Significance
Galanin exerts various physiological functions through galanin receptors, including antinociceptive activity, depression, and sleep. Here, we reveal a distinct binding mode of galanin peptide in galanin receptors from that of the published structures of peptide-bound GPCRs. Moreover, our work shows that the neuromodulator zinc ion negatively modulates galanin signaling in the central nervous system and further advances our understanding of mechanisms of G protein selectivity of GPCRs. These structures will provide a framework for rational design of ligands targeting GALRs for potential therapeutic applications.
Keywords: GPCR signaling, G protein selectivity, negative allosteric modulator
Abstract
Galanin is a biologically active neuropeptide, and functions through three distinct G protein–coupled receptors (GPCRs), namely GALR1, GALR2, and GALR3. GALR signaling plays important roles in regulating various physiological processes such as energy metabolism, neuropathic pain, epileptic activity, and sleep homeostasis. GALR1 and GALR3 signal through the Gi/o pathway, whereas GALR2 signals mainly through the Gq/11 pathway. However, the molecular basis for galanin recognition and G protein selectivity of GALRs remains poorly understood. Here, we report the cryoelectron microscopy structures of the GALR1-Go and the GALR2-Gq complexes bound to the endogenous ligand galanin or spexin. The galanin peptide mainly adopts an alpha helical structure, which binds at the extracellular vestibule of the receptors, nearly parallel to the membrane plane without penetrating deeply into the receptor core. Structural analysis combined with functional studies reveals important structural determinants for the G protein selectivity of GALRs as well as other class A GPCRs. In addition, we show that the zinc ion is a negative allosteric regulator of GALR1 but not GALR2. Our studies provide insight into the mechanisms of G protein selectivity of GPCRs and highlight a potential function of the neuromodulator zinc ion as a modulator of GPCR signaling in the central nervous system.
Galanin is a 29- or 30-amino acid peptide that was isolated from pig intestine in 1983 (1). Through its wide distribution in the nervous system and the endocrine system, galanin is involved in a variety of physiological functions, including regulation of hormones and neurotransmitter release, antinociceptive activity, depression, and sleep/wake homeostasis (2, 3). The endogenous action of galanin is mediated through activation of galanin receptors (GALRs), which belong to class A of G protein–coupled receptor (GPCR) family (4, 5). In addition to galanin, the endogenous galanin-like peptide (GALP) and spexin have recently been identified to activate GALRs (6, 7).
The GALR subfamily consists of three distinct subtypes, GALR1–GALR3. While GALR1 is particularly enriched in the nervous system, GALR2 and GALR3 are broadly distributed in brain as well as peripheral tissues. GALR activation via overexpression or administration of galanin in the nervous system of animals suppresses seizure development and neuropathic pain behavior and shows anxiolytic and antidepressant effects (8–12). A missense mutation in galanin peptide was identified as a cause of temporal lobe epilepsy (TLE) (13). Moreover, galanin expression is up-regulated in the injured neurons, and galanin has been shown to play a role in neuroprotection and neuronal regeneration (14, 15). Therefore, GALRs are potential therapeutic targets for the treatment of pain, epilepsy, depression, neuron injury, and sleep disorders.
GALRs vary in their downstream signaling pathways. GALR1 and GALR3 mainly couple to the inhibitory Gαi/o pathway, leading to the inhibition of adenylyl cyclase activity and the decrease of intracellular adenosine 3′,5′-cyclic monophosphate (cAMP) level. By contrast, GALR2 mainly couples to the stimulatory pathway of Gq/11, inducing the formation of inositol triphosphate (IP3), which in turn increases the cytosolic Ca2+ level (3) (Fig. 1A). However, the molecular basis of G protein selectivity of GALRs remains unknown. Although homology modeling and site-directed mutagenesis studies revealed the essential residues of galanin involved in receptor binding and activation, and the potential galanin binding site of GALRs (16–19), the molecular details of galanin binding of GALRs remain poorly defined at the molecular level. To gain insight into the molecular basis of ligand recognition of GALRs and extend our understanding of G protein selectivity, we sought to determine the cryoelectron microscopy (cryo-EM) structures of GALR1 and GALR2 in complex with Go and Gq heterotrimer, respectively.
Fig. 1.
Overall structures of the galanin-bound GALR1–mini-Go–scFv16 and GALR2–mini-Gq–scFv16 complexes. (A) Schematic representation of GALR receptor signaling. GALR1 and GALR3 primarily couple to Gi/o, while GALR2 mainly signals through Gq. (B) Cryo-EM structures of GALR1–mini-Go–scFv16. (C) Cryo-EM structures of GALR2–mini-Gq–scFv16.
Results
Structure Determination.
To obtain stable GPCR–G protein complexes, we used engineered thermostable mini-G proteins, which only contain the GTPase domain of Gα but still bind to Gβγ heterodimer and recapitulate the pharmacological and structural changes in GPCRs induced by the full-length Gα proteins (20). Moreover, the N-terminal residues of αN in mini-Gαo and mini-Gαq were replaced by the equivalent residues of Gαi to acquire the ability to bind the antibody fragment scFv16 that stabilizes the nucleotide-free GPCR–G protein complex (21). In order to prove the functional relevance of the GPCR–mini-G complex, we performed bioluminescence resonance energy transfer assays (22). Consistent with previous studies (23), the functional activities of mini-Gαo and mini-Gαq are similar to the wild-type Gαi1 and Gαq, respectively (SI Appendix, Figs. S1 A and B). To further improve the complex stability, we introduced a linker that contains a 3C protease cleavage site, between the C terminus of the receptor and the N terminus of the mini-Gα to create a GPCR–G fusion protein. The GALR1–mini-Gαo or GALR2–mini-Gαq fusion protein was transiently expressed in Expi293 cells and assembled with purified Gβ1γ2 and scFv16 in the presence of galanin. The resulting GALR1–mini-Go complexes were coeluted and monodispersed with or without 3C protease treatment from size exclusion chromatography, indicating that GALR1 forms a stable complex with mini-Go (SI Appendix, Fig. S1 C–E). The peak fractions corresponding to the complexes were concentrated and subjected to cryo-EM single particle analysis. Two-dimensional (2D) class average analysis showed that the GALR1–mini-Go fusion protein complex gives more orientations than the GALR1–mini-Go complex without a linker between the receptor and mini-Gα (SI Appendix, Fig. S1 F and G). The combination of the two datasets enables us to obtain a final cryo-EM map of the GALR1–mini-Go complex at a global nominal resolution of 3.3 Å (SI Appendix, Fig. S2 and Table S1). The structures of the galanin- and spexin-bound GALR2–min-Gq fusion complex were determined to a nominal resolution of 3.3 Å and 3.5 Å, respectively (SI Appendix, Figs. S1 H–J and S3 and Table S1). The high-quality EM map allowed us to unambiguously assign side chains of the galanin peptide 1 through 17 and most of the amino acids of the receptors except the extreme terminal residues and some intracellular loops because of their high flexibility (Fig. 1 B and C) (24, 25). The overall structure of the GALR1–Go complex resembles that of the GALR2–Gq complex, with root-mean-square deviation values of 0.886 Å for the Cα atoms of the receptors and 0.604 Å for the Cα atoms of the G proteins.
Comparison of Galanin Binding Pockets of GALR1 and GALR2.
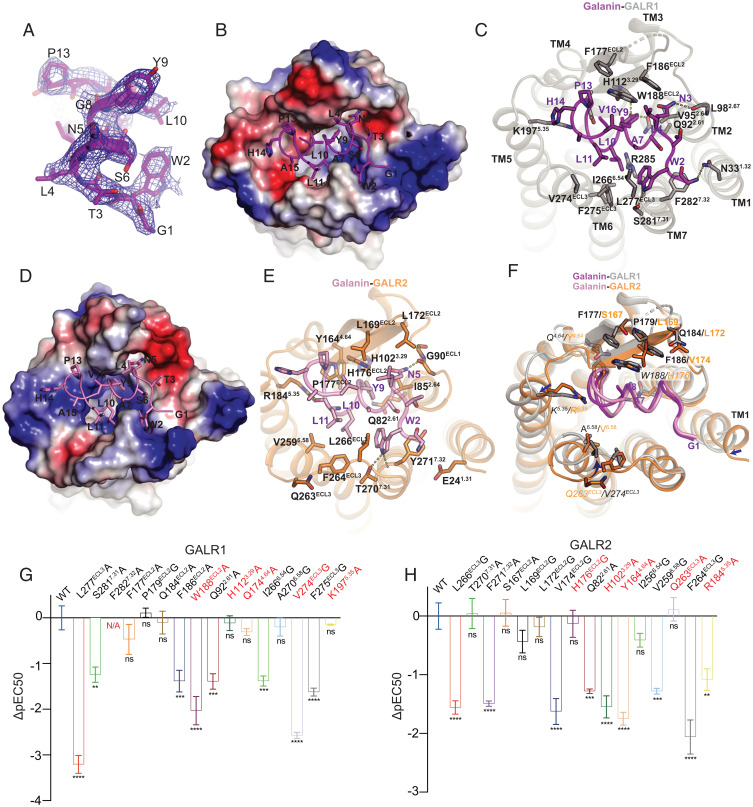
The existence of bulky aromatic amino acids and the high-quality EM density map allowed us to unambiguously assign side chains of galanin (Fig. 2A). The N-terminal portion of galanin (residue 1 through 17) was well resolved due to its direct contact with the receptors, which is consistent with previous studies showing that the binding affinity of the N-terminal region of galanin (1–16) for the receptors is comparable to the full-length galanin (26, 27). Moreover, the N-terminal region (1–16) but not the remaining part is highly conserved in GALP and spexin peptides (SI Appendix, Fig. S4A), both of which are able to activate the GALRs. Galanin mainly forms an alpha helical structure when bound to the receptor as well as in solution itself (28, 29). It occupies the extracellular vestibule of GALRs, which is equivalent to the binding site of a positive allosteric agonist LY2119620 in M2R (30) (SI Appendix, Fig. S4B). It lays on top of the receptor, nearly parallel to the membrane plane and distant from the toggle switch W6.48, the conformational change of which is essential for receptor activation. By contrast, most neuropeptide agonists of class A GPCRs, such as endothelin, orexin, and opioid peptides, bind nearly perpendicular to the membrane plane with one end buried in the helical cavity and the other end interacting with the extracellular loops; and these peptides penetrate in proximity to the toggle switch (31–33) (SI Appendix, Fig. S4B). Galanin contacts all seven transmembrane (TM) helices as well as extracellular loops ECL2 and ECL3, burying a surface area of 866 Å2, which accounts for the high-affinity binding of galanin for GALRs in the subnanomolar range (34). GALR1 and GALR2 use overlapping but a distinct set of residues to contact galanin, mostly via hydrophobic and hydrogen bond interactions (Fig. 2). The first N-terminal residue of galanin (G1) lies between TM1 and TM7 and is closer to TM1 of GALR1 than that of GALR2 (Fig. 2F), which may explain that removal of G1 or N-terminal extension in galanin reduced its binding affinity for GALR1 but not GALR2 (28, 35, 36). W2 is sandwiched between L277ECL3 and F2827.32 of GALR1 and makes an additional hydrogen bond with S2817.31 (Fig. 2C). Therefore, mutation of W2 in galanin or F2827.32 results in significant loss of binding for the receptors (16, 19, 37, 38). F2827.32A mutation in GALR1 almost abolished galanin potency (Fig. 2G), and F2717.32A mutation in GALR2 reduced galanin potency by nearly 100-fold (Fig. 2H). A7E mutation that was identified as a cause of TLE disease likely causes a clash with nearby hydrophobic residues, accounting for reduced binding affinity for GALRs (13) (Fig. 2C). Y9 penetrates into the receptor core, about 10 Å above the toggle switch (SI Appendix, Fig. S4B) and is hydrogen bonded by Q922.61 in GALR1 or Q822.61 in GALR2 (Fig. 2 C and E). Mutation of Q922.61 or Q822.61 to alanine reduced galanin potency by almost 100-fold (Fig. 2 G and H). Our structural observation is also consistent with previous studies showing that Y9 is vital for galanin binding to the receptors (37, 38). However, because of distinct residues of ECL2 and ECL3 involved in binding galanin, the conformations of these regions are different between GALR1 and GALR2 (Fig. 2F). For instance, V274 in the ECL3 of GALR1 engages hydrophobic interaction with L11 (Fig. 2C), while the equivalent residue in GALR2, Q263, rotates away from galanin due to its longer side chain and hydrophilic nature, resulting in the conformational change of ECL3 (Fig. 2F). As a result, V274G mutation reduced agonist potency by about 370-fold, while Q263A mutation showed little effect (Fig. 2 G and H). ECL2 forms an antiparallel β-sheet, which is a characteristic of peptide receptors. It covers galanin as a lid-like structure and forms extensive hydrophobic interactions with L4, P13, and V16. The residues in ECL2 of GALR1 involved in binding galanin have bulkier aromatic side chains than that in GALR2 (Fig. 2F). Mutations of the equivalent residues W188ECL2 and H176ECL2 in GALR1 and GALR2, respectively, had a distinct effect on galanin potency (Fig. 2 G and H), suggesting that ECL2 in GALR1 and GALR2 differently contribute to galanin binding. An endogenous peptide spexin has A7M and G8L mutations in galanin and specifically activates GALR2 and GALR3 (SI Appendix, Fig. S4A). Structure of the spexin-bound GALR2–Gq complex reveals that L8 in spexin likely clashes with the bulkier residue W188ECL2 in GALR1 (SI Appendix, Fig. S4C) (39), accounting for the specific binding of spexin for GALR2 and GALR3 (6). Owing to these conformational differences, R1845.35 in GALR2 but not K1975.35 in GALR1 makes hydrogen bonds with the backbone of galanin (Fig. 2F). As a result, R1845.35A mutation reduces galanin potency by about 10-fold, while K1975.35A mutation shows little effect (Fig. 2 G and H). Previous studies have also shown that mutations of several residues that are distant from galanin significantly reduce its binding affinity for GALRs. These residues include F115 and H264 of GALR1 (19) and F106, H252, H253, I256, W260, and F261 of GALR2 (18). From structural perspectives, most of these residues interact with nearby residues of the receptor, are involved in receptor folding (SI Appendix, Fig. S4 D and E), and their mutations likely disrupt the ligand binding pocket and indirectly influence the ligand binding. Taken together, these results suggest that mechanisms of galanin recognition by GALR1 and GALR2 are not identical, which allows the development of selective ligands targeting a specific subtype.
Fig. 2.
Mechanisms of galanin recognition by GALR1 and GALR2. (A) EM density map for galanin from the structure of the GALR2–Gq complex. (B) Electrostatic potential surface of GALR1 and ribbon representation of galanin (magenta) viewed from the extracellular side. Colors from red to blue represent negatively to positively charged regions. (C) Detailed interaction between GALR1 and galanin. (D) Electrostatic potential of the GALR2–galanin interface is distinct from that of the GALR1–galanin interface. (E) Detailed interaction between GALR2 and galanin. (F) Structural superposition of the GALR1–galanin and the GALR2–galanin complex. The equivalent residues in GALR1 and GALR2 that play distinct roles in galanin binding are shown. Arrows indicate the conformational changes. (G and H) The effects of mutations in GALR1 and GALR2 on galanin potency as measured by the cAMP inhibition assay and the IP1 accumulation assay, respectively. The equivalent residues that play distinct roles in GALR signaling are colored red. The expression levels of these mutants are comparable to the wild type. Data represent mean ± SEM of triplicate measurements in three independent experiments. Significance was analyzed using one-way ANOVA, ****P < 0.0001, ***P < 0.001, **P < 0.01. ns, not significant; N/A, not determined due to no response.
Activation Mechanisms of GALR1 and GALR2.
GALR1 and GALR2 predicted by AlphaFold (40) may represent inactive states, since AlphaFold is biased toward the inactive state of GPCRs (41) and the predicted structures show characteristic features of the inactive state of GPCRs (42) such as the ionic lock between R1333.50 and D1323.49 of the D3.49R3.50Y3.51 motif and the less pronounced outward movement of TM6 compared to the active states (Fig. 3 A–C). Upon galanin binding, the orthosteric site undergoes significant conformational change, as indicated by the inward displacement of the extracellular portions of TM2 and TM6 and the outward shift of the TM1 and TM7 (Fig. 3 A and B). These conformation changes account for the outward motion of TM6 and inward motion of TM7 on the intracellular side (Fig. 3C). GALR1 and GALR2 have consistent movement of TM6 and TM7 as well as similar rearrangement of important motifs for GPCR activation, such as the DRY and NPxxY motifs to other Gi/o- and Gq/11-coupled receptors such as μ-opioid receptor (μOR) and M1 muscarinic receptor (M1R), respectively, suggesting that the conformational changes of the intracellular side of the receptors are important for determining G protein selectivity (SI Appendix, Fig. S4 F and G). The conformational changes of the toggle switch W6.48 and the P5.50I/V3.40F6.44 motif are common features of class A GPCR activation. In contrast to the most class A GPCRs, where the orthosteric sites are in close proximity to the toggle switch, the galanin binding site is distant from it. Hydrophobic interactions between F275 in ECL3 of GALR1 and L10 and L11 in galanin result in the downward shift of F275, which propagates to the downward movement of W2606.48 via I2666.54 and H2636.51 (Fig. 3B). The downward shift of W6.48 is associated with the conformational change of the P5.50I/V3.40F6.44 motif, which allosterically disrupts the conserved ionic lock, leading to the outward displacement of TM6 (Fig. 3C). Inward displacement of TM7 in the intracellular side is observed in the active state of many other class A GPCRs, as indicated by the conformational change of the NPXXY motif, in which Y3037.53 forms a water-meditated hydrogen bond with Y2205.58. The inward displacement of TM7 is coupled by the outward shift of R2857.35 that arises from its interaction with galanin. Although the key residues involved in receptor activation are conserved between GALR1 and GALR2, their conformations vary significantly (Fig. 3D). This is because the hydrophobic interaction between V274 in ECL3 and L11 in galanin exists in GALR1, while this interaction is absent in GALR2 due to the substitution of V274 in Q263, which leads to the upward shift of F264ECL3 as well as the toggle switch W6.48 and PIF motif in GALR2, compared to the equivalent residues in GALR1 (Fig. 3D). Consistent with the important role of F275ECL3/F264ECL3 in GALR receptor activation, mutation of F275 in GALR1 or F264 in GALR2 reduced galanin potency by almost 100-fold (Fig. 2 G and H), and mutation of F264 in GALR2 or the equivalent residue F263 in GALR3 completely abolishes galanin binding (18, 43). The conformation differences of residues involved in receptor activation contribute to the structural variation in the cytoplasmic pocket of GALR1 and GALR2 and may play a role in G protein selectivity.
Fig. 3.
Mechanisms of GALR1 and GALR2 activation. (A) Structural overlay of GALR1 in the active and inactive state (predicted by AlphaFold). (B) Conformational changes of the P5.50I3.40F6.44 motif upon receptor activation. (C) Conformational changes of residues in the cytoplasmic pocket including the D3.49R3.50Y3.51 and the NPY motif upon receptor activation. (D) Structural overlay of the active state of GALR1 and GALR2.
Zn2+ Is a Negative Allosteric Modulator (NAM) of GALR1.
Previous studies have reported that Zn2+ can inhibit galanin binding to the receptors (16). To further investigate the functional role of zinc ion in galanin receptor signaling, we used the NanoBiT complementation-based assay to assess the effect of Zn2+ on activation of receptors by galanin in living cells. Mini-G proteins were used in the NanoBiT assay throughout this study, since they preserve appropriate coupling specificity and can be recruited to the active GPCRs without further dissociation, which increases the signal-to-noise ratio in this assay. As expected, Zn2+ diminished the effect of 1 μM galanin on GALR1 activation in a concentration-dependent manner with an IC50 value of 47.2 μM (Fig. 4A). The diminished effect of Zn2+ is saturable or has a “ceiling” level. In contrast, the diminished effect was observed in GALR2 when the concentration of Zn2+ reached millimolar range that is above the physiological concentration, indicating that Zn2+ had little effect on galanin-induced GALR2 activation. Our structures show that galanin receptors are enriched with histidine residues that may coordinate Zn2+ underneath the orthosteric binding pocket (Fig. 4B). Comparison of primary sequences of GALR1 and GALR2 from different species revealed that H2676.55 but not nearby histidine residues in GALR1 is mutated to isoleucine in GALR2 (SI Appendix, Fig. S5A). As expected, the zinc effect was significantly abrogated in GALR1 when H2676.55 was mutated, while H1123.29A, H2636.51F, or H2646.52F mutation had little influence (Fig. 4A and SI Appendix, Fig. S5B). All these mutants of GALR1 can be activated by galanin, although the potency and efficacy of galanin for these mutants vary (SI Appendix, Fig. S5C). In contrast to Zn2+, other divalent cations such as Mg2+ and Ca2+ do not diminish GALR1 activation by galanin (SI Appendix, Fig. S5D). Furthermore, we tested the effect of Zn2+ on the concentration response curve of galanin. Zn2+ produced the concentration-dependent and saturable rightward shifts in the potency of galanin and decreased the maximum response as well (Fig. 4C). By contrast, Zn2+ had little effect on the galanin concentration-response curve of H2676.55A mutant of GALR1 (Fig. 4D). H2676.55 is located in the TM6 right below the galanin binding site. The extracellular part of TM6 near H2676.55 moves inwards upon galanin binding, which leads to the receptor activation (SI Appendix, Fig. S5E). Moreover, previous studies have shown that H2676.55 mutation reduces the binding affinity of galanin for GALR1 by more than 100-fold (19). As a result, when coordinated by H2676.55 and other nearby residues, Zn2+ likely reduces the binding affinity of galanin, rigidifies the extracellular part of TM6, and restricts its conformational change, attenuating galanin-induced receptor activation. The exact coordination pattern of Zn2+ awaits further investigation. Nevertheless, these results indicate that Zn2+ is a NAM of GALR1.
Fig. 4.
Zinc is a NAM of GALR1. (A) The effect of increasing concentration of zinc on receptor activation induced by 1 μM galanin, as evaluated by the NanoBiT assay, where the small fragment and the large fragment are fused to the C terminus of GALR1 and the N terminus of mini-Go, respectively. The luminescence signals are normalized as percentages of the initial response of GALR1 to galanin without zinc treatment. (B) Histidine residues are enriched underneath the galanin binding pocket of GALR1. (C and D) The actions of increasing concentration of zinc on the galanin dose–response curve of wild type (WT) (C) and H267A mutant (D) of GALR1 measured by the NanoBiT assay. The luminescence signals are normalized to the vehicle treatment as fold change.
Structural Determinants of Gi/o and Gq/11 Selectivity.
A notable difference between structures of the GALR1–Go complex and the GALR2–Gq complex is the relative orientation of Go and Gq to the receptors (Fig. 5 A and B). When aligning the receptors, the α5 of Gαo is rotated around the “wavy hook” of α5 by about 14° toward TM5, compared with Gαq. This orientation difference was also observed in the structures of M1R and M2R bound to G11 and Go, respectively (44). In addition, because of the different interaction interface of the receptor and G protein, the flexibility of intracellular loops (ICLs) in GALR1 and GALR2 differs (Fig. 5A). For instance, ICL1 is ordered in GALR2 owing to the hydrogen bond interaction between D312 in Gβ and the main chain carbonyl group of G53 in ICL1, whereas it is flexible in GALR1 due to the absence of this interaction (SI Appendix, Fig. S6A). The ICL2 of most Gi/o-coupled receptors such as μOR forms an alpha helical structure, where hydrophobic residues at position 34.51 engage weak hydrophobic interactions with the hydrophobic pocket of Gαi/o formed by V34 from the αN–β1 loop, L195 from the β2–β3 loop, and I343 and F336 from α5 (SI Appendix, Fig. S6B). However, the ICL2 of GALR1 is disordered because of the substitution of the hydrophobic residue L13134.51 in arginine and the absence of hydrophobic interaction between the ICL2 of GALR1 and Gi (Fig. 5 C and G). By contrast, L13134.51 in the ICL2 of GALR2 is buried deep in the hydrophobic pocket of Gαq formed by L34 from the αN–β1 loop, V79 from the β2–β3 loop, and F228, K232, and I235 from α5, and engages strong hydrophobic interactions (Fig. 5D). In addition, P13034.50 at the junction of ICL2 and TM3 is stabilized through hydrophobic interactions with I235 and K232 in α5 of Gαq. Consistent with structural observations, the NanoBiT mini-G recruitment assay shows that substitution of L13134.51 or P13034.50 in GALR2 in the equivalent residues in GALR1 impairs the ability of GALR2 to couple Gq (SI Appendix, Fig. S7A), accounting for the inability of GALR1 to couple Gq. However, substitutions of S14034.50, R14134.51, and S1494.49 in ICL2 of GALR1 with the equivalent residues in GALR2 have little effect on coupling efficiency between GALR1 and Gαi (SI Appendix, Fig. S7B), suggesting that ICL2 in GALR1 is not involved in Gi coupling. The important role of ICL2 in Gq coupling but not in Gi/o coupling for GALRs is also demonstrated by the Bioluminescence Resonance Energy Transfer (BRET) G protein dissociation assay using the wild-type G proteins, suggesting that the coupling mode captured using mini-G is functionally relevant (SI Appendix, Fig. S7 D and E). Remarkably, GALR1 acquires the ability to couple Gq, as indicated by the NanoBiT assay as well as the IP1 assay (Fig. 5I and SI Appendix, Fig. S7C), when residues in ICL2 of GALR1 are substituted with that of GALR2, further supporting the important role of ICL2 in Gq coupling.
Fig. 5.
Mechanisms of Go and Gq selectivity in the GALR receptor family. (A and B) Structural superposition of GALR1–Gαo and GALR2–Gαq in two opposite views. Receptors are aligned. (C) Interaction details between ICL2 of GALR1 and Gαo. (D) Interaction details between ICL2 of GALR2 and Gαq. (E) Interaction details between TM5 and TM6 of GALR1 and Gαo. (F) Interaction details between TM5 and TM6 of GALR2 and Gαq. (G) Sequence alignment of ICL2 from the GALR receptor family. (H) Sequence alignment of ICL3 from the GALR receptor family. Residue numbers of GALR2 are indicated above the alignment. (I) The IP1 accumulation assay evaluating the effects of ICL2 substitutions in GALR1 on GALR1–Gq coupling. All mutants are expressed at similar levels as WT. (J) Substitution of ICL3 in GALR2 with that in GALR1 increases coupling efficiency of GALR2 and Go.
To understand the structural mechanism of the inability of GALR2 to couple Gi, we compared the interaction details between the GALR1–Gi and the GALR2–Gq complexes and mainly focused on residues of GALR1 involved in Go coupling that are not conserved in GALR2. R1333.50, I1373.54, L2245.62, L2275.65, L2315.69, and T2456.33 in TM3, TM5, and TM6 of GALR1 engage extensive hydrophobic interactions with the extreme C-terminal part of α5 in Go (Fig. 5E). Most of these residues are conserved in GALR2 (Fig. 5F). Mutations of conserved residues in GALR1 and GALR2 impair the recruitment of Gi and Gq, respectively (SI Appendix, Fig. S7 F–I). Nevertheless, notable differences between GALR1 and GALR2 are in ICL3. S238ICL3 and S235ICL3 in ICL3 of GALR1 make hydrogen bonds with D341 in Gαo, and K237ICL3 engages electrostatic interactions with residues in the GTPase domain of Gαo. All of the three residues are mutated in GALR2, leading to loss of these interactions (Fig. 5 E, F, and H). Mutations of S235ICL3 and K237ICL3 in GALR1 have a modest effect on galanin potency, but dramatically reduced the maximum responses (SI Appendix, Fig. S7 F and G). Remarkably, GALR2 acquired the ability to bind Go, when ICL3 (214 to 225) of GALR2 including the three residues were replaced by the equivalent residues in GALR1 (Fig. 5 H and J). Taken together, our data suggest that ICL2 in GALR2 and ICL3 in GALR1 are critical for determining Gq and Go selectivity, respectively.
Structural Determinants of Gs and Gq Selectivity.
Although it has been shown that interactions between the hydrophobic residue at position 34.51 of ICL2 and the hydrophobic pocket of Gα are essential for the efficient coupling of Gq and Gs (45), it remains unclear how Gq and Gs are selectively recognized. Comparison of structures of D1 dopamine receptor (D1R)–Gs and GALR2–Gq revealed key structural elements in the receptors that determine Gq and Gs selectivity. In the GALR2–Gq complex, the conformation of ICL2 is stabilized by salt bridge interactions between R34.57 (M34.57 in D1R) and D3.49 of the DRY motif as well as a hydrogen bond between R34.57 and Y(−4) in Gαq, while ICL2 of D1R is stabilized by a hydrogen bond between Y34.53 (S34.53 in GALR2) and D3.49, and a potential water-meditated hydrogen bond between Y3.49 and Y(−4) in Gαs (Fig. 6A). Notably, Y34.53M/V34.57 are prevalent in Gs-coupled receptors, while R34.57 is enriched in Gq-coupled receptors (Fig. 6D). Mutations of YM in D1R and RS in GALR2 significantly reduced the potency of dopamine and galanin, respectively (Fig. 6 E and F). Moreover, N(−3) (−1 indicates the last residue of Gα) in Gαq is inserted into a hydrophobic pocket formed by N2.40, F8.50, and other nearby residues, whereas E(−3) flips outside this pocket, probably due to its longer side chain and negative-charge nature. Remarkably, when E(−3) in Gαs but not the nearby residues L(−1) and Q(−5) was substituted with the equivalent residues in Gαq, the coupling efficiency between GALR2 and Gs was significantly increased (Fig. 6G). These different interaction modes of GALR2–Gq and D1R–Gs account for the movement of α5 in Gs toward TM6 and the outward movement of TM6 in D1R, compared to that in GALR2 (Fig. 6B), explaining that most Gs-coupled receptors display a larger TM6 movement in the active state than Gq-coupled receptors. As a result of these conformational changes, Gs is closer to TM5 than Gq, highlighting the important role of TM5 in determining Gs selectivity. Indeed, TM5 in most Gs-coupled receptors has a C-terminal helical extension, and previous studies have shown that the A/V5.65 Q5.68Φ5.69 (Φ represents hydrophobic residues) motif in TM5 is prevalent in receptors that exclusively couples to Gs and is critical for Gs coupling in D1R (46, 47). Residues at position 5.65 in Gs-coupled receptors prefer hydrophobic residues with small side chains such as alanine and valine because of their close distance from the hydrophobic pocket formed by L(−1), L(−2), and L(−7) in Gαs (Fig. 6C). Mutation of A5.65 in leucine would cause a clash with this pocket and impairs the Gs coupling (47). In contrast, leucine is dominant at position 5.65 in Gq-coupled receptors, due to its long distance from the hydrophobic pocket formed by V(−1), L(−2), and L(−7) in Gαq (Fig. 6C). L5.65A mutation in GALR2 weakens the interaction with this hydrophobic pocket and thus significantly decreased galanin potency (Fig. 6F). However, it is noteworthy that Gs- and Gq-coupled receptors show sequence preference at some positions of ICL2 and TM5, but also accommodate various residues at these positions (Fig. 6D), partly because of diverse receptor–G protein interfaces and promiscuous coupling of some GPCRs.
Fig. 6.
Important structural features in class A GPCRs that determining Gs and Gq selectivity. (A) Structural superposition of the GALR2–Gq and the D1R–Gs complexes. Receptors are aligned. (B) N(−3) in Gq is inserted into a hydrophobic pocket formed by TM2, TM7, and H8, while E(−3) in Gs flips outside this pocket. (C) A5.65 in D1R is close to the hydrophobic pocket formed by L(−1), L(−2), and L(−7) in Gs, while L5.65 in GALR2 is distant from that in Gq. (D) Sequence alignment of 41 class A Gs-coupled receptors (Top) and 44 Gq-coupled receptors (Bottom). The dominant residues are indicated below the alignments. (E) Mutations of Y34.53M34.57 in the ICL2 of D1R reduce D1R–Gs coupling efficiency. (F) Mutations of S34.53R34.57 in the ICL2 of GALR2 almost abolish GALR2 and Gq coupling. (G) The effects of mutations of the wavy hook in Gs on coupling efficiency of GALR2–Gαs, as evaluated by the NanoBiT assay.
Discussion
Here, we report cryo-EM structures of the GALR1–Go and GALR2–Gq complex using the GPCR–G protein fusion strategy. The structures revealed distinct mechanisms of galanin recognition and receptor activation for GALR1 and GALR2, which contribute to structural variation in the cytoplasmic pocket of the receptors and may play an important role in determining the G protein selectivity. Moreover, we showed that Zn2+ is a negative allosteric modulator of GALR1 but not GALR2.
Zn2+, known as a neuromodulator, is widely distributed in the central nervous system (CNS), particularly enriched in the synaptic vesicles of glutamatergic neurons (48, 49). It is released to the synaptic cleft upon membrane depolarization and modulates functions of ion channels and receptors on the pre- or postsynaptic membrane. It has been shown that zinc inhibits ionotropic glutamate AMPA and NMAR receptors, fine tuning synaptic transmission in the brain (50, 51). GALR1 is expressed on both glutamatergic and GABAergic postsynaptic neurons. The spatial colocalization of zinc and GALR1 makes it possible for zinc to regulate the function of GALR1. Moreover, the IC50 of zinc on GALR1 activation is 47.2 μM, which is in the range of the physiological concentration of zinc (10 nM to 100 μM) (52). Previous studies have also shown that zinc regulates endogenous ligand binding at several GPCRs, including β2 adrenergic receptors (β2AR) (53), melanocortin receptors (54), and platelet-activating factor receptor (55). In this study, we showed that zinc attenuated GALR1 activation by galanin, possibly through restricting the conformational change of TM6 that leads to receptor activation. Further studies are required to address whether zinc modulates a large number of GPCRs in the CNS and fine tunes GPCR signaling, as does sodium (56).
Combining published structures of the GPCR–Gi complexes, we can roughly divide the class A Gi-coupled receptors into three classes based on the interaction features between ICL2 and G proteins: 1) Receptors that exclusively couple to Gi and have a charge residue at position 34.51 of ICL2, such as GALR1 and sphingosine-1-phosphate receptors (S1PR) (57); 2) receptors that exclusively couple to Gi and have a hydrophobic residue at position 34.51, such as D3 dopamine receptor, M2 muscarinic receptor (M2R), and μ-opioid receptor (32, 44, 58); and 3) receptors that promiscuously couple to Gi and have a large hydrophobic residue at position 34.51, such as the neurotensin receptor 1 (NTSR1), β2AR, and the cholecystokinin A receptor (59, 60). In the first class, when bound to the receptor, ICL2 is disordered, or forms a random coil structure. Since there is no hydrophobic interaction between 34.51 and Gαi, ICL2 in receptors of this class plays a distinct role in determining Gi coupling efficiency (57) (SI Appendix, Fig. S8A). In GALR1, ICL2 is not involved in Gi coupling, whereas in S1PR, ICL2 is involved in hydrophilic interactions with Gi and is important for Gi coupling. In the second class, ICL2 forms an alpha helical structure, and residue 34.51 of ICL2 is located outside and distant from the hydrophobic pocket of Gαi formed by residues from the αN–β1 loop, the β2–β3 loop, and α5, and engages weak hydrophobic interactions (SI Appendix, Fig. S8 B and C). Mutation of this residue had little effect on the Gi coupling or GDP release from Gi/o (61, 62). In the third class, similar to Gs- and Gq-coupled receptors, residue 34.51 is located close to the middle of the hydrophobic pocket of Gαi and engages strong hydrophobic interaction (SI Appendix, Fig. S8 E and F). In addition, some receptors in this class, such as NTSR1, have the other conformation, where residue 34.51 is located outside the hydrophobic pocket (SI Appendix, Fig. S8D). Previous studies have shown that F34.51A mutant of β2AR failed to activate Gi (62), suggesting that the hydrophobic interaction between ICL2 and Gi is very important for Gi coupling in the third class. Owing to the absence of or weak interaction between Gαi and ICL2 in receptors that exclusively couple Gi, the cytoplasmic end of TM5 and TM6, and ICL3 have strong interactions with Gαi and are critical for determining Gi selectivity (SI Appendix, Fig. S8).
It has been recognized that the distal part of α5 in Gα plays a key role in determining G protein selectivity (63–65). We further identified a residue pair N/E(−3) in α5 of Gq/Gs that contributes to structural differences and selective interactions between the D1R–Gs and GALR2–Gq. Substitution of this residue in Gs can promote coupling of GALR2 to noncognate Gs. Moreover, we revealed several signature residues in ICL2 and TM5 that dominate in Gs- and Gq-coupled receptors. Thus, our results provide insights into the molecular mechanisms of G protein selectivity by class A GPCRs.
Materials and Methods
Cloning.
The human GALR1 and GALR2 were cloned into pcDNA3.1(+) vector (Thermo Fisher Scientific) with an N-terminal hemagglutinin (HA) signal sequence and a FLAG epitope tag (DYKDDDDK). An engineered mini-Gαo was fused to the C terminus of GALR1 (1 to 349) with three copies of 3C protease sites between them. GALR2 (1 to 314) was expressed as a fusion protein including two repeats of 3C protease site and a mini-Gαq sequence in the C terminus of GALR2. ScFv16 was cloned into the pFastBac vector (Invitrogen) with an N-terminal GP64 signal sequence and a C-terminal 3C protease site, followed by an octahistidine tag. His6-tagged Gβ1 and Gγ2 (C68S mutation) were cloned in the pFastBac Dual vector for insect cell expression.
Protein Expression and Purification.
The plasmid expressing GALR1–mini-Gαo or GALR1–mini-Gαq was transiently expressed into Expi293F cells (Thermo Fisher Scientific) using polyethyleneimine (PEI, Polysciences). Cells were lysed in the lysis buffer (20 mM HEPES, pH 7.4) supplemented with protease inhibitor mixture (Roche) using a glass dounce grinder and centrifuged at 1,000 × g for 3 min to remove the nucleus. The membrane fraction was pelleted by centrifugation at 65,000 × g, at 4 °C for 1 h and homogenized in the solubilization buffer containing 20 mM HEPES pH 7.4, 150 mM NaCl, 1% lauryl maltose neopentyl glycol (LMNG), 0.2% cholesteryl hemisuccinate (CHS), and 60 nM galanin peptide 1 through 30 (MedChemExpress). After centrifugation to remove debris, the supernatant containing solubilized GALRs–mini-Gα was supplemented with 2 mM CaCl2 and loaded onto the M1 anti-FLAG antibody resin. The resin was washed with wash buffer containing 20 mM HEPES, pH 7.4, 300 mM KCl, 0.01% LMNG, 0.002% CHS, 2 mM CaCl2, 10 mM MgCl2, 2 mM adenosine triphosphate, 6 nM galanin, and eluted with elution buffer containing 20 mM HEPES, pH 7.4, 150 mM NaCl, 0.01% LMNG, 0.002% CHS, 10 mM ethylenediaminetetraacetic acid, 0.5 mg/mL 1× FLAG peptide and 60 nM galanin. Gβ1γ2 (C68S) and scFv16 were expressed and purified as previously described (21, 66).
Complex Assembly.
Purified GALR1–mini-Go or GALR2–mini-Gq proteins, Gβ1γ2 (C68S) and scFv16, were mixed with a molar ratio of 1:1.5:2 in 500 μL of the equilibration buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 0.01% LMNG, 0.002% CHS, 60 nM galanin, 0.5 μM tris(2-carboxyethyl)phosphine) supplemented with 1 μL PNGaseF and 0.5 μL apyrase. The mixture was incubated on ice for 1 h and further purified on a Superose 6 Increase 10/300 column preequilibrated with the equilibration buffer. The peak fractions containing the complex were supplemented with 60 μM galanin and concentrated to about 6 mg/mL. For assembly of the GALR1–miniGαo/Gβ1γ2 (C68S) complex with 3C protease site cleaved, similar procedures were performed as above, except that 3C protease was added before purification on a Superose 6 Increase 10/300 column. For assembly of the spexin-bound GALR2 complexes, the same procedures were performed, except that galanin was replaced by spexin during the purification process.
Cryo-EM Sample Preparation and Data Collection.
The 300-mesh holey carbon grids (Quantifoil Au R1.2/1.3) were glow charged, loaded into a Vitrobot MarkIV instrument chamber (Thermo Fisher Scientific), maintained at 8 °C and 100% humidity. A total of 3.0 μL of GALR complex samples was applied onto the grid, blotted for 3.0 to 4.0 s with a blotting force of 4, before plunge freezing in liquid ethane. Cryo-EM movies were collected on a Titan Krios microscope equipped with a BioQuantum GIF/K3 direct electron detector (Gatan) under accelerating voltage of 300 kV at a nominal magnification of 64,000×. Each movie stack was collected as 32 frames, with a total dose of 50 e-/Å2 for 2.56 s.
Cryo-EM Data Processing.
All movie stacks were collected and processed with MotionCor2 for motion correction (67), with 2× binned to a pixel size of 1.087 Å. Contrast transfer function (CTF) estimation was performed using patch-based CTF estimation in cryoSPARC_v3 (68). All processed images were then subjected to particle picking using Blob picker in cryoSPARC, followed by particle extraction. For the galanin-bound GALR1–mini-Go complex with 3C protease sites cleaved, particles from 1,401 micrographs (dataset A) were subjected to two rounds of 2D classification, generating 248,352 good particles. Ab initio reconstruction and nonuniform refinement were performed to get a reference map for GALR1. For the GALR1–mini-Go fusion protein complex, 886 micrographs (dataset B) were collected, followed by particle picking using Blob picker and particle extraction. Particles from the two datasets were combined and subjected to two rounds of 2D classification, yielding 2,882,487 good particles. These particles were subjected to global three-dimensional (3D) classification in RELION3.1 (69), followed by another round of 3D classification focused on the receptor. A total of 426,045 particles from the best class were run through nonuniform refinement in cryoSPARC, resulting in a final 3.3-Å map.
For the galanin-bound GALR2–mini-Gq fusion protein complex, 1,337 micrographs were collected, and processing procedures were performed as above. In brief, two rounds of 2D classification using autopicked particles resulted in 1,325,739 good particles, which were subjected to two rounds of 3D classification in RELION3.1 using the GALR1–Go complex map as initial model. The 578,453 particles from three classes with clear secondary structure features were selected and subjected to nonuniform refinement in cryoSPARC, resulting in a final 3.29-Å map. All 3D maps were postprocessed with DeepEMhancer (70).
For the spexin-bound GALR2–mini-Gq complex, 1,139 movies were collected and processed as above. A total of 1,015,461 good particles were selected from two rounds of 2D classification and subjected to heterogeneous refinement and nonuniform refinement in cryoSPARC. The final map is about 3.5 Å.
Model Building.
Homology models for GALR1 and GALR2 were generated using the structure of μ-opioid receptor (PDB: 4DKL) in the SWISS-MODEL server. The homology model of GALR1 and the structure of mini-Gαo/Gβγ/scFv16 (PDB: 7D77) were fitted into the EM map in Chimera (71). The structure of mini-Gαq/Gβγ/scFv16 was extracted from the published structure (PDB: 6WHA), and docked into the EM map together with the homology model of GALR2. All the models were manually built in COOT (72) and are subjected to real_space_refinement in Phenix (73) using the reference structure and secondary structure restraints. The statistics for structure refinement are summarized in SI Appendix, Table S1.
cAMP Inhibition Assay.
Chinese hamster ovary (CHO) cells were seeded into six-well plates and cultured overnight until cell confluence reached ∼80%. Plasmids expressing GALR1 or mutants were transfected together with the GloSensor biosensor plasmid following a Lipofectamine cell transfection procedure (Invitrogen). Transfected cells were cultured for 1 d and reseeded into 96-well plates by 3 × 104 cells per well. After 8 h postseeding, the medium was exchanged to CO2-independent medium (Gibco) supplemented with 500 μg/mL of D-luciferin. Cells were stimulated with various concentration gradients of galanin for 5 min and then treated with 1 μM forskolin. The bioluminescence signal was constantly measured for 10 min, and the peak signal was acquired for the inhibitory dose curve fitting and IC50 determination using GraphPad Prism 8 software. Significance analysis was performed using one-way analysis of variance method (one-way ANOVA in Prism 8).
IP1 Accumulation Assay.
Gαq-mediated IP1 accumulation was measured using the IP-ONE Gq HTRF Kit from Cisbio. HEK-293T cells were seeded into 6-well plates, and 2 μg of GALR2 or mutant plasmids were transfected using PEI. After 2 d posttransfection, cells were suspended, washed one time with Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco), resuspended into Hank's Balanced Salt Solution (HBSS) buffer (Beyotine) and seeded into 384-well plates (Greiner) with ∼7,000 cells per well. Transfected cells were stimulated with various concentration gradients of galanin for 1 h and subjected to IP1 accumulation detection following the assay protocol. The inhibitory dose curve was plotted and IC50 was determined using GraphPad Prism 8 (dose–response inhibitory, three parameters). Significance was analyzed using one-way ANOVA.
NanoBiT Assay.
To monitor the interaction between G proteins and GALR1 or GALR2 upon galanin stimulation, a NanoLuc-based enzyme complementation system called NanoBiT assay (74) was used (Promega). The C terminus of GALR1 or GALR2 was fused with the small fragment (smBiT), and the large fragment (LgBit) element was fused to the N terminus of mini-Gα proteins. HEK-293T cells were seeded into six-well plates and transfected with 1 μg of GPCR–smBit and 1 μg of LgBit–mini-Gα. After 2 d posttransfection, cells were suspended, washed once with DPBS and resuspended into the assay buffer containing HBSS supplemented with 0.01% bovine serum albumin (BSA) (Sigma), 10 mM Hepes (Beyotine) and 10 μM coelenterazine-h (Yeasen). The culture was equilibrated at room temperature (RT) for 2 h and subjected to stimulation with various concentration gradients of galanin and instant bioluminescence measurement. The bioluminescence signal was acquired at the time point when the signal went into the stationary phase, and the normalized signal (fold change) was fitted to a three-parameter sigmoidal concentration–response curve in Prism 8 software.
Zn2+ Inhibition Assay.
As zinc produced high background signal in the IP1 accumulation assay and cAMP inhibition assay, the NanoBiT assay was used to measure the effect of Zn2+ effect on GALR signaling.
The same constructs used in the NanoBiT assay were adopted. After 2 d posttransfection, cells were resuspended and washed twice with the assay buffer (20 mM HEPES, pH 7.3, and 150 mM NaCl) and resuspended into the assay buffer supplemented with 10 μM coelenterazine-h and seeded into 96-well plates. After 30 min of incubation at RT, cells were stimulated with various concentration gradients of galanin premixed with a fixed concentration of ZnCl2, or 1 μM of galanin premixed with titrated concentration of ZnCl2. The bioluminescence signals in the stationary phase were acquired and analyzed using three-parameter dose–response-stimulatory or dose–response-inhibitory fitting methods in Prism 8 software.
BRET Assay.
BRET assay was designed and performed as previously described (22). Briefly, for the BRET mini-G protein recruitment assay, GFP2 was tagged at the C-terminal of GALRs while Rluc8 was fused to the N-terminal of mini-Gαo or mini-Gαq. A total of 2 mL of Freestyle 293 cells was seeded into six-well plates at 1.5 million/mL density and transfected with GALRs_GFP and Rluc_mini-G plasmids by a ratio of 1 μg:1 μg per well. After 2 d of culture, cells from each well were collected, washed twice with BRET equilibrium buffer (HBSS supplemented with 20 mM HEPES 7.3, 0.001% BSA), and resuspended in 3 mL BRET equilibrium buffer supplemented with 5 μM coelenterazine 400a (MX4610, maokangbio). A total of 95 μL per well of cell suspensions were seeded into 96-well plates (WHB-96-01, all white) and incubated at room temperature for 5 min, followed by treatment with 5 μL galanin peptide at corresponding concentrations for 5 min. Signals were constantly read six times using a PerkinElmer Multimode microplate reader with 395 nm (coelenterazine 400a) and 512 nm (GFP2) emission filters with 1-s integration time. BRET ratio from the last time reading was calculated as the ratio of GFP2 signal to Rluc8 signal.
For the BRET G protein dissociation assay, Gαi1_Rluc8 or Gαq_Rluc8 and Gγ9_GFP2 fusion constructs were generated as previously described. The same procedures were performed as the mini-G recruitment assay except that a total of 4 μg of GALRs, Gα_Rluc8, Gβ3, and Gγ9_GFP2 plasmids were transfected by the ratio of 1:1:1:1. Data were normalized and analyzed by nonlinear regression using Prism 8 software.
Supplementary Material
Acknowledgments
We thank Dr. Xiangyu Liu at Tsinghua University for providing the plasmid expressing Gβ1γ2. We thank staff at Shuimu BioSciences for their help with cryo-EM data collection. All EM images were collected at Shuimu BioSciences. This work was supported by the Chinese Ministry of Science and Technology, Beijing Municipal Science and Technology Commission (Z201100005320012), and Tsinghua University.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. R.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2121465119/-/DCSupplemental.
Data Availability
The atomic structures have been deposited in the Protein Data Bank (PDB) under the accession codes 7XJJ (24), 7XJK (25), and 7XJL (39). The EM maps have been deposited at the Electron Microscopy Data Bank (EMDB) under the accession nos. EMD-33229, EMD-33230, and EMD-33231. All other study data are included in the article and/or SI Appendix.
References
- 1.Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V., Galanin - A novel biologically active peptide from porcine intestine. FEBS Lett. 164, 124–128 (1983). [DOI] [PubMed] [Google Scholar]
- 2.Šípková J., Kramáriková I., Hynie S., Klenerová V., The galanin and galanin receptor subtypes, its regulatory role in the biological and pathological functions. Physiol. Res. 66, 729–740 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Lang R., et al. , Physiology, signaling, and pharmacology of galanin peptides and receptors: Three decades of emerging diversity. Pharmacol. Rev. 67, 118–175 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Branchek T. A., Smith K. E., Gerald C., Walker M. W., Galanin receptor subtypes. Trends Pharmacol. Sci. 21, 109–117 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Wang S., Gustafson E. L., Galanin receptor subtypes. Drug News Perspect. 11, 458–468 (1998). [PubMed] [Google Scholar]
- 6.Kim D. K., et al. , Coevolution of the spexin/galanin/kisspeptin family: Spexin activates galanin receptor type II and III. Endocrinology 155, 1864–1873 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Mills E. G., Izzi-Engbeaya C., Abbara A., Comninos A. N., Dhillo W. S., Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat. Rev. Endocrinol. 17, 97–113 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Lin E. J., et al. , Recombinant AAV-mediated expression of galanin in rat hippocampus suppresses seizure development. Eur. J. Neurosci. 18, 2087–2092 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Haberman R. P., Samulski R. J., McCown T. J., Attenuation of seizures and neuronal death by adeno-associated virus vector galanin expression and secretion. Nat. Med. 9, 1076–1080 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Millón C., et al. , Role of the galanin N-terminal fragment (1-15) in anhedonia: Involvement of the dopaminergic mesolimbic system. J. Psychopharmacol. 33, 737–747 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Li S. Y., et al. , Involvement of galanin and galanin receptor 1 in nociceptive modulation in the central nucleus of amygdala in normal and neuropathic rats. Sci. Rep. 7, 15317 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokaia M., et al. , Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc. Natl. Acad. Sci. U.S.A. 98, 14006–14011 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guipponi M., et al. , Galanin pathogenic mutations in temporal lobe epilepsy. Hum. Mol. Genet. 24, 3082–3091 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Xu X. F., et al. , Galanin and its receptor system promote the repair of injured sciatic nerves in diabetic rats. Neural Regen. Res. 11, 1517–1526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott-Hunt C. R., Pope R. J., Vanderplank P., Wynick D., Activation of the galanin receptor 2 (GalR2) protects the hippocampus from neuronal damage. J. Neurochem. 100, 780–789 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kask K., Berthold M., Kahl U., Nordvall G., Bartfai T., Delineation of the peptide binding site of the human galanin receptor. EMBO J. 15, 236–244 (1996). [PMC free article] [PubMed] [Google Scholar]
- 17.Church W. B., Jones K. A., Kuiper D. A., Shine J., Iismaa T. P., Molecular modelling and site-directed mutagenesis of human GALR1 galanin receptor defines determinants of receptor subtype specificity. Protein Eng. 15, 313–323 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Lundström L., Sollenberg U. E., Bartfai T., Langel U., Molecular characterization of the ligand binding site of the human galanin receptor type 2, identifying subtype selective interactions. J. Neurochem. 103, 1774–1784 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Kask K., et al. , Mutagenesis study on human galanin receptor GalR1 reveals domains involved in ligand binding. Ann. N. Y. Acad. Sci. 863, 78–85 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Nehmé R., et al. , Mini-G proteins: Novel tools for studying GPCRs in their active conformation. PLoS One 12, e0175642 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda S., et al. , Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 9, 3712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen R. H. J., et al. , TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. 16, 841–849 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K., et al. Structure of a hallucinogen-activated Gq-coupled 5-HT2A serotonin receptor. Cell 182, 1574–1588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.W. Jiang, S. Zheng, Cryo-EM structure of the galanin-bound GALR1-miniGo complex. Protein Data Bank. https://www.rcsb.org/structure/7XJJ. Deposited 18 April 2022. [Google Scholar]
- 25.W. Jiang, S. Zheng, Cryo-EM structure of the galanin-bound GALR2-miniGq complex. Protein Data Bank. https://www.rcsb.org/structure/7XJK. Deposited 18 April 2022. [Google Scholar]
- 26.Smith K. E., et al. , Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J. Biol. Chem. 272, 24612–24616 (1997). [DOI] [PubMed] [Google Scholar]
- 27.Fathi Z., et al. , Cloning, pharmacological characterization and distribution of a novel galanin receptor. Brain Res. Mol. Brain Res. 51, 49–59 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Carpenter K. A., et al. , The glycine residue in cyclic lactam analogues of galanin(1-16)-NH2 is important for stabilizing an N-terminal helix. Biochemistry 38, 15295–15304 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Bárány-Wallje E., Andersson A., Gräslund A., Mäler L., NMR solution structure and position of transportan in neutral phospholipid bicelles. FEBS Lett. 567, 265–269 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Kruse A. C., et al. , Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shihoya W., et al. , Activation mechanism of endothelin ETB receptor by endothelin-1. Nature 537, 363–368 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Koehl A., et al. , Structure of the µ-opioid receptor-Gi protein complex. Nature 558, 547–552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong C., et al. , Structures of active-state orexin receptor 2 rationalize peptide and small-molecule agonist recognition and receptor activation. Nat. Commun. 12, 815 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Florén A., Land T., Langel U., Galanin receptor subtypes and ligand binding. Neuropeptides 34, 331–337 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Runesson J., Saar I., Lundström L., Järv J., Langel U., A novel GalR2-specific peptide agonist. Neuropeptides 43, 187–192 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Sollenberg U. E., Lundstrom L., Bartfai T., Langel U., M871-A novel peptide antagonist selectively recognizing the galanin receptor type 2. Int. J. Pept. Res. Ther. 12, 115–119 (2006). [Google Scholar]
- 37.Land T., et al. , Linear and cyclic N-terminal galanin fragments and analogs as ligands at the hypothalamic galanin receptor. Int. J. Pept. Protein Res. 38, 267–272 (1991). [DOI] [PubMed] [Google Scholar]
- 38.Lundström L., Lu X., Langel U., Bartfai T., Important pharmacophores for binding to galanin receptor 2. Neuropeptides 39, 169–171 (2005). [DOI] [PubMed] [Google Scholar]
- 39.W. Jiang, S. Zheng, Cryo-EM structure of the spexin-bound GALR2-miniGq complex. Protein Data Bank. https://www.rcsb.org/structure/7XJL. Deposited 18 April 2022. [Google Scholar]
- 40.Jumper J., et al. , Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heo L., Feig M., Multi-state modeling of G-protein coupled receptors at experimental accuracy. BioRxiv [Preprint] 10.1101/2021.11.26.470086v1 (2021). Accessed 8 April 2022. [DOI] [PMC free article] [PubMed]
- 42.Weis W. I., Kobilka B. K., The molecular basis of G protein-coupled receptor activation. Annu. Rev. Biochem. 87, 897–919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runesson J., et al. , Determining receptor-ligand interaction of human galanin receptor type 3. Neurochem. Int. 57, 804–811 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Maeda S., Qu Q., Robertson M. J., Skiniotis G., Kobilka B. K., Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moro O., Lameh J., Högger P., Sadée W., Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 (1993). [PubMed] [Google Scholar]
- 46.Xiao P., et al. , Ligand recognition and allosteric regulation of DRD1-Gs signaling complexes. Cell 184, 943–956 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng X., et al. , Structural insights into G protein activation by D1 dopamine receptor. BioRxiv [Preprint] 10.1101/2022.01.18.476830v1 (2022). Accessed 20 January 2022. [DOI] [PMC free article] [PubMed]
- 48.Blakemore L. J., Trombley P. Q., Zinc as a neuromodulator in the central nervous system with a focus on the olfactory bulb. Front. Cell. Neurosci. 11, 297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kay A. R., Tóth K., Is zinc a neuromodulator? Sci. Signal. 1, re3 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson C. T., et al. , Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc. Natl. Acad. Sci. U.S.A. 112, E2705–E2714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalappa B. I., Anderson C. T., Goldberg J. M., Lippard S. J., Tzounopoulos T., AMPA receptor inhibition by synaptically released zinc. Proc. Natl. Acad. Sci. U.S.A. 112, 15749–15754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogt K., Mellor J., Tong G., Nicoll R., The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron 26, 187–196 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Swaminath G., Steenhuis J., Kobilka B., Lee T. W., Allosteric modulation of beta2-adrenergic receptor by Zn(2+). Mol. Pharmacol. 61, 65–72 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Holst B., Elling C. E., Schwartz T. W., Metal ion-mediated agonism and agonist enhancement in melanocortin MC1 and MC4 receptors. J. Biol. Chem. 277, 47662–47670 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Nunez D., Kumar R., Hanahan D. J., Inhibition of [3H]platelet activating factor (PAF) binding by Zn2+: A possible explanation for its specific PAF antiaggregating effects in human platelets. Arch. Biochem. Biophys. 272, 466–475 (1989). [DOI] [PubMed] [Google Scholar]
- 56.van der Westhuizen E. T., Valant C., Sexton P. M., Christopoulos A., Endogenous allosteric modulators of G protein-coupled receptors. J. Pharmacol. Exp. Ther. 353, 246–260 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Yuan Y., et al. , Structures of signaling complexes of lipid receptors S1PR1 and S1PR5 reveal mechanisms of activation and drug recognition. Cell Res. 31, 1263–1274 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu P., et al. , Structures of the human dopamine D3 receptor-Gi complexes. Mol. Cell 81, 1147–1159 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Kato H. E., et al. , Conformational transitions of a neurotensin receptor 1-Gi1 complex. Nature 572, 80–85 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Q., et al. , Ligand recognition and G-protein coupling selectivity of cholecystokinin A receptor. Nat. Chem. Biol. 17, 1238–1244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krishna Kumar K., et al. , Structure of a signaling cannabinoid receptor 1-G protein complex. Cell 176, 448–458 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim H. R., et al. , Structural mechanism underlying primary and secondary coupling between GPCRs and the Gi/o family. Nat. Commun. 11, 3160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conklin B. R., Farfel Z., Lustig K. D., Julius D., Bourne H. R., Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature 363, 274–276 (1993). [DOI] [PubMed] [Google Scholar]
- 64.Conklin B. R., et al. , Carboxyl-terminal mutations of Gq alpha and Gs alpha that alter the fidelity of receptor activation. Mol. Pharmacol. 50, 885–890 (1996). [PubMed] [Google Scholar]
- 65.Semack A., Sandhu M., Malik R. U., Vaidehi N., Sivaramakrishnan S., Structural elements in the Gαs and Gαq C termini that mediate selective G protein-coupled receptor (GPCR) signaling. J. Biol. Chem. 291, 17929–17940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng S., Abreu N., Levitz J., Kruse A. C., Structural basis for KCTD-mediated rapid desensitization of GABAB signalling. Nature 567, 127–131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng S. Q., et al. , MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Punjani A., Rubinstein J. L., Fleet D. J., Brubaker M. A., cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Scheres S. H., RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez-Garcia R., et al. , DeepEMhancer: A deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pettersen E. F., et al. , UCSF Chimera: A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Emsley P., Cowtan K.. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60(Pt 12 Pt 1), 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Adams P. D., et al. , PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoue A., et al. Illuminating G-protein-coupling selectivity of GPCRs. Cell 177, 1933–1947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic structures have been deposited in the Protein Data Bank (PDB) under the accession codes 7XJJ (24), 7XJK (25), and 7XJL (39). The EM maps have been deposited at the Electron Microscopy Data Bank (EMDB) under the accession nos. EMD-33229, EMD-33230, and EMD-33231. All other study data are included in the article and/or SI Appendix.