Abstract
In recent years, several protocols based on the extraction of nucleic acids directly from the soil matrix after lysis treatment have been developed for the detection of microorganisms in soil. Extraction efficiency has often been evaluated based on the recovery of a specific gene sequence from an organism inoculated into the soil. The aim of the present investigation was to improve the extraction, purification, and quantification of DNA derived from as large a portion of the soil microbial community as possible, with special emphasis placed on obtaining DNA from gram-positive bacteria, which form structures that are difficult to disrupt. Furthermore, we wanted to identify and minimize the biases related to each step in the procedure. Six soils, covering a range of pHs, clay contents, and organic matter contents, were studied. Lysis was carried out by soil grinding, sonication, thermal shocks, and chemical treatments. DNA was extracted from the indigenous microflora as well as from inoculated bacterial cells, spores, and hyphae, and the quality and quantity of the DNA were determined by gel electrophoresis and dot blot hybridization. Lysis efficiency was also estimated by microscopy and viable cell counts. Grinding increased the extracellular DNA yield compared with the yield obtained without any lysis treatment, but none of the subsequent treatments clearly increased the DNA yield. Phage λ DNA was inoculated into the soils to mimic the fate of extracellular DNA. No more than 6% of this DNA could be recovered from the different soils. The clay content strongly influenced the recovery of DNA. The adsorption of DNA to clay particles decreased when the soil was pretreated with RNA in order to saturate the adsorption sites. We also investigated different purification techniques and optimized the PCR methods in order to develop a protocol based on hybridization of the PCR products and quantification by phosphorimaging.
Since the Pasteur era, the unculturability of many microorganisms and the lack of specificity and sensitivity of classical detection methods have hampered progress in microbial ecology, particularly with regard to complex ecosystems such as soils and sediments, in which the proportion of culturable cells seldom reaches more than 5% of the total number (2). Nucleic acid-based techniques now provide a range of new tools for exploring parts of this unknown world. A phylogeny-based taxonomy, in which data from nonisolated bacteria are included, is rapidly replacing the former taxonomy based exclusively on morphological, physiological, and biochemical parameters of bacterial isolates (38, 42, 58). Reports now provide preliminary data on unculturable bacteria, allowing the extent of the total diversity in various environments to be better estimated (21, 47, 54). The techniques have, for example, made it possible to study alterations in the composition of soil microbial communities due to various disturbances (5, 41), to identify specific indigenous populations (19, 22) or specific functional genes (17, 44), and to monitor the fate of microorganisms or genes released in the environment (8, 33). They are also essential for addressing fundamental questions concerning the natural spread of genes via mechanisms such as conjugation, transformation, and transduction. However, despite important improvements during recent years, methods for extracting, purifying, amplifying, and quantifying soil DNA still suffer from low efficiency (6, 57, 60).
Analyses of DNA derived from soil microorganisms are based either on in situ lysis of the cells followed by DNA extraction (24, 26, 31, 40, 46, 49, 50) or on the extraction of DNA from cells previously separated from soil (14, 16, 48). The latter strategy specifically targets prokaryote DNA, minimizes the extraction of extracellular DNA, and provides large-fragment DNA with a high degree of purity (20). However, difficulties in processing numerous samples in parallel have limited the use of this technique. Furthermore, the fraction obtained only corresponds to about 25 to 35% of the total number of bacteria present in the soil (2, 14, 45). Different bacterial groups adhere more or less strongly to soil particles (32), which might bias the picture of the composition of the microbial community in the sample. Direct in situ lysis has the potential to circumvent problems of representativity, provided that cells from all groups of microorganisms are lysed in equal proportions. This is a difficulty owing to differences in cell properties and in the extent to which soil structures protect cells from lysis treatments. Moreover, DNA liberated from easily lysed organisms can become adsorbed to soil colloids, leading to an underestimation of the amount of DNA. On the other hand, extracellular DNA, which can remain adsorbed to soil particles for long periods (23, 29, 35, 55), is coextracted with nucleic acids released from recently lysed cells, thus increasing the pool of templates and leading to an overestimation of the number of living bacteria. Another problem related to the extraction of DNA directly from soil is that humic acids and other contaminants are coextracted with the DNA. Since such substances can severely affect subsequent analyses of nucleic acids, it is crucial that the purification step be efficient (46). Preliminary experiments using a range of different soil types demonstrated that extraction and purification protocols have to be carefully adjusted to the soil type under investigation, as was shown by Zhou et al. (60) and van Elsas et al. (50).
The objectives of this study were to identify and quantify the biases of these methods in order to develop the most favorable, or the least unfavorable, protocol. Three types of experiments were conducted by using a range of soils with various contents of clay and organic matter. The first type involved extraction of DNA from indigenous soil microbes in order to compare different lysis treatments. The purification step was improved for quantification of template molecules by PCR-hybridization techniques. The second type of experiment involved extraction of added, purified phage DNA in order to estimate qualitative and quantitative errors related to the adsorption of DNA to soil colloids, and to indicate which effects different lysis treatments could be expected to have on DNA once it is liberated from cells. In the third type of experiment, special emphasis was placed on lysing gram-positive bacteria, since these organisms are ubiquitous in soil and form structures that are difficult to disrupt. To address problems due to localization and cell physiology, DNA was extracted from inoculated vegetative cells, spores, and hyphae of Bacillus anthracis and Streptomyces lividans. Indigenous actinomycetes belonging to the genus Streptosporangium were quantified specifically.
MATERIALS AND METHODS
Soils.
Characteristics of the six soils used in this study are listed in Table 1. Clay and organic matter contents ranged from 9 to 47% and from 1.7 to 4.7%, respectively, while pH varied from 4.3 to 5.8. Soil samples were collected from the upper 5 to 10 cm. All visible roots were removed, and the soils were stored at 4°C for some days when necessary; then they were dried for 24 h at room temperature and sieved (2-mm mesh) prior to being stored for as long as a few months, at 4°C.
TABLE 1.
Sampling locations and characteristics of soils used in the different experiments
| Soil no. | Origin | Texture | Composition (%)
|
Organic matter (g/kg of dry soil) | pH | 109 cells/g (dry wt) of soila
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | Before grindingb | After grindingb | |||||
| 1 | Australia | Sandy clay | 62 | 22 | 16 | 49.7 | 5.8 | 6.5 (0.9) | 2.9 (1.3) |
| 2 | Peyrat le Chateau, France | Sandy clay | 61 | 26 | 13 | 48.2 | 4.9 | 7.3 (0.6) | 5.4 (0.8) |
| 3 | Côte St André, France | Sandy loam | 50 | 41 | 9 | 40.6 | 5.6 | 10.0 (0.7) | 7.5 (1.4) |
| 4 | Chazay d’Azergue, France | Sandy clay loam | 34 | 47 | 19 | 13.9 | 5.8 | 7.8 (1.1) | 4.2 (0.6) |
| 5 | Guadeloupe, France | Clay | 27 | 26 | 47 | 17.0 | 4.8 | 1.4 (0.4) | 0.5 (0.1) |
| 6 | Dombes, France | Sandy clay loam | 20 | 67 | 13 | 30.3 | 4.3 | 7.5 (0.5) | 5.6 (0.9) |
Direct microbial counts using acridine orange staining were performed before and after soil grinding.
n = 3. Numbers within parentheses are standard deviations.
Bacterial strains and culture conditions.
Extracellular DNA as well as bacterial strains providing vegetative cells, spores, and hyphae, used to inoculate soil samples, were chosen so that they could be tracked specifically. To obtain large amounts of extracellular DNA, the lysogenic Escherichia coli strain 1192 Hfr P4X (metB), containing phage lambda cI857 Sam7, was grown in Luria-Bertani (LB) broth for 2 h at 30°C, followed by 30 min at 42°C and 3 h at 37°C. Lambda phage DNA was extracted by the method of Sambrook et al. (36).
The avirulent strain of B. anthracis (Sterne 7700) was used as a bacterial cell inoculum. B. anthracis was grown in Trypticase soy broth (TSB) (Biomérieux, Lyon, France) for about 6 h and monitored to ensure that the optical density at 600 nm (OD600) remained below 0.6. These conditions allow vegetative cells to develop without spore formation (30). Spores of S. lividans OS48.3 (4) were mechanically removed from cultures of the organism on R2YE medium (15), whereupon the spore suspensions were filtered (15). Hyphae from S. lividans OS48.3 were derived from newly pregerminated spores, since the use of only short hyphae was expected to minimize rupture and subsequent DNA leakage. Spores were suspended in TES buffer [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid; Sigma-Aldrich Chimie, St. Quentin Fallavier, France] (0.05 M; pH 8) (15), heat shocked (at 50°C for 10 min, followed by cooling under cold tap water), and added to an equal volume of pregermination medium (yeast extract, 1%; Casamino Acids, 1%; CaCl2, 0.01 M). The solution was incubated at 37°C on a shaker. The proportion of germinated spores was estimated to be ca. 50%, in agreement with Hopwood et al. (15). After centrifugation, the pellets were resuspended in TES buffer, added to 3% TSB, and incubated at 37°C to an OD450 of 0.15 (15). Streptomyces hygroscopicus SWN 736 and Streptosporangium fragile AC1296 (Institute Pushchino, Moscow, Russia) were cultured by the method of Hickey and Tresner (12). DNA from S. lividans spores and hyphae was extracted from pure cultures in accordance with lysis protocol 6 as described below (except that no grinding was done), while S. hygroscopicus and S. fragile spores were extracted by chemical and enzymatic lysis (13).
Choice of extraction buffer.
A TENP buffer (50 mM Tris, 20 mM EDTA, 100 mM NaCl, 1% [wt/vol] polyvinylpolypyrrolidone) developed by Picard et al. (31) was used. Similar buffers have later been used by others (3, 18, 60) for extraction of soil DNA. Tris and EDTA protect the DNA from nuclease activity, NaCl provides a dispersing effect, and polyvinylpolypyrrolidone absorbs humic acids and other phenolic compounds (14, 31). In the present study we further evaluated the extraction efficiency of this buffer at different pHs (6.0 to 10.0) using 20 different soils with a pH range of 5.8 to 8.3 and an organic matter content between 0.2 and 6.3%. These 20 soils (other characteristics not shown) were used only in this experiment. The amount of DNA was determined colorimetrically as described by Richard (34); see below.
In situ lysis and DNA extraction protocols.
Several protocols with an increasing number of steps were tested to evaluate the efficiency of different techniques to lyse soil microbes in situ. For those experiments, the indigenous soil microflora was targeted in six soils. Additional experiments were conducted to study effects of lysis treatments on liberated DNA by analyzing the amounts and quality of added phage lambda DNA recovered from the soils. When an optimized protocol (termed protocol 6) had been developed, this was used to quantify DNA from indigenous actinomycetes and from inoculated gram-positive bacteria in selected soils. In all cases the soil samples were dried and sieved as described above. After grinding, 0.5 ml of TENP buffer was added to 200 mg (dry weight) of soil (except for protocol 1, where buffer was added to unground soil). When the different lysis treatments (see below) had been performed, the soil suspensions were vortexed for 10 min and centrifuged (at 4,000 × g for 5 min), whereupon an aliquot (25 μl) of the supernatant was analyzed by gel electrophoresis (0.8% agarose). Another aliquot representing a known volume, usually 350 μl, of the supernatant was precipitated with isopropanol. Five such portions (representing DNA derived from 1 g of soil) were pooled and resuspended in 100 μl of sterile TE (10 mM Tris–1 mM EDTA, [pH 8.0]) buffer before purification (protocol D; see below) and quantification, either by dot blot hybridization of total DNA or by dot blot hybridization of PCR amplification products (see below). The hybridization signals were quantified by phosphorimaging (for details, see below).
(i) Evaluation of methods for in situ cell lysis.
The quality and quantity of DNA, extracted after an increasing number of lysis treatments (protocols 2 to 5b), were compared to that of extracellular DNA obtained after washing the soil with extraction buffer (protocol 1) (see also Fig. 1). Protocol 1 included no lysis treatment. TENP buffer was added to unground soil, after which the extraction proceeded as described above. Protocol 2 consisted of soil grinding followed by DNA extraction. Two different types of equipment were used for soil grinding. To compare their efficiencies, 5 g of dry soil was ground for 30 s in a grinder containing tungsten rings or for various periods up to 60 min in a soil grinder containing an agate mortar and marbles (diameter, 20 mm). TENP buffer was then added, and the DNA was extracted as described above. Gel electrophoresis showed that 40 min of grinding using agate marbles was necessary to obtain the amounts of DNA extracted after a 30-s grinding using tungsten rings. The size distributions of the DNA fragments were similar with the two methods (results not shown). Thus, these treatments were considered equal, and consequently the one used in each case described below has not been specified. In protocols 3 to 5, the efficiencies of several other lysis treatments were tested, separately or in different combinations, in addition to soil grinding. Protocol 3 was the same as protocol 2 except that it included a homogenization step using an Ultraturrax mixer (Janker & Kunkel, IKA Labortechnik; Staufen, Germany) operating at half the maximum speed for 5 min. Protocols 4a and b were like protocol 3 but with the addition of a sonication step. Two types of sonicators were compared: a titanium microtip sonicator (600-W Vibracell Ultrasonicator; Bioblock, Illkirch, France) (protocol 4a) and a Cup Horn sonicator (protocol 4b). The Vibracell microtip, which produces ultrawaves, is in direct contact with the soil solution. With the Cup Horn sonicator, the soil solution is instead kept in tubes that are put into a water bath through which the waves pass. Preliminary experiments were performed to determine the optimum conditions for the two sonicators (results not shown). The best compromise in terms of amounts of extracted DNA and sizes of the fragments was achieved by 7 or 10 min of sonication with the titanium microtip or the Cup Horn device, respectively, operating at a power setting of 15 W with 50% active cycles. Protocols 5a and b correspond to protocols 4a and b, respectively. After sonication with a titanium microtip or a Cup Horn device, lysozyme and achromopeptidase were added (each to a final concentration of 0.3 mg ml−1). The soil suspensions were incubated for 30 min at 37°C, whereupon lauryl sulfate was added (final concentration, 1%), and the suspensions were incubated for 1 h at 60°C before being centrifuged and precipitated as described above.
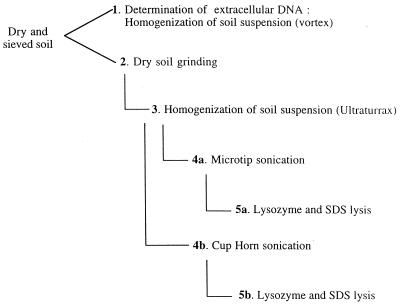
FIG. 1.
Protocols showing successive treatments for direct in situ lysis of soil microorganisms for DNA extraction. For details, see Materials and Methods. Protocol 1 consists of extraction of extracellular DNA from dried soil, with no lysis treatment. Protocol 2 includes grinding of dried soil followed by DNA extraction. Protocol 3 includes soil grinding followed by homogenization in the extraction buffer using an Ultraturrax mixer. In protocols 4a and b, soil grinding and homogenization are followed by sonication using a titanium microtip sonicator (protocol 4a) or a Cup Horn sonicator (protocol 4b). Protocols 5a and b include soil grinding, homogenization, and sonication using a microtip (protocol 5a) or a Cup Horn (protocol 5b) sonicator, followed by chemical and enzymatic lysis.
In addition to the protocols described above, we examined the effects of sonication (with the Cup Horn device; protocol 4b) and thermal shocks (30 s in liquid N2 followed by 3 min in boiling water; this whole treatment was repeated three times) on HindIII-digested phage lambda DNA added to soil (see below). Thermal shocks have been suggested as a means of in situ cell lysis (31). However, since the treatment appeared to have a detrimental effect on free DNA (see Results), it was not included in the protocols described above.
(ii) Optimized protocol.
After evaluation of different lysis treatments, an optimized protocol, termed protocol 6, was obtained. This was the same as protocol 5b except that, before sonication, the soil suspensions were vortexed and then rotated on a wheel for 2 h before being frozen at −20°C. After thawing, they were vortexed for 10 min before sonication. Protocol 6 was used in the experiments where soils were seeded with bacterial cells and in the experiments where indigenous actinomycetes were quantified (see below).
Microscopic counts.
The efficiency of soil grinding as a method for lysing bacterial cells was examined microscopically. Five grams of crude dried soil was mixed in a Waring blender with 50 ml of sterilized ultrapure water for 1.5 min, while 1 g (dry weight) of ground soil (protocol 2) was suspended in 10 ml by agitation for 10 min. The soil suspensions were serially diluted, and acridine orange was added (final concentration, 0.001%). After 2 min the suspensions were filtered through a 0.2-μm-pore-size black Nuclepore membrane. Each filter was rinsed with sterilized water, treated with 1 ml of isopropanol for 1 min to fix the bacterial cells, and rinsed again. Bacterial cells were counted with a Zeiss Universal epifluorescence microscope using a 100× objective. For each soil type, triplicate filters were counted, and at least 200 cells were counted on each filter.
Enumeration of culturable actinomycetes and total CFU.
The actinomycetes surviving the lysis treatments (protocols 1 to 5) were specifically examined in soil 3 (Côte St André; see Table 1). After a 10-fold dilution in a solution of yeast extract (6% [wt/vol]) and sodium dodecyl sulfate (SDS; 0.05%) to induce germination (10), the soil suspensions were serially diluted in sterile water, incubated at 40°C for 20 min, and plated onto HV medium (11). The HV medium was supplemented with actidione and nystatin (each at 50 mg liter−1. Actinomycete colonies were counted after incubation for 15 days at 28°C. A total of about 400 colonies were examined. Identification was based on macro- and microscopical morphological characteristics and on analysis of the diaminopimelic acid content of the isolates (37, 43, 56).
The total amount of culturable bacteria (total CFU) was also assessed for each of the lysis protocols (protocols 1 to 5). The soil suspensions were serially diluted and plated in triplicate onto Bennett agar medium (53) supplemented with nystatin and actidione (each at 50 mg liter−1). Each petri dish was covered with a cellulose nitrate filter (Millipore) and incubated for 3 days at 28°C. After enumeration of the colonies developing on the membranes, the filters were removed, and the plates were reincubated for 7 days at 28°C and then counted again.
Recovery of lambda phage DNA added to the soils.
Lambda phage DNA was digested with HindIII, phenol-chloroform extracted, precipitated, and resuspended in sterile ultrapure water according to standard protocols (36). Dilutions corresponding to 0, 2.5, 5, 7.5, 10, and 15 μg of DNA g (dry weight) of soil−1 were prepared in 60-μl volumes. This was added to 5-g portions of dry soil which were then vigorously mixed by vortexing for 5 min before grinding. Phage DNA was also added to preground soil at concentrations corresponding to 0, 10, and 15 μg of DNA g (dry weight) of soil−1. After grinding, extraction buffer was added and DNA was extracted according to protocol 2 (see above).
Saturation of adsorption sites with RNA.
To determine whether saturation of nucleic acid adsorption sites of soil colloids could increase the DNA recovery, the sandy loam (soil 4) and the clay soil (soil 5) were incubated with an RNA solution prior to any other treatment. Commercial RNA from Saccharomyces cerevisiae (Boehringer Mannheim, Meylan, France) was diluted in phosphate buffer (pH 7.1) and added to the sieved and dried soil samples (2 ml g of soil−1) to final concentrations of 20, 50, and 100 mg of RNA g (dry weight) of soil−1. The tubes containing the soil suspensions were rotated for 2 h at room temperature. After centrifugation, the soil pellets were oven dried (at 50°C overnight). Lambda DNA was then added to the soils (0, 20, or 50 μg g [dry weight] of soil−1) to imitate the fate of DNA released after cell lysis. DNA was extracted according to protocol 2. We later found that the same effect of RNA addition on DNA recovery could be achieved by adding RNA directly to the extraction buffer. This simplified procedure was used for the clay soil (soil 5) in the experiments where microorganisms were inoculated into the soils. RNA was then added at a concentration corresponding to 50 mg of RNA g (dry weight) of soil−1.
Qualitative and quantitative determination of the efficiency of extraction protocols.
DNA quality (absence of degradation) was estimated based on the size of the DNA fragments or the relative position of the DNA smears after electrophoresis of an aliquot of the DNA solution on a 0.8% agarose gel. The fluorescence intensity allowed a semiquantitative estimation of the extraction yields.
Another aliquot was used for quantitative determinations of DNA content by dot blot hybridization and phosphorimaging. The dot blot procedure was performed as described by Simonet et al. (39). Dot blot membranes (GeneScreen Plus; Life Science Products, Boston, Mass.) were prehybridized for at least 2 h in 20 ml of a solution containing 6 ml of 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 1 ml Denhardt’s solution, 1 ml of 10% SDS, and 5 mg of salmon sperm carrier DNA. Hybridization was carried out overnight in the same solution in the presence of the labeled probe before membranes were washed twice in 2× SSC for 5 min at room temperature, then once in 2× SSC–0.1% SDS and once in 1× SSC–0.1% SDS for 30 min at hybridization temperature. Hybridization signals were quantified with a radioanalytical imaging system (Molecular Analyst Software; Bio-Rad, Ivry sur Seine, France).
To quantify the total amount of DNA derived from the indigenous microflora, the different soils were extracted according to protocols 1 to 5. Nonamplified DNA was applied on the dot blot membranes and hybridized with the universal probe FGPS431 (Table 2). This probe, which hybridizes to positions 1392 to 1406 of the E. coli 16S ribosomal DNA (rDNA) gene (1), was end labeled with [α-32P]ATP by using T4 polynucleotide kinase (Boehringer Mannheim). A calibration curve was prepared from E. coli DH5α DNA. Conversion to soil bacteria implied a simplification, assuming that the average rrn copy number is 7, as for E. coli.
TABLE 2.
Primers and probes used for PCR amplification and dot blot hybridization
| Primer or probe | Targeta | Sequence (5′ to 3′) | Source or reference |
|---|---|---|---|
| FGPS431 probe | Universal (1392–1406) | ACGGGCGGTGTGT(A/G)C | 1 |
| FGPS122 primer | Bacteria (6–27) | GGAGAGTTTGATCATGGCTCAG | 1 |
| FGPS350 primer | Streptosporangium (616–635) | CCTGGAGTTAAGCCCCAAGC | This study |
| FGPS643 probe | Streptosporangium (122–142) | GTGAGTAACCTGCCCC(T/C)GACT | This study |
| R499 primer | B. anthracis | TTAATTCACTTGCAACTGATGGG | 30 |
| R500 primer | B. anthracis | AACGATAGCTCCTACATTTGGAG | 30 |
| C501 probe | B. anthracis | TTGCTGATACGGTATAGAACCTGGC | 30 |
| FGPS516 primer | S. lividans OS48.3 | TCCAGATCCTTGACCCGCAG | This study |
| FGPS517 primer | S. lividans OS48.3 | CACGACATTGCACTCCACCG | This study |
| FGPS518 probe | S. lividans OS48.3 | CCGTGAGCCGGATCAG | This study |
Positions on E. coli 16S rRNA gene are given within parentheses. For B. anthracis and S. lividans the primers and probes targeted chromosomal sequences specific to the respective organism. These sequences were not situated within the 16S rRNA gene. The cassette containing the targeted region of S. lividans is described by Clerc-Bardin et al. (4).
HindIII-digested λ DNA was used to quantify the recovery of extracellular DNA. Nonamplified extracts from soils to which phage λ DNA had been added were hybridized at 65°C with HindIII-digested λ DNA, randomly labeled by using the Klenow fragment (Boehringer Mannheim). DNA quantities were calculated by interpolation from a calibration curve prepared with purified DNA.
The total amount of DNA extracted from soils 1, 2, 3, 4, and 6 according to protocol 2 (grinding) was also quantified colorimetrically by the method of Richard (34). Briefly, DNA was mixed with concentrated HClO4 (final concentration of HClO4, 1.5 N). Of this solution, 2.5 volumes were mixed with 1.5 volume of diphenylamine (Sigma-Aldrich) and left to incubate at room temperature for 18 h, after which the OD600 was determined. The DNA in the soil extracts was quantified against a standard curve by using DNA extracted from E. coli DH5α according to standard protocols (36).
Development of a DNA quantification technique using PCR and hybridization.
For PCR amplifications, Taq DNA polymerase (Appligene Oncor, Illkirch, France) was used according to the manufacturer’s recommendations. The PCR program used for all amplifications was as follows: initial denaturation for 3 min at 95°C; then 35 cycles consisting of 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C; and a final extension for 3 min at 72°C.
Isolated and purified DNA from S. fragile was used as a template at concentrations ranging from 100 fg to 100 ng. To specifically amplify DNA from this genus, primers FGPS122 and FGPS350 (Table 2), complementary to part of the 16S rDNA, were chosen after alignment of actinomycete 16S rDNA sequences. Their specificity was tested on a collection of actinomycete strains (from Streptomyces, Streptosporangium, and other closely related genera). PCR products were hybridized with the oligonucleotide probe FGPS643 (Table 2). To mimic the level of purity routinely obtained with DNA extracted from soil, pure DNA templates from S. fragile were mixed with soil extracts resulting from treatments according to lysis protocols 4b and 5b and were purified according to protocol D. Before use, the soil extracts were treated with DNase (1 U μl−1; [Gibco BRL]) for 30 min at room temperature. The DNase was then inactivated by heating at 65°C for 10 min. Verification that inactivation had taken place was done by PCR. Concentrations of humic acids were measured spectrophotometrically (OD280 nm) against a standard curve of commercial humic acids (Sigma). Nondiluted, 10-fold-diluted, and 100-fold diluted DNase-treated soil solutions were mixed with 100 fg to 100 ng of S. fragile DNA before PCR amplification. In another series of experiments, increasing concentrations of DNA from S. hygroscopicus (from 100 pg to 1 μg) were added to the S. fragile DNA to mimic the presence of nontarget DNA and its influence on the PCR process.
Purification of crude DNA extracts.
Four methods of DNA purification were compared. DNA was extracted from 1 g (dry weight) of soil according to protocol 4a and resuspended in 100 μl of TE 8 buffer (50 mM Tris–20 mM EDTA [pH 8.0]). Purification protocol A consisted of elution through two successive Elutip d columns (Schleicher & Schuell, Dassel, Germany) (31). Protocol B consisted of elution through Sephacryl S200 (Pharmacia Biotech, Uppsala, Sweden) followed by an Elutip d column (25). Protocol C involved separation using an aqueous, two-phase system prepared with 17.9% (wt/wt) PEG 8000 (Merck, Darmstadt, Germany) and 14.3% (wt/wt) (NH4)2SO4 (59). After vortexing, the two phases were left to separate at room temperature. One milliliter of each phase was then transferred to another tube, mixed with 100 μl of the sample, and left at 4°C overnight to allow separation. The lower phase was dialyzed for 1 h through a Millipore membrane overlying a TE 7.5 buffer (10 mM Tris–1 mM EDTA at pH 7.5–1 M MgCl2) to remove excess salts. Protocol D consisted of elution through a microspin Sephacryl S400 HR column (Pharmacia Biotech) followed by an Elutip d column. Each protocol ended with an ethanol precipitation step, and the DNA was resuspended in 10 μl of TE 7.5 buffer. The efficiencies of the purification protocols were checked by PCR amplification of undiluted aliquots of the DNA solutions and of aliquots diluted 10- and 100-fold by using standard protocols (see below).
Recovery of DNA from inoculated microorganisms.
Cells, spores, and hyphae were washed twice and enumerated by plate counting or direct microscopic counts. Five-gram portions of dried, sieved soils (soils 2, 3, and 5) were inoculated with 100 μl of a suspension of S. lividans spores and hyphae at concentrations corresponding to 0, 103, 105, 107, and 109 spores g (dry weight) of soil−1 or with B. anthracis vegetative cells at concentrations corresponding to 0, 107, and 109 cells g (dry weight) of soil−1. The amounts of S. lividans hyphae were calculated on the basis of the number of spores from which they originated. After addition of bacterial suspensions, the soil samples were vigorously mixed by vortexing for 5 min before grinding. DNA was extracted according to protocol 6 (see below).
PCR amplification followed by dot blot hybridization and phosphorimaging was used to quantify the amounts of DNA recovered from bacterial cells, spores, and mycelium inoculated into the soils. DNA extraction was performed according to lysis protocol 6. PCR amplification and hybridization were carried out as described above. Primers and probes targeted chromosomal regions outside the 16S region; these were highly specific to the respective organism in order to avoid background signals. For soils seeded with B. anthracis, primers R499 and R500 were used (30), and the amplification products were hybridized with the oligonucleotide probe C501 (Table 2). For soils seeded with S. lividans, PCRs were performed with primers FGPS516 and FGPS517, and the products were hybridized with the oligonucleotide probe FGPS518 (Table 2). The amplified region was part of a cassette specifically constructed to obtain the strain OS48.3 (4). Calibration curves were in all cases obtained by using purified DNA from the target organism.
RESULTS
Choice of extraction buffer.
Twenty different soils were used to determine the optimum pH of the DNA extraction buffer. For all soils, the DNA yield increased with increasing pH of the buffer. The yield for each pH (± standard deviation), calculated as a percentage of the highest value for each soil, was as follows: for pH 6.0, 31 ± 13%; for pH 7.0, 43 ± 16%; for pH 8.0, 60 ± 14%; for pH 9.0, 82 ± 12%; and for pH 10.0, 98 ± 3%). For 16 of the soils, the highest yield was obtained at pH 10.0, while for the other 4 soils, the highest yield was obtained at pH 9.0. However, larger amounts of humic material were released at pH 10.0 than at pH 9.0 (results not shown). Therefore, pH 9.0 was chosen for all experiments reported below.
Efficiencies of DNA extraction protocols.
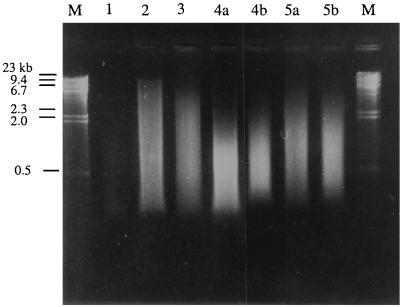
Total DNA from indigenous soil organisms was extracted and quantified in order to evaluate the efficiencies of a number of protocols for in situ cell lysis. Samples from soils 1 to 6 (Table 1) were treated according to lysis protocols 1 to 5 described in Materials and Methods (Fig. 1). After DNA extraction, the soil suspensions were precipitated with isopropanol, and aliquots of the resuspended pellets were, in a first step, analyzed by gel electrophoresis to estimate the quality and amounts of released DNA. However, the color of the DNA extract became darker as the number of lysis steps was increased due to compounds such as humic acids that coextracted with DNA. Some of these dark, crude extracts did not migrate as expected in the agarose gels (data not shown). Consequently, crude DNA solutions were purified (protocol D) prior to quantification. Gel electrophoresis of the purified solutions resulting from the different lysis treatments is exemplified by soil 3 (Fig. 2). Visual comparison under UV light of the intensities of the stained DNA allowed a semiquantitative estimation of the efficiencies of the treatments. Moreover, the presence of smears and the disappearance of long fragments indicated that degradation had taken place. No DNA could be extracted from the clay soil (soil 5).
FIG. 2.
Gel electrophoresis (0.8% agarose) of DNA extracted from 300 mg of soil 3 (Côte St André) after different lysis treatments (protocols 1 to 5 [see Fig. 1]). Lanes M, lambda DNA size marker.
A more precise quantification of DNA from all soils, extracted according to protocols 1 to 5, was made by dot blot hybridization (without prior PCR amplification) using an oligonucleotide probe complementary to a highly conserved sequence in the 16S rDNA region (probe FGPS431 [Table 2]). DNA was detected in extracts from all soils after each of the different lysis steps, with the exception of the clay soil (soil 5). The results were in good agreement with estimations after gel electrophoresis. To compare with an independent method for quantification, DNA extracted according to protocol 2 (all soils except soil 5) was also quantified by a DNA colorimetric method (34). A good correlation (r = 0.88) was found between DNA quantified by this latter technique and results obtained by dot blot hybridization and phosphorimaging (data not shown).
Dot blot hybridization showed that the amounts of extracellular DNA, as determined by extraction without any lysis treatment (protocol 1), ranged from 4 μg g−1 for the acidic soil (soil 6) to 36 μg g−1 for soil 3 (Table 3). Soil grinding (protocol 2) increased the amounts of extracted DNA from all soils (e.g., to 26 μg g of soil−1 for soil 6 and to 59 μg g−1 for soil 3) (Table 3; Fig. 2). With both grinding treatments (see Materials and Methods) a smear was detected on agarose gels, indicating that DNA molecules had been partially degraded (Fig. 2). The sizes of the DNA fragments ranged between 20 and 0.2 kb. The band intensities of the smallest fragments were very weak, indicating that the major part of the fragments were well above 1 kb. Protocol 3 included homogenization in an Ultraturrax mixer after the addition of extraction buffer to the ground soil samples. This resulted in increased amounts of extracted DNA, as determined by dot blotting, for two of the soils (the sandy loam, soil 3, and the acidic soil, soil 6), while the two organic-matter-rich soils (soils 1 and 2) yielded smaller amounts of DNA. Protocols 4a and b evaluated the influence of two types of sonication on the DNA yields from previously ground and homogenized soils. Sonication had no positive effect on the DNA yield compared to protocol 3, except for soil 6. The lysis efficiencies of the two types of sonicators differed somewhat. For soils 2 through 4 the amounts of extracted DNA were highest with the titanium microtip (Table 3; Fig. 2), while for soils 1 and 6 the DNA yield was higher with the Cup Horn device. Contradictory results were also obtained when an enzymatic and chemical lysis step (protocols 5a and b) was added after the sonication: in some cases, amounts of extracted DNA were higher than those recovered by protocols 4a and b, while in other cases they were lower (Table 3).
TABLE 3.
Amount of DNA extracted from different soils after lysis treatments according to protocols 1 to 5a
| Soil no. (origin)b | Amt of DNA (μg/g [dry wt] of soil) ± SD extracted after lysis protocol:c
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4a | 4b | 5a | 5b | |
| 1. (Australia) | 17 ± 2 | 52 ± 2 | 32 ± 5 | 16 ± 3 | 33 ± 2 | 59 ± 1 | 27 ± 0 |
| 2. (Peyrat) | 29 ± 2 | 58 ± 1 | 40 ± 2 | 29 ± 2 | 18 ± 3 | 56 ± 1 | 15 ± 1 |
| 3. (Côte St André) | 36 ± 7 | 60 ± 6 | 148 ± 10 | 94 ± 7 | 38 ± 6 | 73 ± 5 | 47 ± 6 |
| 4. (Chazay) | 9 | 16 | NDd | 32 | 15 | 15 | 70 |
| 6. (Dombes) | 4 ± 2 | 26 ± 3 | 43 ± 1 | 61 ± 1 | 66 ± 1 | 160 ± 7 | 102 ± 5 |
Quantification was performed by phosphorimaging after dot blot hybridization with the universal probe FGPS431 (Table 2).
For soils 1, 2, 3, and 6, n = 3; for soil 4, n = 1.
Protocols: 1, no treatment; 2, dry soil grinding; 3, dry soil grinding plus Ultraturrax homogenization; 4a, dry soil grinding, homogenization, and microtip sonication; 4b, dry soil grinding, homogenization, and Cup Horn sonication; 5a, dry soil grinding, homogenization, microtip sonication, and chemical and enzymatic lysis; 5b, dry soil grinding, homogenization, Cup Horn sonication, and chemical and enzymatic lysis. See also Fig. 1.
ND, not determined.
Direct microbial counts.
Microscopic counts of the total number of bacterial cells after staining with acridine orange were performed for all soils before and after grinding. Before grinding the number of bacteria per gram (dry weight) of soil ranged from 1.4 × 109 (±0.4) in the tropical soil (soil 5) to 10 × 109 (±0.7) in the soil from Côte Saint André (soil 3) (Table 1). After grinding, the cell numbers were 45, 74, 75, 54, 34, and 75% of the initial values for soils 1 through 6, respectively.
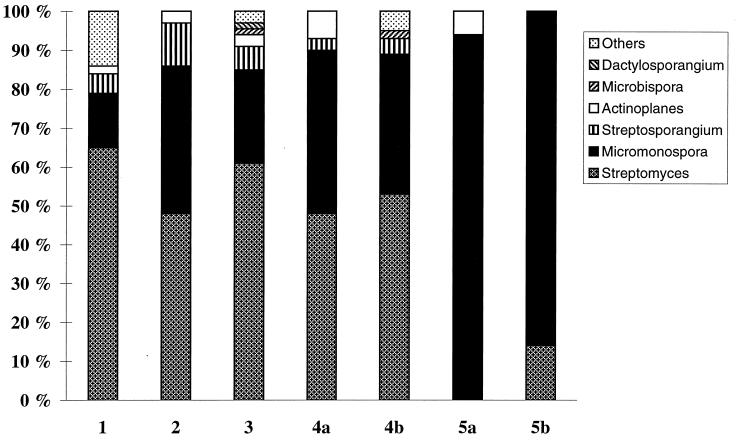
Enumeration of culturable actinomycetes belonging to different genera.
A switch in the actinomycete populations in soil 3 was noticed after the different lysis treatments (Fig. 3). For instance, colonies of Streptomyces spp. dominated the viable actinomycete flora when no lysis treatment was used (protocol 1), comprising 65% of the total number of identified colonies. After grinding, the percentage of Streptomyces colonies decreased to 51%, while the proportion of colonies belonging to the genus Micromonospora increased from 14 to 41%. The chemical and enzymatic lysis (protocols 5a and b) appeared to be particularly efficient in lysing streptomycetes. When all lysis treatments were applied, including chemical and enzymatic lysis (protocols 5a and b), the actinomycete microflora, which still comprised more than 106 CFU g of soil−1, was dominated by species belonging to the genus Micromonospora, while no or very few Streptomyces colonies were recovered. Organisms belonging to genera such as Streptosporangium, Actinomadura, Microbispora, Dactylosporangium, and Actinoplanes appeared on the plates in low numbers (2 to 8% of the total number of identified colonies) following grinding, Ultraturrax homogenization, and sonication but were generally absent when these treatments were combined with chemical and enzymatic lysis.
FIG. 3.
Proportion of actinomycetes belonging to different genera in relation to the total viable actinomycetes after lysis treatments 1 through 5 (see Fig. 1). The number of CFU was determined on a medium selecting for this group of bacteria. A total of about 400 colonies were examined.
The number of total culturable bacteria remaining after each lysis treatment (protocols 2 to 5) was also investigated for soil 4. The results indicate that the number of culturable bacteria did not decrease with the severity of the lysis treatments (about 2 × 106 CFU g of soil−1 in all cases, including when no treatment was applied, i.e., protocol 1). The reason for these low CFU was probably that dry soil was used and that only the most resistant bacteria grew on the plates. The number of colony-forming actinomycetes was generally higher than that of total CFU (all bacteria), owing to the fact that a spore germination step, included in the actinomycete detection protocol, was missing when total bacteria were monitored.
Recovery of added phage lambda.
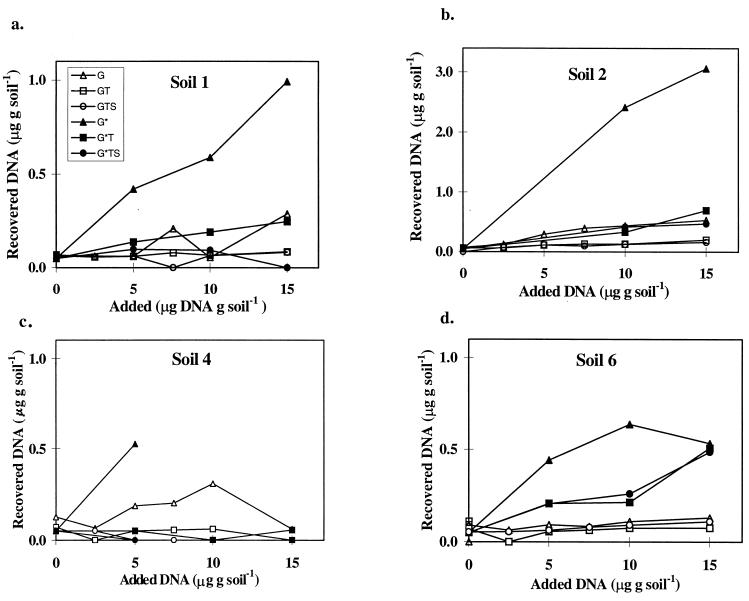
The aims of these experiments were to estimate how successive lysis treatments affect the recovery of naked DNA and whether they contribute to its degradation. This DNA could be either the extracellular DNA fraction released from previously dead organisms, which can persist in soil for months (54), or DNA released from easily lysed organisms during the initial stages of the treatment. To mimic this, HindIII-digested lambda phage DNA was added to the soils at various concentrations before and after grinding. In addition to grinding, a combination of other lysis treatments was tested, including sonication (with the Cup Horn device; protocol 4b) and thermal shocks (see Materials and Methods). After extraction, aliquots that theoretically should contain 25 to 150 ng of lambda DNA were analyzed by gel electrophoresis (data not shown). No lambda phage-specific DNA fragments could be observed when the DNA was inoculated into the soil samples prior to grinding, irrespective of dose or soil type. When DNA was added after grinding and extracted without any additional lysis treatment, lambda phage-specific patterns were detected in the extracts from four of the five soils tested. In all these cases a direct relationship was obtained between the amount of DNA added and the intensities of the signals on agarose gels. The intensities of the signals were, however, lower than the expected signals shown by the molecular standards. Moreover, the 23-kb band was missing in several cases, indicating that long fragments were adsorbed preferentially to soil particles or were more sensitive to degradation than shorter fragments. No bands were detected in samples from the tropical soil (soil 5), which was characterized by a very high clay content (Table 1).
For a more precise quantification, the DNA recovery was determined on a phosphorimager after dot blot hybridization. By using this technique, DNA was detected in all samples, including those that had been inoculated prior to grinding, except for soil 5, in which no DNA could be detected. In all other soils, the amount of DNA extracted increased with increasing inoculum size (Fig. 4). However, recoveries of phage lambda DNA were low. When grinding was the only lysis treatment applied, recoveries ranged between 0.6 and 5.9% of DNA added prior to grinding and between 3.6 to 24% of DNA added after grinding. The highest recoveries were obtained from soil 2.
FIG. 4.
Recovery of HindIII-digested phage lambda DNA added to soils at different concentrations before (G) or after (G∗) grinding. T (thermal shocks) and S (sonication) are additional lysis treatments. Quantification was carried out by phosphorimaging after dot blot hybridization. For each soil, one sample was used for each concentration of phage lambda DNA added. Soil characteristics are described in Table 1. The samples corresponding to 10 and 15 μg of added DNA, soil 4, were lost during preparation.
Gel electrophoresis of aliquots from samples treated with thermal shocks or thermal shocks plus sonication did not result in any bands from any samples, including the trial in which the DNA was added after grinding. Dot blot hybridization confirmed these results. Hybridization signals obtained from soil suspensions that had been treated with thermal shocks or thermal shocks plus sonication were weak at best (data not shown). The sample with the largest amount of DNA (15 μg of DNA g [dry weight] of soil−1) was the only one for which the signal obtained differed somewhat from the background level. No differences or very small differences were observed between samples treated with thermal shocks and those treated with thermal shocks and sonication, indicating that thermal shocks had a detrimental effect on the DNA. Recoveries were highest for soil 2, which had the highest organic-matter content (Table 1), while no DNA was recovered from the clay soil (soil 5).
Additional experiments were conducted with unground samples from soils 4 and 5, which were seeded with 20 and 50 μg of lambda DNA g of soil−1. The samples were extracted either immediately or after a 1-h incubation at 28°C, whereupon the DNA extracts were purified and analyzed by gel electrophoresis. Incubation of soil 4 for 1 h after inoculation did not result in patterns that were qualitatively or quantitatively different from those obtained without incubation or from those observed previously when DNA was added after grinding. This indicates that enzymatic degradation by soil nucleases would not be involved in the low level of DNA recovery. Moreover, omission of the grinding step did not increase the recovery of DNA from soil 5, indicating that soil structure modifications due to grinding did not significantly increase the adsorption of nucleic acids to soil colloids (results not shown).
Saturation of adsorption sites with RNA.
Most of the patterns obtained on agarose gels did not differ significantly from the previous ones in which the RNA treatment had been omitted (data not shown). For instance, we failed to detect any band from the clay-rich soil (soil 5), irrespective of the concentrations of RNA and lambda phage DNA used. Moreover, HindIII-digested lambda DNA-specific bands remained undetectable in RNA-treated sandy loam (soil 4) when lambda DNA was added before grinding. The intensity of the bands from samples seeded with DNA after grinding increased with the RNA concentration, indicating that the treatment could have a positive effect. However, the results after hybridization and phosphorimage analysis did not confirm those of the electrophoresis. For instance, no positive effect of the RNA treatment on the recovery of DNA from the sandy loam was evident when DNA was added after grinding (data not shown). On the other hand, a positive effect of RNA treatment was found for the clay-rich soil (soil 5) when DNA was added after grinding. Although the hybridization signals for control samples did not differ from background levels, significant amounts of DNA were released from RNA-treated samples, and the signals increased with the amount of DNA added as well as with the RNA concentration. However, even for the higher RNA concentrations (100 mg g [dry weight] of soil−1), the recovery rate never exceeded 3%.
Purification of crude DNA extracts.
Of the four purification protocols tested, elution through S400 microspin columns followed by an Elutip d column (protocol D) resulted in the best amplification of undiluted DNA extracts (1 μl of extract in 50 μl of PCR mixture), as determined from gel electrophoresis of the PCR products. DNA purified by the aqueous two-phase system (protocol C) gave smaller amounts of PCR products amplified from undiluted DNA extracts. No amplification products were obtained from undiluted extracts after amplification following protocol A or B. Consequently, protocol D (see Materials and Methods) was used for all experiments in which PCR amplifications and/or dot blot hybridizations were performed.
PCR and hybridization-mediated quantification.
The first step was to determine whether the amounts of PCR product were proportional to the number of template DNA molecules initially present in the reaction tube. S. fragile DNA was used as the template (see Materials and Methods). The primers were FGPS122 and FGPS350 (Table 2). Gel electrophoresis of the PCR products showed that the band intensity increased as the concentration of templates increased. The PCR products were hybridized with the oligonucleotide probe FGPS643 (Table 2), and the signals were quantified by phosphorimaging. A good correlation (r2 = 0.98) was found between the log target number and the log hybridization signal intensity (results not shown).
We then investigated whether the efficiency of the PCR amplification was affected by humic acids and nontemplate DNA. Analysis by gel electrophoresis showed that the increased band intensity of the PCR products, corresponding to different amounts of template DNA, was maintained when amplification was carried out with DNA solutions to which DNase-treated soil extracts, containing humic acids at concentrations up to 8 ng in the 50-μl PCR mix, had been added. With 20 ng of humic acids in the PCR mix, bands corresponding to low template levels disappeared, and at humic acid concentrations of 80 ng and above, no bands were visible (data not shown). The various amounts of S. fragile template DNA provided the expected amounts of PCR products when they were mixed, prior to amplification, with S. hygroscopicus DNA and added to the 50-μl PCR mixture in a range from 100 pg to 1 μg to mimic nontarget DNA released from the soil microflora (data not shown).
Quantification of an indigenous soil actinomycete after different lysis treatments.
Purification protocol D, followed by PCR amplification as described above, was applied to quantify actinomycetes belonging to the genus Streptosporangium in soil 3 after extraction according to protocols 1, 2, 3, 5a, and 5b (Fig. 5). After grinding (protocol 2), the amount of template DNA originating from this actinomycete was estimated by dot blot hybridization and phosphorimaging to be 2.5 ± 1.3 ng g of dry soil−1. Assuming a DNA content of 10 fg cell−1, as for Streptomyces (9), this corresponds to approximately 2.5 × 105 genomes. Similar values were obtained after the other lysis treatments (2.6 ± 1.1 and 1.8 ± 1.3 ng of DNA g of dry soil−1 with protocols 3 and 4b, respectively).
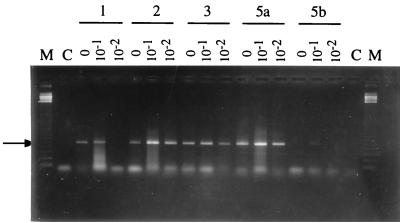
FIG. 5.
PCR amplification of DNA extracted from soil 3 according to lysis protocols 1, 2, 3, 5a, and 5b. Primers FGPS122 and FGPS350 (Table 2) were used to target indigenous Streptosporangium spp. The DNA extract either was undiluted or was diluted 10- or 100-fold. Lanes M, 123-bp molecular marker (Gibco BRL); lanes C, control without DNA. Arrow indicates the 513-bp amplification product.
Efficiency of DNA recovery from soils previously inoculated with bacteria.
Three soils (soils 2, 3, and 5) were inoculated with spores or hyphae from S. lividans at different concentrations (see Materials and Methods). The quantities of mycelium added to the soil (Fig. 6b) corresponded to the number of spores inoculated into the germination medium. Of these, approximately 50% germinated. The exact number of cells in the hyphae of the germinated spores was not determined. Therefore, the amounts of spores and mycelium seeded to the soils are not directly comparable. For each soil sample, extraction protocol 6, purification protocol D, and PCR amplification combined with dot blot hybridization and phosphorimaging were used to enumerate specific DNA targets that had been released. The extracted DNA could be clearly distinguished from the background only when the number of spores added exceeded 105 for soils 3 and 5 and 107 for soil 2 (Fig. 6a). When mycelium was added, extracted DNA could be detected above an amount corresponding to 103 spores g of soil−1 for soils 2 and 3 and above 107 spores g of soil−1 for soil 5 (Fig. 6b). Above the detection level, the hybridization signal increased with increasing amounts of inoculated cells. For the spore inoculum, a 100-fold increase in the number of seeded cells resulted in almost a 100-fold increase in DNA yield. This increase was clearly lower when hyphae were inoculated, especially in soils 2 and 3 (Fig. 6). In contrast to the results obtained when phage lambda DNA was used as the inoculum, DNA was also recovered from the clay-rich soil (soil 5) when bacterial cells were used as the inoculum. RNA treatment increased the recovery of Streptomyces DNA from this soil for both spores and mycelium (Fig. 6). Seeding soils with vegetative cells of B. anthracis resulted in recovery rates similar to those obtained for Streptomyces. Furthermore, for this inoculum also, the recovery rates from soil 5 increased after RNA treatment (results not shown).
FIG. 6.
Amounts of DNA recovered from S. lividans OS48.3 spores (a) and mycelium (b) inoculated into soils at different concentrations. The quantities of mycelium added to the soil corresponded to the number of spores inoculated into the germination medium. Of these, about 50% germinated. The exact number of cells or genomes in the hyphae of the germinated spores was not determined. Therefore, the amounts of inoculated spores and mycelium are not directly comparable. Extraction was carried out by lysis protocol 6 (see Materials and Methods). A prime (′) indicates that RNA was included in the extraction buffer. Target DNA was PCR amplified with primers FGPS516 and FGPS517. Quantification was done by phosphorimaging after dot blot hybridization using the oligonucleotide probe FFPS518. For each soil, one sample was used for each concentration of hyphae or spores. Soil characteristics are described in Table 1.
DISCUSSION
Adsorption of DNA to soil colloids.
The adsorption of DNA to soil colloids (23, 27, 29) probably represents one of the major sources of error when DNA is extracted from soil. To address this problem, we seeded the soil samples with HindIII-digested DNA from phage lambda. This DNA was chosen because large amounts can be easily extracted from E. coli 1192 and because the absence of background signals in nonseeded soils permitted quantification of recoveries after different treatments. Moreover, the quality of recovered DNA could be judged on agarose gels by comparison with the seven fragments ranging from 23 to 0.6 kb. Grinding strongly modifies soil structure and may thereby affect adsorption and desorption properties. It might also affect the physical integrity of nucleic acid molecules. When naked DNA was added to the soils before grinding, no more than a few percent could be recovered. Although adding DNA after grinding increased the recoveries about 10-fold, at least 75 to 90% of the DNA still remained trapped in the soil matrix. It could be argued that the low recoveries were due to the fact that DNA was added to dry soil and that extracellular DNA behaves differently if a water film is present on the soil colloids. However, similar recovery rates were found when lambda DNA was added to a soil slurry (19).
Ogram et al. (27) showed an inverse relationship between adsorption coefficient and DNA fragment length for six of eight soils. This is contradictory to our results, where the intensity of the largest lambda restriction fragment was lower than that of the smaller ones in samples to which phage DNA had been added after grinding, suggesting that adsorption increased with the size of the DNA molecules. One reason for the discrepancy between the results could be that the soils used in the investigation by Ogram et al. (27) were not ground. Another reason for the decreased amounts of long-fragment DNA could, theoretically, be enzymatic degradation or shearing of the DNA molecules. In light of the results from the 1-h incubation experiment using unground soil and the fact that DNA in this case was not subjected to any lysis treatment, it seems unlikely that the observation was due to shearing or degradation. No bands were detected on agarose gels when the lambda DNA was added before grinding, and we were therefore not able to determine whether grinding affected the integrity of the molecules. Even if grinding had resulted in shearing of the DNA, it should still have played only a minor role, since the major part of the DNA remained unrecovered without grinding. Other lysis treatments, such as thermal shocks or sonication, used alone or in combination with grinding, further reduced the recovery. Therefore, if these treatments were applied after grinding, a significant part of the nucleic acids released in the soil solution from actively lysed bacterial cells would escape extraction, thus contributing to serious biases in the results. Moreover, our results indicate that adsorption, and consequently recovery levels, is directly related to soil composition. For instance, we were not able to recover any DNA from the clay-rich soil (soil 5), even when very large amounts (up to 50 μg) were added.
Saturation of adsorption sites.
Certain molecules are known to adsorb to soil colloids in a manner similar to that of DNA (52). We chose RNA so as not to increase the concentration of proteins which might inhibit PCRs (57), since we wanted to avoid increased purification problems. Including RNA in the extraction procedure increased the DNA recovery for soils with a high clay content to the same levels as those obtained with the other soils (Fig. 6). For soils with a lower clay content, the use of RNA did not improve recovery. It can thus be hypothesized that RNA and DNA only partly compete for the same adsorption sites.
Lysis treatments.
An efficient lysis procedure should affect all cell types, including spores and other structures with resistant cell walls. In several studies based on in situ cell lysis, chemical and enzymatic lysis alone or in combination with freeze-thawing has been performed according to the method described by Tsai and Olson (49). There is, however, a risk that these procedures will favor the lysis of gram-negative bacteria (24). Moreover, bacteria might be protected from lysis if localized in inner soil compartments or if strongly adsorbed to soil colloids. In an effort to overcome this, we used successive treatments based on a combination of mechanical and chemical-enzymatic lysis. The efficiency of the treatments was evaluated in terms of both the quantity of extracted DNA and the number of bacteria that escaped lysis.
Grinding permits homogenization of the soil, otherwise characterized by its heterogeneity. It increases the release of bacteria from inner compartments, thus making them available for subsequent lysis treatments, and contributes to the lysis of bacterial cell walls, resulting in the release of their DNA. Efficient soil aggregate disruption and cell lysis can also be accomplished by bead beating using a cell homogenizer (24, 40). The DNA yield from different soils extracted by a protocol based on this technique ranged between 2 and 35 μg g of soil−1 (40, 51). In our study we obtained 16 to 59 μg of DNA g of soil−1 following extraction protocol 2 (grinding only). Although these investigations were based on different soils, this indicates that soil grinding is equal to, or more efficient than, bead beating if a high yield is desired. This is further indicated by comparison of our data from microscopic counts with the findings of Moré et al. (24), who enumerated sediment bacteria before and after glass bead beating. Soil grinding decreased the number of bacteria in different soils to 34 to 75% of the initial value, and bead beating decreased the number of sediment bacteria to 26%. The study by Moré et al. (24) showed that small bacterial cells (size fraction, 1.2 to 0.3 μm) were much more difficult to lyse than larger bacteria (2 to 10 μm). Of these, only the very smallest size fraction reported in the study by Moré et al. (24) are found in soil. The major part of soil bacteria (50 to 70% of total bacterial numbers in nonrhizosphere soil) are <0.5 μm in diameter (28) and thus are comparable to the fraction of nonlysed cells in the study by Moré et al (24). Lysis efficiency estimated by microscopic counts was also reported by Zhou et al. (60) with eight different soils. In that study cells were lysed in situ by an SDS-based extraction which did not include any mechanical treatment. The number of cells decreased 67 to 92%, but the DNA yield was generally several times lower compared to that of the soils in the present investigation (for five of the soils, the yield after purification was <4 μg of soil−1).
The yield of DNA from the different soils (Table 2) was presented in relation to the dry weight of the soils, for comparison with other investigations. If instead it is calculated in relation to the organic-matter content, the values should be very similar for the different soils, since the microbial biomass per gram of organic C is fairly constant, as shown, for example, by the amount of phospholipids extracted from a range of different surface soils (7). For soils 1 and 2 in the present investigation (organic matter content, 5.0 and 4.8%, respectively [Table 1]), the DNA yield ranged from 0.5 to 1.2 and from 0.4 to 1.2 mg g of organic matter−1, respectively, for the different lysis treatments (recalculation of values presented in Table 2). For soils 3, 4, and 6, the corresponding values were 1.2 to 3.7, 1.1 to 5.0, and 0.9 to 5.3 mg of DNA g of organic matter−1. It thus seems that, even though grinding appears to be an efficient method for in situ lysis of soil bacteria, the yield is lower than expected for organic soils. This could be due to less-efficient lysis, but no such conclusion could be drawn from the microscopic counts (Table 1). Instead, it is more probable that the difference is related to the extraction efficiency.
Grinding increased the DNA yield compared with the amount of extracellular DNA obtained when no lysis treatment was performed, but, surprisingly, there was no clear tendency for subsequent treatments of the soil solution to further increase the DNA yield (Fig. 2; Table 3). This was demonstrated when total DNA was hybridized by using a universal probe (Table 3) and also when a specific indigenous organism (S. fragile) was traced (Fig. 5). While thermal shocks did not increase the DNA yield from bacterial cells, they did release humic material. This treatment also decreased the recovery of naked DNA, as shown for inoculated phage lambda (Fig. 4), and was therefore excluded from the final protocol (protocol 6).
Although the bacterial counts remained constant, there was a shift in the community composition of culturable actinomycetes (Fig. 3). This demonstrates the insensitivity of certain microorganisms, such as those belonging to the genus Micromonospora, to most lysis treatments. The only treatment after which no culturable bacteria were found was thermal shocks (data not shown). In addition to differences between bacterial groups in terms of the ease with which they can be lysed, the treatments may release bacteria from different compartments in the soil and/or release bacteria that adhere more or less strongly to soil colloids. Another important factor influencing the viable counts could be that treatments such as homogenization and sonication will disperse bacterial aggregates and thus increase the number of CFU.
The reason for using dried soil in the present study was that grinding is much more powerful in terms of lysis efficiency when dry soil is used compared to fresh soil (results not shown). It is likely that the larger amounts of extracellular DNA obtained in the present study were due to the use of dried soils. Bacteria which are sensitive to drying will be killed, and their DNA will be released. Moreover, drying has physical and chemical implications on the soil which might have affected the results. There was, however, no indication that adsorption of DNA to soil particles increased due to drying, since the same recoveries were obtained as when lambda DNA was added to wet soil (19). Drying of soils is a way to conserve samples for long periods of time that is used by many laboratories. During the development and evaluation of new techniques, it is extremely important to use reference soils which do not change over time. This allows one to compare different techniques without having to perform them all at the same time, while ensuring that observed differences are due to the different techniques (in our case, the lysis treatments) and are not artifacts created by biological and/or chemical changes in the soil over time due to storage. It thus also facilitates repetition of experiments. Another area of interest for the use of dry soils is long-term field experiments in which samples are taken regularly and stored as reference material, permitting studies of temporal or seasonal changes in microbial populations, as well as the possibility for different research groups to perform studies on the same soil samples. Moreover, soil drying is a natural phenomenon which occurs regularly in several climatic regions. The use of dried or freeze-dried soil is therefore relevant for a range of scientific problems.
Quantification.
Targeting an indigenous microorganism (S. fragile), we extracted DNA, which was then amplified and quantified. However, since numerous cells obviously escape lysis, we do not know the extent to which the values were underestimated. In an effort to clarify some of these problems, organisms inoculated into soils were also traced. Although such experiments will not mimic the situation in the field with indigenous microorganisms, they can still be justified because they demonstrate the fate of organisms that have been introduced into soils, either deliberately or accidentally. Also, if no reliable quantitative results are obtained under these conditions, there would be good reason to suspect that serious methodological problems exist. In our experiments the quantities of recovered DNA derived from bacterial vegetative cells, spores, or hyphae increased with the number of seeded cells, but the recoveries were variable and differed between soils (Fig. 6). In some cases only a few percent were recovered. Interestingly, the highest recoveries (from 30 to >100%) were obtained from the high-clay soil (soil 5) when RNA was included in the extraction buffer. Streptomyces DNA could, however, be recovered from this soil even without RNA treatment, in contrast to the results obtained after inoculation with phage lambda DNA. One explanation could be that cell debris somehow protect DNA from adsorption to soil colloids. The results suggest that extraction and quantification of DNA after direct in situ lysis is possible even for soils with a high clay content.
Conclusions.
Methods using in situ cell lysis followed by DNA extraction and quantification offer new ways of studying microorganisms in the environment that make it possible to circumvent biases related to cultivation. However, other problems still prevent the detection of a large part of the soil microbial community. For instance, the lysis treatment is ineffective with some organisms, either because they are resistant to the treatments or because they are protected by soil structures. Grinding, which efficiently homogenizes the soil sample, seems to be the most efficient lysis treatment available today, especially in cases where the device consists of hard material such as tungsten. Moreover, it is a fast method and allows a large number of samples to be handled. For clay soils, the extraction protocol should include an RNA treatment in order to decrease the adsorption of liberated DNA. Purification of the DNA extract is a prerequisite for efficient PCR amplification. Methods of extract purification need to be improved, especially for the study of soils with a high organic-matter content. More effort should be put into optimizing the PCR amplification technique, either by combination with dot blot hybridization and phosphorimaging or by inclusion of internal standards in the PCR, which seem to be promising ways of estimating the size of a targeted population.
ACKNOWLEDGMENTS
The work was supported by the Swedish Council for Forestry and Agricultural Research (Å.F.), EC contract ERBIC18CT970198 (X.N), and by Délégation Général pour l’Armement no. 96/44.108.ETCA/CEB/B (V.R.).
We thank J.-M. Lazzaroni, University of Lyon, for providing E. coli 1992 and M. Mock, Institut Pasteur-Paris, for providing B. anthracis (Sterne 7700).
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakken L R. Separation and purification of bacteria from soil. Appl Environ Microbiol. 1985;49:1482–1487. doi: 10.1128/aem.49.6.1482-1487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clegg C D, Ritz K, Griffiths B S. Direct extraction of microbial community DNA from humified upland soils. Lett Appl Microbiol. 1997;25:30–33. doi: 10.1046/j.1472-765x.1997.00166.x. [DOI] [PubMed] [Google Scholar]
- 4.Clerc-Bardin, S., J.-L. Pernodet, Å. Frostegård, and P. Simonet. Development of a conditional suicide system for a Streptomyces lividans strain and its use to investigate conjugative transfer in soil. Submitted for publication.
- 5.Engelen B, Meinken K, Von Wintzingerode F, Heuer H, Malkomes H-P, Backhaus H. Monitoring impact of a pesticide treatment on bacterial soil communities by metabolic and genetic fingerprinting in addition to conventional testing procedures. Appl Environ Microbiol. 1998;64:2814–2821. doi: 10.1128/aem.64.8.2814-2821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrelly V, Rainey F A, Stackebrandt E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl Environ Microbiol. 1995;61:2798–2801. doi: 10.1128/aem.61.7.2798-2801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frostegård Å, Tunlid A, Bååth E. Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods. 1991;14:151–163. [Google Scholar]
- 8.Giddings G. The release of genetically engineered micro-organisms and viruses into the environment. New Phytol. 1998;140:173–184. doi: 10.1046/j.1469-8137.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 9.Gladek A, Zakrzewska J. Genome size of Streptomyces. FEMS Microbiol Lett. 1984;24:73–76. [Google Scholar]
- 10.Hayakawa M, Ishizawa K, Nonomura H. Distribution of rare actinomycetes in Japanese soils. J Ferment Technol. 1988;66:367–374. [Google Scholar]
- 11.Hayakawa M, Nonomura H. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J Ferment Technol. 1987;65:501–509. [Google Scholar]
- 12.Hickey R J, Tresner H D. A cobalt-containing medium for sporulation of Streptomyces species. J Bacteriol. 1952;64:891–892. doi: 10.1128/jb.64.6.891-892.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hintermann G, Crameri R, Kieser T, Hütter R. Restriction analysis of the Streptomyces glaucescens genome by agarose gel electrophoresis. Arch Microbiol. 1981;130:218–222. [Google Scholar]
- 14.Holben W E, Jansson J K, Chelm B K, Tiedje J M. DNA probe method for the detection of specific microorganisms in the soil bacterial community. Appl Environ Microbiol. 1988;54:703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of streptomyces—a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 16.Jacobsen C S, Rasmussen O F. Development and application of a new method to extract bacterial DNA from soil based on separation of bacteria from soil with cation-exchange resin. Appl Environ Microbiol. 1992;58:2458–2462. doi: 10.1128/aem.58.8.2458-2462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ka J O, Holben W E, Tiedje J M. Analysis of competition in soil among 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl Environ Microbiol. 1994;60:1121–1128. doi: 10.1128/aem.60.4.1121-1128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuske C R, Banton K L, Adorada D L, Stark P C, Hill K K, Jackson P J. Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl Environ Microbiol. 1998;64:2463–2472. doi: 10.1128/aem.64.7.2463-2472.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S-Y, Bollinger J, Bezdicek D, Ogram A. Estimation of the abundance of an uncultured soil bacterial strain by a competitive quantitative PCR method. Appl Environ Microbiol. 1996;62:3787–3793. doi: 10.1128/aem.62.10.3787-3793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leff L G, Dana J R, McArthur J V, Shimkets L J. Comparison of methods of DNA extraction from stream sediments. Appl Environ Microbiol. 1995;61:1141–1143. doi: 10.1128/aem.61.3.1141-1143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liesack W, Janssen P H, Rainey F A, Ward-Rainey N L, Stackebrandt E. Microbial diversity in soil: the need for a combined approach using molecular and cultivation techniques. In: Van Elsas J D, Trevors J T, Wellington E M H, editors. Modern soil microbiology. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 375–439. [Google Scholar]
- 22.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorentz M G, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moré M I, Herrick J B, Silva M C, Ghiorse W C, Madsen E L. Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl Environ Microbiol. 1994;60:1572–1580. doi: 10.1128/aem.60.5.1572-1580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesme X, Picard C, Simonet P. Specific DNA sequences for detection of soil bacteria. In: Trevors J T, van Elsas J D, editors. Nucleic acids in the environment, methods and application. Springer lab manual. Berlin, Germany: Springer-Verlag; 1995. pp. 111–139. [Google Scholar]
- 26.Ogram A, Sayler G S, Barkay T. The extraction and purification of microbial DNA from sediments. J Microbiol Methods. 1987;7:57–66. [Google Scholar]
- 27.Ogram A V, Mathot M L, Harsh J B, Boyle J, Pettigrew C A., Jr Effects of DNA polymer length on its adsorption to soils. Appl Environ Microbiol. 1994;60:393–396. doi: 10.1128/aem.60.2.393-396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen R A, Bakken L R. Viability of soil bacteria: optimization of the plate-counting technique. Microb Ecol. 1987;13:59–74. doi: 10.1007/BF02014963. [DOI] [PubMed] [Google Scholar]
- 29.Paget E, Jocteur Monrozier L, Simonet P. Adsorption of DNA on clay minerals: protection against DNase I and influence on gene transfer. FEMS Microbiol Lett. 1992;97:31–40. [Google Scholar]
- 30.Patra G, Sylvestre P, Ramisse V, Thérasse J, Guesdon J-L. Isolation of a specific chromosomic DNA sequence of Bacillus anthracis and its possible use in diagnosis. FEMS Immunol Med Microbiol. 1996;15:223–231. doi: 10.1111/j.1574-695X.1996.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 31.Picard C, Ponsonnet C, Nesme X, Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992;58:2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priemé A, Sitaula J I B, Klemedtsson Å K, Bakken L R. Extraction of methane-oxidizing bacteria from soil particles. FEMS Microbiol Ecol. 1996;21:59–68. [Google Scholar]
- 33.Prosser J. Molecular marker systems for detection of genetically engineered micro-organisms in the environment. Microbiology. 1994;140:5–17. doi: 10.1099/13500872-140-1-5. [DOI] [PubMed] [Google Scholar]
- 34.Richard G M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974;57:369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- 35.Romanowski G, Lorentz M G, Wackernagel W. Use of polymerase chain reaction and electroporation of Escherichia coli to monitor the persistence of extracellular plasmid DNA introduced into natural soils. Appl Environ Microbiol. 1993;59:3438–3446. doi: 10.1128/aem.59.10.3438-3446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Shirling E B, Gottlieb D. Methods for characterization of Streptomyces species. Int J Syst Bacteriol. 1966;16:313–340. [Google Scholar]
- 38.Siefert J L, Fox G E. Phylogenetic mapping of bacterial morphology. Microbiology. 1998;144:2803–2808. doi: 10.1099/00221287-144-10-2803. [DOI] [PubMed] [Google Scholar]
- 39.Simonet P, Normand P, Moiroud A, Bardin R. Identification of Frankia strains in nodules by hybridization of polymerase chain reaction products with strain-specific oligonucleotide probes. Arch Microbiol. 1990;153:235–240. doi: 10.1007/BF00249074. [DOI] [PubMed] [Google Scholar]
- 40.Smalla K, Cresswell N, Mendonca-Hagler L, Wolters A, van Elsas D J. Rapid DNA extraction protocol from soil for polymerase chain reaction-mediated amplification. J Appl Bacteriol. 1993;74:78–85. [Google Scholar]
- 41.Smit E, Leeflang P, Wernars K. Detection of shifts in microbial community structure and diversity in soil caused by copper contamination using amplified ribosomal DNA restriction analysis. FEMS Microbiol Ecol. 1997;23:249–261. [Google Scholar]
- 42.Stackebrandt E. Phylogenetic relationships vs. phenotypic diversity: how to achieve a phylogenetic classification system of the eubacteria. Can J Microbiol. 1988;34:552–556. doi: 10.1139/m88-094. [DOI] [PubMed] [Google Scholar]
- 43.Staneck J L, Roberts G D. Simplified approach to identification of aerobic Actinomycetes by thin-layer chromatography. Appl Microbiol. 1974;28:226–231. doi: 10.1128/am.28.2.226-231.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stapleton R D, Ripp S, Jimenez L, Cheol-Koh S, Fleming J T, Gregory I R, Sayler G S. Nucleic acid analytical approaches in bioremediation: site assessment and characterization. J Microbiol Methods. 1998;32:165–178. [Google Scholar]
- 45.Steffan R J, Goksøyr J, Bej A K, Atlas R. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988;54:2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tebbe C C, Vahjen W. Interference of humic acids and DNA extracted directly from soil in detection and transformation of recombinant DNA from bacteria and a yeast. Appl Environ Microbiol. 1993;59:2657–2665. doi: 10.1128/aem.59.8.2657-2665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torsvik V, Søorheim R, Goksøyr J. Total bacterial diversity in soil and sediment communities—a review. J Ind Microbiol. 1996;17:170–178. [Google Scholar]
- 48.Torsvik V L. Isolation of bacterial DNA from soil. Soil Biol Biochem. 1980;12:15–21. [Google Scholar]
- 49.Tsai Y-L, Olson B. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Elsas J D, Mäntynen V, Wolters A C. Soil DNA extraction and assessment of the fate of Mycobacterium chlorophenolicum strain PCP-1 in different soils by 16S ribosomal RNA gene sequence based most-probable-number PCR and immunofluorescence. Biol Fert Soils. 1997;24:188–195. [Google Scholar]
- 51.Van Elsas J D, Duarte G F, Rosado A S, Smalla K. Microbiological and molecular biological methods for monitoring microbial inoculants and their effects in the soil environment. J Microbiol Methods. 1998;32:133–154. [Google Scholar]
- 52.Volossiouk T, Robb E J, Nazar R N. Direct DNA extraction for PCR-mediated assays. Appl Environ Microbiol. 1995;61:3972–3976. doi: 10.1128/aem.61.11.3972-3976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waksman S A. The actinomycetes. Classification, identification and description of genera and species. Vol. 2. Baltimore, Md: The Williams and Wilkins Co.; 1961. [Google Scholar]
- 54.Ward D M, Weller R, Bateson M M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990;344:63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- 55.Widmer F, Seidler R J, Watrud L S. Sensitive detection of transgenic plant marker gene persistence in soil microcosms. Mol Ecol. 1996;5:603–613. [Google Scholar]
- 56.Williams S T, Locci R, Beswick A, Kurtböke D I, Kuznetsov V D, Le Monnier F J, Long P F, Maycroft K A, Palma R A, Petrolini B, Quaroni S, Todd J I, West M. Detection and identification of novel actinomycetes. Res Microbiol. 1993;144:653–656. doi: 10.1016/0923-2508(93)90069-e. [DOI] [PubMed] [Google Scholar]
- 57.Wilson I G. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63:3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaslavsky B Y. Separation of biomolecules. In: Zaslavsky Boris Y., editor. Aqueous two-phase partitioning. Physical Chemistry and Bioanalytical Applications. New York: Marcel Dekker, Inc.; 1995. pp. 503–667. , N. Y. [Google Scholar]
- 60.Zhou J, Bruns M A, Tiedje J M. DNA recovery from soils of diverse composition. Appl Environ Microbiol. 1996;62:316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]