Abstract
Background:
Studies evaluating depression’s role in lung cancer risk revealed contradictory findings, partly because of small number of cases, short follow-up periods, and failure to account for key covariates including smoking exposure. We investigated the association of depressive symptoms with lung cancer risk in a large prospective cohort over 24 years, while considering the role of smoking.
Methods:
Women from the Nurses’ Health Study completed measures of depressive symptoms, sociodemographics, and other factors including smoking in 1992 (N=42,913). Depressive symptoms were also queried in 1996 and 2000, whereas regular antidepressant use and physician-diagnosed depression were collected starting in 1996. Multivariable Cox regression models estimated hazard ratios (HR) and 95% confidence intervals (CI) of lung cancer risk until 2016.
Results:
We identified 1,009 cases of lung cancer. Women with the highest versus lowest level of depressive symptoms had an increased lung cancer risk (HRsociodemographics-adjusted=1.62, 95%CI=1.34–1.95; HRfully-adjusted=1.25, 95%CI=1.04–1.51). In a test of mediation, lifetime pack-years of smoking accounted for 38% of the overall association between depressive symptoms and disease risk. When stratifying by smoking status, elevated risk was evident among former smokers but not current or never smokers; however, the interaction term suggested no meaningful differences across groups (p=0.29). Results were similar or stronger when considering time-updated depression status (using depressive symptoms, physician diagnosis, and regular antidepressant use) and chronicity of depressive symptoms.
Conclusions:
These findings suggest that greater depressive symptoms may contribute to lung cancer incidence, directly and indirectly via smoking habits, which accounted for over a third of the association.
Keywords: depression, antidepressant, smoking, cancer, lung cancer
Introduction
Lung cancer is the second most common cancer, with 228,820 new cases estimated in the U.S. in 2020, and the leading cause of cancer death (American Cancer Society, 2020). Although incidence has fallen in recent decades in tandem with declining smoking prevalence, the decline in lung cancer began later and has occurred more slowly in women versus men, and in individuals from lower versus higher socioeconomic status (SES; American Cancer Society, 2020). Among nonsmokers, women are more likely to be diagnosed with lung cancer compared to men; they are also overrepresented among adenocarcinoma cases, the most common histologic subtype of lung cancer (Donington & Colson, 2011). While new cases and deaths from lung cancer are higher in men than women nowadays, these trends are expected to reverse by 2045 (Jeon et al., 2018). Given these disparities and the still significant overall disease burden, it is critical to identify additional modifiable risk factors of lung cancer in women while considering SES characteristics, as well as to identify upstream determinants of smoking.
It has long been suggested that depression plays a role in cancer etiology and progression, with behavioral and psychoneuroimmunological pathways commonly used to explain associations (Blumberg, West, & Ellis, 1954; Green McDonald, O’Connell, & Lutgendorf, 2013; Green McDonald, O’Connell, & Suls, 2015; Spiegel & Giese-Davis, 2003). Depression has been linked with worse lung cancer survival (e.g., Arrieta et al., 2013; Pirl et al., 2012; Sullivan et al., 2014). Yet, only a few studies have investigated the association of depression with lung cancer incidence, and results are mixed. For instance, prospective studies adjusting for age and sex only found greater depressive symptoms were related to increased lung cancer risk (Dalton, Mellemkjaer, Olsen, Mortensen, & Johansen, 2002; Goldacre, Wotton, Yeates, Seagroatt, & Flint, 2007), whereas studies further controlling for SES and lifestyle factors often (Gross, Gallo, & Eaton, 2010; Liang et al., 2011; Penninx et al., 1998) but not always (Knekt et al., 1996) found no association. However, these more rigorous analyses either relied on a small number of lung cancer cases (from 56 to 70; Gross et al., 2010; Penninx et al., 1998) with limited statistical power to detect an association, or had a relatively short follow-up (6 to 8 years; Liang et al., 2011), perhaps failing to capture longer-term processes like carcinogenesis.
Aside from addressing these methodological limitations, elucidating depression’s role in cancer development still deserves further investigation because of depression’s prevalence. Depression is one of the most common mental disorders, twice as common in women than men, and the leading cause of disability worldwide (Kessler, 2003; World Health Organization, 2017). In 2017, 17.3 million U.S. adults reported having experienced ≥1 major depressive episode over the previous year (National Survey on Drug Use and Health, 2017), implying that even more individuals experience non-clinical depressive symptoms. Since depression is modifiable, evaluating its role in cancer incidence may provide valuable insight into avenues for disease prevention and have a substantial impact at the population level (Green McDonald et al., 2015).
Additionally, depression is related to behavioral risk factors for lung cancer, notably cigarette smoking. Evidence shows that depression affects subsequent smoking habits (Cooper, Borland, McKee, Yong, & Dugue, 2016; Trudel-Fitzgerald, Tworoger, Poole, Williams, & Kubzansky, 2016; Weinberger, Pilver, Desai, Mazure, & McKee, 2013); hence, smoking likely mediates the depression-lung cancer association. Alternatively, smoking also increases future risk of depressive symptoms (Boden, Fergusson, & Horwood, 2010; Leventhal & Zvolensky, 2015), suggesting smoking may confound the association of depression with lung cancer risk, or modify the effect of depression on lung cancer risk. In prior studies, when demographics-adjusted models were further controlled for smoking, estimates for effects of depression became lower in some cases (Knekt et al., 1996), but were similar or stronger in other cases (Gross et al., 2010; Penninx et al., 1998). When studying effect modification, studies controlling for several relevant covariates showed the association did not significantly differ across smoking groups (Gross et al., 2010; Penninx et al., 1998). As lung cancer case counts were small in these analyses (from 56 to 70), smoking’s contribution remains uncertain. Because >34 million U.S. adults were smokers in 2018, a substantial proportion of the population is still more likely to develop lung cancer than non-smokers (up to 25-fold; American Cancer Society, 2020). Hence, a thorough examination of smoking’s various roles in the association between depression and lung cancer is required.
Therefore, we investigated the prospective association of depressive symptoms with lung cancer risk in a large cohort of women, and explored smoking’s contribution in this association. We hypothesized higher versus lower depressive symptoms would be associated with increased lung cancer risk, after adjusting for relevant confounders including SES characteristics, exposure to secondhand smoke, and lifetime pack-years of cigarette smoking. We further posited this association would be i) partially mediated by smoking, whereby greater depressive symptoms would relate to higher disease risk directly, and indirectly via greater pack-years, and ii) would not be modified by smoking status, with risk estimates being comparable among smokers and non-smokers.
Methods
Study Population
Data are from the Nurses’ Health Study (NHS) cohort, which began in 1976 with 121,700 female registered nurses ages 30–55 years from across the U.S. Since 1976, participants have returned biennial questionnaires querying health, lifestyle, and psychosocial factors, as well as newly diagnosed medical conditions. Follow-up rates throughout the study exceed 85% (Bao et al., 2016). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Our sample included NHS women who, at the 1992 analytic baseline, did not report a prior cancer diagnosis (except non-melanoma skin cancer) and completed the baseline assessment of self-reported depressive symptoms (see Supplemental Figure 1). Participants who did and did not complete the assessment were similar with regard to age, parents’ occupation and husband’s education, and exposure to secondhand smoke in childhood, as well as smoking levels, diet quality, and shiftwork. However, less physically active participants and those without a familial history of lung cancer were less likely to complete the depression measure. We additionally excluded women with missing data on baseline covariates. To limit the possibility that latent disease experienced prior to diagnosis affected depressive symptoms, lung cancer cases occurring <2 years after baseline depression assessment in 1992 were also excluded, yielding a final analytic sample of N=42,913.
Measures
Figure 1 depicts a timeline representing exposures, covariates, and outcome assessments used in the current study.
Figure 1.
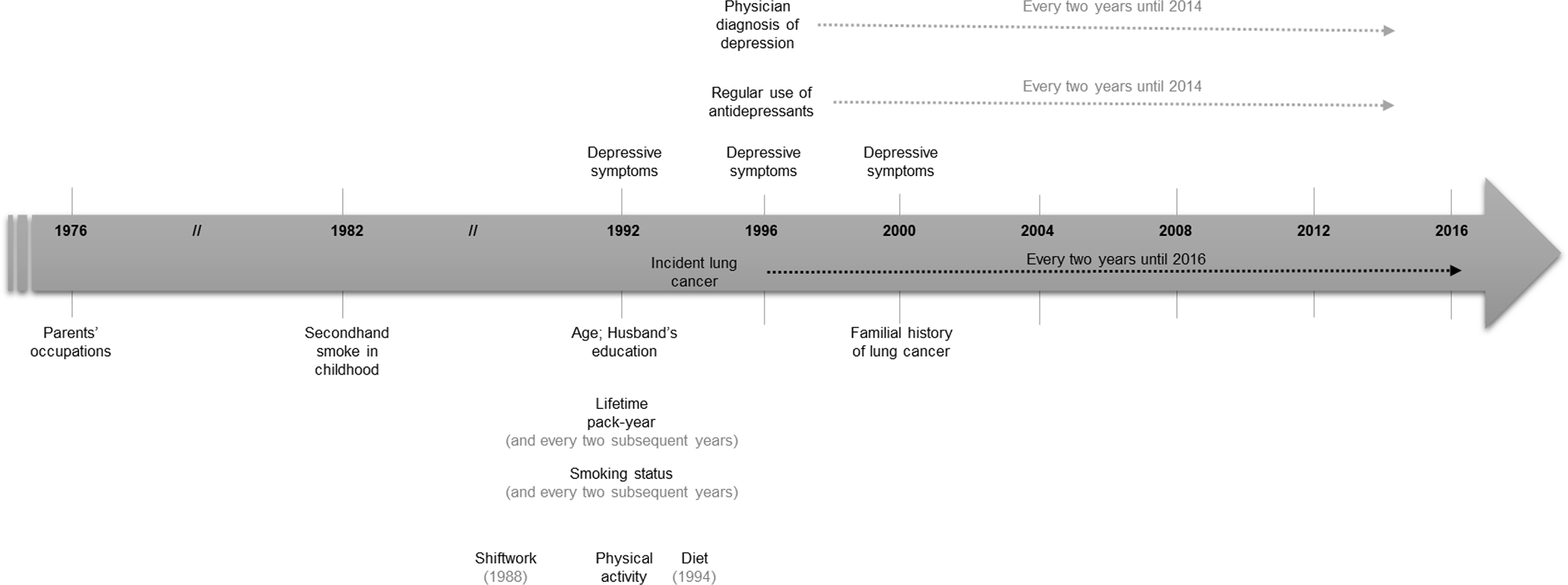
Timeline of exposures, covariates, and outcome assessments throughout the study period.
Note. Variables over the arrow represent the exposures, under the arrow, the covariates, and on the arrow, the outcome.
Depression indicators.
Depressive symptoms experienced in the past month were assessed via the validated 5-item Mental Health Index (MHI-5) from the Medical Outcomes Study Short-Form 36 Health Status Survey (Ware & Sherbourne, 1992) in 1992, 1996, and 2000. Assessed on a Likert scale, item scores were summed and converted to a 100-point scale for a total score, with higher scores indicating lower symptoms. Following prior research (Huang et al., 2015; Kroenke et al., 2005), for the main analyses scores were classified into four categories: 0–60 (severe depressive symptoms), 61–75 (high), 76–85 (moderate) and 86–100 (low or none), allowing assessment of potential threshold effects. In additional analyses (described below), the total score was used in i) a continuous format, with a reverse-coded standardized score (higher scores indicate greater symptoms) to facilitate interpretation, and ii) a dichotomized format, with ≤60 indicating higher and >60 lower symptoms following previous work identifying clinically-relevant cutpoints (Rumpf, Meyer, Hapke, & John, 2001). The MHI-5 has good predictive value for detecting mood disorders/major depression (Berwick et al., 1991; Cuijpers, van Straten, van Schaik, & Andersson, 2009). In our analytic sample, internal consistency reliability is high (α1992=0.82) and scores are fairly stable over the three assessments (ICC=0.53; within-subject variability=0.12).
Starting in 1996, participants reported whether they used regularly “antidepressants (e.g., Elavil, Prozac, Celexa, Paxil)” (yes/no) or received a diagnosis of depression by a physician (yes/no) since the last biennial questionnaire.
Lung cancer.
Information on new diagnoses of lung cancer were queried on each biennial questionnaire. For all reported lung cancer diagnoses and deaths due to lung cancer identified through family members, the National Death Index, or the U.S. Postal Service, NHS researchers contacted study participants or their families to request permission to obtain relevant pathology reports and other medical records, with an excellent confirmation rate (e.g., an estimated 98% of all deaths were captured through the National Death Index; Rich-Edwards, Corsano, & Stampfer, 1994). Physicians blind to study hypotheses ascertained cancer diagnosis and mortality cause from medical records and death certificates, using the International Classification of Diseases, Eighth Revision (World Health Organization, 1967).
Smoking.
Cigarette smoking information was collected on biennial questionnaires, including at study 1992 baseline, via questionnaire-based self-report measures. Similar measures have shown good ability to distinguish between smokers and non-smokers when validated with serum cotinine (Vartiainen, Seppala, Lillsunde, & Puska, 2002). Across models, lifetime pack-years was modeled either a continuous or a categorical variable (never smoker, 1–10 packs/year, 11–30 packs/year, and ≥30 packs/year). In our sample, continuous pack-years was remarkably stable over the follow-up period (ICC=1.00; within-subject variability=0.10). In stratified analyses (described below), smoking status was defined as never, former, or current smokers. These categories were also very stable throughout the study duration (ICC=0.95; within-subject variability=0.22). Given such stability, smoking variables were not time-updated in analyses unless otherwise noted.
Covariates.
Covariates selected following prior evidence (American Cancer Society, 2020; Brenner, Yannitsos, Farris, Johansson, & Friedenreich, 2016; Schernhammer, Feskanich, Liang, & Han, 2013; Sun, Li, Li, Li, & Han, 2016; Wang, Yang, Guo, & Li, 2019) were available at the 1992 baseline unless otherwise stated. Sociodemographics included age (continuous), husband’s education (as a proxy for adult SES; unmarried participant, less than high school, high school graduate, college graduate, graduate school), exposure to secondhand smoke during childhood (queried in 1982 with the item “Did your parents smoke while you were living with them?”; yes/no), parents’ occupations when participants was 16 years old (as a proxy for childhood SES; queried in 1976; both parents dead, farmer, blue collar, white collar), and familial history of lung cancer (queried in 2000; yes/no).
Other factors encompassed diet quality (queried in 1994), physical activity, and shiftwork (queried in 1988). To determine diet quality, participants first completed the 131-item Food Frequency Questionnaire, shown in prior work to have high reproducibility and validity (Giovannucci et al., 1991; Rimm et al., 1992). Then, a continuous summary score (ranging from 0 [poor diet] to 110 [optimal diet]) based on the Alternative Healthy Eating Index was created (McCullough et al., 2002). Physical activity was assessed with a validated self-administered questionnaire (Chasan-Taber et al., 1996); averaged weekly time of moderate-to-vigorous physical activity levels was dichotomized into <150min/week versus ≥150min/week. Shiftwork was evaluated by the total number of years during which participants worked rotating night shifts ≥3 nights/month in addition to days or evenings in that month (never, 1–14 years, 15–29 years, ≥30 years).
Statistical Analyses
Statistical analyses were conducted using SAS, 9.4 (SAS Institute, 2011) at a 0.05 p-level. Descriptive analyses examined the distribution of covariates across 1992 depressive symptom levels, adjusting for age. Cox proportional hazards models estimated the association between four-level depressive symptoms and lung cancer risk over the follow-up period in primary models. Person-years were counted from the 1994 follow-up assessment, with participants contributing person-time until death, diagnosis of lung cancer, or the termination of the follow-up period (2016), whichever came first. Nested models progressively adjusting for sociodemographic and other factors evaluated the role of each group of covariates. The first model was stratified by age and time period, and adjusted for exposure to secondhand smoke in childhood, parents’ occupations when participants were 16, familial history of lung cancer, and husband’s education at study baseline. A second model further controlled for categorical pack-years of smoking. In the third model, other factors (diet quality, physical activity, shiftwork) were added to the first model that adjusted for sociodemographics only. Lastly, the fully-adjusted model accounted for all covariates simultaneously.
To further disentangle the role of smoking in the association of depressive symptoms with lung cancer risk, we conducted two additional analyses. First, causal mediation analysis techniques (Valeri & Vanderweele, 2013; VanderWeele, 2011) were implemented within a Cox proportional hazard framework to assess mediation by pack-years of cigarette smoking. From these models, we estimated the natural direct effect of dichotomized depressive symptoms reported in 1992 on lung cancer risk up to 2016 and the natural indirect effect mediated through continuous pack-years in 1994 among participants with available pack-years data (n=42,906). The natural direct and indirect effects were also used to calculate the proportion of the total effect of that was mediated by pack-years of smoking. Confidence intervals were calculated for all mediation estimates with standard errors obtained via the delta method (VanderWeele, 2013). This analysis was also replicated among women who did not smoke in 1992 (n=37,433) to assure optimal temporality, whereby depressive symptoms would led to subsequent smoking habits (new or relapse).
Second, we assessed 1992 depressive symptoms in relation to lung cancer risk in Cox models stratified by 1992 smoking status (never, former, or current smokers) to evaluate potential effect modification by smoking. Here, we used a continuous measure of depressive symptoms (per 1-standard deviation [SD] increase) to maximize statistical power. We also re-ran primary models including an interaction term (continuous depressive symptoms x categorical smoking status) to determine whether the depressive symptoms-lung cancer association significantly differed across smoking status. Using sensitivity analyses, we repeated these models while i) further adjusting for time-updated continuous pack-years to maximally account for any changes in smoking habits over the follow-up period and ii) using a Poisson distribution, to account for rare events among never smokers.
To determine if observed effects were robust to alternative methods for classifying depressive symptoms, two other additional analyses were conducted. First, we examined the association between chronic exposure to depressive symptoms and subsequent lung cancer risk by using all MHI-5 assessments (i.e., 1992, 1996, 2000; severe depressive symptoms on the MHI-5 at no time points, one time point, or two to three time points). Second, to examine the role of time-updated exposure and alternative depression-related factors on lung cancer risk, a dichotomized depression status (clinical, non-clinical) was created based on a Boolean OR operator approach (Luijendijk et al., 2008; Trudel-Fitzgerald, Chen, Singh, Okereke, & Kubzansky, 2016) that leveraged all available information. Specifically, “clinical depression” was defined as reporting either 1) depressive symptoms reaching clinical significance (dichotomized MHI-5 score at ≤60); 2) physician-diagnosed depression; or 3) regular antidepressant use. Exploratory analyses further stratified these time-updated analyses by smoking status (never, former, or current smokers).
Results
Baseline Characteristics
Over the follow-up period, 42,913 women contributed 763,399 years of person-time, and 1,009 cases of lung cancer were diagnosed. Age-adjusted distribution of covariates are presented across depressive symptoms levels (Table 1). Approximately 15% of the sample had severe depressive symptoms. Compared to women with low/no depressive symptoms, those with severe depressive symptoms were slightly younger and more likely to be unmarried, as well as to have a familial history of lung cancer, parents with blue collar occupations, and been exposed to second-hand smoke in childhood. They were also more likely to report greater lifetime pack-years of smoking, as well as to be current smokers and less physically active.
Table 1.
Age-standardized characteristics of women at the 1992 baseline (N=42,913).
| Levels of depression symptoms | ||||
|---|---|---|---|---|
| Severe (n=6635) | High (n=8416) | Moderate (n=15,196) | Low or none (n=12,666) | |
|
| ||||
| Age, mean (SD)* | 56.7 (7.1) | 57.3 (7.1) | 57.7 (7.0) | 59.1 (6.9) |
| White race, % | 98.1 | 98.2 | 98.3 | 98.1 |
| Non-Hispanic ethnicity, % | 99.1 | 99.3 | 99.3 | 99.4 |
| Husband’s education level | ||||
| - (NHS women not currently married), % | 10.0 | 8.3 | 7.8 | 7.4 |
| - Less than high school, % | 6.5 | 6.1 | 5.4 | 4.7 |
| - High school degree, % | 37.9 | 36.9 | 36.5 | 36.0 |
| - College degree, % | 25.3 | 26.9 | 27.4 | 27.3 |
| - Graduate degree, % | 20.2 | 21.9 | 23.0 | 24.6 |
| Family history of lung cancer, % | 11.5 | 10.8 | 11.0 | 10.9 |
| Second-hand smoking exposure during childhood, % | 70.2 | 68.8 | 68.4 | 66.0 |
| Parents’ occupation | ||||
| - Both parents are dead, % | 0.6 | 0.5 | 0.6 | 0.5 |
| - Farmer, % | 6.2 | 7.1 | 8.3 | 9.2 |
| - Blue collar, % | 23.6 | 22.8 | 21.4 | 20.6 |
| - White collar, % | 69.7 | 69.6 | 69.6 | 69.7 |
| Smoking status | ||||
| - Never smoker, % | 40.7 | 43.0 | 46.2 | 49.6 |
| - Former smoker, % | 42.6 | 42.6 | 42.1 | 39.6 |
| - Current smoker, % | 16.7 | 14.3 | 11.8 | 10.8 |
| Lifetime pack-years, mean (SD) | 15.5 (20.8) | 13.5 (19.4) | 12.0 (18.2) | 10.9 (17.3) |
| Diet quality (AHEI score; range: 0–110), mean (SD) | 51.4 (10.5) | 52.3 (10.3) | 53.0 (10.4) | 53.7 (10.4) |
| 150min/week or more of physical activity, % | 31.5 | 36.4 | 41.4 | 44.9 |
| Years of shiftwork | ||||
| - Never, % | 39.8 | 40.0 | 40.6 | 42.2 |
| - 1–14 years, % | 53.0 | 53.2 | 52.9 | 51.3 |
| - 15–29 years, % | 5.6 | 5.1 | 5.1 | 5.0 |
| - 30 years or more, % | 1.6 | 1.7 | 1.5 | 1.5 |
Values are means (SD) or medians (Q25, Q75) for continuous variables; percentages or ns or both for categorical variables, and are standardized to the age distribution of the study population. Values of polytomous variables may not sum to 100% due to rounding.
Value is not age adjusted
Baseline depressive symptoms and lung cancer risk
In primary models (Figure 2; Table 2), participants with severe versus low/no depressive symptoms had higher lung cancer risk in both sociodemographics-adjusted (HR=1.62, 95%CI=1.34–1.95) and fully-adjusted (HR=1.25, 95%CI=1.04–1.51) models. Women with high versus low/no depressive symptoms also had elevated disease risk (e.g., HRsociodemographics-adjusted=1.30, 95%CI=1.08–1.56), although the estimates were substantially attenuated in models adjusting for pack-years (e.g., HRfully-adjusted=1.11, 95%CI=0.92–1.33). Women reporting moderate depressive symptoms did not have an increased lung cancer risk throughout follow-up (fully-adjusted p-trend=0.01).
Figure 2. Overview of obtained associations between depression indicators and risk of lung cancer over 24 years.
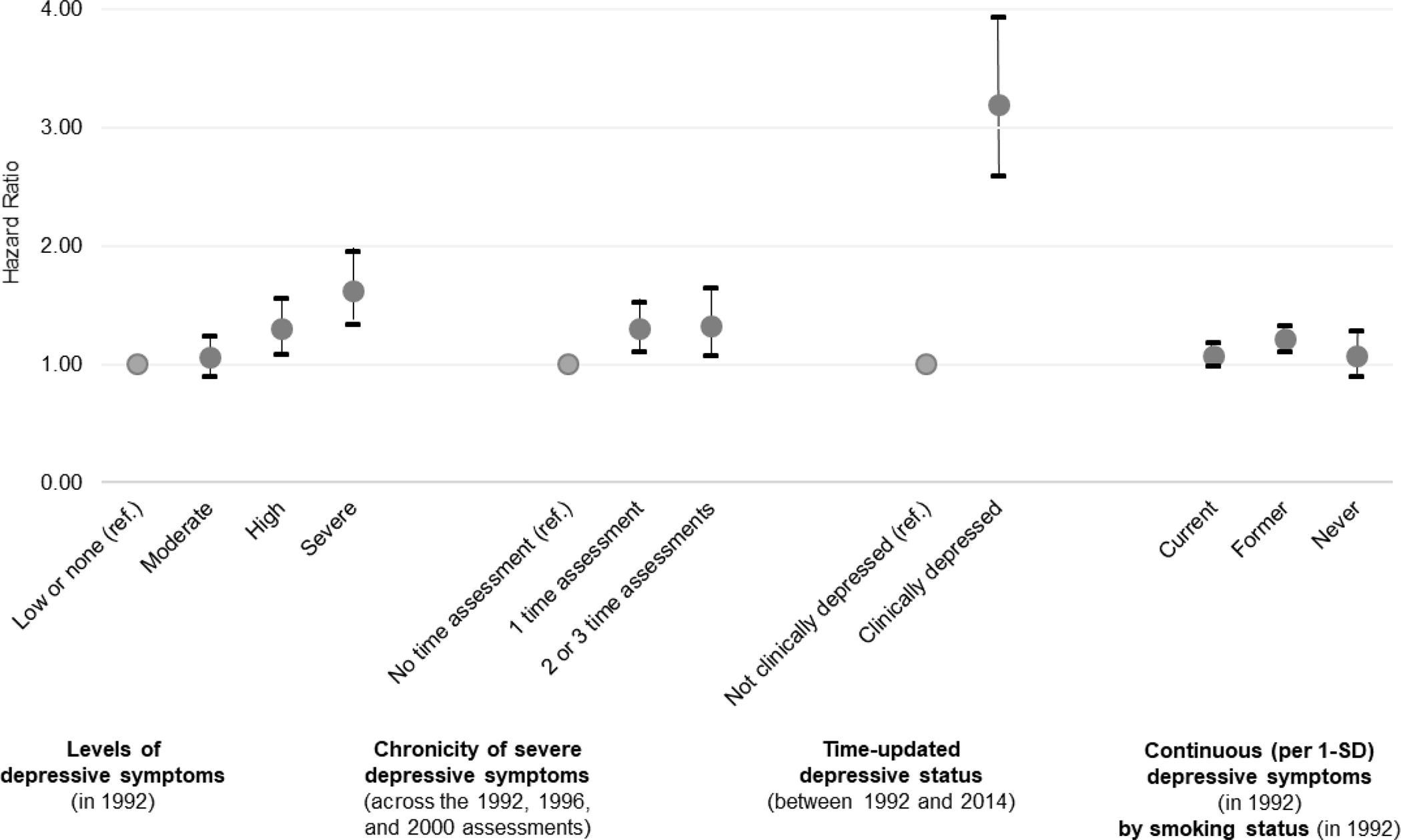
Note. Grey circles represent hazard ratio coefficients; black bars represent 95% confidence intervals. As detailed in the Methods, “depressive symptoms” were assessed with the MHI-5 self-reported scale whereas “depressive status” was assessed with the report of a clinical score on the MHI-5 scale, a physician diagnosis of depression, and antidepressant use. All associations are stratified by age in 1992 and calendar time, and further adjusted for sociodemographic covariates (i.e., exposure to second-hand smoking during childhood, parents’ occupations when participant was 16 years old, familial history of lung cancer, and husband’s education). SD = standard deviation.
Table 2.
Association of level of depressive symptoms with lung cancer incidence (N=42,913; 1009 cases).
| Person-years | Model 1: Sociodemographics | Model 2: Model 1 + smoking | Model 3: Model 1 + other factors | Model 4: All covariates | |||||
|---|---|---|---|---|---|---|---|---|---|
| (cases) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | HR | (95% CI) | |
| Depressive symptoms levels | |||||||||
| 763,399 (1,009) | |||||||||
| Low or none | 224,328 (274) | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) | 1.00 | (ref.) |
| Moderate | 272,950 (323) | 1.06 | (0.90–1.24) | 1.00 | (0.85–1.18) | 1.03 | (0.88–1.22) | 0.99 | (0.84–1.16) |
| High | 149,317 (212) | 1.30 | (1.08–1.56)** | 1.13 | (0.94–1.36) | 1.25 | (1.04–1.49)* | 1.11 | (0.92–1.33) |
| Severe | 116,804 (200) | 1.62 | (1.34–1.95)**** | 1.30 | (1.08–1.57)** | 1.52 | (1.26–1.83)**** | 1.25 | (1.04–1.51)* |
| p-trend | ≤.0001 | 0.003 | ≤.0001 | 0.01 | |||||
p≤ .05
p≤ .01
p≤ .0001.
M1: stratified by age in 1992 (continuous) and calendar time (continuous); further adjusted for exposure to second-hand smoking during childhood (yes, no [reference]); parents’ occupations when participant was 16 years old (both parents dead [reference], farmer, blue collar, white collar); familial history of lung cancer (yes, no [reference]); and husband’s education (participant unmarried [reference], less than high school, high school graduate, college graduate, graduate school)
M2: M1 + average cigarette packs per day per year smoked (not a current smoker [reference], 1–10 packs/year, 11–30 packs/year, 30 or more packs/year)
M3: M1 + diet quality (AHEI score; continuous); physical activity (less than 150min/week of moderate-to-vigorous physical activity [reference], 150min/week or more of moderate-to-vigorous physical activity); years of shiftwork (never [reference], 1–14 years, 15–29 years, 30 years or more)
M4: all covariates mentioned above.
Smoking as a mediator and effect modifier
Mediation analyses suggested the relationship between depressive symptoms and lung cancer risk was at least partly mediated by lifetime pack-years of smoking (Table 3). While there was a significant natural direct effect of severe versus low/no depressive symptoms on disease risk (HRsociodemographics + other factors=1.24, 95%CI=1.06–1.45), the natural indirect effect via smoking was also significant (HRsociodemographics + other factors=1.12, 95%CI=1.10–1.14). Findings suggested 38% of the total effect of depressive symptoms on lung cancer risk was mediated by smoking. The mediating effect became smaller but remained evident when restricting to women who did not smoke in 1992 (models adjusting for sociodemographics and other factors: HRdirect effet=1.38, 95%CI=1.12–1.71; HRindirect effet=1.07, 95%CI=1.05–1.09; proportion mediated by pack-years in 1994=20%).
Table 3.
Mediation by smoking status of the association of dichotomized depressive symptoms with lung cancer incidence (n=42,906; 1009 cases).
| Model 1: Sociodemographics | Model 2: Model 1 + other factors | |||
|---|---|---|---|---|
| HR | (95% CI) | HR | (95% CI) | |
| Natural direct effect | 1.26 | (1.08–1.48)** | 1.24 | (1.06–1.45)** |
| Natural indirect effect | 1.13 | (1.11–1.16)**** | 1.12 | (1.10–1.14)**** |
| Total effect | 1.43 | (1.23–1.68)**** | 1.39 | (1.19–1.63)**** |
| Proportion mediated | 39% | 38% | ||
p≤ .01
p≤ .0001.
M1: adjusted for age (continuous); exposure to second-hand smoking during childhood (yes, no [reference]); parents’ occupations when participant was 16 years old (both parents dead [reference], farmer, blue collar, white collar); familial history of lung cancer (yes, no [reference]); and husband’s education (participant unmarried [reference], less than high school, high school graduate, college graduate, graduate school)
M2: M1 + diet quality (AHEI score; continuous); physical activity (less than 150min/week of moderate-to-vigorous physical activity [reference], 150min/week or more of moderate-to-vigorous physical activity); years of shiftwork (never [reference], 1–14 years, 15–29 years, 30 years or more)
When stratifying by smoking status (Figure 2; Supplemental Table 1), each 1-SD increase in depressive symptoms was associated with a greater lung cancer risk among former smokers (HRfully-adjusted=1.19, 95%CI=1.09–1.30), and a somewhat weaker elevated risk among current smokers (HRfully-adjusted=1.06, 95%CI=0.96–1.16) but confidence intervals were wider among never smokers (HRfully-adjusted=1.07, 95%CI=0.89–1.28). However, the interaction term between smoking status and the continuous depression score was not statistically significant (pfully-adjusted model=0.29), suggesting the association between depressive symptoms and lung cancer risk did not meaningfully vary by smoking status. Further adjusting for time-updated continuous pack-years did not alter the results (interaction term: pfully-adjusted model=0.26) neither did the consideration of a Poisson distribution (interaction term: pfully-adjusted model=0.14), although the estimates were slightly attenuated (Supplemental Table 2).
Alternative specifications of depression
When considering the chronicity of depressive symptoms (Figure 2; Supplemental Table 3), participants who reported severe depressive symptoms at 2–3 versus no time points had a higher lung cancer risk in models adjusted for sociodemographics and other factors (HRsociodemographics-adjusted=1.32, 95%CI=1.07–1.64; HRadjusting for other factors=1.25, 95%CI=1.00–1.55). However, the relationship was substantially attenuated after controlling for smoking. Similarly, participants with severe depressive symptoms at one time point had a higher risk of comparable magnitude in models not adjusting for smoking (HRsociodemographics-adjusted=1.30, 95%CI=1.10–1.52; HRadjusting for other factors=1.26, 95%CI=1.07–1.48). Findings from the analysis using a time-updated exposure based on self-reported depressive symptoms, physician diagnosis, and antidepressant were stronger than those using the baseline self-reported depressive symptom measure only (Figure 2; Supplemental Table 3). For instance, time-varying clinical versus non-clinical depression status was associated with more than two-fold increased risk of lung cancer after statistical adjustment for sociodemographics, smoking, and other factors (HRfully-adjusted=2.47, 95%CI=1.99–3.06). Exploratory analyses further stratifying these models by smoking status showed current, former, and never smokers all had a statistically or marginally significant elevated lung cancer risk if they were categorized as having clinical versus non-clinical depression (Supplemental Table 4), although in some cases the number of lung cases was small (e.g., n=15 among never smokers who were clinically depressed).
Discussion
To our knowledge, this is the largest study to evaluate the association between depression and incident lung cancer among women, and the first to thoroughly consider the role of smoking. Consistent with our hypothesis, greater depressive symptoms were associated, albeit modestly, with increased risk lung cancer risk over the study period. This effect was not only robust to alternative specifications of depressive symptoms, but also yielded, in some cases, stronger estimates than those of the main models (i.e., with time-updated depression status). Lifetime pack-years of cigarette smoking also partly mediated the association of depressive symptoms with incident lung cancer, accounting for up to 38% of the overall effect. Lastly, estimates of depressive symptoms in relation to lung cancer risk stratified by smoking status (never, former, or current smokers), which were of smaller magnitude, did not significantly differ in this sample. These findings may appear inconsistent with prior research that failed to find statistically significant associations between depression and lung cancer risk after adjusting for SES and lifestyle factors (Gross et al., 2010; Liang et al., 2011; Penninx et al., 1998). However, our investigation included substantially more lung cancer cases (n=1,009) and a considerably longer study duration (24 years), compared to these prior investigations (ns from 56 to 70; Gross et al., 2010; Penninx et al., 1998) with follow-ups from 6 to 8 years (Liang et al., 2011), which likely enhanced statistical power to detect existing, albeit modest, associations that may develop over many years.
Findings from mediation analyses suggested the depression-lung cancer association was partly mediated by lifetime pack-years of smoking. As smoking explained approximately a third of the relationship, additional mechanistic pathways are likely at play. Prior research in animal models supports the plausibility of direct endocrine and neuro-immune pathways between depression and cancer mediated by processes like immune dysregulation and DNA damage due to increased oxidative stress (Gidron, Russ, Tissarchondou, & Warner, 2006; Glaser & Kiecolt-Glaser, 2005). Biological factors involved in lung cancer among humans (e.g., telomere length; Karami et al., 2016) (mitochondrial DNA; Meng et al., 2016) would also deserve more empirical attention in studies targeting upstream psychological determinants. In fact, research in the NHS cohort demonstrating an association between depressive symptoms and higher risk of cancers that are less associated with smoking than lung cancer (i.e., colorectal and ovarian cancer; Trudel-Fitzgerald, Chen, et al., 2016) lends support to the presence of direct biological effects of depression that are not mediated by smoking. Moreover, our study did not find that smoking status was a significant effect modifier, consistent with prior multivariable results suggesting similar associations of depression with lung cancer risk among smokers and non-smokers (Gross et al., 2010; Penninx et al., 1998).
The association observed in the main models was also robust to alternative specifications of depression. Notably, women who did versus did not reach the depression scale clinical cutpoint at one or 2–3 time assessments had a similarly significant elevated lung cancer risk. Such consistency across estimates by chronicity period is aligned with the stability of depressive symptoms and low within-person variability observed in the current analytic sample. However, results from these chronicity analyses were no longer significant after adjusting for pack-years, which further reinforces the role of smoking in the depression-lung cancer association. Nonetheless, hazard ratios became stronger when a time-updated exposure combining self-reported depression symptoms, physician-diagnosed, and regular antidepressant use was considered. It is possible this finding is due to reverse causation, as the depression assessment occurred closer to lung cancer diagnosis in this analysis and thus may capture underlying disease processes that affect experience (or report) of mood symptoms. However, given all analyses were lagged by two years, it may be that defining depression using multiple sources of information has actually enhanced its predictive value, therefore contributing to stronger estimates. Lastly, further stratifying these time-updated analyses by smoking status reiterated that depressive symptoms are modestly associated with lung cancer risk similarly for current, former, and never smokers.
The current study has several limitations. Firstly, the depression measure may have captured transient negative affect. However, stability indices obtained from our analytic sample revealed fairly low variation in depressive symptoms across 8 years. Additional analyses also indicated that lung cancer risk was comparable for women who experienced severe depressive symptoms at one versus 2–3 time assessments. Moreover, antidepressant medication may be used for other reasons than depressive symptoms or psychopathologies comorbid to depression. However, the increased lung cancer risk was observed both when including or not antidepressant in the exposure, which lowers the likelihood of misclassification. Depression and anxiety symptoms are also known to be highly comorbid (Hasin et al., 2018) and both linked to worst health outcomes (Cohen, Edmondson, & Kronish, 2015; Trudel-Fitzgerald, Chen, et al., 2016); yet, a comprehensive assessment of anxiety, or other measures of psychopathology, were not available at the study baseline to tease apart depressive symptoms from other forms of psychological distress.
Secondly, the NHS cohort is restricted to female, predominantly White, nurses. To be included in the current analytic sample, women also needed to be healthy enough to survive to the 1992 baseline. Although such homogeneity restraints generalizability of the findings, it does enhances the study internal validity. Prior research has shown that work-related factors (e.g., low job control, long shift length) are associated with greater psychological distress among nurses (National Academies of Sciences Engineering and Medicine, 2019). Moreover, the prevalence of self-reported severe depressive symptoms in this study was higher than the one of diagnosed major depression in U.S. adult women (15% versus 8.7% ; National Survey on Drug Use and Health, 2017). While such higher rates in our sample may facilitate the detection of significant effects, it is unlikely that associations would be drastically different in a less distressed working population of women, which somewhat reduces concerns about generalizability. Nonetheless, additional research must verify whether the observed relationships apply to other populations, particularly different racial and working subgroups, as well as men.
Thirdly, as with all observational research, unmeasured or residual confounding remains possible. We controlled for multiple relevant covariates that may be common prior causes of both depressive symptoms and lung cancer; yet, some measures were somewhat limited (e.g., a single binary item to assess exposure to secondhand smoke in childhood) whereas other potential salient confounders may have been omitted (e.g., exposure to secondhand smoke in adulthood; American Cancer Society, 2020). Although we carefully examined the role of smoking status and lifetime pack-years in our analyses, residual confounding from these constructs may also exist. Nonetheless, given the robustness of associations across models, it is unlikely that unmeasured/residual confounding would substantially change our conclusions.
The current study also had numerous strengths. A primary advantage was its large sample size and long follow-up duration, which facilitated the ascertainment of a substantial number of cases and provided sufficient statistical power to detect existing associations between depressive symptoms and lung cancer risk. Other methodological assets were a thorough consideration of the potential roles of smoking, in order to disentangle its confounding, mediating, and effect modifying influence on the depression-lung cancer association, as well as alternative conceptualizations of depression, both in terms of types of indicators (self-reported symptoms, physician diagnosis, and antidepressant use) and chronicity/recentness of symptoms. Additional strengths of the study included its validation of lung cancer diagnoses via medical records.
In conclusion, this study demonstrates a modest prospective association between greater depressive symptoms and increased lung cancer risk, beyond statistical adjustment for sociodemographics, smoking habits, and other relevant factors. This association was partially mediated by lifetime pack-years and appeared relatively comparable across never, former, and current smokers. Smoking, however, remains the overwhelming cause of lung cancer (American Cancer Society, 2020). In the current sample, 87% of all lung cancer cases were observed among women who were current or former smokers. Thus, smoking prevention and cessation are the most important targets for lung cancer reduction. Depression, nevertheless, is a modifiable risk factor that promotes smoking initiation and interferes with cessation (Cooper et al., 2016; Trudel-Fitzgerald, Tworoger, et al., 2016; Weinberger et al., 2013), and thus should be prioritized as an upstream contributor to behavior. Additionally, because smoking mediated partly but not fully the depression-lung cancer risk association, future research into the potential biological mechanisms for such an effect is warranted. Although depression may not be the main driver of lung cancer absolute risk, given the prevalence of this mental disorder the attributable risk at the population level remains substantial. Altogether, this research points to the necessity of looking beyond behavioral determinants of disease to evaluate the psychosocial factors that may shape the distribution of those determinants as well as potentially exert a direct effect on disease risk.
Supplementary Material
Acknowledgements
We would like to thank the participants and the staff of the Nurses’ Health Study from the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA for their valuable contributions, as well as the following American state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Financial support:
This work was supported by the National Institutes of Health (grant number UM1 CA186107; P01 CA087969) for the Nurses’ Health Study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors declare no potential conflicts of interest.
References
- American Cancer Society. (2020). Cancer Facts & Figures 2020. Retrieved from Atlanta: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
- Arrieta O, Angulo LP, Nunez-Valencia C, Dorantes-Gallareta Y, Macedo EO, Martinez-Lopez D, … Onate-Ocana LF (2013). Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Annals of Surgical Oncology, 20(6), 1941–1948. doi: 10.1245/s10434-012-2793-5 [DOI] [PubMed] [Google Scholar]
- Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE, & Chavarro JE (2016). Origin, methods, and evolution of the three Nurses’ Health Studies. American Journal of Public Health, 106(9), 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DM, Murphy JM, Goldman PA Jr., W. J. E, Barsky AJ, & Weinstein MC (1991). Performance of a five-item mental health screening test. Medical Care, 29(2), 169–176. [DOI] [PubMed] [Google Scholar]
- Blumberg EM, West PM, & Ellis FW (1954). A possible relationship between psychological factors and human cancer. Psychosomatic Medicine, 16(4), 277–286. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM, & Horwood LJ (2010). Cigarette smoking and depression: Tests of causal linkages using a longitudinal birth cohort. British Journal of Psychiatry, 196(6), 440–446. doi: 10.1192/bjp.bp.109.065912 [DOI] [PubMed] [Google Scholar]
- Brenner DR, Yannitsos DH, Farris MS, Johansson M, & Friedenreich CM (2016). Leisure-time physical activity and lung cancer risk: A systematic review and meta-analysis. Lung Cancer, 95, 17–27. doi: 10.1016/j.lungcan.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, … Willett WC (1996). Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology, 7(1), 81–86. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Edmondson D, & Kronish IM (2015). State of the art review: Depression, stress, anxiety, and cardiovascular disease. American Journal of Hypertension, 28(11), 1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J, Borland R, McKee SA, Yong HH, & Dugue PA (2016). Depression motivates quit attempts but predicts relapse: Differential findings for gender from the International Tobacco Control Study. Addiction, 111(8), 1438–1447. doi: 10.1111/add.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, van Straten A, van Schaik A, & Andersson G (2009). Psychological treatment of depression in primary care: a meta-analysis. British Journal of General Practice, 59(559), e51–60. doi: 10.3399/bjgp09X395139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton SO, Mellemkjaer L, Olsen JH, Mortensen PB, & Johansen C (2002). Depression and cancer risk: A register-based study of patients hospitalized with affective disorders, Denmark, 1969–1993. American Journal of Epidemiology, 155(12), 1088–1095. [DOI] [PubMed] [Google Scholar]
- Donington JS, & Colson YL (2011). Sex and gender differences in non-small cell lung cancer. Seminars in Thorac and Cardiovascular Surgery, 23(2), 137–145. doi: 10.1053/j.semtcvs.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Gidron Y, Russ K, Tissarchondou H, & Warner J (2006). The relation between psychological factors and DNA-damage: A critical review. Biological Psychology, 72(3), 291–304. doi: 10.1016/j.biopsycho.2005.11.011 [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, & Willett WC (1991). The assessment of alcohol consumption by a simple self-administered questionnaire. American Journal of Epidemiology, 133(8), 810–817. [DOI] [PubMed] [Google Scholar]
- Glaser R, & Kiecolt-Glaser JK (2005). Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology, 5(3), 243–251. doi: 10.1038/nri1571 [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Wotton CJ, Yeates D, Seagroatt V, & Flint J (2007). Cancer in people with depression or anxiety: Record-linkage study. Social Psychiatry and Psychiatric Epidemiology, 42(9), 683–689. [DOI] [PubMed] [Google Scholar]
- Green McDonald P, O’Connell M, & Lutgendorf SK (2013). Psychoneuroimmunology and cancer: A decade of discovery, paradigm shifts, and methodological innovations. Brain, Behavior, and Immunity, 30 Suppl, S1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green McDonald P, O’Connell M, & Suls J (2015). Cancer control falls squarely within the province of the psychological sciences. American Psychologist, 70(2), 61–74. doi: 10.1037/a0038873 [DOI] [PubMed] [Google Scholar]
- Gross AL, Gallo JJ, & Eaton WW (2010). Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes & Control, 21(2), 191–199. doi: 10.1007/s10552-009-9449-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, & Grant BF (2018). Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry, 75(4), 336–346. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Poole EM, Okereke OI, Kubzansky LD, Eliassen AH, Sood AK, … Tworoger SS (2015). Depression and risk of epithelial ovarian cancer: Results from two large prospective cohort studies. Gynecologic Oncology, 139(3), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Holford TR, Levy DT, Feuer EJ, Cao P, Tam J, … Meza R (2018). Smoking and lung cancer mortality in the United States from 2015 to 2065: A comparative modeling approach. Annals of Internal Medicine, 169(10), 684–693. doi: 10.7326/M18-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami S, Han Y, Pande M, Cheng I, Rudd J, Pierce BL, … Tricl. (2016). Telomere structure and maintenance gene variants and risk of five cancer types. International Journal of Cancer, 139(12), 2655–2670. doi: 10.1002/ijc.30288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2003). Epidemiology of women and depression. Journal of Affective Disorders, 74(1), 5–13. [DOI] [PubMed] [Google Scholar]
- Knekt P, Raitasalo R, Heliovaara M, Lehtinen V, Pukkala E, Teppo L, … Aromaa A (1996). Elevated lung cancer risk among persons with depressed mood. American Journal of Epidemiology, 144(12), 1096–1103. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Bennett GG, Fuchs C, Giovannucci E, Kawachi I, Schernhammer E, … Kubzansky LD (2005). Depressive symptoms and prospective incidence of colorectal cancer in women. American Journal of Epidemiology, 162(9), 839–848. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, & Zvolensky MJ (2015). Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin, 141(1), 176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JA, Sun LM, Muo CH, Sung FC, Chang SN, & Kao CH (2011). The analysis of depression and subsequent cancer risk in Taiwan. Cancer Epidemiology, Biomarkers & Prevention, 20(3), 473–475. doi: 10.1158/1055-9965.EPI-10-1280 [DOI] [PubMed] [Google Scholar]
- Luijendijk HJ, van den Berg JF, Dekker MJ, van Tuijl HR, Otte W, Smit F, … Tiemeier H (2008). Incidence and recurrence of late-life depression. Archives of General Psychiatry, 65(12), 1394–1401. doi: 10.1001/archpsyc.65.12.1394 [DOI] [PubMed] [Google Scholar]
- McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, … Willett WC (2002). Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. American Journal of Clinical Nutrition, 76(6), 1261–1271. [DOI] [PubMed] [Google Scholar]
- Meng S, De Vivo I, Liang L, Hu Z, Christiani DC, Giovannucci E, & Han J (2016). Pre-diagnostic leukocyte mitochondrial DNA copy number and risk of lung cancer. Oncotarget, 7(19), 27307–27312. doi: 10.18632/oncotarget.8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2019). Factors contributing to clinician burnout and professional well-being. In Taking action against clinician burnout: A systems approach to professional well-being. Washington (DC: ): The National Academies Press. [PubMed] [Google Scholar]
- National Survey on Drug Use and Health. (2017). Major depression: Prevalence of major depressive episode among adults. Retrieved from https://www.nimh.nih.gov/health/statistics/major-depression.shtml#part_155033
- Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, & Havlik RJ (1998). Chronically depressed mood and cancer risk in older persons. Journal of the National Cancer Institute, 90(24), 1888–1893. [DOI] [PubMed] [Google Scholar]
- Pirl WF, Greer JA, Traeger L, Jackson V, Lennes IT, Gallagher ER, … Temel JS (2012). Depression and survival in metastatic non-small-cell lung cancer: Effects of early palliative care. Journal of Clinical Oncology, 30(12), 1310–1315. doi: 10.1200/JCO.2011.38.3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Corsano KA, & Stampfer MJ (1994). Test of the National Death Index and Equifax Nationwide Death Search. American Journal of Epidemiology, 140(11), 1016–1019. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, & Willett WC (1992). Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American Journal of Epidemiology, 135(10), 1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- Rumpf HJ, Meyer C, Hapke U, & John U (2001). Screening for mental health: validity of the MHI-5 using DSM-IV Axis I psychiatric disorders as gold standard. Psychiatry Research, 105(3), 243–253. [DOI] [PubMed] [Google Scholar]
- SAS Institute. (2011). The SAS sytem for Windows. Cary, NC: SAS Institute. [Google Scholar]
- Schernhammer ES, Feskanich D, Liang G, & Han J (2013). Rotating night-shift work and lung cancer risk among female nurses in the United States. American Journal of Epidemiology, 178(9), 1434–1441. doi: 10.1093/aje/kwt155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D, & Giese-Davis J (2003). Depression and cancer: Mechanisms and disease progression. Biological Psychiatry, 54(3), 269–282. [DOI] [PubMed] [Google Scholar]
- Sullivan DR, Ganzini L, Duckart JP, Lopez-Chavez A, Deffebach ME, Thielke SM, & Slatore CG (2014). Treatment receipt and outcomes among lung cancer patients with depression. Clinical Oncology (Royal College of Radiologists), 26(1), 25–31. doi: 10.1016/j.clon.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Sun Y, Li Z, Li J, Li Z, & Han J (2016). A healthy dietary pattern reduces lung cancer risk: A systematic review and meta-analysis. Nutrients, 8(3), 134. doi: 10.3390/nu8030134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Chen Y, Singh A, Okereke OI, & Kubzansky LD (2016). Psychiatric, psychological, and social determinants of health in the Nurses’ Health Study cohorts. American Journal of Public Health, 106(9), 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel-Fitzgerald C, Tworoger SS, Poole EM, Williams DR, & Kubzansky LD (2016). Prospective changes in healthy lifestyle among midlife women: When psychological symptoms get in the way. American Journal of Preventive Medicine, 51(3), 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, & Vanderweele TJ (2013). Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychological Methods, 18(2), 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ (2011). Causal mediation analysis with survival data. Epidemiology, 22(4), 582–585. doi: 10.1097/EDE.0b013e31821db37e [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ (2013). A three-way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology, 24(2), 224–232. doi: 10.1097/EDE.0b013e318281a64e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen E, Seppala T, Lillsunde P, & Puska P (2002). Validation of self reported smoking by serum cotinine measurement in a community-based study. Journal of Epidemiology and Community Health, 56(3), 167–170. doi: 10.1136/jech.56.3.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yang T, Guo XF, & Li D (2019). The associations of fruit and vegetable intake with lung cancer risk in participants with different smoking status: A meta-analysis of prospective cohort studies. Nutrients, 11(8). doi: 10.3390/nu11081791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr., & Sherbourne CD (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30(6), 473–483. [PubMed] [Google Scholar]
- Weinberger AH, Pilver CE, Desai RA, Mazure CM, & McKee SA (2013). The relationship of dysthymia, minor depression, and gender to changes in smoking for current and former smokers: Longitudinal evaluation in the U.S. population. Drug and Alcohol Dependence, 127(1–3), 170–176. doi: 10.1016/j.drugalcdep.2012.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (1967). International Statistical Classification of Diseases, Injuries, and Causes of Death, Eighth Revision. Retrieved from Geneva: https://www.who.int/news-room/spotlight/international-classification-of-diseases
- World Health Organization. (2017). Depression and other common mental disorders: Global health estimates. Retrieved from Geneva: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


