Abstract
PURPOSE:
Aging associated with progressive declines in physical function is well-known; however, it is unclear how breast cancer diagnosis affects the trajectories of physical function over a long period of time. The current study examined the trajectories in objective measures of physical function over 20 years for women with breast cancer and matched controls.
METHODS:
8,687 community-dwelling women aged 65 years or older enrolled in the Study of Osteoporotic Fractures between 1986 and 1988 were followed for 20 years. Objective physical function was assessed up to 9 times, including gait speed and handgrip strength. Cognitive impairment states was defined by the Modified Mini-Mental State Examination.
RESULTS:
We observed all measures of physical function declined over time. While no differences in trends between cases and controls during the pre-diagnosis period were observed, after cancer diagnosis, grip strength and gait speed declined significantly faster in cases than controls. Quadriceps strength significantly decreased ~7 pounds shortly after breast cancer diagnosis, and then improved over time.
CONCLUSION:
Our study revealed that older breast cancer survivors relative to older women without cancer had significantly worse declines in grip strength and gait speed. Breast cancer survivors also had a sharp, short-term drop followed by gradual improvement over time in quadriceps strength. These findings suggest exercise training targeting muscle strength and mobility would be beneficial among older breast cancer survivors.
Keywords: Breast cancer, cancer survivors, physical function trajectories, physical function decline
Background
Breast cancer is the most common cancer among women in the US and worldwide [1]. With advances in early detection and treatment, the 5-year survival rate for all stages combined has reached more than 90% in the United States (US) [2]. As a result, there are more than 3.8 million breast cancer survivors in the US with further increases projected due to an aging population and higher risk of breast cancer with an increasing age. Despite improving survival, a growing body of evidence suggests that breast cancer survivors are uniquely confronted with an array of physical and psychosocial health problems after cancer treatment [3–8].
Several studies reported that women diagnosed with breast cancer experience a significant loss in physical function beyond the age-related decline observed in women without cancer diagnosis [9–12]. Other studies have indicated that the negative effects of breast cancer appeared to decrease with time [13–15]. However, the assessment of physical function in these studies employed self-report, known to be subject to bias. Alternatively, objectively measured physical function may include more accurate indicators of physical function better suited to reflect changes in function before problems are reported by an individual. Furthermore, objective measures of physical function can assess different components of status including gait speed and grip strength. To our knowledge, few prospective studies have examined objectively measured physical function changes among older breast cancer survivors [16, 17].
Herein, secondary analyses were performed on the Study of Osteoporotic Fractures, a longitudinal investigation with more than 20 years of follow-up and repeated measures of objectively measured physical function to examine whether breast cancer occurrence contributes to a decline in physical function. Cancer-free women were also included to account for the attendant effects of usual aging. Given that accelerated aging may be attributed to the direct and indirect effects of cancer treatment, we hypothesized that the trajectory of age-related physical function declines would be greater among breast cancer survivors compared with women without cancer [18, 19]. Understanding the trajectory of breast cancer on objectively measured physical function can inform supportive interventions to improve specific components of health status among the growing population of older breast cancer survivors.
Methods
Study of Osteoporotic Fractures
The Study of Osteoporotic Fractures (SOF) is a prospective study of risk factors for fractures and other health outcomes among community-dwelling older women. Details of the SOF cohort and study procedures have been published elsewhere [20]. Briefly, 9704 community-dwelling, ambulatory white women aged 65 years or older were recruited between 1986 and 1988 from population-based lists in Baltimore, Minneapolis, Pittsburgh and Portland. Those unable to walk without assistance or with bilateral hip replacements were excluded. Examination, interviews and questionnaires were performed at enrollment and at follow-up visits approximately every 2 years over 20 years of follow-up with a total of 9 measures. At each site, the institutional review boards approved the study, and all participants provided written informed consent.
Study population
For the purposes of our analysis, we considered all 9704 women who were initially recruited between 1986 and 1988 to ensure enough repeated measures. We excluded 31 women who had a non-released high value for age>90 at baseline, 724 women who were lost to follow-up, 281 women who ever had a stroke or prevalent cancer at baseline, and 158 women with missing covariates. Of the remaining 8510 aged 65–90 at baseline, 452 women were diagnosed with an incident, invasive breast cancer during follow-up, confirmed through physician review of medical records. Each breast cancer case was matched based on age at baseline and person-time of follow-up with five controls who were free of cancer during the entire follow-up. Controls must have been followed at least as long as the case to be considered as a match [21]. A total of 2712 nested case-control individuals (452 breast cancer cases and 2260 controls) were included for further analysis. For each control, we assigned an index time as the date of breast cancer event for her matched case.
Objectively measured physical function
The SOF measured both upper and lower extremity function. Handgrip performance has been used as an objective measurement of upper limb performance since it has been recognized as a simple, accurate, and economical screening tool for the measurement of upper body strength. Handgrip strength has been associated with health-related quality of life, disability, morbidity, and mortality among older people [22, 23]. The lower extremity physical performance measurements were based on the Short Physical Performance Battery, comprising gait speed and ability to rise from a chair [24, 25]. Since quadriceps strength is essential for physical performance and quadriceps muscle weakness is an important risk factors in the development of knee osteoarthritis and disability in the elderly populations [26, 27], measurements on quadriceps strength have been added in the SOF starting with visit 2. Hand-grip strength, gait speed and timed chair stand were measured during every visit with 9 repeated assessments. Grip strength was measured in both hands at each clinical site visit using a hand-held isometric dynamometer (Sparks Instruments and Academics, Coralville, Iowa) and a standard protocol. Both the max and the average of both hands (in kilograms) were used in the analysis. Timed chair stand (in seconds) was measured as the time to rise from a 16-inch height chair and sit down as quickly as possible five times without the use of arms according to a standard protocol. Gait speed (in meters per second) and step length (in meters) were assessed by a timed 6-meter walk according to a standard protocol. Participants were asked to walk at their usual pace and at their rapid pace, and the time and number of steps for both of their usual pace and their rapid pace were recorded. Quadriceps strength was measured at visit 2,3,4, 6 and 7, measured in pounds as the peak and the average force of the right and left limbs (Body Masters; Lafayette Instruments, Lafayette, Ind).
All repeated measurements on physical function were used. For participants who were diagnosed with breast cancer during follow-up, measures preceding the cancer diagnosis were defined as pre-diagnosis measures, and measures following cancer diagnosis were defined as post-diagnosis measures. For the matched controls, we defined the pre- and post- diagnosis measures in the same way as their corresponding cases at their index date corresponding to cases.
In addition, we also identified pre and post-diagnosis measures that were closest to the breast cancer diagnosis date for each individual to assess short-term change associated with cancer diagnosis.
Covariates
Covariates included age (in continuous years), education level (<12 years, 12 years, 13–16 years, 17 years or more), physical activity (measured in total calories per week expended in past year), alcohol consumption (measured in total number drinks over lifetime), smoking (measured in pack-years: never smoke, <10, 1–−<30, 30–<50, >=50), history of hypertension (yes, no) and diabetes (yes, no). Age was assessed at cancer diagnosis. Other covariates were self-reported at baseline. Time-varying body mass index was measured by weight in kilograms divided by the square of height in meters. Waist circumference was measured in centimeters at baseline.
Statistical Analysis
First, t tests and chi-square tests were used to test differences of baseline characteristics between participants with and without breast cancer. Further we compared the average number of repeated measurements by breast cancer status overall and by pre- and post-diagnosis period.
Second, the difference-in-difference method (DID) [28] was used to assess the short-term impact of breast cancer diagnosis on physical function measures. Pre- and post- physical function assessments that were closest to the cancer diagnosis for women with breast cancer and the comparison group were analyzed using linear mixed models, with period (pre- or post-diagnosis), group (breast cancer or non-cancer controls) and their interaction term as fixed factors and individuals as random factors in the model. The mixed effects regression analysis takes account of the within-person correlation among the repeated measurements of our outcomes. The interaction term in the model was used to address the main question of whether the two groups (cases and controls) changed the same amount and in the same direction from pre to post-diagnosis of breast cancer.
Third, generalized DID models [28] were used to assess the long-term impact of breast cancer diagnosis on physical function measures. All physical function measures were used in the model. In the models, a Time variable (measured in years since the breast cancer diagnosis, the index date), and Time interacting with other variables (pre-post diagnosis period, group and period*group) were added to the model to assess the secular time trends of physical function; whether the time trends differed between the two groups; and differed before and after breast cancer diagnosis using a formular as follows:
Where Yit – physical function; Time: years since breast cancer diagnosis (the index date); Group (cases or controls); Period (pre- or post- diagnosis period). β – coefficient.
Statistical tests were evaluated at p<.05 for main effects and p<.10 for interactions.
Results
Differences of basic characteristics between older breast cancer survivors (cases) and matched controls
Compared with controls, cases were slightly higher in body mass index and waist circumference, and more likely to have higher education level and be never smokers. There were no significant baseline differences between cases and controls for other characteristics including age, total calories per week expended in past year, total number of alcoholic drinks over lifetime, and history of hypertension or diabetes. There were no significant differences between cases and controls for physical function measured at baseline or visit 2. More than 93% of cases were in breast cancer stage I-III (Table 1).
Table 1.
Baseline characteristics between breast cancer cases and their matched cancer-free controls *
| variable label | Overall N=2712 | Controls (N=2260) | Cases (N=452) | P-value |
|---|---|---|---|---|
| Age at baseline | 70.5 ± 4.4 | 70.5 ± 4.4 | 70.5 ± 4.4 | 1.0000 |
| Body mass index (kg/m2) (mean, std) | 26.5 ± 4.5 | 26.4 ± 4.4 | 27.1 ± 4.9 | 0.001 |
| Waist circumference (cm) (mean, std) | 83.3 ± 11.0 | 83.1 ± 11.0 | 84.5 ± 11.3 | 0.02 |
| Education | 0.01 | |||
| <12 years | 619 (22.8%) | 524 (23.2%) | 95 (21.0%) | |
| 12 years | 1133 (41.8%) | 953 (42.2%) | 180 (39.8%) | |
| 13–16 years | 719 (26.5%) | 600 (26.5%) | 119 (26.3%) | |
| 17 or more | 241 (8.9%) | 183 (8.1%) | 58 (12.8%) | |
| Smoke packyear | 0.02 | |||
| Never smoker | 1620 (59.7%) | 1327 (58.7%) | 293 (64.8%) | |
| <10 | 330 (12.2%) | 285 (12.6%) | 45 (10.0%) | |
| 10–<30 | 351 (12.9%) | 302 (13.4%) | 49 (10.8%) | |
| 30–<50 | 251 (9.3%) | 203 (9.0%) | 48 (10.6%) | |
| ≥ 50 | 160 (5.9%) | 143 (6.3%) | 17 (3.8%) | |
| Total kcal/wk expended in past year | 1645 ± 1645 | 1628 ± 1644 | 1729 ± 1648 | 0.23 |
| Total # alcoholic drinks over lifetime | 5157 ± 12472 | 5151 ± 12967 | 5187 ± 9635 | 0.96 |
| Hypertension | 0.58 | |||
| 0 | 1751 (64.6%) | 1454 (64.3%) | 297 (65.7%) | |
| 1 | 961 (35.4%) | 806 (35.7%) | 155 (34.3%) | |
| Diabetes | 0.66 | |||
| 0 | 2550 (94.0%) | 2127 (94.1%) | 423 (93.6%) | |
| 1 | 162 (6.0%) | 133 (5.9%) | 29 (6.4%) | |
| Objectively measured physical function | ||||
| Grip strength (kilograms) | ||||
| Average grip strength | 21.3 ± 4.3 | 21.3 ± 4.4 | 21.5 ± 4.1 | 0.34 |
| Max grip strength | 22.6 ± 4.4 | 22.6 ± 4.4 | 22.8 ± 4.1 | 0.30 |
| Timed chair stand (seconds) | 12.1 ± 4.3 | 12.1 ± 4.4 | 12.0 ± 4.0 | 0.72 |
| Gait speed/stride length | ||||
| Regular gait speed (meters per second) | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.17 |
| Rapid gait speed (meters per second) ** | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 0.21 |
| Regular stride length (meters) | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.10 |
| Rapid stride length (meters) ** | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.81 |
| Quadriceps strength (pounds) ** | ||||
| Average quadriceps strength | 64.0 ± 27.0 | 63.7 ± 26.7 | 65.2 ± 28.1 | 0.33 |
| Peak quadriceps strength | 60.1 ± 25.8 | 59.9 ± 25.7 | 61.5 ± 26.5 | 0.27 |
| Cancer stage | ||||
| I | 290 (64.2%) | |||
| II | 56 (12.4%) | |||
| III | 59 (13.1%) | |||
| IV | 17 (3.8%) | |||
| Unknown | 30 (6.6%) |
Values expressed as n (%), mean ± standard deviation. P-value comparisons across breast categories are based on Chi-square test for categorical variables; T test for continuous variables.
These physical functions were not measured at baseline. They were based on measurements at visit 2.
The average number of repeated measurements (times) were very similar between controls and cases: 6.25 vs 6.75 overall, 4.18 vs 4.22 for pre-diagnosis period and 2.57 vs 2.85 for post-diagnosis period (Supplemental Table 1).
Short-term impact of breast cancer diagnosis on physical function measures
Table 2 shows the coefficients of interest from the analysis for pre-and post- diagnosis (two time points that were closest to breast cancer diagnosis) of physical function. The results indicate no significant difference for most of the pre-diagnosis measures between cases and controls (Group) except for relative better gait speed in cases than controls. Physical function significantly declined for all physical function measures comparing post-diagnosis period to pre- diagnosis period except for rapid stride length in controls (Period), and the declines were significantly worse among breast cancer cases for gait speed and quadriceps strength (Group*Period) (Table 2).
Table 2.
Coefficients of the association between breast cancer diagnosis and changes in physical function between pre- and post-diagnosis
| Group (cases vs controls) | Period (Post vs Pre) | Group*Period | |
|---|---|---|---|
| Objectively measured physical Function | |||
| Grip strength (kilograms) | |||
| Average grip strength | 0.14 | −1.43 *** | −0.18 |
| Max grip strength | 0.008 | −1.45 *** | 0.02 |
| Timed chair stand (seconds) | 0.002 | 0.77 *** | 0.35 |
| Gait speed/stride length | |||
| Regular gait speed (meters per second) | 0.03** | −0.06 *** | −0.03 * |
| Rapid gait speed (meters per second) | 0.04* | −0.07 *** | −0.05 * |
| Regular stride length (meters) | 0.008 | −0.02 *** | −0.01 |
| Rapid stride length (meters) | 0.01 | −0.007 | −0.02 |
| Quadriceps strength (pounds) | |||
| Average quadriceps strength | 2.43 | −8.43 *** | −3.18 * |
| Peak quadriceps strength | 2.29 | −8.44 *** | −3.25 * |
P value <0.05;
p value <0.01;
p value <0.0001.
All the models were adjusted for age at diagnosis (in continuous years), education level (<12 years, 12 years, 13–16 years, 17 years or more), physical activity (measured in total calories per week expended in past year), alcohol consumption (measured in total number drinks over lifetime), smoking (measured in pack-years: never smoke, <10, 10–−<30, 30–<50, >=50), history of hypertension (yes, no), diabetes (yes, no) and body mass index.
Long-term impact of breast cancer diagnosis on trajectories of physical function measures
Timed chair stand, gait speed, and stride length at regular pace
In the analysis fitting all the repeated measurements of physical function over 20 years of follow-up, we observed that all the physical function components declined significantly over time in both case and control groups. However, there were no significantly different patterns between cases and controls for timed chair stand, gait speed or stride length at regular pace.
Average grip strength and rapid gait speed
For average grip strength and rapid gait speed, there was no significant difference between cases and controls (Group) and time trend before cancer diagnosis (Group*Time). After cancer diagnosis, both grip strength and rapid gait speed among breast cancer cases exhibited a significantly greater decline compared to controls (Group*Period*Time) (Table 3). Figure 1 (a) and (b) illustrates the grip strength and rapid walking speed changes over time in cases and controls.
Table 3.
Coefficients of physical function trajectories in relation to breast cancer diagnosis *
| Average grip strength (kilograms) | Rapid gait speed (meters per second) | Average quadriceps strength (pounds) | ||||
|---|---|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | Estimate | P-value | |
| Group (cases vs controls) | 0.40 | 0.28 | 0.01 | 0.65 | 4.76 | 0.05 |
| Period (Post vs Pre) | −0.68 | <0.0001 | −0.02 | 0.04 | 0.08 | 0.95 |
| Group*Period | −0.31 | 0.38 | 0.01 | 0.73 | −6.90 | 0.04 |
| Time (years) | −0.39 | <0.0001 | −0.02 | <0.0001 | −1.62 | <0.0001 |
| Group*Time | 0.04 | 0.32 | 0.002 | 0.65 | 0.24 | 0.54 |
| Period* Time | 0.06 | 0.04 | −0.002 | 0.56 | −1.44 | <0.0001 |
| Group*Period*Time | −0.11 | 0.09 | −0.01 | 0.04 | 1.54 | 0.07 |
All the models were adjusted for age at diagnosis (in continuous years), education level (<12 years, 12 years, 13–16 years, 17 years or more), physical activity (measured in total calories per week expended in past year), alcohol consumption (measured in total number drinks over lifetime), smoking (measured in pack-years: never smoke, <10, 10–−<30, 30–<50, >=50), history of hypertension (yes, no), diabetes (yes, no) and body mass index.
Figure 1.
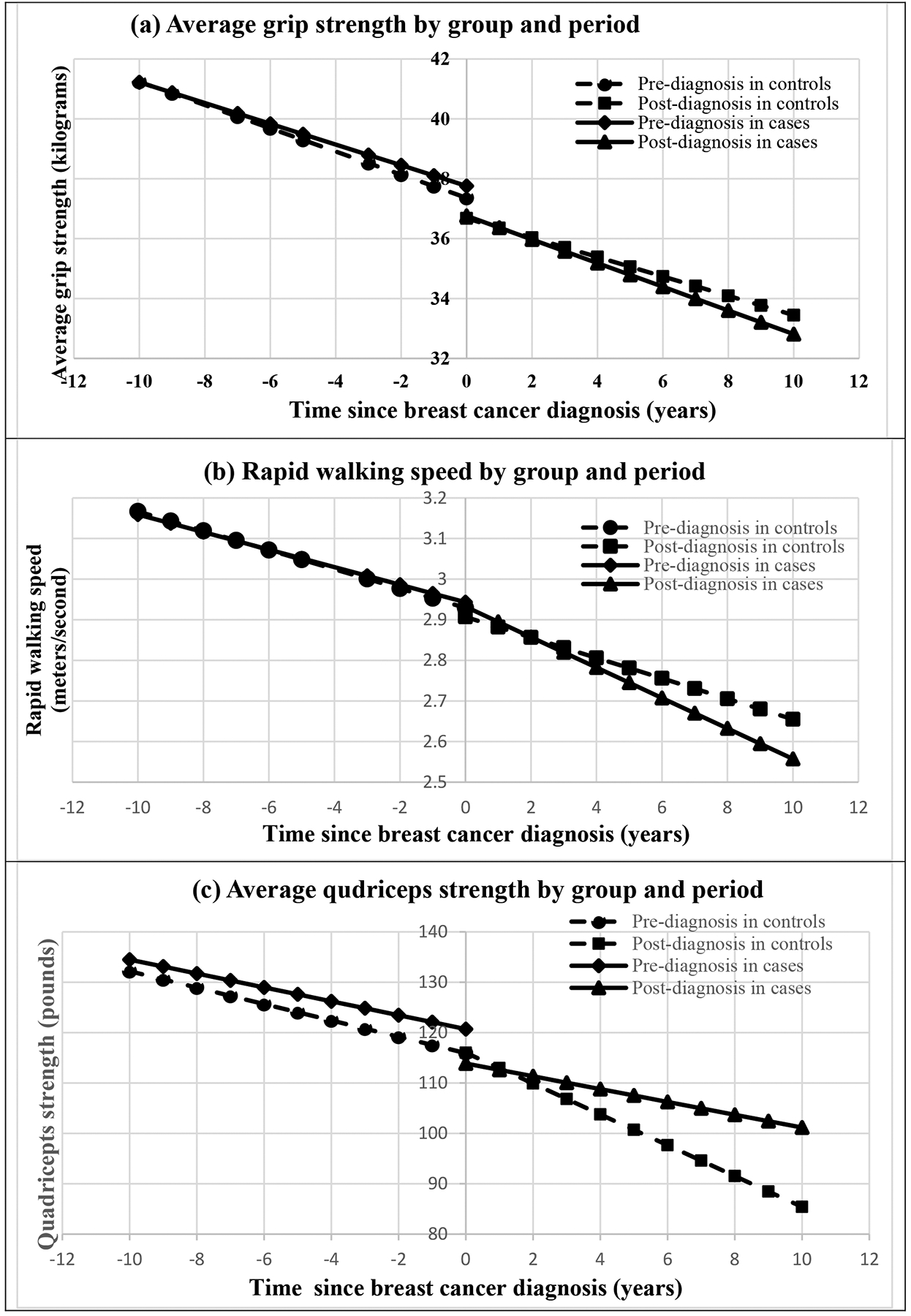
Physical function over time for cases and controls.
Average quadriceps strength
For average quadriceps strength, there was a slightly better initial measurement in cases than controls (Period) but similar time trends during pre-diagnosis period (Group*Time) between cases and controls. However, after cancer diagnosis, there was a significant strength drop shortly after breast cancer diagnosis in cases (around 7 pounds drop for Group*Period) and then the strength recovered over time (coefficient = 1.54 pounds per year for Group*Period*Time) (Table 3). In other words, there was a precipitous drop around cancer diagnosis in cases (referring to the drop when time since breast cancer diagnosis=0), and then the decline in cases was less steep compared to the controls after cancer diagnosis. The patterns for the quadricep strength trajectories by group and period are illustrated in Figure 1 (c). All the results remained similar after further adjusting for stage of breast cancer.
Discussion
Our study using objectively measured physical function with 20 years of follow-up data revealed that breast cancer survivors aged 65 and over at baseline demonstrated worse decline in physical function compared to women without cancer and such changes varied depending on the physical function component examined. As expected, physical function significantly declined over time across all components measured for both cases and controls during the pre-diagnosis period. After cancer diagnosis, there was a significantly greater decline for grip strength and rapid walking speed among breast cancer survivors relative to cancer-free controls. For quadriceps strength, there was a sharp, short-term loss after breast cancer diagnosis followed by a gradual improvement over time. We did not observe significant differences in the rate of change for other measures (including timed chair stand, grip speed at regular pace or stride length) between cases and controls.
When pre-/post- diagnosis measures from two time points closest to breast cancer diagnosis were analyzed, declines were significantly worse in breast cancer survivors than controls for gait speed and quadriceps strength. While not measured in this study, the short-term physical function declines between pre- and post-diagnosis may likely be due to the direct and indirect effects of cancer treatment. Indeed, such changes can precipitate sedentary physical activity patterns that can exacerbate mobility difficulties [29] and systemic deconditioning [30]. Moreover, breast cancer survivors are often treated with multiple therapies that are known to contribute to functional declines in older adults [31–34]. Minimal clinically important differences for these physical function measures are not well-established. However, the difference in quadriceps strength between cases and controls by the end of the follow-up period was more than 10 pounds, which probably exceeds a minimal clinically important difference based on a recent research in a different clinical population [35].
The short-term physical function decline we observed in older breast cancer survivors is consistent with findings from some studies [11, 12, 34, 36], while other studies reported non-significant differences in older breast cancer survivors compared with their cancer-free peers [9, 37–39]. However, all the previous studies were based on self-reported physical function and most did not include an appropriate cancer-free control group to represent ‘usual aging,’ such that it is hard to determine whether physical function changes are attributable to breast cancer and its treatments. This is especially problematic in studies of older women, given the high prevalence of aging symptoms.
Among several studies that included pre-/post-breast cancer measures and cancer-free women, Satariano et al. [39] found that at 3 months after diagnosis, cases were approximately twice as likely as age-matched controls to report upper-body limitation, especially for women younger than 75 years. At one year after diagnosis, younger breast cancer patients showed the greatest improvement [39]. Kroenke et al. [9] reported a similar finding in the Nurses’ Health Study: younger (<65 years) but not older (65 or older) breast cancer survivors experienced worse functional limitations compared with women without cancer. However, both studies were based on assessments conducted at two points in time without examining the long-term impacts of the disease on trajectories of functional aging. One longitudinal study based on 15 years of follow-up of self-reported physical function on both pre-/post-diagnosis measurements observed that breast cancer survivors had dramatic physical function declines one-year post-diagnosis and then began to return to levels of physical function similar to their pre- diagnosis status [40]. These findings are consistent with the long-term trends we observed for quadriceps strength.
Although self-reported physical function has been used widely to monitor treatment-related symptoms and physical function decrements in cancer survivors, it is subjective and may be prone to bias. Furthermore, it cannot easily quantify and distinguish various physical components and thus it is hard to evaluate which aspects of function may be most affected by the cancer diagnosis and therefore which components are targets for interventions. Using objective measurements of physical function can overcome these limitations. However, we only identified one small cross-sectional study that examined objective measures of physical function in breast cancer survivors [16], and one cohort study based on older adults with any cancer [17]. The small cross-section study with one-time measurement reported that breast cancer survivors had significantly lower short Physical Performance Battery score, longer chair stand times, and lower handgrip strength than controls, but similar walk speed [16]. The cohort study observed that a steeper decline in gait speed prior to a cancer diagnosis and an accelerated declines in appendicular lean mass after a cancer diagnosis, compared with cancer-free controls [17].
Our study observed a worse decline in hand grip strength and rapid walking speed but not regular pace of gait speed in breast cancer survivors. This suggests that the maximum gait speed (walk as fast as safely possible) may be more sensitive to disease-related function decline than regular pace. Gait speed is a well-known indicator of functional decline and predictor of mortality in older adults and is frequently used in geriatric settings as a quick and reliable way of monitoring the functional capacity of older adults including cognitive ability [41–43]. Gait speed depends on the function and coordination of the musculoskeletal, visual, central nervous, and peripheral nervous systems [43]. It reflects mobility and dynamic balance, and quadriceps strength reflects lower body muscle function. Lower-limb muscle strength is linked to walking speed. Gait speed should be considered not only as a motor function but an integrative measure of health. Our findings indicate that the impacts of breast cancer on physical function may include both upper and lower body muscle function and mobility and dynamic balance. Our findings suggest that interventions to build skeletal muscle strength may improve physical function thereby positively affecting mobility among breast cancer survivors. Studies have suggested that structured training programs for older adults including resistance exercise can increase muscle strength and physical functioning [44]. Our study also highlights a need for routine assessment of physical function including grip strength, gait speed and leg strength by primary care physicians to help monitor and prevent physical function decline. As most individuals complete the intensive phase of cancer treatment during the first year after diagnosis, interventions to help them return to their pre-cancer status more quickly could be beneficial.
Strengths of our study include use of age-matched cancer-free comparison groups, objective assessment of physical function and using multiple assessments of physical function over time to assess long term impact of breast cancer. Several limitations also deserve mention. First, we examined incident breast cancer during the follow-up; thus, the length of measurement of physical function between pre and post- diagnosis varied, which may affect assessment of physical function. Second, we were unable to adjust for information on cancer treatment. Furthermore, the SOF study was launched more than 30 years ago, and considerable changes concerning cancer treatment have taken place over time. Given that the tendency of major cancer treatment changes to be more conservative and to minimize or lessen side effects, we would expect that changes in physical function may be less likely to be due to cancer treatment today. Third, our sample includes only older, non-Hispanic white women and thus may not generalize to other populations. Fourth, we had fewer repeated quadriceps strength measurements than other measurements. Although there was no difference in the average number of repeated measurements for quadriceps strength pre- and post-diagnosis period between cases and controls, the fewer measures may introduce greater imprecision to the model, especially for the post-diagnosis period. Another limitation is there were no direct measures of cardiorespiratory fitness which is known to be linked to mobility and physical activity. In addition, although we have adjusted for tumor stage in our model, it is possible that some cancer stage related residual confounding may affect physical function.
In conclusion, our study using objectively measured physical function with 20 years of follow-up data revealed that older breast cancer survivors had significantly worse declines in grip strength and rapid walking speed and a sharp short-term drop followed by gradual improvement over time in quadriceps strength compared to women without cancer. Our findings suggest that targeted exercise training among older breast cancer survivors focused on developing body muscle strength and mobility would improve physical functioning and thereby improve the healthy life span of this growing population. Further work is needed to understand how cancer treatments may impact the trajectories of specific physical function components in this growing population.
Supplementary Material
Acknowledgement
We would like to acknowledge the contribution of the Study of Osteoporotic Fractures (SOF) Investigators and the National Institute on Aging (NIA) AgingResearchBiobank (https://agingresearchbiobank.nia.nih.gov/) where the SOF collection of biospecimens and data is maintained.
Funding
Dr. Luo is partially supported by the National Institutes of Health (R03CA256238). Dr. Cespedes is partially supported by K01CA226155; R01AG065334; R01CA240394; R01CA251589. Dr. Carter receives support from the National Cancer Institute (R01CA235598) and the Indiana Clinical and Translational Sciences Institute (UL1TR002529).
Footnotes
Conflicts of Interest
The authors declare no potential conflicts of interest.
Ethics approval
This is a secondary data analysis. In accordance with 45 CFR 46.101(b) and/or IU HRPP Policy, the study is granted exemption (category 4): Secondary research for which consent is not required
Availability of data and material
The data are available from the National Institute on Aging (NIA) AgingResearchBiobank upon reasonable request (https://agingresearchbiobank.nia.nih.gov/).
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.ASC (2020) Survival Rates for Breast Cancer (https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html). In:American Cancer Society (ASC) [Google Scholar]
- 3.Jordan JH, Thwin SS, Lash TL, Buist DS, Field TS, Haque R, Pawloski PA, Petersen HV, Prout MN, Quinn VP, Yood MU, Silliman RA, Geiger AM (2014) Incident comorbidities and all-cause mortality among 5-year survivors of Stage I and II breast cancer diagnosed at age 65 or older: a prospective-matched cohort study. Breast Cancer Res Treat 146:401–409. doi: 10.1007/s10549-014-3021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L, Weiner LS, Hartman SJ, Horvath S, Jeste D, Mischel PS, Kado DM (2019) Breast cancer treatment and its effects on aging. J Geriatr Oncol 10:346–355. doi: 10.1016/j.jgo.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnuson A, Lei L, Gilmore N, Kleckner AS, Lin FV, Ferguson R, Hurria A, Wittink MN, Esparaz BT, Giguere JK, Misleh J, Bautista J, Mohile SG, Janelsins MC (2019) Longitudinal Relationship Between Frailty and Cognition in Patients 50 Years and Older with Breast Cancer. J Am Geriatr Soc 67:928–936. doi: 10.1111/jgs.15934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahles TA, Root JC, Ryan EL (2012) Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 30:3675–3686. doi: 10.1200/JCO.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T (2013) A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev 39:297–304. doi: 10.1016/j.ctrv.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee YH, Hussin MG, Jacobsen PB, Small BJ (2012) Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 30:3578–3587. doi: 10.1200/JCO.2011.39.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD (2004) Functional impact of breast cancer by age at diagnosis. Journal of Clinical Oncology 22:1849–1856. doi: Doi 10.1200/Jco.2004.04.173 [DOI] [PubMed] [Google Scholar]
- 10.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA (2000) The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer 89:2176–2186. doi: [DOI] [PubMed] [Google Scholar]
- 11.Karlsen RV, Frederiksen K, Larsen MB, von Heymann-Horan AB, Appel CW, Christensen J, Tjonneland A, Ross L, Johansen C, Bidstrup PE (2016) The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among middle-aged to elderly women with and without breast cancer. Acta Oncol 55:720–727. doi: 10.3109/0284186X.2015.1127415 [DOI] [PubMed] [Google Scholar]
- 12.Michael YL, Wu C, Pan K, Seguin-Fowler RA, Garcia DO, Zaslavsky O, Chlebowski RT (2020) Postmenopausal Breast Cancer and Physical Function Change: A Difference-in-Differences Analysis. J Am Geriatr Soc 68:1029–1036. doi: 10.1111/jgs.16323 [DOI] [PubMed] [Google Scholar]
- 13.Hsu T, Ennis M, Hood N, Graham M, Goodwin PJ (2013) Quality of life in long-term breast cancer survivors. J Clin Oncol 31:3540–3548. doi: 10.1200/JCO.2012.48.1903 [DOI] [PubMed] [Google Scholar]
- 14.Peuckmann V, Ekholm O, Rasmussen NK, Moller S, Groenvold M, Christiansen P, Eriksen J, Sjogren P (2007) Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast Cancer Res Treat 104:39–46. doi: 10.1007/s10549-006-9386-6 [DOI] [PubMed] [Google Scholar]
- 15.Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, Pozet A, Guizard AV, Henry-Amar M, Velten M (2011) Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat 129:125–134. doi: 10.1007/s10549-011-1408-3 [DOI] [PubMed] [Google Scholar]
- 16.Winters-Stone KM, Medysky ME, Savin MA (2019) Patient-reported and objectively measured physical function in older breast cancer survivors and cancer-free controls. J Geriatr Oncol 10:311–316. doi: 10.1016/j.jgo.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams GR, Chen Y, Kenzik KM, McDonald A, Shachar SS, Klepin HD, Kritchevsky S, Bhatia S (2020) Assessment of Sarcopenia Measures, Survival, and Disability in Older Adults Before and After Diagnosis With Cancer. JAMA Netw Open 3:e204783. doi: 10.1001/jamanetworkopen.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guida JL, Ahles TA, Belsky D, Campisi J, Cohen HJ, DeGregori J, Fuldner R, Ferrucci L, Gallicchio L, Gavrilov L, Gavrilova N, Green PA, Jhappan C, Kohanski R, Krull K, Mandelblatt J, Ness KK, O’Mara A, Price N, Schrack J, Studenski S, Theou O, Tracy RP, Hurria A (2019) Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst 111:1245–1254. doi: 10.1093/jnci/djz136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Wang F, Shi L, Cai H, Zheng Y, Zheng W, Bao P, Shu XO (2020) Accelerated aging in breast cancer survivors and its association with mortality and cancer recurrence. Breast Cancer Res Treat 180:449–459. doi: 10.1007/s10549-020-05541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P, et al. (1990) Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 263:665–668. [PubMed] [Google Scholar]
- 21.Grandits G, Neuhaus J (2010) Using SAS® to Perform Individual Matching in Design of Case-Control Studies https://support.sas.com/resources/papers/proceedings10/061-2010.pdf. In: SAS Global Forum. [Google Scholar]
- 22.Jakobsen LH, Rask IK, Kondrup J (2010) Validation of handgrip strength and endurance as a measure of physical function and quality of life in healthy subjects and patients. Nutrition 26:542–550. doi: 10.1016/j.nut.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 23.Bohannon RW (2008) Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 31:3–10. doi: 10.1519/00139143-200831010-00002 [DOI] [PubMed] [Google Scholar]
- 24.Penninx BWJH, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM (2000) Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol a-Biol 55:M691–M697. doi: DOI 10.1093/gerona/55.11.M691 [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:M85–94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 26.Heidari B (2011) Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med 2:205–212. [PMC free article] [PubMed] [Google Scholar]
- 27.Ito Y, Aoki T, Sato T, Oishi K, Ishii K (2020) Comparison of quadriceps setting strength and knee extension strength tests to evaluate lower limb muscle strength based on health-related physical fitness values in elderly people. Bmj Open Sport Exerc 6. doi: ARTN e000753 10.1136/bmjsem-2020-000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warton EM (2020) Time after time: difference-in-differences and interrupted time series models in SAS. In: SAS Global Forum 2020. [Google Scholar]
- 29.Carter SJ, Hunter GR, Norian LA, Turan B, Rogers LQ (2018) Ease of walking associates with greater free-living physical activity and reduced depressive symptomology in breast cancer survivors: pilot randomized trial. Support Care Cancer 26:1675–1683. doi: 10.1007/s00520-017-4015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers LQ, Courneya KS, Carter SJ, Anton PM, Verhulst S, Vicari SK, Robbs RS, McAuley E (2016) Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res Treat 159:283–291. doi: 10.1007/s10549-016-3945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, Faithfull S (2017) Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. Journal of Clinical Oncology 35:2604-+. doi: 10.1200/Jco.2016.71.3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney C, Schmitz KH, Lazovich D, Virnig BA, Wallace RB, Folsom AR (2006) Functional limitations in elderly female cancer survivors. J Natl Cancer Inst 98:521–529. doi: 10.1093/jnci/djj130 [DOI] [PubMed] [Google Scholar]
- 33.Avis NE, Deimling GT (2008) Cancer survivorship and aging. Cancer 113:3519–3529. doi: 10.1002/cncr.23941 [DOI] [PubMed] [Google Scholar]
- 34.Hurria A, Soto-Perez-de-Celis E, Allred JB, Cohen HJ, Arsenyan A, Ballman K, Le-Rademacher J, Jatoi A, Filo J, Mandelblatt J, Lafky JM, Kimmick G, Klepin HD, Freedman RA, Burstein H, Gralow J, Wolff AC, Magrinat G, Barginear M, Muss H (2019) Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc 67:920–927. doi: 10.1111/jgs.15493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwakura M, Okura K, Kubota M, Sugawara K, Kawagoshi A, Takahashi H, Shioya T (2021) Estimation of minimal clinically important difference for quadriceps and inspiratory muscle strength in older outpatients with chronic obstructive pulmonary disease: a prospective cohort study. Phys Ther Res 24:35–42. doi: 10.1298/ptr.E10049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Euhus DM, Addae JK, Snyder CF, Canner JK (2019) Change in health-related quality of life in older women after diagnosis of a small breast cancer. Cancer 125:1807–1814. doi: 10.1002/cncr.31993 [DOI] [PubMed] [Google Scholar]
- 37.Cohen HJ, Lan L, Archer L, Kornblith AB (2012) Impact of age, comorbidity and symptoms on physical function in long-term breast cancer survivors (CALGB 70803). J Geriatr Oncol 3:82–89. doi: 10.1016/j.jgo.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazovich D, Robien K, Cutler G, Virnig B, Sweeney C (2009) Quality of Life in a Prospective Cohort of Elderly Women With and Without Cancer. Cancer 115:4283–4297. doi: 10.1002/cncr.24580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satariano WA, Ragland DR (1996) Upper-body strength and breast cancer: a comparison of the effects of age and disease. J Gerontol A Biol Sci Med Sci 51:M215–219. doi: 10.1093/gerona/51a.5.m215 [DOI] [PubMed] [Google Scholar]
- 40.Petrick JL, Reeve BB, Kucharska-Newton AM, Foraker RE, Platz EA, Stearns SC, Han XS, Windham BG, Irwin DE (2014) Functional status declines among cancer survivors: Trajectory and contributing factors. J Geriatr Oncol 5:359–367. doi: 10.1016/j.jgo.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peel NM, Kuys SS, Klein K (2013) Gait Speed as a Measure in Geriatric Assessment in Clinical Settings: A Systematic Review. J Gerontol a-Biol 68:39–46. doi: 10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 42.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J (2011) Gait speed and survival in older adults. JAMA 305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yates T, Zaccardi F, Dhalwani NN, Davies MJ, Bakrania K, Celis-Morales CA, Gill JMR, Franks PW, Khunti K (2017) Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur Heart J 38:3232–3240. doi: 10.1093/eurheartj/ehx449 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the National Institute on Aging (NIA) AgingResearchBiobank upon reasonable request (https://agingresearchbiobank.nia.nih.gov/).


