Abstract
The severity of COVID-19 is largely determined by the inflammatory response, a “Cytokine storm,” that involves both pro- and anti-inflammatory cytokines. In the current study we investigated the balance of pro- and anti-inflammatory status as represented by the levels of IL-6/IL-10 in severe to critical COVID-19 patients. 66 confirmed COVID-19 patients admitted to the ICU were categorized into groups according to the mortality and respiratory failure. Data were collected retrospectively in ICU, including a peripheral immune cells and infection-related biomarker CRP. The measurements of cytokine levels were performed by Immulite analyzer for IL-6 and ELISA sandwich for IL-10. In addition, longitudinal measurement of IL-6 was performed during 5 days post admission. Longitudinal assays showed that IL-6 was sustained at a medium level within 5 days post admission in severe cases who survived or not requiring mechanical ventilation, whereas it was sustained at high levels throughout the disease course in either deceased cases or who developed respiratory failure. The ratio of IL-6/lymphocytes was positively correlated with the risk of mortality, while IL-10/lymphocytes ratio could predict respiratory failure in ICU. IL-6/IL-10 profiling revealed that deceased patients have different magnitudes of both IL-6 and IL-10 cytokine release. Notably, excessive levels of IL-6 concomitant with high levels of IL-10 were more common in diseased COVID-19 patients. Taking into account the IL-6/IL-10 profiling may help clinicians to identify the right time of anti-inflammation treatment and select patients who will respond to anti-cytokine therapies and maintain an adequate inflammatory response for SARS-CoV-2 clearance.
Keywords: COVID-19, Cytokine storm, Interleukin 6, Interleukin 10, SARS-CoV-2
1. Introduction
Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) rapidly spread worldwide and was declared a pandemic in early 2020 (Ludwig and Zarbock, 2020). Although most SARS-CoV-2 infected patients display mild-to-moderate symptoms and undergo spontaneous regression, approximately 10–20% of cases develop severe symptoms. Some patients suffer from multi-organ dysfunction, including acute respiratory distress syndrome (ARDS), vasodilatory shock, acute kidney injury, coagulopathy, and impaired brain or heart function; which also represent the common clinical manifestations that characterize sepsis (Scientific Societies, 2021). In order to better understand the pathogenesis of this disease it will be useful to discover effective biomarkers to distinguish which subgroup(s) of patients should be given priority in ICU admission.
To elucidate the pathophysiological mechanisms underlying this heterogeneous disease, much attention has been paid to inflammatory response. Accumulating evidence suggests that a subgroup of patients with severe COVID-19 might have a disrupted and disproportionate inflammatory response, particularly exhibiting excessive cytokine production leading to a « cytokine storm. » (Chen et al., 2021b, Li et al., 2021, Liu et al., 2021). A cytokine storm is observed in many pathologies such as sepsis and also recently in COVID-19 pathology (Fajgenbaum et al., 2020). It’s associated with uncontrolled hyperinflammation leading to tissue damage, apoptosis of immune cells, and impaired cytotoxic function (Jesenak et al., 2020, Vabret et al., 2020). Cytokine storm is amplified by the key pro-inflammatory cytokine IL-6, whereas anti-inflammatory cytokines such as IL-10 control the pro- inflammatory response (Belaid et al., 2022). It is widely accepted that pro- and anti-inflammatory mechanisms should be balanced while maintaining an adequate inflammatory response for pathogen clearance (Cicchese et al., 2018). Therefore, we argue that deciphering the balance of pro- and anti-inflammatory status in COVID-19 patients is a prerequisite for the development of efficient therapeutics and outcome-prediction tools.
The aim of this study was to investigate the value of IL-6/IL-10 profiling to discriminate balanced from unbalanced inflammatory responses in a Tunisian cohort of SARS-CoV-2 infected patients with severe to critical disease. In addition, the study explored the IL-6/lymphocyte ratio and IL-10/lymphocyte ratio as a prognosis tool to predict the mortality and respiratory failure in severe to critical COVID-19 patients.
2. Patients and methods
2.1. Patients
A cohort of 66 patients diagnosed with severe pneumonia by SARS-COV-2 infection upon admission in the intensive care unit (ICU) of Military Principal Hospital of Tunisia (HPMT) was investigated retrospectively from medical records. The period of admission was between September 16, 2020 and May 29, 2021, corresponding to the 4th wave of infection, dominated by the delta variant. Patients were tested positive for SARS-CoV-2 by nasopharyngeal swab or rapid antigenic test and pneumonia was documented by chest x-ray or computed tomography. The ages of the patient ranged between 7 and 88 years old.
Disease severity of COVID-19 patients was classified according to the interim guidelines from the WHO and the National Health Commission of China (World Health Organization, 2020): (i) The mild disease group was defined as patients displaying mild, clinical symptoms with no pneumonia on computerized tomography (CT) imaging. (ii) Patients with a moderate illness were characterized by fever, respiratory symptoms, and a CT imaging indicating the presence of pneumonia. (iii) Patients belonging to the severe disease group were those who met at least one of the following criteria: shortness of breath and respiratory rate ≥ 30 breaths/min; SpO2 ≤ 93% at a rest state; PaO2/FiO2 ≤ 300 mmHg; and/or lung infiltrates > 50% of the lung field within 24–48 h. (iv) Critical patients were defined as those meeting at least one of the following conditions: patients with respiratory failure who were in need of mechanical ventilation; patients displaying signs of cardiovascular shock; and patients with other organ failures, which required monitoring in the intensive care unit. Therefore, the cases enrolled in this study are severe group (severe to critical).
This study was approved by the Ethics Commission of Military Principal Hospital of Tunisia (HPMT) (61/2020). Ethical consent was obtained from the family of patients.
2.2. Data collection
We collected data on age, sex, exposure history, chronic medical histories (hypertension, diabetes and asthma), symptoms from onset to hospital admission (fever, cough, dyspnea, headache, asthenia and flu syndrome), vital signs at ICU admission (heart rate and respiratory rate), treatment (vitamin therapy, glucocorticoids, antibiotic treatment, anticoagulation treatment, oxygen therapy), as well as living status.
Clinical information including clinical laboratory measurements and computed tomographic (CT) scans were collected at the moment of their admission in ICU. Initial clinical laboratory investigation included a complete blood count, serum biochemical test, coagulation profile (including Troponin, NT-proBNP, Fibrinogen and D-dimer) and infection-related biomarkers (Procalcitonin, Ferritin and CRP).
2.3. IL-6 and IL-10 measurements
Blood samples were processed according to hospital’s standard procedures, including a blood sample withdrawn into a vacutainer tube containing coagulant for serum collection. The samples were centrifuged for 20 min at 2000 rpm. Serum was then collected and tested within 4–6 h. All procedures were performed under Biosafety level 3 protection.
Cytokines IL-6 and IL-10 were assessed in serum samples. IL-6 was assessed in serum samples drawn shortly at each time point by chemiluminescence immunoassay (CLIA) performed on a fully automated analyzer (Immulite (Immulite ®1000,Siemens). IMMULITE 1000 IL-6 kit (#L2K6P2) is a solid-phase enzyme-labeled chemiluminescent sequential immunometric assay. The concentrations of IL-10 were measured by ELISA assay (#DE4699) following the manufacturer’s instructions (Demeditec Diagnostics GmbH, Kiel, Germany). Quantification of the levels of IL-6 and IL-10 were performed on the first day of admission to ICU. Moreover, longitudinal assay was performed for the levels of IL-6 during 5 days post admission in ICU.
2.4. Statistical analysis
All statistical analyses were performed by GraphPad Prism software 5 (GraphPad PRISMA 5.0 computer program) and MedCalc® software (version 20.022, Belgium). Shapiro-Wilk normality test was conducted to estimate the distribution of the data. Categorical variables were expressed as frequency rates or percentages and significance was detected by χ2 or Fisher’s exact test. Continuous variables were expressed as mean and range or medians and interquartile range (IQR) values. For normally distributed continuous variables, differences between groups were compared using Unpaired t-test; conversely, the Mann-Whitney U test was used for continuous variables that were not normally distributed. Correlations were determined using Spearman rank correlation analysis. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the ability of the immunologic parameters in predicting severe disease. The optimal cutoff points were obtained by calculating Youden’s index. Multivariate logistic regression analysis was used to estimate Odds Ratios of biomarkers. Survival analysis was performed using Kaplan-Meier method. For all statistical analysis, P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics and treatments of severe COVID-19 among groups of patients
In our study, a total of 66 patients having a severe COVID-19 disease were admitted in the intensive care unit (ICU). Patients were tested positive for SARS-CoV-2 by nasopharyngeal swab or rapid antigenic test and pneumonia was documented by chest x-ray or computed tomography. The most common radiological findings were bilateral pulmonary infiltration, ground glass opacities and consolidations.
The study participant demographics and summary of clinical information are shown in Table 1 . The mean age of the study population was 62.94 (7–88) years. The most common age group has been ≥ 60 years with 64% males and 36% females. The mean length of hospital stay was 11 (1–34) days.
Table 1.
Comparison of demographics, clinical characteristics and treatments between severe COVID-19 patients groups.
| All patients (n = 66) | Survivors (n = 23) | Non-Survivors (n = 43) | P | Non Mechanical Ventilation (n = 26) | Mechanical Ventilation (n = 40) | P | |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age, years | 62.94 (7–88) | 60.61 (37–87) | 64.19 (7–88) | 0.0066 | 61.04 (7–88) | 64.18 (42–84) | 0.1506 |
| <40 years | 2 (3.03 %) | 1 (4.34%) | 1 (2.32%) | 0.584 | 2 (7.69%) | 0 (0%) | 0.1659 |
| 40–60 years | 24 (36.36%) | 11 (47.82%) | 13 (30.23%) | 0.232 | 10 (38.46%) | 14 (35%) | 0.5163 |
| ≥60 years | 40 (60.6 %) | 11 (47.82%) | 29 (67.44%) | 0.0148 | 14 (53.84%) | 26 (65%) | 0.4046 |
| Sex | |||||||
| Female | 24 (36.36%) | 8 (34.78%) | 16 (37.2%) | 0.5321 | 10 (38.46%) | 14 (35%) | 0.5163 |
| Male | 42 (63.63%) | 15 (65.21%) | 27 (62.79%) | 16 (61.53%) | 26 (65%) | ||
| Comorbidities | |||||||
| Hypertension | 31 (46.96%) | 5 (21.73%) | 26 (60.46%) | 0.0012 | 7 (26.92%) | 24 (60%) | 0.001 |
| Diabetes | 23 (34.84%) | 5 (21.73%) | 18 (41.86%) | 0.0164 | 6 (23.07%) | 17 (42.5%) | 0.0493 |
| Asthma | 6 (9.09%) | 2 (8.69%) | 4 (9.3%) | 0.4367 | 2 (7.69%) | 4 (10%) | 0.4367 |
| Signs and symptoms at disease onset | |||||||
| Fever | 30 (45.45%) | 12 (52.17%) | 18 (41.86%) | 0.2512 | 12 (46.15%) | 18 (45%) | 0.2512 |
| Cough | 25 (37.87%) | 6 (26.08%) | 19 (44.18%) | 0.0268 | 7 (26.92%) | 18 (45%) | 0.0572 |
| Headache | 3 (4.54%) | 1 (4.34%) | 2 (4.65%) | 0.5952 | 1 (3.84%) | 2 (5%) | 0.5952 |
| Asthenia | 13 (19.69%) | 6 (26.08%) | 7 (16.27%) | 0.5453 | 6 (23.07%) | 7 (17.5%) | 0.5453 |
| Dyspnea | 31 (46.96%) | 12 (52.17%) | 19 (44.18%) | 0.2096 | 14 (53.84%) | 17 (42.5%) | 0.4133 |
| Flu Syndrome | 15 (22.72%) | 4 (17.39%) | 11 (25.58%) | 0.1197 | 4 (15.38%) | 11 (27.5%) | 0.1197 |
| Vital signs on admission | |||||||
| Heart rate, bpm | 91.02 (52–200) | 89.26 (60–200) | 91.97 (52–123) | 0.5421 | 90.9 (60–200) | 90.09 (52–123) | 0.4578 |
| Respiratory rate, per min | 27.71 (14–55) | 24.28 (14–40) | 29.71 (15–55) | 0.2782 | 25.56 (14–53) | 28.97 (15–55) | 0.3489 |
| SpO2, % | 92.81 (76–100) | 93.3 (50–100) | 91 (60–100) | 0.4584 | 92.91 (50–100) | 91.13 (60–100) | 0.5415 |
| PaCO2 | 39.58 (13–95) | 38.18 (13–76) | 40.31 (20–95) | 0.5643 | 38.16 (13–76) | 40.49 (20–95) | 0.5643 |
| PaO2 | 72.45 (36–190) | 74.96 (36–190) | 71.07 (37–136) | 0.4531 | 74.65 (36–190) | 70.97 (37–136) | 0.4531 |
| Lung Injury (%) | 61 (10–90) | 60 (10–90) | 62 (10–90) | 0.0008 | 56 (10–90) | 64 (15–90) | < 0.0001 |
| Oxygen support | |||||||
| Non-invasive ventilation | 58 (87.87%) | 17 (73.91%) | 41 (95.34%) | 0.3213 | 21 (80.76%) | 37 (92.5%) | 0.0588 |
| Mask oxygen | 63 (95.45%) | 22 (95.65%) | 41 (95.34%) | 0.5697 | 25 (96.15%) | 38 (95%) | 0.4771 |
| High flow oxygen | 35 (53.03%) | 12 (52.17%) | 23 (53.48%) | 0.5674 | 10 (38.46%) | 25 (62.5%) | 0.0289 |
| Mechanical ventilation (Invasive) | 40 (60.6%) | 3 (13.04%) | 37 (86.04%) | 0.001 | |||
| Treatment | |||||||
| Vitaminotherapy | 66 (100%) | 23 (100%) | 43 (100%) | 0.5724 | 26 (100%) | 40 (100%) | 0.5706 |
| Glucocorticoids | 54 (81.81%) | 16 (69.56%) | 38 (88.37%) | 0.3413 | 19 (73.07%) | 35 (87.5%) | 0.3888 |
| Antibiotic treatment | 62 (93.93%) | 21 (91.3%) | 41 (95.34%) | 0.528 | 23 (88.46%) | 39 (97.5%) | 0.4662 |
| Anticoagulation treatment | 55 (83.33%) | 15 (65.21%) | 40 (93.02%) | 0.2433 | 18 (69.23%) | 37 (92.5%) | 0.01 |
| Onset to admission, days | 14 (2–89) | 12 (2–74) | 15 (2–89) | 0.2933 | 14 (2–89) | 15 (2–76) | 0.3999 |
| Hospital stay, days | 11 (1–34) | 9 (1–30) | 11 (1–34) | 0.3761 | 9 (1–30) | 13 (2–34) | 0.183 |
| <14 | 41 (62.12%) | 17 (73.91%) | 24 (55.81%) | 0.3139 | 20 (76.92%) | 21 (52.5%) | 0.2256 |
| 14–21 | 18 (27.27%) | 4 (17.39%) | 14 (32.55%) | 0.2355 | 4 (15.38%) | 14 (35%) | 0.0461 |
| >21 | 7 (33.33%) | 2 (8.69%) | 5 (11.62%) | 0.5488 | 2 (7.69%) | 5 (12.5%) | 0.3235 |
| Survivors | 23 (34.84%) | 21 (80.76%) | 2 (5%) | 0.0005 | |||
| Non-Survivors | 43 (65.15%) | 5 (19.23%) | 38 (95%) | <0.0001 | |||
Data are presented as n (%) or mean (range), where n is the total number of patients with available data. p values comparing survivors and non-survivors or comparing non-mechanical ventilation and mechanical ventilation severe COVID-19 patients are from χ2 or Fisher’s exact test or the Unpaired t-test.
More than half of the patients had underlying comorbidities (Table 1), with hypertension (47%) being the most common comorbidity followed by diabetes (35%). The most common symptoms at disease onset were dyspnea (47%) and fever (45%), followed by cough (38%). The mean of onset symptoms to admission was 14 days (2–89).
With regard to treatment during course of hospitalization (Table 1), all patients were given vitamino therapy and most patients were given antibiotic treatment (94%). Systematic corticosteroid and anticoagulation treatment were used in most patients (82% and 83%, respectively).
Oxygen support was used in all patients (Table 1). 58/66 (88%) patients used non-invasive ventilator. In total, 40/66 (61%) patients deteriorated during hospitalization and required mechanical ventilation.
In total, 23/66 (35%) patients were discharged and 43/66 (65%) patients died (Table 1). The group of non-survivors were older respect to discharged patients (64.19 years vs 60.61 years; P = 0.0066), presented a higher prevalence of hypertension (60.46% vs 21.73%; P = 0.0012), and also had higher percentage of lung injury (60.46% vs 21.73%; P = 0.0008) respect to the survivors (Table 1). Corticosteroid and anticoagulation treatment were used in most deceased patients (88.37% and 93.02, respectively). Among those who expired 86.04% required mechanical ventilation, while only 13.04% who recovered needed that.
Table 1 also summarizes clinical characteristics and treatments among groups of COVID-19 patients requiring mechanical ventilation (n = 40), or not (n = 26). Among the group of patients who required intubation the most common age group was ≥60 years (65%). Most of patients who required intubation were of male sex (65%); compared to 35 % of females in the same group. The group that requires intubation were older with respect to the group that did not require intubation (64.18 years vs 61.04 years; P = 0.1506), presented a higher prevalence of hypertension (60% vs 26.92%; P = 0.001), and also had higher percentage of lung injury (64% vs 56%; P < 0.0001), when compared to the group that did not require intubation. Anticoagulation treatment was used in most patients who required intubation compared to those did not require intubation (92.5% vs 69.23; P = 0.01) (Table 1).
3.2. Laboratory parameters of severe COVID-19 among groups of patients
Laboratory findings in survivors (n = 23) and deceased (n = 43) severe COVID-19 patients were shown in Table 2 . Amongst the patients admitted in the ICU, there were significant differences in Troponin (P = 0.026), D-dimer (P = 0.0257), Procalcitonin (P = 0.0148) and Ferritin (P = 0.04) between recovered and deceased patients. Levels of NT-pro BNP and Fibrinogen were markedly higher than normal range (>300 pg/ml and >4 g/l; respectively) in both groups. In addition, mean serum levels of C-reactive protein (CRP) tended to be higher in all severe cases without significant differences between recovered and deceased patients (136.9 mg/l vs 154.9 mg/ml; P = 0.055) (Table 2).
Table 2.
Comparison of laboratory results between severe SARS-CoV-2 patients groups.
| All patients (n = 66) | Normal range | Survivors (n = 23) | Non-Survivors (n = 43) | P | Non Mechanical Ventilation (n = 26) | Mechanical Ventilation (n = 40) | P | |
|---|---|---|---|---|---|---|---|---|
| Blood routine | ||||||||
| Mean (range) Hemoglobin (g/dl) | 12.03 (6–19) | 11.5–16 | 12.21 (6–19) | 11.94 (6.4–19) | 0.3155 | 12.35 (10.25–13.75) | 12.07 (6–19) | 0.4348 |
| Mean (range) Red blood cell count (x106/µl) | 4.405 (1.96–6.38) | 4.5–5.6 | 4.38 (2.65–6.38) | 4.27 (1.96–6.38) | 0.4278 | 4.31 (1.96–6.38) | 4.31 (3.69–4.75) | 0.4991 |
| Mean (range) Platelet (x103/µl) | 262.6 (7–621) | 150–450 | 266.9 (23–344) | 260.2 (7–621) | 0.2573 | 243.8 (7–430) | 275.1 (23–621) | 0.2984 |
| Median (IQR) White blood cell count (x103/µl) |
11.5 (9.25–14.5) | 4.5–10 | 10.1 (8.5–13.8) | 11.7 (9.3–14.6) | 0.2235 | 10.2 (8.37–14.1) | 12 (9.32–14.45) | 0.1254 |
| Median (IQR) Lymphocyte count (x103/µl) | 0.7 (0.54–0.93) | 1.125–4 | 0.8 (0.5–1) | 0.7 (0.59–0.9) | 0.353 | 0.7 (0.5–0.94) | 0.7 (0.59–0.94) | 0.2684 |
| Median (IQR) Monocyte count (x103/µl) | 0.42 (0.2–0.65) | 0.09–0.8 | 0.5 (0.2–0.7) | 0.4 (0.2–0.58) | 0.309 | 0.35 (0.2–0.6) | 0.44 (0.3–0.7) | 0.071 |
| Median (IQR) Neutrophil count (x103/µl) | 9.22 (7.82–12.6) | 2.025–7 | 8.8 (6.5–12.6) | 10.18 (8.1–12.85) | 0.086 | 8.7 (6.45–12.77) | 10.09 (8.13–12.73) | 0.2647 |
| Mean (range) Eosinophil count (x103/µl) | 0.0147 (0–0.25) | <0.3 | 0.015 (0–0.18) | 0.0146 (0–0.25) | 0.4523 | 0.01 (0–0.18) | 0.017 (0–0.25) | 0.1211 |
| Mean (range) Basophil count (x103/µl) | 0.0187 (0–0.2) | <0.115 | 0.014 (0–0.1) | 0.02 (0–0.2) | 0.3745 | 0.018 (0–0.2) | 0.018 (0–0.14) | 0.4987 |
| Median (IQR) Neutrophil to lymphocyte ratio (NLR) |
13.61 (9.19–20.56) | 12.86 (7.2–18.11) | 15 (11.51–21.9) | 0.0685 | 15 (9.59–21.6) | 13.6 (8.98–21.6) | 0.4721 | |
| Median (IQR) Lymphocyte to monocyte ratio (LMR) |
1.68 (1.08–2.61) | 1.66 (1.15–2.55) | 1.71 (1.04–3) | 0.3962 | 1.54 (1.07–2.86) | 1.8 (1.11–2.53) | 0.2962 | |
| Coagulation function | ||||||||
| Median (IQR) Troponin (ng/l) | 7.5 (1.5–38) | <1.5 | 6 (1.5–17) | 8 (2–87) | 0.026 | 12.5 (2–22.5) | 5 (1.5–84) | 0.059 |
| Median (IQR) NT-proBNB (pg/ml) | 0.88 (0.05–255) | <300 | 1.25 (0.17–234) | 0.85 (0.13–290.8) | 0.4324 | 1.25 (0.15–382.5) | 0.85 (0.14–215.8) | 0.2724 |
| Mean (range) Fibrinogen (g/l) | 5.92 (1.5–10) | 2–4 | 5.99 (2.7–9.85) | 5.89 (1.5–10) | 0.4781 | 5.47 (1.5–9.65) | 6.23 (2.1–10) | 0.075 |
| Median (IQR) PT (%) | 80 (70–97) | >70 | 86 (69–93) | 80 (70.5–97) | 0.413 | 89.5 (73.5–98) | 79 (67–97) | 0.0807 |
| Median (IQR) INR | 1.13 (1.05–1.2) | 1–1.3 | 1.13 (1.05–1.18) | 1.14 (1.04–1.21) | 0.4881 | 1.08 (1.04–1.18) | 1.14 (1.05–1.23) | 0.1808 |
| Median (IQR) D-dimer (ng/ml) |
2203 (918–3963) | < 500 | 1693 (590–3100) | 2307 (1279–5426) | 0.0257 | 1819 (660–3164) | 2300 (1133–5730) | 0.0198 |
| Infection-related biomarkers | ||||||||
| Median (IQR) Procalcitonin (ng/ml) | 0.29 (0.17–1.28) | <0.05 | 0.16 (0.05–0.59) | 0.33 (0.19–3.69) | 0.0148 | 0.21 (0.07–0.7) | 0.3 (0.17–3.09) | 0.1465 |
| Mean (range) Ferritin (ng/ml) | 1904 (72–13235) | 30–280 | 564.2 (93–934) | 2440 (72–13235) | 0.04 | 1312 (93–7086) | 2348 (72–13235) | <0.0001 |
| Mean (range) C-reactive protein (mg/l) (CRP) | 148.6 (10–441) | <6 | 136.9 (15–320) | 154.9 (10–441) | 0.055 | 146.3 (15–329) | 150.2 (10–441) | 0.4828 |
| Blood biochemistry | ||||||||
| Mean (range) Hematocrit (%) | 36.64 (18.3–57.1) | 36.96 (20–57.1) | 36.47 (18.3–57.1) | 0.4169 | 36.28 (18.3–57.1) | 36.89 (26.5–57.1) | 0.3645 | |
| Mean (range) MCV(fl) | 85 (71.8–103.7) | 84.01 (72–95.2) | 85.56 (71.8–103.7) | 0.3246 | 84.05 (72–95.2) | 85.65 (71.8–103.7) | 0.165 | |
| Mean (range) MCH(pg) | 28.13 (20.4–34.3) | 27.75 (21–31.9) | 28.30 (20.4–34.3) | 0.4534 | 28.24 (21–32.7) | 28.04 (20.4–34.3) | 0.5748 | |
| Mean (range) MCHC (g/dl) | 32.84 (24.4–36.3) | 32.83 (27–36.3) | 32.85 (24.4–35.9) | 0.4798 | 33.05 (27–36.3) | 32.67 (24.4–35.9) | 0.5053 | |
| Mean (range) Reticulocyte (x109/l) | 52.06 (21.8–106) | 54.31 (21.8–106) | 51.18 (23.7–80) | 0.4565 | 47.1 (21.8–86.9) | 54.54 (23.7–106) | 0.1992 | |
Data are presented as mean (range) or median (Interquartile range (IQR)). p values comparing survivors and non-survivors or comparing non-mechanical ventilation and mechanical ventilation severe COVID-19 patients are from Unpaired t-test or the non-parametric Mann-Whitney U test.
Compared with the normal range, the blood counts of patients on admission showed increased whole blood count (WBC) in most of the severe cases. WBC was above the upper limit of normal in deceased and recovered patients (>10x103/µl) (Table 2). Lymphopenia (lymphocyte count < 1.125x103/µl) was frequent in both survivors and deceased patients without significant differences between groups (P = 0.353). Increased neutrophil counts above the upper limit of normal (>7x103/µl) was observed in both deceased and recovered patients without significant differences between groups (P = 0.086) (Table 2).
The laboratory parameters of enrolled individuals who required intubation (n = 40) and those did not required intubation (n = 26) was also listed in Table 2. No significant difference of Troponin, NT-proBNP and Fibrinogen were found among the two groups (P > 0.05). Concentrations of serum C-reactive protein (CRP) also showed no significant difference among two COVID-19 groups (Table 2). Levels of Ferritin (P < 0.0001) and D-dimer levels (P = 0.0198) were markedly higher in patients who required mechanical ventilation than those did not required mechanical ventilation.
Amongst the patients admitted in the ICU, there were no significant differences in mean hemoglobin (P = 0.4348), total leukocyte count (P = 0.1254), absolute neutrophil and lymphocyte counts (P > 0.05), absolute monocyte count (P = 0.071), NLR and LMR (P > 0.05), as shown in Table 2. The requirement of mechanical ventilation was independent of lymphopenia, increased WBC and neutrophil count (Table 2).
3.3. IL-6 versus IL-10 and their associations with CRP and circulatory immune cells in severe COVID-19 patients
Possible associations of relevant cytokines (IL-6 and IL-10) with CRP and circulatory immune cells were evaluated among severe COVID-19 patients (n = 66). To examine the relationship between serum IL-6 and CRP, we performed Spearman rank correlation analysis, and the results showed that CRP was significantly positively correlated with IL-6 (r = 0.237, P = 0.0052) (Fig. 1 A). We further examined the relationship between serum IL-10 and CRP by performing Spearman rank correlation analysis, and the results showed no significant difference correlation between CRP and IL-10 (r = 0.019, P = 0.4383) (Fig. 1B).
Fig. 1.
Correlation analysis between IL-6 and CRP (A), IL-10 and CRP (B), IL-6 and neutrophils (C), IL-10 and neutrophils (D), IL-6 and lymphocytes (E), IL-10 and lymphocytes (F), IL-6 and IL-10 (G) quantified in the sera of severe SARS-CoV-2 patients (n = 66). p < 0.05; r = Spearman coefficient.
We next examined the correlation between serum IL-6 level and immune cells in peripheral blood of severe COVID-19 patients. As shown in Fig. 1C, Positive correlation with IL-6 was found for neutrophil count (r 0.168, P = 0.0383). Analysis of the correlation between serum IL-10 level and immune cells in peripheral blood of severe COVID-19 patients demonstrated a positive correlation between IL-10 and total lymphocytes (r = 0.227, P = 0.0334) (Fig. 1F).
Interestingly, a positive correlation was demonstrated between serum IL-6 and IL-10 among severe COVID-19 patients admitted to the ICU (r = 0.315, P = 0.0049) (Figure 1G). To clarify the importance of this correlation in COVID-19 disease, we examined the ratio of IL-6/IL-10 in associations with CRP, D-dimer and circulatory immune cells. As shown in Figure 2A, a positive correlation was demonstrated between IL-6/IL-10 ratio and CRP in severe patients (r = 0.225, P = 0.0404). Nevertheless, no significant correlation was seen with IL-6/IL-10 ratio for D-dimer (r = 0.117, P = 0.1858) (Fig. 2 B). Importantly, a positive correlation with IL-6/IL-10 ratio was demonstrated for neutrophils count (r = 0.32, P = 0.0067) and white blood cells (WBC) count (r = 0.328, P = 0.0049) among all severe COVID-19 patients (Fig. 2C, 2E).
Fig. 2.
Correlation analysis between IL-6/IL-10 ratio and CRP (A), IL-6/IL-10 ratio and D-dimer (B), IL-6/IL-10 ratio and neutrophils (C), IL-6/IL-10 ratio and lymphocytes (D), IL-6/IL-10 ratio and WBC (White Blood Cells) (E), quantified in the sera of severe SARS-CoV-2 patients (n = 66). p < 0.05; r = Spearman coefficient.
3.4. IL-6 versus IL-10 among recovered and deceased patients
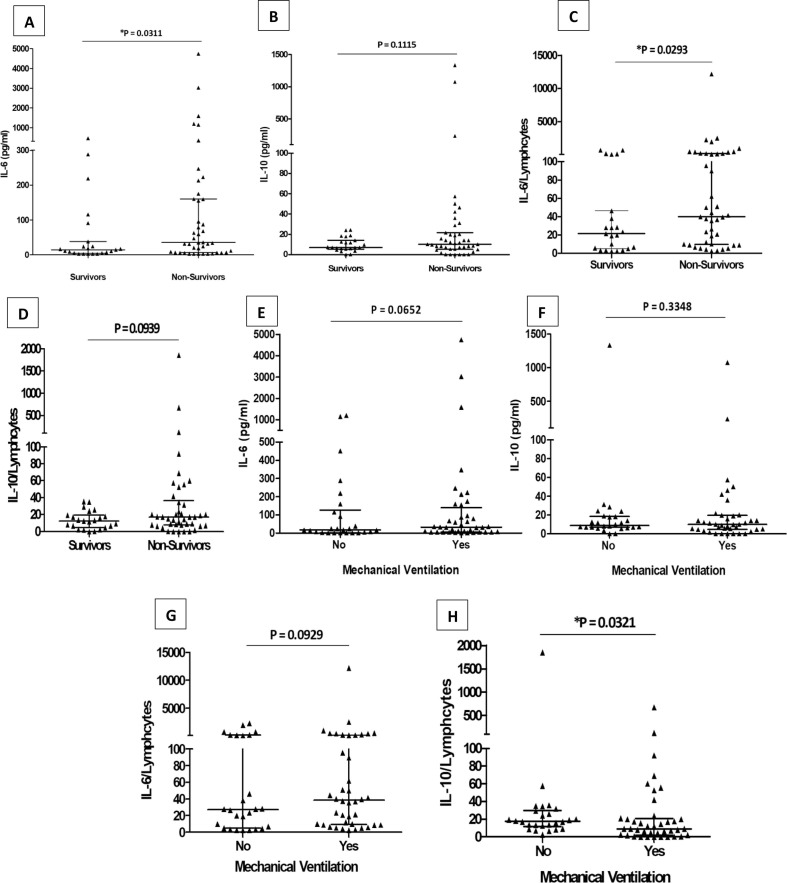
We first compared the levels of IL-6 and IL-10 in serum samples from survivors (n = 23) and deceased (n = 43) COVID-19 patients. Evaluation of serum cytokines on admission revealed that level of IL-6 was markedly higher in deceased than in survivor’s cases (P = 0.0311). The median concentration of IL-6 in the survivor’s group was 14.2 pg/ml (IQR 4.29–38.2 pg/ml), while that in the deceased group was 35 pg/ml (IQR 6.25–160 pg/ml) (Fig. 3 A). Although deceased patients had higher serum IL-10 level, no difference was reported with respect to the recovered group (P = 0.1115). The median serum IL-10 concentration in the survivor’s group was 6.86 pg/ml (IQR 5.43–14 pg/ml), while that in the deceased group was 10.19 pg/ml (IQR 5.43–21.62 pg/ml) (Fig. 3B).
Fig. 3.
Quantification of IL-6 (A) and IL-10 (B) IL-6/Lymphocytes ratio (C) and IL-10/Lymphocytes ratio (D) were compared dividing samples in survivors (n = 23) and non-survivors (n = 43). Based on respiratory failure, quantification of IL-6 (E) and IL-10 (F) IL-6/Lymphocytes ratio (G) and IL-10/Lymphocytes ratio (H) were compared. No Mechanical Ventilation (n = 26), Mechanical Ventilation (n = 40). Differences were analyzed with the Mann-Whitney U test at p < 0.05.
We next assessed the ratios of IL-6/lymphocytes and IL-10/lymphocytes among survivors (n = 23) and deceased (43) COVID-19 patients. As shown in Fig. 3E, the IL-6/lymphocyte ratio was markedly higher in the deceased group compared to those who recovered (P = 0.0293). The median IL-6/lymphocyte ratio in the survivors group was 21.4 (IQR 5–46.4), while that in the deceased group was 40 (IQR 10–223.9) (Fig. 3C). No significant difference has been observed for IL-10/lymphocyte ratio between recovered and deceased patients (P = 0.0939) (Fig. 3D).
3.5. IL-6 versus IL-10 among patients requiring or not mechanical ventilation
Severe COVID-19 patients were classified as patients requiring mechanical ventilation (n = 40) and those who did not require mechanical ventilation (n = 26) (Table 1, Fig. 3). Analysis of serum IL-6 levels according to respiratory failure demonstrated elevated IL-6 with the need for mechanical ventilation. Nevertheless, no significant difference was seen for IL-6 serum levels between the two groups (P = 0.0652) (Fig. 3E). We further examined IL-10 serum levels according to respiratory failure. As shown in Fig. 3F, IL-10 serum levels decreased between the two groups with no significant difference (P = 0.3348). Next, we explored the ratios of IL-6/lymphocytes and IL-10/lymphocytes among patients requiring or not requiring mechanical ventilation. As shown in Fig. 3G, no difference has been observed in the ratio of IL- 6/lymphocytes between the two groups (P = 0.0929). Interestingly, the ratio of IL-10/lymphocytes decreased significantly between groups of patients who did not require mechanical ventilation and those requiring mechanical ventilation (P = 0.0321) (Fig. 3H).
3.6. Longitudinal changes of IL-6 in COVID-19 patients with mortality and respiratory failure
To further evaluate the longitudinal change of IL-6 with respect to mortality, serum IL-6 levels were measured among recovered (n = 23) and deceased patients (n = 43) during five days of hospitalization from the day of their admission to the ICU. Serum concentrations of IL-6 during hospitalization were significantly higher in the deceased patients than in those who recovered (Fig. 4 A). More importantly, IL-6 concentration remained high without decrease in deceased patients during hospitalization, whereas the sustained level of this pro-inflammatory cytokine was at a median less than 21 pg/ml in the survivors group during hospitalization (Fig. 4A). The deceased group showed markedly increased IL-6 levels on hospital day 1, 4 and 5 as compared to recovered cases (Fig. 4A).
Fig. 4.
Dynamic profiles of IL-6 serum value were compared dividing samples in survivors (n = 23) and non-survivors (n = 43) (A) or non-mechanical (n = 26) and mechanical ventilation (n = 40) (B) during 5 days after hospitalization. Data are expressed as median and interquartile range (IQR). *P < 0.05 indicates difference between survivor’s patients versus non-survivors patients, # P < 0.05 indicates difference between patients did not requiring mechanical ventilation versus patients requiring mechanical ventilation. The Mann-Whitney U test was used to compare groups at p < 0.05.
Next, we examined the longitudinal change of IL-6 with respect to respiratory failure. Serum IL-6 levels were measured among patients who did not require mechanical ventilation (n = 26) and those requiring intubation (n = 40) during five days of hospitalization after their admission in ICU. The level of the pro-inflammatory cytokine IL-6 was gradually decreased from day 1 to day 3 in patients with respiratory failure. However, IL-6 concentration was significantly increased at day 4 of hospitalization in patients requiring mechanical ventilation. More importantly, IL-6 concentration remained high without decrease in patients requiring mechanical ventilation after five days of hospitalization (Fig. 4B). As described in Fig. 4B, patients who did not require intubation showed unchanged IL-6 levels that remained at medium level (median less than 22 pg/ml) during 5 days of hospitalization.
3.7. Potential immunologic markers to predict mortality and respiratory failure in ICU
ROC curve and area under ROC curve (AUC) were generated to evaluate the potential use of age and immunologic markers as prognosis tools to predict mortality and respiratory failure in patients admitted in ICU. As shown in Fig. 5 A and Table 3 , age, IL-6 levels, IL-6/lymphocyte ratio and CRP/IL-6 ratio have the highest prognosis efficiency to predict mortality in ICU.
Fig. 5.
Area under the receiving operating curves of biomarkers as prognosticators of mortality (A) and mechanical ventilation (B) in ICU. Legend: CRP = C-reactive protein.
Table 3.
Predictive power of biomarkers as prognosticator of mortality in ICU.
| Variable | AUC | 95% CI | Criterion associated with best sensitivity and specificity at Youden index | Sensitivity | Specificity | Significance level P (Area = 0.5) |
|---|---|---|---|---|---|---|
| Age (years) | 0.644 | 0.550 to 0.730 | >63 | 59,76% | 66,67% | 0.0112 |
| D-dimer (ng/ml) | 0.607 | 0.511 to 0.697 | >1132 | 77.5% | 47.06% | 0.0711 |
| IL-6 (pg/ml) | 0.694 | 0.602 to 0.775 | >17.7 | 73.17% | 62.86% | 0.0003 |
| IL-10 (pg/ml) | 0.592 | 0.464 to 0.711 | >24.28 | 23.26% | 100% | 0.1910 |
| IL-6/Lymphocytes | 0.695 | 0.602 to 0.778 | >28.4 | 66.67% | 66.67% | 0.0005 |
| IL-10/Lymphocytes | 0.628 | 0.495 to 0.749 | >35.62 | 25.64% | 100% | 0.0773 |
| CRP/IL-6 | 0.663 | 0.569 to 0.748 | ≤8.411 | 75.61% | 54.55% | 0.0023 |
| CRP/IL-10 | 0.548 | 0.416 to 0.676 | ≤24.239 | 76.92% | 36.36% | 0.5310 |
| IIL-6/IL-10 | 0.551 | 0.419 to 0.679 | >2.52 | 51.28% | 72.73% | 0.5063 |
Multivariate regression analysis showed that age, hypertension, IL-6 levels, IL-6/lymphocyte ratio and CRP/IL-6 ratio were positively correlated with the risk of mortality. Interestingly, hypertension (OR: 2.8574, P = 0.007), IL-6 > 17.7 pg/ml (OR: 3.1, P = 0.0002) and CRP/IL-6 ratio ≤ 8.411 (OR: 2.9048, P = 0.0003) are considered independent prognostic factors for COVID-19 mortality (Table 4 ).
Table 4.
Logistic regression multivariate analysis for the prognosis of mortality in ICU.
| Variable | Odds Ratio | 95% confidence interval | P |
|---|---|---|---|
| Age (>63 years) | 1.0239 | 0.9902 to 1.0239 | 0.038 |
| Comorbidities | |||
| Hypertension | 2.8574 | 1.1209 to 7.2845 | 0.007 |
| Diabetes | 1.0493 | 0.4078 to 2. 6996 | 0.2424 |
| Asthma | 1.2893 | 0.1964 to 8.4636 | 0.9087 |
| Sex (male) | 1.2940 | 0.5055 to 3.3127 | 0.9315 |
| IL-6 (>17.7 pg/ml) | 3.1 | 1.7189 to 5.5907 | 0.0002 |
| IL-6/Lymphocytes (>28.4) | 2.037 | 1.1717 to 3.5414 | 0.0117 |
| CRP/IL-6 (≤8.411) | 2.9048 | 1.6219 to 5.2023 | 0.0003 |
As shown in Fig. 5B and Table 5 , IL-10/lymphocyte ratio and D-Dimer have the highest prognosis efficiency to predict respiratory failure in ICU.
Table 5.
Predictive power of biomarkers as prognosticator of mechanical ventilation in ICU.
| Variable | AUC | 95% CI | Criterion associated with best sensitivity and specificity at Youden index | Sensitivity | Specificity | Significance level P (Area = 0.5) |
|---|---|---|---|---|---|---|
| Age (years) | 0.556 | 0.449 to 0.663 | >61 | 66.15% | 49.06% | 0.3087 |
| D-dimer (ng/ml) | 0.612 | 0.517 to 0.702 | >1132 | 79.37% | 41.18% | 0.0355 |
| IL-6 (pg/ml) | 0.582 | 0.487 to 0.672 | >24 | 64.62% | 55.77% | 0.1277 |
| IL-10 (pg/ml) | 0.532 | 0.405 to 0.656 | ≤5.43 | 35% | 84.62% | 0.6592 |
| IL-6/Lymphocytes | 0.564 | 0.468 to 0.656 | >32.43 | 64.06% | 54% | 0.2488 |
| IL-10/Lymphocytes | 0.705 | 0.599 to 0.796 | ≤2.033 | 50% | 92.31% | 0.0002 |
| CRP/IL-6 | 0.561 | 0.466 to 0.654 | ≤6.88 | 69.23% | 50% | 0.2591 |
| CRP/IL-10 | 0.540 | 0.408 to 0.668 | >8.8138 | 66.67% | 60% | 0.6021 |
| IL-6/IL-10 | 0.588 | 0.454 to 0.712 | >0.272 | 94.44% | 28% | 0.2643 |
Multivariate regression analysis showed that hypertension, IL-10/lymphocytes ratio and D-Dimer were associated with respiratory failure. In addition, hypertension (OR: 2.4706, P = 0.0189) and D-Dimer (OR: 4, P = 0.0001) were considered as independent prognostic factors for respiratory failure (Table 6 ).
Table 6.
Logistic regression multivariate analysis for the prognosis of mechanical ventilation in ICU.
| Variable | Odds Ratio | 95% confidence interval | P |
|---|---|---|---|
| Age (>61 years) | 1.0189 | 0.9906 to 1.0479 | 0.1932 |
| Comorbidities | |||
| Hypertension | 2.4706 | 1.1608 to 5.2581 | 0.0189 |
| Diabetes | 1.1930 | 0.5639 to 2.5237 | 0. 6444 |
| Asthma | 1.0929 | 0.2336 to 5.1131 | 0.9102 |
| Sex (male) | 0.8623 | 0.3997 to 1.8603 | 0.7057 |
| D-dimer (>1132 ng/ml) | 4 | 1.9898 to 8.0411 | 0.0001 |
| IL-10/Lymphocytes (≤2.033) | 1.1667 | 0.6424 to 2.1187 | 0.6126 |
3.8. Co-expression of IL-6 and IL-10 among groups of COVID-19 patients
Based on Youden’s index, the optimal cutoff value was 17.7 pg/ml for IL-6 and 24.28 pg/ml for IL-10 (Table 3). In the light of these data, we further classified patients depending to high and low levels of each cytokine as fellow: IL-6 High: ≥17.7 pg/ml; IL-6 Low: IL-6 < 17.7 pg/ml; IL-10 High: ≥ 24.28 pg/ml and IL-10 Low: <24.28 pg/ml. In order to investigate the interplay between IL-6 and IL-10 in severe COVID-19 patients, the study assigned patients into four groups: Group I: (IL-6 High/ IL-10 High), n = 25; Group II: (IL-6 High/ IL-10 Low), n = 19; Group III: (IL-6 Low/ IL-10 High), n = 6 and Group IV: (IL-6 Low/ IL-10 Low), n = 16 (Fig. 6 ).
Fig. 6.
IL-6 and IL-10 subsets depending to clinical outcomes of COVID-19 patients. Kaplan-Meier survival curves (A) and Kaplan-Meier analysis graphing the percentage of patients receiving mechanical ventilation (B) in four groups of COVID-19 patients. Group I: (IL-6 High/ IL-10 High), n = 25. Group II: (IL-6 High/ IL-10 Low), n = 19. Group III: (IL-6 Low/ IL-10 High), n = 6. Group IV: (IL-6 Low/ IL-10 Low), n = 16. Log-rank test shows significant difference in survival curve among the four IL-6/IL-10 groups. Survivors (n = 23); Non-survivors (n = 43). Non-mechanical (n = 26); Mechanical ventilation (n = 40). Time = hospitalization once admission to death or discharged. IL-6 High: ≥17.7 pg/ml; IL-6 Low: IL-6 < 17.7 pg/ml; IL-10 High: ≥ 24.28 pg/ml and IL-10 Low: <24.28 pg/ml.
We first examined the co-expression of IL-6 and IL-10 depending to the mortality of COVID-19 patients with Kaplan-Meier survival curves analysis (Fig. 6A). The Kaplan-Meier survival curves revealed that compared to the patients from the other groups, group I had worse survival rates (P = 0.018). In line with these findings, the study found the survival rates of groups I to IV were, respectively: 24%, 42.1%, 50%, and 37.5% (Fig. 6A).
We further explored the co-expression of IL-6 and IL-10 depending to the respiratory failure COVID-19 patients with Kaplan-Meier curves (Fig. 6B). A Kaplan-Meier analysis graphing the percentage of patients requiring mechanical ventilation showed that the probability of respiratory failure depends of IL-6/IL-10 profile (P = 0.021) (Fig. 6B). Percentage of patients with respiratory failure was greater than those not requiring mechanical ventilation in Group I and Group II. All patients in Group III had received mechanical ventilation (6/6 (100%)). For the last Group with (IL-6 Low/ IL-10 Low) profile, the percentage of patients not requiring mechanical ventilation was greater than those receiving invasive mechanical ventilation (10/16 (62.5%) and 6/16 (37.5%), respectively) (Fig. 6B).
4. Discussion
The “cytokine storm” with aberrant inflammatory cytokines IL-6 and IL-10 is a hallmark of severe SARS-CoV-2 infection (Fajgenbaum, 2020, Tang et al., 2020). Most therapeutic strategies against advanced COVID-19 disease target the overactive cytokine response with anticytokine therapies or immunomodulators, but this must be balanced with maintaining an adequate inflammatory response for pathogen clearance (Tang et al.,2021). Understanding the interplay between IL-6 and IL-10 which reflects the balance between pro- and anti-inflammatory cytokines is of crucial importance to identify patients under hyperinflammatory state allowing a better stratification of patients and will help to rationally determine the best treatment options for each patient. This study presents cytokine profiling in a cohort of 66 COVID-19 patients requiring ICU care with a particular emphasis on pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10.
In line with previous reports, our data showed that the majority of patients, especially those who developed a severe disease with a fatal outcome, exhibited a significantly increased concentration of IL-6 (Santa Cruz et al., 2021). Gorham and colleagues in a retrospective study evaluated IL-6 concentrations between survivors and non-survivors in patients admitted to intensive care unit (ICU). The authors noticed a big gap in IL-6 levels between the two groups, represented by considerably higher level in non-survivors compared with survivors. They suggested repeated evaluation of IL-6 as a marker of poor prognosis in critically ill patients with SARS CoV-2 induced disease (Gorham et al., 2020). Varchetta and colleagues also noticed that higher level of serum IL-6 in deceased versus survivors from SARS CoV-2 induced disease. The pro-inflammatory activity of this cytokine is linked to the pneumonia (Varchetta et al.,2021). IL-6 has multiple effects in regulating inflammation. Cifaldi et al. (2015) has reported that elevated IL-6 level was linked to impaired cytolytic function by overstimulating the immune system and finally might result in multiple organ failure.
Yang et al., (2021) suggested that IL-6/lymphocyte ratio could serve as an indicator of poor prognosis of COVID-19. The dynamic changes of IL-6/lymphocyte ratio in patients showed that the magnitude of the immune response dysregulation was related to the severity of COVID-19 patients. In line with these findings, our data revealed that IL-6/lymphocyte ratio positively correlated with the risk of mortality among severe COVID-19 patients. Indeed, ROC curve analyses showed that IL-6/lymphocyte ratio, IL-6 levels and CRP/IL-6 ratio have the highest prognosis efficiency to predict mortality in ICU. Previous studies reported that depending on the value of IL-6/lymphocyte ratio the physicians could identify the specific subpopulations of COVID-19 patients who were at greater risk for unfavorable outcomes at an early stage (Yang et al., 2021). Elevated IL-6/lymphocyte ratio in deceased compared to recovered patients was resulted from the increased IL-6 and the decreased lymphocyte counts. Recent studies’ results provide some explanations. Despite uncertainty over the exact pathophysiological, much evidence indicates that high levels of IL-6 and low lymphocyte count are biomarkers of cytokine storm and immune response in COVID-19 patients (Santa Cruz et al., 2021, Chen et al., 2020a, Chen et al., 2020b). As shown in another study, IL-6 could suppress the T cell activation, which may explain the decrease of lymphocytes (Belaid et al., 2022). Through analyzing the immune cells and inflammatory cytokines in the severe COVID-19 patients, Zhou et al. (2020) noted that the Th1 cells (GMCSF+IFN-γ+) and inflammatory monocytes (CD14+CD16+ with high expression of IL-6) existed particularly in ICU patients. Therefore, lots of these pathogenic T cells and inflammatory monocytes may get sequestrated into the pulmonary circulation and arouse inflammatory storm which probably prevents alveolar gas exchange and contributes to the high mortality of severe COVID-19 patients and is consistent with the relative lymphopenia.
Moreover, potential mortality risk factors for COVID-19 were analyzed by multivariable logistic regression analysis. The results indicated that age, hypertension, IL-6 levels, IL-6/lymphocyte ratio and CRP/IL-6 ratio were positively correlated with the risk of mortality. Among the aforementioned parameters, hypertension, IL-6 > 17.7 pg/ml and CRP/IL-6 ratio ≤ 8.411 seem to better predict fatal outcome than IL-6/lymphocyte ratio. Furthermore, IL-6 levels, IL-6/lymphocyte ratio and CRP/IL-6 ratio could not predict respiratory failure. Indeed, no significant difference was shown for these biomarkers between patients with respiratory failure and those not requiring mechanical ventilation. Our findings were in discordance with another study which reported that IL-6/lymphocytes ratio has the best predictive power and that between the analyzed biomarkers, it was the only independent risk factor for mortality and respiratory failure (Yanget al., 2021). Furthermore, our study identified hypertension and D-Dimer > 1132 ng/ml as two reliable prognosis indicators that accurately stratify patients into risk categories and predict COVID-19 respiratory failure. Consistent with the current findings, it has been reported that elderly COVID-19 patients had higher mortality than young patients. Higher mortality and respiratory failure were attributed to a higher rate of chronic comorbidities among elderly patients, such as hypertension which was considered as an independent risk factor of severity and mortality in COVID-19 adult patients (Albitar et al., 2020, Chen et al., 2021a). Early descriptions of COVID-19 demonstrated that increased concentration of D-dimer was associated with worse clinical outcome. In mechanically ventilated patients with COVID-19-ARDS, D-dimer value higher than 1880 ng/ml increased risk of death in ICU (Tonetti et al., 2021).
The information about correlation of longitudinal changes of inflammatory parameters with disease severity in COVID-19 patients is scarce. In this study, the longitudinal assays showed sustained levels of the pro-inflammatory cytokine IL-6 at medium concentration within 5 days post admission in severe cases requiring mechanical ventilation who survived or not, whereas it was sustained at high levels throughout the disease course in either deceased cases and those who developed respiratory failure. Consistent with those findings, Zeng et al. (2020) reported that IL-6 was elevated with illness deterioration and was markedly higher on admission, suggesting that vigilant monitoring and early intervention aiming to control overactive inflammation may be useful to prevent the further deterioration of COVID-19 (Zeng et al., 2021). IL-6 is widely recognized as an important potent initiator of inflammatory responses. IL-6 may promote inflammation by recruiting immune cells to the lung, which may directly contribute to the pathogenesis of ARDS (McGonagle et al.,2020). Since deceased patients more often developed systematic inflammation than did recovered patients, it seems that IL-6 had a crucial role of exuberant inflammatory responses in SARS-CoV-2 infection pathogenesis. A surge in the secretion of this cytokine can promote a state of hyperinflammation that is harmful for host cells (Huang et al., 2021).
Considering the central role of the pro-inflammatory and anti-inflammatory balance during the course of SARS-CoV-2 infection (Han et al., 2020), here the study analyzes the profile of IL-6/IL-10 according to the mortality and respiratory failure. Based on Youden’s index, the optimal cutoff value was 17.7 pg/ml for IL-6 and 24.28 pg/ml for IL-10. Therefore, we identified four (IL-6, IL-10) profiles: (IL-6 High, IL-10 High), (IL-6 High,IL-10 Low), (IL-6 Low,IL-10 High) and (IL-6 Low,IL-10 Low). A Kaplan-Meier analysis graphing the percentage of patients requiring mechanical ventilation showed that the probability of respiratory failure depends of IL-6/IL-10 profile. In (IL-6 High, IL-10 High) and (IL-6 High,IL-10 Low) profiles, more patients with respiratory failure were observed than among those not requiring mechanical ventilation. Nevertheless, all patients with (IL-6 Low, IL-10 High) profile required mechanical ventilation. In addition, in (IL-6 Low, IL-10 Low) profile, patients not requiring mechanical ventilation were more observed than those with respiratory failure.
Interestingly, our study revealed that most deceased patients have excessive IL-6 levels concomitant with high levels of IL-10. In addition, COVID-19 patients with the aforementioned profile (IL-6 High, IL-10 High) had worse survival rates compared to COVID-19 patients with other IL-6/IL-10 profiles. Similar results were documented in other studies which showed that aberrant inflammatory cytokines IL-6 and IL-10 associated with cytokine storm are hallmarks of severe SARS-CoV-2 infection resulting in progress with a disease course requiring ICU care (Tang et al., 2021, Belaid et al., 2022). Similar to our results, Luo et al. (2020) detected the IL-6 and IL-10 levels above reference ranges in the serum of COVID-19 patients in ICU. Following the association with severity, Jiménez-Gastélum et al. (2021) found an association of increased levels of the IL-6 and the IL-10 with mechanical ventilation and death. However, Herold et al. (2021) previously reported that IL-6 has been linked to the necessity of mechanical ventilation but IL-10 has not. Plasma and/or bronchoalveolar levels of IL-6 and IL-10 have been identified as early biomarkers of lung injury and predictive factors of prolonged mechanical ventilation, organ dysfunctions, morbidity and mortality in lung diseases. In addition, it was reported that in SARS-COV-2 infection, IL-6 and IL-10 were highly abundant, especially during the adaptive immune response (Han et al., 2020). In this respect, Diao et al. (2020) reported that the high levels of IL-6 and IL-10, observed in patients with severe COVID-19, correlate with a decrease in numbers and function of T helper (Th) 1 and T CD8+ cells promoting an impaired adaptive response against SARSCoV-2 infection This finding was recently corroborated by the research of Gil-Etayo et al. (2021) in which it is also reported that the IL-6 and IL-10 could relate to an overactivation of the Th2 cell subset leading to disease worsening or even death.
In line with previous studies, IL-6 along with other cytokines such as IL-10 is produced in significant quantities during inflammatory episodes, including SARS-CoV-2 infection (Notz et al., 2020, Chen et al., 2020a, Chen et al., 2020b, Belaid et al., 2022). The increased levels of IL-10 in the plasma of COVID-19 patients probably reflect a host response to prevent the harmful effect of the cytokine storm (Chen et al., 2020a, Chen et al., 2020b, Belaid et al., 2022). However, the induction of IL-10 expression to dampen the overexaggerated inflammation seems to be inefficient as the fatal outcome is associated with the highest levels of IL-6 and IL-10. Similar to that in MERS-CoV infection, in SARS-CoV-2, IL-10 level was continuously elevated in critically ill patients and deceased cases with COVID- 19, while IL-10 concentration transiently increased during hospitalization in severe cases and survivors and then fell to lowest level before discharge. Therefore, the transient increase of IL-10 level may reflect a compensatory anti-inflammatory or counter-regulatory reaction in response to a heightened level of pro-inflammatory cytokines, and sustained elevation of IL-10 is probably correlated with the poor prognosis (Zeng et al., 2020). Several studies have confirmed the biological involvement and immuno-regulatory role of IL-10 in patients with sepsis, where overproduction of IL-10 was shown to be a predictor of severity and poor outcome (Matsumoto et al., 2018, Li et al., 2015). However, unregulated increase of IL-10 might induce immunosuppression which may negatively impact the outcomes (Kumar et al., 2019). Consistent with these findings, we suggested an immunosuppressive role of IL-10 in most of deceased COVID-19 patients.
Although IL-6 is an available and attractive therapy targets, blocking IL-6 may not be beneficial in most of severe COVID-19 patients. Inhibitors of pro-inflammatory cytokine IL-6 used for controlling hyperinflammation may adversely cause immunosuppression. Such immunosuppression may negatively impact the outcomes (Colaneri et al., 2020, Kimmig et al., 2020). Based on these findings, we suggested that targeting IL-6 in severe patients with (IL-6 High, IL-10 High) profile may contribute to accentuation of immunosuppression environment generated by overproduction of IL-10 in those patients. Interestingly, deceased patients have different magnitudes of both IL-6 and IL-10 cytokine release. Indeed, those patients may have excessive IL-6 combined with low IL-10 or low IL-6 combined with high IL-10 or low IL-6 and low IL-10. Therefore, anticytokine therapies or immunomodulators used to control hyperinflammation must take into account IL-6/IL-10 profiling to maintain an adequate inflammatory response for SARS-CoV-2 clearance.
Based on IL-6/IL-10 profiling, physicians could identify patients who are in hyperinflammatory status from those in immunosuppressive status before starting anti-inflammation therapy. Supporting this hypothesis, data from several studies have revealed that the balance between hyperinflammatory response and anti-inflammatory response play a key role in the course of the disease (Han et al., 2020, Liu et al., 2020). Depending to the IL-6/IL-10 profiling, severe COVID-19 patients will respond to blocking IL-6 alone or to both blocking of IL-6 and IL-10 or to blocking IL- 10 alone. In addition, a timely anti-inflammation treatment initiated at the right window time is of pivotal importance and should be tailored in individual patients to achieve the most favorable effects.
This study had some limitations. First, the retrospective design may cause an unavoidable and inherent selection bias in enrolling diagnosed participants. Second, our measurements were delayed due to late admission because of hospital congestion which could underestimate the level of cytokines.
5. Conclusion
Our study presents here the first description of immunological characteristics of a cohort of Tunisian patients with COVID-19 that confirms some data described in previous reports. The study also showed that the IL-6/lymphocyte ratio could be used as potential immunologic markers to predict mortality; whereas the IL-10/lymphocyte ratio may help to predict possible requirements for mechanical ventilation. Importantly, SARS-CoV-2 infection appears to elicit differential immune response. Indeed, deceased COVID-19 patients have different magnitudes of both IL-6 and IL-10 cytokine release. Interestingly our study reveals a more common cytokine profile for diseased COVID-19 patients with excessive levels of IL-6 concomitant with high levels of IL-10. Nevertheless, severe COVID is still a challenging disease, further prospective investigations of immunological profiles during the course of the disease are necessary to find the right” biological signature” reflecting the balance between immune activation and inflammatory inhibition. It will better enable effective treatment choices to target key pathogenic molecules and is an adjustable strategy for a personalized treatment.
6. Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The Authors thank Professor Darryl Macer and Professor Gharbi Mohamed for their encouragement. We thank Professor James B.Cole and Darry Macer for English check They also thank the staff of COVID unit, particularly, Mr Naceur Jazi, the technical staff of the laboratory of immunology, Ms Chama kdous and Mr Wassim Dkhil and the scientific student Jihen Ayachi for the technical support.
References
- Albitar O., Ballouze R., Ooi J.P., Sheikh Ghadzi S.M. Risk factors for mortality among COVID-19 patients. Diabetes Res. Clin. Pract. 2020;166 doi: 10.1016/j.diabres.2020.108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaid B., Lamara Mahammad L., Mihi B., Rahali S.Y., Djidjeli A., Djidjik R.T. Cell counts and IL-6 concentration in blood of North African COVID-19 patients are two independent prognostic factors for severe disease and death. J. Leukoc. Biol. 2022;111(1):269–281. doi: 10.1002/JLB.4COVA1020-703R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Liu,. Y, Qin, J., Ruan, C., Zeng,. X, Xu, A., Yang, R., Li, J., Cai, H., Zhang, Z., 2021. Hypertension as an independent risk factor for severity and mortality in patients with COVID-19: a retrospective study. Postgrad. Med. J. Oct 5:postgradmedj-140674. doi: 10.1136/postgradmedj-2021-140674. [DOI] [PubMed]
- Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur. Respir. J. 2020;56(4):2003006. doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R., Lan, Z., Ye, J., Liu, Y., Zhang, P., 2021. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 28(12),589095. doi: 10.3389/fimmu.2021.589095. PMID: 33995341; PMCID: PMC8115911. [DOI] [PMC free article] [PubMed]
- Cicchese J.M., Evans S., Hult C., Joslyn L.R., Wessler T., Kirschner D.E. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. 2018;285(1):147–167. doi: 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., Benedetti F.D., Strippoli R. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis. Rheumatol. 2015;67(11):3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo Covid19 registry (SMACORE) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;1(11):827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajgenbaum, D.C, C.H., 2020. Cytokine Storm. N. Engl. J. Med. 383(23), 2255-227. PubMed| Google Scholar. [DOI] [PMC free article] [PubMed]
- Gil-Etayo F.J., Suàrez-Fernández P., CabreraMarante O., Arroyo D., Garcinuño S., Naranjo L., et al. T-helper cell subset response is a determining factor in COVID-19 progression. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.624483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham J., Moreau A., Corazza F., Peluso L., Ponthieux F., Taccone F.S. Interleukine-6 in critically ill COVID-19 patients: a retrospective analysis. PLoS One. 2020;15(12):e0244628. doi: 10.1371/journal.pone.0244628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Ma Q., Li C., Liu R., Zhao L., Xia Y. Profiling serum cytokines in COVID- 19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes. Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Li M., Luo G., Wu X., Su B., Zhang D. The inflammatory factors associated with disease severity to predict COVID-19 progression. J. Immunol. 2021;206(7):1597–1608. doi: 10.4049/jimmunol.2001327. [DOI] [PubMed] [Google Scholar]
- Jesenak, M., Brndiarova, M., Urbancikova, I et al., 2020. Immune parameters and COVID-19 infection—associations with clinical severity and disease prognosis. Front. Infect Microbiol. 10,1–10. Iran J. Immunol.. Dec;18(4):331-337. doi: 10.22034/IJI.2021.89905.1978. [DOI] [PMC free article] [PubMed]
- Kimmig, L.M., Wu, D., Gold, M., Pettit, N.N. Pitrak, D., Mutlu, E.A., Mutlu. G.M., 2020. IL-6 Inhibition in Critically Ill COVID-19 Patients Is Associated With Increased Secondary Infections. Front. Med. (Lausanne).28(7), 583897. [DOI] [PMC free article] [PubMed]
- Kumar, V., 2019.Immunometabolism:Another Road to Sepsis and Its Therapeutic Targeting. Inflammation. 42(3),765-788. [DOI] [PubMed]
- Li J., Li M., Su L., Wang H., Xiao K., Xie L. Alterations of T helper lymphocyte subpopulations in sepsis, severe sepsis, and septic shock: a prospective observational study. Inflammation. 2015;38(3):995–1002. doi: 10.1007/s10753-014-0063-3. [DOI] [PubMed] [Google Scholar]
- Li, C.H., Chiou, H.C., Lin, M.H., Kuo, C.H., Kuo, Y.H., 2021. Immunological map in COVID-19. J. Microbiol. Infect. 54(4),547-556. doi: 10.1016/j.jmii.2021.04.006. Epub 2021 May 12. PMID: 34023234; PMCID: PMC8114810. [DOI] [PMC free article] [PubMed]
- Liu, J., Li, S., Liu, J., 2020. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBio. Medicine 55. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- Liu, K., Yang, T., Peng, X.M., Ye, X.L., Liu, W., 2021 .A systematic meta-analysis of immune signatures in patients with COVID-19. Rev. Med. Virol. 31(4),2195. doi: 10.1002/rmv.2195. Epub 2020 Nov 20. PMID: 34260780; PMCID: PMC7744845. [DOI] [PMC free article] [PubMed]
- Ludwig S., Zarbock A. Coronaviruses and SARS-CoV-2: a brief overview. Anesth. Analg. 2020;13:193–196. doi: 10.1213/ANE.0000000000004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Zhang J.W., Zhang W., Lin Y.L., Wang Q. Circulating levels of IL-2, IL-4, TNF-α, IFN-γ, and C-reactive protein are not associated with severity of COVID-19 symptoms. J. Med. Virol. 2020:1–3. doi: 10.1002/jmv.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Ogura H., Shimizu K., Ikeda M., Hirose T., Shimazu T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018;8(1):13995. doi: 10.1038/s41598-018-32275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;19(6) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notz Q., Schmalzing M., Wedekink F., Schlesinger T., Gernert M., Lotz C. Pro- and anti- inflammatory responses in severe COVID-19-induced acute respiratory distress syndrome-an observational pilot study. Front. Immunol. 2020;6(11) doi: 10.3389/fimmu.2020.581338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Cruz A., Mendes-Frias A., Oliveira A.I., Dias L., Matos A.R., Silvestre R. Interleukin- 6 is a biomarker for the development of fatal severe acute respiratory syndrome Coronavirus 2 pneumonia. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.613422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Yin Z., Hu Y., Mei H. Controlling cytokine storm is vital in COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Y., Sun., J., Pan, H. F., Yao, Y.,Yuan ., Li, Y., 2021.Aberrant cytokine expression in COVID-19 patients: Associations between cytokines and disease severity. Cytokine 143, 155523. [DOI] [PMC free article] [PubMed]
- Tonetti T., Grasselli G., Rucci P., Alessandri F., Dell'Olio A., Ranieri V.M. Synergistic effect of static compliance and d-dimers to predict outcome of patients with COVID-19-ARDS: a prospective multicenter study. Biomedicines. 2021;9(9):1228. doi: 10.3390/biomedicines9091228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varchetta S., Mele D., Oliviero B., Mantovani S., Ludovisi S., Mondelli M.U. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021;18(3):604–612. doi: 10.1038/s41423-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization.2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance 28 January. WHO 10.
- Yang, B., Chang, X, J., Huang, W.. Pan, Z. Si., Zhang, C., H. Li., 2021. The role of IL-6/lymphocyte ratio in the peripheral blood of severe patients with COVID-19. Int .Immunopharmacol. 97, 107569. [DOI] [PMC free article] [PubMed]
- Zeng Z., Yu H., Chen H., Qi W., Chen L., Wu D. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit. Care. 2020;24(1):525. doi: 10.1186/s13054-020-03255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Wei H. Pathogenic T- cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]