Significance
Aging animals, particularly females, suffer from diminished reproductive ability, likely due to high costs of germline maintenance. Potential remedies may be found in signals exchanged by members of opposite sexes to promote reproductive success. We show that in the nematode Caenorhabditis elegans, male pheromone facilitates healthy oocyte aging. This pheromone increases germline proliferation and physiological cell death, which is required to maintain oocyte quality. We show that young adults that have not yet commenced reproduction are particularly sensitive to signals from mates and nutrients, likely because during this narrow time window, they set an environment-appropriate balance between germline and soma investment. We advocate the study of social signals as a productive avenue for identifying regulators of physiology and aging.
Keywords: germline aging, germline precursor proliferation, physiological germline cell death, pheromone, C. elegans
Abstract
Pheromones exchanged by conspecifics are a major class of chemical signals that can alter behavior, physiology, and development. In particular, males and females communicate with potential mating partners via sex pheromones to promote reproductive success. Physiological and developmental mechanisms by which pheromones facilitate progeny production remain largely enigmatic. Here, we describe how a Caenorhabditis elegans male pheromone, ascr#10, improves the oogenic germline. Before most signs of aging become evident, C. elegans hermaphrodites start producing lower-quality gametes characterized by abnormal morphology, increased rates of chromosomal nondisjunction, and higher penetrance of deleterious alleles. We show that exposure to the male pheromone substantially ameliorates these defects and reduces embryonic lethality. ascr#10 stimulates proliferation of germline precursor cells in adult hermaphrodites. Coupled to the greater precursor supply is increased physiological germline cell death, which is required to improve oocyte quality in older mothers. The hermaphrodite germline is sensitive to the pheromone only during a time window, comparable in duration to a larval stage, in early adulthood. During this period, prereproductive adults assess the suitability of the environment for reproduction. Our results identify developmental events that occur in the oogenic germline in response to a male pheromone. They also suggest that the opposite effects of the pheromone on gamete quality and maternal longevity arise from competition over resource allocation between soma and the germline.
In many species, fecundity declines, particularly in females, before other signs of organismal aging become prominent. For example, in a nematode Caenorhabditis elegans, reproductive senescence is separable from somatic aging and occurs earlier than the overt onset of organismal decline and death (1–4). As in other species (5, 6), oocyte quality is compromised in older individuals (7, 8). Soma plays an important role in regulating germline aging (2, 8), likely balancing it with organismal aging and coordinating reproductive output with environmental conditions (9, 10).
The relative simplicity of the C. elegans reproductive system makes it a tractable model to study the effects of aging. In hermaphrodites, the germline is tightly packed in a gonad that consists of two arms that converge on a shared uterus. The distal (from the uterus) to proximal axis reflects the sequential order of differentiation from mitotic germline precursors to mature gametes (11). Hermaphrodites produce a cache of ∼300 sperm before irreversibly switching to oogenesis (12). In this respect, sexually mature adult hermaphrodites are similar to females of many animal species that can store sperm following mating (13). In the absence of males, C. elegans hermaphrodites reproduce by selfing during the first ∼5 d of adulthood but could remain fertile for an additional ∼5 d if mated (3). Aging hermaphrodites start producing overtly defective oocytes (7, 8, 14) and deplete stores of germline precursor cells (GPCs) in the distal gonad (4, 8, 9), a population that contains germline stem cells and other mitotic cells, as well as cells that are just entering meiosis (12, 15). Mated—and, thus, sperm-replete—hermaphrodites older than ∼12 d of age do not appear capable of reproduction (3), even though they retain GPCs (9), indicating that oocyte quality loss may predate complete exhaustion of oocyte precursors.
In multiple species, male pheromones can affect reproduction-related traits in females (16). This is the case for the prominent male-biased pheromone in C. elegans, ascr#10 (17). In addition to altering aspects of behavior (18, 19), this small-molecule signal induces changes in the reproductive system: it improves the ability of hermaphrodites to facilitate sperm guidance (20) and increases the number of GPCs in the distal gonad (21, 22). The effect of physiologically relevant concentrations (22) of ascr#10 on the distal germline in hermaphrodites (Fig. 1 A and B) appears to counteract the effects of aging, in that germlines of older hermaphrodites exposed to male pheromone resemble the germlines of their younger, untreated counterparts. We sought to identify developmental processes in the hermaphrodite germline that are altered in response to the male pheromone and to test whether this social signal improves germline quality in aging individuals.
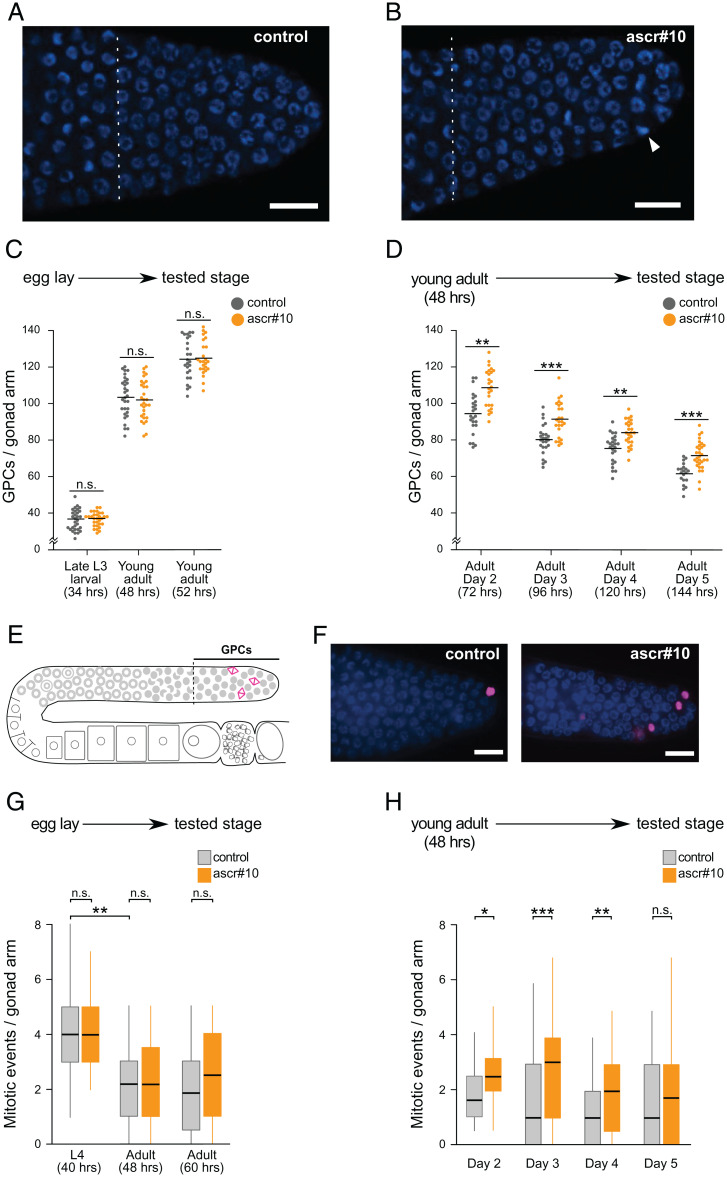
Fig. 1.
ascr#10 increases proliferation of germline precursors. (A and B) Representative images of DAPI-stained progenitor zones in day 5 N2 hermaphrodites aged on (A) control or (B) ascr#10 plates. (Scale bars, 10 μm.) Proximal are to the left, and distal are to the right. Dashed vertical lines delimit proximal boundaries of progenitor zones, as defined by Crittenden et al. (70). Notice the higher number of mitotic figures (arrowhead) in B. (C and D) Experimental scheme and results are shown for (C) number of GPCs in N2 hermaphrodites reared on or off ascr#10 from egg lay to indicated stage (in hours after hatching) and (D) number of GPCs in N2 hermaphrodites reared on or off ascr#10 from 48 h following release from L1 arrest to indicated stage. In C and D, each dot is the number of GPCs in one individual; horizontal bars represent means. (E) Schematic representation of one arm of hermaphrodite gonad showing the GPC population and mitotically dividing cells (magenta). (F) Representative DAPI-stained progenitor zones from hermaphrodites aged on or off ascr#10. pH3-positive cells are shown in magenta. (Scale bars, 10 μm.) (G and H) Mitotic events in gonads of (G) larvae and young adults exposed to ascr#10 since early embryogenesis and (H) adult hermaphrodites exposed to ascr#10 since early adulthood. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. See Table S1 for primary data and details of statistical analyses.
Results
Male Pheromone Increases Germline Proliferation in the Adults.
A hallmark of germline aging in C. elegans hermaphrodites is the decline of the population of GPCs (4, 8, 9). Exposure to ascr#10 results in an increase in the numbers of GPCs (21) (Fig. 1 A and B). Because the number of GPCs is the most development-proximal and highly reproducible (22) manifestation of the effects of the male pheromone on the hermaphrodite germline, we used this phenotype as an entry point to study the underlying mechanisms.
During larval development and the first few hours of adulthood, the number of cells in the progenitor zone increases to a maximum of ∼120 to 130 per gonad arm, while subsequently this population is gradually spent down as animals age (23). Therefore, the observation of more GPCs in animals exposed to ascr#10 may be due to increased acquisition of precursors during larval development or to a slower decline of this cell population in adults. To distinguish between these two possibilities, we varied the time during which hermaphrodites were exposed to ascr#10. Developing in the presence of the pheromone from embryogenesis to young adulthood did not alter the number of GPCs (Fig. 1C), regardless of the age at which this number was assessed (SI Appendix, Fig. S1). In contrast, exposure that started soon (∼3 h) after the molt separating the last larval stage and adulthood consistently resulted in higher GPC numbers (Fig. 1D). Therefore, ascr#10 exerts its effects on the germline during adulthood.
In adult hermaphrodites, cells in the progenitor zone continue to divide, albeit at a lower rate than in larvae (24). An increase of GPC proliferation rate in adults in the presence of ascr#10 could result in a greater number of germline precursors. Consistent with this hypothesis, we noticed an increased number of mitotic figures in animals aged in the presence of the pheromone (Fig. 1B). To obtain additional evidence of increased proliferation, we examined the distal portion of the gonad (Fig. 1E), the progenitor zone (12, 15), using antibodies against phospho-histone H3 (pH3) that mark nuclei in M-phase (25). We found that adult hermaphrodites exposed to ascr#10 had consistently more pH3-positive cells in the progenitor zone compared to untreated controls (Fig. 1F). Exposure to ascr#10 during larval stages did not increase germline proliferation (Fig. 1G), consistent with the results shown in Fig. 1C. Proliferative activity in young (48 h), prereproductive adults was lower than in larvae, as previously reported (24), and it was not increased on ascr#10. Only in 60-h-old egg-laying adults was there an indication (not significant) of increased GPC proliferation in the presence of the male pheromone (Fig. 1G). These findings are consistent with the idea that the germline response to ascr#10 requires egg laying (18), which starts ∼8 h after the L4-to-adult transition (see below). Importantly, we found that exposing adult hermaphrodites to ascr#10 significantly increased the number of proliferating germline cells (Fig. 1H), mirroring results shown in Fig. 1D.
Male Pheromone Increases Physiological Cell Death in the Germline.
We reasoned that the increased number of mitotic nuclei in the germlines of ascr#10-exposed worms may lead to higher incidence of germline cell deaths because in C. elegans hermaphrodites, physiological apoptosis is a common fate that consumes over 50% (26, 27) and likely ∼85% (15, 28) of cells that enter meiosis. In the C. elegans germline, apoptosis affects cells in late pachytene that are located in the vicinity of the gonad bend (26) (Fig. 2A). We observed a significantly increased number of dying cells, particularly on day 2 of adulthood and thereafter, likely because excess GPCs require some time to arrive into the region of the gonad where cell deaths take place (Fig. 2 B and C and SI Appendix, Fig. S2A). Loss of caspase CED-3, a core component of the cell death machinery (29), prevented the ascr#10-dependent increase in cell deaths (Fig. 2D). Conducting this experiment in the background of a ced-1 mutation that precludes corpse engulfment allowed for easier visualization of dying cells (26) but did not interfere with ascr#10 effects on cell death (SI Appendix, Fig. S2B).
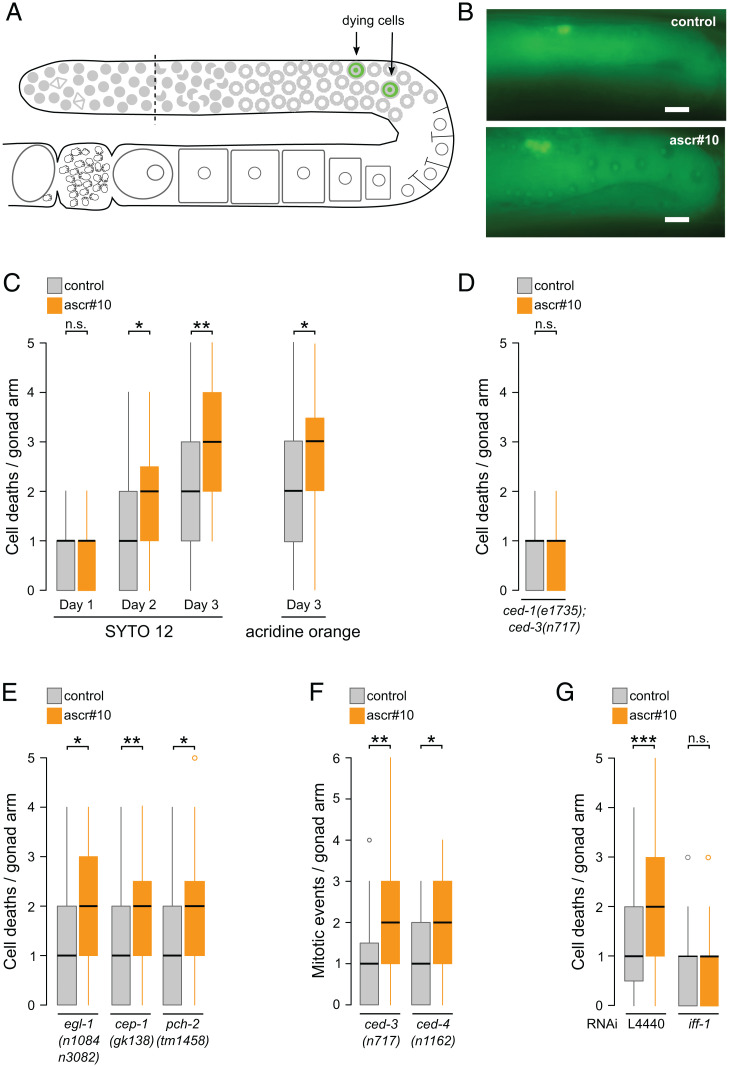
Fig. 2.
ascr#10 increases physiological cell death in the germline. (A) Schematic representation of one arm of hermaphrodite gonad showing approximate location of dying cells (green). (B) Representative SYTO12-stained gonads from hermaphrodites aged on or off ascr#10. (Scale bars, 10 μm.) (C) Numbers of dying cells, identified using two different methods, in gonads of adult hermaphrodites exposed to ascr#10 since early adulthood. (D) Increased germline cell death on ascr#10 requires CED-3. (E) ascr#10 increases incidence of cell death in mutants defective in several types of germline apoptosis. (F) Mutations that block cell death do not prevent increased GPC proliferation in the presence of ascr#10. (G) Blocking GPC proliferation prevents increased cell death on ascr#10. D through F show results from hermaphrodites on day 3 of adulthood, and G shows day 2 of adulthood. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. See Table S1 for primary data and details of statistical analyses.
In C. elegans, physiological germline cell death is independent of the activity of a BH3-only protein EGL-1 (26). We found that ascr#10-induced increase in cell death was egl-1 independent and also unaltered in animals carrying mutations in cep-1 (a p53/p63 homolog) or pch-2 genes (Fig. 2E) that are required for apoptosis due to DNA damage (30, 31) and unsynapsed chromosomes (32), respectively.
Loss-of-function mutations in the core components of the apoptotic machinery, ced-3 and ced-4 (Apaf-1), did not affect the increase of proliferating GPCs seen in the presence of ascr#10 (Fig. 2F and SI Appendix, Fig. S2C). In contrast, blocking germline proliferation (SI Appendix, Fig. S2D) in adult hermaphrodites by RNA interference (RNAi) knockdown of iff-1 (initiation factor 5A; ref. 33) precluded the ascr#10-induced increase in cell death (Fig. 2G). We concluded that exposure to the male pheromone increased the number of mitotic GPCs, thereby increasing the supply of cells that could undergo physiological cell death.
Male Pheromone Improves the Quality of the Oogenic Germline.
Next, we investigated the consequences of continuous exposure to ascr#10 during adulthood on the quality of the hermaphrodite germline. Beyond ∼day 5 of adulthood, both selfing and mated (i.e., sperm-replete) hermaphrodites show evidence of aging-related oogenesis defects (1, 4, 8). We noticed that embryonic lethality in the self-offspring of older mothers was lower in the presence of ascr#10 (SI Appendix, Fig. S3A), yet we sought more direct evidence that this was due to improvements in the oogenic germline. Mating self-sperm-depleted (day 5 of adulthood) hermaphrodites to young adult males is an efficient test of oocyte quality. We found that whereas aging substantially compromised the ability of oocytes to sustain normal development, exposure to ascr#10 during oogenesis relieved underlying defects (Fig. 3A).
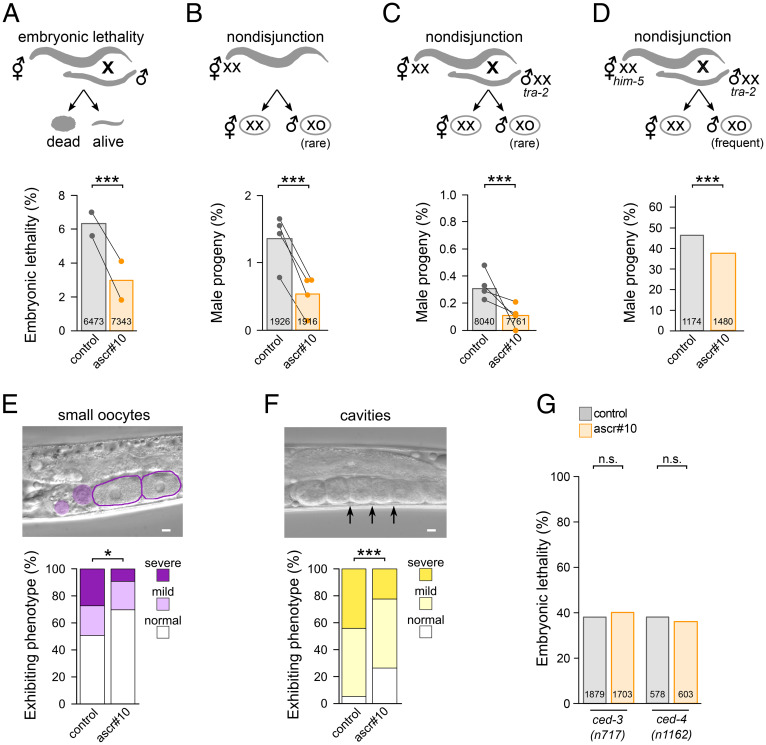
Fig. 3.
ascr#10 improves quality of oogenic germline. (A–C) Experimental scheme and results are shown for (A) embryonic lethality in progeny from day 5 adult N2 hermaphrodites aged on or off ascr#10 and mated to young N2 males; (B) percentage of males in self-progeny from day 5 adult N2 hermaphrodites aged on or off ascr#10; and (C) percentage of males in progeny from day 5 adult N2 hermaphrodites aged on or off ascr#10 and mated to young tra-2(q276) males. The tra-2(q276) males produce XX sperm. In A–C, dots represent means of experimental replicates, each paired with its control. (D) Percentage of males in progeny from day 5 adult him-5(e1490) hermaphrodites aged on or off ascr#10 mated to young tra-2(q276) males. (E and F) Day 7 adult N2 hermaphrodites aged on or off ascr#10 and examined for (E) abnormally sized oocytes and (F) gaps between oocytes are shown. (Scale bars, 10 μm.) (G) Mutants defective in cell death execution do not show reduced embryonic lethality (offspring of mated day 5 adults are shown) in the presence of ascr#10. In A through D and G, numbers inside bars represent totals of scored embryos. *P < 0.05; ***P < 0.001; n.s., not significant. See Table S1 for primary data and details of statistical analyses.
A hallmark of germline aging is an increased incidence of gametes with chromosomal aberrations (34). In selfing C. elegans hermaphrodites with the XX karyotype, one manifestation of this phenomenon is a higher frequency of rare, spontaneously arising male (XO) offspring (8, 35) that result from nondisjunction (36). We therefore compared the frequency of male offspring in the self-broods of hermaphrodites (day 5 of adulthood) on versus off ascr#10. Exposure to ascr#10 significantly reduced the occurrence of male progeny produced by older mothers (Fig. 3B).
It is possible that the observed increase in the incidence of male offspring in self-broods is not due exclusively to nondisjunction during oogenesis, but to a combination of factors including sperm defects. All sperm are produced during the L4 larval stage (12)—that is, before the onset of ascr#10 treatment (see Materials and Methods)—yet we sought independent evidence implicating oocyte defects in increased production of male offspring by aging mothers. We took advantage of the tra-2(q276) allele that masculinizes XX individuals into sperm-producing males (37). We mated young day 1 tra-2 (XX) males with aged (day 5 of adulthood) wild-type N2 (XX) hermaphrodites that exhausted their self-sperm supply; this cross is expected to generate almost exclusively hermaphrodite (XX) offspring except for rare XO males that are due to nondisjunction. We found that when hermaphrodites in this cross were aged in the presence of ascr#10, the rate of male offspring was approximately twofold lower (Fig. 3C), and embryonic lethality was likewise decreased (SI Appendix, Fig. S3B), further supporting the idea that this male pheromone could improve oocyte quality.
Given the somewhat low frequency of nondisjunction events even in older wild-type N2 C. elegans hermaphrodites, overall incidence of males was low. To overcome this limitation, we mated him-5(e1490) hermaphrodites (day 5 of adulthood), a strain characterized by high frequency of nondisjunction (36), to young tra-2(q276) males. Although the frequency of male offspring in this cross was ∼150 times higher than when wild-type N2 mothers were used (compare to Fig. 3C), mothers exposed to ascr#10 showed a lower rate of nondisjunction (Fig. 3D).
Morphological defects can be seen in the germlines of older hermaphrodites. We found that occurrence of small oocytes (7, 8) and cavities between proximal oocytes (8) were significantly lower on ascr#10 (Fig. 3 E and F).
Physiological germline apoptosis plays a role in maintaining oocyte quality in aging C. elegans (7). Because exposure to ascr#10 increased cell death (Fig. 2), we tested whether the ability to execute the cell death program was required for the beneficial effects of this pheromone. We found that ascr#10 did not decrease lethality in the offspring of aged hermaphrodites carrying loss-of-function mutations in ced-3 or ced-4 genes that block cell death (29) (Fig. 3G). Mutations in ced-3 and ced-4 increase lethality in the offspring (compare Fig. 3 G to A) and have other organismal effects (38). Still, we interpret the results in Figs. 2 and 3 as an indication that improvement of oocyte quality on ascr#10 involves core components of the apoptosis signaling pathway.
Offspring of Older Mothers Show Increased Penetrance of Lethal Alleles, while the Male Pheromone Suppresses This Effect.
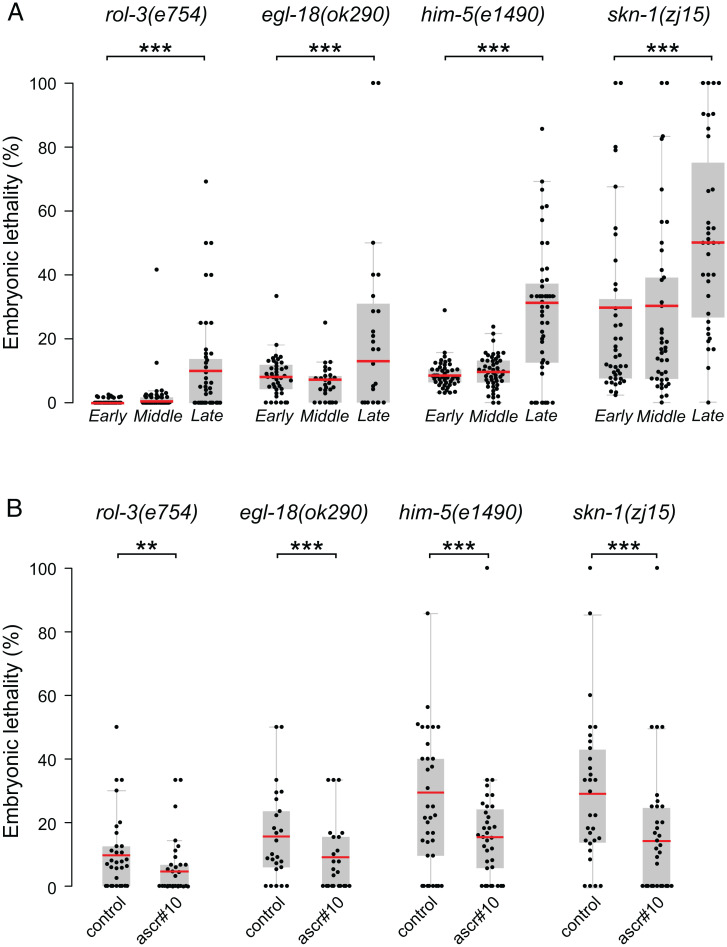
We reasoned that lower gamete quality in older mothers may exacerbate developmental defects. To test this idea, we selected mutant alleles of four genes that cause weakly penetrant embryonic lethality. We collected samples of early, middle, and late broods (produced on day 1, day 3, and days 4 and 5 of adulthood, respectively) from singled hermaphrodites and evaluated fractions of embryos that failed to hatch. In all four genetic backgrounds, late broods (approximately last 5% of total progeny production) displayed considerably higher levels of embryonic lethality (Fig. 4A). For comparison, embryonic lethality among comparably late progeny of wild-type N2 mothers is ∼4.8% (SI Appendix, Fig. S3A). Late progeny of the mutant strains displayed higher embryonic lethality than seen in late N2 broods, even when the somewhat-elevated lethality among early offspring was considered. We inferred that an age-related decline in gamete quality, combined with the defects caused by the mutant alleles, resulted in a notable decline in successful embryonic development in the progeny of older mothers. In all four genetic backgrounds, late broods (days 4 and 5) of hermaphrodites that were maintained in the presence of ascr#10 showed significantly lower offspring lethality (Fig. 4B). Combining these results with the data presented in the previous section, we concluded that by multiple criteria, ascr#10 improves the quality of the hermaphrodite germline, and certainly of oocytes, in the way that counteracts the effects of reproductive aging.
Fig. 4.
ascr#10 reduces penetrance of lethal alleles. (A) Fractions of unhatched embryos measured during “early” (day 1), “middle” (day 3), and “late” (days 4 and 5) progeny in rol-3(e754), egl-18(ok290), him-5(e1490), and skn-1(zj15). (B) Fractions of unhatched embryos in the progeny of day 4 and 5 hermaphrodites aged on or off ascr#10. Each dot represents the fraction of unhatched embryos from one hermaphrodite. Red bars denote means. **P < 0.01; ***P < 0.001. See Table S1 for primary data and details of statistical analyses.
A Narrow Window of Sensitivity of the Hermaphrodite Germline to the Male Pheromone.
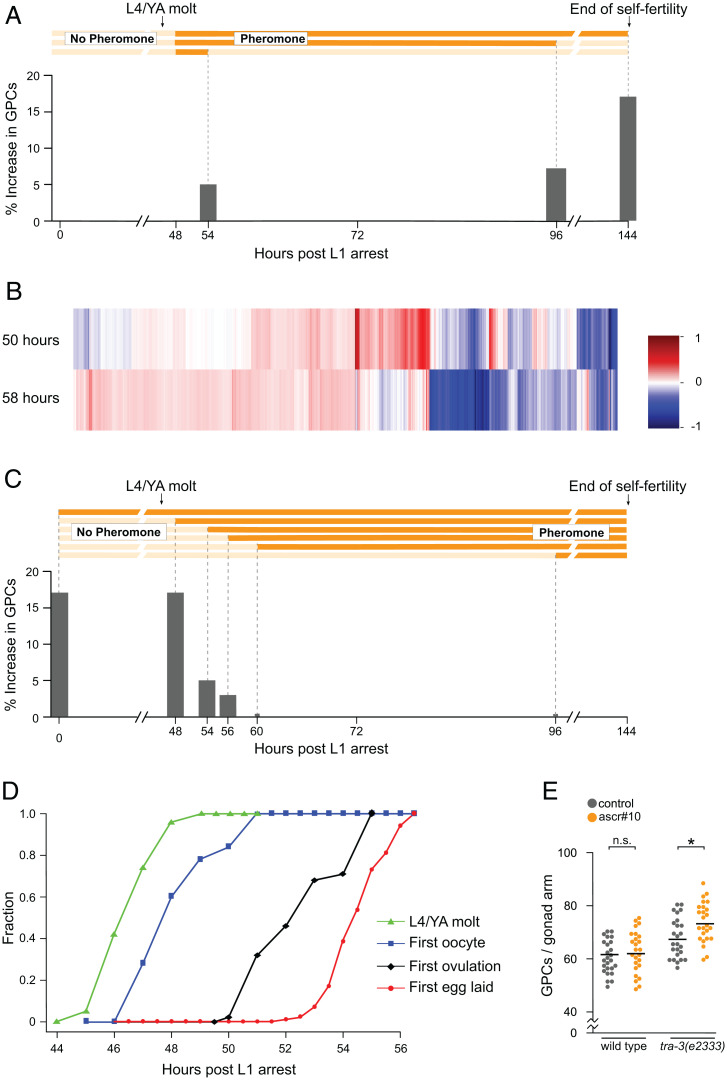
Because at least some developmental processes are sensitive to pheromones only during precisely defined periods (39), we investigated the time dependence of germline response to ascr#10. First, we found that for maximal response, exposure needs to be continuous throughout adulthood (Fig. 5A).
Fig. 5.
ascr#10 increases the number of germline precursors in aging adults. (A) Increase (relative to paired controls) in the number of GPCs following exposure to ascr#10 from 48 h until different time points. In all experiments, GPCs were counted in day 5 mothers. Temporal extent of exposure is represented by the schematics above. The greatest effect, at 144 h, shows an increase in GPCs equivalent to Fig. 1D. (B) A heatmap representing differentially expressed genes (on vs. off ascr#10) in 50- and 58-h-old adults. (C) Increase (relative to paired controls) in the number of GPCs following exposure to ascr#10 from different starting points until day 5. The temporal extent of exposure is represented in the schematics above. The greatest effects (exposure starting at 0 or 48 h) show an increase in GPCs equivalent to Fig. 1D. Organization of A and C is inspired by Schaedel et al. (39). (D) Fractions of worms that attained four developmental milestones: molting from the L4 larval stage to adult, producing the first oocyte in the gonad, ovulating the first embryo, and laying the first egg. (E) Comparison of the number of GPCs on versus off ascr#10 in N2 and tra-3(e2333) hermaphrodites with pheromone exposure starting at 56 h postrelease from the L1 arrest. *P < 0.05; n.s., not significant. See Table S1 for primary data and details of statistical analyses.
Second, we showed previously that several hermaphrodite responses to ascr#10, including the increase in the number of GPCs, require ongoing egg laying (18). This requirement of reproduction for pheromone response sharply distinguishes between late larvae and prereproductive adults, on the one hand, and actively reproducing adults. One possible mechanism to account for this difference is that the pheromone receptor or other components required for the response are expressed only in older individuals. The identity of the ascr#10 receptor is not currently known, and much remains to be learned about other genes involved in reception and signal transduction (22). We therefore compared transcriptional response to physiological amounts of ascr#10 between young adults that were immediately prereproductive and those that just initiated egg laying. We found that 1,667 genes were differentially expressed in prereproductive worms exposed to ascr#10. We also found 3,627 genes that changed expression in the young adults that started to lay eggs. The two conditions shared a highly significant (P < 10−45, hypergeometric test) overlap of 741 genes (Fig. 5B). When we compared fold-changes across the union of 4,553 genes, regardless of significance, we observed that the majority of genes were measured to change in the same direction across both conditions, suggesting that the reason for the relatively small overlap between both groups is likely due to insufficient statistical power with which to call significance. We will report a detailed analysis of the genome-wide response to ascr#10 elsewhere. Here, we concluded that the lack of behavioral (18) and germline (Fig. 1) responses to this male pheromone in prereproductive adults is not because these worms do not express the receptor or other components essential for transduction of the signal. Rather, the absence of the response is likely due to the lack of a signal that reproduction has commenced, which must exert a potent modulatory effect.
Third, not only does the ascr#10 response in the germline require the onset of egg laying (even if exposure began during an early larval stage), but the exposure to the pheromone also must commence by no later than ∼56 h post-release from the L1 arrest (Fig. 5C), which is ∼8 to 10 h after the L4-to-adult transition. This time closely corresponds to the onset of egg laying (Fig. 5D), demonstrating that the germline response to ascr#10 requires exposure before egg laying commences, whereas the response itself is conditioned on active egg laying (18). To test whether the apparent inability of the germlines of N2 hermaphrodites to respond to ascr#10 when exposure started at 56 h was due to their age or functional state, we evaluated the ability of comparably old tra-3(e2333) hermaphrodites to respond to ascr#10. The tra-3(e2333) hermaphrodites delay the switch from spermatogenesis to oogenesis by several hours (40); therefore, whereas most ∼56-h-old N2 hermaphrodites have already initiated egg laying, almost none of the tra-3(e2333) hermaphrodites have. We found that unlike their N2 counterparts, the tra-3(e2333) hermaphrodites increased the number of GPCs on ascr#10 when exposure starts at 56 h (Fig. 5E). These data further support the notion that it is not age, per se, but instead whether egg laying has commenced that determines the age at which the germline is no longer responsive to ascr#10.
Different Sensitivity of the Reproductive System to Brief Food Deprivation Just before and Just after the Onset of Reproduction.
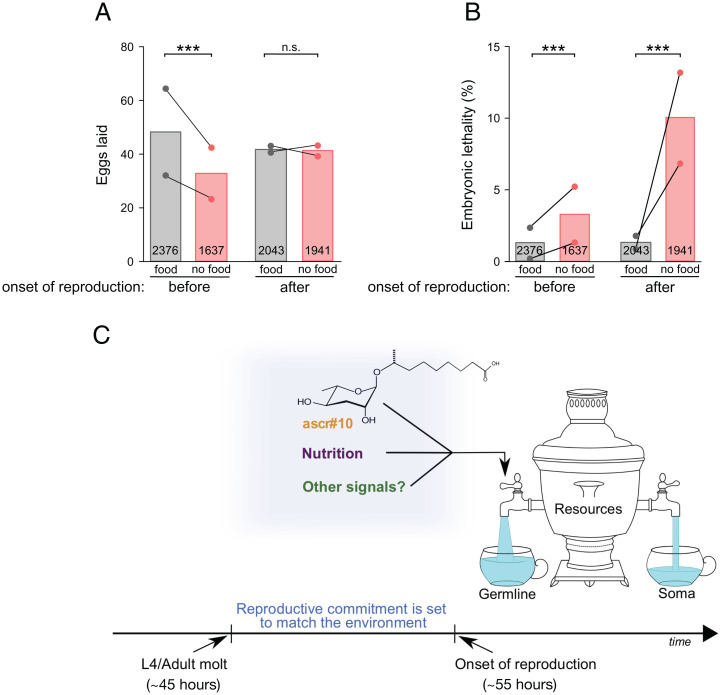
We hypothesized that the short period between the beginning of adulthood and the onset of reproduction represents not only a window of pheromone sensitivity, but also, more generally, a critical time during which prereproductive adult hermaphrodites conduct the final assessment of the environment’s suitability for reproduction. To explore this idea, we briefly (4 h) deprived hermaphrodites of food either just before or just after the onset of egg laying. Imposing this stress immediately before the onset of reproduction substantially reduced progeny production in the following 24 h, whereas starvation after the onset of reproduction had no discernable effect (Fig. 6A). In contrast, whereas brief food deprivation during the sensitive period appeared to have modest effect on progeny quality, starvation early during reproduction was associated with substantially increased embryonic lethality (Fig. 6B). We interpret these results to mean that prior to irreversible commitment to reproduction, mothers experiencing adverse environmental conditions can lower their reproductive output to preserve offspring quality. After the onset of reproduction, the ability to adjust reproductive physiology in response to the environment is reduced, and adverse conditions result in poorer offspring quality.
Fig. 6.
Predictions and model. (A) Total number and (B) embryonic lethality of progeny produced in the first 24 h by young N2 hermaphrodite mothers that were briefly deprived of food either just before (48 to 52 h) or just after (56 to 60 h) the onset of reproduction. In A and B, numbers inside bars represent totals of scored embryos. (C) Model summarizing the role of serotonin signaling in regulating the flow of resources into somatic versus germline maintenance. Graphical depiction of the model benefited from Maklakov and Friberg (85). ***P < 0.001; n.s., not significant. See Table S1 for primary data and details of statistical analyses.
Discussion
Results reported here support five main conclusions. First, previously we showed that the germlines of adult C. elegans hermaphrodites appeared more youthful in the presence of male sex pheromone ascr#10 (20, 21). Here, we demonstrate that by several measures—the ability to sustain development, rates of nondisjunction, and morphology—exposure to ascr#10 improved the quality of the oogenic germline, thus ameliorating the effects of aging. The beneficial effects are particularly notable in older mothers who have exhausted the cache of self-sperm; in the absence of the male pheromone, these animals would show pronounced reproductive defects. Higher concentrations of ascr#10 relative to other pheromones signal the presence of males (22). Therefore, our finding that ascr#10 improves oocyte maintenance supports the idea that this pheromone communicates a higher demand for quality oocytes in anticipation of mating to promote reproductive success (19, 21). In other species, female reproductive physiology is substantially altered by mating, at least in part due to components of the seminal fluid (41) that can alter the germline (42) as well as organ systems required to sustain the germline (43, 44).
Second, we argue that the major effect of ascr#10 on the germline is to increase proliferation of GPCs. Action of the pheromone appears to be restricted to the germlines of adult, not larval, hermaphrodites, strengthening the case for the somewhat distinct mechanisms of germline maintenance during different life stages (45). The adult-specific effect appears sensible because a plausible male strategy would be to manipulate gamete quality in likely mating partners, not in sexually immature larvae. In Drosophila melanogaster, ovaries develop more germline precursors in response to male-produced signals (46), suggesting that dynamic regulation of germline proliferation in response to sex-specific signals may be a common phenomenon.
Third, the male pheromone causes increased incidence of germline cell death that is due to a greater supply of cells that could die. The dying cells bear the hallmarks of physiological apoptosis, a process that eliminates the majority of oocyte precursors in C. elegans (26) and other animals (47–50). The dependence of the pheromone-caused oocyte improvement on the core apoptosis genes reflects the important role of physiological germline apoptosis in maintaining oocyte quality in aging C. elegans (7). Physiological germ cell death in vertebrates and invertebrates (51) could remove defective oocytes or cells that, instead of becoming mature gametes, provision developing oocytes (52, 53). In C. elegans, there is evidence that cell death does preferentially remove defective germline precursors (32, 54, 55), although selective culling of defective oocytes is unlikely to account for all observed deaths (7). Therefore, the plausible primary “purpose” of cell death in the germline may be to salvage resources (e.g., nutrients, metabolites, organelles) from the dying cells to improve oocyte quality. Our finding that exposure of hermaphrodites to ascr#10 increases proliferation of mitotic germline precursors is consistent with the idea that this pheromone promotes resource allocation to the germline.
Fourth, continuous exposure to ascr#10 causes germline effects as long as it is initiated prior to the first egg being laid. Because larval germlines do not appear to respond, the window of pheromone responsiveness extends for ∼8 to 10 h between the L4-to-adult molt and the onset of reproduction. This period is as long as a larval stage and is used by the adults to “run a final check” of the environmental conditions prior to initiating reproduction. Food availability and quality as well as the presence of potential mates are just some of the factors that animals must consider to optimally match their reproductive strategy to the environment. Our results suggest that a study of salient sensory inputs during very early (i.e., prereproductive) adulthood has the potential to elucidate important components of the brain–germline axis.
Finally, the apparent balancing of demands of reproduction versus survival (56) may be due, at least in part, to a tradeoff between somatic and reproductive maintenance (57–60). In C. elegans, male pheromones in general (61–63), and ascr#10 in particular (21, 63), shorten hermaphrodite lifespan. Germline hyperactivity, such as extra cell deaths induced by the male pheromone, are known to cause senescent pathologies (64) and could, thus, directly reduce hermaphrodite longevity. An additional cause of shorter hermaphrodite lifespan in the presence of male signals may be due to the differential regulation of the flow of resources into somatic versus germline maintenance, depicted by the “samovar hypothesis” (Fig. 6C). Male pheromones and other environmental inputs signifying conditions conducive to reproduction would result in increased proliferation of GPCs, thus causing preferential channeling of resources toward the germline. The reduced resource allocation toward the soma may manifest as a shortened lifespan seen in the presence of ascr#10 (21, 63) and may represent one possible cause of a detrimental effect of mating on female physiology in C. elegans (65) that has also been seen in other species (43).
Materials and Methods
Nematode Strain Maintenance.
All strains (complete list in Table S2) were obtained from the Caenorhabditis Genetics Center and were maintained at 20 °C on OP50 under standard nematode growth conditions (66). Populations were synchronized by alkaline hypochlorite treatment of gravid hermaphrodites. Isolated eggs were allowed to hatch overnight in M9 buffer with rotation at 20 °C (67). The arrested L1 larvae were pipetted onto lawn plates of OP50 the following morning at a density of 30 to 60 larvae per plate. Based on our experience staging N2 hermaphrodites, 48 h after release from larval arrest was designated as day 1 of adulthood (68). At this stage, worms are adults but have not yet begun to lay eggs. Some strains were slightly delayed in their development compared to the N2 wild type. Timing of experiments using these strains was adjusted to accommodate for their developmental delay. On day 1 of adulthood, hermaphrodites were transferred in populations of 30 worms per plate to either control or treatment plates. Adult hermaphrodites were moved to fresh plates every other day.
Conditioning Plates with Ascarosides.
Concentrated ascr#10 was stored in ethanol at −20 °C. This stock was diluted with water, and a total of 100 μL (for 60-mm plates) or 50 μL (for 35-mm plates) of ascaroside solution (containing 2.2 or 1.1 femtograms per plate, respectively) was applied to the plate and spread evenly with a sterile bent glass rod. Plates were incubated at 20 °C overnight to allow the ascaroside to be absorbed. Control plates were prepared in the same manner using water as the control. The following day, the plates were seeded with 20 μL of a 1:10 dilution of OP50 overnight culture and allowed to grow at 20 °C before use.
Counting GPCs.
Hermaphrodites were aged in small populations of 30 per plate on either ascr#10 or control plates. At the indicated times, they were stained with DAPI as described (21) using a variation of the protocol by Pepper et al. (69). Briefly, following washes in M9 and fixation with 95% ethanol, animals were incubated with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and mounted on 2% agarose pads for visualization. We counted the number of nuclei in the progenitor zone, as defined by Crittenden et al. (70). The slides were imaged on a Leica DM5000B microscope using a Retiga 2000R camera.
Immunohistochemistry.
Worm dissection and antibody staining were modified from Crittenden et al. (71). Hermaphrodites were picked and their gonads dissected in 200 μL phosphate-buffered saline (PBS)-0.1%Tween 20 with 0.25 mM levamisole in a large glass Petri dish. The dissected animals were incubated with 3% paraformaldehyde in PBS-Tween in 1.5 mL microcentrifuge tubes for 30 min at 20 °C with rocking. This was followed by washes, fixation in methanol at −20 °C for 30 min, washes, and blocking with 3% bovine serum albumin in PBS-Tween for 30 min at 20 °C with rocking. The blocking agent was washed off, and the worms were incubated overnight with the primary antibody (Abcam rabbit polyclonal against Histone H3 phospho serine 10 diluted 1:100 in blocking solution) at 4 °C with rocking. The following morning, the worms were washed 3× with PBS-Tween at 20 °C with rocking for more than 10 min. After washing, the worms were incubated with secondary antibody (Abcam goat anti-rabbit-Alexa Fluor 555 diluted 1:1,000) for 2 h at 20 °C with rocking. The worms were washed again 3× with PBS-Tween as above and suspended in 15 μL Vectashield with DAPI (Vector Laboratories, Burlingame, CA). A glass micropipet was used to transfer the worms in Vectashield to 2% agarose pads, where they were covered with a glass coverslip. All experiments were processed with paired controls.
Staining for Cell Death.
The response to ascr#10 as an increase in apoptotic cells was determined in three ways. Animals were stained with SYTO12 (Invitrogen) using the protocol of Gumienney et al. (26). An alternative stain, acridine orange (Invitrogen) was also used to monitor cell deaths as described by Lant and Derry (72). Both protocols require the hermaphrodites to take up the dye and, later, to consume unstained bacteria to clear the stain from the intestine. Since the worms are alive during the staining protocol, staining began in the morning and finished in the afternoon of the day specified. In addition, the increase of apoptotic cells when exposed to ascr#10 was observed in a strain carrying the CED-1::GFP transgene. This strain cannot be used for quantifying the number of apoptotic corpses, however, because it has been noted that not all cells surrounded by CED-1::GFP go on to form apoptotic corpses (73). All experiments were processed with paired controls.
RNAi Knockdown of iff-1.
Adult (48 h post-release from L1 arrest) N2 wild-type hermaphrodites were fed HT115 E. coli containing an empty vector (L4440) or expressing iff-1 double-stranded RNAi (obtained from the Ahringer RNAi Library and verified by sequencing). On day 2, animals were stained with SYTO12 to assess the number of apoptotic cells in the germline. A second batch of animals from the same experiment were stained with anti-pH3 antibody to demonstrate that knockdown of iff-1 reduced proliferation in the germline.
Embryonic Lethality Measurements.
N2 hermaphrodites were aged in synchronized populations of ∼30 to day 5 of adulthood (when most of the worms were depleted of self-sperm), singled, and allowed to mate with three young adult N2 males for 1 h. After mating, the hermaphrodites were singled to fresh plates. Hatched progeny and unhatched fertilized eggs were scored for 3 d after the mating.
Unlike N2 wild type, the mutants in Fig. 4 had high-enough levels of embryonic lethality to be detected without mating to extend the reproductive span. At 48 h after release from L1 arrest, synchronized hermaphrodites were singled to seeded plates and subsequently transferred to fresh seeded plates every 12 h. Hatched larvae and dead embryos were counted on each plate and designated as “early” [48 to 84 h; 48 to 96 h for skn-1(zj15)], “middle” (102 to 120 h), or “late” (120 to 168 h).
To assess the effect of ascr#10 on embryonic lethality, hermaphrodites were aged in small populations on ascr#10 or control plates. At 120 h post-release from L1 arrest, 35 worms from each population were singled to seeded ascr#10 or control plates. Hatched larvae and dead embryos were counted until the end of self-reproduction. Experiments to determine embryonic lethality in ced-3(n717) and ced-4(n1162) in Fig. 3G used day 5 hermaphrodites that were mated to young N2 males as described above.
Male Frequency Experiments.
Hermaphrodites were maintained in populations of 30 per plate (both control and treatment) and were transferred every other day to fresh plates. On day 4 of adulthood, the hermaphrodites were singled to fresh plates, and the fraction of male offspring generated during the last 2 d of self-progeny production (days 5 and 6 of adulthood) was noted. Data from these experiments were also used to assess the percentage of embryonic lethality in the progeny of selfing hermaphrodites. The strain JK987 tra-2(q276)/mnC1[dpy-10(e128) unc-52(e444)] segregates phenotypic males with the XX karyotype (74). Synchronized day 5 N2 wild-type or him-5(e1490) hermaphrodites were mated with one young adult JK987 male for 2 h and singled to fresh plates. Progeny production was monitored for 4 d after the mating, and the fraction of male progeny was scored.
Germline Morphology in Aged Hermaphrodites.
On day 7 of adulthood, hermaphrodites were assessed for defects in the proximal gonad. To assess oocyte morphology, we used two phenotypes—abnormally small oocytes in the proximal gonad and the presence of atypical cavities between oocytes in the proximal gonad—as described by Luo et al. (8). In young adult hermaphrodites, the cellular volume of the oocytes in the proximal gonad increases prior to oocyte maturation so that the most proximal oocytes fill the gonadal lumen. Smaller oocytes have been correlated with decreased oocyte quality (7). Also prior to maturation, the oocyte shape changes from cylindrical to ovoid (11). This shape change causes a small gap between the most proximal oocyte in the gonad and the next oocyte. The presence of cavities between more distal oocytes is abnormal. The presence of small oocytes or cavities in one arm of the gonad was scored as a “mild” defect, and the presence in both arms of the gonad was counted as “severe.”
RNA Sequencing.
Synchronized populations of L1s were obtained by hypochlorite treatment as above and pipetted onto control or ascr#10 plates that had been seeded with 20 μL drops of OP50 the previous day. Care was taken to keep the density between 30 and 50 L1s per plate. In total, 16 plates (8 control and 8 ascr#10 plates) were prepared in this manner for the “50 h” sample, and 12 plates (6 control and 6 ascr#10 plates) were prepared for the “58 h” sample. Worms were allowed to develop on these plates at 20 °C until the allotted times. Just prior to harvesting the worms, we closely examined each plate for developmentally delayed or abnormal worms, which were removed. Plates that contained males were excluded. At 50 h and 58 h after the worms were dispensed on the plates, they were collected, washed four times with PBS, and snap-frozen in liquid nitrogen. Total RNA was extracted with Qiagen RNeasy Mini Kit (Cat. No. 74104) and on-column DNase digestion done with Qiagen RNase-Free DNase (Cat. No. 79254) according to the manufacturer’s protocols. Three independent samples were prepared and analyzed for each condition each time the experiment was performed. In total, 1 μg total RNA was used in library preparation with NEBNext UltraTM II Directional RNA Library Prep with Sample Purification Beads Kit for Illumina (NEB#E7765). Libraries were sequenced on a HiSEq 4000, 50-bp single-read system at the Northwestern University Sequencing Core facility (NUSeq). Due to technical issues that resulted in lower than desired read depth, the 58-h timepoint was collected a second time.
We used a publicly available Nextflow (75) pipeline (https://github.com/nf-core/rnaseq) to carry out quality control (FASTQC; ref. 76), alignment, and quantification (Salmon; ref. 77) of our RNA sequencing reads. The pipeline used MultiQC to assemble the final HTML report (78). We used R 4.1.0 and DESeq2 version 1.32.0 (79) to perform pairwise differential expression analyses of each timepoint comparing control animals to animals exposed to ascr#10. We called a gene differentially expressed if its false discovery rate (q value) was less than or equal to 0.05. Since we had six replicates across two batches of the 58-h timepoint, we pooled the 58-h replicates together including a batch correction term in the general linear model to maximize statistical power. We report only those differentially expressed genes that were measured across the 50-h and 58-h timepoints. The DESeq2 results are shown in Table S3. We generated the heatmap using Python (80) version 3.7.10, Matplotlib (81) version 3.3.4, and seaborn (82) version 0.11.2. The hypergeometric P value and other calculations were carried out using SciPy (83) version 1.6.2 and NumPy (84) version 1.19.2.
Removal of Food Experiments.
Synchronized hermaphrodites grown in small populations (30–50) were washed off, rinsed 5× in M9, deposited on either seeded or unseeded nematode growth medium (NGM) plates, and incubated for 4 h. In total, 25 worms for each condition were singled onto seeded plates, and progeny production was monitored for 24 h (and an extra 24 h to confirm unhatched embryos). The “56 to 60 h” cohort was treated in the same way, except all worms were examined to ensure that egg laying had begun.
Data Analysis.
Experiments were compared against matched controls that were processed in parallel. For details of quantification and statistical methods used, see studies by Aprison and Ruvinsky (18, 19, 22). Statistical tests were performed in R or Excel. Tests performed for each comparison are shown in Table S1.
Note Added in Proof.
When our paper was in press, a study by Wu and colleagues (86) reported on a protein in C. elegans that contributes to the tradeoff between reproduction and longevity. The loss of this TRL-1 protein upregulates vitellogenin translation thus increasing yolk provision to oocytes and brood size, but at the cost of reduced lifespan. This is precisely the kind of molecular mechanism that could mediate the germline vs. soma balance depicted in our Figure 6C.
Supplementary Material
Acknowledgments
I.R. is profoundly grateful to Erin Sherman for theoretical and practical inspiration of this project. We thank Rick Morimoto for generous hospitality, David Greenstein and Hannah Seidel for advice, and Frank Schroeder for ascarosides. This work was funded in part by NIH Grant R01GM126125 to I.R. We thank WormBase and the Caenorhabditis Genetics Center (CGC). WormBase is supported by Grant U41 HG002223 from the National Human Genome Research Institute at the NIH, the UK Medical Research Council, and the UK Biotechnology and Biological Sciences Research Council. The CGC is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015576119/-/DCSupplemental.
Data Availability
All scripts and parameters to reproduce our analyses can be found at GitHub (https://github.com/dangeles/MalePheromoneRNAseq). Raw sequence reads and processed counts can be found in the Gene Expression Omnibus database (accession no. GSE193636). All other study data are included in the article and/or supporting information.
References
- 1.Hughes S. E., Evason K., Xiong C., Kornfeld K., Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 3, e25 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo S., Shaw W. M., Ashraf J., Murphy C. T., TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet. 5, e1000789 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickett C. L., Dietrich N., Chen J., Xiong C., Kornfeld K., Mated progeny production is a biomarker of aging in Caenorhabditis elegans. G3 (Bethesda) 3, 2219–2232 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocsisova Z., Kornfeld K., Schedl T., Rapid population-wide declines in stem cell number and activity during reproductive aging in C. elegans. Development 146, dev173195 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igarashi H., Takahashi T., Nagase S., Oocyte aging underlies female reproductive aging: Biological mechanisms and therapeutic strategies. Reprod. Med. Biol. 14, 159–169 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan F. E., et al. , Age-associated dysregulation of protein metabolism in the mammalian oocyte. Aging Cell 16, 1381–1393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andux S., Ellis R. E., Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 4, e1000295 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo S., Kleemann G. A., Ashraf J. M., Shaw W. M., Murphy C. T., TGF-β and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 143, 299–312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Z., Hubbard E. J., Non-autonomous DAF-16/FOXO activity antagonizes age-related loss of C. elegans germline stem/progenitor cells. Nat. Commun. 6, 7107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinquin A., et al. , Intermittent stem cell cycling balances self-renewal and senescence of the C. elegans germ line. PLoS Genet. 12, e1005985 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarter J., Bartlett B., Dang T., Schedl T., On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 205, 111–128 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Kimble J., Crittenden S. L., Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 23, 405–433 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Neubaum D. M., Wolfner M. F., Wise, winsome, or weird? Mechanisms of sperm storage in female animals. Curr. Top. Dev. Biol. 41, 67–97 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Garigan D., et al. , Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics 161, 1101–1112 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox P. M., et al. , Cyclin E and CDK-2 regulate proliferative cell fate and cell cycle progression in the C. elegans germline. Development 138, 2223–2234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyatt T. D., Pheromones and Animal Behavior: Chemical Signals and Signatures (Cambridge University Press, ed. 2, 2014). [Google Scholar]
- 17.Izrayelit Y., et al. , Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 7, 1321–1325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aprison E. Z., Ruvinsky I., Dynamic regulation of adult-specific functions of the nervous system by signaling from the reproductive system. Curr. Biol. 29, 4116–4123.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aprison E. Z., Ruvinsky I., Coordinated behavioral and physiological responses to a social signal are regulated by a shared neuronal circuit. Curr. Biol. 29, 4108–4115.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aprison E. Z., Ruvinsky I., Sex pheromones of C. elegans males prime the female reproductive system and ameliorate the effects of heat stress. PLoS Genet. 11, e1005729 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aprison E. Z., Ruvinsky I., Sexually antagonistic male signals manipulate germline and soma of C. elegans hermaphrodites. Curr. Biol. 26, 2827–2833 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Aprison E. Z., Ruvinsky I., Counteracting ascarosides act through distinct neurons to determine the sexual identity of C. elegans pheromones. Curr. Biol. 27, 2589–2599.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Killian D. J., Hubbard E. J., Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev. Biol. 279, 322–335 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Roy D., et al. , Cell cycle features of C. elegans germline stem/progenitor cells vary temporally and spatially. Dev. Biol. 409, 261–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendzel M. J., et al. , Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Gumienny T. L., Lambie E., Hartwieg E., Horvitz H. R., Hengartner M. O., Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126, 1011–1022 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Jaramillo-Lambert A., Ellefson M., Villeneuve A. M., Engebrecht J., Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Dev. Biol. 308, 206–221 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Agarwal I., et al. , HOP-1 presenilin deficiency causes a late-onset notch signaling phenotype that affects adult germline function in Caenorhabditis elegans. Genetics 208, 745–762 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gartner A., Boag P. R., Blackwell T. K., “Germline survival and apoptosis” In WormBook: The Online Review of C. elegans Biology (WormBook, Pasadena, CA, 2008), pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gartner A., Milstein S., Ahmed S., Hodgkin J., Hengartner M. O., A conserved checkpoint pathway mediates DNA damage--induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5, 435–443 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Schumacher B., Hofmann K., Boulton S., Gartner A., The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Bhalla N., Dernburg A. F., A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science 310, 1683–1686 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Hanazawa M., et al. , The Caenorhabditis elegans eukaryotic initiation factor 5A homologue, IFF-1, is required for germ cell proliferation, gametogenesis and localization of the P-granule component PGL-1. Mech. Dev. 121, 213–224 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Nagaoka S. I., Hassold T. J., Hunt P. A., Human aneuploidy: Mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose A. M., Baillie D. L., The effect of temperature and parental age on recombination and nondisjunction in Caenorhabditis elegans. Genetics 92, 409–418 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodgkin J., Horvitz H. R., Brenner S., Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics 91, 67–94 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okkema P. G., Kimble J., Molecular analysis of tra-2, a sex determining gene in C. elegans. EMBO J. 10, 171–176 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yee C., Yang W., Hekimi S., The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell 157, 897–909 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schaedel O. N., Gerisch B., Antebi A., Sternberg P. W., Hormonal signal amplification mediates environmental conditions during development and controls an irreversible commitment to adulthood. PLoS Biol. 10, e1001306 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodgkin J., Barnes T. M., More is not better: Brood size and population growth in a self-fertilizing nematode. Proc. Biol. Sci. 246, 19–24 (1991). [DOI] [PubMed] [Google Scholar]
- 41.Avila F. W., Sirot L. K., LaFlamme B. A., Rubinstein C. D., Wolfner M. F., Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 56, 21–40 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDonough-Goldstein C. E., Pitnick S., Dorus S., Drosophila oocyte proteome composition covaries with female mating status. Sci. Rep. 11, 3142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed S. M. H., et al. , Fitness trade-offs incurred by ovary-to-gut steroid signalling in Drosophila. Nature 584, 415–419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White M. A., Bonfini A., Wolfner M. F., Buchon N., Drosophila melanogaster sex peptide regulates mated female midgut morphology and physiology. Proc. Natl. Acad. Sci. U.S.A. 118, e2018112118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hubbard E. J. A., Schedl T., Biology of the Caenorhabditis elegans germline stem cell system. Genetics 213, 1145–1188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ameku T., Niwa R., Mating-induced increase in germline stem cells via the neuroendocrine system in female Drosophila. PLoS Genet. 12, e1006123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller M. A., Technau U., Smith K. M., Steele R. E., Oocyte development in Hydra involves selection from competent precursor cells. Dev. Biol. 224, 326–338 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Matova N., Cooley L., Comparative aspects of animal oogenesis. Dev. Biol. 231, 291–320 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Pepling M. E., Spradling A. C., Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 (2001). [DOI] [PubMed] [Google Scholar]
- 50.McCall K., Eggs over easy: Cell death in the Drosophila ovary. Dev. Biol. 274, 3–14 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Baum J. S., St George J. P., McCall K., Programmed cell death in the germline. Semin. Cell Dev. Biol. 16, 245–259 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Tilly J. L., Commuting the death sentence: How oocytes strive to survive. Nat. Rev. Mol. Cell Biol. 2, 838–848 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Chartier N. T., et al. , A hydraulic instability drives the cell death decision in the nematode germline. Nat. Phys. 17, 920–925 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chu H. P., et al. , Germline quality control: eEF2K stands guard to eliminate defective oocytes. Dev. Cell 28, 561–572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raiders S. A., Eastwood M. D., Bacher M., Priess J. R., Binucleate germ cells in Caenorhabditis elegans are removed by physiological apoptosis. PLoS Genet. 14, e1007417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maklakov A. A., Chapman T., Evolution of ageing as a tangle of trade-offs: Energy versus function. Proc. Biol. Sci. 286, 20191604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maklakov A. A., Immler S., The expensive germline and the evolution of ageing. Curr. Biol. 26, R577–R586 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Ezcurra M., et al. , C. elegans eats its own intestine to make yolk leading to multiple senescent pathologies. Curr. Biol. 28, 2544–2556.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stieglitz J., et al. ; HORUS Study Team, Computed tomography shows high fracture prevalence among physically active forager-horticulturalists with high fertility. eLife 8, e48607 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H. Y., Jolly C., Bublys K., Marcu D., Immler S., Trade-off between somatic and germline repair in a vertebrate supports the expensive germ line hypothesis. Proc. Natl. Acad. Sci. U.S.A. 117, 8973–8979 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maures T. J., et al. , Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science 343, 541–544 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi C., Runnels A. M., Murphy C. T., Mating and male pheromone kill Caenorhabditis males through distinct mechanisms. eLife 6, e23493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ludewig A. H., et al. , An excreted small molecule promotes C. elegans reproductive development and aging. Nat. Chem. Biol. 15, 838–845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de la Guardia Y., et al. , Run-on of germline apoptosis promotes gonad senescence in C. elegans. Oncotarget 7, 39082–39096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi C., Murphy C. T., Mating induces shrinking and death in Caenorhabditis mothers. Science 343, 536–540 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner S., The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sulston J., Hodgkin J., “Methods” in The Nematode Caenorhabditis elegans, Wood W. B., Ed. (Cold Spring Harbor Laboratory Press, 1988), pp. 587–606. [Google Scholar]
- 68.Aprison E. Z., Ruvinsky I., Balanced trade-offs between alternative strategies shape the response of C. elegans reproduction to chronic heat stress. PLoS One 9, e105513 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pepper A. S., Killian D. J., Hubbard E. J., Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics 163, 115–132 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crittenden S. L., Leonhard K. A., Byrd D. T., Kimble J., Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol. Biol. Cell 17, 3051–3061 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crittenden S. L., Seidel H. S., Kimble J., Analysis of the C. elegans germline stem cell pool. Methods Mol. Biol. 1463, 1–33 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Lant B., Derry W. B., Fluorescent visualization of germline apoptosis in living Caenorhabditis elegans. Cold Spring Harb. Protoc. 2014, 420–427 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Craig A. L., Moser S. C., Bailly A. P., Gartner A., Methods for studying the DNA damage response in the Caenorhabdatis elegans germ line. Methods Cell Biol. 107, 321–352 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Hodgkin J., Exploring the envelope. Systematic alteration in the sex-determination system of the nematode Caenorhabditis elegans. Genetics 162, 767–780 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Tommaso P., et al. , Nextflow enables reproducible computational workflows. Nat. Biotechnol. 35, 316–319 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Andrews S., Fast Q. C., A Quality Control Tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
- 77.Patro R., Duggal G., Love M. I., Irizarry R. A., Kingsford C., Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 14, 417–419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ewels P., Magnusson M., Lundin S., Käller M., Multi Q. C., MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Rossum G., Drake F. L., Python 3 Reference Manual (Scotts Valley, CA, 2009). [Google Scholar]
- 81.Hunter J. D., Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007). [Google Scholar]
- 82.Waskom M. L., Seaborn: Statistical data visualization. J. Open Source Softw. 6, 3021 (2021). [Google Scholar]
- 83.Virtanen P., et al. ; SciPy 1.0 Contributors, SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harris C. R., et al. , Array programming with NumPy. Nature 585, 357–362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maklakov A. A., Friberg U., Ageing: Why males curtail the longevity of their mates. Curr. Biol. 26, R929–R932 (2016). [DOI] [PubMed] [Google Scholar]
- 86.D. Wu et al., An antagonistic pleiotropic gene regulates the reproduction and longevity tradeoff. Proc. Natl. Acad. Sci. U.S.A. 119, e2120311119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All scripts and parameters to reproduce our analyses can be found at GitHub (https://github.com/dangeles/MalePheromoneRNAseq). Raw sequence reads and processed counts can be found in the Gene Expression Omnibus database (accession no. GSE193636). All other study data are included in the article and/or supporting information.