Abstract
Background
Hypoxic-ischemic encephalopathy (HIE) brain damage is related to inflammatory responses and oxidative stress. Interleukin (IL)-35 is an antioxidant and anti-inflammatory cytokine. Thus, the effect of IL-35 treatment on neonatal rats with hypoxic-ischemic brain injury was investigated.
Methods
A total of 96 7-day-old Sprague Dawley rats were randomly divided into three groups: sham group, HIE group, and IL-35 group. After left common carotid occlusion and 2.5 h hypoxia (HI injury), IL-35 (20 µg/g) was intraperitoneally (i.p.) administered to the pups. In vitro, BV2 cells were treated with or without IL-35 6 h before oxygen-glucose deprivation (OGD) insult and the microglia culture medium (MCM) was co-cultured with b.End3 cerebral vascular endothelial cells. Microglial polarization and activation were assessed by real-time quantitative polymerase chain reaction (RT-qPCR), Western blot, and enzyme-linked immunosorbent assay (ELISA). Endothelial cell dysfunction was measured by cell counting kit-8 and Western blot assays.
Results
Administration of IL-35 alleviated neurological deficiencies, decreased brain edema, ameliorated cerebral infarction, and limited M1 microglial polarization in HI-injured pups. Meanwhile, IL-35 decreased pro-inflammatory cytokines, tumor necrosis factor-α, IL-1β, and reactive oxygen species generation in OGD-induced bEnd.3 cells. Furthermore, IL-35 treatment could reverse the vascular endothelial cell injury induced by microglial polarization. Finally, IL-35 markedly suppressed the activation of hypoxia-inducible factor-1α (HIF-1α) and the nuclear factor-κB (NF-κB) signaling pathway in vivo and in vitro.
Conclusions
IL-35 relieved hypoxic-ischemic-induced brain injury and inhibited the inflammatory response by suppressing microglial polarization and activation. These results suggest that IL-35 might have potential applications for the treatment of HIE.
Keywords: Hypoxic-ischemic encephalopathy (HIE), interleukin-35 (IL-35), microglia
Introduction
Hypoxic-ischemic encephalopathy (HIE) is caused by insufficient cerebral blood flow and oxygen supply, which directly leads to approximately 24% of neonatal deaths and results in severe neurological disabilities (1). Currently, therapeutic hypothermia, performed immediately after birth, has been demonstrated to have neuroprotective effects, as evidenced by improved functions and minimized symptoms induced by HIE (2,3). However, many infants still die or suffer from severe neurological impairment (3). Therefore, there is an urgent need to clarify the molecular mechanisms of HIE and develop more effective therapeutic strategies.
Hypoxic ischemia is considered to be a powerful stimulus that initiates a series of events including rapid activation of resident microglia (4), infiltration of circulating inflammatory cells into ischemic lesions, and blood brain barrier (BBB) damage (5). Microglia are resident immune cells of the central nervous system and crucial mediators of neuroinflammation (6). Microglial activation and aggregation are pathological markers of HIE in human infants (6). After hypoxia-ischemia, microglia can be activated to a pro-inflammatory (M1) or anti-inflammatory (M2) phenotype depending on the stimuli they received and the progression of injury (3). M1 microglia are related to increased protein expression of inflammatory mediators [interleukin-6 (IL-6), IL-1β, tumor necrosis factor (TNF)-α, CD32, and CD86, among others] and reactive oxygen species (ROS) production (7). In contrast, M2 microglia are associated with anti-inflammatory mediators [arginase-1 (Arg-1), CD206, and chitinase-like protein 3 (Ym-1)] and enhanced expression of genes related to inflammation resolution, scavenging, and homeostasis (4). Uncontrolled activation of M1 microglia can injure healthy neurons and reduce the survival rate of injured neurons. In contrast, M2 microglia inhibit inflammation and contribute to recovery after brain injury (4). Corroborating these findings, growing evidence suggests that the regulation of microglia polarization is an important target for HIE treatment (4,8).
Interleukin-35 (IL-35) is a novel immunosuppressive cytokine, consisting of P35 and EBI3 subunits (9). It has been found that IL-35 exerts strong immunosuppressive effects in inflammatory diseases, bacterial and viral infectious diseases, several autoimmune diseases, and tumors (10). For example, IL-35 decreases the ratio of M1/M2-like macrophages and the total number of macrophages, thereby inhibiting the inflammatory process in psoriasis (11). In an experimental study by Xu et al., IL-35 was shown to exert anti-inflammatory effects and improve neurological function in mice with cerebral ischemia (12). Importantly, Kang et al. reported that enteritis symptoms were significantly alleviated by exosome-mediated IL-35 overexpression in the experimental autoimmune uveitis mouse model (13). However, the therapeutic effect of IL-35 on HIE needs further research.
Here, we asked whether IL-35 indeed exerts a protective effect in the cerebral injury of HIE, and if so, whether the M1 polarization of microglia might be ameliorated by treatment with IL-35. To test this hypothesis, the effects of IL-35 on a rat model of neonatal HIE and an in vitro oxygen-glucose deprivation (OGD) model were investigated. In addition, considering that hypoxia-inducible factor-1α (HIF-1α), regulated by the activation of nuclear factor-κB (NF-κB) p65, is a major player in hypoxia-ischemia and inflammation (14,15), the effects of IL-35 on the NF-κB/HIF-1α signaling pathway were also investigated. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-100/rc).
Methods
Animals
In this study, unsexed Sprague Dawley rat pups and their mothers were purchased from Nanjing Medical University. Seven-day-old rat pups (n=96, weight =11–17 g) were used. All animals were maintained on a 12-h light/dark cycle in a controlled room environment with free access to breast milk, water, and food. Animal experiments were performed at Laboratory Animal Center of Nantong University under a project license (No. 20210166) granted by the Animal Experimentation Committee of Nantong University and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals in Neuroscience Research (16).
Neonatal HIE model
The animal model of neonatal hypoxic-ischemic brain injury was established as previously described (17). Briefly, the pups were anesthetized with 3% isoflurane (2.5% maintenance) in air during surgery and the temperature was controlled using a heated blanket. Rats’ necks were wiped with alcohol and procedures were performed using standard sterile techniques. A small lateral incision (5 mm in length) was made and the right common carotid artery (CCA) was separated. Next, the right CCA was double ligated using a 5.0 surgical suture and transected between the ligatures. The pups were then allowed to regain consciousness for 1 h. The pups were then placed in a hypoxia chamber (8% O2/92% N2) at 37 ℃ for 2 h. The sham pups were anesthetized and the right CCA was exposed, but no ligation, cutting, or exposure to hypoxia was performed. Thereafter, the pups were returned to their mothers.
Experimental design and treatment
A total of 96 7-day-old pups were used for this study. Eighteen pups were used in each experiment (n=6/group), except for the evaluation of neurological deficit (n=8/group), as follows: (I) infarct volume assessment; (II) brain water content; (III) Western blotting; (IV) real-time quantitative polymerase chain reaction (RT-PCR) analysis. Pups died before sampling (n=10) were excluded from total numbers.
Pups were randomly and blindly separated into three groups: (I) HI + PBS group; (II) HI + IL-35 group; (III) sham-operated group. IL-35 (St. Louis, MO, USA) was diluted with phosphate-buffered saline (PBS, 0.01 M, pH 7.4) and pups in the IL-35 treatment groups were intravenously administered with IL-35 (20 μg/g body weight) immediately and 24 h post HI. The pups in the HI and sham groups were injected with the same volume of PBS (Figure 1A). All researchers conducting animal testing were blinded to the treatment conditions.
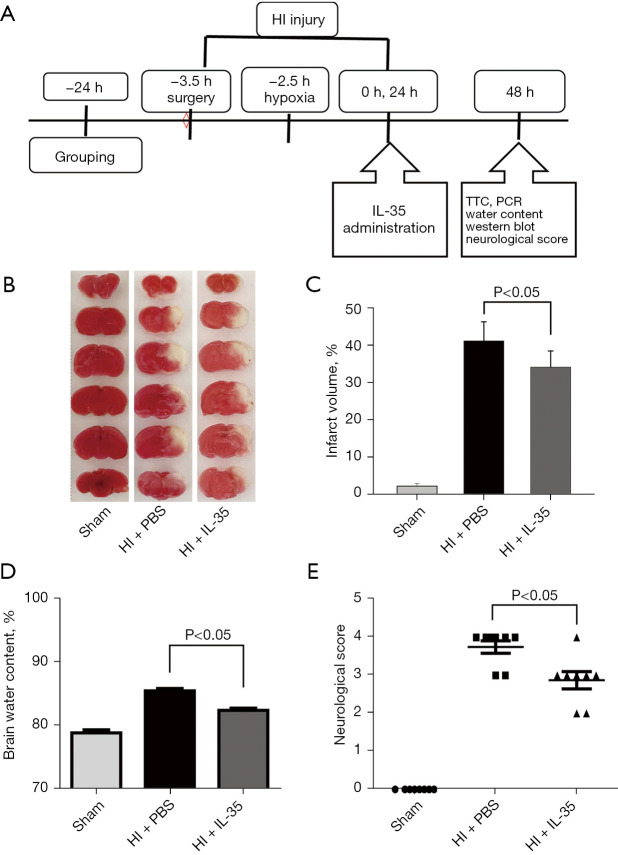
Figure 1.
IL-35 reduced brain injury in pups subjected to HI insult. Intraperitoneal injection of IL-35 (20 µg/g BW) in HIE pups immediately and 24 h post HI. (A) Diagram of the in vivo experiment; (B) representative images of TTC staining of each group at 48 h post HI; (C) results of infarct volume at 48 h following HI insult. n=6 in each group; (D) brain water content was assessed at 48 h following HI insult. n=6 in each group; (E) neurological scores were recorded at 48 h post HI. n=8 in each group. Error bars represent the mean ± SD. IL, interleukin; HIE, hypoxic-ischemic encephalopathy; HI, hypoxic ischemia; TTC, 2,3,5-triphenyltetrazolium chloride; BW, body weight; PCR, polymerase chain reaction; PBS, phosphate-buffered saline.
Evaluation of neurological deficits
Neurological deficits of the experimental pups were evaluated 48 h after HI injury (n=8/group), as previously described (18). Briefly, neurological scores of the pup were assessed as follows: 0 (normal motor function), 1 (forelimb weakness and torso turning to the affected side when held by the tail), 2 (circling to the affected side when held by the tail), 3 (circling to the ipsilateral side or unable to bear weight on the ipsilateral side), 4 (rolling to the affected side), and 5 (no barrel rolling or spontaneous motor activity). In this evaluation method, a lower score indicates less neurological deficits.
Infarct volume assessment
Cerebral infarction was measured by 2,3,5-triphenyltetrazolium chloride (TTC) (Yi Fei Xue Biotechnology, Nanjing, China) staining 48 h after HI injury. Briefly, the pups (n=6/group) were anesthetized and euthanized, followed by transcardiac perfusion using chilled PBS (20 mL, 0.01 M, pH 7.4). The brains were removed and separated into serial coronal sections (2 mm thickness). Then, these sections were soaked in TTC phosphate buffer (2%, 37 ℃) for 15 min and paraformaldehyde phosphate buffer (4%) for 30 min in a dark place. Areas of red (normal brain tissues) and white (infarct tissues) staining were measured using an image analysis system (Image-Pro Plus6.0, Media Cybernetics, Wyoming, USA). The infarction volume = infarct area (white pale area)/total area of slice × 100%.
Brain water content
The wet-dry method was used to determine brain water content 48 h after HI injury. Briefly, the pups (n=6/group) were anesthetized and euthanized. Then, the brains were dissected out and sliced. The sections were quickly weighed (wet weight), and then placed in an oven (100 ℃) for 24 h to assess dry weight. The water content was calculated as follows: [(wet weight) – (dry weight)]/(wet weight) ×100%.
Cell culture
The brain endothelial cell line bEnd.3 and microglia BV2 cells were purchased from Procell Life Science and Technology, Co., Ltd. (Wuhan, Hubei, China). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (4×105 cells per well) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin and streptomycin, and L-glutamine (2.0 mM), and incubated in a humidified atmosphere containing 5% CO2 at 37 ℃.
OGD insult and IL-35 treatment
With the purpose of evaluating the effects of IL-35 on BV2 cells, cells were treated with IL-35 6 h before and during OGD, an accepted in vitro HI cell model (19). Briefly, cells were transferred to glucose- and serum-free medium inside a hypoxia chamber (H35; Don Whitley Scientific Ltd., Shipley, UK), and maintained at 37 ℃ in 5% CO2 and 95% N2 for 6 h. Then, cells were cultured with fresh DMEM and moved to an incubator with 5% CO2 and 95% air at 37 ℃ for 24 h. Cells in the control group were cultured with DMEM under normal conditions. Finally, BV2 cells were collected and cell polarization was assessed by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) and Western blot analysis.
Co-culture of endothelial cells and BV2 cells
Co-culture of IL-35-treated microglia culture medium (MCM) and endothelial cells was used to test the indirect impact of microglia polarization on endothelial cells. The MCM was mixed with complete DMEM at a ratio of 1:1. Endothelial cells were then cultured with this mixed culture medium under normal culture conditions for 24 h. Then, a cell counting kit-8 (CCK8) (Dojindo Laboratories, Kumamoto, Japan) was used to evaluate endothelial cell viability, and the experimental steps were strictly performed according to the manufacturer’s manual. The concentration of TNF-α and IL-1β in the culture supernatant was assessed using enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s protocol (Yi Fei Xue Biotechnology, Nanjing, China). The expression levels of zonula occludens-1 (ZO-1) and occludin in endothelial cells were measured using Western blots.
Real-time quantitative PCR (RT-qPCR)
Pups were sacrificed after prior anesthesia 48 h after HI injury (n=6/group) and the cortical tissue in the peri-infarct was obtained. Total RNA was extracted from cerebral cortices and BV2 cells using Trizol Reagent (Invitrogen, Grand Island, NY, USA) and reverse transcribed to cDNA using a cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, WA, USA). RT-qPCR was performed by the SYBR Green method (K1622, Applied Biosystems, CA, USA). The reaction was performed at 95 ℃ for 5 min followed by 40 cycles of 95 ℃ for 15 s and 55 ℃ for 40 s on an IQ5 PCR machine (Bio-Rad, Hercules, CA, USA). The relative expression of the targeted mRNA was normalized to the expression of β-actin. Sequence-specific primers were designed according to previous literature (20).
Western blot analysis
Pups were sacrificed after prior anesthesia 48 h after HI injury (n=6/group). The cortical tissue in the peri-infarct was dissected out, washed in cold PBS, and homogenized below 4 ℃. The total protein was lysed using radioimmunoprecipitation assay (RIPA) lysis buffer. Then, equal amounts of proteins (30 μg) were separated on 10% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA, USA) and transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore Corporation, Billerica, USA). After blocking with 5% nonfat dry milk for 2 h, the membranes were incubated overnight at 4 ℃ with primary antibodies against ZO-1 (1:1,000, Abcam, Cambridge, MA, USA), occludin (1:1,000, Abcam, Cambridge, MA, USA), NF-kB pp65, and HIF-α (both 1:1,000, CST, Danvers, MA, USA). Next, the blots were incubated for 1 h with horseradish peroxidase (HRP)-conjugated secondary antibodies (dilution 1:10,000, Yi Fei Xue Biotechnology, Nanjing, China) at room temperature. Finally, the bands were visualized on a ChemiDoc™ MP System (Bio-Rad, Hercules, CA, USA). The results were quantified by normalizing the relative intensity to glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical analysis
All statistical analyses were carried out with GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA). Normally distributed data was expressed as mean ± SD. Comparisons between two groups were performed using the Student’s t-test. Multiple comparisons among three groups were performed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. A two-tailed P<0.05 was considered statistically significant.
Results
IL-35 alleviated brain injury following neonatal HI insult
To evaluate the effect of IL-35 treatment on brain injury post HI, IL-35 was injected immediately post HI and infarct volume was assessed by TTC stains 48 h post HI (Figure 1A). As expected, extensive infarctions were detected in the PBS groups subjected to HI in comparison with sham groups (P<0.05; Figure 1B,1C). Importantly, IL-35 treatment markedly reduced infarct volume compared to the PBS group (P<0.05; Figure 1B,1C). In the pathological condition of brain injury post HI, the BBB is mainly impacted. Therefore, brain water content assessment was performed to evaluate BBB permeability 48 h after HI. The results indicated that cerebral HI caused an increase in brain water content (vs. sham, P<0.05) and IL-35 administration resulted in a marked reduction in brain edema (Figure 1D).
In addition, we also performed Longa’s scoring to examine neurological deficits 48 h after HI injury. Corresponding to tissue loss, pups subjected to HI displayed obvious behavioral deficits compared to the sham groups (P<0.05; Figure 1E). Moreover, IL-35 treatment dramatically attenuated HI-induced increases in behavioral deficits (P<0.05, Figure 1E). Together, IL-35 treatment alleviated brain injury and improved functional recovery after HI injury.
IL-35 treatment suppressed M1 microglial polarization after HI injury
Microglia are major mediators of neuroinflammation and can be activated after HI injury. To further reveal the mechanism by which IL-35 modulates microglia, representative markers of M1 microglia (IL-1β, TNF-α, and CD32) and M2 microglia (Arg-1, CD206, and YM-1) were assessed using RT-qPCR analysis in the cortical tissue of the penumbra 48 h after HI. The results showed that HI injury enhanced M1 microglial polarization, whereas IL-35 treatment inhibited HI-induced M1 polarization and promoted M2 polarization (Figure 2A-2F).
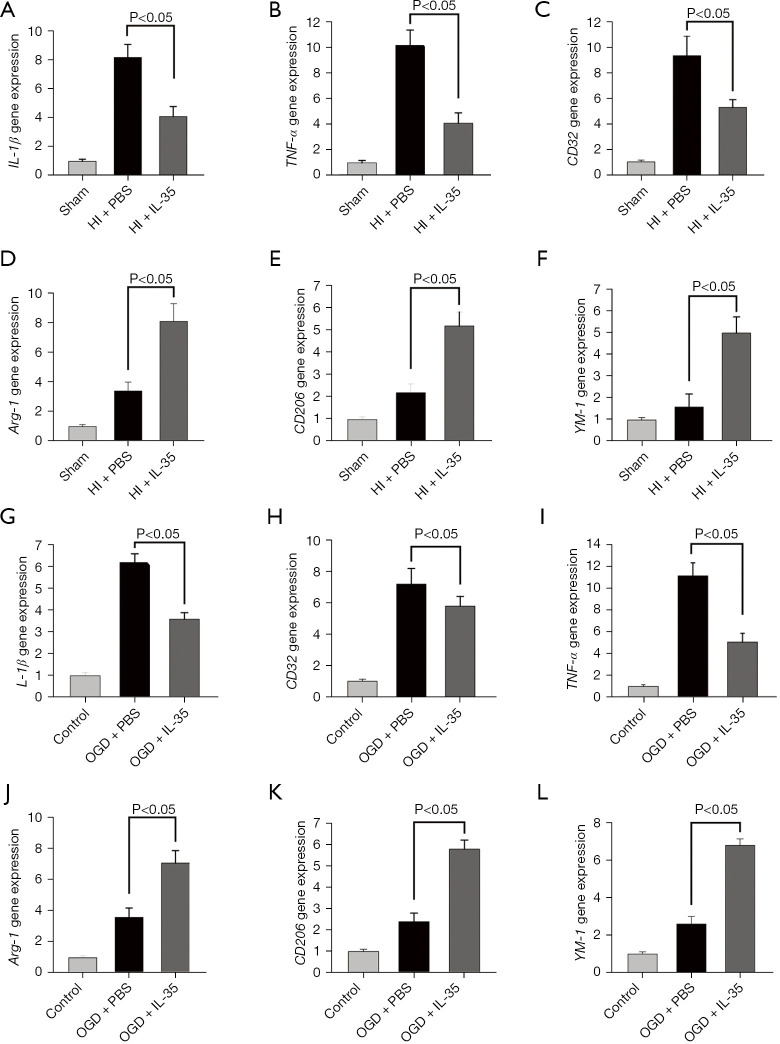
Figure 2.
IL-35 treatment suppressed M1 microglial polarization after HI injury. Intraperitoneal injection of IL-35 (20 µg/g BW) in HIE pups immediately and 24 h post HI. Microglial polarization was assessed at 48 h following HI insult. (A-C) M1 markers (IL-1β, TNF-α, and CD32) were assessed by RT-qPCR analysis; (D-F) M2 markers (Arg-1, CD206, and YM-1) were assessed by RT-qPCR analysis. n=6 in each group. Error bars represent the mean ± SD. BV2 cells were cultured with IL-35 (20 ng/mL) for 6 h and then subjected to 6 h OGD and 24 h reoxygenation; (G-I) M1 markers (IL-1β, CD32, and TNF-α) were assessed at 24 h post-reoxygenation by RT-qPCR analysis; (J-L) M2 markers (Arg-1, CD206, and YM-1) were assessed 24 h post-reoxygenation by RT-qPCR analysis. Error bars represent the mean ± SD of at least 3 independent experiments. IL, interleukin; HIE, hypoxic-ischemic encephalopathy; RT-qPCR, real-time quantitative polymerase chain reaction; OGD, oxygen-glucose deprivation; TNF-α, tumor necrosis factor-α; Arg-1, arginase-1; Ym-1, chitinase-like protein 3; BW, body weight; PBS, phosphate-buffered saline; HI, hypoxic ischemia.
Furthermore, the effect of IL-35 on OGD-induced polarization of BV2 cells was examined. As expected, the results showed that OGD injury enhanced IL-1β, TNF-α, and CD32 gene expression, whereas IL-35 treatment inhibited OGD-induced IL-1β, TNF-α, and CD32 gene expression and promoted Arg-1, CD206, and YM-1 gene expression (Figure 2G-2L). Overall, IL-35 suppressed HI-induced M1 microglia polarization and promoted M2 microglia polarization.
IL-35-treated MCM alleviated vascular endothelial cell injury
To better reveal the effect of BV2 cell polarization on BBB dysfunction, co-culturing of endothelial cells with MCM was performed. BV2 cells were treated with or without IL-35 6 h before OGD insult and the MCM was collected 24 h after OGD insult. Then, MCM was mixed with DMEM (1:1) and this mixed medium was used to culture vascular endothelial cells under normal conditions for 24 h (Figure 3A). We first investigated whether IL-35 modulated OGD-induced microglial release of TNF-α and IL-1β cytokines by ELISA analysis. The results showed that OGD induced high concentrations of TNF-α and IL-1β in the culture supernatant, which were decreased in response to IL-35 treatment (Figure 3B,3C).
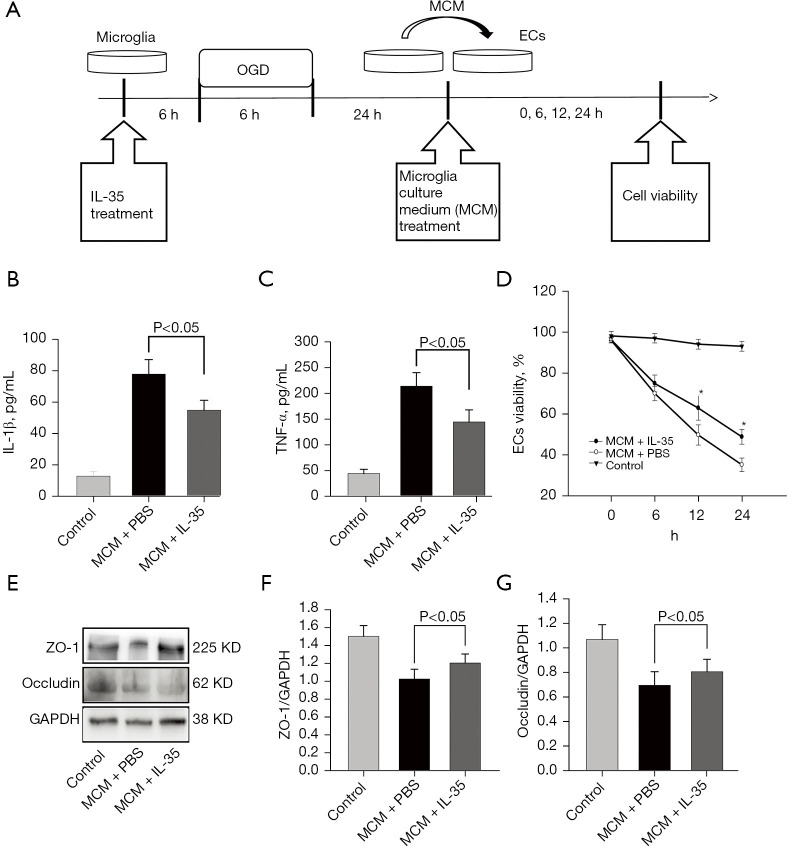
Figure 3.
IL-35-treated microglia culture medium alleviated vascular endothelial cell injury. BV2 cells were cultured with IL-35 for 6 h and then subjected to 6 h OGD and 24 h reoxygenation. The culture medium was mixed with complete DMEM at a ratio of 1:1. Vascular endothelial cells were cultured with this mixed culture medium under normal culture conditions for 24 h. (A) Diagram of the in vitro experiment; (B,C) ELISA was performed to detect the concentration of IL-1β and TNF-α in the supernatant; (D) the viability of ECs was assessed using CCK8 assays; (E) the expression levels of ZO-1 and occludin were measured using Western blots; (F,G) Western blot assay revealed that IL-35 could preserve ZO-1 and occludin protein expression. Error bars represent the mean ± SD of at least 3 independent experiments. *, P<0.05, vs. MCM without IL-35. IL, interleukin; OGD, oxygen-glucose deprivation; ECs, Endothelial cells; CCK8, cell counting Kit-8; MCM, microglia culture medium; ZO-1, zonula occludens-1; ELISA, enzyme-linked immunosorbent assay; TNF-α, tumor necrosis factor-α; MCM, microglia culture medium; DMEM, Dulbecco’s Modified Eagle’s Medium; PBS, phosphate-buffered saline.
The results of endothelial cell viability showed that OGD-induced endothelial cell injury was increased after MCM treatment without IL-35, whereas IL-35 significantly reduced MCM-induced endothelial cell death (Figure 3D). Furthermore, similar results were found in tight junction protein expression. Compared with the untreated group, MCM treatment without IL-35 resulted in decreased ZO-1 and occludin expression (Figure 3E-3G). However, the damage of tight junction proteins was reversed by the addition of IL-35 (Figure 3E-3G, all P<0.05). Taken together, these results suggested that IL-35 treatment could reverse vascular endothelial cell injury induced by microglia polarization.
IL-35 inhibited ROS production in OGD-induced BV2 cells
It is widely known that excessive accumulation of free radicals can lead to M1 polarization of microglia. Due to its antioxidant effect, we hypothesized that IL-35 can inhibit M1 microglia polarization by reducing ROS. To verify our hypothesis, we assessed ROS levels in BV2 cells induced by OGD. The results of the dichlorofluorescin diacetate (DCFH-DA) fluorescence assay demonstrated that treatment with IL-35 reduced the production of ROS (Figure 4A,4B). In addition, our data showed that IL-35 reduced the concentration of malondialdehyde (MDA) in BV2 cells (vs. OGD group, P<0.05, Figure 4C).
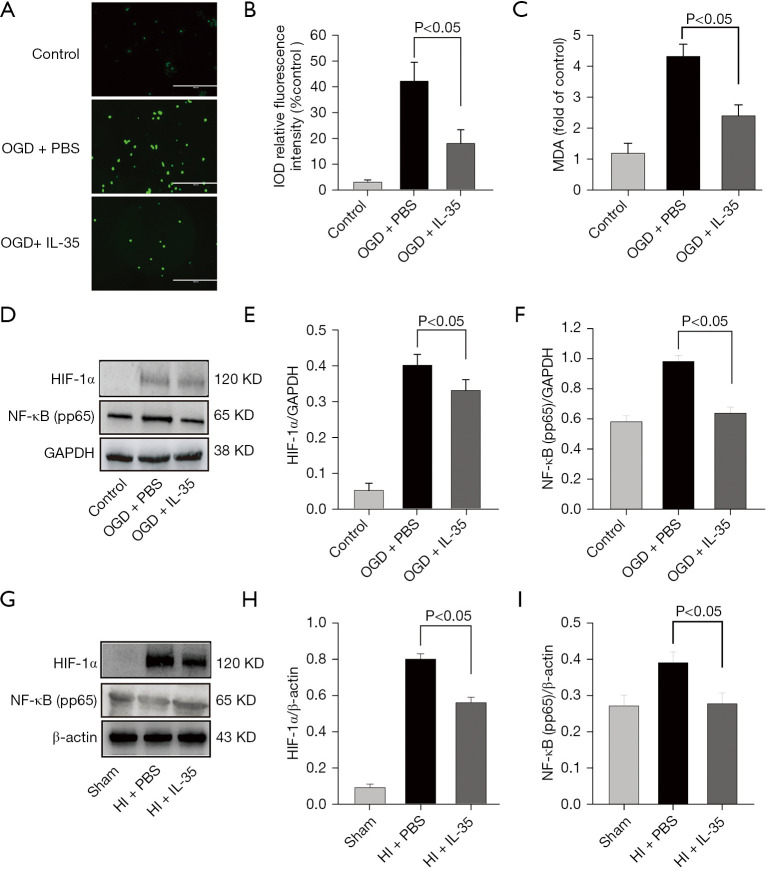
Figure 4.
IL-35 inhibited ROS production in OGD-induced BV2 cells. BV2 cells were cultured with IL-35 for 6 h and then subjected to 6 h OGD and 24 h reoxygenation. (A) Intracellular ROS was viewed with fluorescence microscopy. Scale bar =100 µm. (B) The resulting fluorescence intensity was quantified using ImageJ. (C) The MDA concentration in cell lysates was determined. (D) Representative Western blot bands of NF-κB pp65 and HIF-1α protein expression in BV2 cells induced by OGD-R. (E,F) Western blot assay revealed that IL-35 could suppress the NF-κB pp65 and HIF-1α protein expression in BV2 cells induced by OGD-R. The data were normalized to GAPDH expression (n=3). Error bars represent the mean ± SD of at least 3 independent experiments. (G) Intraperitoneal injection of IL-35 (20 µg/g BW) in HIE pups immediately and 24 h post HI. The cortical tissue in the penumbra was obtained at 48 h post HI. Representative blots of NF-κB pp65 and HIF-1α protein expression in each group. (H,I) Western blot assay revealed that IL-35 could suppress the NF-κB pp65 and HIF-1α protein expression. The data were normalized to β-actin expression (n=6 in each group). IL, interleukin; ROS, reactive oxygen species; OGD, oxygen-glucose deprivation; MDA, malondialdehyde; HIE, hypoxic-ischemic encephalopathy; HI, hypoxic ischemia; HIF-1α, hypoxia-inducible factor-1α; NF-κB, nuclear factor-κB; BW, body weight; PBS, phosphate-buffered saline.
High ROS levels could trigger the phosphorylation of NF-kB p65 and then upregulate transcription of HIF-1α. Furthermore, NF-κB and HIF-1α play important roles in ischemic stroke and M1 microglial polarization (21). Consistent with previous study (21), the Western blot results showed that NF-kB p65 phosphorylation and HIF-1α were significantly increased after HI injury, and IL-35 treatment limited these processes in vivo and in vitro (Figure 4D-4I). Taken together, our data demonstrated that IL-35 may inhibit the activation of M1 microglia at least partly through regulating the ROS/NF-κB/HIF-1α signal pathway.
Discussion
In this study, we investigated the efficacy of IL-35 in HI-induced pups and OGD-insulted BV2 cells. In vivo results showed that IL-35 treatment could reduce the infarct volume, attenuate neuronal degeneration, ameliorate neurological outcomes, and suppress M1 microglial polarization in HI-injured rats. Furthermore, in vitro results demonstrated that IL-35 could reduce OGD-insulted microglia polarization and modulate cytokine release. These effects might be partly associated with its ability to inhibit the ROS/NF-κB/HIF-1α pathway.
Early after HI insult, glucose and oxygen deprivation in cells leads to a drop in adenosine triphosphate availability, resulting in increased excitotoxicity, neurotoxicity, and oxidative stress (22). After successful resuscitation, restoring blood flow triggers the production of ROS, which causes local inflammatory responses (23). Accordingly, many anti-inflammatory drugs have been considered to be useful for the treatment of cerebral ischemia/reperfusion (I/R) injury, such as dimethyl fumarate and minocycline (24). In this study, IL-35 treatment alleviated brain damage and improved functional recovery after HI injury.
It is well known that microglia polarization plays a key role in brain damage in neonates with HIE (25). Compared to adults, neonatal microglia activation occurs more swiftly after ischemic insults and continues for weeks (25). Furthermore, regulation of microglia activation can provide effective neuroprotection after HI insult (1,26). In our in vitro and in vitro experiments, we found that IL-35 limited M1 polarization and enhanced M2 polarization, as evidenced by changes in M1/M2-related mRNA and proteins. In line with these findings, IL-35 has been demonstrated to decrease the ratio of M1/M2 macrophages in diabetic neuropathic pain (27).
In the context of HI injury, activated microglia release various pro-inflammatory factors, such as TNF-α, IL-1β, and ROS, eventually causing tissue damage (28-30). Under hypoxic conditions, the HIF-1α and NF-κB signaling pathways are activated and operate synergistically to regulate the transcription of genes associated with pro-inflammatory chemokines, cytokines, and adhesion molecules (31-33). The activation of HIF-1α also enhances M1 macrophage polarization and inflammation (34). In this study, we confirmed that treatment with IL-35 limited the upregulation of NF-κB pp65 and HIF-1α, as well as the production of TNF-α, IL-1β, and ROS in OGD-induced BV2 cells, thus providing an indirect protective effect on endothelial cells.
Our study has 4 limitations. Firstly, we cannot rule out that IL-35 also works by modulating other cells, such as monocytes, macrophages, and Th17 cells. Secondly, the dose, therapeutic window, and intervention methods of IL-35 deserve further study. Thirdly, further studies are needed to investigate the dynamic changes of IL-35 levels in the brain tissues of HIE pups. Finally, very little is known about the long-term effects of IL-35 on HIE.
In summary, we demonstrated that IL-35 alleviated brain injury and improved neurological outcomes in a rat model of neonatal HIE. We showed that this role involves regulating microglial polarization. The ameliorating effect of IL-35 against the polarization of M1 microglia was partly realized through the ROS, HIF-1α, and NF-κB signaling pathways, thereby suppressing endothelial cell dysfunction. Therefore, our findings show that IL-35 may have important applications in the clinical treatment of HIE.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by a project of Medical and Health Technology Development Program in Yancheng City, China (YK2016065).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed at Laboratory Animal Center of Nantong University under a project license (No. 20210166) granted by the Animal Experimentation Committee of Nantong University and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals in Neuroscience Research.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-100/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-100/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-100/coif). The authors have no conflicts of interest to declare.
References
- 1.Le K, Song Z, Deng J, et al. Quercetin alleviates neonatal hypoxic-ischemic brain injury by inhibiting microglia-derived oxidative stress and TLR4-mediated inflammation. Inflamm Res 2020;69:1201-13. 10.1007/s00011-020-01402-5 [DOI] [PubMed] [Google Scholar]
- 2.Gunn AJ, Thoresen M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb Clin Neurol 2019;162:217-37. 10.1016/B978-0-444-64029-1.00010-2 [DOI] [PubMed] [Google Scholar]
- 3.Wassink G, Davidson JO, Dhillon SK, et al. Therapeutic Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Curr Neurol Neurosci Rep 2019;19:2. 10.1007/s11910-019-0916-0 [DOI] [PubMed] [Google Scholar]
- 4.Guo J, Zhang XL, Bao ZR, et al. Gastrodin Regulates the Notch Signaling Pathway and Sirt3 in Activated Microglia in Cerebral Hypoxic-Ischemia Neonatal Rats and in Activated BV-2 Microglia. Neuromolecular Med 2021;23:348-62. 10.1007/s12017-020-08627-x [DOI] [PubMed] [Google Scholar]
- 5.Gussenhoven R, Klein L, Ophelders DRMG, et al. Annexin A1 as Neuroprotective Determinant for Blood-Brain Barrier Integrity in Neonatal Hypoxic-Ischemic Encephalopathy. J Clin Med 2019;8:137. 10.3390/jcm8020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou D, Ji L, Chen Y. TSPO Modulates IL-4-Induced Microglia/Macrophage M2 Polarization via PPAR-γ Pathway. J Mol Neurosci 2020;70:542-9. 10.1007/s12031-019-01454-1 [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Ying M, Gu S, et al. Cysteinyl Leukotriene Receptor 2 is Involved in Inflammation and Neuronal Damage by Mediating Microglia M1/M2 Polarization through NF-κB Pathway. Neuroscience 2019;422:99-118. 10.1016/j.neuroscience.2019.10.048 [DOI] [PubMed] [Google Scholar]
- 8.Al Mamun A, Yu H, Mirza MA, et al. Myeloid cell IRF4 signaling protects neonatal brains from hypoxic ischemic encephalopathy. Neurochem Int 2019;127:148-57. 10.1016/j.neuint.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Zhang Y, Wang Q, et al. Interleukin-35 in immune-related diseases: protection or destruction. Immunology 2019;157:13-20. 10.1111/imm.13044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo M, Peng H, Chen P, et al. The immunomodulatory role of interleukin-35 in fibrotic diseases. Expert Rev Clin Immunol 2019;15:431-9. 10.1080/1744666X.2019.1564041 [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Lin Y, Li C, et al. IL-35 Decelerates the Inflammatory Process by Regulating Inflammatory Cytokine Secretion and M1/M2 Macrophage Ratio in Psoriasis. J Immunol 2016;197:2131-44. 10.4049/jimmunol.1600446 [DOI] [PubMed] [Google Scholar]
- 12.Xu C, Zhu H, Shen R, et al. IL-35 is a Protective Immunomodulator in Brain Ischemic Injury in Mice. Neurochem Res 2018;43:1454-63. 10.1007/s11064-018-2560-5 [DOI] [PubMed] [Google Scholar]
- 13.Kang M, Choi JK, Jittayasothorn Y, et al. Interleukin 35-Producing Exosomes Suppress Neuroinflammation and Autoimmune Uveitis. Front Immunol 2020;11:1051. 10.3389/fimmu.2020.01051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y. Role of HIF-1a in regulating autophagic cell survival during cerebral ischemia reperfusion in rats. Oncotarget 2017;8:98482-94. 10.18632/oncotarget.21445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzhalilova DS, Kosyreva AM, Diatroptov ME, et al. Dependence of the severity of the systemic inflammatory response on resistance to hypoxia in male Wistar rats. J Inflamm Res 2019;12:73-86. 10.2147/JIR.S194581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research (2010) Guide for the Care and Use of Laboratory Animals. Prepublication Draft. 8th Edition, The National Academies Press, Washington DC, 6, 47. [Google Scholar]
- 17.Chen D, Dixon BJ, Doycheva DM, et al. IRE1α inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J Neuroinflammation 2018;15:32. 10.1186/s12974-018-1077-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A, Xu Y, Yuan J. Ginkgolide B ameliorates NLRP3 inflammasome activation after hypoxic-ischemic brain injury in the neonatal male rat. Int J Dev Neurosci 2018;69:106-11. 10.1016/j.ijdevneu.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 19.Liu LL, Qiao S, Wang ML, et al. MiR224-5p Inhibitor Restrains Neuronal Apoptosis by Targeting NR4A1 in the Oxygen-Glucose Deprivation (OGD) Model. Front Neurosci 2020;14:613. 10.3389/fnins.2020.00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B, Huang B, Hu G, et al. Isovitexin-Mediated Regulation of Microglial Polarization in Lipopolysaccharide-Induced Neuroinflammation via Activation of the CaMKKβ/AMPK-PGC-1α Signaling Axis. Front Immunol 2019;10:2650. 10.3389/fimmu.2019.02650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu R, Liao XY, Pan MX, et al. Glycine Exhibits Neuroprotective Effects in Ischemic Stroke in Rats through the Inhibition of M1 Microglial Polarization via the NF-κB p65/Hif-1α Signaling Pathway. J Immunol 2019;202:1704-14. 10.4049/jimmunol.1801166 [DOI] [PubMed] [Google Scholar]
- 22.Cardinali DP. An Assessment of Melatonin's Therapeutic Value in the Hypoxic-Ischemic Encephalopathy of the Newborn. Front Synaptic Neurosci 2019;11:34. 10.3389/fnsyn.2019.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu MY, Yiang GT, Liao WT, et al. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol Biochem 2018;46:1650-67. 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Huang Q, Hu Z, et al. Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front Neurosci 2019;13:1036. 10.3389/fnins.2019.01036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel AR, Ritzel R, McCullough LD, et al. Microglia and ischemic stroke: a double-edged sword. Int J Physiol Pathophysiol Pharmacol 2013;5:73-90. [PMC free article] [PubMed] [Google Scholar]
- 26.Le K, Chibaatar Daliv E, Wu S, et al. SIRT1-regulated HMGB1 release is partially involved in TLR4 signal transduction: A possible anti-neuroinflammatory mechanism of resveratrol in neonatal hypoxic-ischemic brain injury. Int Immunopharmacol 2019;75:105779. 10.1016/j.intimp.2019.105779 [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Wang J, Li H, et al. IL-35 promotes microglial M2 polarization in a rat model of diabetic neuropathic pain. Arch Biochem Biophys 2020;685:108330. 10.1016/j.abb.2020.108330 [DOI] [PubMed] [Google Scholar]
- 28.Torres-Cuevas I, Corral-Debrinski M, Gressens P. Brain oxidative damage in murine models of neonatal hypoxia/ischemia and reoxygenation. Free Radic Biol Med 2019;142:3-15. 10.1016/j.freeradbiomed.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 29.Li C, Bian Y, Feng Y, et al. Neuroprotective Effects of BHDPC, a Novel Neuroprotectant, on Experimental Stroke by Modulating Microglia Polarization. ACS Chem Neurosci 2019;10:2434-49. 10.1021/acschemneuro.8b00713 [DOI] [PubMed] [Google Scholar]
- 30.Velagapudi R, Jamshaid F, Lepiarz I, et al. The tiliroside derivative, 3-O-[(E)-(2-oxo-4-(p-tolyl) but-3-en-1-yl] kaempferol produced inhibition of neuroinflammation and activation of AMPK and Nrf2/HO-1 pathways in BV-2 microglia. Int Immunopharmacol 2019;77:105951. 10.1016/j.intimp.2019.105951 [DOI] [PubMed] [Google Scholar]
- 31.Yu LM, Zhang WH, Han XX, et al. Hypoxia-Induced ROS Contribute to Myoblast Pyroptosis during Obstructive Sleep Apnea via the NF-κB/HIF-1α Signaling Pathway. Oxid Med Cell Longev 2019;2019:4596368. 10.1155/2019/4596368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korbecki J, Kojder K, Kapczuk P, et al. The Effect of Hypoxia on the Expression of CXC Chemokines and CXC Chemokine Receptors-A Review of Literature. Int J Mol Sci 2021;22:843. 10.3390/ijms22020843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B, Dasgupta C, Huang L, et al. MiRNA-210 induces microglial activation and regulates microglia-mediated neuroinflammation in neonatal hypoxic-ischemic encephalopathy. Cell Mol Immunol 2020;17:976-91. 10.1038/s41423-019-0257-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang L, Li X, Zhang Y, et al. Microarray and bioinformatics analyses of gene expression profiles in BALB/c murine macrophage polarization. Mol Med Rep 2017;16:7382-90. 10.3892/mmr.2017.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as