerland.
The trial was registered as NCT00268476 (ClinicalTrials.gov) and ISRCTN78818544 (ISRCTN.com). The trial was done in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki and had relevant ethics (West Midlands–Edgbaston Research Ethics Committee) and regulatory approvals. All patients gave written informed consent. The rationale and design, including sample size calculations, have been described previously [2,3]. Full details are in the protocol at www.stampedetrial.org.
Procedures
All patients received lifelong hormone therapy as gonadotrophin-releasing hormones (GnRHs) agonists or antagonists or orchidectomy. In addition, docetaxel was permitted after it became available for this setting in the UK. Docetaxel, when used, was given as six 3 weekly cycles of 75mg/m2 with or without prednisolone 10 mg daily.
External beam RT to the prostate was given as 1 of 2 schedules nominated prior to randomisation: 36 Gy in 6 consecutive weekly fractions of 6 Gy or 55 Gy in 20 daily fractions of 2.75 Gy over 4 weeks. Treatment was given with the patient supine, with a full bladder and an empty rectum. The planning target volume consisted of the prostate only with an 8-mm margin posteriorly and a 10-mm margin elsewhere. RT was to commence as soon as practicable after randomisation, and, if the patient was having docetaxel as part of standard of care (SOC), within 3 to 4 weeks after the last docetaxel dose.
Patients were followed up 6 weekly until 6 months after randomisation, 12 weekly to 2 years, 6 monthly to 5 years, and then annually. Toxicities and symptoms were reported at regular follow-up visits or when an AE was categorised as “serious.” These were graded with Common Terminology Criteria for Adverse Events (CTCAE) v4·0. Separately, bowel and bladder adverse effects during RT and long-term possible RT effects were recorded using the RTOG scale [4]. Participants were asked to complete the EORTC QLQ-C30 at each scheduled follow-up appointment.
Metastatic burden at randomisation was evaluated retrospectively through central imaging review of whole body scintigraphy and computerized tomography (CT) or MRI staging scans. Metastatic burden was classified according to the definition used in the CHAARTED trial [5] as either high (polymetastatic; ≥4 bone metastases with ≥1 outside the vertebral bodies or pelvis and/or visceral metastases) or low (oligometastatic). Patients with only lymph node metastases, in the absence of bone or visceral disease, were therefore classified as low metastatic burden regardless of the number of nodal metastases.
Randomisation and masking
Patients were randomised centrally using a computerised algorithm, developed and maintained by the trials unit. Minimisation with a random element of 20% was used (80% probably of allocation to a minimising treatment), stratifying for hospital, age at randomisation (<70 versus ≥70 years), nodal involvement (negative versus positive versus indeterminate), the World Health Organization (WHO) performance status (0 versus 1 or 2), planned form of ADT (orchidectomy versus LHRH (leuteinising hormone-releasing hormone) agonist versus LHRH antagonist versus dual androgen blockade), and regular aspirin or nonsteroidal anti-inflammatory drug (NSAID) use (yes or no). Planned docetaxel use was added as a stratification factor after use was permitted as part of SOC. Allocation was 1:1 to SOC only or SOC+RT. There was no blinding to treatment allocation.
Primary and secondary outcomes
The primary efficacy outcome measure was OS, defined as time from randomisation to death from any cause. Secondary outcomes for this long-term efficacy analysis included local intervention–free survival (LIFS)—consisting of time from randomisation to the first report on case report forms of TURP, ureteric stent, surgery for bowel obstruction, urinary catheter, nephrostomy, colostomy, death from prostate cancer—and symptomatic local event-free survival (SLEFS), comprising any of these LIFS events or acute kidney injury, urinary tract infection, or urinary tract obstruction. Cause of death was determined by the site investigator, with some cases reclassified as prostate cancer death according to predefined criteria which suggested this to be the likely cause. Patients without the event of interest were censored at the time last known to be event free. QoL analyses focused on Global QoL % and QLQ-30 Summary Score %, as derived from patient reports at scheduled assessment time points in the first 2 years after randomisation (see S2 Text).
Statistical analysis
The primary outcome measure, OS, was assessed across all patients and separately within patient subgroups characterised by baseline metastatic burden (low versus high) and nominated RT schedule (daily versus weekly).
Standard survival analysis methods were used to analyse time-to-event data in Stata v16.1 (College Station, Texas, United States of America). A nonparametric stratified log-rank test was used to assess any difference in survival between treatment groups; this was stratified across the minimisation factors used at randomisation (except hospital and planned form of hormone therapy) plus protocol-specific time periods defined by other arms recruiting to STAMPEDE or changes to SOC which could affect the population being randomised. Cox proportional hazards (PHs) regression models adjusting for the same stratification factors and stratified by time period were used to estimate relative treatment effect; a HR less than 1·00 favoured the research arm. Unadjusted estimates of treatment effect are also presented. Flexible parametric models (FPMs) were fitted with degrees of freedom (5.5) and adjusted for stratification factors and time periods [6]. Medians and 5-year survival estimates are presented from the FPM fitted to the data. Kaplan–Meier curves, using the KMunicate format [7], show estimated survival over time. Following the fitting of Cox models, the PHs assumption was tested using a global Grambsch–Therneau test with log-transformed time; restricted mean event-free (“survival”) time (RMST) was emphasised in the presence of nonproportionality, using a t-star of 91 months as determined by the Royston and Parmar method [6]. Cox and Fine and Gray regression models [8] were used for cause-specific and competing risk analyses, respectively; competing risks were non-prostate cancer–related death for prostate cancer–specific survival and death from any cause for SLEFS and LIFS. Evidence for different treatment effect across subgroups was assessed using the likelihood ratio p-value for an interaction term added to the relevant adjusted Cox/FPM model, or Wald test p-value from Fine and Gray model. Sensitivity analyses of local event outcomes examined the impact of excluding death from prostate cancer and a competing risks approach with death from any cause specified as a competing event. All tests are presented as 2 sided, with 95% CIs and the relevant p-value.
Median follow-up was estimated using the Kaplan–Meier method with reverse censoring on death. All patients were included in the efficacy and QoL analyses according to allocated treatment on an intention-to-treat (ITT) basis; sensitivity analyses exclude patients who did not explicitly fulfill all of the eligibility criteria. AE data are shown for the safety population, in patients with at least 1 follow-up assessment and analysed according to whether RT was received within 1 year of randomisation (SOC+RT) or not (SOC).
Analyses of the QoL outcomes included partly conditional and composite approaches, building on the approaches previously used in the trial [9]. For the former, missing values were multiply imputed using observed data using chained equations. Imputed values for assessments dating after a patient had died were restored to missing. Generalised estimating equations with an independence correlation matrix were used to estimate the expected value of the outcome for each treatment arm at each assessment time point. For the composite approach, observations following the death of a patient were set to 0% (corresponding to the lowest possible QoL state). Mixed linear regression with random intercept and slope (with unstructured correlation specification) was used to model the outcome. Additional cross-sectional analyses estimated the difference in average QoL associated with treatment allocation in patients alive and with data available at a given assessment time point, controlling for baseline state.
This trial is reported per the Consolidated Standards of Reporting Trials (CONSORT; see S3 Text).
Results
Patients
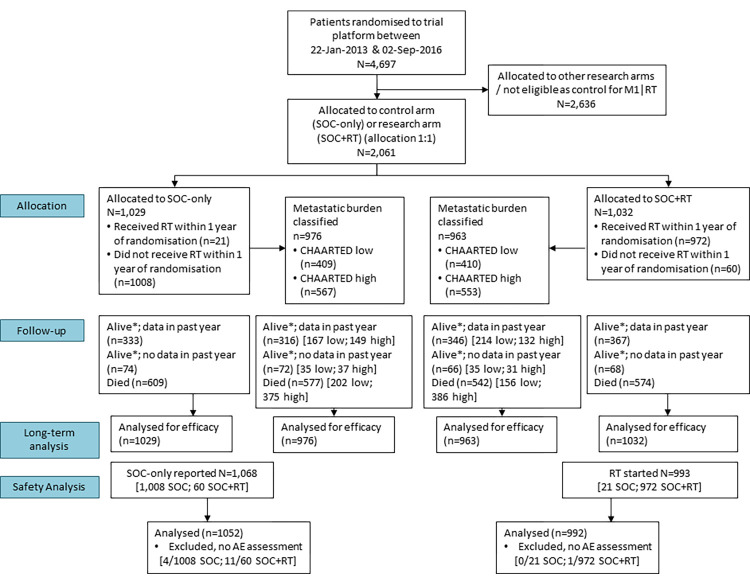
Between January 22, 2013 and September 2, 2016, 2,061 patients were randomised from 117 hospitals in UK and Switzerland: 1,029 to SOC and 1,032 to SOC+RT. The data set was frozen on March 17, 2021 and included information up to November 30, 2020. Fig 1 shows the CONSORT flow diagram for analyses presented in this paper. Table 1 shows baseline characteristics balanced across the allocated treatment groups. Table A in S1 Text shows baseline characteristics in 1,939 (94%) patients who were evaluable for disease burden, 819 (40%) with low- and 1,120 (54%) with high-burden disease.
Fig 1. CONSORT diagram.
AE, adverse event; CONSORT, Consolidated Standards of Reporting Trials; RT, radiotherapy to the prostate, SOC, standard of care. *Alive, no withdrawal of permission for continued data collection.
Table 1. Baseline characteristics of all patients in the comparison.
| Characteristic | SOC (n = 1,029) | SOC+RT (n = 1,032) | |
|---|---|---|---|
| Age at randomisation (years) | Median (IQR) | 68 (63 to 73) | 68 (63 to 73) |
| Range | 37 to 86 | 45 to 87 | |
| WHO performance status | 0 | 732 (71%) | 734 (71%) |
| 1 to 2 | 297 (29%) | 298 (29%) | |
| Pain from prostate cancer | Absent | 826 (81%) | 855 (83%) |
| Present | 198 (19%) | 172 (17%) | |
| Missing | 5 | 5 | |
| Previous notable health issues | Myocardial infarction | 67 (7%) | 58 (6%) |
| Cerebrovascular disease | 29 (3%) | 32 (3%) | |
| Congestive heart failure | 5 (<1%) | 8 (1%) | |
| Angina | 46 (4%) | 52 (5%) | |
| Hypertension | 408 (40%) | 444 (43%) | |
| T-category at randomisation | T0 | 0 (0%) | 1 (<1%) |
| T1 | 12 (1%) | 12 (1%) | |
| T2 | 84 (9%) | 89 (9%) | |
| T3 | 585 (62%) | 603 (63%) | |
| T4 | 260 (28%) | 247 (26%) | |
| TX | 88 | 80 | |
| N-category at randomisation | N0 | 345 (36%) | 344 (36%) |
| N+ | 620 (64%) | 620 (64%) | |
| NX | 64 | 68 | |
| Metastatic burden | Low metastatic burden* | 409 (42%) | 410 (43%) |
| High metastatic burden | 567 (58%) | 553 (57%) | |
| Not classified | 53 | 69 | |
| Sites of metastases | Bone | 919 (89%) | 917 (89%) |
| Liver | 23 (2%) | 19 (2%) | |
| Lung | 42 (4%) | 48 (5%) | |
| Distant lymph nodes | 295 (29%) | 304 (29%) | |
| Other | 35 (3%) | 32 (3%) | |
| Gleason sum score | < = 7 | 173 (17%) | 175 (18%) |
| 8 to 10 | 826 (83%) | 820 (82%) | |
| Unknown | 30 | 37 | |
| PSA pre-ADT (ng/ml) | Median (IQR) | 98 (30 to 316) | 97 (33 to 313) |
| Range | 1 to 20,590 | 1 to 11,156 | |
| Time from diagnosis (days) | Median (IQR) | 73 (55 to 94) | 73 (55 to 93) |
| Missing | 1 | 2 | |
| Days from starting hormones | Median (IQR) | 53 (35 to 70) | 55 (34 to 70) |
| Range | -3 to 84 | 0 to 86 | |
| Missing | 17 | 13 | |
| Planned for SOC docetaxel | No | 845 (82%) | 849 (82%) |
| Yes | 184 (18%) | 183 (18%) | |
| Nominated RT schedule | 36 Gy/6 f over 6 weeks | 482 (47%) | 498 (48%) |
| 55 Gy/20 f over 4 weeks | 547 (53%) | 534 (52%) |
*Note: One patient classified with low-burden disease was subsequently restaged as nonmetastatic by the randomising site. They remain in the low metastatic burden subgroup for this analysis.
ADT, androgen deprivation therapy; IQR, interquartile range; PSA, prostate specific antigen; RT, radiotherapy to the prostate; SOC, standard of care; WHO, World Health Organization.
Median duration of follow-up was 61.3 months (interquartile range [IQR] = 53.8 to 73.1) and was similar in both treatment groups: SOC 61.0 (IQR = 53.8 to 72.6) and SOC+RT 61.6 (IQR = 53.8 to 73.1).
OS by allocated treatment and metastatic burden
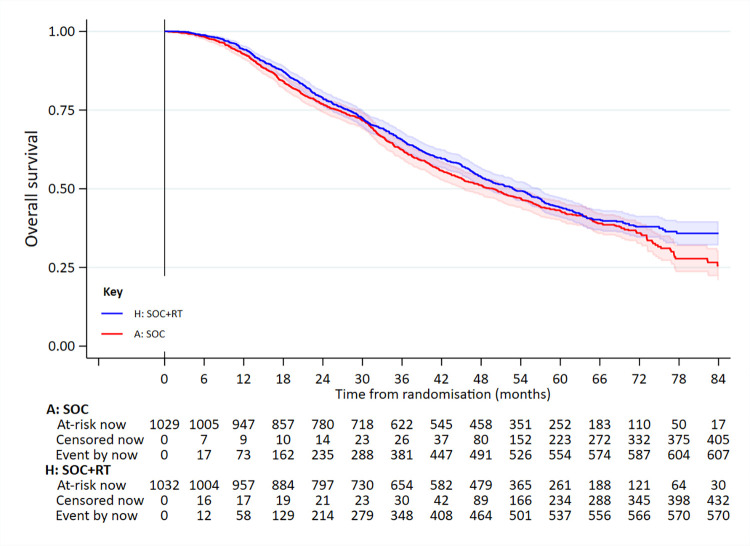
A total of 1,183 deaths were reported, 609 in patients allocated to SOC and 574 in those allocated to SOC+RT (Fig 2, Table 2).
Fig 2. OS in all patients.
Adjusted HR = 0.90 (95% CI 0.81 to 1.01; p = 0.081). HR, hazard ratio; OS, overall survival; RT, radiotherapy to the prostate; SOC, standard of care.
Table 2. Summary of estimated treatment effect for main outcome measures: all patients and metastatic burden subgroups.
| Outcome measure | Patient group | Adjusted HR~ | Unadjusted HR^ | Event free at 5 years+ | RMST+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| SOC | SOC+RT | SOC | SOC+RT | Difference | |||||
| OS | All patients | 0.90 (0.81 to 1.01) | 0.90 (0.81 to 1.01) | 42% | 45% | 52.9 | 55.5 | 2.5 (−0.2 to 5.2) | |
| Low metastatic burden | 0.64 (0.52 to 0.79) | 0.66 (0.54 to 0.82) | 53% | 65% | 60.6 | 69.0 | 8.4 (4.5 to 12.2) | ||
| High metastatic burden | 1.11 (0.96 to 1.28) | 1.08 (0.94 to 1.25) | 35% | 30% | 47.7 | 45.5 | −2.2 (−5.7 to 1.2) | ||
| Weekly RT (36 Gy/6 f) | 1.00 (0.85 to 1.18) | 1.01 (0.86 to 1.19) | 44% | 42% | 53.9 | 53.6 | −0.3 (−3.4 to 2.8) | ||
| (Low metastatic burden) | 0.67 (0.49 to 0.93) | 0.71 (0.52 to 0.97) | 54% | 64% | 61.3 | 68.2 | 6.9 (0.6 to 13.2) | ||
| (High metastatic burden) | 1.22 (0.99 to 1.50) | 1.19 (0.97 to 1.46) | 37% | 29% | 48.9 | 44.5 | −4.3 (−9.6 to 0.9) | ||
| Daily RT (55 Gy/20 f) | 0.83 (0.71 to 0.97) | 0.81 (0.69 to 0.95) | 41% | 47% | 52.2 | 57.2 | 5.0 (1.1 to 8.9) | ||
| (Low metastatic burden) | 0.62 (0.47 to 0.83) | 0.63 (0.48 to 0.84) | 52% | 66% | 59.9 | 69.5 | 9.6 (4.0 to 15.2) | ||
| (High metastatic burden) | 1.02 (0.83 to 1.25) | 0.99 (0.81 to 1.21) | 33% | 32% | 46.8 | 46.6 | −0.2 (−4.5 to 4.0) | ||
| Prostate cancer–specific survival * | All patients | 0.92 (0.81 to 1.04) | 0.92 (0.81 to 1.04) | 49% | 51% | 57.6 | 59.5 | 1.9 (−1.1 to 5.0) | |
| Low metastatic burden | 0.62 (0.49 to 0.79) | 0.64 (0.50 to 0.81) | 62% | 72% | 65.7 | 73.7 | 8.0 (4.0 to 12.0) | ||
| High metastatic burden | 1.12 (0.96 to 1.31) | 1.10 (0.94 to 1.28) | 41% | 35% | 51.8 | 49.0 | −2.8 (−6.6 to 1.0) | ||
| SLEFS # | All patients | 1.00 (0.90 to 1.13) | 1.00 (0.90 to 1.12) | 39% | 40% | 49.2 | 48.9 | −0.3 (−3.5 to 2.8) | |
| Low metastatic burden | 0.72 (0.59 to 0.88) | 0.73 (0.60 to 0.90) | 46% | 58% | 54.5 | 61.8 | 7.2 (2.5 to 11.9) | ||
| High metastatic burden | 1.23 (1.06 to 1.42) | 1.21 (1.05 to 1.40) | 33% | 26% | 45.1 | 39.4 | −5.8 (−9.7 to −1.9) | ||
| LIFS # | All patients | 0.94 (0.83 to 1.06) | 0.93 (0.83 to 1.05) | 44% | 47% | 53.5 | 55.1 | 1.6 (−1.5 to 4.7) | |
| Low metastatic burden | 0.62 (0.49 to 0.77) | 0.63 (0.50 to 0.78) | 54% | 67% | 59.7 | 69.1 | 9.5 (5.2 to 13.8) | ||
| High metastatic burden | 1.18 (1.01 to 1.37) | 1.16 (1.00 to 1.34) | 38% | 32% | 49.0 | 44.7 | −4.4 (−8.4 to −0.4) | ||
Note: HR and RMST difference are for SOC+RT relative to SOC.
*Cause-specific treatment × metastatic burden interaction test p < 0.001 [p = 0.0000977]. Competing risks analysis: overall adjusted sub-HR = 0.93 (95% CI 0.82 to 1.05; p = 0.260); low-burden adjusted sub-HR = 0.66 (95% CI 0.52 to 0.83; p = 0.001); high-burden adjusted sub-HR = 1.11 (95% CI 0.95 to 1.29; p = 0.189); treatment × metastatic burden interaction test p < 0.001 [p = 0.000350].
#SLEFS: treatment × metastatic burden interaction test p < 0.001 [p = 0.0000314]. LIFS interaction p < 0.001 [p = 2.53 × 10−6].
~Estimates from Cox models adjusting for age, nodal involvement, WHO performance status, regular aspirin or NSAID use, and planned SOC docetaxel at randomisation, stratified by randomisation time period.
^Estimates from unadjusted, unstratified Cox models.
+Survival probabilities and RMST estimates are taken from FPMs with t-star = 91 months.
HR, hazard ratio; LIFS, local intervention–free survival; NSAID, nonsteroidal anti-inflammatory drug; RMST, restricted mean event-free (“survival”) time; RT, radiotherapy to the prostate; SLEFS, symptomatic local event–free survival; SOC, standard of care; WHO, World Health Organization.
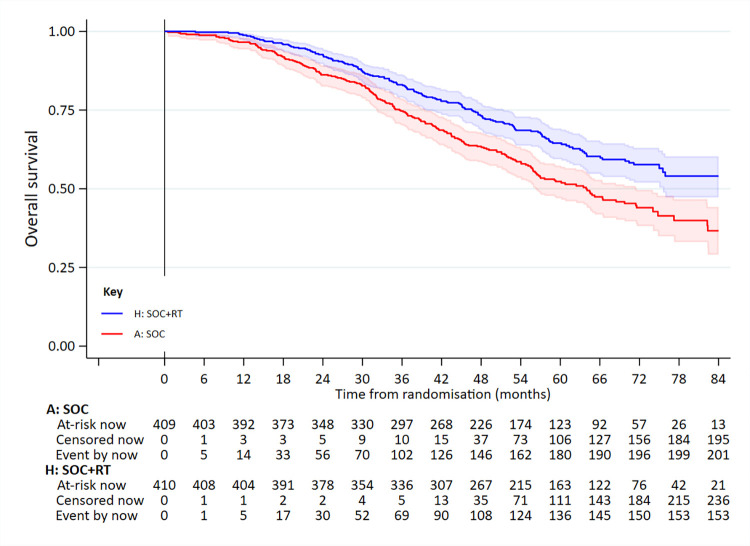
In the low metastatic burden group, 358 had died: 202/409 SOC and 156/410 SOC+RT. Median survival was 63.6 months for SOC and 85.5 months for SOC+RT (5-year survival 53% versus 65%); adjusted HR = 0.64 (95% CI 0.52 to 0.79; p < 0.001 [p = 0.00004]) (Fig 3, Table 2). There was no evidence of non-PHs.
Fig 3. OS in patients in the low-burden metastatic disease group.
Adjusted HR = 0.64 (95% CI 0.52 to 0.79; p < 0.001 [p = 0.00004]). HR, hazard ratio; OS, overall survival; RT, radiotherapy to the prostate; SOC, standard of care.
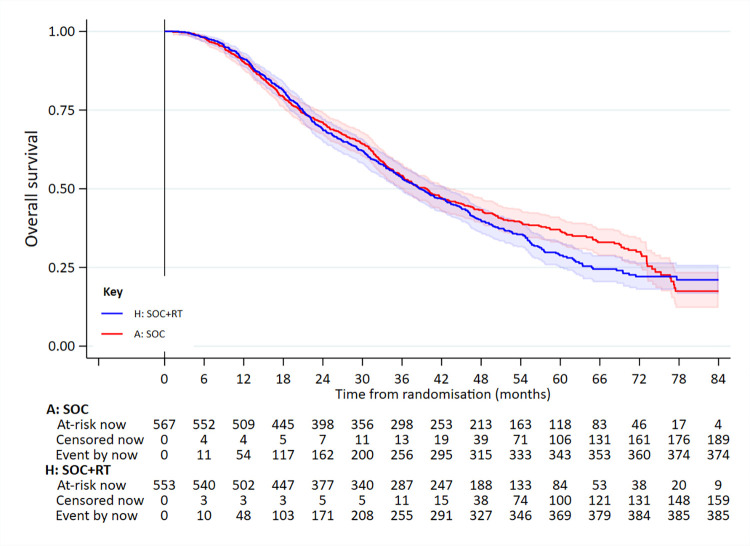
In the high-burden disease group, 761 had died: 375/567 SOC and 386/553 SOC+RT. Median survival was 41.2 months in SOC and 38.8 months in SOC+RT (5-year survival 35% versus 30%): adjusted HR = 1.11 (95% CI 0.96 to 1.28; p = 0.164) (Fig 4, Table 2). There was no evidence of non-PHs.
Fig 4. OS in patients in the high-burden metastatic disease group.
Adjusted HR = 1.11 (95% CI 0.96 to 1.28; p = 0.164). HR, hazard ratio; OS, overall survival; RT, radiotherapy to the prostate; SOC, standard of care.
There was clear evidence of differential treatment effect according to metastatic burden: interaction test p < 0.001 [p = 0.00005].
Similar results were obtained from cause-specific and competing risk analyses (Table 2). A participant audit found 36 (<2%) patients with baseline data or documented protocol deviation inconsistent with the comparison’s full eligibility criteria. Sensitivity analyses found no impact from excluding these patients (Tables B and C in S1 Text). Analysis of time from randomisation to reported second-line treatments indicates no confounding of RT treatment effect on OS by postprogression abiraterone or enzalutamide therapy (S10 and S11 Figs).
Exploration of OS by elected RT schedule
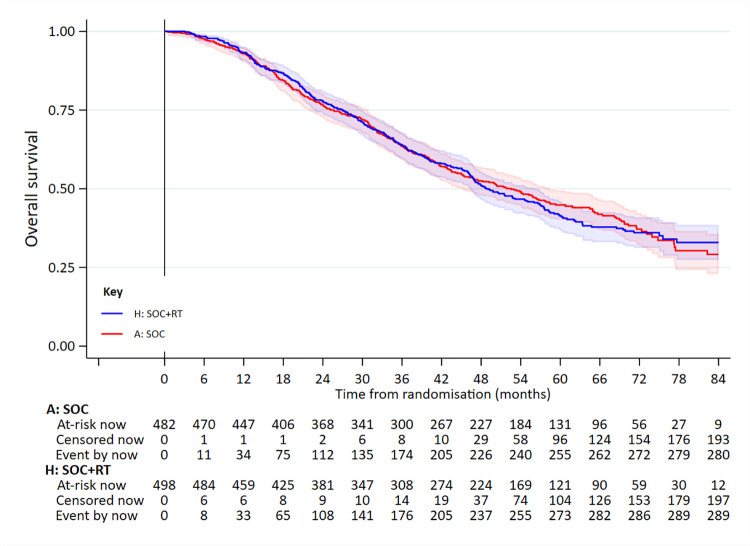
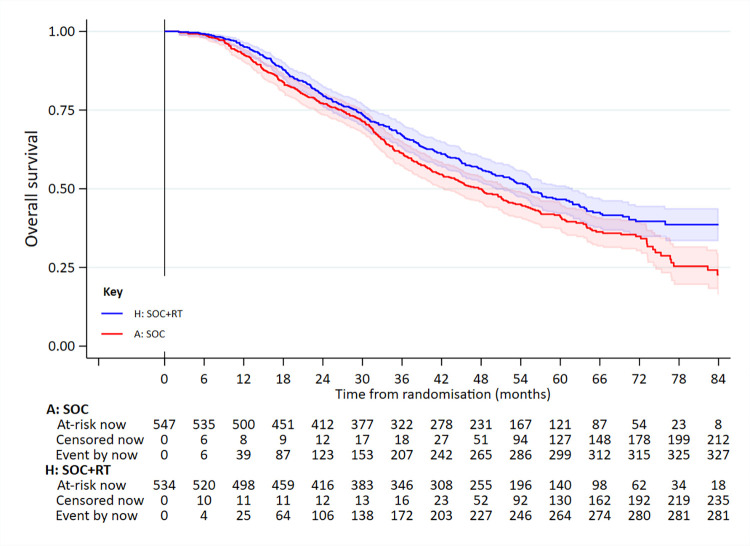
In 980 patients nominated prior to randomisation for weekly RT, 575 had died: 282/482 SOC and 293/498 SOC+RT. Median survival was 52.2 months for SOC and 49.9 months for SOC+RT (5-year survival: 44% versus 42%); adjusted HR = 1.00 (95% CI 0.85 to 1.18); p = 0.974 (Fig 5, Table 2). In 1,081 patients nominated prior to randomisation for daily RT, 608 died: 327/547 SOC and 281/534 SOC+RT. Median survival was 47.8 months in SOC and 55.5 months in SOC+RT (5-year survival 41% versus 47%); adjusted HR = 0.83 (95% CI 0.71 to 0.97; p = 0.022) (Fig 6, Table 2). There was no good evidence of interaction in the treatment effect by RT schedule: interaction p = 0.088.
Fig 5. OS in patients nominated for weekly RT (36 Gy/6 f) prior to randomisation.
Adjusted HR = 1.00 (95% CI 0.85 to 1.18; p = 0.974). HR, hazard ratio; OS, overall survival; RT, radiotherapy to the prostate; SOC, standard of care.
Fig 6. OS in patients nominated for daily RT (55 Gy/20 f) prior to randomisation.
Adjusted HR = 0.83 (95% CI 0.71 to 0.97; p = 0.022). HR, hazard ratio; OS, overall survival; RT, radiotherapy to the prostate; SOC, standard of care.
Given that RT improved OS in the low metastatic burden patients, RT schedule was further explored in this subgroup. In 360 patients nominated for weekly RT, 162 had died: 94/190 SOC and 68/170 SOC+RT; adjusted HR = 0.67 (95% CI 0.49 to 0.93; p = 0.015 [p = 0.0155]). In 459 patients nominated for daily RT, 196 had died: 108/219 SOC and 88/240 SOC+RT; adjusted HR = 0.62 (95% CI 0.47–0.83; p = 0.001 [p = 0.00112]). There was no good evidence of interaction in the treatment effect by RT schedule: interaction p = 0.732.
SLEFS by allocated treatment
A total of 1,209 (59%) patients were reported as experiencing at least 1 symptomatic local event: 608 SOC and 601 SOC+RT. In 789 cases (400 SOC and 389 SOC+RT), death from prostate cancer was the only event recorded. Table 3 summarises the reported incidence of each type of event. There was no evidence of a difference in time to first reported event by treatment arm: adjusted HR = 1.00 (95% CI 0.90 to 1.13; p = 0.931); median symptomatic local event–free survival 43.8 months SOC, 43.3 months SOC+RT (5-year SLEFS survival 39% versus 40%) (S1 Fig, Table 2).
Table 3. First symptomatic local event reported (patients with event reported).
| Type of event | SOC (n = 608) | SOC+RT (n = 601) |
|---|---|---|
| Urinary tract infection | 57 (9%) | 80 (13%) |
| Urinary catheter | 52 (9%) | 44 (7%) |
| Acute kidney injury | 33 (5%) | 34 (6%) |
| TURP | 24 (4%) | 24 (4%) |
| Urinary tract obstruction | 15 (2%) | 15 (3%) |
| Ureteric stent | 19 (3%) | 8 (1%) |
| Nephrostomy | 5 (1%) | 2 (<1%) |
| Colostomy | 3 (<1%) | 3 (1%) |
| Surgery for bowel obstruction | 0 (0%) | 2 (<1%) |
| PCa death | 400 (66%) | 389 (65%) |
PCa, prostate cancer; RT, radiotherapy to the prostate; SOC, standard of care; TURP, transurethral resection of the prostate.
A total of 1,086 (53%) patients had 1 or more local intervention events reported, 556 SOC and 530 SOC+RT, of which death from prostate cancer was the only event in 78% and 81% of cases. Median local intervention event–free survival was 51.1 months in SOC and 53.6 months in SOC+RT (5-year survival 44% versus 47%); adjusted HR = 0.94 (95% CI 0.83 to 1.06; p = 0.286) (S2 Fig, Table 2, Table D in S1 Text). Table E in S1 Text presents the results of sensitivity analyses.
AEs by allocated treatment
Urinary-related late AEs of grade 3 were reported for 20 (2%) patients who received RT within 1 year after randomisation; 10 (2%) were planned for weekly and 10 (2%) for daily treatment; no grade 4 or 5 urinary-related events were reported. Bowel-related late AEs of grade 3 or 4 were reported for 26 (3%) patients, 15 (3%) planned for weekly and 11 (2%) daily treatment (Table 4, Table F in S1 Text). For 610 patients with data available at 2 years, grade 3 urinary AEs were reported for 3 (0.5%) and grade 3 bowel AEs for 6 (1%) (Table G in S1 Text). At 4 years, 2/467 (0.4%) patients had grade 3 or 4 bowel toxicity (Table H in S1 Text).
Table 4. Patients with grade 3/4 worst late RT toxicity score reported over entire time on trial.
| Toxicity area | SOC+RT | |
|---|---|---|
| Weekly, 36 Gy/6 f (n = 473) |
Daily, 55 Gy/20 f (n = 517) |
|
| Urinary | 10 (2%) | 10 (2%) |
| Hematuria | 4 (1%) | 4 (1%) |
| Urethral stricture | 3 (1%) | 4 (1%) |
| Cystitis | 3 (1%) | 4 (1%) |
| Bowel | 15 (3%) | 11 (2%) |
| Proctitis | 9 (2%) | 5 (1%) |
| Diarrhea | 6 (1%) | 6 (1%) |
| Rectal–anal stricture | 0 (0%) | 0 (0%) |
| Rectal ulcer | 0 (0%) | 1 (<1%) |
| Bowel obstruction | 1 (<1%) | 1 (<1%) |
Note: SOC+RT in safety population (RTOG scale; patients with RT started within 1 year of randomisation). There were no reported grade 5 late RT toxicity events.
RT, radiotherapy to the prostate; SOC, standard of care.
Over the entire reported follow-up period, at least 1 grade 3 to 5 AE was reported for 458 (44%) of SOC and 451 (45%) SOC+RT patients. Areas of focus for this long-term analysis were endocrine disorders: 160/1,052 (15%) SOC versus 155/992 (16%) SOC+RT; musculoskeletal disorders: 112/1,052 (11%) SOC, 104/992 (10%) SOC+RT; blood and bone marrow disorders: 56/1,052 (5%) SOC, 49/992 (5%) SOC+RT; cardiovascular disorders: 46/1,052 (4%) SOC, 56/992 (6%) SOC+RT; renal disorders: 50/1,052 (5%) SOC, 52/992 (5%) SOC+RT; general disorders: 57/1,052 (5%) SOC, 43/992 (4%) SOC+RT; gastrointestinal disorders: 47/1,052 (4%) SOC, 52/992 (5%) SOC+RT; lab abnormalities: 49/1,052 (5%) SOC, 48/992 (5%) SOC+RT (Table I in S1 Text, S7 Fig). At 2 years, of 715 patients with data available, a grade 3 to 5 AE was reported for 52/320 (16%) SOC and 54/395 (14%) SOC+RT (Table J in S1 Text, S8 Fig). At 4 years, based on 358 patients, this was 12/133 (9%) SOC versus 29/225 (13%) SOC+RT (Table K in S1 Text, S9 Fig).
QoL
There was no evidence of a difference in QoL scores over time between the allocated treatment groups. Average Global QoL in the first 2 years after randomisation across all patients was 73.2% SOC and 72.4% SOC+RT; absolute difference −0.8% (95% CI −2.5% to 0.9%), p = 0.349 (partly conditional analysis) (Table 5, S3 Fig). When including patients who had died prior to an assessment as having a Global QoL score of 0% at that assessment, average Global QoL was 60.3% SOC versus 61.6% SOC+RT; absolute difference 1.3% (95% CI -1.1% to 3.8%), p = 0.287 (composite outcome analysis) (Table 5, S4 Fig).
Table 5. Summary of QoL analyses.
| Outcome measure | Analysis | Average over first 2 years on trial | Difference (95% CI) | |
|---|---|---|---|---|
| SOC | SOC+RT | |||
| Global QoL (%) | Partly conditional | 73.2% | 72.4% | −0.8% (−2.5% to 0.9%) |
| Composite outcome | 60.3% | 61.6% | 1.3% (−1.1% to 3.8%) | |
| Cross-sectional: 12 weeks | n/a | n/a | −2.9% (−4.8% to −1.0%) | |
| Cross-sectional: 24 weeks | n/a | n/a | −0.9% (−3.1% to 1.3%) | |
| Cross-sectional: 60 weeks | n/a | n/a | −1.4% (−4.1% to 1.3%) | |
| Cross-sectional: 104 weeks | n/a | n/a | 1.8% (−2.4% to 6.0%) | |
| QLQ-30 Summary Score (%) | Partly conditional | 85.4% | 84.2% | −1.2% (−2.4% to 0.0%) |
| Composite outcome | 70.6% | 71.7% | 1.2% (−1.3% to 3.6%) | |
| Cross-sectional: 12 weeks | n/a | n/a | −2.0% (−3.2% to −0.8%) | |
| Cross-sectional: 24 weeks | n/a | n/a | −1.0% (−2.3% to 0.4%) | |
| Cross-sectional: 60 weeks | n/a | n/a | −1.0% (−2.8% to 0.7%) | |
| Cross-sectional: 104 weeks | n/a | n/a | 0.9% (−1.8% to 3.6%) | |
Note: Partly conditional estimates are based on observed and multiply imputed data from patients alive at scheduled assessments within the first 2 years since randomisation. Composite outcome estimates are based on observed data and implied imputation of missing data from scheduled assessments when a patient was alive, and the assumption of a patient’s Global QoL/QLQ-30 Summary Score being 0% at all scheduled assessments after they have died. Cross-sectional analyses estimate the difference in average Global Qol/QLQ-30 Summary Score between SOC+RT and SOC treatment groups at the specified scheduled assessment, controlling for response at baseline, in complete cases only (i.e., in patients with outcome data provided at baseline and who have survived and for who outcome data is available at the specified scheduled assessment).
QoL, quality of life; RT, radiotherapy to the prostate; SOC, standard of care.
Average QLQ-30 Summary Score in the first 2 years across all patients was 85.4% SOC and 84.2% SOC+RT; absolute difference −1.2% (95% CI −2.4% to 0.0%), p = 0.050 (partly conditional analysis) (Table 5, S5 Fig). When assuming a value of 0% for assessments after a patient had died, average Summary Score was 70.6% SOC and 71.7% SOC+RT; absolute difference 1.2% (95% CI −1.3% to 3.6%), p = 0.365 (composite outcome analysis) (Table 5, S6 Fig).
Cross-sectional analyses of both Global QoL and QLQ-30 Summary Score indicated evidence of poorer QoL at week 12 after randomisation for patients allocated to SOC+RT—Global QoL absolute difference −2.9% (95% CI −4.8% to −1.0%, p = 0.003); Summary Score absolute difference −2.0% (95% CI −3.2% to −0.8%, p = 0.001)—but not at other assessments (Table 5).
Discussion
This final analysis has confirmed that prostate RT improves OS in men with newly diagnosed, low-burden metastatic prostate cancer, but not in men with high-burden disease. The magnitude of the survival benefit is substantial and clinically relevant, particularly given that prostate RT is a relatively cheap, widely accessible, and well-tolerated treatment.
These results of the final analysis confirm the findings from the initial analysis. The additional 2 years of follow-up, and the subsequent increase in the number of events for analysis, has reduced the CIs around the point estimate of the HR of the OS benefit for prostate RT. However, the point estimate itself has changed very little, improving from 0.68 to 0.64 for men in the low metastatic disease risk group. This result is consistent with that from the smaller HORRAD trial [10]. Our new data strongly support those guidelines already recommending the use of prostate RT in men with low-burden metastatic disease. We have not found any benefit for prostate RT in men with high-burden disease, either in OS or in preventing interventions for local disease progression.
We found no compelling evidence of a difference in efficacy or toxicity between the 2 RT dose-schedules tested. The weekly schedule of 36 Gy in 6 fractions over 6 weeks has an obvious practical advantage in terms of convenience and may be preferred for that reason. A daily schedule might be preferred if pelvic nodal RT were to be used in addition or if RT dose escalation was thought to be appropriate. Prostate RT did not have any long-term impact on QoL either in this trial, or in the HORRAD trial [11]. The risk of toxicity from prostate RT, although low, could be further reduced by the use of more contemporary intensity modulated techniques [12].
The criteria used in the trial to classify cases as low or high burden were taken from those used in the CHAARTED trial [5]. These criteria are based on the presence or absence of visceral disease on CT scan, together with the number and the location of bone metastases on bone scan. Patients with only lymph node metastases have low-burden disease, regardless of the extent of nodal disease. There is no good reason to think that these criteria are optimal for identifying those patients with metastatic disease who stand to benefit from prostate RT. The initial analysis of STAMPEDE suggested that the survival benefit from prostate RT gradually decreased in magnitude as the number of bone metastases visible on a baseline bone scan increased [13]. One could decide to identify patients suitable for prostate RT based solely on the number of bone metastases visible on baseline bone scan, regardless of location. A count-of-metastases approach would be simpler to use in the clinic than the CHAARTED definition and would likely increase the number of men considered suitable for prostate RT.
The trial has several strengths, including the randomised design, the large number of events for analysis, and recruitment from over 100 centres, which adds to the generalisability of the results. The main limitations of the study are the changes in clinical practice since the trial started, particularly with regard to imaging techniques and systemic treatment. The trial recruited between 2013 and 2016 and, while this has the benefit of long follow-up, it also means that newer imaging techniques, such as PSMA (Prostate-specific membrane antigen) PET and whole body MRI, were unavailable. It is important to note that low-burden disease in the trial was defined according to bone scan and CT scan. There is no agreed definition of metastatic disease burden based solely on PSMA PET or on whole body MRI. In patients without visceral disease but who have more than 4 bone metastases on PET or MRI, a bone scan may be required in addition, in order to determine suitability for prostate RT. If this is not practicable, and there remains uncertainty as to whether a patient has high- or low-burden disease, there is a strong argument for using prostate RT.
The systemic treatment of metastatic prostate cancer has changed since the trial recruited. Standard treatment for men with low-burden metastatic disease now includes one of the newer hormone agents (abiraterone or apalutamide or enzalutamide) in addition to ADT. The effect of these agents on the survival benefit of prostate RT is unknown. Similarly, the effect of prostate RT on the survival benefit of the newer hormonal agents is also unknown. Based on current evidence, it is reasonable to assume that both prostate RT and one of the newer hormonal agents should be considered SOC for low-burden metastatic disease, in addition to ADT. The PEACE-1 Trial is testing the use of prostate RT in men receiving ADT + abiraterone.
In summary, this final analysis confirms that prostate RT improves OS in men with low-burden, newly diagnosed, metastatic prostate cancer, indicating that it should be recommended as a SOC.
Supporting information
Adjusted HR = 1.00 (95% CI 0.90 to 1.13; p = 0.931). HR, hazard ratio; RT, radiotherapy to the prostate; SLE, symptomatic local event; SOC, standard of care.
(TIF)
Adjusted HR = 0.94 (95% CI 0.83 to 1.06; p = 0.286). HR, hazard ratio; LI, local intervention; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
Difference in weighted average: −0.8% (95% CI −2.5% to 0.9%; p = 0.349). QoL, quality of life; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
Difference in weighted average: 1.3% (95% CI −1.1% to 3.8%; p = 0.287). QoL, quality of life; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
Difference in weighted average: −1.2% (95% CI −2.4% to 0.0%; p = 0.050). RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
Difference in weighted average: 1.2% (95% CI −1.3% to 3.6%; p = 0.365). RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
FFS, failure-free survival; RT, radiotherapy to the prostate; SOC, standard of care.
(TIF)
ADT, androgen deprivation therapy; IQR, interquartile range; PSA, prostate specific antigen; RT, radiotherapy to the prostate; SOC, standard of care; WHO, World Health Organization. Table B in S1 Text. Eligibility status following participant audit. RT, radiotherapy to the prostate; SOC, standard of care. Table C in S1 Text. Sensitivity analyses on OS based on explicit eligibility. ITT, intention-to-treat; OS, overall survival; RT, radiotherapy to the prostate; SOC, standard of care. Table D in S1 Text. First local intervention event reported (patients with event reported). PCa, prostate cancer; RT, radiotherapy to the prostate; SOC, standard of care; TURP, transurethral resection of the prostate. Table E in S1 Text. Summary of analyses of time to local event outcomes. *Subdistribution HR for competing risks models. ^Cox model, adjusting for age, nodal involvement, WHO performance status, regular aspirin or NSAID use and planned SOC docetaxel at randomisation, stratified by randomisation time period. +Fine and Gray model with outcome excluding PCa death and death from any cause as competing risk. NSAID, nonsteroidal anti-inflammatory drug; PCa, prostate cancer; SOC, standard of care; WHO, World Health Organization. Table F in S1 Text. Grade 3 to 5 late RT toxicities reported over entire time on trial (RTOG). Note: Treatment arms correspond to safety population; patients with ≥1 Follow-Up CRF returned. RT, radiotherapy to the prostate; RTOG, Radiation Therapy Oncology Group; SOC, standard of care. Table G in S1 Text. Grade 3 to 5 late RT toxicities reported at 2 years (RTOG). Note: Treatment arms correspond to safety population; patients with ≥1 Follow-Up CRF returned and no reported progression at 2 years. RT, radiotherapy to the prostate; RTOG, Radiation Therapy Oncology Group; SOC, standard of care. Table H in S1 Text. Grade 3 to 5 late RT toxicities reported at 4 years (RTOG). Note: Treatment arms correspond to safety population; patients with ≥1 Follow-Up CRF returned and no reported progression at 4 years. RT, radiotherapy to the prostate; RTOG, Radiation Therapy Oncology Group; SOC, standard of care. Table I in S1 Text. Grade 3 to 5 AEs reported over entire time on trial, overall and for selected body systems (CTCAE). Note: Treatment arms correspond to safety population; patients with ≥1 Follow-Up/SAE CRF returned. AE, adverse event; RT, radiotherapy to the prostate; RTOG, Radiation Therapy Oncology Group; SOC, standard of care. Table J in S1 Text. Grade 3 to 5 AEs reported at 2 years, overall and for selected body systems (CTCAE). Note: Treatment arms correspond to safety population; patients with ≥1 Follow-Up/SAE CRF returned and no reported progression at 2 years. AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; RT, radiotherapy to the prostate; SOC, standard of care. Table K in S1 Text. Grade 3 to 5 AEs reported at 4 years, overall and for selected body systems (CTCAE). Note: Treatment arms correspond to safety population; patients with ≥1 Follow-Up/SAE CRF returned and no reported progression at 4 years. AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; RT, radiotherapy to the prostate; SOC, standard of care.
(DOCX)
(PDF)
CONSORT, Consolidated Standards of Reporting Trials.
(PDF)
(PDF)
Acknowledgments
Large-scale trials do not happen without huge collaborations. Thanks to all central and site staff who have made the STAMPEDE trial happen. See S4 Text for full list of investigators, oversight committees, and contributors. In particular, thanks to all the people who have chosen to participate in STAMPEDE and their families and friends who have supported them.
The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Investigators and collaborators
See Credit List included as S4 Text and on the STAMPEDE trial website: http://www.stampedetrial.org/media-section/presentation-repository/trial-recognition.
Abbreviations
- ADT
androgen deprivation therapy
- AE
adverse event
- CONSORT
Consolidated Standards of Reporting Trials
- CTCAE
Common Terminology Criteria for Adverse Events
- FPM
flexible parametric model
- GnRH
gonadotrophin-releasing hormone
- HR
hazard ratio
- IQR
interquartile range
- ITT
intention-to-treat
- LIFS
local intervention–free survival
- NSAID
nonsteroidal anti-inflammatory drug
- OS
overall survival
- PH
proportional hazard
- QoL
quality of life
- RMST
restricted mean event-free (“survival”) time
- RT
radiotherapy
- SLEFS
symptomatic local event-free survival
- SOC
standard of care
- WHO
World Health Organization
Data Availability
Data will be available to successful applications for clearly specified research projects following the MRC CTU at UCL standard data sharing processes: https://www.mrcctu.ucl.ac.uk/our-research/other-research-policy/data-sharing/ Discussion with the trial team is encouraged to determine whether the relevant data to support the application are available. Email to: mrcctu.datareleaserequest@ucl.ac.uk.
Funding Statement
Research support for this comparison and other comparisons in the STAMPEDE protocol was awarded by Cancer Research UK (CRUK_A12459) www.cancerresearchuk.org (for this comparison, co-authors CCP, DPD, MDM, MKBP, MR, MRS, NDJ; and additionally for other comparisons DG, DM, GA, REL, RM, WC); Medical Research Council (MRC_MC_UU_12023/25, MC_UU_00004/01 and MC_UU_00004/02) www.ukri.org/councils/mrc (to authors MKBP, MRS, REL); and Swiss Group for Clinical Cancer Research, www.sakk.ch (to co-author SG). Other research support for the STAMPEDE protocol was awarded by Astellas www.astellas.com, Clovis Oncology www.clovisoncology.com, Janssen www.janssen.com, Novartis www.novartis.com, Pfizer www.pfizer.com, Sanofi-Aventis www.sanofi.com. CCP, DPD and NDJ are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parker C, Castro E, Fizazi K, Heidenreich A, Ost P, Procopio G, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–34. Epub 2020/07/01. doi: 10.1016/j.annonc.2020.06.011 . [DOI] [PubMed] [Google Scholar]
- 2.Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–66. Epub 2018/10/26. doi: 10.1016/S0140-6736(18)32486-3 ; PubMed Central PMCID: PMC6269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker CC, Sydes MR, Mason MD, Clarke NW, Aebersold D, de Bono JS, et al. Prostate radiotherapy for men with metastatic disease: a new comparison in the STAMPEDE trial. Clin Oncol. 2013;25(5):318–20. Epub 2013/03/16. doi: 10.1016/j.clon.2013.01.005 . [DOI] [PubMed] [Google Scholar]
- 4.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31 (5):1341–6. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 5.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373 (8):737–46. doi: 10.1056/NEJMoa1503747 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royston P, Parmar MKB. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med. 2011;30:2409, Epub 2421–21. doi: 10.1002/sim.4274 [DOI] [PubMed] [Google Scholar]
- 7.Morris TP, Jarvis CI, Cragg W, Phillips PPJ, Choodari-Oskooei B, Sydes MR. Proposals on Kaplan-Meier plots in medical research and a survey of stakeholder views: KMunicate. BMJ Open. 2019;9(9):e030215. Epub 2019/10/03. doi: 10.1136/bmjopen-2019-030215 ; PubMed Central PMCID: PMC6773317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray R. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16 (3):1141–54. [Google Scholar]
- 9.Rush HL, Murphy L, Morgans AK, Clarke NW, Cook AD, Attard G, et al. Quality of life for men with prostate cancer contemporaneously randomly allocated to receive either docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol. 2021. doi: 10.1200/JCO.21.00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeve LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410–8. Epub 2018/09/30. doi: 10.1016/j.eururo.2018.09.008 . [DOI] [PubMed] [Google Scholar]
- 11.Boeve L, Hulshof M, Verhagen P, Twisk JWR, Witjes WPJ, de Vries P, et al. Patient-reported Quality of Life in Patients with Primary Metastatic Prostate Cancer Treated with Androgen Deprivation Therapy with and Without Concurrent Radiation Therapy to the Prostate in a Prospective Randomised Clinical Trial; Data from the HORRAD Trial. Eur Urol. 2021;79(2):188–97. Epub 20200922. doi: 10.1016/j.eururo.2020.08.023 . [DOI] [PubMed] [Google Scholar]
- 12.Wilkins A, Mossop H, Syndikus I, Khoo V, Bloomfield D, Parker C, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605–16. Epub 2015/11/03. doi: 10.1016/S1470-2045(15)00280-6 ; PubMed Central PMCID: PMC4664817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali A, Hoyle A, Haran AM, Brawley CD, Cook A, Amos C, et al. Association of Bone Metastatic Burden With Survival Benefit From Prostate Radiotherapy in Patients With Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2021;7(4):555–63. Epub 2021/02/19. doi: 10.1001/jamaoncol.2020.7857 ; PubMed Central PMCID: PMC7893550. [DOI] [PMC free article] [PubMed] [Google Scholar]