Abstract
Randomly amplified polymorphic DNA (RAPD) analysis and the PCR assay were used in combination with dilution plating on a semiselective medium to detect and enumerate propagules of Trichoderma hamatum 382, a biocontrol agent utilized in compost-amended mixes. Distinct and reproducible fingerprints were obtained upon amplification of purified genomic DNA of T. hamatum 382 with the random primers OPE-16, OPH-19, and OPH-20. Three amplified DNA fragments of 0.35 (OPE-160.35), 0.6 (OPH-190.6), and 0.65 (OPH-200.65) kb were diagnostic for T. hamatum 382, clearly distinguishing it from 53 isolates of four other Trichoderma spp. tested. Some isolates of T. hamatum shared these low-molecular-weight fragments with T. hamatum 382. However, RAPD analysis of isolates of T. hamatum with all three random primers used in consecutive PCR tests distinguished T. hamatum 382 from other isolates of T. hamatum. These three RAPD amplicons were cloned and sequenced, and pairs of oligonucleotide primers for each cloned fragment were designed. Use of the primers in the PCR assay resulted in the amplification of DNA fragments of the same size as the cloned RAPD fragments from genomic DNA of T. hamatum 382. A combination of dilution plating on a semiselective medium for Trichoderma spp. and PCR, with the RAPD primers OPH-19, OPE-16, and OPH-20 or the three sequence-characterized primers, was used successfully to verify the presence of T. hamatum 382 propagules in nine different soil, compost, and potting mix samples. All 23 Trichoderma isolates recovered on semiselective medium from commercial potting mixes fortified with T. hamatum 382 were identified as T. hamatum 382, whereas 274 Trichoderma isolates recovered from the other nine samples were negative in the PCR assay. Thus, this highly specific combination of techniques allowed detection and enumeration of propagules of T. hamatum 382 in fortified compost-amended potting mixes. Sequence-characterized amplified region markers also facilitated the development of a very simple procedure to amplify DNA of T. hamatum 382 directly from fortified compost-amended potting mixes.
Trichoderma hamatum 382 and Flavobacterium balustinum 299 have been identified as effective biocontrol agents in compost-amended substrates (13, 24). They consistently induce suppression of diseases caused by a broad spectrum of soilborne plant pathogens if inoculated into compost after peak heating but before substantial recolonization with mesophilic microorganisms has occurred (10). The presence of significant populations of T. hamatum 382 and F. balustinum 299 in fortified commercial mixes is one of several factors crucial to efficacy (13). Therefore, verification of their propagule densities in fortified potting mixes at various stages after mix formulation is critical to prediction of biological control activity. Methods for monitoring populations of F. balustinum 299 were described earlier (13, 25). Until now, however, propagules of T. hamatum 382 have been monitored on a semiselective medium for Trichoderma spp. (4, 6). Unfortunately, this approach does not distinguish T. hamatum 382 in commercial mixes from other indigenous isolates of T. hamatum that may not have equivalent biocontrol efficacy (12, 17, 18). Techniques such as isozyme analysis and serology are nonspecific at the isolate level for most fungi (16), including species of Trichoderma (23). On the other hand, nucleic acid-based genetic markers can be highly specific, and high sensitivity has been achieved with PCR-based assays in the identification and detection of many species of fungi (16).
Random markers as products of the PCR-based randomly amplified polymorphic DNA (RAPD) technique (26) have been developed to differentiate numerous fungi, including Trichoderma species (1, 5, 7–9, 21, 27). This technique, which utilizes a single, short (10 nucleotide bases) primer of arbitrary sequence to amplify DNA fragments, requires no prior knowledge of the target site sequence. Since the genome of T. hamatum 382 is poorly understood, RAPD analysis may prove to be an ideal method for DNA fingerprinting. Recently, sequence-characterized amplified region (SCAR) markers (11, 15, 19, 20) or sequence-tagged site markers (3, 11) have been derived from RAPD markers. SCAR and sequence-tagged site markers offer advantages over, and are distinct from, RAPD markers because they are PCR amplified with specific primers and may represent a single locus in the genome.
We report the use of the RAPD-PCR technique to develop markers for reliable differentiation of T. hamatum 382 from other isolates of T. hamatum and Trichoderma spp. These RAPD markers were converted into SCAR markers. We also demonstrate the use of a combination of dilution plating and RAPD or specific PCR analysis for verifying the propagule densities or CFU of T. hamatum 382 in compost-amended potting mixes fortified with T. hamatum 382. We also report the development of a very simplified procedure for the direct amplification of DNA of T. hamatum 382 from fortified compost-amended potting mixes.
MATERIALS AND METHODS
Fungal isolates and DNA extraction.
Trichoderma isolates, retrieved from a stock culture collection of five species originally recovered from composts and potting mixes (12, 18), identified according to the system of Bissett (2), and stored on silica gel crystals at −70°C according to the method Sleesman and Leben (22) or received from H. Bolkan (Table 1), were cultured for 10 days at 25°C on potato dextrose agar (Difco Laboratories, Detroit, Mich.) slants. Single spore cultures were then prepared from each isolate and maintained at 4°C (16). DNA was isolated from mycelial mats of each single spore isolate grown for 4 days at 24°C in 50 ml of potato dextrose broth (PDB) (Difco) in 250-ml Erlenmeyer flasks on an orbital shaker (125 rpm). The mycelial mats were collected by vacuum filtration, frozen immediately in liquid nitrogen, and lyophilized. The lyophilized mats were ground with a mortar and pestle in liquid nitrogen and stored immediately at −70°C. DNA was extracted from these preparations according to the method of Lee and Taylor (14). The DNA concentration was determined by measuring the absorbance at 260 nm.
TABLE 1.
Description of Trichoderma spp. used in this study
| Species and isolates | Sourcea |
|---|---|
| T. hamatum | |
| 20, 29, 46, 100, 104, 331, 337, 362, 382, 395, 402, 513, 546, 559, 610, 644, 665, 666, 672, 687, 691, 719, 728, 729, 753, 769, 781, 784, 794, 809, 897, 899, 945 | Suppressive potting mix |
| 204, 212, 229, 239, 255, 263 | Conducive potting mix |
| 961, 964, 1037, 1045, 1057, 1119, 1183 | Potting mix of unknown suppressiveness |
| T. harzianum | |
| 8, 71, 76, 77 | Campbell Research and Development, Davis, Calif. |
| 120, 311, 716, 887, 1220, 1224, 1238, 1241, 1246, 1269, 1323, 1346, 1357, 1359, 1364 | Suppressive potting mix |
| 84, 458, 478, 738, 743, 820, 852, 855, 1100 | Conducive potting mix |
| 955, 956, 972, 996, 1025, 1190, ss-bl | Potting mix of unknown suppressiveness |
| T. koningii | |
| 45, 107, 320, 569, 1351 | Suppressive potting mix |
| 232, 254 | Conducive potting mix |
| 973, 1086 | Potting mix of unknown suppressiveness |
| T. viride 123, 1409, 1410, 1415 | Suppressive potting mix |
| T. virens 611, 917, 1243, 1305, 1446 | Suppressive potting mix |
Isolated from composted hardwood bark mixes obtained from two commercial nurseries (Warner Nursery, Willoughby, Ohio, and Paygro Nursery, South Charleston, Ohio). Suppressive and conducive mixes suppress or do not suppress, respectively, Rhizoctonia damping-off in a radish bioassay.
RAPD analysis and PCR conditions.
Ten-nucleotide random primers of arbitrary sequence (kits A to I) were obtained from Operon Technologies, Inc., Alameda, Calif. A total of 180 random primers were screened against purified genomic DNA of 10 randomly selected isolates of T. hamatum, including T. hamatum 382. Primers that directed amplification of clear, distinct, reproducible, and polymorphic bands in T. hamatum 382 were included in the RAPD analyses of all isolates of five Trichoderma species. The three selected primers, OPH-19, OPE-16, and OPH-20, were screened against genomic DNA of all 107 isolates of Trichoderma spp. The sequences of these three primers are 5′-CTGACCAGCC (OPH-19), 5′-GGTGACTGTG (OPE-16), and 5′-GGGAGACATC (OPH-20). Amplification reactions were performed in 0.6-ml microcentrifuge tubes in a 25-μl reaction volume containing 16.2 mM Tris-HCl, pH 8.3, 50 mM KCl, 0.8 mM NaCl, 1.5 mM MgCl2, 0.8 μM EDTA, 40 μM dithiothreitol, 0.22% Triton X-100, 0.4% glycerol, 200 μM (each) deoxynucleoside triphosphate, 0.2 μM single random primer, 1.0 U of Taq DNA polymerase (Promega Corp., Madison, Wis.), and 10 to 15 ng of genomic DNA. The reaction mixtures were overlaid with 50 μl of sterilized mineral oil to avoid evaporation. PCR amplification was performed in a Thermolyne Temp-Tronic thermal cycler (Barnstead/Thermolyne, Dubuque, Iowa) programmed for 45 cycles of denaturation at 94°C for 30 s, low-stringency annealing at 30°C for 1.0 min, and polymerization at 72°C for 1.0 min, with a final extension step of 72°C for 10 min. The reaction tubes were held at 4°C following the final amplification cycle. The amplified products were analyzed within 8 h or stored at −20°C.
Analysis of the amplification products.
The PCR amplification products were resolved by loading 10 μl of the reaction mixture in 1.4% (wt/vol) agarose (Sigma, St. Louis, Mo.) gels followed by electrophoresis in 1 × TBE (Tris-borate-EDTA; pH 8.0) buffer at 6 V/cm. A 1-kb DNA ladder (GIBCO-BRL Life Technologies, Gaithersburg, Md.) was used as a molecular size marker for comparison. The ethidium bromide-stained gels were analyzed with the NightHawk image analysis system (pdi, Huntington Station, N.Y.).
Cloning and sequencing of RAPD amplicons.
Three diagnostic DNA fragments amplified with OPH-19, OPH-20, and OPE-16 were excised from agarose gels, and DNA was electroeluted with an Elutrap (Schleicher & Schuell, Inc., Keene, N.H.). The eluted DNA was reamplified with the corresponding RAPD primers to verify its identity. The reamplified DNA was blunt ended and purified by extraction with an equal volume of a chloroform-isoamyl alcohol (24:1 ratio) mixture followed by precipitating the extract with 2 volumes of ethanol. This blunt-ended DNA was phosphorylated and ligated into a pBluescribe(+) vector (Vector Cloning Systems, San Diego, Calif.) that had been linearized with EcoRV. XL1-Blue competent cells (Stratagene) were transformed with ligated DNA, plated on Luria-Bentami agar containing ampicillin (100 μg/ml) and IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM), and incubated overnight at 37°C. Standard procedures were followed to isolate plasmid DNA from transformants and to verify the presence of inserts by restriction double digestion with HindIII and XhoI. The cloned fragments were sequenced manually from both ends with a Sequenase sequencing kit (U.S. Biochemical Corp., Cleveland, Ohio) by the dideoxy chain termination method with M-13 and T-3 universal primers. These DNA fragments were also sequenced completely by the Biopolymer Facility at The Ohio State University, Columbus.
Design of SCAR primers and PCR amplification conditions.
The sequences from both ends of each cloned RAPD fragment were used to design pairs of oligonucleotide primers (Table 2). SCAR primers were synthesized by Oligos Etc., Wilsonville, Oreg. Genomic DNA was amplified with these primers in a PCR containing 2 mM MgCl2, 10× PCR buffer, 2.4 mM (each) deoxynucleoside triphosphate, 25 pmol of each primer, and 1.0 U of Taq polymerase (Promega) in a total volume of 25 μl. The amplification conditions consisted of 35 cycles of 35 s at 94°C, 35 s at 55°C, and 55 s at 72°C, with a final extension step of 7.0 min at 72°C. All of the reactions were held at 4°C at the end of the PCR cycles. The amplified products were resolved electrophoretically in a 1.2% agarose gel.
TABLE 2.
Sequence of 24-mer oligonucleotide SCAR primers derived from RAPD markers that distinguish T. hamatum 382 from other T. hamatum isolates and Trichoderma spp.
| SCAR markersa | Primerb | Sequencec |
|---|---|---|
| SCH19588 | OPH19-F | CTGACCAGCCTGTTAAAATCAT |
| OPH19-R | CTGACCAGCCCAAAGACTCCC | |
| SCE16347 | OPE16-F | GGTGACTGTGGCCTTGTTTGCATA |
| OPE16-R | GGTGACTGTGAATGGCGAAGCTAC | |
| SCH20624 | OPH20-F | GGGAGACATCGCATCTGCATGTAA |
| OPH20-R | GGGAGACATCAACGATGATTCAGC |
Subscript number refers to size of amplified product from genomic DNA of T. hamatum 382.
Letters H and E refer to the kit, and numbers 19, 16, and 20 refer to the random primer number from Operon Technologies. The letters F and R refer to forward and reverse primers from both ends of the RAPD fragment cloned.
Sequences of random primers used in RAPD analysis are underlined.
Combined dilution plating and RAPD or PCR analysis.
The presence of T. hamatum 382 propagules was verified in soil, compost, and potting mix samples collected from various locations in Ohio. In addition, the identity of T. hamatum 382 and its propagule densities were determined in samples randomly collected from commercial potting mixes fortified with this biocontrol agent. Ten-gram samples from a blended mix were blended for 30 s in 0.1% water agar in a Waring blender, and a 10-fold dilution series in sterilized double-distilled water was then plated on a semiselective medium for Trichoderma (4, 6). After 7 to 10 days of incubation at 24°C, the total number of Trichoderma propagules was determined. Individual colonies were purified on potato dextrose agar plates, and mycelia from the edges of colonies were transferred to PDB to produce mycelial mats on a rotary shaker as described above. Finally, DNA was isolated and RAPD or PCR analysis was performed for each isolate, also as described above. RAPD analysis was first done with primer OPH-19; those samples containing the OPH-190.6 (see below) fragment were further subjected to RAPD analysis with OPE-16. Samples containing the OPE-160.35 fragment (see below) were then tested by using OPH-20 primer. For specific PCR analysis, individual colonies were directly analyzed from the dilution plates. A loopful of fungal propagules was scraped from the plates and transferred to 1.5-ml microcentrifuge tubes in 500 μl of lysis buffer (120 mM sodium phosphate, 1% sodium dodecyl sulfate (SDS), 5 M NaCl). Quartz sand (0.2 g) was added to these tubes to facilitate grinding. The samples were ground directly in microcentrifuge tubes for 1 to 2 min with a microcentrifuge pestle attached to a drill. The tubes were incubated for 30 min at 70°C while being mixed occasionally with a vortex and were then centrifuged at 12,000 (Eppendorf centrifuge model 5415C; Brinkman Instruments, Inc., Westbury, N.Y.) rpm for 10 min. DNA in the supernatant was extracted with chloroform-isoamyl alcohol (24:1) and precipitated with ethanol. The washed pellet was resuspended in Tris-EDTA (TE) buffer and diluted to 1:50. Specific PCR analysis of these samples was performed with the SCAR primers SCH-19 F/R, SCE-16 F/R, and SCH-20 F/R in sequence, as described above.
Direct extraction and amplification of T. hamatum 382 DNA from fortified compost-amended potting mixes.
One-gram bulk samples of potting mix fortified with T. hamatum 382 taken randomly from commercially prepared mix batches were ground in liquid nitrogen and suspended in SDS buffer (120 mM sodium phosphate, 1% SDS). The tubes were incubated for 30 min at 70°C and centrifuged at 12,000 rpm (Eppendorf centrifuge model 5415C) for 10 min. Each pellet was resuspended in SDS buffer and heat-treated a second time. DNA was extracted with high salt concentration (5 M NaCl) and chloroform-isoamyl alcohol (24:1) by mixing and centrifugation. DNA was then precipitated from the aqueous phase with ethanol, and the washed pellet was resuspended in TE buffer. DNA was then diluted 1:100 and amplified by PCR with the SCAR primers SCH-19 F/R, SCE-16 F/R, and SCH-20 F/R, as described above.
Nucleotide sequence accession numbers.
The nucleotide sequences of the three cloned RAPD fragments OPH-190.6, OPE-160.35, and OPH-200.65 have been deposited in the GenBank database under accession no. AF182638, AF182639, and AF182640, respectively.
RESULTS
RAPD analysis of purified DNA.
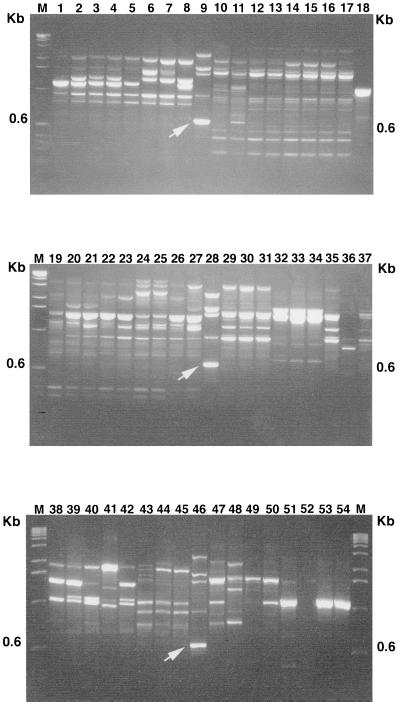
The use of the random primers OPE-16, OPH-19, and OPH-20 resulted in the amplification of 5 to 10 distinct fragments from the genomic DNA of T. hamatum 382. Three amplicons (OPH-190.6, OPE-160.35, and OPH-200.65) were generated reproducibly and were polymorphic in the original screening of 10 randomly selected isolates of T. hamatum. The reproducibility of these amplicons was confirmed by repeating RAPD-PCR analysis of the same and new DNA preparations. The amplicon OPH-190.6 clearly distinguished T. hamatum 382 from isolates of other Trichoderma spp. (Fig. 1). The other two amplicons, OPE-160.35 and OPH-200.65, were also diagnostic for T. hamatum 382 (data not shown). The approximate sizes of the amplicons OPH-190.6, OPE-160.35, and OPH-200.65 were 600, 350, and 650 bp, respectively. They were always present in T. hamatum 382 but not in isolates of other Trichoderma spp. with the exception of one isolate of Trichoderma harzianum, which produced a weak band at approximately the same location in the gel as OPH-19. However, these RAPD markers were also shared by some other isolates of T. hamatum. Of the 46 isolates of T. hamatum analyzed, 12 shared OPH-190.6, 20 shared OPE-160.35, and 12 shared OPH-200.65 amplicons with T. hamatum 382. However, T. hamatum 382 could be distinguished from other isolates of T. hamatum when all three primers were used in sequential RAPD analyses (Fig. 2). For example, 12 isolates of T. hamatum containing the OPH-190.6 fragment (Fig. 2A) could be distinguished from T. hamatum 382 by further analysis with OPE-16 (Fig. 2B) and OPH-20 (Fig. 2C). As shown in Fig. 2B, only 4 of the 12 T. hamatum isolates shared the OPE-160.35 fragment with T. hamatum 382. None of these four isolates also contained the OPH-200.65 fragment (Fig. 2C).
FIG. 1.
RAPD marker (OPH-190.6) differentiates T. hamatum 382 from other Trichoderma spp. Lanes 9, 28, and 46, T. hamatum 382; lanes 1 to 8, 10 to 27, and 29 to 37, T. harzianum; lanes 38 to 45, Trichoderma koningii; lanes 47 to 50, Trichoderma viride; lanes 51 to 54, Trichoderma virens. The OPH-190.6 amplicons are indicated by arrows. M, molecular size standards.
FIG. 2.
Differentiation of T. hamatum 382 from other T. hamatum isolates by sequential use of three random primers. (A) Twelve T. hamatum isolates (lanes 1 to 12) share the OPH-190.6 amplicon with T. hamatum 382 (lane 13). (B) Of the 12 T. hamatum isolates (lanes 1 to 12) sharing OPH-190.6 with T. hamatum 382 (lane 13), only 4 also share the OPE-160.35 amplicon. (C) Of the four isolates shown in panel B (lanes 1 and 3 to 5), none share the OPH-200.7 amplicon with T. hamatum 382 (lane 2). Lane 14 in panel A and lane 6 in panel C are negative controls. M, molecular size standards.
Development and use of SCAR markers.
In order to develop a rapid, simple, and specific diagnostic PCR assay for T. hamatum 382 and to predict T. hamatum 382 propagule densities in the fortified compost-amended substrates, the three RAPD markers were converted into SCAR markers. For each of the three cloned RAPD fragments (OPH-190.6, OPE-160.35, and OPH-200.65), two 24-mer oligonucleotide primers were synthesized (Table 2). The 5′ terminus of each primer consisted of the 10-base random primer and the next 14 bases of the amplified DNA sequence, unless otherwise specified. Use of these primers in PCR with the genomic DNA of T. hamatum 382 resulted in amplification of single bands of the same size as the cloned RAPD fragments (Fig. 3). When aligned to each other, the sequences of the three DNA fragments showed no homology.
FIG. 3.
RAPD and SCAR markers diagnostic for T. hamatum 382. Shown are the PCR products amplified with OPH-19 (lane 1), SCH19 F/R (lane 2), OPE-16 (lane 4), SCE16 F/R (lane 5), OPH-20 (lane 7), and SCH20 F/R (lane 8). Lanes 3 and 6 are blank. M, molecular size standards.
Distribution of T. hamatum 382 CFU in soil and compost samples.
Total Trichoderma propagules in various soil and compost samples ranged from 3.0 to 5.04 log10 CFU/g (dry weight) of the sample (Table 3). All of the 23 Trichoderma-like colonies from the T. hamatum 382-fortified potting mix selected for RAPD analysis were positive for T. hamatum 382. These isolates contained the DNA fragments OPH-190.6, OPE-160.35, and OPH-200.65 (Table 3) and thus were identified as T. hamatum 382. Of the remaining 274 colonies selected from the soil, forest litter, compost, and nonfortified potting mix samples, only one colony, isolated from Earthgro pine bark potting mix, contained OPH-190.6 and OPE-160.35 DNA fragments. This isolate did not contain OPH-200.65 and was thus ruled out as T. hamatum 382. Fragments 588, 347, and 624 bp in size were amplified from the less purified DNA preparation extracted directly from all five individual Trichoderma-like colonies recovered from commercial potting mixes fortified with T. hamatum 382 with the SCAR primers SCH-19 F/R, SCE-16 F/R, and SCH-20 F/R, respectively (Fig. 4).
TABLE 3.
RAPD analysis of individual Trichoderma-like colonies isolated from different soil, compost, and potting mix samples
| Sample description | Amt of Trichoderma propagules (log10 CFU/g)i | No. of colonies tested | No. of T. hamatum 382-like colonies with:
|
% T. hamatum 382 | ||
|---|---|---|---|---|---|---|
| OPH-19 | OPE-16 | OPH-20 | ||||
| Nursery soil 1a | 3.34 ± 0.50 | 13 | 0 | 0 | 0 | 0 |
| Nursery soil 2b | 4.05 ± 0.36 | 23 | 0 | 0 | 0 | 0 |
| Warner compostb | 3.01 ± 0.11 | 106 | 0 | 0 | 0 | 0 |
| Bark compostc | 3.00 ± 0.29 | 21 | 0 | 0 | 0 | 0 |
| Forest litterd | 4.52 ± 0.42 | 21 | 0 | 0 | 0 | 0 |
| Container mix 1e | 3.52 ± 0.25 | 21 | 0 | 0 | 0 | 0 |
| Container mix 2f | 5.04 ± 0.01 | 42 | 0 | 0 | 0 | 0 |
| Container mix 3g | 3.80 ± 1.05 | 27 | 1 | 1 | 0 | 0 |
| Container mix 4h | 5.00 ± 0.18 | 23 | 23 | 23 | 23 | 100 |
Forestry nursery at Ohio Agricultural Research and Development Center, Wooster, Ohio.
Warner Nursery, Willoughby, Ohio.
Composted hardwood bark, Warner Nursery.
Native hardwood forest in Wayne County, Ohio.
Composted pine bark from Warner Nursery.
Composted pine bark from Paygro Inc., South Charleston, Ohio.
Composted pine bark mix from Earthgro Inc., Lebanon, Conn.
Composted pine bark mix from Earthgro Inc. fortified with T. hamatum 382 and F. balustinum 299.
Mean log10 CFU/gram (dry weight) of replicate samples ± standard deviation.
FIG. 4.
Direct analysis of individual Trichoderma-like colonies by PCR with the SCAR primers SCH19 F/R (A), SCE16 F/R (B), and SCH20 F/R (C). Shown are amplified products of purified DNA of T. hamatum 382 (lanes 1) and less-purified DNA preparation extracted directly from five individual Trichoderma-like colonies isolated from fortified compost-amended potting mixes (lanes 2 to 6). M, molecular size standards.
DNA amplification from compost samples.
The three sequence-characterized markers, SCH-19588, SCE-16347, and SCH-20625, facilitated the amplification of crude DNA preparations extracted directly from compost-amended substrates and diluted 1:100 in TE buffer. All six batches of the fortified potting mixes analyzed in the direct PCR assay were positive for T. hamatum 382 (Fig. 5). DNA extraction and PCR amplification procedures were done twice.
FIG. 5.
Amplification of DNA extracted directly from compost-amended potting mix samples fortified with T. hamatum 382 with the SCAR primers SCH19 F/R, SCE16 F/R, and SCH20 F/R. Shown are the amplified products of crude DNA preparation extracted directly from six batches of fortified compost-amended potting mixes and diluted 1:100 in TE (lanes 1 to 6) and purified DNA of T. hamatum 382 (lanes 7). M, molecular size standards.
DISCUSSION
RAPD-PCR fingerprinting has proven to be a valuable tool for studying DNA polymorphisms in fungal organisms. It is a simple and reliable technique that can be used to detect genetic differences at the isolate level. Using this approach, we have identified molecular markers that can be used to detect a specific isolate of T. hamatum (isolate 382) that is an effective biocontrol agent against several soilborne pathogens (13, 24). These RAPD markers discriminate this isolate from other isolates of T. hamatum and Trichoderma spp. Zimand et al. (27) also used the RAPD procedure for the specific identification of isolate T-39 in the T. harzianum group. They found that a set of nine primers was necessary to distinguish T-39 from other isolates of the group, in contrast to the three RAPD markers needed to distinguish T. hamatum 382 from other T. hamatum isolates in our work. While this differentiation procedure requires three separate PCR experiments, isolates not identical to T. hamatum 382 are eliminated at each step, resulting in a final analysis of only a few strains. The combination of RAPD-PCR and dilution plating on semiselective medium was very useful for detecting T. hamatum 382 and for verifying its propagules or CFU in fortified compost-amended substrates. Although it is likely that the majority of CFU originated from spores, it is also possible that some fraction originated from mycelial fragments. Furthermore, the germination efficiency of propagules on the semiselective medium has not been characterized, although it is reasonable to assume that the culturability of propagules is uniform from sample to sample. Nevertheless, there is a clear relationship between compost suppresiveness and CFU density (13, 18). Thus, the propagule density measurements made, expressed as CFU per gram, have practical value.
Although we successfully applied RAPD analysis to detect differences at the isolate level, this technique may not be effective to accomplish our long-term objective of developing a sensitive, rapid, and reliable monitoring system for T. hamatum 382 in compost-amended substrates fortified with this biocontrol agent. We chose to convert these RAPD markers into SCAR markers to avoid some of the disadvantages associated with the RAPD technique. We showed that SCAR primers could be used to amplify the specific DNA fragments of T. hamatum 382 from less-purified genomic DNA or DNA from a mixture of organisms.
Conversion of RAPD markers into SCAR markers facilitated this detection and monitoring process by enabling us to directly evaluate the individual Trichoderma-like colonies from the dilution plates without purifying the individual colonies, growing them in PDB and obtaining the purified DNA for RAPD analysis. In our laboratory, a combination of dilution plating on Trichoderma semiselective medium and RAPD or PCR analysis has become routine for monitoring CFU of T. hamatum 382 in fortified mixes. This represents a significant improvement over the dilution plating method alone, where only total Trichoderma propagules can be assessed.
We applied this combination of techniques to verify the presence of T. hamatum 382 in soil, compost, and potting mix samples from various locations. All of the isolates recovered from soil and nonfortified compost-amended potting mix samples were different from T. hamatum 382. This enormous variability and uniqueness among Trichoderma isolates from various locations suggests that a single RAPD or SCAR marker is sufficient to rule out most, if not all, non-T. hamatum 382 isolates. It is noteworthy that we collected soil samples from the same location where T. hamatum 382 had been recovered originally 20 years ago and we did not recover a Trichoderma isolate identical to T. hamatum 382. Even so, some of the T. hamatum isolates in our culture collection shared the three RAPD markers with T. hamatum 382. They were also recovered by baiting from composted hardwood bark mixes suppressive of Rhizoctonia damping-off (18). Any linkage between these commonly shared markers and suppressiveness of Rhizoctonia damping-off needs to be studied.
SCAR markers also enabled us to develop a very simple procedure for amplification of DNA of T. hamatum 382 extracted directly from fortified compost samples, thus eliminating the time-consuming culturing step. The procedure was consistent, and the results were reproducible. DNA extraction and PCR amplification procedures were repeated, and the same results were obtained. We believe that most of the inhibitors of the PCR in the composted material were eliminated in the first DNA extraction step. Dilution of template DNA in the final extraction step further reduced inhibition of PCR amplification. This simplified PCR assay for T. hamatum 382 can be used to analyze a large number of samples and to confirm the presence of T. hamatum 382 in commercial mix batches in less than 12 h. However, methods to quantify T. hamatum 382 propagules or CFU in a given batch of fortified mix based on DNA amplification need to be developed.
In summary, isolate-specific markers were identified and converted into SCAR markers. Both RAPD and SCAR markers were successfully used to discriminate T. hamatum 382 from other isolates of T. hamatum and other Trichoderma spp. These markers were also very useful for predicting propagules or CFU of T. hamatum 382 in the fortified commercial potting mixes. This was achieved by RAPD-PCR or specific-PCR analysis of individual Trichoderma-like colonies grown on semiselective medium for Trichoderma spp. and isolated from fortified mixes. The SCAR primers also facilitated the development of a simplified and cost-effective procedure for direct amplification of DNA of T. hamatum 382 from fortified compost-amended substrates. The use of SCAR markers as a diagnostic tool may prove very useful for tagging a particular isolate or strain of interest in a complex system without transforming the organism.
ACKNOWLEDGMENTS
We gratefully acknowledge Sophien Kamoun and Kevin Simcox for their thoughtful criticism of the manuscript. We also acknowledge M. S. Krause and C. A. Musselman for technical assistance.
Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, The Ohio State University. This work was also funded by grants from USDA/IR-4, Rutgers, N.J., Earthgro Inc. Lebanon, Conn., and Strong-lite, Pine Bluff, Ark.
REFERENCES
- 1.Arisan-Atac I, Heidenreich E, Kubicek C P. Randomly amplified polymorphic DNA fingerprinting identifies subgroups of Trichoderma viride and other Trichoderma spp. capable of chestnut blight biocontrol. FEMS Microbiol Lett. 1995;126:249–256. doi: 10.1111/j.1574-6968.1995.tb07426.x. [DOI] [PubMed] [Google Scholar]
- 2.Bissett J. A revision of the genus Trichoderma. III. Section Pachybasium. Can J Bot. 1991;69:2373–2417. [Google Scholar]
- 3.Brisbane P G, Neate S M, Pankhurst C E, Scott N S, Thomas M R. Sequence-tagged site markers to identify Rhizoctonia solani AG4 or 8 infecting wheat in south Australia. Phytopathology. 1995;85:1423–1427. [Google Scholar]
- 4.Chung Y R, Hoitink H A J. Interactions between thermophilic fungi and Trichoderma hamatum in suppression of Rhizoctonia damping-off in a bark compost-amended container medium. Phytopathology. 1990;80:73–77. [Google Scholar]
- 5.Crowhurst R N, Hawthorne B T, Rikkerink E H A, Templeton M D. Differentiation of Fusarium solani f. sp. cucurbitae race 1 and 2 by random amplification of polymorphic DNA. Curr Genet. 1991;20:391–396. doi: 10.1007/BF00317067. [DOI] [PubMed] [Google Scholar]
- 6.Elad Y, Chet I, Henis Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica. 1981;9:59–67. [Google Scholar]
- 7.Fujimori F, Okuda T. Application of the random amplified polymorphic DNA using the polymerase chain reaction for efficient elimination of duplicate strains in microbial screening. J Antibiot. 1994;47:173–182. doi: 10.7164/antibiotics.47.173. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin P H, Annis S L. Rapid identification of genetic variation and pathotype of Leptosphaeria maculans by random amplified polymorphic DNA assay. Appl Environ Microbiol. 1991;57:2484–2486. doi: 10.1128/aem.57.9.2482-2486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guthrie P A I, Magill C W, Frederiksen R A, Odvody G N. Random amplified polymorphic DNA markers: a system for identifying and differentiating isolates of Colletotrichum graminicola. Phytopathology. 1992;82:832–835. [Google Scholar]
- 10.Hoitink H A J, Inbar Y, Boehm M J. Status of compost-amended potting mixes naturally suppressive to soilborne diseases of floricultural crops. Plant Dis. 1991;75:869–873. [Google Scholar]
- 11.Kaplan D T, Vanderspool M C, Garrett C, Chang S, Opperman C H. Molecular polymorphisms associated with host range in the highly conserved genomes of burrowing nematodes, Radopholus spp. Mol Plant-Microbe Interact. 1996;9:32–68. doi: 10.1094/mpmi-9-0032. [DOI] [PubMed] [Google Scholar]
- 12.Kuter G A, Nelson E B, Hoitink H A J, Madden L V. Fungal populations in container media amended with composted hardwood bark suppressive and conducive to Rhizoctonia damping-off. Phytopathology. 1983;73:1450–1456. [Google Scholar]
- 13.Kwok O C H, Fahy P C, Hoitink H A J, Kutter G A. Interactions between bacteria and Trichoderma hamatum in suppression of Rhizoctonia damping-off in bark compost media. Phytopathology. 1987;77:1206–1212. [Google Scholar]
- 14.Lee S B, Taylor J W. Isolation of DNA from fungal mycelia and single spores. In: Innis M A, Gelfand D H, Sninsky J T, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press; 1990. [Google Scholar]
- 15.Maisonneuve B, Bellec Y, Anderson P, Michelmore R W. Rapid mapping of two genes for resistance to downy mildew from Lactuca serriola to existing clusters of resistance genes. Theor Appl Genet. 1994;89:96–104. doi: 10.1007/BF00226989. [DOI] [PubMed] [Google Scholar]
- 16.Miller S A. Detecting propagules of plant pathogenic fungi. In: De Boer S H, editor. Advances in plant pathology: pathogen indexing techniques. London, United Kingdom: Academic Press Ltd.; 1996. pp. 73–102. [Google Scholar]
- 17.Nelson E B, Hoitink H A J. The role of microorganisms in suppression of Rhizoctonia solani in container media amended with composted hardwood bark. Phytopathology. 1983;73:274–278. [Google Scholar]
- 18.Nelson E B, Kuter G A, Hoitink H A J. Effects of fungal antagonists and compost age on suppression of Rhizoctonia damping-off in container media amended with composted hardwood bark. Phytopathology. 1983;3:1457–1462. [Google Scholar]
- 19.Ohmori T, Murata M, Motoyoshi F. Molecular characterization of RAPD and SCAR markers linked to the Tm-1 locus in tomato. Theor Appl Genet. 1996;92:151–156. doi: 10.1007/BF00223369. [DOI] [PubMed] [Google Scholar]
- 20.Paran I, Michelmore R W. Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theor Appl Genet. 1993;85:985–993. doi: 10.1007/BF00215038. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y L, Loomis P, Christian D, Carris L M, Leung H. Analysis of the genetic relationships among the wheat bunt fungi using RAPD and ribosomal DNA markers. Phytopathology. 1996;86:311–318. [Google Scholar]
- 22.Sleesman J P, Leben C. Preserving phytopathogenic bacteria at −70°C or with silica gel. Plant Dis Rep. 1978;62:910–913. [Google Scholar]
- 23.Thornton C R, Dewey F M, Gilligan C A. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for the detection of live propagules of Trichoderma harzianum in a peat-bran medium. Soil Biol Biochem. 1994;26:909–920. [Google Scholar]
- 24.Trillas-Gay M I, Hoitink H A J, Madden L V. Nature of suppression of Fusarium wilt of radish in a container medium amended with composted hardwood bark. Plant Dis. 1986;70:1023–1027. [Google Scholar]
- 25.Tunlid A, Hoitink H A J, Low C, White D C. Characterization of bacteria that suppress Rhizoctonia damping-off in bark compost media by analysis of fatty acid biomarkers. Appl Environ Microbiol. 1989;55:1368–1374. doi: 10.1128/aem.55.6.1368-1374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams J G K, Kubelic A R, Livak J, Rafalski A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimand G, Valinsky L, Elad Y, Chet I, Manulis S. Use of RAPD procedure for the identification of Trichoderma strains. Mycol Res. 1994;98:531–534. [Google Scholar]