Abstract
Microplastics can act as carriers of heavy metals and may enter humans through ingestion and threaten human health. However, the bioaccessibility of heavy metals associated with microplastics and its implications for human health risk assessments are poorly understood. Therefore, in this study, four typical heavy metals (As(V), Cr(VI), Cd(II), and Pb(II)) and one typical microplastic (polyvinyl chloride, PVC) were chosen to estimate the human health risk of microplastic-associated heavy metals by incorporating bioaccessibility. Significant adsorption of heavy metals was observed with the following order for adsorption capacity: Pb(II) > Cr(VI) > Cd(II) > As(V); the efficiencies for desorption of these four heavy metals from PVC microplastics were all below 10%. The Fourier transform infrared spectroscopy results indicated that the functional groups on the surface of the virgin PVC microplastics did not play an important role in the capture process. Heavy metals in both gastric and small intestinal phases were prone to release from PVC microplastics when bioaccessibility was evaluated with the in vitro SBRC (Soluble Bioavailability Research Consortium) digestion model. In addition, Pb(II) bioaccessibility in the gastric phase was significantly higher than those in the other phases, while As(V), Cr(VI), and Cd(II) bioaccessibilities showed the opposite trend. After incorporating bioaccessibility adjustments, the noncarcinogenic hazards and carcinogenic risks determined were lower than those based on total metal contents. The individual hazard quotients (HQ) and carcinogenic risks (CR) for ingestion of these four heavy metals from PVC microplastics were all lower than the threshold values for adults and children. In summary, this study will provide a new view of the human health risks of heavy metals associated with microplastics.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-022-20983-8.
Keywords: Microplastics, Heavy metals, Bioaccessibility, In vitro model, Human health risk assessment
Introduction
Plastics have been extensively used in a wide array of applications all over the world due to their durability, cost-effectiveness, and versatility. However, long-term and large-scale application of plastics and insufficient management and disposal of their wastes have released enormous amounts of these polymers into the environment and caused worldwide plastic pollution (Gallo et al. 2018). Subsequently, plastics can be broken down into tiny particles by a range of aging factors, such as UV radiation, physical weathering, biodegradation, and heat stress in the environment (Luo et al. 2022). Furthermore, the small particles of plastics are also anthropogenically produced for personal care, household, and industrial materials (Fendall and Sewell 2009). These synthetic solid particles or polymeric matrices with sizes ranging from 1 μm to 5 mm and of either primary or secondary manufacturing origin are defined as microplastics, and microplastic pollution has gained global attention in recent years (Frias et al. 2019).
Currently, microplastics are ubiquitous in almost all matrices of the environment (Cao et al. 2021). Moreover, plastics usually contain various hazardous chemical ingredients and can adsorb a variety of contaminants from the surrounding environment (Khalid et al. 2021). Among the toxic contaminants adsorbed on microplastics, heavy metals are key inorganic contaminants, and they exhibit biotoxicity enrichment and difficult biodegradation (Gupta et al. 2020; Deore et al. 2021). Recently, both environmental monitoring projects and laboratory experiments have confirmed that microplastics have high affinities for heavy metals (Cao et al. 2021; Guo et al. 2021; Khalid et al. 2021). Most of the existing studies have focused on the behaviors, influencing factors, and mechanisms of heavy metal adsorption onto microplastics (Gao et al. 2021; Kutralam-Muniasamy et al. 2021; Liu et al. 2022; Luo et al. 2022). Moreover, microplastics were recently reported to act as a vector for heavy metals in aquatic and terrestrial systems and increase the chance of their bioaccumulation in organisms (Brennecke et al. 2016; Bradney et al. 2019; Abbasi et al. 2020; Godoy et al. 2019; Naqash et al. 2020). Furthermore, desorption of heavy metals from microplastics in the environments and guts of organisms that inadvertently ingest microplastics can cause microplastics to pose more risks of heavy metals (Munier and Bendell 2018; Zhu et al. 2018). In particular, significant exposure of humans to microplastics has been verified with water, food, and so on (Cox et al. 2019; Ebere et al. 2019; Smith et al. 2018). It could be easily expected that microplastics could provide a pathway for transfer of heavy metals from the environment to the food chain and even to human bodies. Hence, the human health risks of heavy metals associated with microplastics should be considered.
Classical determinations of human health risks from heavy metals are usually based on the total content of heavy metals and use of the human health risk assessment (HHRA) model developed by the US Environmental Protection Agency (USEPA 1996) and the Dutch National Institute of Public Health and Environmental Protection (Van den Berg 1995). However, an assessment based on total content may lead to overestimation of the health risk and may also identify high-risk sources and priority pollutants inaccurately (Liu et al. 2019). This is mainly because only some of the total heavy metal content ingested is bioaccessible to humans. In particular, previous studies have clearly shown that only some of the heavy metal content is desorbed from microplastics in the guts of marine birds (Holmes et al. 2020) as well as terrestrial invertebrates (Hodson et al. 2017). To this end, bioaccessibility of heavy metals derived from in vitro simulation of the human gastrointestinal tract is considered reliable in quantifying the risk to organisms of the presence of microplastics. However, information on the bioaccessibility of microplastic-associated heavy metals is quite limited (Liao et al. 2020; Godoy et al. 2020), as is information on the implications for human health risk assessments.
Arsenic, chromium, cadmium, and lead are the most common toxic heavy metals, which can enter the food web causing a threat to human health. Therefore, in this study, these four typical heavy metals (As(V), Cr(VI), Cd(II), and Pb(II)) and one typical microplastic (polyvinyl chloride, PVC) were chosen to incorporate the bioaccessibility of heavy metals into a human health risk assessment for microplastic-associated heavy metals. First, the adsorption and desorption behaviors of these four heavy metals with PVC microplastics were investigated. Second, the bioaccessibility of heavy metals from PVC microplastics was determined by introducing the in vitro SBRC (Soluble Bioavailability Research Consortium) model. Finally, both the noncarcinogenic hazard and carcinogenic risk for heavy metals were assessed by incorporating bioaccessibility.
Materials and methods
Microplastic particles
PVC microplastics with a mean particle diameter of 150 μm were purchased from Dongguan Qingtian Plastic Products Co., Ltd. (Guangdong, China). The microplastics were purified by shaking with 10% HNO3 (liquid–solid ratio = 1:50 w/v) for 48 h and then treated with ultrasound for 30 min to remove potential heavy metals on the surface. After separation with a filter paper (medium speed, pore size 30–50 μm), microplastics were air-dried and then stored for subsequent experiments.
Adsorption experiments
Adsorption experiments were carried out to simulate adsorption of the heavy metals onto microplastic in natural water. First, 100 μg·L−1 stock solutions with the pH of 7.0 of four heavy metals (As(V), Cr(VI), Cd(II), and Pb(II)) were separately prepared by dilution of the 1000 μg·L−1 standard solutions. Second, PVC microplastics were added to the heavy metal stock solutions (1:300 w/v) in 50-mL centrifuge tubes and then shaken at 150 rpm and 25 °C (Liao et al., 2020). Six time points (1, 3, 6, 12, 24, and 48 h) were chosen to determine the equilibrium time for adsorption of heavy metals on PVC microplastics. Third, the solutions were centrifuged at 4000 rpm for 10 min and then filtered through 0.45-μm filters. Finally, the concentrations of heavy metals in the filtrates were determined with an inductively coupled plasma-mass spectrometer (ICP–MS; Agilent 7800, Agilent Technologies, USA) at the Instrumental Analysis Center of Huaqiao University. The detection limits for As, Cr, Cd, and Pb were 0.01523, 0.01814, 0.01340, and 0.01225 μg·L−1, respectively. As a control, adsorption studies with PVC microplastics were conducted in ultra-pure water under the same conditions. All treatments were conducted in triplicate. The adsorption capacities of PVC for As(V), Cr(VI), Cd(II), and Pb(II) were calculated based on the following Eq. (1):
| 1 |
where qe is the amount of heavy metals adsorbed on PVC microplastics at a predetermined time (μg·g−1); C0 and Ct are the initial and equilibrium concentrations of heavy metals (μg·L−1); V is the volume of the solution (L); and W is the mass of PVC microplastics (g).
In addition, a scaled-up study of heavy metal (As(V), Cr(VI), Cd(II), and Pb(II)) adsorption on PVC microplastics was also conducted to prepare heavy metal-loaded microplastics for subsequent desorption and in vitro digestion studies. The adsorption time was set as the determined equilibrium time to ensure maximum As(V), Cr(VI), Cd(II), and Pb(II) adsorption on PVC microplastics. Scaled-up adsorption in ultra-pure water was also conducted for control. Furthermore, the PVC microplastics before and after As(V), Cr(VI), Cd(II), and Pb(II) adsorption were characterized by Fourier transform infrared spectroscopy (FTIR, Nicolet iS50, Thermo Fisher, USA) to better understand the adsorption processes.
Desorption experiments
To estimate release of the four heavy metals from polluted PVC microplastics, As(V), Cr(VI), Cd(II), and Pb(II)-loaded PVC microplastics were added to 0.01 M CaCl2 solution (1:100 w/v) and then shaken at 150 rpm at 25 °C. The desorption time was set as 24 h, which was equal to the duration of food digestion in the normal human gut, to make the desorption results comparable (Liao et al. 2020). After filtration through 0.45-μm filters, the balanced solutions were analyzed for As, Cr, Cd, and Pb by using ICP–MS. The desorption amounts were calculated based on Eq. (2):
| 2 |
where qe’ is the amount of heavy metals desorbed from PVC microplastics (μg·g−1); Ct is the concentration of As(V), Cr(VI), Cd(II), and Pb(II) in the solution at 24 h (μg·L−1); V is the volume of the solution (L); and W is the mass of microplastics (g).
Bioaccessibility of heavy metals on PVC microplastics
Bioaccessibilities of the four heavy metals on PVC microplastics were determined using in vitro SBRC gastrointestinal models, including both the gastric and small intestinal phases (Feng et al. 2018). Gastric juice was prepared by dissolving 45.045 g of glycine in 1500 mL ultra-pure water, and the pH was subsequently adjusted to 1.5 by dropwise addition of HCl. For juice from the small intestine, a high concentration of concentrated solution was prepared (0.875 g cholate and 2.5 g trypsin were added to 10 mL). The pH of the solution was adjusted to 7.0 with saturated sodium carbonate solution (Na2CO3) (Wen et al. 2020). Briefly, the simulated in vitro digestion process was initiated by adding 4 mL of artificial gastric juice to 0.04 g of PVC microplastics, and the mixture was shaken at 37 °C and 100 rpm for 1 h to represent digestion in the gastric phase. Then, 80 μL of highly concentrated small intestine solution was added to the mixture, and the mixture was further shaken at 37 °C and 100 rpm for another 4 h to represent digestion in the small intestine. After digestion, the mixture was centrifuged at 12,000 rpm for 2 min, and the supernatants were filtered through a 0.45-μm filter. Heavy metal concentrations were measured with ICP–MS. The bioaccessibility of heavy metals was calculated as the percentage of the metal contents in the soluble fraction relative to the total concentration on the microplastics (Ruby et al. 1999) using Eq. (3):
| 3 |
where BA is the bioaccessibility (%), Cs is the dissolved concentration of heavy metals in the gastric or small intestinal phase (μg·g−1), and Ctotal is the total concentration of heavy metals on microplastics (μg·g−1).
Health risk assessment of heavy metals on PVC
To evaluate the human health hazard of the four heavy metals on PVC microplastics, both the noncarcinogenic and carcinogenic risks resulting from ingestion were assessed with the modified human health risk assessment (HHRA) model based on bioaccessibility (Yu and Yang 2019). For the risk assessment, the average daily intake (ADD, mg·kg−1·day−1) of heavy metals on PVC microplastics was first calculated using Eq. (4):
| 4 |
where c is the total concentration of the four heavy metals on PVC microplastics (mg·kg−1); IngR is the ingestion rate, which was estimated as 714 mg·day−1 and 449 mg·day−1 for adults and children, respectively; EF is the exposure frequency, which was set as 350 day−1; and ED is the exposure duration. According to the Ministry of Ecology and Environment of the People’s Republic of China, the exposure durations were set as 6 years and 24 years for children and adults, respectively; BW is the average body weight, which was set as 18.9 kg for children and 58.3 kg for adults; AT is the averaging time (for noncarcinogenic risk: ED × 365 days; for carcinogenic risk: 70 × 365 days); and 1 × 10−6 is the conversion factor (Table S1).
The noncarcinogenic risks due to ingestion of As(V), Cr(VI), Cd(II), and Pb(II) on PVC microplastics were assessed based on the hazard quotient (HQ), which was calculated using Eq. (5):
| 5 |
where ADD is the average daily intake of heavy metals on PVC microplastics (mg kg−1 day−1); BA is the bioaccessibility of As(V), Cr(VI), Cd(II), and Pb(II) on PVC microplastics; and RfD is the reference dose of daily intake. The RfDs for As(V), Cr(VI), Cd(II), and Pb(II) are 3.0 × 10−4, 3.0 × 10−3, 1.0 × 10−3, and 3.5 × 10−3 mg·(kg·day)−1, respectively (Table S2). When the HQ value < 1, there is no noncarcinogenic risk to humans. The HQ value ≥ 1 indicates that the level of exposure is likely to cause chronic noncarcinogenic risks (USEPA 2016).
Since only As(V) and Cr(VI) were considered carcinogenic through ingestion, the carcinogenic risk (CR) for ingestion of As(V) and Cr(VI) on PVC microplastics was calculated using Eq. (6):
| 6 |
where ADD is the average daily intake of the four heavy metals on PVC microplastics (mg kg−1 day−1); BA is the bioaccessibility of As(V) and Cr(VI) on PVC microplastics; and SF is the carcinogenic slope factor. The SFs for As(V) and Cr(VI) are 1.5 and 5.0 10−1 kg·day·mg−1, respectively (Table S2). When the CR value < 1.0 × 10−6, there is no carcinogenic risk to humans. When CR > 1.0 × 10−4, there is carcinogenic risk to humans. The CR value between 1.0 × 10−6 ~ 1.0 × 10−4 indicates a carcinogenic risk within the acceptable range.
Statistical analysis
All experiments were conducted in triplicate, and all the data are presented as the means ± standard deviation (SD). Analysis of variance (ANOVA) was performed with SPSS 20.0. All illustrations were drawn with Origin 2018.
Results and discussion
Adsorption and desorption of heavy metals on PVC microplastics
The adsorption capacities of PVC microplastics for heavy metals (As(V), Cr(VI), Cd(II), and Pb(II)) at different adsorption times are shown in Fig. 1. The amounts of these four heavy metals adsorbed by the PVC microplastics increased rapidly in the initial phase (1 ~ 6 h). The adsorption amounts did not increase significantly over time after 24 h, indicating that dynamic equilibrium was achieved. These results revealed that the interactions between heavy metals and PVC microplastics were relatively fast, which was possibly because the heavy metals were mainly adsorbed on the outer surfaces of the microplastic particles (Gao et al. 2019) and the even and chemically clean surfaces of virgin microplastics (Liao et al. 2020). Similarly, many other studies also noted that adsorption of heavy metals (e.g., Cd(II) and Pb(II)) by microplastics reached equilibrium within 24 h (Lang et al. 2020; Liao et al. 2020; Tang et al. 2020; Shen et al. 2021). In addition, the maximum capacity for adsorption of Pb (1.869 μg·g−1) on PVC microplastics was significantly higher than those of the other three heavy metals (Cr (1.184 μg·g−1), Cd (1.117 μg·g−1), and As (0.833 μg·g−1)) (t test, p < 0.05). Metal adsorption presumably proceeded via electrostatic interactions between the metal ions and charged or polar regions of the microplastic surface (Holmes et al. 2012). Usually, As(V) and Cr(VI) exist as oxyanions (e.g., AsO43− and CrO42−), while Cd(II) and Pb(II) exist as bivalent cations (e.g., Pb2+ and Cd2+). In addition, the low zeta potential of PVC microplastics was indicated in previous reports, which showed negatively charged microplastic surfaces (Jiang et al. 2020; Wang et al. 2020). Therefore, it is reasonable to expect microplastics with negative charges to facilitate adsorption of cationic metals due to electrostatic attractions (Gao et al. 2021). Furthermore, the electrostatic adsorption affinities of heavy metals with equal charges are inversely related to the hydrated ion radius (Saha et al. 2002; Covelo et al. 2011). Therefore, Pb2+, which has a smaller hydrated ionic radius (0.401 nm) than Cd2+ (0.426 nm), unsurprisingly showed a higher capacity for adsorption on PVC microplastics (Zou et al. 2020). To better understand the adsorption mechanisms, and especially the interactions between metals and PVC microplastics, FTIR spectra for PVC microplastics were compared with those determined after loading As(V), Cr(VI), Cd(II), and Pb(II) (Figure S1). The appearance of a series of peaks between 500 and 3000 cm−1 suggested numerous C-H stretching and C–Cl stretching vibrations of the PVC microplastics (Table S3). However, there was no significant difference in the FTIR spectra of PVC microplastics measured before and after capture of As(V), Cr(VI), Cd(II), and Pb(II), which could indicate that the functional groups on the surface of virgin PVC microplastics did not play an important role in the capture process. However, the capture process of natural-aged microplastics was mainly controlled by the organic films containing oxygen functional groups rather than their own structures, which may explain why the heavy metal adsorption capacities determined herein for virgin microplastics were lower than those of a previous study using aged microplastics (Fu et al. 2021).
Fig. 1.
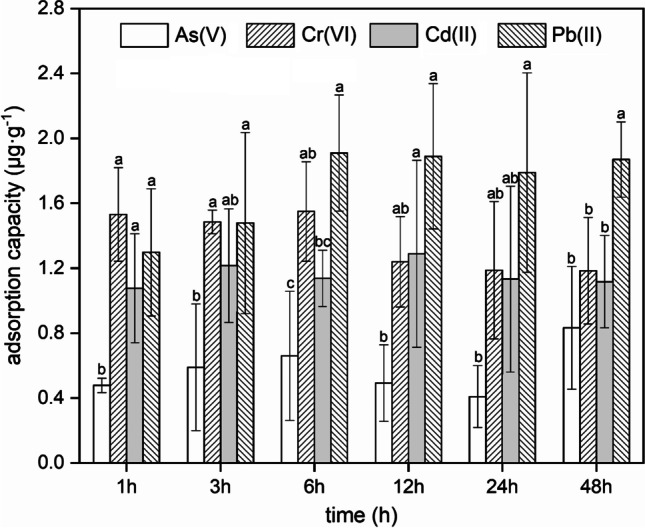
The adsorption capacity of heavy metals on PVC microplastics. Error bars represent the standard deviation of triplicates. Values with different letters differ significantly (p<0.05)
Desorption capacities of these four heavy metals from PVC microplastics were also determined in CaCl2 solution. As shown in Fig. 2, after 24 h of desorption, the amounts of As(V), Cr(VI), Cd(II), and Pb(II) released from PVC microplastics were 0.010 μg·g−1, 0.023 μg·g−1, 0.009 μg·g−1, and 0.155 μg·g−1, respectively. Notably, the heavy metal desorption ratios for all these heavy metals were less than 10%. Therefore, the high adsorption and low desorption capacities for heavy metals on PVC microplastics suggested that PVC may be capable of accumulating heavy metals effectively and carrying them into the environment and even transferring them along the food chain. These results were in agreement with those of previous studies showing that microplastics can act as a vector for heavy metals and increase levels in organisms within both aquatic and terrestrial environments, which may result in the additional potential for toxicity (Brennecke et al. 2016; Hodson et al. 2017; Godoy et al. 2019). Furthermore, the bioaccessibilities of heavy metals adsorbed on PVC microplastics were further determined by simulating a human gastrointestinal tract to develop a more accurate human health risk assessment, and this is discussed in the following sections.
Fig. 2.
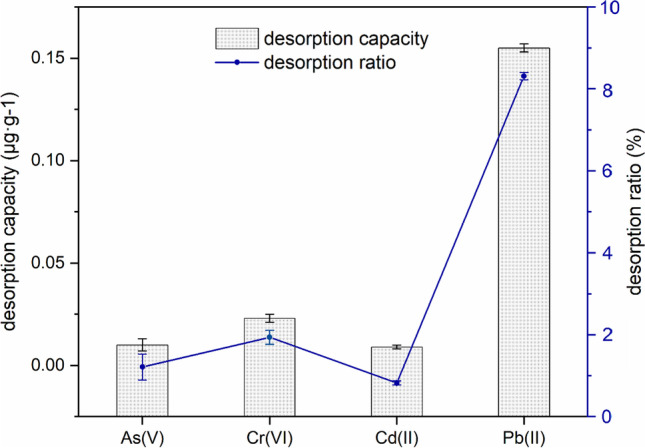
The desorption capacity of heavy metals from PVC microplastics. Error bars represent the standard deviation of triplicates
Bioaccessibility of heavy metals from PVC microplastics
The bioaccessibilities of heavy metals (As(V), Cr(VI), Cd(II), and Pb(II)) from PVC microplastics were estimated by using both gastric and small intestinal phases of the SBRC assay. As shown in Fig. 3, the average As, Cr, Cd, and Pb bioaccessibilities in PVC microplastics in both the gastric phase and small intestinal phase were higher than the corresponding desorption ratios in the CaCl2 solution. This suggested that PVC microplastics were prone to release more heavy metals into the gastrointestinal fluid than into the CaCl2 solution, and they could thus serve as vectors transporting heavy metals into the human digestive system. Similarly, desorption of both inorganic (e.g., Zn) and organic pollutants (e.g., phenanthrene, perfluorooctanoic acid, and ethylhexyl phthalate) was enhanced under simulated gut conditions (Bakir et al. 2014; Hodson et al. 2017). Remarkably, significant increases (p < 0.05) in As, Cr, and Cd and a decrease (p < 0.05) in Pb bioaccessibility were observed from the gastric phase (pH 1.5) to the small intestinal phase (pH 7.0) of the SBRC assay. Since Cr(VI) and As(V) compounds existed as oxyanions and were mainly negatively charged, the negative potential on the surface of PVC microplastics would be increased with a higher solution pH. Therefore, the higher pH in juice from the small intestine could inactivate the adherence of As(V) and Cr(VI) on PVC microplastics and lead to extensive As(V) and Cr(VI) desorption from the surface, which may be the dominant reason for the increased As and Cr bioaccessibility in neutral intestinal conditions (Jiang et al. 2020; Wang et al. 2020). Similarly, the capacity for adsorption of Cr(VI) on PE microplastics also decreased as the pH was adjusted from 1.6 to 8.9 (Zhang et al. 2020). In contrast, the higher Pb bioaccessibility in the gastric phase could be attributed to the high acid condition, which may enhance adsorption of Pb2+ cations on PVC microplastics. More importantly, as a cation, Pb could easily precipitate under neutral intestinal conditions. In particular, the solubility of Pb acetate was reported to decrease from 100% at pH 1.5 in the gastric phase to 14% at pH 7.0 in the small intestine phase of the SBRC assay (Juhasz et al. 2009). Thus, Pb precipitation is likely responsible for the decreased bioaccessibility of Pb on PVC microplastics, as indicated by the small intestinal phase of the SBRC assay. However, although Cd(II) also existed as a divalent cation, Cd bioaccessibility increased in the small intestinal phase of the SBRC assay, which suggested that the increase in pH may not cause a significant decrease in the capacity of PVC microplastics to adsorb Cd2+ cations. In accordance with this, Zhou et al. (2020) reported that Cd(II) adsorption onto microplastics first increased and then gradually decreased with increasing solution pH, and charge repulsion may have inhibited adsorption. Additionally, fluid for the small intestine phase consisted of bile and pancreatin, which can serve as biosurfactants and complexants to dissolve Cr and increase its bioaccessibility in the small intestine (Maldonado-Valderrama et al. 2011; Zhang et al. 2018).
Fig. 3.
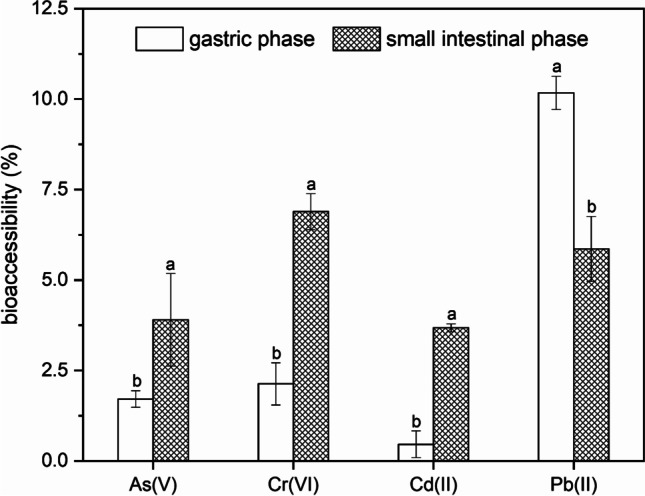
Bioaccessibility of heavy metals from PVC microplastics in the gastric and small intestinal phases of SBRC model. Error bars represent the standard deviation of triplicates. Values with different letters differ significantly (p<0.05)
Human health risk assessment for heavy metals from PVC microplastics
Since PVC microplastics can transport heavy metals to human digestive systems, both the noncarcinogenic hazards and carcinogenic risks were characterized for children and adults (Table 1).
Table 1.
Noncarcinogenic hazard quotient (HQ) and carcinogenic risk (CR) of heavy metals on PVC microplastics
| Adults | Children | |||||
|---|---|---|---|---|---|---|
| Noncarcinogenic risk | ||||||
| HQT | HQB-GP | HQB-IP | HQT | HQB-GP | HQ B-IP | |
| As | 3.26 × 10−2 | 5.58 × 10−4 | 1.27 × 10−3 | 6.32 × 10−2 | 1.08 × 10−3 | 2.47 × 10−3 |
| Cr | 4.63 × 10−3 | 9.86 × 10−5 | 3.19 × 10−4 | 8.99 × 10−3 | 1.91 × 10−4 | 6.19 × 10−4 |
| Cd | 1.31 × 10−2 | 6.05 × 10−5 | 4.82 × 10−4 | 2.54 × 10−2 | 1.17 × 10−4 | 9.35 × 10−4 |
| Pb | 6.27 × 10−3 | 6.38 × 10−4 | 3.68 × 10−4 | 1.22 × 10−2 | 1.24 × 10−3 | 7.13 × 10−4 |
| Carcinogenic risk | ||||||
| CRT | CRB-GP | CRB-IP | CRT | CRB-GP | CRB-IP | |
| As | 5.03 × 10−6 | 8.61 × 10−8 | 1.96 × 10−7 | 2.44 × 10−6 | 4.17 × 10−8 | 9.52 × 10−8 |
| Cr | 2.38 × 10−6 | 5.07 × 10−8 | 1.64 × 10−7 | 1.16 × 10−6 | 2.46 × 10−8 | 7.96 × 10−8 |
Note: HQT, HQB-GP, and HQB-IP are the noncarcinogenic risk based on total (HQT) and bioaccessible concentrations in the gastric (HQB-GP) and small intestinal phases (HQB-IP) of heavy metals on PVC microplastics, respectively. The values for noncarcinogenic risk larger than the threshold value (1) were marked in bold. CRT, CRB-GP, and CRB-IP are the noncarcinogenic risk based on total (CRT) and bioaccessible concentrations in the gastric (CRB-GP) and small intestinal phases (CRB-IP) of heavy metals on PVC microplastics, respectively. The values for carcinogenic risk larger than the threshold value (10−6) were marked in bold
Although PVC microplastics showed the highest adsorption capacity for Pb, the noncarcinogenic HQs of individual heavy metals based on the total heavy metal concentrations from PVC microplastics decreased in the order As(V) > Cd(II) > Pb(II) > Cr(VI) due to the high toxicities of As(V) and Cd(II). Moreover, As(V), Cr(VI), Cd(II), and Pb(II) HQs for both adults and children were much lower than the safe level (< 1.0), indicating that ingestion of PVC microplastics containing As(V), Cr(VI), Cd(II), and Pb(II) will not pose a noncarcinogenic risk to humans. In determining carcinogenic risks, only As(V) and Cr(VI) were included. The mean CRs for adults were 5.03 × 10−6 for As(V) and 2.38 × 10−5 for Cr(VI), and those for children were 2.44 × 10−6 for As(V) and 1.16 × 10−5 for Cr(VI), which indicated that As contributed most to the overall cancer risk for children. Furthermore, since all of the mean CR values for both adults and children exceeded the precautionary criterion (10−6), the carcinogenic risks for oral ingestion of heavy metals, especially As(V), from microplastics need attention.
Furthermore, human health risks have been widely reported to be overestimated by using total heavy metal contents (Luo et al. 2012; Li et al. 2018; Liu et al. 2019; Ma et al. 2021); thus, risk assessments incorporating the in vitro bioaccessibility of heavy metals from PVC microplastics were also performed. It is not surprising that all the HQ and CR values based on the bioaccessible fractions in both gastric and small intestinal phases were lower than the corresponding values determined by using total concentrations. Interestingly, the order of HQs changed after adjustment with bioaccessibility in the gastric phase to Pb(II) > As(V) > Cr(VI) > Cd(II), and Pb was identified as the main driver of noncarcinogenic risk. This order could be explained by noting that Pb(II) had highest bioaccessibility in the gastric phase. Moreover, after incorporating the bioaccessibilities of heavy metals, all of the CR values for both adults and children were lower than the precautionary criterion (10−6), and the carcinogenic risk from each individual heavy metal was predicted to be negligible.
It must be noted that the negligible health risks determined in this study were based on the use of PVC microplastics from the laboratory adsorption experiment to simulate microplastic adsorption of heavy metals in natural water, and this was done to reduce interferences from other factors as much as possible. However, the different types, densities, shapes, and particle sizes of microplastics vary in the amounts of heavy metals they contain or can adsorb from the environment (Li et al. 2020; Naqash et al. 2020; Cao et al. 2021; Khalid et al. 2021). For instance, Pb concentrations determined for foamed polyurethane (PU) plastics from Whitsand Bay reached 16,000 μg·g−1 (Turner and Lau 2016), which may result in tremendous health risks. Furthermore, small, especially nanosized, plastic particles could be capable of absorbing higher concentrations of heavy metals and could also cross cell membranes and enter the circulatory systems of organisms (Davranche et al. 2019; Shen et al. 2019). Likewise, aging/weathering could also increase the adsorption capacity of microplastics for heavy metals (Wagner et al. 2014; Mao et al. 2020; Fu et al. 2021), which may lead to more significant health risks. Therefore, due to the complexity of microplastic-associated heavy metals, this study preliminarily explored the use of an in vitro gastrointestinal model for assessment of human health risks due to heavy metals from microplastics. A more comprehensive approach for evaluating the risks and hazard potentials of heavy metals adsorbed on microplastics with various properties should be considered from this perspective.
Conclusions
The results of this study revealed significant adsorption of heavy metals, especially Pb(II), on microplastics. Desorption of heavy metals from PVC microplastics was negligible in CaCl2 solution, which guaranteed accumulation of heavy metals on PVC microplastics. In the SBRC in vitro model, the heavy metals, especially Pb(II) in the gastric phase, tended to be desorbed more efficiently from PVC microplastics, and this indicated relatively high oral bioaccessibility of heavy metals. These results suggested that PVC microplastics could accumulate heavy metals in heavy metal-contaminated aquatic environments and then migrate and release them in the human digestive system. Moreover, carcinogenic risks were detected in the human health risk assessment for oral ingestion of both Cr(VI) and As(V) from microplastics when total contents were used, but no significant noncarcinogenic or carcinogenic risks were observed for heavy metals after incorporating bioaccessibility adjustments. Overall, this study provides insights into assessments of human health risks arising for heavy metals associated with microplastics. In particular, due to increased use of plastics in healthcare during the current COVID-19 pandemic, more representative microplastics with different properties (e.g., type, size, shape, and weathered/aging condition) and more realistic exposure scenarios should be taken into account to allow for better health risk assessments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Instrumental Analysis Center of Huaqiao University for analysis support.
Author contribution
Xue-juan Chen: methodology, investigation, writing original draft. Jin-jin Ma: investigation, resources. Rui-lian Yu: supervision, writing review and editing, funding acquisition. Gong-ren Hu: supervision, funding acquisition. Yu Yan: conceptualization, methodology, project administration, writing review and editing, funding acquisition.
Funding
This work was supported by the National Natural Science Foundation of China (42177103, 41807410, 21477042).
Data availability
Present study data are available with corresponding author and are available on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi S, Moore F, Keshavarzi B, Hopke PK, Naidu R, Rahman MM, Oleszczuk P, Karimi J. PET-microplastics as a vector for heavy metals in a simulated plant rhizosphere zone. Sci Total Environ. 2020;744:140984. doi: 10.1016/j.scitotenv.2020.140984. [DOI] [PubMed] [Google Scholar]
- Bakir A, Rowland SJ, Thompson RC. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ Pollut. 2014;185:16–23. doi: 10.1016/j.envpol.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Bradney L, Wijesekara H, Palansooriya KN, Obadamudalige N, Bolan NS, Ok YS, Rinklebe J, Kim KH, Kirkham MB. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ Int. 2019;131:104937. doi: 10.1016/j.envint.2019.104937. [DOI] [PubMed] [Google Scholar]
- Brennecke D, Duarte B, Paiva F, Caçador I, Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar Coast Shelf Sci. 2016;178:189–195. doi: 10.1016/j.ecss.2015.12.003. [DOI] [Google Scholar]
- Cao Y, Zhao M, Ma X, Song Y, Zuo S, Li H, Deng W. A critical review on the interactions of microplastics with heavy metals: mechanism and their combined effect on organisms and humans. Sci Total Environ. 2021;788:147620. doi: 10.1016/j.scitotenv.2021.147620. [DOI] [PubMed] [Google Scholar]
- Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ Sci Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517-. [DOI] [PubMed] [Google Scholar]
- Davranche M, Veclin C, Pierson-Wickmann AC, El Hadri H, Grassl B, Rowenczyk L, Dia A, Ter Halle A, Blancho F, Reynaud S, Gigault J. Are nanoplastics able to bind significant amount of metals? The lead example. Environ Pollut. 2019;249:940–948. doi: 10.1016/j.envpol.2019.03.087. [DOI] [PubMed] [Google Scholar]
- Deore MS, Keerthana S, Naqvi S, Kumar A, Flora SJS. Alpha-lipoic acid protects co-exposure to lead and zinc oxide nanoparticles induced neuro, immuno and male reproductive toxicity in rats. Front Pharmacol. 2021;12:626238. doi: 10.3389/fphar.2021.626238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebere EC, Wirnkor VA, Ngozi VE. Uptake of microplastics by plant: a reason to worry or to be happy? World Scientific News. 2019;131:256–267. [Google Scholar]
- Fendall LS, Sewell MA. Contributing to marine pollution by washing your face: microplastics in facial cleansers. Mar Pollut Bull. 2009;58:1225–1228. doi: 10.1016/j.marpolbul.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Frias JPGL, Nash R. Microplastics: finding a consensus on the definition. Mar Pollut Bull. 2019;138:145–147. doi: 10.1016/j.marpolbul.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Fu Q, Tan X, Ye S, Ma L, Gu Y, Zhang P, Chen Q, Yang Y, Tang Y. Mechanism analysis of heavy metal lead captured by natural-aged microplastics. Chemosphere. 2021;270:128624. doi: 10.1016/j.chemosphere.2020.128624. [DOI] [PubMed] [Google Scholar]
- Gallo F, Fossi C, Weber R, Santillo D, Sousa J, Ingram I, Nadal A, Romano D. Marine litter plastics and microplastics and their toxic chemicals components: the need for urgent preventive measures. Environ Sci Eur. 2018;30:1–14. doi: 10.1186/s12302-018-0139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Li J, Sun C, Zhang L, Jiang F, Cao W, Zheng L. Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar Pollut Bull. 2019;144:61–67. doi: 10.1016/j.marpolbul.2019.04.039. [DOI] [PubMed] [Google Scholar]
- Gao X, Hassan I, Peng Y, Huo S, Ling L. Behaviors and influencing factors of the heavy metals adsorption onto microplastics: a review. J Cleaner Prod. 2021;319:128777. doi: 10.1016/j.jclepro.2021.128777. [DOI] [Google Scholar]
- Godoy V, Blázquez G, Calero M, Quesada L, Martín-Lara MA. The potential of microplastics as carriers of metals. Environ Pollut. 2019;255:113363. doi: 10.1016/j.envpol.2019.113363. [DOI] [PubMed] [Google Scholar]
- Godoy V, Martinez-Ferez A, Martin-Lara MA, Vellido-Perez JA, Calero M, Blazquez G. Microplastics as vectors of chromium and lead during dynamic simulation of the human gastrointestinal tract. Sustainability. 2020;12:4792. doi: 10.3390/su12114792. [DOI] [Google Scholar]
- Guo X, Wang J. Projecting the sorption capacity of heavy metal ions onto microplastics in global aquatic environments using artificial neural networks. J Hazard Mater. 2021;402:123706. doi: 10.1016/j.jhazmat.2020.123709. [DOI] [PubMed] [Google Scholar]
- Gupta A, Kumar A, Naqvi S, Flora SJS. Chronic exposure to multi-metals on testicular toxicity in rats. Toxicol Mech Method. 2020;31:53–66. doi: 10.1080/15376516.2020.1828522. [DOI] [PubMed] [Google Scholar]
- Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol. 2017;51:4714–4721. doi: 10.1021/acs.est.7b00635. [DOI] [PubMed] [Google Scholar]
- Holmes LA, Thompson RC, Turner A. In vitro avian bioaccessibility of metals adsorbed to microplastic pellets. Environ Pollut. 2020;261:114107. doi: 10.1016/j.envpol.2020.114107. [DOI] [PubMed] [Google Scholar]
- Jiang M, Hu L, Lu A, Liang G, Lin Z, Zhang T, Xu L, Li B, Gong W. Strong sorption of two fungicides onto biodegradable microplastics with emphasis on the negligible role of environmental factors. Environ Pollut. 2021;267:115496. doi: 10.1016/j.envpol.2020.115496. [DOI] [PubMed] [Google Scholar]
- Juhasz AL, Weber J, Smith E, Naidu R, Marschner B, Rees M, Rofe A, Kuchel T, Sansom L. Evaluation of SBRC–gastric and SBRC–intestinal methods for the prediction of in vivo relative lead bioavailability in contaminated soils. Environ Sci Technol. 2009;43:4503–4509. doi: 10.1021/es803238u. [DOI] [PubMed] [Google Scholar]
- Khalid N, Aqeel M, Noman A, Khan SM, Akhter N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environment. Environ Pollut. 2021;290:118104. doi: 10.1016/j.envpol.2021.118104. [DOI] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G, Perez-Guevara F, Martinez IE, Shruti VC. Overview of microplastics pollution with heavy metals: analytical methods, occurrence, transfer risks and call for standardization. J Hazard Mater. 2021;415:125755. doi: 10.1016/j.jhazmat.2021.125755. [DOI] [PubMed] [Google Scholar]
- Lang M, Yu X, Liu J, Xia T, Wang T, Jia H, Guo X. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics. Sci Total Environ. 2020;722:137762. doi: 10.1016/j.scitotenv.2020.137762. [DOI] [PubMed] [Google Scholar]
- Li J, Huang W, Xu Y, Jin A, Zhang D, Zhang C. Microplastics in sediment cores as indicators of temporal trends in microplastic pollution in Andong salt marsh, Hangzhou Bay. China Reg Stud Mar Sci. 2020;35:101149. doi: 10.1016/j.rsma.2020.101149. [DOI] [Google Scholar]
- Li T, Song Y, Yuan X, Li J, Ji J, Fu X, Zhang Q, Guo S. Incorporating bioaccessibility into human health risk assessment of heavy metals in rice (Oryza sativa L.): a probabilistic-based analysis. J Agric Food Chem. 2018;66:5683–5690. doi: 10.1021/acs.jafc.8b01525. [DOI] [PubMed] [Google Scholar]
- Liao Y, Yang J. Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Sci Total Environ. 2020;703:134805. doi: 10.1016/j.scitotenv.2019.134805. [DOI] [PubMed] [Google Scholar]
- Liu S, Huang J, Zhang W, Shi L, Yi K, Yu H, Zhang C, Li S, Li J. Microplastics as a vehicle of heavy metals in aquatic environments: a review of adsorption factors, mechanisms, and biological effects. J Environ Manage. 2021;302:113995. doi: 10.1016/j.jenvman.2021.113995. [DOI] [PubMed] [Google Scholar]
- Liu X, Ouyang W, Shu Y, Tian Y, Feng Y, Zhang T, Chen W. Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ Pollut. 2019;254:113113. doi: 10.1016/j.envpol.2019.113113. [DOI] [PubMed] [Google Scholar]
- Luo H, Liu C, He D, Xu J, Sun J, Li J, Pan X. Environmental behaviors of microplastics in aquatic systems: a systematic review on degradation, adsorption, toxicity and biofilm under aging conditions. J Hazard Mater. 2021;423:126915. doi: 10.1016/j.jhazmat.2021.126915. [DOI] [PubMed] [Google Scholar]
- Luo X, Ding J, Xu B, Wang Y, Li H, Yu S. Incorporating bioaccessibility into human health risk assessments of heavy metals in urban park soils. Sci Total Environ. 2012;424:88–96. doi: 10.1016/j.scitotenv.2012.02.053. [DOI] [PubMed] [Google Scholar]
- Ma J, Yan Y, Chen X, Niu Z, Yu R, Hu G. Incorporating bioaccessibility and source apportionment into human health risk assessment of heavy metals in urban dust of Xiamen. China Ecotoxicol Environ Saf. 2021;228:112985. doi: 10.1016/j.ecoenv.2021.112985. [DOI] [PubMed] [Google Scholar]
- Maldonado-Valderrama J, Wilde P, Macierzanka A, Mackie A. The role of bile salts in digestion. Adv Colloid Interface Sci. 2011;165:36–46. doi: 10.1016/j.cis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Mao R, Lang M, Yu X, Wu R, Yang X, Guo X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J Hazard Mater. 2020;393:122525. doi: 10.1016/j.jhazmat.2020.122515. [DOI] [PubMed] [Google Scholar]
- Naqash N, Prakash S, Kapoor D, Singh R. Interaction of freshwater microplastics with biota and heavy metals: a review. Environ Chem Let. 2020;18:1813–1824. doi: 10.1007/s10311-020-01044-3. [DOI] [Google Scholar]
- Senathirajah K, Attwood S, Bhagwat G, Carbery M, Wilson S, Palanisami T. Estimation of the mass of microplastics ingested - a pivotal first step towards human health risk assessment. J Hazard Mater. 2021;404:124004. doi: 10.1016/j.jhazmat.2020.124004. [DOI] [PubMed] [Google Scholar]
- Shen M, Song B, Zeng G, Zhang Y, Teng F, Zhou C. Surfactant changes lead adsorption behaviors and mechanisms on microplastics. Chem Eng J. 2021;405:126989. doi: 10.1016/j.cej.2020.126989. [DOI] [Google Scholar]
- Shen M, Zhang Y, Zhu Y, Song B, Zeng G, Hu D, Wen X, Ren X. Recent advances in toxicological research of nanoplastics in the environment: a review. Environ Pollut. 2019;252:511–521. doi: 10.1016/j.envpol.2019.05.102. [DOI] [PubMed] [Google Scholar]
- Smith M, Love DC, Rochman CM, Neff RA. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018;5:375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Lin L, Wang X, Feng A, Yu A. Pb(II) uptake onto nylon microplastics: interaction mechanism and adsorption performance. J Hazard Mater. 2020;386:121960. doi: 10.1016/j.jhazmat.2019.121960. [DOI] [PubMed] [Google Scholar]
- Turner A, Lau KS. Elemental concentrations and bioaccessibilities in beached plastic foam litter, with particular reference to lead in polyurethane. Mar Pollut Bull. 2016;112:265–270. doi: 10.1016/j.marpolbul.2016.08.005. [DOI] [PubMed] [Google Scholar]
- USEPA (1996) Soil screening guidance: technical background document. Publication 9355:4-17A
- USEPA (2016) EPA ExpoBox (A Toolbox for Exposure Assessors): exposure assessment tools by routes. https://www.epa.gov/expobox
- Van den Berg R (1995) Human exposure to soil contamination: a qualitative and quantitative analysis towards proposals for human toxicological intervention values. National Institute of Public Health and Environmental Protection (RIVM), Bilthoven, the Netherlands.
- Wagner M, Scherer C, Alvarez-Munoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, Klasmeier J, Marti T. Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur. 2014;26:1–9. doi: 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang X, Zhang Y, Wang N, Wang Y, Meng G, Chen Y. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci Total Environ. 2020;749:141603. doi: 10.1016/j.scitotenv.2020.141603. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang L, Hua T, Li Y, Zhou X, Wang W, You Z, Wang H, Li M. The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment. Environ Pollut. 2020;257:113440. doi: 10.1016/j.envpol.2019.113440. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang Y, Liu G, He G, Liu W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: the roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020;184:116209. doi: 10.1016/j.watres.2020.116209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Present study data are available with corresponding author and are available on reasonable request.


