Abstract
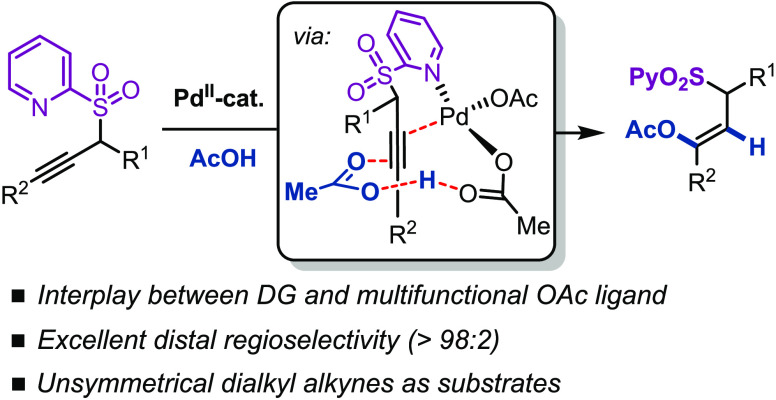
The cooperative action of the acetate ligand, the 2-pyridyl sulfonyl (SO2Py) directing group on the alkyne substrate, and the palladium catalyst has been shown to be crucial for controlling reactivity, regioselectivity, and stereoselectivity in the acetoxylation of unsymmetrical internal alkynes under mild reaction conditions. The corresponding alkenyl acetates were obtained in good yields with complete levels of β-regioselectivity and anti-acetoxypalladation stereocontrol. Experimental and computational analyses provide insight into the reasons behind this delicate interplay between the ligand, directing group, and the metal in the reaction mechanism. In fact, these studies unveil the multiple important roles of the acetate ligand in the coordination sphere at the Pd center: (i) it brings the acetic acid reagent into close proximity to the metal to allow the simultaneous activation of the alkyne and the acetic acid, (ii) it serves as an inner-sphere base while enhancing the nucleophilicity of the acid, and (iii) it acts as an intramolecular acid to facilitate protodemetalation and regeneration of the catalyst. Further insight into the origin of the observed regiocontrol is provided by the mapping of potential energy profiles and distortion–interaction analysis.
Keywords: multifunctional ligand, ligand-assisted proton shuttle, acetoxylation of alkynes, unsymmetrical dialkyl alkynes, metal−ligand cooperativity, directing group
Introduction
Catalytic reactions controlled by the action of ligands playing active roles besides stabilizing and tuning the metal, commonly known as multifunctional ligands, are capturing increasing attention in synthetic chemistry.1 The ability of carboxylate ancillary ligands to switch denticity, thereby facilitating reactions alongside the metal, has been widely exploited in some catalytic transformations.2 For example, acetate ligands have enabled mechanisms for C–H activation alternative to direct oxidative addition by acting as an inner-sphere base to assist deprotonation of the C–H bond concomitant with M–C bond formation, which is referred to as concerted metalation–deprotonation (CMD) or amphiphilic metal–ligand activation (AMLA).3 Another general class of related mechanism refers to proton transfer between two different ligands without involving any metal hydride known as ligand-to-ligand hydrogen transfer (LLHT).4 However, in this type of reaction, most often the protonated ligand subsequently leaves the metal to afford the product. Alternatively, it could remain bonded to the metal and still has active participation as an intramolecular acid at other stages of the catalytic cycle, a mechanistic variation termed as a ligand-assisted proton shuttle (LAPS).5 This strategy is neither restricted to carboxylate ligands nor to C–H activation. Capitalizing on this concept, the application of metal–ligand interaction in the catalytic functionalization of alkynes would be translated into more efficient and selective transformations (Scheme 1a). Recently, Zhang and co-workers have described a gold-catalyzed anti-carboxylation of alkynes using an amide-containing phosphine ligand, which directs the attack of the incoming carboxylic acid via hydrogen bonding.6 This ligand–reagent interaction not only makes the addition reaction pseudointramolecular in nature but also enhances the nucleophilicity of the acid (Scheme 1b). In addition, the protonated amide that remains at the coordination sphere of the metal subsequently serves as an intramolecular proton source for protodeauration, thereby accelerating the catalyst turnover.7 Despite some emerging examples, reactions that occur via a LAPS mechanism remain rare.8
Scheme 1. Catalytic anti-Hydro-oxycarbonylation of Alkynes via Metal/Ligand Cooperativity.
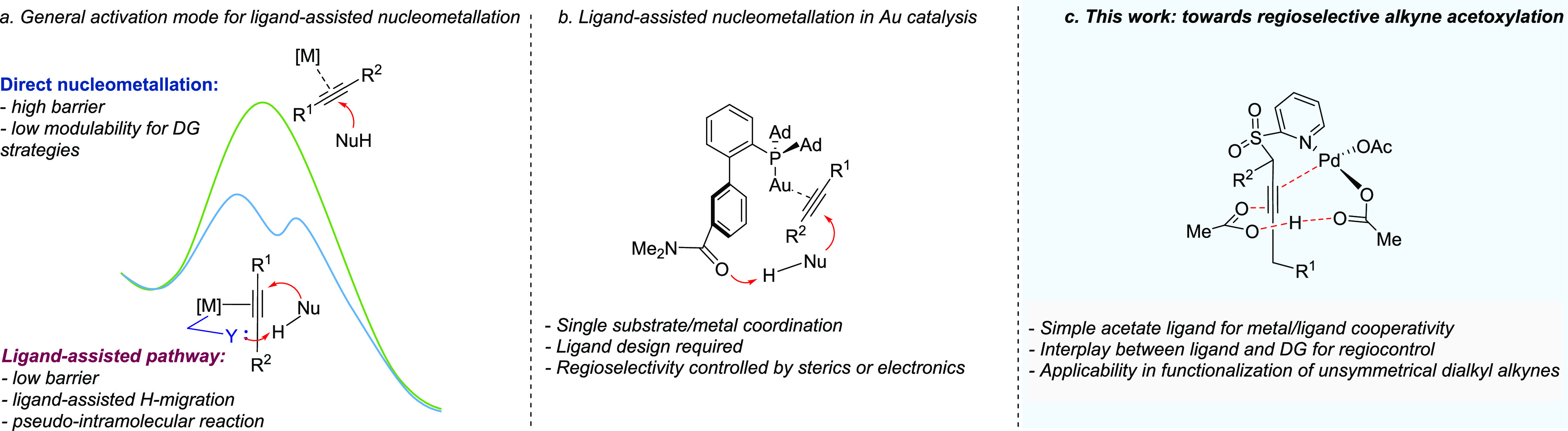
Despite the wealth of reactivity that has been established in the catalytic functionalization of alkynes toward the construction of stereochemically defined olefins,9−11 unsymmetrical dialkyl-substituted alkynes are noticeably absent from most contributions and, when present, typically provide unsatisfactory levels of reactivity and/or regioselectivity.12 In an effort to enhance the involvement of this class of alkynes, our group has reported an indirect solution that relies on the use of a propargylic SO2Py directing group for achieving site-selectivity control.13 In our previously reported Pd-catalyzed hydroarylation reaction, it was found that monodentate phosphine ligands significantly outperformed their bidentate analogues because they allow the alkyne substrate to accommodate through bidentate coordination in the ligand sphere of the metal, facilitating the regioselective insertion of the Pd–Ar σ-bond.13c Guided by this knowledge, we envisaged that Pd-catalyzed acetoxylation could benefit from the readiness of carboxylate ligands to switch their denticity, thus generating a coordination position on the metal center for substrate activation. This transformation, also known as hydro-oxycarbonylation, is typically proposed to occur via an anti-carboxymetalation pathway in which the carboxylic acid attacks the C–C triple bond coordinated to an alkynophilic metal.14−18 To the best of our knowledge, only two single isolated examples of fully regioselective carboxylation of unsymmetrical dialkyl alkynes have been disclosed.19 Recently, Li has reported the use of the 2-pyridyloxy moiety as a directing group under Ru catalysis, but the method is limited to aryl-containing alkynes.19b On the other hand, examples of palladium catalysis in carboxylation of internal alkynes16 are scarce compared to ruthenium14 or gold catalysis,17 and the mechanism of the reaction has not been investigated in detail before. This paper describes the viability, as well as a combined experimental and computational mechanistic study, of the SO2Py-directed regiocontrolled anti-acetoxylation of unsymmetrical dialkyl alkynes. This study has revealed multiple roles of the acetate ligand throughout the catalytic cycle. These include creating an acetic acid-binding pocket close to the metal center to favor regioselective addition, enhancing the nucleophilicity of the acid, and serving as a proton shuttle to facilitate protodemetalation (Scheme 1c).
Results and Discussion
Exploration of the Viability of anti-Acetoxylation
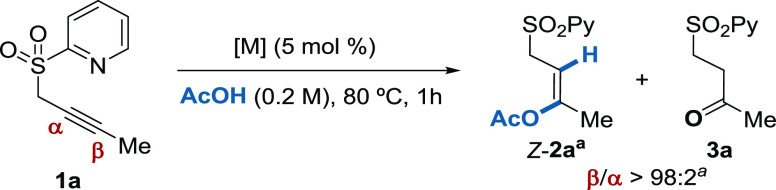
Based on previous studies for the anti-acetoxylation of alkynes,20 we initially tested model substrate 1a under a different survey of metal catalysts using AcOH both as a solvent and reagent (Table 1). To our delight, we observed that Pd(OAc)2 delivered the corresponding acetoxylated product Z-2a in 86% isolated yield, with complete β-regio- and anti-stereoselectivities after 1 h at 80 °C (entry 1). Other precious metals with a high affinity toward alkyne activation such as Pt(CH3CN)2Cl2 and Au(PPh3)Cl/AgOTf delivered the product Z-2a along with considerable amounts of the byproduct 3a (entries 2 and 3, respectively). When AgSbF6 was used as a catalyst, complete decomposition was observed (entry 4), while other metal salts such as Zn(ClO4)2 did not promote any reaction (entry 5). Having identified Pd(OAc)2 as a suitable catalyst, we explored lower loadings but the reduced yield of Z-2a was observed when using 3 mol % catalyst (41% yield, entry 6). A slight reduction in temperature to 60 °C afforded the product Z-2a in synthetically useful 76% yield, but longer reaction times were necessary to achieve full conversion (5 h, entry 7). Other palladium(II) sources lacking any acetate ligand such as PdBr2, Pd(acac)2, Pd(TFA)2, and Pd(CH3CN)2Cl2 provided the product in very low yield or completely suppressed the reaction, resulting in decomposition of the starting material (entries 8–11), which points toward an important role of the acetate ligand. In addition, no product was detected if palladium was omitted in the reaction (entry 12).
Table 1. Optimization Studies for the Acetoxylation of Substrate 1a.
| entry | catalyst | Z-2a/3aa | yield (%)b |
|---|---|---|---|
| 1 | Pd(OAc)2 | >98:2 | 86 |
| 2 | Pt(CH3CN)2Cl2 | 44:56 | 72 |
| 3 | AuCl(PPh3)/AgOTf | 62:38 | 66 |
| 4 | AgSbF6 | decomp | |
| 5 | Zn(ClO4)2·6H2O | nr | |
| 6c | Pd(OAc)2 | >98:2 | 41 |
| 7d | Pd(OAc)2 | >98:2 | 76 |
| 8 | PdBr2 | nr | |
| 9 | Pd(acac)2 | >98:2 | 10 |
| 10 | Pd(TFA)2 | nr | |
| 11 | PdCl2(CH3CN)2 | decomp | |
| 12 | none | nr | |
| 13 | Pd/Aue | 94:6 | 79 |
| 14 | Pd(OAc)2,H2Of | 86:14 | 81 |
| 15 | Pd/Aue,H2Of | 52:48 | 85 |
Determined by 1H NMR spectroscopy from the crude mixture.
Determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
3 mol % Pd(OAc)2 was used.
Reaction performed at 60 °C during 5 h for full completion.
A combination of Pd(OAc)2 (5 mol %) and AuCl(PPh3)/AgOTf (5 mol %) was used as a catalyst.
Extra water (10% v/v) was added to the reaction mixture. nr: no reaction (starting material recovered).
Formation of ketone 3a led us to question whether it arises from enol acetate deprotection or from a competing attack of water onto the corresponding π-complex intermediate. To gain an insight into this aspect, we performed the reaction of 1a catalyzed by AuCl(PPh3)/AgOTf but in the presence of Pd(OAc)2 as a cocatalyst (5 mol %). Interestingly, this change led to a dramatic increase in selectivity toward the acetoxylation product 2a from 2a/3a = 62:38 to 94:6 (Table 1, entry 13). This result provides compelling evidence that ketone 3a arises from the attack of water present in AcOH onto the π-complex intermediate, followed by protodemetalation and also suggests that the acetate ligand on Pd(OAc)2 may play an important role in controlling chemoselectivity. Even when extra 10% v/v water was added to the reaction mixture under Pd(OAc)2 catalysis, the acetoxylation product was formed with good selectivity (2a/3a = 86:14, entry 14). The addition of extra water (10% v/v) in the presence of a combination of gold and palladium catalysis under otherwise identical conditions revealed significant loss of selectivity, likely due to competing Au-catalyzed hydration (2a/3a = 52:48, entry 15).
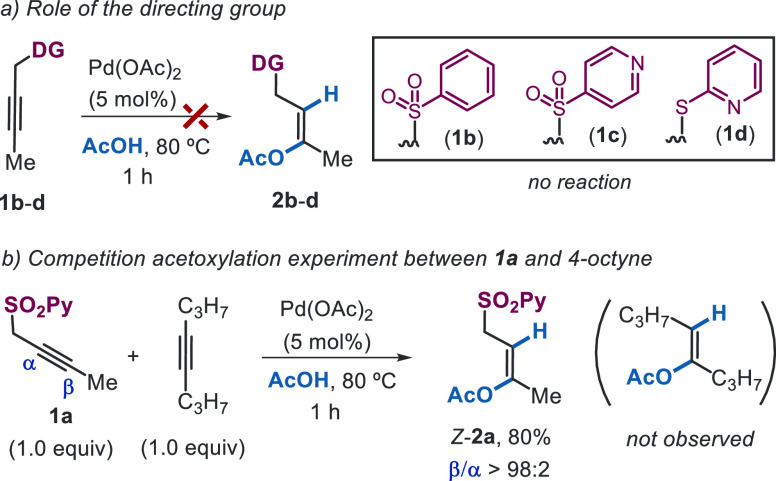
Then, to test the role of the SO2Py directing group in controlling reactivity and selectivity, substrates bearing at the propargylic position related groups with different electronic and coordinating properties were screened under the optimized conditions (Scheme 2a). The SO2Ph analogue 1b proved to be unreactive, being fully recovered after 1 h, and an identical result was found for alkyne 1c, an electronically similar isomer of 1a carrying the 4-pyridyl sulfonyl group. These results highlight the essential role of the coordinating 2-pyridyl unit for ensuring not only regiocontrol but also reactivity. Interestingly, the lack of reactivity showed by the 2-pyridyl sulfonyl alkyne 1d emphasizes the cooperative directing role of both the sulfonyl-tethering group and the 2-pyridyl moiety, presumably by weakening its metal-coordinating ability to facilitate catalytic turnover.
Scheme 2. Importance of the Directing Group and Competition Experiment.
The strong reliance of alkyne acetoxylation on the directing effect of the SO2Py moiety could be exploited to achieve chemoselective functionalization of alkynes. This hypothesis was validated in an intermolecular competition reaction between 1a and 4-octyne under the standard reaction conditions, which revealed that only 1a underwent smooth acetoxylation, providing 2a in 80% yield and complete β-regioselectivity (Scheme 2b), with no acetoxylation of 4-octyne being detected. This result points toward an essential role of the SO2Py group in promoting acetoxylation.
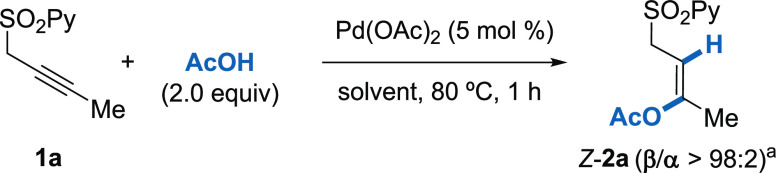
With suitable conditions for the acetoxylation of substrate 1a in our hands, we next questioned the need for using AcOH as a solvent by examining the effect of solvent in the reaction of 1a in the presence of 2 equiv of AcOH (Table 2). In comparison with the use of AcOH (entry 1), a dramatic decrease in reactivity was observed under nonpolar solvents such as toluene (7%, entry 2). Slightly better reactivity was observed when switching to more polar solvents such as 4-CF3-C6H5 or 1,2-dichloroethane, albeit the yield was not synthetically useful (21%, entries 3 and 4). Interestingly, highly polar solvents such as dimethylformamide (DMF) or iPrOH that could act as a stabilizing ligand occupying open coordination sites on the metal proved to be detrimental to reactivity, completely inhibiting the product formation (entries 5 and 6). In this case, we hypothesized that the catalytically active Pd(OAc)2 species could interact with the solvent through hydrogen bonding, thus precluding the activation of the incoming carboxylic acid during the nucleometalation of the alkyne.21 The same negative result was observed when 1,4-dioxane was used as a solvent (entry 7). However, when 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) was employed, the product Z-2a was obtained in 46% yield (entry 8).22 Using these conditions, the isolated yield of Z-2a could be further improved to 61% upon increasing the reaction time to 18 h (entry 9). These results strongly suggest that a drastic depletion of the reactivity was observed when using a solvent different from AcOH, with the exception of HFIP, thus proving the challenging nature of this transformation.
Table 2. Solvent Effect in the Acetoxylation of 1a.
| entry | solvent | yield (%)a |
|---|---|---|
| 1 | AcOH | 86 |
| 2 | toluene | 7 |
| 3 | 4-CF3-C6H5 | 21 |
| 4 | DCE | 21 |
| 5 | DMF | |
| 6 | iPrOH | |
| 7 | 1,4-dioxane | |
| 8 | HFIP | 46 |
| 9b | HFIP | 61 |
Determined by 1H NMR spectroscopy in the reaction crude.
Reaction run for 18 h.
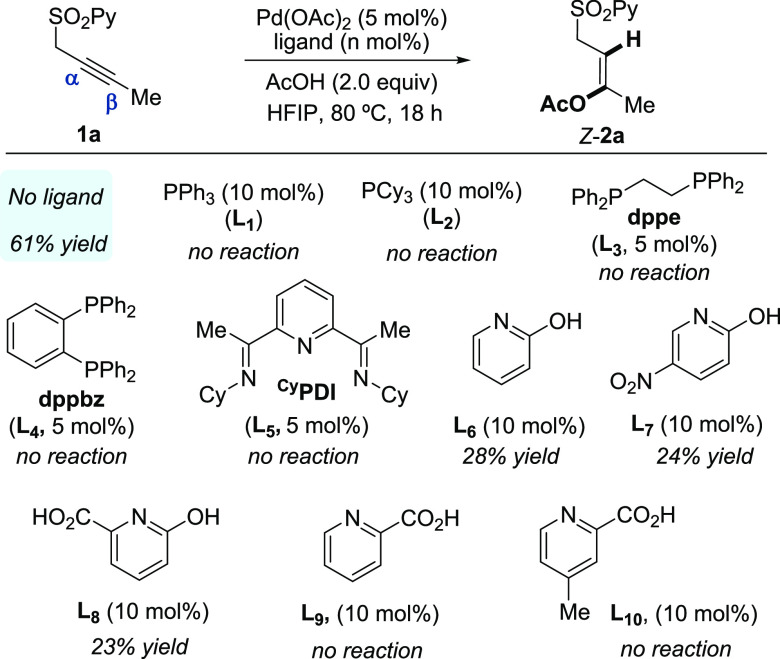
The effect of ligands in the acetoxylation of 1a was also investigated (Scheme 3). In this study, we decided to use HFIP as a solvent (conditions of entry 9 in Table 2) to favor the complexation of the metal to the added ligand and minimize possible interference caused by a large excess of AcOH. The examination of a variety of mono- (PPh3 and PCy3) and bidentate (dppe and dppbz) phosphines commonly used in organic synthesis and pincer-type nitrogen ligands such as bis(imino)pyridines (CyPDI) completely inhibited the reaction. Interestingly, X-type pyridine-based ligands such as 2-hydroxypyridines/2-pyridones delivered the product Z-2a but only in low yield (L6–L8, 23–28%). This type of ligand is able to assist the dissociation of trimeric palladium acetate [Pd3(OAc)6].23 In contrast, pyridine-2-carboxylic acid derivatives were totally ineffective ligands (L9–L10). We speculate that the external ligand might hinder the simultaneous coordination of palladium to the substrate (in a bidentate fashion) and the acetate ligand, which could be key to enabling catalysis. Consequently, the ligandless conditions found initially in Table 1 (entry 1) were selected as optimal for the acetoxylation reaction.
Scheme 3. Ligand Effect in the Acetoxylation Reaction.
Structural Scope for the anti-Acetoxylation
Having established an efficient catalytic system for the β-acetoxylation of substrate 1a, we set out to investigate the versatility of the reaction toward more challenging substrates (Scheme 4). Gratifyingly, this method is compatible with the presence of an alkyl substituent at the propargylic position without erosion of the reaction yield (83%, Z-4). The use of substrates with larger alkynyl substituents was also well-tolerated, maintaining the exceptional levels of regio- and stereoselectivities (products Z-5 and Z-6, 74 and 72% yield, respectively). Larger alkyl groups at the propargylic position, such as nBu, phenethyl, or even tBu, were also amenable to the reaction without affecting the regio- or stereoselectivity (Z-7-9, 53–73%). Importantly, potentially sensitive functional groups such as alkyl halide or nitrile were also accommodated with no observable impact in yield or selectivity (products Z-10–12, 61–79% yield). Interestingly, the SO2Py directing group is capable to override the inherent electronic bias due to conjugation imposed by the aromatic substituent in internal aryl-substituted alkynes, since this class of alkynes typically undergoes insertion with opposite regioselectivity.9 As illustrated for products Z-13–16, the excellent β-regioselectivity was maintained for some representative aryl alkynes regardless of the electronic character of the aromatic ring. In all cases studied, the corresponding acetoxylation products were isolated as single regioisomers in synthetically useful yields, even for substrates containing strong electron-withdrawing groups (Z-15 and Z-16, 52 and 50%, respectively). Unfortunately, terminal alkynes were incompatible, largely producing catalyst deactivation. On the other hand, primary hydroxyl- and carboxylic acid-containing alkynes proved not to be suitable in this reaction, resulting in deactivation of the catalyst or decomposition of the starting material (not shown, see the Supporting Information, SI for further details).
Scheme 4. Representative Substrate Scope for the Acetoxylation of Unsymmetrical Internal Alkynes Employing the SO2Py Group as a Regiocontroller.
Regio- and stereochemistry of all products were determined in the reaction crude by 1H NMR spectroscopy. Complete levels of selectivity (β/α > 98:2; Z/E > 98:2) were observed in all cases studied. Reaction yields refer to products isolated after purification by flash column chromatograpy.
Kinetic Analysis
To further understand the role of acetic acid in the reaction, we performed a kinetic analysis to determine the presence of a primary kinetic isotope effect (KIE) by monitoring the acetoxylation of substrate 1a in AcOH and AcOD-d4 by independent experiments (Figure 1). From this study, we observed a clear effect in the reaction rate for the acetoxylation of propargyl sulfone 1a, in which a slower formation of the acetoxylated product took place when acetic acid-d4 was employed both as a solvent and reagent (Figure 1a,b). In addition, the plot of the ln(100-conversion) vs time for the nondeuterated and deuterated kinetic profiles led to a linear fit, resulting in a pseudo-first-order kinetics from which a primary KIE of 5.5 was obtained (Figure 1c, see the SI for details). These results suggest that either the addition of the acid or the protodepalladation step could be the rate-determining step of the reaction.
Figure 1.
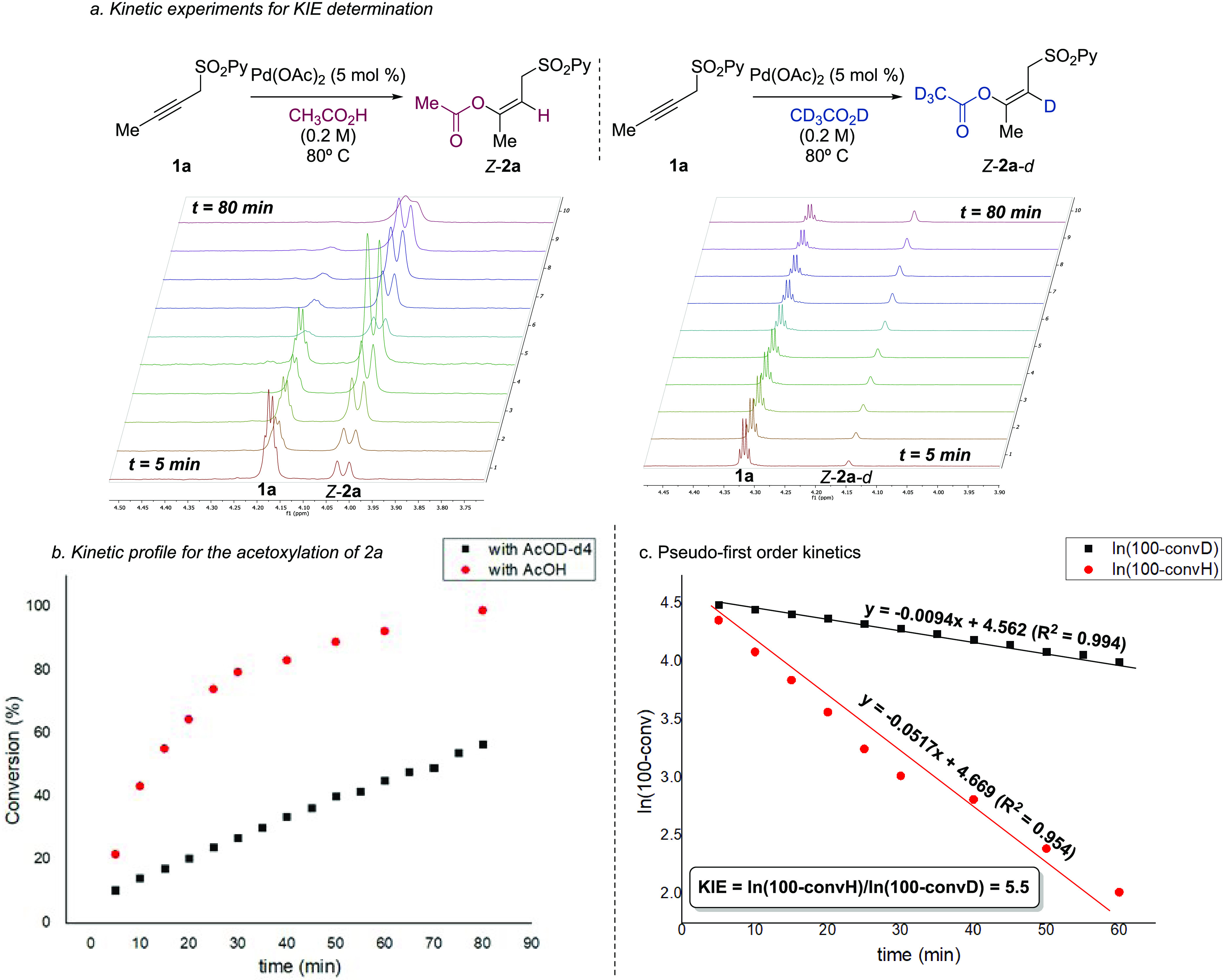
(Top) Time course of the acetoxylation of 1a by 1H NMR spectroscopy in the presence of acetic acid (left) or acetic acid-d4 (right). (Bottom) Determination of the KIE for the acetoxylation of 1a.
Computational Analysis
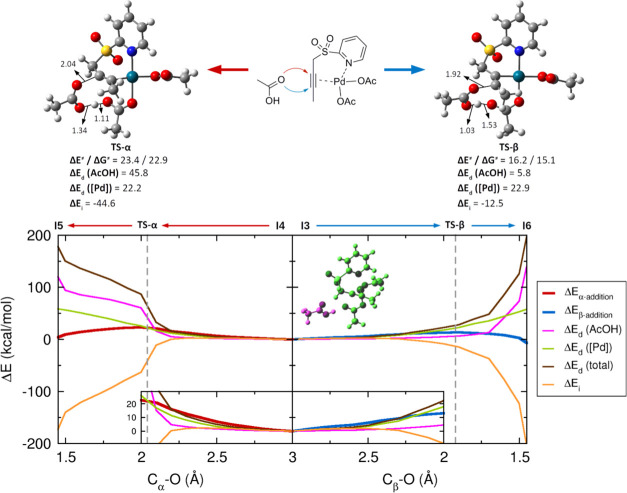
To gain insight into the reaction mechanism, we conducted a computational analysis considering several pathways. These calculations were carried out using the M06-L functional. For gas-phase geometry optimizations and frequency calculations, the def2-SVP basis set with effective core potential (ECP) was used for Pd, and the cc-pVDZ basis set was used for all other atoms (C, H, N, O, S). Single-point energy calculations with an SMD continuum solvation model were performed using the def2-TZVP basis set with ECP for Pd, and the cc-pVTZ basis set for all other atoms (see the SI for further details). To analyze the intrinsic reactivity of the propargyl sulfone 1a (I1 in Figure 2) either free in solution or bound to Pd(OAc)2, we first calculated the condensed Fukui functions24 at the Cα and Cβ positions ( Figure 2). These results showed that, albeit the triple bond is naturally polarized, it becomes more electrophilic upon Pd coordination (see the SI); in both cases, the Cβ position is more electrophilic. However, the rather low difference between the two positions (+0.028 for Cα and +0.051 for Cβ) suggests that the selectivity toward the β-adduct cannot be exclusively accounted for by electrophilicity. Thus, we have calculated the complete reaction profiles for the α- and β-acetoxylation (Figure 2). We found that the reaction follows a stepwise mechanism in both cases. Initially, we observed that the complexation of propargyl sulfone 1a with Pd(OAc)2 is thermodynamically feasible to form the corresponding chelate species I2. From this intermediate, we further explored the addition of AcOH to both positions of the C–C triple bond. The first step corresponds to the AcOH addition to the triple bond and the concomitant proton migration from the incoming AcOH molecule to one of the acetate ligands bound to Pd. From both alkenyl-palladium(II) intermediates (I5 and I6), intramolecular protodepalladation can take place to form the final alkenes (I9 and I10) and regenerate Pd(OAc)2 upon de-coordination from the product. The selectivity is determined at the first step, given that the formation of the 6-membered palladacycle (β-addition) is kinetically (ΔΔG≠ = (TS-β-I3)-(TS-α-I4) = −7.7 kcal/mol) and thermodynamically (exergonic, ΔΔGreac = (I6-I3)-(I5-I4) = −9.9 kcal/mol) more favorable. In contrast, the protodepalladation step proceeds very similarly for both pathways (ΔΔG≠ = (TS-Hβ-I6)-(TS-Hα-I5) = +1.7 and ΔΔGreac = (I8-I6)-(I7-I5) = +2.1 kcal/mol) in an intramolecular fashion since one AcOH is coordinated to the palladium center after the addition of the carboxylic acid. Alternative reaction pathways (coordination of an additional AcOH molecule and intramolecular syn insertion of the alkyne into the Pd—OAc bond) are discussed in the SI, none of them were found to be more favorable than the β-acetoxylation presented here. These conclusions help to understand the key role of the acetate ligand since it assists in the formation of the C–O bond during the acetoxypalladation event through proton migration. This reactivity closely resembles the more typical concerted metalation–deprotonation (CMD) pathway observed in some C–H activation processes.2b Therefore, the acetate ligand behaves as a multifunctional ligand during the addition of the carboxylic acid and further protodemetalation. This observation is in accordance with the lack of reactivity observed when different strong coordinating ligands are present since they are not expected to be active in the proton migration event. In contrast, when 2-hydroxypyridines were employed, the reaction delivered the acetoxylated product in low yields, pointing out to the potential role as acid activators of these N-coordinating species during the proton migration.25 Additionally, this activation mode is observed in the transition state of the rate-determining step (the acetoxypalladation), thus explaining the KIE obtained when compared with the reaction kinetics in AcOH and AcOD-d4.
Figure 2.
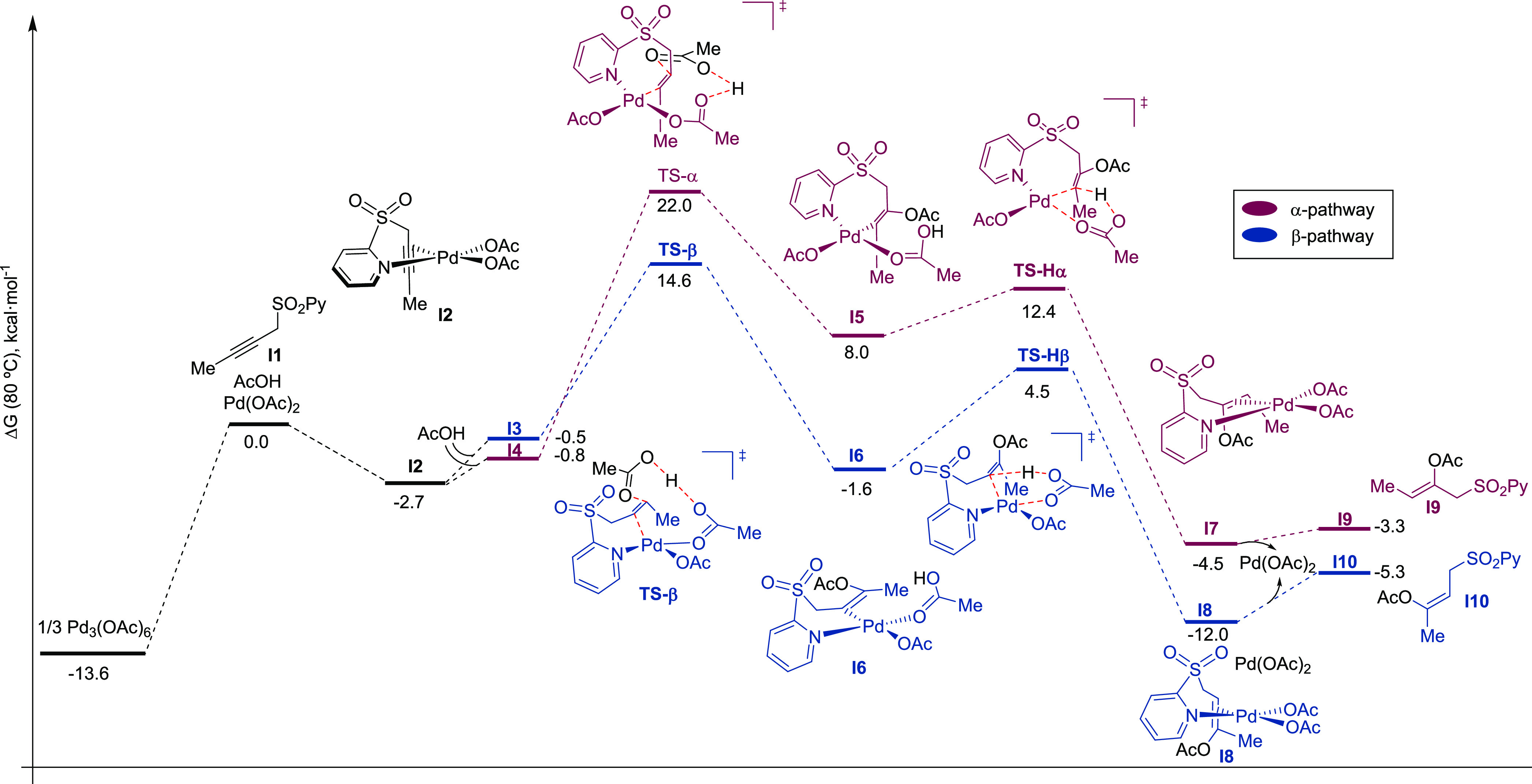
Reaction profiles for the intermolecular α- (magenta) and β-acetoxylation (blue). Gibbs free energies in kcal/mol at 353.15 K are given relative to the sum of all reagents (1a + AcOH + Pd(OAc)2) at an infinite distance.
To further understand the origin of the differences in the activation energies of the first step of the reaction, we analyzed the energy profiles by means of the distortion/interaction model.26 In short, this model proposes that the reaction energy at any point of the reaction coordinate can be divided into two components, one associated with the reagents’ geometry deformations (the distortion energy, ΔEd), and the stabilization produced when the distorted reagents interact (the interaction energy, ΔEi). Figure 3 shows the distortion/interaction diagrams for the α- (left) and β-acetoxylation (right). To carry out this analysis, we divided the reaction complex into two subunits, the incoming AcOH molecule and the 1a-Pd(OAc)2 complex. While the ΔEd ([Pd]) curves (the distortion energy associated with the 1a-Pd(OAc)2 complex) are similar, the main difference between both reaction pathways lies in ΔEd (AcOH), which starts to increase earlier in the α-addition than in the β-addition. In fact, at the position of TS-α (recall Figure 3), ΔEd (AcOH) is not only larger than ΔEd ([Pd]) but also slightly larger (in absolute values) than ΔEi, making it the predominant term for the activation energy. In contrast, for TS-β, the contribution of ΔEd (AcOH) is minimal. This critical difference can be related to the moment at which proton migration takes place. For the α-addition, the lower electrophilicity of the Cα position requires that O–H dissociation occurs in an early stage to increase AcOH nucleophilicity and to facilitate Cα–O bond formation, but the cost of the O–H bond cleavage is larger than the gain in reactivity. In contrast, as the Cβ position is more reactive, O–H dissociation can develop after Cβ–O bond formation and would occur after the TS-β, so the energy required to O–H dissociation is clearly compensated by ΔEi along the whole pathway.
Figure 3.
(Top) Activation energies and Gibbs free energies and distortion–interaction energy contributions at the TSs. Energies in kcal/mol. (Bottom) Distortion/interaction diagrams for the intermolecular α- (left) and β-acetoxylation (right) projected onto the C–O distance. Vertical dashed lines mark the position of TS-α or TS-β. [Pd] accounts for the 1a-Pd(OAc)2 complex. A zoom of the region comprised between a C–O distance value of 2 and 3 Å is provided in the inset. Distances are given in Å, energies in kcal/mol.
The above-mentioned factor determines that the proton migration plays a critical role in favoring the β-acetoxylation over the α-, as for the α-addition the O–H cleavage is required in an early stage and thus increases ΔEd (AcOH) to a point that it cannot be compensated by ΔEi, while for the β-addition, it occurs at the end of the reaction. This can be proved easily by plotting the O–H distance against the C–O (Figure 4); in the α-addition (red pathway), the O–H dissociation occurs before C–O formation and starts before the TS, while the opposite behavior is observed for the β-addition.
Figure 4.
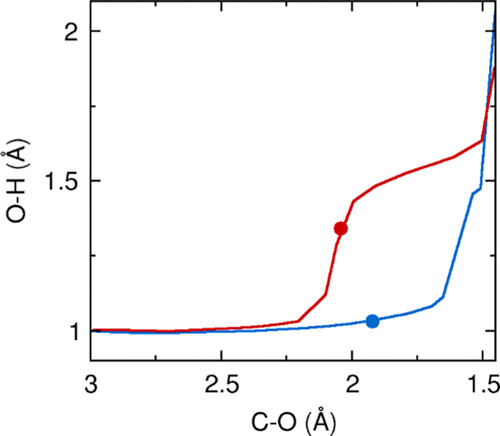
Evolution of the O–H and C–O distances along the α- (red) and β-acetoxylation (blue) reaction pathways. Full dots indicate the position of TS-α and TS-β.
To further corroborate our hypothesis on the differential asynchronicity in the bond formation at TS-α and TS-β, we have resorted to a NBO analysis to evaluate/quantify the interaction between the two approximating moieties (Figure 5, top and bottom, respectively). For both transition states, the Cα–Cβ is no longer a triple bond, given that the C–Pd bond is already formed, and a virtual p orbital can be located over the other carbon center. However, the formation of the new C–O bond is in a more advanced state in TS-β, as reflected by the greater second-order interaction energy between the oxygen lone pair and the carbon p orbital of the 1a-Pd(OAc)2 complex (Table 3, 107 kcal/mol in TS-β vs 68 kcal/mol in TS-α). In a similar vein, the original O–H bond of the incoming acetic acid is broken, while the new one (with the acetate ligand) is yet to be formed. As was discussed before, the proton transfer takes place earlier in the α-addition than in the β-addition. Consequently, the second-order interaction energy between the empty 1s orbital of the transferred proton and the oxygen lone pair of the acetate ligand is greater in TS-α (276 kcal/mol) than that in TS-β (49 kcal/mol). On the other hand, the second-order interaction energy between the 1sH and the oxygen lone pair of acetic acid is greater in TS-β (354 kcal/mol) than that in TS-α (129 kcal/mol).
Figure 5.
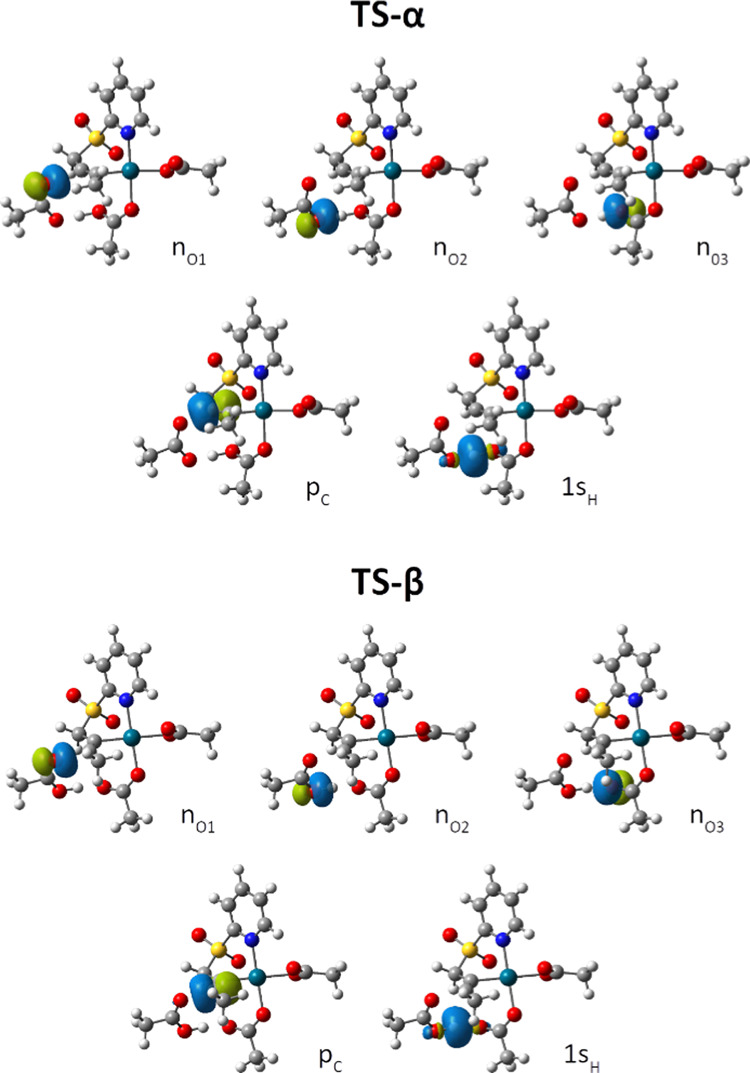
NBO orbitals of TS-α (top) and TS-β (bottom) considered in this study.
Table 3. Second-Order Interaction Energies (in kcal/mol) between Selected NBO Orbital Pairs for TS-α and TS-β.
| orbital pair | TS-α | TS-β |
|---|---|---|
| nO1 → pC | 68 | 107 |
| nO2 → 1sH | 129 | 354 |
| nO3 → 1sH | 276 | 49 |
Conclusions
In summary, this study of the acetoxylation reaction of unsymmetrical dialkyl alkynes with AcOH shows that the cooperative effects of the palladium metal, the SO2Py directing group at the propargylic position, and the acetate ligand play essential roles in reaction efficiency and selectivity control. The corresponding alkenyl acetates are obtained with good yields and excellent level of regioselectivity (acetate delivered distal to the directing group) and anti-addition stereoselectivity. Experimental and computational mechanistic analyses suggest that the acetate ligand plays multiple important roles. It brings the AcOH near the metal–substrate complex through hydrogen bonding, enhances its nucleophilicity, and directs the attack to the alkyne in a regioselective fashion. Additionally, once protonated, it also facilitates protodematallation acting as a proton shuttle to protonate the alkenyl-Pd intermediate intramolecularly. These studies revealed that the anti-acetoxypalladation of the alkyne is both a rate-determining and regioselectivity-determining step of the catalytic cycle. The two regiochemical outcomes have been analyzed in detail using the distortion/interaction model, showing that the proton migration plays a critical role in favoring the β-acetoxylation over the α-attack. These observations provide a blueprint for the rational design of more efficient methods to attain regiocontrol in the functionalization of internal alkynes through the addition of polar X—H bonds. Further studies regarding the synthetic potential of the resulting alkenyl acetates and directing group transformations are under study in our laboratories and will be reported in due course.
Acknowledgments
We thank the Ministerio de Ciencia e Innovación (MICINN) and Fondo Europeo de Desarrollo Regional (FEDER, UE) for financial support (Agencia Estatal de Investigación/Projects PGC2018-098660-B-I00 and PGC2018-094644-B-C21). J.C. and I.C. thank MECD and MINECO for an FPU fellowship and a Ramón y Cajal contract, respectively. We also acknowledge the Centro de Computación Científica of the Universidad Autónoma de Madrid for computational time. We thank Inés Manjón for generous donation of precursors for the synthesis of starting materials. We also thank the referees for their careful and insightful feedback, which helped us to improve our manuscript and to gain a better understanding of the reaction.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscatal.2c00710.
Experimental procedures; 1H and 13C NMR spectra for all new compounds; and DFT calculations (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Crabtree R. H. Multifunctional ligands in transition metal catalysis. New J. Chem. 2011, 35, 18–23. 10.1039/C0NJ00776E. [DOI] [Google Scholar]
- a Musaev D. G.; Liebeskind L. S. On the Mechanism of Palladium(0) Catalyzed, Copper(I) Carboxylate Mediated Thioorganic-Boronic Acid Desulfitative Coupling. A Noninnocent Role for the Carboxylate Ligand. Organometallics 2009, 28, 4639–4642. 10.1021/om900602b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ackermann L. Carboxylate-Assisted Transition-Metal-Catalyzed C–H Bond Functionalizations: Mechanism and Scope. Chem. Rev. 2011, 111, 1315–1345. 10.1021/cr100412j. [DOI] [PubMed] [Google Scholar]
- a García-Cuadrado D.; de Mendoza P.; Braga A. A. C.; Maseras F.; Echavarren A. M. Proton-Abstraction Mechanism in the Palladium-Catalyzed Intramolecular Arylation: Substituent Effects. J. Am. Chem. Soc. 2007, 129, 6880–6886. 10.1021/ja071034a. [DOI] [PubMed] [Google Scholar]; b Altus K. M.; Love J. A. The Continuum of Carbon–Hydrogen (C–H) Activation Mechanisms and Terminology. Commun. Chem. 2021, 4, 173 10.1038/s42004-021-00611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Guihaumé J.; Halbert S.; Eisenstein O.; Perutz R. N. Hydrofluoroarylation of Alkynes with Ni Catalysts. C–H Activation via Ligand-to-Ligand Hydrogen Transfer, an Alternative to Oxidative Addition. Organometallics 2012, 31, 1300–1314. 10.1021/om2005673. [DOI] [Google Scholar]; b Nett A. J.; Montgomery J.; Zimmerman P. M. Entrances, Traps, and Rate-Controlling Factors for Nickel-Catalyzed C–H Functionalization. ACS Catal. 2017, 7, 7352–7362. 10.1021/acscatal.7b02919. [DOI] [Google Scholar]
- a Johnson D. G.; Lynam J. M.; Slattery J. M.; Welby C. E. Insights into the Intramolecular Acetate-Mediated Formation of Ruthenium Vinylidene Complexes: a Ligand-Assisted Proton Shuttle (LAPS) Mechanism. Dalton Trans. 2010, 39, 10432–10441. 10.1039/c0dt00431f. [DOI] [PubMed] [Google Scholar]; b Breit B.; Gellrich U.; Li T.; Lynam J. M.; Milner L. M.; Pridmore N. E.; Slattery J. M.; Whitwood A. C. Mechanistic Insight into the Ruthenium-Catalysed anti-Markovnikov Hydration of Alkynes Using a Self-Assembled Complex: a Crucial Role for Ligand-Assisted Proton Shuttle Processes. Dalton Trans. 2014, 43, 11277–11285. 10.1039/C4DT00712C. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang Z.; Li Y.; Wu G.; Cao Z.; Zhang L. A General Ligand Design for Gold Catalysis Allowing Ligand-Directed anti-Nucleophilic Attack of Alkynes. Nat. Commun. 2014, 5, 3470 10.1038/ncomms4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.; Zhang L. Designed Bifunctional Ligands in Cooperative Homogeneous Gold Catalysis. CCS Chem. 2021, 3, 1989–2002. 10.31635/ccschem.020.202000454. [DOI] [Google Scholar]
- For recent examples, see:; a Leeb N. M.; Drover M. W.; Love J. A.; Schafer L. L.; Slattery J. M. Phosphoramidate-Assisted Alkyne Activation: Probing the Mechanism of Proton Shuttling in a N,O-Chelated Cp*Ir(III) Complex. Organometallics 2018, 37, 4630–4638. 10.1021/acs.organomet.8b00656. [DOI] [Google Scholar]; b de Aguirre A.; Díez-Gonzalez S.; Maseras F.; Martín M.; Sola E. The Acetate Proton Shuttle between Mutually Trans Ligands. Organometallics 2018, 37, 2645–2651. 10.1021/acs.organomet.8b00417. [DOI] [Google Scholar]; c Galiana-Cameo M.; Urriolabeitia A.; Barrenas E.; Passarelli V.; Pérez-Torrente J. J.; Di Giuseppe A.; Polo V.; Castarlenas R. Metal–Ligand Cooperative Proton Transfer as an Efficient Trigger for Rhodium-NHC-Pyridonato Catalyzed gem-Specific Alkyne Dimerization. ACS Catal. 2021, 11, 7553–7567. 10.1021/acscatal.1c00602. [DOI] [Google Scholar]
- For recent general reviews on alkyne functionalization, see:; a Zeng X. Recent Advances in Catalytic Sequential Reactions Involving Hydroelement Addition to Carbon-Carbon Multiple Bonds. Chem. Rev. 2013, 113, 6864–6900. 10.1021/cr400082n. [DOI] [PubMed] [Google Scholar]; b Ansell M. B.; Navarro O.; Spencer J. Transition Metal Catalyzed Element-Element’ Additions to Alkynes. Coord. Chem. Rev. 2017, 336, 54–77. 10.1016/j.ccr.2017.01.003. [DOI] [Google Scholar]; c Chen J.; Guo J.; Lu Z. Recent Advances in Hydrometallation of Alkenes and Alkynes via the First Row Transition Metal Catalysis. Chin. J. Chem. 2018, 36, 1075–1109. 10.1002/cjoc.201800314. [DOI] [Google Scholar]; d Corpas J.; Mauleón P.; Arrayás R. G.; Carretero J. C. Transition-Metal-Catalyzed Functionalization of Alkynes with Organoboron Reagents: New Trends, Mechanistic Insights, and Applications. ACS Catal. 2021, 11, 7513–7551. 10.1021/acscatal.1c01421. [DOI] [Google Scholar]
- For selected reviews on catalytic alkyne functionalization, see:; a Cheng Z.; Guo J.; Lu Z. Recent Advances in Metal-Catalysed Asymmetric Sequential Double Hydrofunctionalization of Alkynes. Chem. Commun. 2020, 56, 2229–2239. 10.1039/D0CC00068J. [DOI] [PubMed] [Google Scholar]; b Rocchigiani L.; Bochmann M. Recent Advances in Gold(III) Chemistry: Structure, Bonding, Reactivity, and Role in Homogeneous Catalysis. Chem. Rev. 2021, 121, 8364–8451. 10.1021/acs.chemrev.0c00552. [DOI] [PubMed] [Google Scholar]; c Rasool J. U.; Ali A.; Ahmad Q. N. Recent Advances in Cu-Catalyzed Transformations of Internal Alkynes to Alkenes and Heterocycles. Org. Biomol. Chem. 2021, 19, 10259–10287. 10.1039/D1OB01709H. [DOI] [PubMed] [Google Scholar]; d Ghosh S.; Chakrabortty R.; Ganesh V. Dual Functionalization of Alkynes Utilizing the Redox Characteristics of Transition Metal Catalysts. ChemCatChem 2021, 13, 4262–4298. 10.1002/cctc.202100838. [DOI] [Google Scholar]
- Alonso F.; Beletskaya I. P.; Yus M. Transition-Metal-Catalyzed Addition of Heteroatom–Hydrogen Bonds to Alkynes. Chem. Rev. 2004, 104, 3079–3160. 10.1021/cr0201068. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Lin C.; Shen L. Recent Progress in Transition Metal-Catalyzed Regioselective Functionalization of Unactivated Alkenes/Alkynes Assisted by Bidentate Directing Groups. ChemCatChem 2018, 11, 961–968. 10.1002/cctc.201801625. [DOI] [Google Scholar]; b Kawasaki Y.; Ishikawa Y.; Igawa K.; Tomooka K. Directing Group-Controlled Hydrosilylation: Regioselective Functionalization of Alkyne. J. Am. Chem. Soc. 2011, 133, 20712–20715. 10.1021/ja209553f. [DOI] [PubMed] [Google Scholar]; c Liu P.; Fukui Y.; Tian P.; He Z.-T.; Sun C.-Y.; Wu N.-Y.; Lin G.-Q. Cu-Catalyzed Asymmetric Borylative Cyclization of Cyclohexadienone-Containing 1, 6-Enynes. J. Am. Chem. Soc. 2013, 135, 11700–11703. 10.1021/ja404593c. [DOI] [PubMed] [Google Scholar]; d Sun J.; Zheng G.; Xiong T.; Zhang Q.; Zhao J.; Li Y.; Zhang Q. Copper-Catalyzed Hydroxyl-Directed Aminoarylation of Alkynes. ACS Catal. 2016, 6, 3674–3678. 10.1021/acscatal.6b00759. [DOI] [Google Scholar]; e Mailig M.; Hazra A.; Armstrong M. K.; Lalic G. Catalytic Anti-Markovnikov Hydroallylation of Terminal and Functionalized Internal Alkynes: Synthesis of Skipped Dienes and Trisubstituted Alkenes. J. Am. Chem. Soc. 2017, 139, 6969–6977. 10.1021/jacs.7b02104. [DOI] [PubMed] [Google Scholar]; f Das M.; Kaicharla T.; Teichert J. F. Stereoselective Alkyne Hydrohalogenation by Trapping of Transfer Hydrogenation Intermediates. Org. Lett. 2018, 20, 4926–4929. 10.1021/acs.orglett.8b02055. [DOI] [PubMed] [Google Scholar]; g Huang C.; Qian H.; Zhang W.; Ma S. Hydroxy Group-Enabled Highly Regio- and Stereoselective. Hydrocarboxylation of Alkynes. Chem. Sci. 2019, 10, 5505–5512. 10.1039/C8SC05743E. [DOI] [PMC free article] [PubMed] [Google Scholar]; For examples including anti-addition reactions, see:; h Derosa J.; Cantu A. L.; Boulous M. N.; O’Duill M. L.; Turnbull J. L.; Liu Z.; De La Torre D. M.; Engle K. M. Directed, Regiocontrolled Hydroamination of Unactivated Alkenes via Protodepalladation. J. Am. Chem. Soc. 2017, 139, 5183–5193. 10.1021/jacs.7b00892. [DOI] [PubMed] [Google Scholar]; i Pang Y.; Liu G.; Huang C.; Yuan X.-A.; Li W.; Xie J. A Highly Efficient Dimeric Manganese-Catalyzed Selective Hydroarylation of Internal Alkynes. Angew. Chem., Int. Ed. 2020, 59, 12789–12794. 10.1002/anie.202004950. [DOI] [PubMed] [Google Scholar]
- a Moure A. L.; Arrayás R. G.; Cárdenas D. J.; Alonso I.; Carretero J. C. Regiocontrolled CuI-Catalyzed Borylation of Propargylic-Functionalyzed Internal Alkynes. J. Am. Chem. Soc. 2012, 134, 7219–7222. 10.1021/ja300627s. [DOI] [PubMed] [Google Scholar]; b García-Rubia A.; Romero-Revilla J. A.; Mauleón P.; Arrayás R. G.; Carretero J. C. Cu-Catalyzed Silylation of Alkynes: A Traceless 2-Pyridylsulfonyl Controller Allows Access to Either Regioisomer on Demand. J. Am. Chem Soc. 2015, 137, 6857–6865. 10.1021/jacs.5b02667. [DOI] [PubMed] [Google Scholar]; c Corpas J.; Quirós M. T.; Mauleón P.; Arrayás R. G.; Carretero J. C. Metal- and Photocatalysis to Gain Regiocontrol and Stereodivergence in Hydroarylations of Unsymmetrical Dialkyl Alkynes. ACS Catal. 2019, 9, 10567–10574. 10.1021/acscatal.9b02768. [DOI] [Google Scholar]
- For methods based on Ru-catalysis, see:; a Ruppin C.; Dixneuf P. H. Synthesis of Enol Esters from Terminal Alkynes Catalyzed by Ruthenium Complexes. Tetrahedron Lett. 1986, 27, 6323–6324. 10.1016/S0040-4039(00)87798-9. [DOI] [Google Scholar]; b Rotem M.; Shvo Y. Addition of carboxylic acids to alkynes catalysed by ruthenium complexes. J. Organomet. Chem. 1993, 448, 189–204. 10.1016/0022-328X(93)80084-O. [DOI] [Google Scholar]; c Kawatsura M.; Namioka J.; Kajita K.; Yamamoto M.; Tsuji H.; Itoh T. Ruthenium-Catalyzed Regio- and Stereoselective Addition of Carboxylic Acids to Aryl and Trifluoromethyl Group Substituted Unsymmetrical Internal Alkynes. Org. Lett. 2011, 13, 3285–3287. 10.1021/ol201238u. [DOI] [PubMed] [Google Scholar]; d Jeschke J.; Gäbler C.; Lang H. Regioselective Formation of Enol Esters from the Ruthenium-Catalyzed Markovnikov Addition of Carboxylic Acids to Alkynes. J. Org. Chem. 2016, 81, 476–484. 10.1021/acs.joc.5b02293. [DOI] [PubMed] [Google Scholar]; e Jeschke J.; Engelhardt T. B.; Lang H. Ruthenium-Catalyzed Hydrocarboxylation of Internal Alkynes. Eur. J. Org. Chem. 2016, 2016, 1548–1554. 10.1002/ejoc.201501583. [DOI] [Google Scholar]; f Liu G.; Zhang X.; Kuang G.; Lu N.; Fu Y.; Peng Y.; Xiao Q.; Zhou Y. Phosphine-Free Ru-Catalyzed Regio- and Stereoselective Addition of Benzoic Acids to Trifluoromethylated Alkynes toward Facile Access to Trifluoromethyl Group-Substituted (E)-Enol Esters. ACS Omega 2020, 5, 4158–4166. 10.1021/acsomega.9b03936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methods based on Rh-catalysis:; Lumbroso A.; Vautravers N. R.; Breit B. Rhodium-Catalyzed Selective anti-Markovnikov Addition of Carboxylic Acids to Alkynes. Org. Lett. 2010, 12, 5498–5501. 10.1021/ol102365e. [DOI] [PubMed] [Google Scholar]
- For methods based on Pd-catalysis, see:; a Lu X.; Zhu G.; Ma S. A Novel Regio- and Stereo-Specific Hydroacetoxylation Reaction of 2-Alkynoic Acid Derivatives. Tetrahedron Lett. 1992, 33, 7205–7206. 10.1016/S0040-4039(00)60873-0. [DOI] [Google Scholar]; b Wakabayashi T.; Ishii Y.; Murata T.; Mizobe Y.; Hidai M. Stereoselective Addition of Carboxylic Acids to Electron Deficient Acetylenes Catalyzed by the PdMo3S4 Cubane-Type Cluster. Tetrahedron Lett. 1995, 36, 5585–5588. 10.1016/00404-0399(50)1067R-. [DOI] [Google Scholar]; c Smith D. L.; Goundry W. R. F.; Lam H. W. Palladium-Catalyzed Hydroacyloxylation of Ynamides. Chem. Commun. 2012, 48, 1505–1507. 10.1039/C1CC13595C. [DOI] [PubMed] [Google Scholar]; d Tsukada N.; Takahashi A.; Inoue Y. Hydrocarboxylation of Unactivated Internal Alkynes with Carboxylic Acids Catalyzed by Dinuclear Palladium Complexes. Tetrahedron Lett. 2011, 52, 248–250. 10.1016/j.tetlet.2010.11.046. [DOI] [Google Scholar]; e Wu J.; Deng X.; Hirao H.; Yoshikai N. Pd-Catalyzed Conversion of Alkynyl-λ3-Iodanes to Alkenyl-λ3-iodanes via Stereoselective 1,2-Iodine(III) Shift/1,1-Hydrocarboxylation. J. Am. Chem. Soc. 2016, 138, 9105–9108. 10.1021/jacs.6b06247. [DOI] [PubMed] [Google Scholar]
- Examples using Au-catalysis, see:; a Roembke P.; Schmidbaur H.; Cronje S.; Raubenheimer H. Application of (Phosphine)Gold(I) Carboxylates, Sulfonates and Related Compounds as Highly Efficient Catalysts for the Hydration of Alkynes. J. Mol. Catal. A: Chem. 2004, 212, 35–42. 10.1016/j.molcata.2003.11.011. [DOI] [Google Scholar]; b Chary B. C.; Kim S. Gold(I)-Catalyzed Addition of Carboxylic Acids to Alkynes. J. Org. Chem. 2010, 75, 7928–7931. 10.1021/jo101543q. [DOI] [PubMed] [Google Scholar]; c González-Liste P. J.; León F.; Arribas I.; Rubio M.; García-Garrido S. E.; Cadierno V.; Pizzano A. Highly Stereoselective Synthesis and Hydrogenation of (Z)-1-Alkyl-2-arylvinyl Acetates: a Wide Scope Procedure for the Preparation of Chiral Homobenzylic Esters. ACS Catal. 2016, 6, 3056–3060. 10.1021/acscatal.6b00282. [DOI] [Google Scholar]; d León F.; González-Liste P. J.; García-Garrido S. E.; Arribas I.; Rubio M.; Cadierno V.; Pizzano A. Gold-Catalyzed Regio- and Stereoselective Addition of Carboxylic Acids to Iodoalkynes: Access to (Z)-β-Iodoenol Esters and 1,4-Disubstituted (Z)-Enynyl Esters. J. Org. Chem. 2017, 82, 5852–5867. 10.1021/acs.joc.7b00710. [DOI] [PubMed] [Google Scholar]; e González-Liste P. J.; García-Garrido S. E.; Cadierno V. Gold(i)-Catalyzed Addition of Carboxylic Acids to Internal Alkynes in Aqueous Medium. Org. Biomol. Chem. 2017, 15, 1670–1679. 10.1039/C6OB02800D. [DOI] [PubMed] [Google Scholar]; For examples using Ag as catalyst, see:; f Ishino Y.; Nishiguchi I.; Nakao S.; Hirashima T. Novel Synthesis of Enols Esters Through Silver-Catalyzed Reaction of Acetylenic Compounds with Carboxylic Acids. Chem. Lett. 1981, 10, 641–644. 10.1246/cl.1981.641. [DOI] [Google Scholar]; g Yin J.; Bai Y.; Mao M.; Zhu G. Silver-Catalyzed Regio- and Stereoselective Addition of Carboxylic Acids to Ynol Ethers. J. Org. Chem. 2014, 79, 9179–9185. 10.1021/jo501615a. [DOI] [PubMed] [Google Scholar]
- For synthetic versatility of alkenyl acetates, see:; a Mukaiyama T. The Directed Aldol Reaction. Org. React. 1982, 28, 203–331. [Google Scholar]; b Gooßen L. J.; Paetzold J. Decarbonylative Heck Olefination of Enol Esters: Salt-Free and Environmentally Friendly Access to Vinyl Arenes. Angew. Chem., Int. Ed. 2004, 43, 1095–1098. 10.1002/anie.200352357. [DOI] [PubMed] [Google Scholar]; c Isambert N.; Cruz M.; Arévalo M. J.; Gómez E.; Lavilla R. Enol esters: Versatile substrates for Mannich-Type multicomponent reactions. Org. Lett. 2007, 9, 4199–4202. 10.1021/ol701717z. [DOI] [PubMed] [Google Scholar]; d Nishimoto Y.; Onishi Y.; Yasuda M.; Baba A. α-Alkylation of Carbonyl Compounds by Direct Addition of Alcohols to Enols Acetates. Angew. Chem., Int. Ed. 2009, 48, 9131–9134. 10.1002/anie.200904069. [DOI] [PubMed] [Google Scholar]; e Sun C.-L.; Wang Y.; Zhou X.; Wu Z.-H.; Li B.-J.; Guan B.-T.; Shi Z.-J. Construction of Polysubstituted Olefins through Ni-Catalyzed Direct Activation of Alkenyl C—O of Substituted Alkenyl Acetates. Chem. - Eur. J. 2010, 16, 5844–5847. 10.1002/chem.200902785. [DOI] [PubMed] [Google Scholar]; f González-Liste P. J.; Francos J.; García-Garrido S. E.; Cadierno V. The Intermolecular Hydro-oxycarbonylation of Internal Alkynes: Current State of the Art. ARKIVOC 2018, 2008, 17–39. 10.24820/ark.5550190.p010.188. [DOI] [Google Scholar]
- a Dupuy S.; Gasperini D.; Nolan S. P. Highly Efficient Gold (I)-Catalyzed Regio- and Stereoselective Hydrocarboxylation of Internal Alkynes. ACS Catal. 2015, 5, 6918–6921. 10.1021/acscatal.5b02090. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang Q.; Shi Y.; Huang X.; Wang Y.; Jiao J.; Tang Y.; Li J.; Xu S.; Li Y. Ru(II)-Catalyzed Difunctional Pyridyloxy-Directed Regio- and Stereospecific Addition of Carboxylic Acids to Internal Alkynes. Org. Lett. 2022, 24, 379–384. 10.1021/acs.orglett.1c04052. [DOI] [PubMed] [Google Scholar]
- For selected examples, see:; a Zhang Q.; Lu X. Highly Enantioselective Palladium(II)-Catalyzed Cyclization of (Z)-4′-Acetoxy-2′-butenyl 2-Alkynoates: An Efficient Synthesis of Optically Active γ-Butyrolactones. J. Am. Chem. Soc. 2000, 122, 7604–7605. 10.1021/ja001379s. [DOI] [Google Scholar]; b Zhang Q.; Lu X. PdII-Catalyzed Cyclization of Alkynes Containing Aldehyde, Ketone, or Nitrile Groups Initiated by the Acetoxypalladation of Alkynes. Angew. Chem., Int. Ed. 2002, 41, 4343–4345. . [DOI] [PubMed] [Google Scholar]; c Tello-Aburto R.; Kalstabakken K. A.; Harned A. H. Org. Biomol. Chem. 2013, 11, 5596–5604. 10.1039/c3ob27491h. [DOI] [PubMed] [Google Scholar]
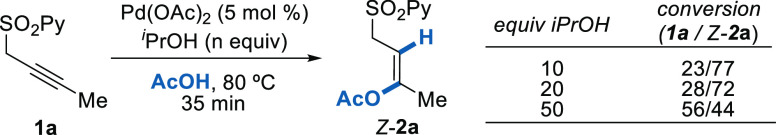
- The acetoxylation of
substrate 1a in AcOH under different amounts of iPrOH demonstrated an inverse relationship
between conversion toward the acetoxylation product and the amount
of iPrOH added, suggesting that a competing
interaction between the acetate ligand and the iPrOH via hydrogen-bonding could disrupt the activation of the
incoming carboxylic acid:
- It is known that the use of HFIP has a beneficial effect in hydro-oxycarbonylation reactions, see:; Bai Z.; Bai Z.; Song F.; Wang H.; Chen G.; He G. Palladium-Catalyzed Amide-Directed Hydrocarbofunctionalization of 3-Alkenamides with Alkynes. ACS Catal. 2020, 10, 933–940. 10.1021/acscatal.9b04285. [DOI] [Google Scholar]
- Mandal N.; Datta A. Harnessing the Efficacy of 2-Pyridone Ligands for Pd-Catalyzed (β/γ)-C(sp3)–H Activations. J. Org. Chem. 2020, 85, 13228–13238. 10.1021/acs.joc.0c02210. [DOI] [PubMed] [Google Scholar]
- Ayers P. W.; Morrison R. C.; Roy R. K. Variational Principles for Describing Chemical Reactions: Condensed Reactivity Indices. J. Chem. Phys. 2002, 116, 8731–8744. 10.1063/1.1467338. [DOI] [Google Scholar]
- For examples on the use of 2-pyridones in C—H activation processes, see:; a Wang P.; Farmer M. E.; Huo X.; Jain P.; Shen P.-X.; Ishoey M.; Bradner J. E.; Wisniewski S. R.; Eastgate M. D.; Yu J.-Q. Ligand-Promoted Meta-C—H Arylation of Anilines, Phenols, and Heterocycles. J. Am. Chem. Soc. 2016, 138, 9269–9276. 10.1021/jacs.6b04966. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang P.; Verma P.; Xia G.; Shi J.; Qiao J. X.; Tao S.; Cheng P. T. W.; Poss M. A.; Farmer M. E.; Yeung K.-S.; Yu J.-Q. Ligand Accelerated non-Directed C–H Functionalization of Arenes. Nature 2017, 551, 489–493. 10.1038/nature24632. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang P.; Farmer M. E.; Yu J.-Q. Ligand-Promoted meta-C—H Functionalization of Benzylamines. Angew. Chem. 2017, 129, 5207–5211. 10.1002/ange.201701803. [DOI] [PMC free article] [PubMed] [Google Scholar]; d St John-Campbell S.; White A. J. P.; Bull J. A. Methylene C(sp3)–H β,β′-Diarylation of Cyclohexanecarbaldehydes Promoted by a Transient Direction Group and Pyridone Ligand. Org. Lett. 2020, 22, 1807–1812. 10.1021/acs.orglett.0c00124. [DOI] [PubMed] [Google Scholar]
- Bickelhaupt F. M.; Houk K. N. Analyzing Reaction Rates with the Distortion/Interaction-Activation Strain Model. Angew. Chem., Int. Ed. 2017, 56, 10070–10086. 10.1002/anie.201701486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.