Significance
Transmission of malarial parasites occurs via the bites of Anopheles mosquitoes, whose blood-feeding behavior modulates the risk of infection. In many malaria endemic regions, eradication strategies rely on reducing transmission by targeting nocturnal blood-feeding Anopheles with insecticidal nets. However, a proportion of mosquitoes may naturally feed when humans are not protected by nets, setting a ceiling to the efficacy of massive net-based interventions. In Bangui, Central African Republic, 20 to 30% of daily exposure to indoor bites occurs during daytime, and this fraction may correspond to mosquitoes escaping exposure to current vector control measures. Knowledge about the daily rhythmicity of mosquito biting is therefore crucial to adjust vector control tactics to protect people at places where they spend daytime.
Keywords: Anopheles, biting behavior, residual malaria, vector control, Central African Republic
Abstract
Malaria control interventions target nocturnal feeding of the Anopheles vectors indoors to reduce parasite transmission. Mass deployment of insecticidal bed nets and indoor residual spraying with insecticides, however, may induce mosquitoes to blood-feed at places and at times when humans are not protected. These changes can set a ceiling to the efficacy of these control interventions, resulting in residual malaria transmission. Despite its relevance for disease transmission, the daily rhythmicity of Anopheles biting behavior is poorly documented, most investigations focusing on crepuscular hours and nighttime. By performing mosquito collections 48-h around the clock, both indoors and outdoors, and by modeling biting events using circular statistics, we evaluated the full daily rhythmicity of biting in urban Bangui, Central African Republic. While the bulk of biting by Anopheles gambiae, Anopheles coluzzii, Anopheles funestus, and Anopheles pharoensis occurred from sunset to sunrise outdoors, unexpectedly ∼20 to 30% of indoor biting occurred during daytime. As biting events did not fully conform to any family of circular distributions, we fitted mixtures of von Mises distributions and found that observations were consistent with three compartments, corresponding indoors to populations of early-night, late-night, and daytime-biting events. It is not known whether these populations of biting events correspond to spatiotemporal heterogeneities or also to distinct mosquito genotypes/phenotypes belonging consistently to each compartment. Prevalence of Plasmodium falciparum in nighttime- and daytime-biting mosquitoes was the same. As >50% of biting occurs in Bangui when people are unprotected, malaria control interventions outside the domiciliary environment should be envisaged.
Transmission of parasites of the genus Plasmodium that are the causative agents of human malaria is considered to occur mainly from sunset to sunrise, when their vectors, mosquitoes of the genus Anopheles, feed on human hosts that are at rest or asleep (1, 2). This principle is so firmly established that sampling protocols to measure the strength of transmission usually disregard diurnal Anopheles feeding (3). This has not always been so: sampling routines implemented in the early 20th century by medical entomologists generally covered the whole 24-h biting cycle of different mosquito species (4). It is generally assumed that nighttime blood-feeding evolved because hosts are less active at this time, so that mosquitoes incur a lower risk of being swatted or chased away, enabling higher feeding success (2). Human hosts, however, most often rest indoors; many human-biting anophelines, therefore, penetrate inside households in order to have access to and feed on humans. Current mainstay vector control tactics exploit these behaviors in order to reduce transmission by means of two interventions: protecting humans under long-lasting insecticidal bed nets (LLINs), and spraying houses with residual insecticides (i.e., indoor residual spraying, IRS) (5). Additional benefits of IRS come from the habit of some malaria vectors to rest inside dwellings, using them as refugia either before or after blood-feeding (1).
Despite the incontestable success of these control interventions in reducing the burden of malaria (6), a plateau in the incidence rate of malaria cases has been observed in Africa during the last years (7). This could be at least in part explained by the intensive selection pressure put up by insecticides (8). Indeed, mutations conferring insecticide resistance have rapidly emerged (9), and resistance to different classes of insecticides is presently widespread in most malaria vector populations (10, 11). Moreover, there is evidence that some vector populations are changing their behavior in response to control interventions by feeding progressively more at places and at times when humans are less likely to be protected (12, 13). So far, behavioral modifications have resulted in more biting during the evening and early morning and out-of-doors (14–21). Recent studies reporting significant amounts of daytime biting, however, remain somewhat anecdotal, with few exceptions. Overall, these modifications increase the window of opportunity for human–vector contact, producing what is called residual malaria transmission (22, 23): this expression implies that transmission will persist even if LLINs and IRS are fully effective. It is feared, therefore, that the malaria elimination target set upon current control interventions may be compromised in the long term by residual malaria transmission (24). Countering this phenomenon should be based on better knowledge of the biology and behavior of the vectors, with the aim to develop more suitable interventions (12, 23, 25).
In the Central African Republic, malaria remains a major public health problem and the main cause of deaths among children <5 y old (26). In 2006, the National Malaria Control Program implemented the first phase of the Global Fund Program for Malaria based on free distribution of LLINs to pregnant women and children <5 y old, with moderate results (26, 27). A new campaign of mass LLIN distribution was deployed in 2015. Yet, malaria incidence was not significantly reduced (28). Several studies reported the presence in the country of populations of the major malaria vectors Anopheles gambiae, Anopheles coluzzii, and Anopheles funestus that are resistant to pyrethroids, the class of insecticides used for impregnation of bed nets (29–31). It is not known, however, the degree to which insecticide resistance is responsible for such moderate reductions in malaria incidence in this country, above and beyond what can be accounted for by a national health system weakened by years of civil war and unrest.
In order to appreciate the potential of residual malaria transmission in this epidemiological context, we investigated the biting behavior of the malaria vectors occurring in urban settings of Bangui, Central African Republic. We implemented two significant modifications to the sampling protocol and analytical procedures that are usually applied in this kind of investigation. First, the sampling plan consisted of monthly sessions of 48-h around-the-clock collections of mosquitoes coming to feed on human hosts both indoors and outdoors. Second, the daily rhythmicity of the observed biting events was analyzed quantitatively using a circular statistics framework that models these events on a circumference rather than along the usual linear representation (32–34). Unexpectedly, we found that 20 to 30% of malaria vector biting occurred at full daytime indoors. These results suggest that current vector control interventions may not be enough to achieve sufficient reductions in malaria transmission in Bangui. Perhaps even more significant, these observations suggest that Anopheles mosquitoes may have the potential to achieve fundamental modifications in the temporal organization and circadian control of their feeding behavior, with major impacts on malaria control strategies in Africa. We elaborate these results as follows.
Results
Diversity and Biting Rates of Malaria Mosquitoes.
In total, 7,982 Anopheles mosquitoes were collected in Bangui between June 2016 and May 2017 at four sites within the city (Fig. 1A and Dataset S1). Morphological and molecular analyses identified 5,187 A. gambiae, 991 A. coluzzii, 812 Anopheles pharoensis, 774 A. funestus, 182 Anopheles nili, 20 A. gambiae × coluzzii hybrids, 15 Anopheles ziemanni, and 1 Anopheles moucheti (Dataset S1). All of these species are competent malaria vectors, and three of them (A. gambiae, A. coluzzii, and A. funestus) are the most important malaria vectors in tropical Africa (35). The yearly average biting rate was 22.7 daily bites per person, with the highest peak in September (35.3), and the lowest in July (14.8), corresponding to the end and the peak of the rainy season, respectively. When considering only the night period (from 1800 to 0600 hours), the yearly average biting rate was 17.9 bites per person per nighttime (range, 12.2 to 27.8). Among the collected species, A. gambiae contributed the most to the overall biting rate (11.7 bites per person per nighttime). Analysis of the feeding location showed that ∼60% of the sampled mosquitoes were caught indoors, despite the presence of LLINs in the houses where collections were carried out. Among the four predominant species, A. funestus was the most endophagic (71% of indoor collections), while A. pharoensis was the most exophagic (68% of outdoor collections) (Table 1). Unsurprisingly, >90% of the collected mosquitoes were unfed (Dataset S1).
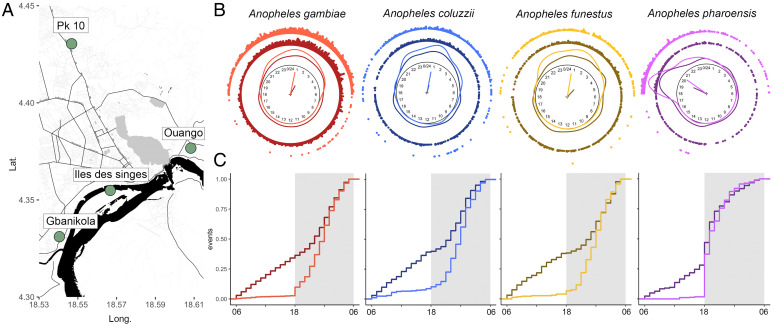
Fig. 1.
Overview of diel biting events by malaria vectors in the Central African Republic. (A) Mosquito collection sites in the city of Bangui (source: https://OpenStreetMap.org). (B) Circular representation of biting events, kernel distributions (lines), sample mean directions ⍬, and resultant lengths R depicted as arrow vectors on the unit circle for each species and location. Darker colors represent indoor collections, and lighter colors outdoor collections. (C) Linear representation of biting events during the day expressed by the cumulative sample distribution curves of the relative frequency of landings on human collectors. The gray areas represent nighttime.
Table 1.
Circular summary statistics by mosquito species and location of collection
| Uniformity | Reflective symmetry | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Location | n | ⍬ | R | Statistic | P | z | P |
| A. gambiae | Indoors | 3,167 | 1:53 | 0.332 | 0.332 | <0.001 | 4.952 | <0.001 |
| Outdoors | 2,020 | 0:46 | 0.766 | 0.766 | <0.001 | 1.470 | 0.142 | |
| A. coluzzii | Indoors | 609 | 0:57 | 0.245 | 0.246 | <0.001 | 0.743 | 0.458 |
| Outdoors | 382 | 0:43 | 0.687 | 0.687 | <0.001 | 1.781 | 0.075 | |
| A. funestus | Indoors | 551 | 2:54 | 0.300 | 0.301 | <0.001 | 3.123 | 0.002 |
| Outdoors | 223 | 0:38 | 0.737 | 0.738 | <0.001 | 0.025 | 0.980 | |
| A. pharoensis | Indoors | 345 | 19:40 | 0.465 | 0.467 | <0.001 | 1.145 | 0.252 |
| Outdoors | 467 | 20:19 | 0.796 | 0.796 | <0.001 | 7.124 | <0.001 | |
n: number of biting events; ⍬: bias-corrected sample mean direction, expressed as hour:minutes. R: bias-corrected sample mean resultant length. Uniformity tests whether biting events are evenly distributed around the 24-h circle. Reflective symmetry tests whether the distribution of biting events is symmetrical about the central direction; when the test is not rejected (marked in boldface) the sample is compatible with a symmetric distribution.
Diel Rhythms of Biting Events.
In total, 6,292 and 1,690 Anopheles were collected during nighttime (1800 to 0600 hours) and daytime (0600 to 1800 hours), respectively (Dataset S1), highlighting that we would have underestimated human exposure to vector bites by ∼22% had we resorted to the “standard” way of measuring transmission with collections carried out solely during the nighttime. In order to ensure enough statistical power, in what follows we focus the analysis on the most abundant species (n = 7,764 mosquitoes; 6,086 during nighttime and 1,678 during daytime) (Table 1 and Dataset S1). Our approach was to seek the best circular probability model compatible with the data for the four most abundant species. The nature of the model informs about how mosquito-biting rates change according to the hour of the day, and this in turn can provide a quantitative framework to investigate the processes underlying the overt manifestation of this periodic behavior. Circular statistics allow us to estimate descriptive statistics summarizing circular data, and set a formal inferential ground to test for differences in rhythms among species or populations, something not feasible with a linear approach.
In this report, we first looked and tested for fundamental properties of circular distributions—like uniformity, modality, and reflective symmetry (34)—in order to characterize the shape of the distribution of biting events. Second, we calculated sample trigonometric moments to provide estimates of the mean direction, concentration, skewness, and kurtosis of biting events. Then, we looked for differences among species, locations, months, and sites in the sample distribution of biting events using nonparametric tests. Finally, we fitted different circular probability models, and their mixtures, in order to evaluate the strength of evidence for compatibility with the data. For any given species, diel biting rhythmicity is usually presumed to remain constant in time and space within a given site or locality (24). We then tested this assumption by analyzing monthly variations in the diel distribution of biting events and second in each of the four collection sites across Bangui.
Results from these analyses indicate that there exists a variability in the number and distribution of biting events across monthly samples and through the four sites in Bangui (SI Appendix, Figs. S1–S5). This variation is mostly associated with changes in mosquito abundance due to population dynamics (SI Appendix, Tables S1 and S2 and Dataset S1), although further research is needed. Therefore, we assumed that daily rhythmicity of biting remained similar across the city and seasons. Accordingly, it seems justified to pool monthly samples of the four sites in the city by species and location in order to increase power and obtain more precise estimates of statistics and parameters. All the samples departed significantly from uniformity (Table 1 and SI Appendix, Table S4), and in three of eight cases, also from reflective symmetry (Table 1 and SI Appendix, Table S5). The sample distribution of biting events was generally complex and often not unimodal (Fig. 1B). Overall, these results indicate that the 24-h distribution of biting events does not conform to simple circular distribution models. This will be tested more formally later on.
Bias-corrected mean direction estimates of biting events showed that the mean direction of blood-feeding activity of A. gambiae, A. coluzzii, and A. funestus was at night later than midnight (⍬, between 0000 and 0300 hours) (Fig. 1B, Table 1, and SI Appendix, Table S3), while for A. pharoensis it was at dusk, with a mean direction around 2000 hours (Fig. 1B, Table 1, and SI Appendix, Table S3). We use this distinction to differentiate between the former group of species, which we designate as “nocturnal,” and A. pharoensis, which we designate as “crepuscular.” This nomenclature is only intended as a shorthand to facilitate the discussion of results, and not to define a distinct temporal window of activity given that all species could be active throughout the day. Interestingly, no major differences were observed between indoor and outdoor mean directions (darker and lighter colors, respectively, in Fig. 1B) in all four species; the biting mean direction outdoors, however, consistently preceded by ∼0.2 to 2.5 h the indoor mean direction for the more endophagic nocturnal species, whereas the reverse was observed for the more exophagic crepuscular species. As found during the analysis of the monthly samples and sites (SI Appendix, Figs. S1–S5 and Table S1), the distribution of R values showed a consistent and higher concentration of biting events outdoors than indoors in all species (Fig. 1B, Table 1, and SI Appendix, Fig. S6).
We used the nonparametric Mardia–Watson–Wheeler test for circular homogeneity to verify whether further pooling of samples across species or locations was warranted by the data. This test verifies whether several samples are drawn from a common distribution. Homogeneity of distributions was rejected for most comparisons (Table 2). However, the indoor-biting activity of A. gambiae and A. funestus, and the outdoor biting activity of A. gambiae, A. funestus, and A. coluzzii were compatible with a common circular distribution. Unsurprisingly, the differently shaped samples of the crepuscular species A. pharoensis (Fig. 1B) differed significantly from those of the other species (Table 2). According to the results of this analysis, we further considered the following groups of populations of biting events: the outdoor-biting nocturnal species, the outdoor-biting crepuscular species, and their respective species-specific indoor-biting counterparts. All tests for a common median direction between the outdoor vs. indoor compartments were statistically significant [randomization version of Fisher’s nonparametric test (36), P < 0.001] (SI Appendix, Table S6), except for A. coluzzii and A. pharoensis, for which the difference in mean direction between compartments was only ∼15 and ∼30 min, respectively (Table 1). Similarly, all tests for a common concentration parameter were statistically significant [Wallraff’s nonparametric test (36), P < 0.002] (SI Appendix, Table S7), except for those tests comparing each pair of the nocturnal species indoors.
Table 2.
Mardia-Watson–Wheeler tests for circular homogeneity among samples
| A. gambiae | A. coluzzii | A. funestus | A. pharoensis | |||||
|---|---|---|---|---|---|---|---|---|
| Species | Indoors | Outdoors | Indoors | Outdoors | Indoors | Outdoors | Indoors | Outdoors |
| A. gambiae | ||||||||
| Indoor | 0.000 | 0.024 | 0.000 | 0.283 | 0.000 | 0.000 | 0.000 | |
| Outdoor | 386.141 | 0.000 | 1.000 | 0.000 | 1.000 | 0.000 | 0.000 | |
| A. coluzzii | ||||||||
| Indoor | 14.155 | 129.120 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | |
| Outdoor | 91.610 | 1.629 | 64.230 | 0.000 | 1.000 | 0.000 | 0.000 | |
| A. funestus | ||||||||
| Indoor | 9.188 | 170.999 | 20.598 | 88.640 | 0.000 | 0.000 | 0.000 | |
| Outdoor | 63.404 | 0.579 | 50.077 | 0.168 | 69.853 | 0.000 | 0.000 | |
| A. pharoensis | ||||||||
| Indoor | 222.922 | 356.771 | 142.090 | 234.323 | 183.412 | 187.367 | 0.000 | |
| Outdoor | 567.820 | 457.453 | 402.129 | 289.264 | 457.353 | 196.330 | 36.688 | |
Below the main diagonal: W, the test statistic; above the main diagonal: P value after Bonferroni correction. Boldface font identifies cases where the null hypothesis is not rejected, indicating comparable circular distributions.
These results suggest that the timing of outdoor blood feeding in the nocturnal vectors is similar, and differences among species emerge only once the indoor compartment is considered. Such delays in the timing of indoor biting may represent differences in the rate at which each species is able to penetrate into human dwellings. According to this explanation, A. coluzzii would be the most rapid and A. funestus the least rapid of the nocturnal species. These differences may be accounted for by specific responses to ambient illumination and delays imposed by the necessity to find a way into buildings, which may be more time-consuming later at night. Above and beyond differences in mean direction, however, it was the concentration of events that was markedly different between locations, the nocturnal species showing the highest degree of dispersion indoors.
Linear Representation of Daily Accumulation of Bites.
In order to appreciate the proportion of biting events also along the linear 24-h representation of the day, we plotted the cumulative frequency of biting events expressing the Zeitgeber time (Zt) in hours after the lights-on signal (i.e., “dawn,” hence Zt0 = 0600 hours) (Fig. 1C). In the three nocturnal species, the rate of accumulation of the proportion of indoor bites was similar during daytime, with slight differences around lights-off (Zt12 = 1800 hours): the rate somewhat decreased at Zt12 in A. coluzzii and A. funestus, whereas it remained approximately constant until ∼2200 hours in A. gambiae (Fig. 1C). After ∼2200 hours, the rate of accumulation markedly increased in all three species to level-off again just before lights-on at Zt0 (Fig. 1C). In the crepuscular species A. pharoensis, the rate of accumulation indoors during daytime was lower than in the nocturnal species (shallower slope of the portion of the curve falling at daytime in Fig. 1C), and then suddenly increased and maintained a greater rate after lights off. The shape of the cumulative distribution curve shows that the accumulation rate then progressively declined until lights-on (Fig. 1C). The rate of accumulation of outdoor biting was markedly lower during daytime in all four species, reaching a maximum proportion of bites of 9% in A. coluzzii. Conversely, the accumulation rate substantially increased at lights-off: abruptly for A. pharoensis, markedly in A. gambiae, and more progressively for A. coluzzii and A. funestus (Fig. 1C). The cumulative distribution curves for outdoor biting were significantly different from indoor biting in each species (Kolmogorov–Smirnov nonparametric two-tailed tests: D > 0.5, P < 0.001 in all cases).
Fitting Circular Distributions.
The empirical analysis above has demonstrated that the circular distributions of biting events were not uniform, and were sometimes asymmetrical and somewhat multimodal, indicating complex structural patterns. To explore the extent to which the sample distributions conformed or departed from circular probability models, biting events were fitted first to the von Mises distribution, which is a symmetric circular analogous of the normal distribution for linear data, and it is the best-supported model in circular data analysis (36). The battery of goodness-of-fit tests (Kuiper, Rayleigh, Rao, or Watson) rejected conformance to the von Mises distribution for all samples, except A. funestus outdoors (SI Appendix, Table S8). Because the distribution of biting events failed to conform in most cases to the von Mises probability model, we fitted the data to families of increasingly more complex yet flexible circular distribution models: the Jones–Pewsey and the inverse Batschelet (36). The goodness-of-fit tests indicated that samples conformed to Jones–Pewsey distributions in all cases (SI Appendix, Table S9). However, because asymmetries in the sample circular distributions were detected in the data (Table 1), and considering that both the von Mises and Jones–Pewsey distributions are reflectively symmetric, we contemplated also the fit of the inverse-Batschelet distribution given that this family of unimodal distributions display the widest range of both skewness and peakedness. The battery of goodness-of-fit tests, however, indicated that only the Rayleigh test provided evidence for conformance to the inverse Batschelet (SI Appendix, Table S10).
Accordingly, we sought evidence for decreasing the number of parameters necessary to explain the distribution of biting events by reducing the full family models (four parameters) to nested submodels containing progressively fewer parameters: three parameters (skew-von Mises and symmetric models), or two parameters (von Mises model). The Akaike information criterion (AIC) identified the full family models as those better explaining the data (Table 3). Only two exceptions were observed: A. funestus outdoors, for which the von Mises was the better model, and A. pharoensis indoors, for which the three-parameter symmetric model was the better fit (Table 3). The Bayesian information criterion (BIC) criterion, however, returned half of the times a different “best” model than that identified by the AIC (Table 3). In some cases, the discrepancy could be explained by the similar plausibility of alternative models (e.g., A. gambiae outdoors and A. funestus indoors, where the best model by BIC is the second-ranked best model by AIC with ΔAIC < 2). In another case, A. coluzzii indoors, the BIC did not resolve two alternative models (ΔBIC = 0.03 for the second-ranked full family model). Overall, these results indicate that the distribution of biting events of the four mosquito species were, with few exceptions, asymmetric, showed variable degrees of skewness and peakedness, and were not always unimodal. These complexities could not be accounted for in full by any of the tested circular probability models, which produced inconsistent results across species, locations, and statistical inference tests. In the following section we consider the possibility that the complexity of the distribution of biting events in each sample may result from the aggregation of different underlying circular distribution models.
Table 3.
Model comparison and reduction assessed by the AIC and BIC for the family of inverse Batschelet circular distributions, and maximum-likelihood estimates of the parameters of the corresponding reduced distributions
| Species | Location | Model | AIC | BIC | ξ | Κ | ν | λ |
|---|---|---|---|---|---|---|---|---|
| A. gambiae | Indoors | von Mises | 10926.462 | 10938.583 | 0.494 | 0.704 | 0.000 | 0.000 |
| Symmetric | 10819.187 | 10837.368 | 0.389 | 0.795 | 0.000 | 0.668 | ||
| Skew-von Mises | 10815.291 | 10833.472 | 1.596 | 0.763 | 0.776 | 0.000 | ||
| Full family | 10762.609 | 10786.852 | 1.447 | 0.798 | 0.639 | 0.493 | ||
| A. gambiae | Outdoors | von Mises | 4500.969 | 4512.191 | 0.203 | 2.511 | 0.000 | 0.000 |
| Symmetric | 4467.363 | 4484.195 | 0.197 | 3.077 | 0.000 | −0.237 | ||
| Skew-von Mises | 4500.688 | 4517.521 | 0.095 | 2.514 | −0.074 | 0.000 | ||
| Full family | 4466.847 | 4489.291 | 0.090 | 3.076 | −0.078 | −0.236 | ||
| A. coluzzii | Indoors | von Mises | 2128.405 | 2137.196 | 0.256 | 0.521 | 0.000 | 0.000 |
| Symmetric | 2084.585 | 2097.771 | 0.230 | 0.811 | 0.000 | 1.000 | ||
| Skew-von Mises | 2094.456 | 2107.641 | 1.774 | 0.638 | 1.000 | 0.000 | ||
| Full family | 2080.217 | 2097.798 | 1.394 | 0.816 | 0.633 | 1.000 | ||
| A. coluzzii | Outdoors | von Mises | 977.193 | 985.062 | 0.181 | 1.937 | 0.000 | 0.000 |
| Symmetric | 973.132 | 984.937 | 0.172 | 1.803 | 0.000 | 0.233 | ||
| Skew-von Mises | 975.039 | 986.844 | 0.523 | 1.953 | 0.227 | 0.000 | ||
| Full family | 972.416 | 988.156 | 0.482 | 1.830 | 0.195 | 0.199 | ||
| A. funestus | Indoors | von Mises | 1889.123 | 1897.710 | 0.734 | 0.642 | 0.000 | 0.000 |
| Symmetric | 1877.299 | 1890.179 | 0.619 | 0.717 | 0.000 | 0.614 | ||
| Skew-von Mises | 1867.706 | 1880.586 | 1.977 | 0.717 | 1.000 | 0.000 | ||
| Full family | 1866.160 | 1883.334 | 1.680 | 0.729 | 0.694 | 0.350 | ||
| A. funestus | Outdoors | von Mises | 505.844 | 512.557 | 0.165 | 2.264 | 0.000 | 0.000 |
| Symmetric | 507.109 | 517.178 | 0.164 | 2.406 | 0.000 | −0.098 | ||
| Skew-von Mises | 507.839 | 517.909 | 0.150 | 2.264 | −0.010 | 0.000 | ||
| Full family | 509.106 | 522.533 | 0.153 | 2.406 | −0.007 | −0.098 | ||
| A. pharoensis | Indoors | von Mises | 1083.893 | 1091.533 | −1.130 | 1.070 | 0.000 | 0.000 |
| Symmetric | 1033.532 | 1044.992 | −1.162 | 1.361 | 0.000 | 1.000 | ||
| Skew-von Mises | 1084.185 | 1095.645 | −0.831 | 1.077 | 0.185 | 0.000 | ||
| Full family | 1037.293 | 1052.573 | 0.240 | 1.354 | 0.792 | 1.000 | ||
| A. pharoensis | Outdoors | von Mises | 913.813 | 922.010 | −0.965 | 2.872 | 0.000 | 0.000 |
| Symmetric | 905.687 | 917.981 | −1.076 | 2.415 | 0.000 | 0.304 | ||
| Skew-von Mises | 804.238 | 816.532 | 0.804 | 3.476 | 1.000 | 0.000 | ||
| Full family | 769.283 | 785.676 | 0.704 | 2.580 | 1.000 | 0.499 |
ξ: location parameter; κ: concentration parameter; ν: skewness parameter; λ: peakedness parameter. The lowest AIC and BIC for each combination of species/location is highlighted in boldface.
Fitting von Mises Distribution Mixtures.
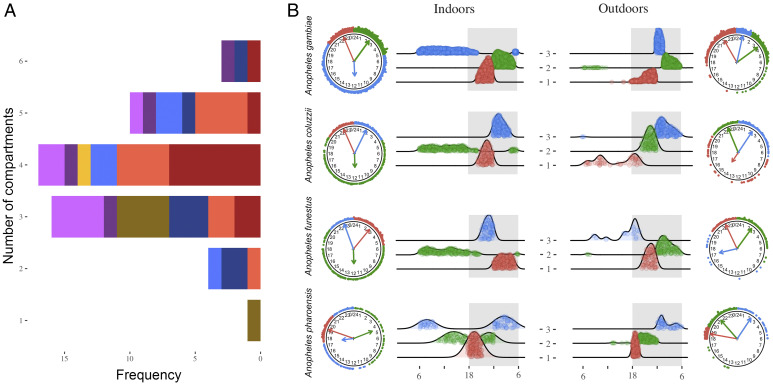
The previous analyses revealed that in most cases the circular distribution of biting events was complex, and in only one case it conformed to the von Mises “null,” despite sizeable sample sizes (Table 3 and SI Appendix, Table S7). The von Mises distribution is a useful probabilistic model for circular data, whose utility in statistical inference and modeling is analogous to the normal distribution for linear data (33). It may be desirable, therefore, to reduce complex multimodal distributions by considering them as mixtures of underlying simple probability models. Thus, to determine whether the observed circular distribution of biting events could be modeled as a mixture of two or more von Mises distributions, we sought for the number of “compartments” K, their parameters and mixture proportions, that best fitted the biting activity of each mosquito, per location and per month. Selection of the optimal number of compartments by the BIC identified K values ranging between 2 and 4 for >80% of samples, with K = 3 being the mode for ∼50% of samples (Fig. 2A and SI Appendix, Table S11). Only one sample had K = 1 (A. funestus indoors, June 2016) (Fig. 2A and SI Appendix, Table S11).
Fig. 2.
Fitting mixtures of von Mises circular distributions. (A) Frequency distribution of number of compartments that best fit the observed circular distribution of biting events in monthly samples. Colors pertain to the nature of the sample: A. gambiae (red/dark red), A. coluzzii (blue/dark blue), A. funestus (yellow/brown), and A. pharoensis (violet/purple). Darker colors represent indoor collections, lighter colors outdoor collections. (B) Circular and linear representations of assignment of biting events to compartments assuming conservatively K = 3 across species and locations. Blue, cluster 1; green, cluster 2; and red, cluster 3. Arrows represent mean directions of each cluster, with lengths indicating the concentration parameter. x axis, time in hours. Gray areas visualize nighttime.
These results suggest that the observed complexity in the distribution of biting events could be accounted for by the occurrence of different populations of biting events in the dataset. When, for visualization and inference, each biting event was assigned to a compartment, assuming the conservative estimate K = 3 for all species and locations across the year (Fig. 2B), A. gambiae, A. coluzzii, and A. funestus showed a similar stable pattern indoors, with two distinct populations biting early on (2000 to 2400 hours) and later at night (0000 to 0500 hours) and a third compartment of biting events during the daytime (Fig. 2B). Interestingly, biting events occurring around Zt0 or Zt12 were more likely associated with the daytime population. A similar, although less manifest, pattern was found for outdoor biting, the main difference being in the association of biting events occurring at twilight: those around Zt12 were associated with early-night biting and those around Zt0 were associated with late-night biting. Conversely, for A. pharoensis, there was a clear difference between the fractions of the population biting indoors and outdoors, due to the rarity of daytime biting outdoors for this species. The distinction between compartments was mainly between biting at dusk (1800 to 2000 hours), early-night biting (before midnight), and late-night biting (from midnight to dawn). As observed for outdoor biting in the nocturnal species, the fraction of the daytime-biting population around Zt12 was associated with early-night biting and that around Zt0 was associated with late-night biting. A characteristic common to all four species and locations was the much greater dispersion of daytime-biting events compared to nighttime biting. Altogether, these results confirm the heterogeneity and complexity of the temporal structure of mosquito feeding behavior, as well as the existence of more than one statistical population of biting events occurring across 24 h.
Residual Malaria Transmission.
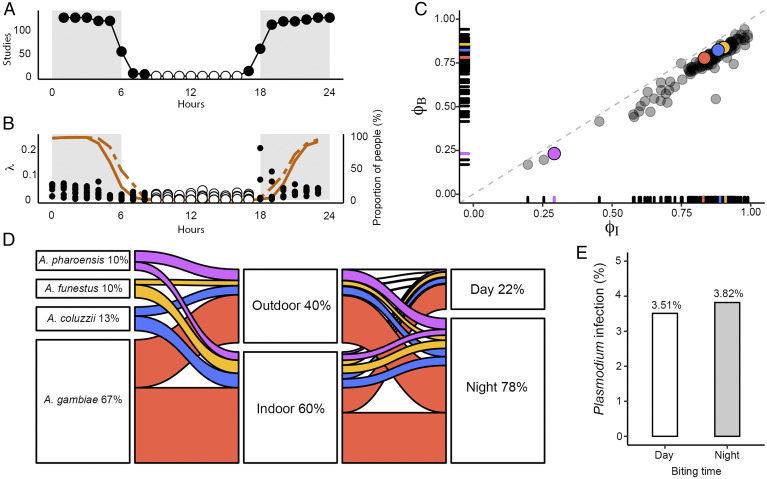
Plasmodium transmission intensity is directly proportional to the human biting rates of Anopheles mosquitoes (37). We have therefore related the spatiotemporal structure of mosquito biting observed in Bangui to their potential exposure to indoor vector control interventions, such as IRS and LLINs. For this aim we have used the approach and systematic review of Sherrard-Smith et al. (24), who estimated the proportion of mosquito bites received while indoors or in bed assuming average human behavior. To put our results in context with respect to other studies, we have plotted the hour-by-hour coverage of the studies included in the systematic review (Fig. 3A), showing that of 127 studies from which starting and ending mosquito collection times could be extracted, none of them accounts for biting behavior in the central hours of daytime (from 0900 to 1700 hours) (white dots in Fig. 3A, corresponding to zero number of studies).
Fig. 3.
Impact of diurnal biting activity on residual malaria transmission in Bangui. (A) Sampling coverage of the studies reviewed by Sherrard-Smith et al. (24) denoted by the hour-by-hour frequency of recorded biting activity. Gray areas represent nighttime. None of the reviewed studies cover the period from 0900 to 1700 hours (white dots). (B) Hourly distribution along the day of the proportion of mosquitoes’ bites (λ, dots in the figure) in relation to the average proportion of people indoors (orange dashed line) or in bed (orange continuous line). White dots designate the period when biting occurs when people are not in households. (C) Combined mosquito and human activity data estimating mosquito biting risk expressed by the mean proportion of bites (black dots in B) taken while humans are indoors (ΦI) or in bed (ΦB). A. gambiae (red), A. coluzzii (blue), A. funestus (yellow), and A. pharoensis (violet). Each gray dot represents the corresponding values of individual studies of the systematic review (24). (D) Summary of the observed proportion of mosquito bites by species according to location and period of the day: A. gambiae (red), A. coluzzii (blue), A. funestus (yellow), and A. pharoensis (violet). (E) Prevalence of P. falciparum DNA in the head/thorax of a subset of Anopheles specimens (n = 271) randomly chosen from the dataset according to the period of the day when they were collected.
Following the same approach of the systematic review, we have combined measures of the proportional diel distribution of mosquito bites (dots in Fig. 3B), with estimates of the average proportion of people inside houses (dashed line in Fig. 3B) or protected by bed nets (continuous line in Fig. 3B), and calculated indices ΦI and ΦB for Bangui (Fig. 3C) to provide measures of mosquito-biting risk (24). These indices indicate the maximum impact that malaria control interventions based on IRS or long-lasting insecticide-impregnated bed nets, respectively, can achieve for any observed set of human and mosquito behaviors. Fig. 3C shows that ΦI and ΦB do not cluster away from the values reported in the systematic review. The outlier (magenta dot in Fig. 3C) corresponds to A. pharoensis, which was not included in the systematic review. These results may suggest that indoor vector control interventions remain effective despite substantial diurnal exposure of people to malaria vector bites, but alternative interpretations are offered later on.
To sum up these findings, we have plotted the proportional contribution of each species to total transmission exposure according to location and time of biting (Fig. 3D). This compartmentalization allows one to visualize potential exposure to transmission in relation to vector control interventions. Fig. 3D shows that ∼40% of bites occurred outdoors, where malaria vectors are not exposed to indoor control interventions. However, the vast majority of outdoor biting occurred during the night, when people are for the most part indoors, hence protected by LLINs or IRS. Nevertheless, ∼22% of indoor biting occurred during the day, when people are not protected by bed nets. This compartment can be considered as a “gray zone” of vector control, because IRS may not be effective if partial coverage is implemented only in structures where people occur at night (i.e., dwellings). The relative contribution of the four malaria vectors to this transmission exposure compartment was similar to their relative abundance in the total population.
Finally, we verified whether daytime biting effectively exposes people to transmission of Plasmodium. We screened for the presence of Plasmodium falciparum sporozoites in two random subsets of mosquitoes that were collected biting during daytime and nighttime, respectively (Fig. 3E and Dataset S1), and found no significant difference in parasite prevalence in the two subsets (3.51% daytime vs. 3.82% nighttime; χ2 test, P = 1). The presence of the knockdown insecticide resistance (kdr) mutations was assessed in 361 randomly selected mosquitoes (A. gambiae n = 247, A. coluzzii n = 114). The kdr mutations were detected in ∼98% and in ∼76% of A. gambiae and A. coluzzii, respectively (SI Appendix, Table S12, and Dataset S1).
Discussion
Nocturnal feeding in anopheline mosquitoes has modulated their biology and evolution. This specialization affects not only reproductive strategies, host choice, and survival, but also transmission of the parasites that anophelines carry. Vector control interventions have drastically reduced malaria incidence rates in the last decades by specifically targeting such nocturnal blood-feeding behavior (6, 7). Our analysis of mosquito-biting activity in the capital of the Central African Republic by 48-h around-the-clock sampling, however, showed that the major malaria vectors A. gambiae, A. coluzzii, A. funestus, and A. pharoensis substantially bite also during the daytime inside human dwellings. The analysis of the 24-h diel biting rhythm of these species by circular statistics modeling revealed complex patterns of distribution of biting events, highlighting the likely occurrence of distinct populations of biting events in each species. Finally, by estimating that ∼22% of anopheline biting occurred at daytime during hours that are not covered by routine entomological surveillance protocols, our study pinpoints significant weaknesses in the current evaluation paradigm for residual malaria transmission that may be biasing inferences about the nature and extent of the problem and the impact of control interventions, with important consequences on the implementation of malaria control strategies.
Monitoring mosquito-biting activity in relation to human behavior is essential to determine the risk of transmission of mosquito-borne diseases and the efficacy of vector control campaigns (24, 38–40). In Bangui, 13% (A. pharoensis) to 28% (A. funestus) of anopheline biting occurred during daytime; this fraction was higher when considering only indoor collections (28 to 39%). Importantly, daytime biting was not limited to a few hours just before sunset or after sunrise, but was widely scattered across the whole daytime period. In Afrotropical malaria vectors, conventional sampling plans to study biting exposure only cover the nocturnal period of the day, generally from sunset to sunrise. This is because it is implicitly assumed that, with only few exceptions, diurnal biting is negligible in the genus Anopheles. From a historical perspective, the consequences of this assumption produced in the second half of the 20th century a paradigm shift in the implementation of sampling protocols to assess malaria transmission, presumably due to the realization that the bulk of biting activity in the major vectors is generally between midnight and sunrise (41). Before this paradigm shift, routine mosquito biting collections were generally implemented over the whole 24-h diel cycle (4, 42). Accordingly, some older entomological studies have recorded variable degrees of daytime-biting activity in anophelines across Africa (43–47). The common features of these studies are that: 1) they cover essentially a circumscribed geographical area in understudied central Africa; 2) they concern a limited number of species among which A. funestus is among the few main malaria vectors observed biting during daylight [noteworthy, a study from the 1970s in the region of Bangui found that 18% of A. funestus bites were recorded during daytime (48)]; and 3) plasticity in the diel structure of biting behavior appears influenced by the degree of shading in the proximate environment, with diurnal biting occurring mostly in lowly lit habitats. This feature is consistent with results from this study, whereby daytime biting was essentially limited to the darker indoor compartment and was not observed in full sunlight outdoors. Indeed, exposure to light has potent inhibitory effects on the activity of nighttime-biting mosquitoes (49, 50), and modifies the circadian structure of spontaneous locomotion in A. coluzzii (51).
All these features, in combination with the heterogeneous nature of this behavioral polymorphism across the full geographical range of main malaria vectors, have probably contributed to neglect diurnal Anopheles biting over the years. On the other hand, recent studies have specifically addressed the question of changes in vector biting rhythmicity after the implementation of control interventions, which can have impactful consequences on residual malaria transmission. Preliminary experimental evidence that about a third of variation in biting time is due to additive genetic variance in another major Afrotropical malaria vector, Anopheles arabiensis (52), indicates that disruptive selection imposed by insecticide-treated bed nets may produce shifts toward earlier and later biting times, as shown by simulation models (53). The genetic and molecular determinants of these phenotypes, however, are likely to be complex (50, 54, 55). Nevertheless, by extending routine sampling plans of a few hours beyond sunrise and before sunset (16, 38, 39, 56), some field studies have indeed found evidence of changes in feeding behavior at places and times when human hosts are more readily available (16–19, 21).
In Bangui, we do not have conclusive evidence that substantial daytime biting occurred either before the implementation of LLINs during the last years, or in response to it. Based on what we have outlined above, we suspect that it is by building on standing phenotypic variation in local anopheline populations that a response to the massive distribution of LLINs may have increased the fraction of daytime biting in malaria vectors compared to what found in 1974 in the same region, in as much as what has been reported elsewhere in Africa. The strength of the evidence for this interpretation, however, admittedly rests on results from a single study (48), as well as limited knowledge about the impact of LLINs on the demography of mosquito populations in Bangui. Nevertheless, a major difference compared to other reports of behavioral shifts in biting time across Africa is that in Bangui we observed that the dispersion of the biting events throughout the whole daylight photoperiod is substantial, and not just of a few hours before sunset and after sunrise, demonstrating that natural populations of Anopheles mosquitoes have the potential (i.e., naturally occurring or selected after human pressure, such as upon bed-net deployment) to adjust the temporal organization and circadian control of their feeding behavior.
A major outstanding question is the extent of this phenomenon. Some studies have reported >20% of the total number of bites occurring during the first few hours of daytime (Fig. 3B); we could expect that this percentage may be even higher if the sampling protocol of these studies covered the whole diel cycle. Further studies, therefore, will need to take into account Anopheles diurnal biting to investigate residual malaria transmission. Statistical modeling of the temporal distribution of biting events during 24 h revealed that the samples were compatible with mixtures of multiple circular von Mises distributions. The number of distinct compartments that fitted the data were three or four, sometimes more. Taking the conservative assumption of three compartments, we observed two biting populations corresponding to periods at nighttime, when people are indoors or under bed nets, and a third biting population corresponding to daytime when people are unprotected. It is not known whether these statistical populations of biting events correspond also to distinct biological populations of mosquitoes, and whether such distinction implies that biting occurs in population-specific temporal windows. In other words, it remains to be verified whether diurnal biters invariably bite during daytime, and the degree to which this behavior is the result of environmental plasticity (SI Appendix, Fig. S6) vs. genetic determination. If diurnal biters invariably do so, this fraction of vectors would elude exposure to insecticide-impregnated bed nets, with consequent impacts on the efficacy of malaria control based upon this intervention. A corollary is that if the observed distribution of diel biting events in Bangui is the result of exposure of malaria vectors to LLINs, the ensuing behavioral shift has produced a distinct daytime-biting subpopulation, leaving the behavior of other “constitutive” nighttime-biting subpopulations unchanged.
Natural populations of Anopheles mosquitoes can display heterogeneities in biting rhythmicity (38), which sometimes can be accounted for by physiological and environmental features. It is important to differentiate them from night-to-night variations, which may affect sample size and therefore the studies’ conclusions. For example, some studies have shown that parous females tend to bite later than nulliparous mosquitoes (57, 58), possibly because of delays due to oviposition by gravid females. Host accessibility and vector control measures can also influence biting rhythms, and genetic determinants like mutations conferring insecticide resistance can affect biting behavior (13). In Bangui, however, kdr resistance alleles in A. gambiae and A. coluzzii were almost fixed; the occurrence of multiple populations of biting events, therefore, cannot be imputed to the kdr background. Many processes involved in mosquito feeding are strongly regulated by circadian rhythms. For example, activities related to oviposition, blood-feeding, and sugar-feeding, and modulated by olfaction, follow distinct rhythms that affect metabolic and immunity processes (2). In a recent study, higher infection rates in A. stephensi were observed when feeding during daytime, with consequences upon vectorial capacity (59). Different subpopulations of mosquitoes feeding at different periods of the diel cycle, therefore, may exhibit distinct physiological, behavioral, and genetic features affecting their transmission potential. Future studies need to determine the underpinning biological mechanisms associated with differences in biting rhythms.
Vector competence is strongly regulated by genetic and environmental factors that determine the intensity and efficacy of transmission of malarial parasites (60). Recently, Suh et al. (61) showed that the probability of mosquitoes being infected is higher earlier in the evening than in the morning. This could be the effect of daily temperature fluctuations that interact with the mosquito immune system (62). Moreover, in avian malaria, mosquito density can affect parasite transmission, whereby a lower number of bites during the day can result in lower transmission (63). Our study does not address vector competence for Plasmodium; however, results show equivalent P. falciparum infectious rates in A. gambiae and A. coluzzii during daytime and nighttime (Fig. 3E). Accordingly, it will be important to consider and integrate diurnal biting into malaria transmission-risk models. A major question raised by diurnal biting is the impact that such behavior can have on malaria control based on indoor interventions, like IRS and LLINs, which are the mainstay of the world eradication strategy (64). By putting results from Bangui in context with other studies that have evaluated how mosquito feeding behavior influences residual malaria transmission (24), we found that indices quantifying the risk of mosquito biting remained in the range of studies that did not report the occurrence of diurnal biting in malaria vector populations. This result can be interpreted in alternative ways: at face value, it implies that substantial diurnal biting by vectors does not effectively modify the impact that IRS and LLINs provide on transmission, which is rather good news (Fig. 3C). The main reason underlying such an outcome is that human occurrence in households, and hence exposure to mosquito bites, is virtually nil during the daytime (dashed line in Fig. 3B). In an urban context, however, people spend significant amounts of time indoors away from their domestic environment. Even in rural areas, schoolchildren spend a substantial portion of the day indoors. If indoor diurnal biting does not occur exclusively in domestic households, the alternative interpretation is that the ΦI and ΦB indices in Fig. 3C do not adequately capture and represent the extent of affordable malaria control provided by insecticide-impregnated bed nets when diurnal biting by vectors is substantial. When further taking into account the significant sampling bias upon which results in Fig. 3C are based upon (Fig. 3A), the implications resulting from an uncritical interpretation of Fig. 3C might be too optimistic. It is worth noting that the analysis in Sherrard-Smith et al. (24) considered behaviors in the absence of mosquito nets, whereas in this study collection households harbored insecticide-impregnated bed nets, although this difference does not modify the interpretation of results.
IRS is a strategy alternative or complementary to insecticide-impregnated bed nets in our arsenal of malaria control tools (65). The World Health Organization defines IRS as the application of a long-lasting residual insecticide to the surfaces of all houses or structures (including animal shelters) where malaria vectors might come into contact with the insecticide (66). IRS, therefore, is particularly effective in areas where vectors preferentially feed and rest indoors. Some vectors that feed indoors but rest outdoors can also be impacted by IRS if they rest, even briefly, indoors before exiting the house. The vectors feeding outdoors may come into contact with the insecticide if they rest postprandially, at least in part, inside sprayed structures. In accordance with this definition, considering the nocturnal biting behavior of malaria vectors, and accounting for cost/effectiveness, buildings and structures where people or livestock occur only during the day may not be targeted by spraying operations (67). Our results indicate that the operational selection criteria of target structures for IRS should take into account explicitly the potential for diurnal biting of malaria vectors. Moreover, above and beyond entomological and epidemiological considerations, IRS is not suitable under all circumstances. In sparsely inhabited or difficult to access areas it may not be feasible to implement an IRS campaign. In general, IRS is inappropriate in most urban situations because spraying a large number of structures may be challenging and not cost-effective (66). Accordingly, reliance on LLINs for cost-effective malaria control exacerbates the threats posed by residual malaria transmission, especially in urban areas. Considering the insecticide resistance status of the local vector populations, the substantial proportion of outdoor/diurnal biting, and the challenging urban context, our results suggest that Bangui is a case study for which residual malaria transmission may be well “out of control.”
Conclusions
As the malaria elimination strategy still largely relies on reducing transmission (64), the biology of the vectors will determine our ability to achieve the final goal of malaria eradication (68). Indeed, the complexity, diversity, and plasticity of mosquito behavior can hamper disease control (12, 13). Diurnal biting activity in Anopheles has been neglected during the last century of malaria research; however, as current vector control interventions will continue to put pressures on the timing and location of mosquito biting, the impact of diurnal biting needs to be fully appreciated in order to develop appropriate interventions, including implementation of explicit guidelines for vector surveillance and control (67) at places where people spend significant amounts of time during daytime.
Materials and Methods
Ethical Approval.
This study and the methods employed for mosquito collections, including the human landing catch, were approved by the Institutional Review Board of the Health Sciences Faculty of the University of Bangui (authorization number 01/UB/FACSS/CSCVPER/17).
Study Sites.
Mosquitoes were collected at four sites in Bangui: Gbanikola, Îles des Singes, Ouango, and Pk10 (Fig. 1A and Dataset S1). These sites were selected on the basis of previous entomological studies to take into account vector diversity (30). Bangui is located at the country southern border and lies on the northern banks of the Ubangi River. The climate is partly tropical. The average annual relative humidity is 61%, and the average monthly relative humidity ranges from 47% in December to 72% in August. Temperatures range from 21 °C to 34 °C. The rainy season extends from May to October, and the dry season from November to April.
Mosquito Collections and Processing.
From June 2016 to May 2017, adult mosquitoes were captured while landing on human collectors: this is the golden standard to assess the strength of transmission of mosquito-borne pathogens (3). Each sampling session lasted 48 h around the clock, with two collectors at each site rotating between sites, and working shift schedules of 6 h each to reduce fatigue. For logistic reasons, collections could not be performed in December 2016. At each site, two houses equipped with LLINs, separated by at least 20 m, were selected. Collections were carried out both inside and outside each house. Site, position, and collection time were varied for each volunteer to avoid bias due to differences among collectors in attractiveness to mosquitoes and collection ability. This sampling protocol corresponds to a collection effort of 352 human-days. Nighttime collections refer to mosquitoes caught between 1800 and 0600 hours, whereas daytime collections refer to mosquitoes caught between 0600 and 1800 hours. Mosquitoes were morphologically identified using taxonomical identification keys (69, 70). After morphological identification, each mosquito was classified according to gonotrophic status (unfed, fed, half-gravid, and gravid) under a dissecting scope, and then stored individually in a 1.5-mL Eppendorf tube at –20 °C for subsequent molecular analyses.
Molecular Analyses.
Total genomic DNA from each mosquito was extracted using 2% cetyl trimethyl ammonium bromide (71). DNA pellets were dissolved in 100 μL of sterile water at 4 °C for 24 h and stored at –20 °C. Specimens belonging to cryptic taxonomic groups (A. gambiae, A. nili, and A. funestus) were identified using species-specific PCR assays (72–74). The presence of P. falciparum DNA in salivary glands of Anopheles females and carriage of the target-site L1014F (kdr-w) and L1014S (kdr-e) mutations was determined in randomly selected specimens (n = 271) using TaqMan assays (75, 76).
Statistical Analysis.
Circular statistical analyses were carried out in R following Pewsey et al. (36) and functions available therein. Additional functions available in the R package circular were used for plotting and calculating basic circular statistics (77). First, a variable of class circular was created. As mosquitoes were aggregated over 1-h time slots at collection, biting events were transformed into a pseudocontinuous variable by adding a uniform random component over the [0, 1] interval of each time slot. Comparison of the diel biting rhythmicity at the four collection sites did not show significant differences; therefore, biting events were pooled prior to further analyses. Central location and dispersion metrics in circular data were provided by the mean direction θ and the mean resultant length R; the latter provides an estimate of the variability in the concentration of events around the mean direction (36). These are measures analogous to the sample mean and SD on the linear scale. Larger values of R (i.e., values closer to 1) indicate a greater concentration of events, while lower values of R (i.e., values closer to zero) indicate sparser events. When R = 0, events are uniformly distributed around the circumference and the mean direction is not defined (33): that is, the circular distribution is uniform, meaning that the rate of arrival of mosquitoes at a host is constant throughout the 24-h diel cycle.
Tests of conformance to circular probability models considered three circular distribution families: the von Mises, the Jones–Pewsey, and the inverse Batschelet (36). The von Mises distribution is a two-parameter unimodal and symmetric distribution characterized by a location parameter μ and a concentration parameter κ. The Jones–Pewsey is a three-parameter family of unimodal symmetrical distributions. The first two parameters are the usual location and concentration parameters μ and κ of the von Mises distribution, and the third parameter is a shape parameter ψ ∈ [−∞, ∞] controlling the extent of peakedness. The von Mises distribution is obtained when ψ = 0. The inverse Batschelet is a four-parameter family of unimodal distributions characterized by ξ, a location parameter, κ ≥ 0, a concentration parameter, ν ∈ [−1, 1], a skewness parameter, and λ ∈ [−1, 1], a parameter that regulates peakedness. Nested submodels of the inverse Batschelet are obtained by reducing the number of parameters to three or two. For the three parameters case, the skew-von Mises model is obtained when λ = 0, and the symmetric model is obtained when ν = 0. The von Mises is a particular case of the inverse-Batschelet distribution when ν = 0 and λ = 0. To test whether the distribution of biting events conformed to mixtures of von Mises distributions, the expectation-maximization algorithm for maximum-likelihood estimation implemented in the movMF package was used to identify different compartments, distributions, and their mixture proportions (78). As there could be differences in the circular distribution parameters at the sampling month level, the analysis was carried out for each combination of species, location, and month. To fit mixtures of von Mises distributions, the BIC was used to select the number of compartments that best fit the data. Then, the data were refitted to a mixture of von Mises–Fisher distribution. To avoid convergence problems, the analysis was limited to samples with n > 40 specimens. Finally, mixtures of von Mises distributions that pooled samples across months were plotted by assuming that month-by-month differences are random realizations of the same underlying mixture of distributions.
Supplementary Material
Acknowledgments
We thank the volunteers of Gbanikola, Îles des Singes, Pk10, and Ouango in Bangui for their collaboration and their compliance during the field investigations; and Vincent Robert, Basile Kamgang, Ousmane Ndiath, Karine Mouline, and Gilbert Le Goff for their assistance and discussions. Funding was provided by the Division International of Institut Pasteur Paris, Institut Pasteur in Bangui, the Agence Universitaire de la Francophonie (Grant Bangui-PAL), the Institut de Recherche pour le Développement, and the Agence Nationale de la Recherche project ANORHYTHM (ANR-16-CE35-0008). The funders had no role in the study design, data collection, or analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2104282119/-/DCSupplemental.
Data Availability
All study data are included in the main text and supporting information.
References
- 1.Gillies M., “Anopheline mosquitoes: Vector behaviour and bionomics” in Malaria: Principles and Practices of Malariology, Wernsdorfer W. H., McGregor I. S., Eds. (Churchill Livingston, London, 1988), pp. 453–485. [Google Scholar]
- 2.Rund S. S., O’Donnell A. J., Gentile J. E., Reece S. E., Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7, 14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver J., Mosquito Ecology (Springer Netherlands, ed. 3, 2008). [Google Scholar]
- 4.Haddow A. J., Studies of the biting habits of African mosquitoes. An appraisal of methods employed, with special references to the twenty-four hour catch. Bull. Entomol. Res. 45, 199–242 (1954). [Google Scholar]
- 5.Pates H., Curtis C., Mosquito behavior and vector control. Annu. Rev. Entomol. 50, 53–70 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Bhatt S., et al. , The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO, World Malaria Report (WHO, 2020). https://www.who.int/publications/i/item/9789240015791. Accessed 14 April 2022.
- 8.Ranson H., Lissenden N., Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Antonio-Nkondjio C., et al. , Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit. Vectors 10, 472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock P. A., et al. , Associated patterns of insecticide resistance in field populations of malaria vectors across Africa. Proc. Natl. Acad. Sci. U.S.A. 115, 5938–5943 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock P. A., et al. , Mapping trends in insecticide resistance phenotypes in African malaria vectors. PLoS Biol. 18, e3000633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatton M. L., et al. , The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution 67, 1218–1230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrasco D., et al. , Behavioural adaptations of mosquito vectors to insecticide control. Curr. Opin. Insect Sci. 34, 48–54 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Charlwood J. D., Graves P. M., The effect of permethrin-impregnated bed nets on a population of Anopheles farauti in coastal Papua New Guinea. Med. Vet. Entomol. 1, 319–327 (1987). [DOI] [PubMed] [Google Scholar]
- 15.Curtis C., et al. , “Impregnated bed nets and curtains against malaria mosquitoes” in Control of Disese Vectors in the Community, Curtis C. F., Ed. (Wolfe Publishing Ltd, London: ), pp 5–46. (1991). [Google Scholar]
- 16.Moiroux N., et al. , Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 206, 1622–1629 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Sougoufara S., et al. , Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: A new challenge to malaria elimination. Malar. J. 13, 125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomsen E. K., et al. , Mosquito behavior change after distribution of bed nets results in decreased protection against malaria exposure. J. Infect. Dis. 215, 790–797 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doucoure S., et al. , Anopheles arabiensis and Anopheles funestus biting patterns in Dielmo, an area of low level exposure to malaria vectors. Malar. J. 19, 230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perugini E., et al. , Behavioural plasticity of Anopheles coluzzii and Anopheles arabiensis undermines LLIN community protective effect in a Sudanese-savannah village in Burkina Faso. Parasit. Vectors 13, 277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degefa T., Githeko A. K., Lee M. C., Yan G., Yewhalaw D., Patterns of human exposure to early evening and outdoor biting mosquitoes and residual malaria transmission in Ethiopia. Acta Trop. 216, 105837 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durnez L., Coosemans M., “Residual transmission of malaria: An old issue for new approaches” in Anopheles Mosquitoes—New Insights into Malaria Vectors, Manguin S., Ed. (InTech Open Books, 2013) pp. 671–704. [Google Scholar]
- 23.Killeen G. F., Characterizing, controlling and eliminating residual malaria transmission. Malar. J. 13, 330 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherrard-Smith E., et al. , Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc. Natl. Acad. Sci. U.S.A. 116, 15086–15095 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw W. R., Catteruccia F., Vector biology meets disease control: Using basic research to fight vector-borne diseases. Nat. Microbiol. 4, 20–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Médecins Sans Frontières, In times of COVID-19, malaria remains the number one killer of children in CAR, (MSF, 2020). https://www.msf.org/malaria-deadly-killer-children-during-coronavirus-car. Accessed 14 April 2022.
- 27.Médecins Sans Frontières, Rapport Médecins Sans Frontières. Une Crise silencieuse République Centrafricaine, (MSF, 2011). https://www.msf.fr/communiques-presse/rapport-republique-centrafricaine-une-crise-silencieuse. Accessed 14 April 2022.
- 28.Moyen J., Aawi M., Rapport de Situation (SITREP) (The Alliance for Malaria Prevention, 2021). [Google Scholar]
- 29.Kamgang B., et al. , Exploring insecticide resistance mechanisms in three major malaria vectors from Bangui in Central African Republic. Pathog. Glob. Health 112, 349–359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ndiath M. O., et al. , Composition and genetics of malaria vector populations in the Central African Republic. Malar. J. 15, 387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangba M. L., et al. , Insecticide resistance status of the Anopheles funestus population in Central African Republic: A challenge in the war. Parasit. Vectors 9, 230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batschelet E., Circular Statistics in Biology (Academic Press, 1981). [Google Scholar]
- 33.Fisher N., Statistical Analysis of Circular Data (Cambridge University Press, 1993). [Google Scholar]
- 34.Mardia K., Jupp P., Directional Statistics (John Wiley & Sons, Ltd, 1999). [Google Scholar]
- 35.Sinka M. E., et al. , The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors 3, 117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pewsey A., Neuhäuser M., Ruxton G. D., Circular Statistics in R (Oxford University Press, 2013). [Google Scholar]
- 37.Smith D. L., McKenzie F. E., Statics and dynamics of malaria infection in Anopheles mosquitoes. Malar. J. 3, 13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huho B., et al. , Consistently high estimates for the proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int. J. Epidemiol. 42, 235–247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moiroux N., et al. , Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS One 9, e104967 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soma D. D., et al. , Quantifying and characterizing hourly human exposure to malaria vectors bites to address residual malaria transmission during dry and rainy seasons in rural Southwest Burkina Faso. BMC Public Health 21, 251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamon J., Les moustiques anthropophiles de la région de Bobo-Dioulasso (République de Haute-Volta); Cycles d’agressivité et variations saisonnières. Ann. Soc. Entomol. Fr. 132, 85–144 (1963). [Google Scholar]
- 42.Haddow A. J., The mosquitoes of Bwamba County, Uganda. II. Biting activity with special reference to the influence of microclimate. Bull. Entomol. Res. 36, 33–73 (1946). [Google Scholar]
- 43.Hamon M. J., Adam J. P., Grjebine A., [Observations on the distribution and behaviour of anophelines in French Equatorial Africa, the Cameroons and West Africa.] [in French] Bull. World Health Organ. 15, 549–591 (1956). [PMC free article] [PubMed] [Google Scholar]
- 44.Cavalié P., Mouchet J., Les campagnes expérimentales d’éradication du paludisme dans le Nord de la République du Cameroun. I. Les vecteurs de l’épidemiologie du paludisme dans le Nord-Cameroun. Med. Trop. (Mars.) 21, 847–870 (1961). [Google Scholar]
- 45.Haddow A., Ssenkubuge Y., The mosquitoes of Bwamba County, Uganda.IX. Further studies on the biting behaviour of an outdoor population of the Anopheles gambiae Giles complex. Bull. Entomol. Res. 62, 407–414 (1973). [Google Scholar]
- 46.White G., Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae). III. The distribution, ecology, behaviour and vectorial importance of species D in Bwamba County, Uganda, with an analysis of biological, ecological, morphological and cytogenetical relationships of Ugandan species D. Bull. Entomol. Res. 63, 65–97 (1973). [Google Scholar]
- 47.McCrae A. W. R., Boreham P. F. L., Ssenkubuge Y., The behavioural ecology of host selection in Anopheles implexus (Theobald) (Diptera, Culicidae). Bull. Entomol. Res. 66, 587–631 (1976). [Google Scholar]
- 48.Cordellier R., Geoffroy B., Contribution à l’étude des culicidés de la République Centrafricaine: Rythmes d’activités en secteur préforestier. Cahiers ORSTOM. Série Entomologie Médicale et Parasitologie 12, 19–48 (1974). [Google Scholar]
- 49.Sheppard A. D., et al. , Light manipulation of mosquito behaviour: Acute and sustained photic suppression of biting activity in the Anopheles gambiae malaria mosquito. Parasit. Vectors 10, 255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baik L. S., et al. , Circadian regulation of light-evoked attraction and avoidance behaviors in daytime- versus nighttime-biting mosquitoes. Curr. Biol. 30, 3252–3259.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traoré A. S., et al. , Effects of insemination and blood-feeding on locomotor activity of wild-derived females of the malaria mosquito Anopheles coluzzii. Parasit. Vectors 14, 457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Govella N. J., Johnson P. C. D., Killeen G. F., Ferguson H. M., Heritability and phenotypic plasticity of biting time behaviors in the major African malaria vector Anopheles arabiensis. bioRxiv [Preprint] (2021). https://www.biorxiv.org/content/10.1101/2021.05.17.444456v1.abstract (Accessed 14 April 2022).
- 53.Ferreira C. P., Lyra S. P., Azevedo F., Greenhalgh D., Massad E., Modelling the impact of the long-term use of insecticide-treated bed nets on Anopheles mosquito biting time. Malar. J. 16, 373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rund S. S., Hou T. Y., Ward S. M., Collins F. H., Duffield G. E., Genome-wide profiling of diel and circadian gene expression in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. U.S.A. 108, E421–E430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maliti D. V., et al. , Investigating associations between biting time in the malaria vector Anopheles arabiensis Patton and single nucleotide polymorphisms in circadian clock genes: Support for sub-structure among An. arabiensis in the Kilombero valley of Tanzania. Parasit. Vectors 9, 109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geissbühler Y., et al. , Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar. J. 6, 126 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bockarie M. J., et al. , The late biting habit of parous Anopheles mosquitoes and pre-bedtime exposure of humans to infective female mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 90, 23–25 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Hamon J., Chauvet G., Thelin L., [Observations on the methods of evaluation of the physiological age of female Anopheles]. [in French]. Bull. World Health Organ. 24, 437–443 (1961). [PMC free article] [PubMed] [Google Scholar]
- 59.O’Donnell A. J., Rund S. S. C., Reece S. E., Time-of-day of blood-feeding: Effects on mosquito life history and malaria transmission. Parasit. Vectors 12, 301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beerntsen B. T., James A. A., Christensen B. M., Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 64, 115–137 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suh E., et al. , The influence of feeding behaviour and temperature on the capacity of mosquitoes to transmit malaria. Nat. Ecol. Evol. 4, 940–951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shapiro L. L. M., Whitehead S. A., Thomas M. B., Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 15, e2003489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pigeault R., Caudron Q., Nicot A., Rivero A., Gandon S., Timing malaria transmission with mosquito fluctuations. Evol. Lett. 2, 378–389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WHO, Global Technical Strategy for Malaria 2016-2030, 2021 Update. (WHO, 2021). https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf. Accessed 14 April 2022.
- 65.Sherrard-Smith E., et al. , Systematic review of indoor residual spray efficacy and effectiveness against Plasmodium falciparum in Africa. Nat. Commun. 9, 4982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO, Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination. (WHO, 2nd Ed., 2015). [Google Scholar]
- 67.Najera J., Zaim M., Malaria Vector Control. Decision Making Criteria and Procedures for Judicious Use of Insecticides (World Health Organization, Geneva, 2002). [Google Scholar]
- 68.WHO, Strategic Advisory Group on Malaria Eradication: Benefits, Future Scenarios and Feasibility-Executive Summary (World Health Organization, Geneva, 2019). [Google Scholar]
- 69.Gillies M. T., De Meillon B., The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region). (South African Institute for Medical Researach, 1968). [Google Scholar]
- 70.Gillies M. T., Coetzee M., A Supplement to the Anophelinae of Africa South of the Sahara (South African Institute for Medical Researach, 1987). [Google Scholar]
- 71.Morlais I., Ponçon N., Simard F., Cohuet A., Fontenille D., Intraspecific nucleotide variation in Anopheles gambiae: New insights into the biology of malaria vectors. Am. J. Trop. Med. Hyg. 71, 795–802 (2004). [PubMed] [Google Scholar]
- 72.Cohuet A., et al. , Species identification within the Anopheles funestus group of malaria vectors in Cameroon and evidence for a new species. Am. J. Trop. Med. Hyg. 69, 200–205 (2003). [PubMed] [Google Scholar]
- 73.Kengne P., Awono-Ambene P., Antonio-Nkondjio C., Simard F., Fontenille D., Molecular identification of the Anopheles nili group of African malaria vectors. Med. Vet. Entomol. 17, 67–74 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Santolamazza F., et al. , Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 7, 163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bass C., et al. , PCR-based detection of Plasmodium in Anopheles mosquitoes: A comparison of a new high-throughput assay with existing methods. Malar. J. 7, 177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bass C., et al. , Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: A comparison of two new high-throughput assays with existing methods. Malar. J. 6, 111 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Agostinelli C., Lund U., R Package Circular: Circular Statistics. Version 0.4-93. (Department of Environmental Sciences, Informatics, Statistics, CA, 2017). [Google Scholar]
- 78.Hornik K., Grün B., On maximum likelihood estimation of the concentration parameter of von Mises-Fisher distributions. Comput. Stat. 29, 945–957 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and supporting information.